Abstract
Incorporation of a nitric oxide (NO)-releasing moiety in aspirin can overcome its gastric side effects.
We investigated the NO-release patterns and antiplatelet effects of novel furoxan derivatives of aspirin (B8 and B7) in comparison to existing antiplatelet agents.
Cyclooxygenase (COX) activity was investigated in purified enzyme using an electron paramagnetic resonance-based technique. Concentration–response curves for antiplatelet agents±the soluble guanylate cyclase inhibitor, ODQ (50 μM) were generated in platelet-rich plasma (PRP) and washed platelets (WP) activated with collagen using turbidometric aggregometry. NO was detected using an isolated NO electrode.
The furoxan derivatives of aspirin (B8, B7) and their NO-free furazan equivalents (B16, B15; all 100 μM) significantly inhibited COX activity (P<0.01; n=6) in vitro and caused aspirin-independent, cGMP-dependent inhibition of collagen-induced platelet aggregation in WP. B8 was more potent than B7 (PRP IC50=0.62±0.1 μM for B8; 400±89 μM for B7; P<0.0001. WP IC50s=0.6±0.1 and 62±10 μM, respectively). The NO-free furazan counterparts were less potent antiplatelet agents (WP IC50s=54±3 μM and 62±10 μM, respectively; P<0.0001, B8 vs B16). Of the hybrids investigated, only B8 retained antiplatelet activity in PRP.
NO release from furoxan–aspirin hybrids was undetectable in buffer alone, but was accelerated in the presence of either plasma or plasma components, albumin (4%), glutathione (GSH; 3 μM) and ascorbate (50 μM), the effects of which were additive for B7 but not B8. NO generation from furoxans was greatly enhanced by platelet extract, an effect that could largely be explained by the synergistic effect of intracellular concentrations of GSH (3 mM) and ascorbate (1 mM).
We conclude that the decomposition of furoxan–aspirin hybrids to generate biologically active NO is catalysed by endogenous agents which may instil a potential for primarily intracellular delivery of NO. The blunting of the aspirin effects of furoxan hybrids is likely to be due to loss of the acetyl moiety in plasma; the observed antiplatelet effects are thereby primarily mediated via NO release. Compounds of this class might represent a novel means of inhibiting platelet aggregation by a combination of NO generation and COX inhibition.
Keywords: Nitric oxide, platelets, furoxan, nitroaspirin, thrombosis, antithrombotic
Introduction
Aspirin is a nonsteroidal anti-inflammatory drug that has long been used as a prophylactic against thrombotic coronary events (ISIS-2, 1988; PHS-Committee, 1989; Manson et al., 1991). It reduces cardiovascular risk, primarily through irreversible inhibition of prostaglandin H synthase-1 (also termed cyclooxygenase-1, COX-1)-mediated platelet aggregation (for review see, Patrono, 1994). Aspirin selectively and irreversibly acetylates a serine residue (Ser 530) of COX-1 (Roth & Majerus, 1975; Roth et al., 1975; DeWitt & Smith, 1988). COX-1 inhibition results in reduced production of thromboxane A2, a vital element in the induction of irreversible platelet aggregation in response to stimuli such as collagen (FitzGerald, 1991; Hamberg et al., 1975). The anucleate nature of platelets makes them unable to synthesize new proteins and replace inhibited enzyme; recovery of full platelet activity only takes place as a function of platelet turnover (Burch et al., 1978). Aspirin also acts to inhibit the formation of thrombin (Kessels et al., 1994; Szczeklik et al., 1992), a unique action that also prevents platelet aggregation and impacts on the coagulation pathway. Taken together, these properties offer a degree of platelet selectivity in the action of aspirin.
Unfortunately, gastrointestinal disorders, including ulceration, are a common side effect of aspirin, limiting its use (Cameron, 1975; Wallace, 1997; Tramer et al., 2000; Seager & Hawkey, 2001). The effect is due to inhibition of prostaglandins that normally protect the gastric mucosa (Whittle, 1977; Robert et al., 1979; Schoen & Vender, 1989; Wallace, 1997). Aspirin esters containing a nitric oxide (NO)-donor nitrooxy function (e.g. NCX4016) are thought to overcome the gastric side effects through the protective actions of drug-derived NO. NO increases blood flow in the gastric mucosa, promoting repair and removal of toxins (Wallace & Miller, 2000). NO also increases secretion of protective gastric mucus (Brown et al., 1993) and is thought to promote healing of gastric ulcers by promoting angiogenesis (Ma & Wallace, 2000). Alternatively, the protective effects of NO aspirin could be due to masking of the aspirin carboxylic acid moiety by the ester function (Rainsford & Whitehouse, 1976; Cena et al., 2003).
While NO hybrids of aspirin were primarily designed to protect against damage to the gastric mucosa, there may be additional benefits of drug-derived NO through its many protective effects in the vascular system. NO is a powerful endogenous vasodilator (Palmer et al., 1987) which acts to keep the vasculature in an active state of dilatation by stimulating cGMP-mediated relaxation of vascular smooth muscle cells. NO also opposes the adherence of monocytes to the vessel wall (Tsao et al., 1997) and displays antithrombotic actions through its ability to inhibit platelet adhesion (Radomski et al., 1987b, 1987c) and aggregation (Radomski et al., 1987a; 1990; Pasqui et al., 1991). Inhibition of platelet aggregation occurs primarily via stimulation of cGMP; the platelet aggregation response to sodium nitroprusside has been shown to be entirely cGMP dependent (Sogo et al., 2000). However, cGMP-independent signalling mechanisms have also been identified (Gordge et al., 1998; Trepakova et al., 1999; Sogo et al., 2000; Homer & Wanstall, 2002; Crane et al., 2005). Reduced NO synthesis or availability is heavily implicated as a key factor in the initiation and progression of atherogenesis (Anderson et al., 1995; Maxwell, 2002; Shaul, 2003).
The release at NO from NCX4016 and glyceryl trinitrate has been reported to occur through identical mechanisms (Grosser & Schroder, 2000). Platelets have a poor capability to release NO from organic nitrates (Weber et al., 1996) and it is possible that compounds such as NCX4016 will fail to show additional, NO-mediated effects in platelets, at least in vitro. A new range of drugs, in which a NO-donating moiety (furoxan group) is joined by an ester linkage to the aspirin molecule (B8, B7; Figure 1) appear to overcome the problem of gastric lesions (Cena et al., 2003). However, the NO-release mechanism is unlikely to require the same cellular machinery as that for organic nitrates, suggesting that they might offer a degree of NO-mediated antiplatelet effects to complement those of the aspirin moiety.
Figure 1.
Structural formulae of furoxan hybrids (B8 and B7), furazan hybrids (B16 and B15) and the nitrooxy-ester NCX4016.
In the present study, we investigated the mechanism of action of two examples (3-cyanofuroxan-4-yl)methyl 2-acetoxybenzoate (B8) and (3-carbamoylfuroxan-4-yl)methyl 2-acetoxybenzoate (B7); Figure 1), with different NO-releasing properties (Cena et al., 2003) in human platelets in vitro. Here, we characterize the NO-releasing and COX-inhibiting effects of these novel furoxan derivatives of aspirin and compare their actions to related antiplatelet agents.
Methods
Materials
2-(N,N-diethyamino)-diazenolate-2-oxide (DEA/NO; Axxora, Nottingham, U.K.) was dissolved in 0.01 M NaOH. 1H-[1,2,4]oxadiazolo[4,3-α]quinoxalin-1-one (ODQ; Cookson, Langford, Bristol, U.K.) was dissolved in dimethylsulphoxide (DMSO) and stored at −20°C. Collagen was purchased from Labmedics (Stockport, U.K.). 1-Hydroxy-3-carboxy-2,2,5,5-tetramethylpyrrolidine. HCl (CPH) was purchased from Axxora. All other chemicals were purchased from Sigma (Poole, Dorset, U.K.). All nitro-aspirins were synthesized at the Università degli Studi di Torino, as described (Cena et al., 2003). Compound purity was assessed by high-performance liquid chromatography before biological assays (over 98% for all compounds; Merck Purosphere RP-18 column (Merck, Darmstadt, Germany; 250 × 4 mm2, 5 μm particle size) eluting with flow rate of 1 ml min−1. Mobile phase consisted of 0.1% aqueous trifluoroacetic acid (TFA) and acetonitrile containing 0.1% TFA in different ratios according with compounds properties. The column effluent was monitored at 224/254 nm). Compound identity was chemically confirmed by nuclear magnetic resonance.
The nitro-aspirins were dissolved in DMSO, then diluted in PBS to give a final concentration of 0.1% DMSO, which pilot studies had determined not to affect platelet aggregation. No precipitation of any drug was observed following dilution.
COX activity assay
The ability of novel and established compounds to inhibit COX-1 was measured using an adaptation of a published method (Schreiber et al., 1989). The assay relies on detection of oxidizing free radicals produced as a by-product by the peroxidase element of the COX enzyme. A spin-trapping agent, CPH, was utilized to trap the short-lived radicals, forming a stable adduct, 3-carboxy-proxyl. The assay makes use of commercially available purified COX-1 from ovine seminal vesicles (Sigma), pretreated with haematin for 5 min.
The COX activity assay was performed at 37°C in 1 ml of Tyrode's buffer. In all, 100 U ml−1 purified COX-1 was incubated with 3 nM haematin (5 min, 37°C) prior to the assay. Aspirin, salicylic acid, a furoxan, a furazan, NCX4016 (100 μM) or a vehicle control (DMSO, 1%) was added and left to incubate for a further 10 min prior to addition of the spin-trap, CPH (1 mM). At this point (time zero), a baseline EPR measurement was taken (MS200, Magnettech, Germany. Instrument settings: B0-field, 3356 G; sweep width; 50 G, sweep time, 30 s; modulation amplitude, 1500 mG; microwave power, 20 mW; microwave frequency, 9.3 GHz). After 2 min, 0.5 mM AA (sodium salt) was added; EPR measurements were made (50 μl samples in micropipette tubes) after a further 1.5 min incubation. The suicidal nature of COX-1 activation means that the period of activation is complete within ∼1 min of AA addition (as confirmed by pilot experiments).
The EPR results were corrected for any auto-oxidation of spin-trap by subtraction of values recorded from a duplicate sample run in the absence of AA. The intensity scale on the y-axis of all graphs is an arbitrary scale based upon the area under the curve (AUC) of the first derivative traces generated. Results graphs show the timepoint 1.5 min after the addition of AA, as this was determined in pilot experiments to be the most appropriate point at which to compare free radical generation between control and furoxan-treated COX-1, given that spin-adduct generation in response to AA had peaked – subsequent adduct formation was at an equivalent rate in control and AA-treated samples and was likely to be due to nonspecific auto-oxidation of CPH.
Blood preparation
Peripheral venous blood was drawn from the antecubital fossa of human volunteers aged 20–45 years who were non-smokers and had not taken any platelet-active agents during the previous 10 days. Blood was collected into tubes containing 3.8% sodium citrate and centrifuged at 200 × g for 10 min at room temperature to obtain platelet-rich plasma (PRP). An aliquot of PRP was further centrifuged at 1200 × g for 10 min to obtain platelet-poor plasma (PPP). Washed platelets (WP) samples were obtained by adding 300 ng ml−1 prostacyclin to a 2 ml PRP sample before centrifuging at 1200 × g for 10 min. Prostacyclin is commonly used at this concentration in this type of study (Giuliano & Warner, 1999; Kobzar et al., 2001; Crane et al., 2002). The effect of the prostacyclin is only temporary due to its short half-life. The supernatant was then discarded and the pellet resuspended in Tyrode's buffer (137 mM NaCl, 2.7 mM KCl, 1.05 mM MgSO4, 0.4 mM NaH2PO4, 12.5 mM NaHCO3, 5.6 mM Glucose, 10 mM HEPES and 0.8 mM CaCl in dH2O at pH 7.4). Prostacyclin (300 ng ml−1) was again added before a second 10 min centrifugation (1200 × g). Finally, the pellet was resuspended in 2 ml Tyrode's buffer. For aggregation, platelet counts were determined using a Coulter Ac.T 8 Hematology Analyzer (Coulter Electronics, Luton, U.K.) and standardized to 250 × 109 l−1 via dilution with PPP (PRP) or Tyrode's buffer (WP).
Aggregation
Inhibition of platelet aggregation was measured using optical platelet aggregometry in a 4-channel aggregometer (Chrono-Log, Labmedics, Stockport, U.K.) and data captured via an analogue-digital converter (Maclab 4e, AD Instruments, Sussex, U.K.). The instrument was calibrated such that the difference in light transmission between test (PRP or WP) and reference (PPP or Tyrode's buffer, respectively) samples was set to generate an 80 mV signal. Typically, maximal aggregation caused ∼60 mV change in signal. Briefly, PRP or WP samples were equilibrated at 37°C and stirred continuously at 1000 r.p.m. The samples were then treated with either B8 (10 nM–3 μM), B7 (10 μM–1 mM), their respective NO-free structurally related furazan derivatives, (4-cyanofurazan-3-yl)methyl 2-acetoxybenzoate (B16; 10 nM–3 μM) or (4-carbamoylfurazan-3-yl)methyl 2-acetoxybenzoate (B15; 10 μM–1 mM), NCX4016 (3 μM–0.3 mM) or aspirin (acetylsalicylic acid; 3 μM–1 mM) for 10 min before induction of aggregation with supramaximal concentrations of collagen (2.5 μg ml−1). The aggregation was then recorded turbidometrically over 5 min against a reference PPP sample. Experiments were also performed with the addition of 50 μM (supramaximal concentration (Crane et al., 2005)) of the cGMP inhibitor ODQ 15 min before addition of the drug. Aggregation was expressed as a percentage inhibition of control aggregation obtained with 2.5 μg ml−1 collagen.
NO release
Samples of PRP, PPP or Tyrode's buffer (2 ml) were incubated in the aggregometer at 37°C and stirred continuously at 1000 r.p.m. An isolated NO electrode (ISO-NO MARKII, World Precision Instruments, Stevenage, U.K.), calibrated using DEA/NO (0.1–1.6 μM), was introduced into the cuvette and allowed to stabilize for 10 min. B8 (100 μM) or B7 (500 μM) was then added to the cuvette and the release of NO recorded for 10 min before addition of haemoglobin (10 μM) to scavenge any generated NO. Experiments were also carried out in Tyrode's buffer±4% albumin, with the addition of glutathione (3 μM or 3 mM), ±50 μM ascorbate before B8 or B7. Ascorbate (1 mM) was also used, but only in the presence of 10 mM HEPES to buffer its acidic pH. The concentrations of albumin, ascorbate and glutathione studied are all physiologically relevant. The detection limit for the electrode under the conditions of the experiment was found to be ∼10 nM NO.
A platelet extract was also prepared from samples of PRP and platelet count was determined using the Coulter Ac.T 8 Hematology Analyzer. The platelets were washed as above in the presence of prostacyclin to obtain a platelet pellet. The total platelet volume of the pellet was calculated by multiplying the number of platelets by the average platelet volume (5.14 × 10−15 l; obtained from the hematology analyzer). The pellet was resuspended in 1 ml of 0.5% Triton X in Tyrode's buffer for 1 h and the dilution of cell contents estimated using the total platelet volume from the above calculation. This was then homogenized by hand for 15 min and centrifuged at 12,000 × g for 10 min to give a cell extract. B8 (100 μM) or B7 (500 μM) was added to 1 ml of this supernatant in the assay as described above.
A further set of experiments was carried out following a 10 min preincubation of the platelet extract with the thiol alkylator, 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB; 500 μM). The NO release from B8 (100 μM) or B7 (500 μM) was then recorded as above. The samples were collected and frozen (−20°C) and later used for thiol determination by colorimetric analysis at 405 nm. Thiol levels of the extract samples were determined from a standard curve created using 10 μM–30 mM glutathione in 500 μM DTNB.
Statistics
All results are expressed as the mean±s.e.m. Concentration–response curves were analysed by two-way analysis of variance (two-way ANOVA). EPR data were analysed by 1-way ANOVA and NO electrode data by two-tailed paired Student's t-test. Dunnett's or Bonferroni post-tests were carried out where appropriate. P<0.05 was considered to be statistically significant.
Results
COX activity
Aspirin (100 μM) significantly reduced the EPR signal generated by AA-stimulated purified COX-1 in Tyrode's buffer compared to a vehicle control (Figure 2; P<0.01; n=8). A significant inhibition of COX activity was also observed with the furoxan–aspirins, B8 and B7 (100 μM; P<0.05 and P<0.01 respectively, n=6). Even greater inhibition was demonstrated with the NO-free furazan equivalents, B16 and B15 (100 μM, P<0.01 for both, n=6). The furazan compounds demonstrated a significantly greater inhibition of COX than aspirin (P<0.01). The nitrooxy-ester, NCX4016 (100 μM) also abolished COX activity (P<0.01, n=6). Salicylic acid (100 μM, n=6) failed to significantly inhibit generation of the spin-adduct, as did the NO donor DEA/NO (100 μM, n=6; Figure 2b).
Figure 2.
EPR-based COX-activity assay. (a) Typical 3-peak EPR spectra obtained in the absence (control; COX+AA) and presence of aspirin or hybrid compound (all 100 μM) after correction for background autoxidation. EPR settings: B0-field, 3356 G; sweep width; 50 G, microwave frequency, 9.3 GHz; sweep time, 30 s; modulation amplitude, 1500 mG; microwave power, 20 mW. (b) Effect of aspirin, salicylic acid, furoxans, furazans and NCX4016 (all 100 μM) on EPR signals generated from COX-1 after treatment with substrate (AA). In each case, drug incubations were for 10 min prior to the baseline EPR reading. Readings shown were taken 1.5 min after the addition of AA. *P<0.05, **P<0.01; one-way ANOVA with Dunnet's post hoc test vs control: n=6–10. Values are mean±s.e.m.
Effect of aspirin, salicylic acid and NCX4016 on platelet aggregation
Aspirin (3–300 μM) caused concentration-dependent inhibition of collagen-induced platelet aggregation in PRP and the effect was enhanced in WP, but salicylic acid failed to show an inhibitory effect, even at concentrations of 300 μM (Figure 3; n=6–8). NCX4016 (3–300 μM) had no effect on collagen-induced platelet aggregation in PRP (Figure 3a; n=6), but did cause concentration-dependent inhibition of platelet aggregation in WP that was significantly more potent than aspirin (Figure 3b; P=0.002; n=6). Responses to NCX4016 were insensitive to the soluble guanylate cyclase inhibitor, ODQ (15 min preincubation; P=0.88, NCX4016+ODQ vs NCX4016 alone in WP; Figure 3b).
Figure 3.
Collagen (2.5 μg ml−1)-induced platelet aggregation in PRP and WP. (a) Effect of NCX4016, salicylic acid and aspirin on collagen-induced platelet aggregation in PRP. ***P<0.0001, n=6–7. (b) The effect of the guanylate cyclase inhibitor ODQ (50 μM; 15 min preincubation) on responses to NCX4016 in WP. **P=0.002, n=6. Values are mean±s.e.m. Statistical analysis by two-way ANOVA.
Effect of B8 and B7 on platelet aggregation
B8 (10 nM–3 μM) caused concentration-dependent inhibition of collagen-induced platelet aggregation in PRP at concentrations ∼100-fold lower than for aspirin (B8 IC50 in PRP=0.62±0.1 μM; Figure 4a, (see Figure 3a for aspirin effect)). ODQ significantly inhibited the responses to B8 in PRP (Figure 4a; n=6–8; P<0.0001), while the NO-free, structurally related furazan derivative of B8 (B16; 10 nM–1 mM) was considerably less effective at inhibiting platelet aggregation than B8 in PRP (Figure 4a; P<0.0001; n=8).
Figure 4.
Collagen (2.5 μg ml−1)-induced platelet aggregation in PRP and WP. (a) Effect of B8 (±ODQ; 50 μM, 15 min preincubation) and its NO-free equivalent, B16, on collagen-induced platelet aggregation in PRP. P<0.0001 (B8+ODQ vs B8 alone, n=6–9). (b) The effect of ODQ (50 μM) on responses to B8 in WP. P<0.0001 (+ODQ vs B8 alone), n=6–9. (c) Effect of B7 (±ODQ; 50 μM) and its NO-free equivalent B15 on collagen-induced platelet aggregation in PRP. ***P<0.0001, n=6–7. (d) The effect of ODQ (50 μM) on the B7 response in WP. ***P<0.0001, n=6–7. Values are mean±s.e.m. Statistical analysis by two-way ANOVA.
The effects of B8 in PRP were largely mirrored in WP: B8 was found to be a powerful inhibitor of collagen-induced aggregation (IC50=0.6±0.1 μM) and its actions were significantly inhibited by ODQ (Figure 4b; P<0.0001; n=6–8). B16 had a significantly greater inhibitory effect on platelet aggregation in WP compared to PRP (IC50=54±3 μM; Figure 4b; P<0.0001; PRP B16 vs WP B16; n=6–8).
B7 (10 μM–1 mM) was less effective than B8 and aspirin at inhibiting collagen-induced platelet aggregation in PRP (B7 IC50=0.36±0.1 mM). ODQ significantly inhibited the response of B7 in PRP (Figure 4c; n=6–7; P<0.0001). B15 was ineffective in PRP.
In order to make a direct comparison, concentrations studied with WP were dictated by the PRP response curve. B7 (10 μM–1 mM) was considerably more effective in WP (IC50=24±8 μM) than PRP. The inhibitory effects of B7 in WP were significantly attenuated by ODQ (P=0.002, two-way ANOVA; n=6–7). In stark contrast to the findings in PRP, B15 was found to be a powerful inhibitor of platelet aggregation in WP (B15 IC50 in WP=62±10 μM); indeed, at concentrations of 100 μM or more, it was as effective as B7 under these conditions.
NO release from furoxan–aspirin hybrids: effect of endogenous antioxidants
Sample recordings of NO generation from B7 and B8 in the presence and absence of GSH and ascorbate are shown in the inserts to Figure 5a and c. Owing to the different profile of NO release by the different drugs, it was established that AUC was more representative of NO release than peak concentration in these experiments; subsequent values quoted are all AUC over the 10 min incubation (mmol min), but sample peak values are also given for information.
Figure 5.
NO release recorded over 10 min from B8 (100 μM) or B7 (500 μM) in various media. (a) NO release from B8 in media related to plasma conditions: Tyrode's buffer was reconstituted with approximate plasma concentrations of the plasma constituents, albumin (4%), GSH (3 μM) and ascorbate (50 μM). Inset shows typical 10 min traces of NO release recorded via the NO electrode in Tyrode's buffer with or without ascorbate (1 mM) and GSH (3 mM). (b) Shows typical NO release from B8 in media related to platelet conditions: Tyrode's buffer 3 and 1 mM are approximate intracellular concentrations of glutathione and ascorbate, respectively; n=6–7. GSH=Glutathione. Ascorb=ascorbate. Values are mean±s.e.m. (c) Shows NO release from B7 in media related to plasma conditions. Inset shows typical 10 min traces of NO release from B7 recorded via the NO electrode in Tyrode's buffer with or without ascorbate (1 mM) and GSH (3 mM) (d) shows NO release from B7 in media related to platelet conditions.
NO release from NCX4016 (100 μM) was undetectable in Tyrode's buffer, PRP or WP (n=6–8 for each). Likewise, NO release from the furoxans B8 and B7, was undetectable in Tyrode's buffer alone (Figure 5a and c). However, it was detected in samples of PRP, WP, PPP or Tyrode's buffer in the presence of approximate plasma concentrations of albumin (4%), GSH (3 μM) or ascorbate (50 μM; Figure 5b and d).
NO was released from B8 in platelet-containing samples (PRP and WP; mean NO release=33.2±2.9 and 33.0±2.5 mmol min, respectively, with both peaking at ∼1.25 μM NO; Figure 5b), and in PPP (mean NO release for B8=21.0±2.8 mmol min, peak at ∼0.6 μM NO; Figure 5a).
Reconstitution of Tyrode's buffer with approximate plasma concentrations of either albumin (4%), glutathione (3 μM) or ascorbate (50 μM) enhanced NO generation from B8. A combination of these three constituents failed to enhance NO release from B8 beyond the effects seen with the individual components alone (2.7±0.7 mmol min, peak at ∼0.040 μM for GSH alone and ∼0.038 μM NO in the presence of plasma concentrations of albumin, GSH and ascorbate; Figure 5a; P=0.78 vs GSH alone, P=0.13 vs ascorbate alone, both unpaired Student's t-test).
Reconstitution of Tyrode's buffer with approximately intracellular concentrations of GSH (3 mM) generated marginally less NO from B8 (14.4±2.5 mmol min; Figure 5b; peak at ∼0.124 μM NO) compared with samples containing platelets. Ascorbate (1 mM) in Tyrode's buffer caused minimal NO release from B8 (=2.2±0.9 mmol min; Figure 5b; peak at ∼0.004 μM NO), but a combination of GSH and ascorbate caused a considerable increase in NO generation from B8 (247±19 mmol min, n=6, Figure 5b, P<0.0001 vs individual components alone, unpaired Student's t-test; peak at ∼2.31 μM NO).
Relatively low levels of NO were released from B7 in PRP and WP (1.6±0.4 and 3.8±0.7 mmol min, respectively; n=6, Figure 5d. Peaks at ∼0.085 and ∼0.120 μM NO, respectively) despite the higher drug concentration used. Reconstitution of the Tyrode's buffer with approximate plasma concentrations of either albumin (4%), glutathione (3 μM) or ascorbate (50 μM) enhanced NO generation from B7 and a combination of these three constituents had an additive effect on the release of NO from B7 (3.8±0.8 mmol min; peak at ∼0.295 μM NO), where release was equivalent to that seen in PPP (unpaired Student's t-test P=0.628; Figure 5c).
Reconstitution of Tyrode's buffer with approximately intracellular concentrations of GSH (3 mM) increased NO release from B7 (8.5±1.1 mmol min; peak at ∼0.504 μM NO). Ascorbate (1 mM) in Tyrode's buffer caused minimal NO release from B7 (3.1±1.6 mmol min; peak at ∼0.004 μM NO), but a combination of GSH and ascorbate caused a considerable increase in NO generation from B7 (47±4 mmol min n=6, Figure 5d; peak at ∼0.642 μM NO).
Effect of platelet extract on NO generation from B8 and B7
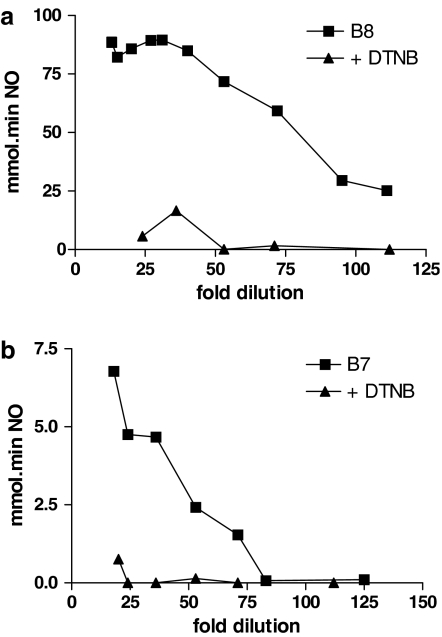
There was a relationship between the dilution factor of platelet extract and the amount of NO released from both B8 and B7 (Figure 6a and b). NO generation by B8 (100 μM) failed to increase at platelet extract dilutions below ∼1 in 30, with a maximum NO generation of ∼90 mmol min. Pretreatment of platelet extracts with the thiol alkylator, DTNB (500 μM), all but abolished NO generation.
Figure 6.
A sample of WP was suspended in 1 ml 0.5% Triton X and then homogenized to release platelet extract before centrifugation to remove membrane fraction. The dilution factor on x-axis was calculated by volume Triton X (1 ml)/(number of platelets × average platelet volume). (a) NO release from 16 platelet extracts treated with 100 μM B8. The triangles show samples treated in the same way but with a 10 min preincubation with 500 μM DTNB before addition of B8. (b) NO release from 13 platelet extracts treated with 500 μM B7. The triangles show samples treated in the same way but with a 10 min preincubation with 500 μM DTNB before addition of B7.
A similar pattern was observed with B7 (500 μM), but the amount of NO generated in the presence of platelet extracts was >10-fold lower than from B8, despite the higher drug concentration. In the case of B7, dilutions of platelet extract lower than ∼1 in 75 failed to generate detectable NO, but there was a direct relationship between NO generation and dilution factor at dilutions of 1 in 75 to 1 in 25 (Figure 6b). DTNB again showed a marked inhibitory effect on NO generation in the presence of platelet extract. Colorimetric analysis of DTNB-treated samples yielded intracellular total reduced thiol concentrations of 22±4 mM (corrected for dilution; n=5).
Discussion
Our results show that two novel furoxan–aspirin hybrids drugs effectively inhibit collagen-induced platelet aggregation and the relative contribution of NO to the inhibitory effect was dependent on the characteristics of the specific furoxan involved. The potent effects of B8 were considered to be largely NO dependent on account of the fact that the closely related NO-free furazan, B16, had only a very weak antiplatelet effect. B7 was a less potent inhibitor of aggregation than B8 but the effects were also primarily NO mediated in PRP, where the furazan counterpart was largely ineffectual. However, in WP, it was apparent that the antiplatelet activity of B7 comprised both NO-dependent and -independent components. The existing nitrooxy ester, nitro-aspirin (NCX4016), was also an effective antiplatelet agent in WP but the effects were entirely sGC independent and were altogether lost in PRP. The release of NO from the furoxans was found to be critically dependent on the presence of endogenous reducing agents. NO generation was most striking in the presence of intracellular levels of ascorbate+GSH, which acted synergistically to release NO. By contrast, the nitrooxy ester, NCX4016, failed to generate detectable NO in samples containing either platelets or plasma.
Both the nitrooxy ester, NCX4016, and the furoxan–aspirin hybrids were demonstrated to significantly inhibit COX-1. The alterations to the chemical structure in the hybrids perhaps play a role in bringing about the enhanced inhibition that was observed compared to the unaltered aspirin compound. The powerful inhibitory effect of the furoxan–aspirin hybrids in the purified COX-1 assay, together with the equally powerful effects of the furazan equivalents and the lack of significant inhibition observed with the NO donor DEA/NO, suggests that the COX-1-inhibitory activity of B8 and B7 is more likely to be aspirin-mediated than NO-mediated. This assay demonstrates that, in addition to their ability to inhibit platelet aggregation via NO, the furoxan–aspirin hybrids retain an aspirin-like action in vitro. At face value, these data would indicate that any antiplatelet effects of the furoxan–aspirin hybrids should be at least partially mediated by aspirin-mediated inhibition of COX-1. However, it is important to recognize that this effect might be lost in biological media or in vivo, depending on how these compounds are hydrolysed under physiologically relevant conditions. Previous studies (Cena et al., 2003) have demonstrated complete hydrolysis of the furoxan–aspirin acetyl group in serum. This feature would suggest that in plasma, there is at least a partial loss of the acetyl group, resulting in formation of the salicylic acid equivalent. As salicylic acid was demonstrated to have no effect in this assay of COX activity and failed to affect platelet aggregation, modifications to the chemical structure to improve retention of the acetyl group in plasma is likely to be necessary to avoid loss of the COX-inhibiting effects of the compounds. Other groups have also demonstrated that COX inhibition by the hybrid NCX4016 has a similar requirement for the acetyl group but not the NO moiety (Corazzi et al., 2005).
The present study extends findings from preliminary aggregation studies which have been published previously (Cena et al., 2003). Here, we further investigated the relative contribution of the NO- and aspirin moieties to the antiplatelet effect. The furoxan–aspirin hybrid drugs inhibited platelet aggregation in both WP and PRP, although the effects of both compounds, and B7 in particular, were attenuated in PRP. The comparatively weak inhibitory effects of the NO-free furazan counterparts is indicative of a major role for NO in platelet inhibition, while the inhibitory effect of ODQ confirmed that a major component of the effects is sGC dependent, especially in PRP. The impact of ODQ on responses was less pronounced in WP, particularly in the case of B7, due in part to the greater influence of NO-independent effects, as illustrated by increased sensitivity of WP to the furazan counterparts of B7 and B8. The effects of the furazan derivatives were weak compared to aspirin, but were nevertheless more potent than salicylic acid under the conditions of these experiments. The lower activity of these agents in PRP compared to WP is in keeping with the complete hydrolysis of the furoxan–aspirin acetyl group in serum (Cena et al., 2003), resulting in formation of relatively ineffectual salicylic acid. These hybrid molecules are likely to undergo hydrolysis at two positions: firstly, at the acetyl group, converting aspirin to salicylic acid and secondly at the other ester linkage to release the furoxan. The order and rates at which hydrolysis occurs will greatly influence the ability of these compounds to retain aspirin activity.
The high levels of NO release by B8 and the loss of its aspirin effect in PRP imply that its antiplatelet actions are mainly NO mediated. By comparison, B7, which releases NO more slowly, has an IC50 closer to that of aspirin. These characteristics are more suitable for a NO–aspirin hybrid drug because they provide potential for a more balanced action between NO and aspirin antiplatelet effects. The unfortunate loss of the aspirin effect in PRP, however, is an issue that needs to be addressed by appropriate modification of the chemical structure.
The finding that NCX4016 is an effective inhibitor of platelet aggregation in WP is in keeping with previous in vitro studies using NCX4016 (Lechi et al., 1996) and the related drug, NCX4125 (Minuz et al., 1995; Wallace et al., 1995). However, our results go on to show that the effect is lost in PRP, possibly due to sequestration by a plasma constituent such as albumin or due to breakdown in the plasma to the inactive salicylic acid. Interestingly, the inhibitory effect of NCX4016 was significantly enhanced compared to aspirin but was not affected by the sGC inhibitor, ODQ. While a cGMP-independent effect of NCX4016-derived NO cannot be ruled out, our inability to detect NO generation from this compound, coupled with the known inability of platelets to effect NO release from organic nitrates (Weber et al., 1996), and the COX assay data demonstrating powerful anti-COX activity of NCX4016, suggests that the antiplatelet action of NCX4016 is most likely NO independent. Although our study shows NCX4016 to be a poor inhibitor of platelet function, its effects in other cell types may still make it a useful cardiovascular drug. Antiplatelet effects may still be retained in vivo through remote nitrooxy-ester activation in cells other than platelets (e.g. smooth muscle cells), although this would appear to be a rather inefficient method of NO delivery specifically to platelets. Nevertheless, antiplatelet effects of NCX4016 have been demonstrated ex vivo, in animals and humans (Wainwright et al., 2002; Fiorucci et al., 2003; 2004; Momi et al., 2005). Furthermore, NCX4016 has beneficial effects in vivo, where it has been demonstrated to protect the vascular endothelium in diabetic rats (Pieper et al., 2002), to reduce blood pressure in hypertensive rats (Muscara et al., 2001), to prevent restenosis in hypercholesterolemic mice (Napoli et al., 2001) and to reduce infarct size in a model of cardiac ischaemia in pigs (Wainwright et al., 2002).
The furoxan drugs are very stable compounds that appear only to decompose to release significant NO when they encounter appropriate media. There was no release of NO from the furoxans in Tyrode's buffer, but NO generation was detected in PPP, WP and PRP. These observations suggest that some elements of plasma can stimulate decomposition of the furoxans and that the platelets themselves enhance the effect to a greater extent for B8 than B7.
The plasma components, albumin, glutathione and ascorbate, chosen for their reducing properties, were all found to have a limited capacity to stimulate furoxan decomposition to release NO. In the case of B7, these constituents could individually stimulate moderate NO release, and the additive effect of all three could fully emulate NO release in PPP. The effect of these constituents on B8 were similar, if less dramatic, for the individual reducing agents, but here there was no additive effect when coincubated, suggesting that an as yet unidentified plasma constituent is responsible for a proportion of NO release from B8.
Reconstitution of Tyrode's buffer with intracellular levels of GSH and ascorbate demonstrated that both these reducing agents were mildly effective in stimulating release of NO from the compounds. Interestingly, however, they acted synergistically in coincubation experiments to massively increase NO generation. The separate experiments using platelet extracts established that there was a relationship between the concentration of platelet extract and amount of NO generated from the furoxans. The ability of platelet extracts to generate NO from both furoxans was found to be dependent on the presence of reduced thiol groups and, in keeping with our other NO release data and the platelet aggregation experiments, B8 generated considerably more NO in the 10 min incubation period than B7. Taken together, these results indicate that low-level decomposition of B7 in plasma can be explained by the additive effects of albumin, GSH and ascorbate but that there is also likely to be much greater NO release inside platelets mediated by the synergistic action of ascorbate and reduced intracellular thiols. B8 is more sensitive to the same agents, and its plasma decomposition is also affected by an as yet unidentified plasma component. Given the reactive nature of NO, the apparent preferential release inside target cells is a likely advantage for successful NO delivery over compounds that can only generate NO remotely in cell types other than platelets.
Previous studies have shown that both furoxan–aspirins (Cena et al., 2003) and NCX4016 (Fiorucci et al., 1999; 2003; Fiorucci & Del Soldato, 2003) have a preferential gastrotoxicity profile over aspirin, suggesting either a masking of the toxic aspirin effect or an active NO element in the gastrointestinal tract. However, the activity of both the NO and aspirin elements of the hybrids in platelets is questionable. NCX4016 has no antiplatelet action in PRP, and in WP it fails to demonstrate aspirin-independent effects, possibly due to sequestration by a plasma constituent. Unlike NCX4016, the furoxans demonstrate aspirin-independent effects on platelet aggregation in both PRP and WP in vitro, but their ability to retain the action of aspirin is compromised, particularly in PRP. The furoxans appear to display a more effective antiplatelet activity over NCX4016, but further molecular modifications are necessary in an effort to retain the aspirin actions, in addition to achieving the added benefits of NO.
In summary, furoxan–aspirin hybrids are stable antiplatelet compounds, the decomposition of which is catalysed by endogenous agents which may instil a potential for primarily intracellular delivery of NO, on account of the differential distribution of glutathione and ascorbate within cells compared to plasma and extracellular fluid. While the furoxan hybrid examples tested in these experiments carry a number of limitations, they also highlight the therapeutic potential of future exponents of this class of drugs.
Acknowledgments
C.M.T. is supported by a BHF Student Fellowship (FS/03/068). The EPR miniscope is supported by a BHF core facility Grant (CUI/99010).
Abbreviations
- AA
arachidonic acid
- AUC
area under the curve
- cGMP
cyclic 5′-guanosine monophosphate
- COX-1
cyclooxygenase-1
- CPH
1-hydroxy-3-carboxy-2,2,5,5-tetramethylpyrrolidine.HCl
- DEA/NO
2-(N,N-diethyamino)-diazenolate-2-oxide
- DMSO
dimethylsulphoxide
- DTNB
5,5′-dithio-bis(2-nitrobenzoic acid)
- EPR
electron paramagnetic resonance
- GSH
glutathione
- NO
nitric oxide
- ODQ
1H-[1,2,4]oxadiazolo[4,3-α]quinoxalin-1-one
- PPP
platelet poor plasma
- PRP
platelet-rich plasma
- sGC
soluble guanylate cyclase
- TFA
trifluoroacetic acid
- WP
washed platelets
References
- ANDERSON T.J., GERHARD M.D., MEREDITH I.T., CHARBONNEAU F., DELAGRANGE D., CREAGER M.A., SELWYN A.P., GANZ P. Systemic nature of endothelial dysfunction in atherosclerosis. Am. J. Cardiol. 1995;75:71B–74B. doi: 10.1016/0002-9149(95)80017-m. [DOI] [PubMed] [Google Scholar]
- BROWN J.F., KEATES A.C., HANSON P.J., WHITTLE B.J. Nitric oxide generators and cGMP stimulate mucus secretion by rat gastric mucosal cells. Am. J. Physiol. 1993;265:G418–G422. doi: 10.1152/ajpgi.1993.265.3.G418. [DOI] [PubMed] [Google Scholar]
- BURCH J.W., STANFORD N., MAJERUS P.W. Inhibition of platelet prostaglandin synthetase by oral aspirin. J. Clin. Invest. 1978;61:314–319. doi: 10.1172/JCI108941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMERON A.J. Aspirin and gastric ulcer. Mayo Clin. Proc. 1975;50:565–570. [PubMed] [Google Scholar]
- CENA C., LOLLI M.L., LAZZARATO L., GUAITA E., MORINI G., CORUZZI G., MCELROY S.P., MEGSON I.L., FRUTTERO R., GASCO A. Antiinflammatory, gastrosparing, and antiplatelet properties of new NO-donor esters of aspirin. J. Med. Chem. 2003;46:747–754. doi: 10.1021/jm020969t. [DOI] [PubMed] [Google Scholar]
- CORAZZI T., LEONE M., MAUCCI R., CORAZZI L., GRESELE P. Direct and Irreversible Inhibition of Cyclooxygenase-1 by Nitroaspirin (NCX 4016) J. Pharmacol. Exp. Ther. 2005;315:1331–1337. doi: 10.1124/jpet.105.089896. [DOI] [PubMed] [Google Scholar]
- CRANE M.S., OLLOSSON R., MOORE K.P., ROSSI A.G., MEGSON I.L. Novel role for low molecular weight plasma thiols in nitric oxide-mediated control of platelet function. J. Biol. Chem. 2002;277:46858–46863. doi: 10.1074/jbc.M208608200. [DOI] [PubMed] [Google Scholar]
- CRANE M.S., ROSSI A.G., MEGSON I.L. A potential role for extracellular nitric oxide generation in cGMP-independent inhibition of human platelet aggregation: biochemical and pharmacological considerations. Br. J. Pharmacol. 2005;144:849–859. doi: 10.1038/sj.bjp.0706110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEWITT D.L., SMITH W.L. Primary structure of prostaglandin G/H synthase from sheep vesicular gland determined from the complementary DNA sequence. Proc. Natl. Acad. Sci. U.S.A. 1988;85:1412–1416. doi: 10.1073/pnas.85.5.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FIORUCCI S., DEL SOLDATO P. NO-aspirin: mechanism of action and gastrointestinal safety. Dig. Liver Dis. 2003;35 Suppl 2:S9–S19. doi: 10.1016/s1590-8658(03)00047-1. [DOI] [PubMed] [Google Scholar]
- FIORUCCI S., ANTONELLI E., SANTUCCI L., MORELLI O., MIGLIETTI M., FEDERICI B., MANNUCCI R., DEL SOLDATO P., MORELLI A. Gastrointestinal safety of nitric oxide-derived aspirin is related to inhibition of ICE-like cysteine proteases in rats. Gastroenterology. 1999;116:1089–1106. doi: 10.1016/s0016-5085(99)70012-0. [DOI] [PubMed] [Google Scholar]
- FIORUCCI S., MENCARELLI A., MENEGUZZI A., LECHI A., RENGA B., DEL SOLDATO P., MORELLI A., MINUZ P. Co-administration of nitric oxide-aspirin (NCX-4016) and aspirin prevents platelet and monocyte activation and protects against gastric damage induced by aspirin in humans. J. Am. Coll. Cardiol. 2004;44:635–641. doi: 10.1016/j.jacc.2004.03.079. [DOI] [PubMed] [Google Scholar]
- FIORUCCI S., SANTUCCI L., GRESELE P., FACCINO R.M., DEL SOLDATO P., MORELLI A. Gastrointestinal safety of NO-aspirin (NCX-4016) in healthy human volunteers: a proof of concept endoscopic study. Gastroenterology. 2003;124:600–607. doi: 10.1053/gast.2003.50096. [DOI] [PubMed] [Google Scholar]
- FITZGERALD G.A. Mechanisms of platelet activation: thromboxane A2 as an amplifying signal for other agonists. Am. J. Cardiol. 1991;68:11B–15B. doi: 10.1016/0002-9149(91)90379-y. [DOI] [PubMed] [Google Scholar]
- GIULIANO F., WARNER T.D. Ex vivo assay to determine the cyclooxygenase selectivity of non-steroidal anti-inflammatory drugs. Br. J. Pharmacol. 1999;126:1824–1830. doi: 10.1038/sj.bjp.0702518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDGE M.P., HOTHERSALL J.S., NORONHA-DUTRA A.A. Evidence for a cyclic GMP-independent mechanism in the anti-platelet action of S-nitrosoglutathione. Br. J. Pharmacol. 1998;124:141–148. doi: 10.1038/sj.bjp.0701821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSSER N., SCHRODER H. A common pathway for nitric oxide release from NO-aspirin and glyceryl trinitrate. Biochem. Biophys. Res. Commun. 2000;274:255–258. doi: 10.1006/bbrc.2000.3121. [DOI] [PubMed] [Google Scholar]
- HAMBERG M., SVENSSON J., SAMUELSSON B. Thromboxanes: a new group of biologically active compounds derived from prostaglandin endoperoxides. Proc. Natl. Acad. Sci. U.S.A. 1975;72:2994–2998. doi: 10.1073/pnas.72.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOMER K.L., WANSTALL J.C. Inhibition of rat platelet aggregation by the diazeniumdiolate nitric oxide donor MAHMA NONOate. Br. J. Pharmacol. 2002;137:1071–1081. doi: 10.1038/sj.bjp.0704971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISIS-2 Randomized trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2.ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. J. Am. Coll. Cardiol. 1988;12:3A–13A. doi: 10.1016/0735-1097(88)92635-6. [DOI] [PubMed] [Google Scholar]
- KESSELS H., BEGUIN S., ANDREE H., HEMKER H.C. Measurement of thrombin generation in whole blood – the effect of heparin and aspirin. Thromb. Haemost. 1994;72:78–83. [PubMed] [Google Scholar]
- KOBZAR G., MARDLA V., JARVING I., SAMEL N. Comparison of anti-aggregatory effects of PGI2, PGI3 and iloprost on human and rabbit platelets. Cell. Physiol. Biochem. 2001;11:279–284. doi: 10.1159/000047814. [DOI] [PubMed] [Google Scholar]
- LECHI C., ANDRIOLI G., GAINO S., TOMMASOLI R., ZULIANI V., ORTOLANI R., DEGAN M., BENONI G., BELLAVITE P., LECHI A., MINUZ P. The antiplatelet effects of a new nitroderivative of acetylsalicylic acid – an in vitro study of inhibition on the early phase of platelet activation and on TXA2 production. Thromb. Haemost. 1996;76:791–798. [PubMed] [Google Scholar]
- MA L., WALLACE J.L. Endothelial nitric oxide synthase modulates gastric ulcer healing in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;279:G341–G346. doi: 10.1152/ajpgi.2000.279.2.G341. [DOI] [PubMed] [Google Scholar]
- MANSON J.E., STAMPFER M.J., COLDITZ G.A., WILLETT W.C., ROSNER B., SPEIZER F.E., HENNEKENS C.H. A prospective study of aspirin use and primary prevention of cardiovascular disease in women. JAMA. 1991;266:521–527. [PubMed] [Google Scholar]
- MAXWELL A.J. Mechanisms of dysfunction of the nitric oxide pathway in vascular diseases. Nitric Oxide. 2002;6:101–124. doi: 10.1006/niox.2001.0394. [DOI] [PubMed] [Google Scholar]
- MINUZ P., LECHI C., TOMMASOLI R., GAINO S., DEGAN M., ZULIANI V., BONAPACE S., BENONI G., ADAMI A., CUZZOLIN L., LECHI A. Antiaggregating and vasodilatory effects of a new nitroderivative of acetylsalicylic acid. Thromb. Res. 1995;80:367–376. doi: 10.1016/0049-3848(95)00189-x. [DOI] [PubMed] [Google Scholar]
- MOMI S., PITCHFORD S.C., ALBERTI P.F., MINUZ P., DEL SOLDATO P., GRESELE P. Nitroaspirin plus clopidogrel versus aspirin plus clopidogrel against platelet thromboembolism and intimal thickening in mice. Thromb. Haemost. 2005;93:535–543. doi: 10.1160/TH04-07-0464. [DOI] [PubMed] [Google Scholar]
- MUSCARA M.N., LOVREN F., MCKNIGHT W., DICAY M., DEL SOLDATO P., TRIGGLE C.R., WALLACE J.L. Vasorelaxant effects of a nitric oxide-releasing aspirin derivative in normotensive and hypertensive rats. Br. J. Pharmacol. 2001;133:1314–1322. doi: 10.1038/sj.bjp.0704209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAPOLI C., CIRINO G., DEL SOLDATO P., SORRENTINO R., SICA V., CONDORELLI M., PINTO A., IGNARRO L.J. Effects of nitric oxide-releasing aspirin versus aspirin on restenosis in hypercholesterolemic mice. Proc. Natl. Acad. Sci. U.S.A. 2001;98:2860–2864. doi: 10.1073/pnas.041602898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PALMER R.M., FERRIGE A.G., MONCADA S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- PASQUI A.L., CAPECCHI P.L., CECCATELLI L., MAZZA S., GISTRI A., LAGHI PASINI F., DI PERRI T. Nitroprusside in vitro inhibits platelet aggregation and intracellular calcium translocation. Effect of haemoglobin. Thromb. Res. 1991;61:113–122. doi: 10.1016/0049-3848(91)90238-r. [DOI] [PubMed] [Google Scholar]
- PATRONO C. Aspirin as an antiplatelet drug. N. Engl. J. Med. 1994;330:1287–1294. doi: 10.1056/NEJM199405053301808. [DOI] [PubMed] [Google Scholar]
- PHS-COMMITTEE Final report on the aspirin component of the ongoing Physicians' Health Study. Steering Committee of the Physicians' Health Study Research Group. N. Engl. J. Med. 1989;321:129–135. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- PIEPER G.M., SIEBENEICH W., OLDS C.L., FELIX C.C., DEL SOLDATO P. Vascular protective actions of a nitric oxide aspirin analog in both in vitro and in vivo models of diabetes mellitus. Free Radic. Biol. Med. 2002;32:1143–1156. doi: 10.1016/s0891-5849(02)00832-8. [DOI] [PubMed] [Google Scholar]
- RADOMSKI M.W., PALMER R.M., MONCADA S. The anti-aggregating properties of vascular endothelium: interactions between prostacyclin and nitric oxide. Br. J. Pharmacol. 1987a;92:639–646. doi: 10.1111/j.1476-5381.1987.tb11367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RADOMSKI M.W., PALMER R.M., MONCADA S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet. 1987b;2:1057–1058. doi: 10.1016/s0140-6736(87)91481-4. [DOI] [PubMed] [Google Scholar]
- RADOMSKI M.W., PALMER R.M., MONCADA S. The role of nitric oxide and cGMP in platelet adhesion to vascular endothelium. Biochem. Biophys. Res. Commun. 1987c;148:1482–1489. doi: 10.1016/s0006-291x(87)80299-1. [DOI] [PubMed] [Google Scholar]
- RADOMSKI M.W., PALMER R.M., MONCADA S. An L-arginine/nitric oxide pathway present in human platelets regulates aggregation. Proc. Natl. Acad. Sci. U.S.A. 1990;87:5193–5197. doi: 10.1073/pnas.87.13.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAINSFORD K.D., WHITEHOUSE M.W. Gastric irritancy of aspirin and its congeners: anti-inflammatory activity without this side-effect. J. Pharm. Pharmacol. 1976;28:599–601. doi: 10.1111/j.2042-7158.1976.tb02807.x. [DOI] [PubMed] [Google Scholar]
- ROBERT A., NEZAMIS J.E., LANCASTER C., HANCHAR A.J. Cytoprotection by prostaglandins in rats. Prevention of gastric necrosis produced by alcohol, HCl, NaOH, hypertonic NaCl, and thermal injury. Gastroenterology. 1979;77:433–443. [PubMed] [Google Scholar]
- ROTH G.J., MAJERUS P.W. The mechanism of the effect of aspirin on human platelets. I. Acetylation of a particulate fraction protein. J. Clin. Invest. 1975;56:624–632. doi: 10.1172/JCI108132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTH G.J., STANFORD N., MAJERUS P.W. Acetylation of prostaglandin synthase by aspirin. Proc. Natl. Acad. Sci. U.S.A. 1975;72:3073–3076. doi: 10.1073/pnas.72.8.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHOEN R.T., VENDER R.J. Mechanisms of nonsteroidal anti-inflammatory drug-induced gastric damage. Am. J. Med. 1989;86:449–458. doi: 10.1016/0002-9343(89)90344-6. [DOI] [PubMed] [Google Scholar]
- SCHREIBER J., FOUREMAN G.L., HUGHES M.F., MASON R.P., ELING T.E. Detection of glutathione thiyl free radical catalyzed by prostaglandin H synthase present in keratinocytes. Study of co-oxidation in a cellular system. J. Biol. Chem. 1989;264:7936–7943. [PubMed] [Google Scholar]
- SEAGER J.M., HAWKEY C.J. ABC of the upper gastrointestinal tract: indigestion and non-steroidal anti-inflammatory drugs. BMJ. 2001;323:1236–1239. doi: 10.1136/bmj.323.7323.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAUL P.W. Endothelial nitric oxide synthase, caveolae and the development of atherosclerosis. J. Physiol. 2003;547:21–33. doi: 10.1113/jphysiol.2002.031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOGO N., MAGID K.S., SHAW C.A., WEBB D.J., MEGSON I.L. Inhibition of human platelet aggregation by nitric oxide donor drugs: relative contribution of cGMP-independent mechanisms. Biochem. Biophys. Res. Commun. 2000;279:412–419. doi: 10.1006/bbrc.2000.3976. [DOI] [PubMed] [Google Scholar]
- SZCZEKLIK A., KRZANOWSKI M., GORA P., RADWAN J. Antiplatelet drugs and generation of thrombin in clotting blood. Blood. 1992;80:2006–2011. [PubMed] [Google Scholar]
- TRAMER M.R., MOORE R.A., REYNOLDS D.J., MCQUAY H.J. Quantitative estimation of rare adverse events which follow a biological progression: a new model applied to chronic NSAID use. Pain. 2000;85:169–182. doi: 10.1016/s0304-3959(99)00267-5. [DOI] [PubMed] [Google Scholar]
- TREPAKOVA E.S., COHEN R.A., BOLOTINA V.M. Nitric oxide inhibits capacitative cation influx in human platelets by promoting sarcoplasmic/endoplasmic reticulum Ca2+-ATPase-dependent refilling of Ca2+ stores. Circ. Res. 1999;84:201–209. doi: 10.1161/01.res.84.2.201. [DOI] [PubMed] [Google Scholar]
- TSAO P.S., WANG B., BUITRAGO R., SHYY J.Y., COOKE J.P. Nitric oxide regulates monocyte chemotactic protein-1. Circulation. 1997;96:934–940. doi: 10.1161/01.cir.96.3.934. [DOI] [PubMed] [Google Scholar]
- WAINWRIGHT C.L., MILLER A.M., WORK L.M., DEL SOLDATO P. NCX4016 (NO-aspirin) reduces infarct size and suppresses arrhythmias following myocardial ischaemia/reperfusion in pigs. Br. J. Pharmacol. 2002;135:1882–1888. doi: 10.1038/sj.bjp.0704646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLACE J.L., MILLER M.J. Nitric oxide in mucosal defense: a little goes a long way. Gastroenterology. 2000;119:512–520. doi: 10.1053/gast.2000.9304. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L. Nonsteroidal anti-inflammatory drugs and gastroenteropathy: the second hundred years. Gastroenterology. 1997;112:1000–1016. doi: 10.1053/gast.1997.v112.pm9041264. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L., MCKNIGHT W., DEL SOLDATO P., BAYDOUN A.R., CIRINO G. Anti-thrombotic effects of a nitric oxide-releasing, gastric-sparing aspirin derivative. J. Clin. Invest. 1995;96:2711–2718. doi: 10.1172/JCI118338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBER A.A., NEUHAUS T., SEUL C., DUSING R., SCHROR K., SACHINIDIS A., VETTER H. Biotransformation of glyceryl trinitrate by blood platelets as compared to vascular smooth muscle cells. Eur. J. Pharmacol. 1996;309:209–213. doi: 10.1016/0014-2999(96)00338-x. [DOI] [PubMed] [Google Scholar]
- WHITTLE B.J. Mechanisms underlying gastric mucosal damage induced by indomethacin and bile-salts, and the actions of prostaglandins. Br. J. Pharmacol. 1977;60:455–460. doi: 10.1111/j.1476-5381.1977.tb07522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]