Abstract
• Background and Aims Polyploidization plays an important role in the evolution of many plant genera, including Koeleria. The knowledge of ploidy, chromosome number and genome size may enable correct taxonomic treatment when other features are insufficient as in Koeleria. Therefore, these characteristics and their variability were determined for populations of six central European Koeleria taxa.
• Methods Chromosome number analysis was performed by squashing root meristems, and ploidy and 2C nuclear DNA content were estimated by flow cytometry.
• Key Results Three diploids (K. glauca, K. macrantha var. macrantha and var. pseudoglauca), one tetraploid (K. macrantha var. majoriflora), one decaploid (K. pyramidata) and one dodecaploid (K. tristis) were found. The 2C nuclear DNA content of the diploids ranged from 4·85 to 5·20 pg. The 2C DNA contents of tetraploid, decaploid and dodecaploid taxa were 9·31 pg, 22·89 pg and 29·23 pg, respectively. The DNA content of polyploids within the K. macrantha aggregate (i.e. within K. macrantha and K. pyramidata) was smaller than the expected multiple of the diploid genome (K. macrantha var. macrantha). Geography-correlated variation of DNA content was found for some taxa. Czech populations of K. macrantha var. majoriflora had a 5·06 % smaller genome than the Slovak ones. An isolated eastern Slovakian population of K. tristis revealed 8·04 % less DNA than populations from central Slovakia. In central and north-west Bohemia, diploid and tetraploid cytotypes of K. macrantha were sympatric; east from this region diploid populations, and towards the west tetraploid populations were dominant.
• Conclusions Remarkable intra-specific inter-population differences in nuclear DNA content were found between Bohemian and Pannonian populations of Koeleria macrantha var. majoriflora and between geographically isolated central and eastern Slovakian populations of K. tristis. These differences occur over a relatively small geographical scale.
Keywords: Chromosome number, polyploidy, nuclear DNA content, genome size variation, flow cytometry, grasses, Poaceae, Koeleria
INTRODUCTION
The genus Koeleria Pers. comprises 35–40 species of perennial, xerophilous to mesophilous and commonly heliophilous grasses widely distributed in Europe, North America, Australia, New Zealand and non-tropical regions of Africa, Asia and South America (Meusel et al., 1965). Some Koeleria species play an important role in many plant communities. The most widely distributed K. macrantha (Ledeb.) Schult. is considered to be one of the essential species in the steppes of Eurasia and prairies of the North American Great Plains (Looman, 1978; Tzvelev, 1983; Arnow, 1994). Other species are considered to be rare and/or threatened, e.g. K. tristis Domin is endemic in the West Carpathians and K. arenaria (Dumort.) Conert is an endangered taxon restricted to the coastal sand dunes of western Europe (Soják and Chrtek, 1963; Conert, 1998).
Although Koeleria has been subjected to several taxonomic revisions during the 20th century, many essential systematic questions remain unanswered (Domin, 1907; Ujhelyi, 1972; Holub et al., 1972; Tzvelev, 1983; Molina, 1993). The main reason is the high morphological similarity of many taxa, especially in the K. macrantha aggregate (from the taxa discussed in this paper, the aggregate contains K. macrantha and K. pyramidata), accompanied by their large intraspecific phenotypic variability. Misunderstanding these facts and considering phenotypes as genotypes led some taxonomists to describe hundreds of intraspecific taxa (Domin, 1907; Ujhelyi, 1972), and thus provided the basis for many recent taxonomic problems. These problems seem to be very current, especially in central Europe, from where most of the taxa were described. Therefore this paper focuses in detail on this area. It also became obvious that for Koeleria a classification system based only on morphology was inadequate.
The basic chromosome number of Koeleria is x = 7. As shown in many studies, polyploidization has played a major role in the evolution of this genus. Karyologic analysis of approx. 20 taxa yielded a wide range of chromosome numbers 2n = 14, 28, 42, 56, 70, 84 representing ploidy levels from 2x to 12x (Fedorov, 1969; Holub et al., 1972; Ujhelyi, 1972; Arnow, 1994). In general, auto- and allopolyploidization can lead to genotype changes during plant evolution. As a consequence of genome duplications in polyploids, new phenotypes showing altered gene expression may arise (in part this is caused by gene dosage regulation). These novel types may differ from their progenitors and successfully occupy new niches (reviewed in Osborn et al., 2003). Chromosome number, ploidy and C-values (1C corresponds to the DNA content of the unreplicated reduced chromosome complement n, while the 1Cx value represents the DNA content of one non-replicated monoploid genome with the chromosome number x; Greilhuber et al., 2005) represent important characteristics for plants (reviewed in Bennett, 1998; Bennett et al., 2000). Moreover, genomic data can contribute in combination with morphological characters to intergeneric classification, taxa delimitation or hybrid identification (Zonneveld, 2001; Bureš et al., 2004; Morgan-Richards et al., 2004). For some plant groups positive correlations between nuclear DNA content and various features such as life-styles (Barow and Meister, 2002; Bureš et al., 2004; Jakob et al., 2004), environmental conditions and/or geographical distribution (reviewed in Price et al., 1988a, b; Ceccarelli et al., 1992; Bottini et al., 2000; Schmuths et al., 2004) were found.
This paper reports on the determination of chromosome numbers, flow cytometric analysis of 2C nuclear DNA content, and ploidy level of six central European Koeleria taxa to answer the following questions: (a) What is the nuclear genome size, ploidy level and chromosome number of investigated taxa? (b) What is the inter-population variability of the nuclear DNA content within the analysed taxa? (c) Is the variability positively correlated with their geographical distribution? (d) What are the implications for the taxonomic treatment of the genus?
MATERIALS AND METHODS
Experimental material
Six native Koeleria taxa from central Europe were examined: K. glauca (Spreng.) DC., K. macrantha (Ledeb.) Schult. subsp. macrantha var. macrantha, K. macrantha subsp. macrantha var. pseudoglauca (Schur) Trávnícek et Pecinka ined. (= K. gracilis var. glabra Domin, nom. illeg.), K. macrantha subsp. macrantha var. majoriflora (Borbás) Trávníček et Pecinka ined., K. pyramidata (Lam.) Beauv. and K. tristis Domin. Based on morphological characters, these taxa are placed into three—perhaps unnatural—lineages, usually treated as sections or subsections (Domin, 1905; Soják and Chrtek, 1963). Koeleria macrantha and K. pyramidata belong to nominal section Koeleria. Koeleria glauca belongs in subsection Glaucae Domin and K. tristis in subsection Splendentes Domin, both within section Bulbosae Domin. Classification of K. tristis as a synonym of K. pyramidata (cf. Humpries, 1980) is not acceptable. Both species can be distinguished on the basis of their morphology (K. tristis: leaf blades glabrous, serrate on the margins, dry leaf blades are convoluted, firm and nitid; K. pyramidata: leaf blades pubescent, with a prominent line of >2 mm long hairs on the sides, dry leaf blades are usually not convoluted, soft and glareless), have different chromosome numbers, ecology and geographical distribution. Koeleria tristis differs also from K. eriostachya Pančić (by looser panicles without shortened branches and by lemmas only rarely covered by long hairs). However, the exact relationship of K. tristis with other Koeleria taxa is unknown.
In total 286 plants from 126 populations were collected in Czech Republic, Germany, Hungary, Poland and Slovakia [Supplementary information; voucher herbarium specimens are deposited at the University of Olomouc (herbarium code: OL)]. The plants used for ploidy analysis were cultivated in the experimental garden of the Faculty of Biological Sciences, Palacký University, Olomouc. Plants that were used for chromosome number and nuclear DNA content analysis were grown in pots filled with perlite in a greenhouse at the Institute of Experimental Botany, Olomouc.
Chromosome counts
Young roots were incubated in a saturated solution of paradichlorbenzene in distilled water for 4 h, rinsed twice for 15 min in distilled water and fixed in ethanol–acetic acid (3 : 1) for at least 24 h. Fixed material was hydrolysed in 5 m HCl for 30 min and rinsed in distilled water. After Feulgen staining of chromosomes, roots were rinsed in distilled water, macerated in 10 % pectinase (Serva) for approx. 25 min at 37 °C and gently squashed in a drop of 45 % acetic acid. Chromosomes were counted in at least five intact metaphase plates per plant analysed.
Flow cytometric analysis
The analysis was performed by a Partec PAS II flow cytometer (Partec GmbH, Münster, Germany) equipped with a high pressure mercury arc lamp (100 W). Suspensions of intact nuclei were prepared by chopping approx. 0·05 g of basal parts of young leaves with a razor blade in 0·5 mL of Otto I buffer (Otto, 1990) and filtered through a 50-µm nylon mesh. Subsequently, 1 mL of Otto II buffer (Otto, 1990) containing propidium iodide and RNase (both 50 µg mL−1) was added. After incubation for 10 min at 20 °C, relative fluorescence intensity of nuclei was analysed. For the ploidy analysis, the sample of unknown ploidy was measured together with a plant with known chromosome number. For 2C nuclear DNA content estimation, each plant was measured at least three times together with Secale cereale L. var. cereale (‘Dankovske’, 2C = 16·19 pg; Doležel et al., 1998) as an internal standard (Fig. 1A). The position of nuclei G0/G1 peaks in histograms of relative fluorescence intensity was used to estimate nuclear DNA content. The 2C DNA content of Koeleria was calculated as:
 |
Fig. 1.
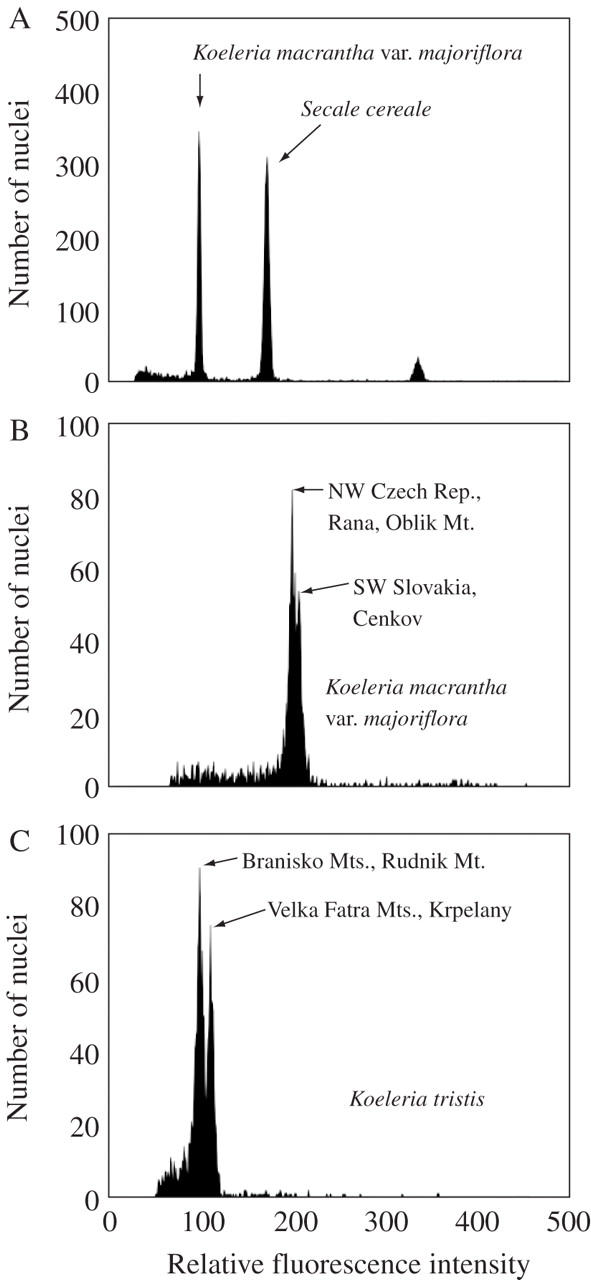
Histograms of relative fluorescence intensity obtained after analysis of propidium-iodide stained nuclei of: (A) Koeleria macrantha var. majoriflora and Secale cereale ‘Dankovske’ (2C = 16·19 pg), which served as internal reference standard; (B) K. macrantha var. majoriflora; and (C) K. tristis. The simultaneous analysis of material from two populations of K. macrantha var. majoriflora with significantly different genome sizes resulted in a double peak, confirming the results of individual measurements (B). The same was true for K. tristis (C). Peaks of nuclei in G0/G1 phase of the cell cycle are marked by arrows.
Intraspecific differences in genome size between populations of K. macrantha var. majoriflora and K. tristis were further tested to exclude the possibility that they represent an experimental artefact (e.g. due to presence of inhibitors in one sample). Material from two populations was co-chopped and analysed as described above. In the case of real differences, a double peak would be expected (Fig. 1B and C).
Statistical analysis
Nuclear DNA content data were analysed using the NCSS 97 statistics software (Statistical Solutions Ltd, Cork, USA). Hierarchical analysis of variance was performed to determine the variability within each taxon. The Tukey–Kramer and Bonferroni tests were used as multiple comparison tests of DNA values to discriminate dissimilar populations within each taxon. Using hierarchical cluster analysis, the relationships, based on nuclear DNA content, between individual populations of different Koeleria taxa were analysed and the unweighted pair-group method was chosen.
RESULTS
Within the 126 populations analysed (see Supplementary information) belonging to six Koeleria taxa, three diploids (2n = 14), one tetraploid (2n = 28), one decaploid (2n = 70) and one dodecaploid (2n = ∼84) (Table 1) have been found. Chromosome numbers 2n = 56 and 2n = 70 previously reported for K. tristis from Rudnik Mt in the Branisko Mts (Májovský et al., 1987; Murín and Májovský, 1987) could not be confirmed in the present study even when plants from the same population were examined.
Table 1.
Chromosome numbers, ploidy and nuclear DNA content in central European Koeleria taxa
| Absolute 2C DNA content |
||||||
|---|---|---|---|---|---|---|
| No. of populations analysed (chromosomes/ploidy) | 2n | Ploidy | No. of populations/samples/measurements | Mean ± s.d. (pg) | 1Cx genome size (Mbp)* | |
| K. glauca | 4/6 | 14 | 2x | 4/5/15 | 5·20 ± 0·06 | 2543 |
| K. macrantha | ||||||
| var. macrantha | 4/59 | 14 | 2x | 9/28/86 | 4·95 ± 0·08 | 2421 |
| var. pseudoglauca | 1/4 | 14 | 2x | 1/4/12 | 4·85 ± 0·05 | 2372 |
| var. majoriflora | 1/22 | 28 | 4x | 8/26/83 | 9·31 ± 0·25 | 2277 |
| K. pyramidata | 4/30 | 70 | 10x | 10/26/79 | 22·89 ± 0·50 | 2239 |
| K. tristis | 1/7 | ∼84 | 12x | 5/13/44 | 29·23 ± 1·12 | 2382 |
*1 pg DNA = 978 Mbp (Doležel et al., 2003).
Flow cytometric analyses revealed 2C DNA contents ranging from 4·85 to 29·23 pg. The mean 2C nuclear DNA content of K. glauca was 5·20 pg with only a small inter-population difference of 2·84 %. The low variation between four analysed populations was consistent with their phenotypic uniformity in the field and might reflect their close geographical origin.
Ploidy analysis and chromosome counting revealed diploid and tetraploid populations of K. macrantha. Within diploid populations, two different races were distinguished: a widely distributed type with pubescent leaves (var. macrantha) and a rare type with glabrous leaves occurring in relic vegetation on (ultra)basic soils in the Carpathians (var. pseudoglauca). The mean 2C nuclear DNA content estimated for nine populations of diploid K. macrantha var. macrantha was 4·95 pg without a significant inter-population difference (4·89 %, P > 0·05). For K. macrantha var. pseudoglauca only one population was analysed, therefore genome size variation could not be estimated. A morphometric study of diploid and tetraploid populations revealed significant differences as to quantitative characters between both cytotypes, i.e. length of spikelets, lower glume, anthers and stomata (Pecinka, 2001). Therefore the tetraploid populations were classified as a separate taxon, var. majoriflora. The mean 2C nuclear DNA content of eight populations of K. macrantha var. majoriflora was 9·31 pg and showed a significant inter-population difference correlated with their geographic distribution in ANOVA and multiple comparison tests (P < 0·05). Six populations from the Czech Republic had significantly smaller genomes (5·06 %) than two populations from southern Slovakia (9·19 pg vs. 9·68 pg) (Fig. 1B).
The mean 2C nuclear DNA content of decaploid K. pyramidata was 22·89 pg with statistically significant interpopulation differences (P < 0·05) in ANOVA. The multiple comparison Tukey–Kramer test found differences between the populations: (a) Nové Dobrkovice vs. Rviště (also confirmed by Bonferroni test), Kurdějov and Krnov; (b) Rviště vs. Abrod. However, it is difficult to explain these differences on the basis of geographical or reproductive isolation, since all populations are located within the continuous distribution area of the species.
The mean 2C nuclear DNA content of the five populations of dodecaploid K. tristis analysed was 29·23 pg. The multiple comparison tests revealed significant differences between the population from Rudník Mt and all the other populations (P < 0·05). The plants from Rudník Mt (27·32 pg; eastern Slovakia) had significantly less nuclear DNA (8·04 %) than those from the Vel'ká Fatra Mts and the Chočské vrchy Mts (29·71 pg; central Slovakia) (Fig. 1C).
DISCUSSION
Within the known range of genome size in plants (Bennett et al., 2000), the analysed Koeleria taxa occupy an intermediate position. As far as is known, only four Koeleria species have been analysed previously for genome size. Using Feulgen microdensitometry, Grime et al. (1985) determined 2C nuclear DNA content of K. macrantha from Scotland to be 9·20 pg; the ploidy and chromosome number of the plants analysed were not mentioned. Later on, the value was included in the ‘Plant C-values database’ and annotated as 2C nuclear DNA content of the diploid (Bennett and Leitch, 2003). However, so far, only tetraploid populations of K. macrantha have been reported from the British Isles (Dixon, 2000). Therefore it is assumed that the analysed plants were most likely tetraploid and their 2C nuclear DNA content is comparable with the value of 9·31 pg found for tetraploid populations of central European K. macrantha by flow cytometry (this study). Recently, the 2C nuclear DNA content of three tetraploid species, K. novozelandica Domin, K. cheesemanii (Hack.) Petrie and K. riguorum Edgar et Gibb, endemic to New Zealand, has been estimated by flow cytometry to be 9·95 pg, 9·95–9·82 pg and 9·82 pg, respectively (Murray et al., 2005). The nuclear DNA content of 5·90 pg previously reported for K. novozelandica (Murray et al., 2003) was not confirmed. The genome size of 9·82–9·95 pg of tetraploid New Zealand species corresponds rather well to the genome size of central European tetraploids (9·31 pg).
The genome sizes of polyploid taxa belonging to the K. macrantha aggregate (Domin, 1907; Holub et al., 1972; Tzvelev, 1983): K. macrantha var. majoriflora (9·31 pg) and K. pyramidata (22·89 pg) were found to be lower than expected on the basis of the K. macrantha var. macrantha genome (5·96 % and 7·51 %, respectively). A similar decrease in genome size of polyploids in comparison to diploids has also been observed for other genera (Lysák and Doležel, 1998; Hörandl and Greilhuber, 2002; Kotseruba et al., 2003). The possible reason could be partial elimination of repetitive DNA sequences in the polyploid genomes (Greilhuber, 1998; Kotseruba et al., 2003). Several recent studies have shown that sequence elimination by various mechanisms may occur rapidly in newly established polyploid plant genomes (Song et al., 1995; Voytas and Naylor, 1998; Ozkan et al., 2001; Devos et al., 2002).
European diploid and tetraploid cytotypes in K. macrantha show distribution correlated with longitude. In eastern Slovakia, only diploid populations were found, whereas, in central Germany tetraploid populations seem to be dominant. According to previous flow cytometric analyses and the study of voucher herbarium specimens (Pecinka, 2001), central and north-west Bohemia appears to be a transition zone with a frequent occurrence of both cytotypes, sometimes even with mixed populations in one locality. In spite of this, no triploids indicating hybridization between var. macrantha and var. majoriflora were observed within >100 plants sampled in this region. However, occurrence of tetraploid hybrid plants originating from a fusion of a non-reduced gamete of a diploid and a reduced gamete of a tetraploid cannot be excluded.
Within the populations of tetraploid K. macrantha var. majoriflora analysed, the populations from Czech Republic had significantly lower nuclear DNA than those from southern Slovakia (Fig. 1B). This difference was paralleled by minute differences in their morphology such as colour and hairiness of lemmas (Pecinka, 2001), and might indicate their different evolutionary history. Geography-correlated inter-population variation in nuclear DNA content was also found for K. tristis. This West Carpathian endemic occurs only in 14 localities, unevenly distributed within a small area of 150 × 25 km (Soják and Chrtek, 1963; Pecinka, 2001). All populations except one are clustered in central Slovakia (Vel'ká Fatra Mts, Chočské vrchy Mts and the western part of the Nízké Tatry Mts). The remaining single population, which is located ∼110 km eastwards in the Branisko Mts (eastern Slovakia), had a significantly lower genome size (8·04 %) than those from central Slovakia (Fig. 1C). This difference suggests a long-term isolation of central and eastern Slovakian populations, which could be maintained over time by extreme substrate and vegetation requirements of this species. Koeleria tristis occurs exclusively on dolomites or dolomitic-limestones which are quite rare in Slovakia. Moreover, it grows only in spatially relatively small isolates of perialpine and dealpine vegetation which are separated by large areas of continuous forest at least since the end of the last glaciation, i.e. approx. 10 000 years ago (Krippel, 1986). The difference in genome size observed between populations of K. tristis is in contrast with their uniformity in morphological characters (Pecinka, 2001). Inter-population variation in genome size has also been reported for more-or-less distant populations of other plants like Armeria maritima, Prospero autumnale, Hordeum or Arabidopsis thaliana (Ebert et al., 1996; Vekemans et al., 1996; Jakob et al., 2004; Schmuths et al., 2004). However, it does not seem to be a general rule, since it was not found for isolated populations of other species such as Sesleria albicans or Abies fraseri (Lysak et al., 2000; Auckland et al., 2001).
SUPPLEMENTARY MATERIAL
Supplementary information available online (http://aob.oxfordjournals.org) includes a list of all populations investigated, numbers of plants analysed for chromosome number, ploidy and genome size from each population and the mean population genome size.
Acknowledgments
We thank Z. Dočkalová, R. Vašut, M. Dančák, L. Filipová and M. Pečinka for collecting plant material, J. Weiserová and J. Vrána for excellent technical assistance, J. Číhalíková for help with the preparation of mitotic chromosomes, M. Duchoslav for advice concerning statistical analyses, I. Schubert and J. Bartoš for helpful comments to the manuscript and B. Murray for discussion about the genome size of K. novozelandica.
LITERATURE CITED
- Arnow LA. 1994. Koeleria macrantha and K. pyramidata (Poaceae): nomenclatural problems and biological distinctions. Systematic Botany 19: 6–20. [Google Scholar]
- Auckland LD, Johnston JS, Price HJ, Brigwater FE. 2001. Stability of nuclear DNA content among divergent and isolated populations of Fraser fir. Canadian Journal of Botany 79: 1375–1378. [Google Scholar]
- Barow M, Meister A. 2002. Lack of correlation between AT frequency and genome size in higher plants and the effect of nonrandomness of base sequences on dye binding. Cytometry 47: 1–7. [DOI] [PubMed] [Google Scholar]
- Bennett MD. 1998. Plant genome values: how much do we know? Proceedings of the National Academy of Sciences of the USA 95: 2011–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MD, Leitch IJ. 2003. Angiosperm DNA C-values database (release 4·0, Jan. 2003) http://www.rbgkew.org.uk/cval/homepage.html
- Bennett MD, Bhandol P, Leitch IJ. 2000. Nuclear DNA amounts in angiosperms and their modern uses—807 new estimates. Annals of Botany 86: 859–909. [Google Scholar]
- Bottini MCJ, Greizerstein EJ, Aulicino MB, Poggio L. 2000. Relationships among genome size, environmental conditions and geographical distribution in natural populations of NW Patagonian species of Berberis L. (Berberidaceae). Annals of Botany 86: 565–573. [Google Scholar]
- Bureš P, Wang Y-F, Horová L, Suda J. 2004. Genome size variation in central European species of Cirsium (Compositae) and their natural hybrids. Annals of Botany 94: 353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli M, Falistocco E, Cionini PG. 1992. Variation of genome size and organization within hexaploid Festuca arundinacea. Theoretical and Applied Genetics 83: 273–278. [DOI] [PubMed] [Google Scholar]
- Conert HJ. 1998. 35. Koeleria. In: Conert HJ, Eckehart JJ, Kadereit JW, Schultze-Motel W, Wagnitz G, Weber HE, eds. G. Hegi, Illustrierte Flora von Mittel-Europa. München: Blackwell Wissenschafts, 261–277.
- Devos KM, Brown JKM, Bennetzen JL. 2002. Genome size reduction through illegitimate recombination counteracts genome expansion in Arabidopsis. Genome Research 12: 1075–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doležel J, Bartoš J, Voglmayr H, Greilhuber J. 2003. Nuclear DNA content and genome size of trout and human. Cytometry 51A: 127–128. [DOI] [PubMed] [Google Scholar]
- Doležel J, Greilhuber J, Lucretti S, Meister A, Lysák MA, Nardi L, et al. 1998. Plant genome size estimation by flow cytometry: inter-laboratory comparison. Annals of Botany 82 (Suppl. A):17–26. [Google Scholar]
- Dixon JM. 2000. Biological flora of the British Isles no. 212. Koeleria macrantha (Ledeb.) Schultes [K. alpigena Domin, K. cristata (L.) Pers. pro parte, K. gracilis Pers., K. albescens auct. non DC.]. Journal of Ecology 88: 709–726. [Google Scholar]
- Domin K. 1907. Monographie der Gattung Koeleria. Bibliotheca Botanica 65: 1–354. [Google Scholar]
- Ebert I, Greilhuber J, Speta F. 1996. Chromosome banding and genome size differentiation in Prospero (Hyacinthaceae): diploids. Plant Systematics and Evolution 203: 143–177. [Google Scholar]
- Fedorov AA. 1969. Chromosome numbers of flowering plants. Leningrad: Komarov Botanical Institute, Academy of Sciences of the USSR, 537–538.
- Greilhuber J. 1998. Intraspecific variation in genome size: a critical reassessment. Annals of Botany 82: 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greilhuber J, Lysák M, Doležel J, Bennett MD. 2005. The origin, evolution and proposed stabilization of the terms ‘genome size’, and ‘C-value’ to describe nuclear DNA contents. Annals of Botany 95: 255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grime JP, Shacklock JML, Band SR. 1985. Nuclear DNA contents, shoot phenology and species co-existence in a limestone grassland community. New Phytologist 100: 435–445. [Google Scholar]
- Holub J, Měsíček J, Javůrková V. 1972. Annotated chromosome counts of Czechoslovak plants (31–60). Folia Geobotanica et Phytotaxonomica 7: 167–202. [Google Scholar]
- Hörandl E, Greilhuber J. 2002. Diploid and autotetraploid sexuals and their relationships to apomicts in the Ranunculus cassubicus group: insights from DNA content and isozyme variation. Plant Systematics and Evolution 234: 85–100. [Google Scholar]
- Humpries CJ. 1980. 68. Koeleria Pers. In: Tutin TG, Heywood VH, Burges NA, Valentine DH, Walters SM, Webb DA (eds), Flora Europaea, Vol. 5. Cambridge: Cambridge University Press, 218–220.
- Jakob SS, Meister A, Blattner FR. 2004. The considerable genome size variation of Hordeum species (Poaceae) is linked to phylogeny, life form, ecology, and speciation rates. Molecular Biology and Evolution 21: 860–869. [DOI] [PubMed] [Google Scholar]
- Kotseruba V, Gernand D, Meister A, Houben A. 2003. Uniparental loss of ribosomal DNA in the allotetraploid grass Zingeria trichopoda (2n = 8). Genome 46: 156–163. [DOI] [PubMed] [Google Scholar]
- Krippel E. 1986. Post-glacial development of flora in Slovakia. Bratislava: VEDA, 156–175 [in Slovak].
- Looman J. 1978. Biological flora of the Canadian prairie provinces. V. Koeleria gracilis Pers. Canadian Journal of Plant Science 58: 459–466. [Google Scholar]
- Lysák MA, Doležel J. 1998. Estimation of nuclear DNA content in Sesleria (Poaceae). Caryologia 52: 123–132. [Google Scholar]
- Lysák MA, Rostková A, Dixon JM, Rossi G, Doležel J. 2000. Limited genome size variation in Sesleria albicans. Annals of Botany 86: 399–403. [Google Scholar]
- Májovský J, Murín A, Feráková V, Hindáková M, Schwarzová T, Uhríková A, et al. 1987. Karyotaxonomický prehl'ad flóry Slovenska. Bratislava: VEDA SAV, 395.
- Meusel et al. 1965. Koeleria Vergleichende Chorologie der Zentraleuropäischen Flora. Jena: Gustav Fischer Verlag, 46.
- Molina AM. 1993. Koeleria Las especies del género Koeleria (Gramineae: Poeae) de Sudamérica. Parodiana 8: 37–67. [Google Scholar]
- Morgan-Richards M, Trewick SA, Chapman HM, Krahulcová A. 2004. Interspecific hybridization among Hieracium species in New Zealand: evidence from flow cytometry. Heredity 93: 34–42. [DOI] [PubMed] [Google Scholar]
- Murín A, Májovský J. 1987. Karyological study of Slovakian flora. IV. Acta Facultatis Rerum Naturalium Universitatis Comenianae Botanica 34: 3–20. [Google Scholar]
- Murray BG, Weir IE, Ferguson AR, de Lange PJ. 2003. Variation in DNA C-value and haploid genome size in New Zealand native grasses. New Zealand Journal of Botany 41: 63–69. [Google Scholar]
- Murray BG, de Lande PJ, Ferguson AR. 2005. Nuclear DNA variation, chromosome numbers and polyploidy in the endemic and indigenous grass flora of New Zealand. Annals of Botany 96: 1293–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn TC, Pires JC, Birchler JA, Auger DL, Chen ZJ, Lee HS, et al. 2003. Understanding mechanisms of novel gene expression in polyploids. Trends in Genetics 19: 141–147. [DOI] [PubMed] [Google Scholar]
- Otto FJ. 1990. DAPI staining of fixed cells for high resolution flow cytometry of nuclear DNA. In: Darzynkiewicz Z, Crissman HA, eds. Methods in cell biology, Vol. 33. New York: Academic Press, 105–110. [DOI] [PubMed]
- Ozkan H, Levy AA, Feldman M. 2001. Allopolyploidy-induced rapid genome evolution in the wheat (Aegilops-Triticum) group. The Plant Cell 13: 1735–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecinka A. 2001. Taxonomy and chorology of the genus Koeleria Pers. (Poaceae) in the Czech Republic and Slovakia. MSc Thesis, Palacký University in Olomouc, Czech Republic.
- Price HJ. 1988a. Nuclear DNA content variation within angiosperm species. Evolutionary Trends in Plants 2: 53–60. [Google Scholar]
- Price HJ. 1988b. DNA content variation among higher plants. Annals of the Missouri Botanical Gardens 75: 1248–1257. [Google Scholar]
- Schmuths H, Meister A, Horres R, Bachman K. 2004. Genome size variation among accessions of Arabidopsis thaliana. Annals of Botany 93: 317–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soják J, Chrtek J. 1963. Koeleria tristis Domin, ein beachtenswerter Endemit der Slowakischen Flora. Biológia 18: 916–922. [Google Scholar]
- Song K, Lu P, Tang K, Osborn TC. 1995. Rapid genome change in synthetic polyploids of Brassica and its implications for polyploid evolution. Proceedings of the National Academy of Sciences of the USA 92: 7719–7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzvelev NN. 1983. Grasses of the Soviet Union, Part 1. New Delhi and Calcutta: Oxonian Press, 394–411.
- Ujhelyi J. 1972. Evolutionary problems of European koelerias. Symposia Biologica Hungarica 12: 163–176. [Google Scholar]
- Vekemans X, Lefébvre C, Couland J, Blaise S, Gruber W, Siljak-Yakovlev S, et al. 1996. Variation in nuclear DNA content at the species level in Armeria maritima. Hereditas 124:237–242. [Google Scholar]
- Voytas DF, Naylor GJP. 1998. Rapid flux in plant genomes. Nature Genetics 20: 6–7. [DOI] [PubMed] [Google Scholar]
- Zonneveld BJM. 2001. Nuclear DNA content of all species of Helleborus (Ranunculaceae) discriminate between species and sectional divisions. Plant Systematics and Evolution 229: 125–130. [Google Scholar]


