Abstract
1. Glucose is one of the most important substrates for generating metabolic energy required for the maintenance of cellular functions. Glucose-mediated changes in neuronal firing pattern have been observed in the central nervous system of mammals. K+ channels directly regulated by intracellular ATP have been postulated as a linkage between cellular energetic metabolism and excitability; the functional roles ascribed to these channels include glucose-sensing to regulate energy homeostasis and neuroprotection under energy depletion conditions. The hippocampus is highly sensitive to metabolic insults and is the brain region most sensitive to ischemic damage. Because the identity of metabolically regulated potassium channels present in hippocampal neurons is obscure, we decided to study the biophysical properties of glucose-sensitive potassium channels in hippocampal neurons.
2. The dependence of membrane potential and the sensitivity of potassium channels to glucose and ATP in rat hippocampal neurons were studied in cell-attached and excised inside-out membrane patches.
3. We found that under hypoglycemic conditions, at least three types of potassium channels were activated; their unitary conductance values were 37, 147, and 241 pS in symmetrical K+, and they were sensitive to ATP. For K+ channels with unitary conductance of 37 and 241, when the membrane potential was depolarized the longer closed time constant diminished and this produced an increase in the open-state probability; nevertheless, the 147-pS channels were not voltage-dependent.
4. We propose that neuronal glucose-sensitive K+ channels in rat hippocampus include subtypes of ATP-sensitive channels with a potential role in neuroprotection during short-term or prolonged metabolic stress.
KEY WORDS: Glucose, Potassium channels, ATP, Hippocampal neurons
INTRODUCTION
Glucose is one of the most important substrates for generating the metabolic energy required for the maintenance of cellular functions. Therefore, living organisms must be endowed with mechanisms to regulate the availability of this metabolic source to maintain the energy requirements. Fluctuations of metabolic factors (substrates, intermediates, or end products) modify neuronal function by regulating ion channels, a critical property for controlling homeostasis in the mammalian brain. Several authors have demonstrated that glucose modifies neuronal membrane potential by decreasing potassium conductance (Tang et al., 2004). A member of the inwardly rectifying K+ channel family that is directly regulated by the intracellular ATP/ADP ratio, has been postulated as a linkage between excitability and cellular energetic metabolism in different tissues (Jiang et al., 1994; Aguilar-Bryan and Bryan, 1999; Seino and Miki, 2003). The study of the so-called KATP channels is a rapidly expanding field; from a molecular point of view, KATP channels are heteromultimers of two types of subunits; a pore forming subunit (either Kir6.1 or Kir6.2) and a sulfonylurea receptor (SUR1, SUR2A, SUR2B, or SUR2C) (Ashcroft and Gribble, 1998). This K+ channel family is heterogeneous regarding its functional role and biophysical properties in different tissues, but it is distinguished by the blockade upon the increase of intracellular concentration of ATP and the modulation by sulfonylureas. On the whole, the single-channel conductance of ATP-sensitive K+ channels is relatively large, ranging from 50 to 150 pS when exposed to symmetrical K+ gradient (Ashcroft, 1988; Noma and Takano, 1991). Intracellular ATP, but not membrane potential, does regulate the open-state probability of this channel; however, voltage-dependence of single-channel kinetics has been postulated by several authors (Jiang et al., 1994; Spruce et al., 1985). Intracellular concentration of ATP that produces half-maximal inhibition of the channel has been reported from 13 to 500 μM depending on cell type (Ashcroft, 1988); the range of sensitivity to ATP in different isoforms of the KATP channel as well as their distribution in the brain is necessary to understand their functional relevance.
By using high affinity binding to sulfonylureas (Zini et al., 1993) and in situ hybridization analysis (Karschin et al., 1997; Zawar et al., 1999), it has been confirmed that KATP channels are widely distributed in the nervous system. In some regions, like the midbrain (Stanford and Lacey, 1995), neocortex (Jiang and Haddad, 1997), substantia nigra (Jiang et al., 1994; Stanford and Lacey, 1996), and hypothalamus (Dunn-Meynell et al., 1997; Hall et al., 1997; Routh et al., 1997; Ibrahim et al., 2003; Allen and Brown, 2004), the biophysical and pharmacological profile of KATP channels have been broadly characterized. However, the information about their functional properties in the hippocampus is limited (Tromba et al., 1992, 1994; Zawar et al., 1999), even when hippocampal neurons express high density of glibenclamide binding sites.
Using pharmacological and histological approaches it has been suggested that KATP channels could be involved in cytoprotective mechanisms in hippocampal neurons during cerebral ischemia (Herteaux et al., 1995). Under physiological conditions an increase in glucose metabolism stimulates ATP production and therefore the closure of KATP channels, leading to a rapid membrane depolarization. However, energy depletion caused by hypoxia or ischemia results in hyperpolarization due to the opening of KATP channels, which may prevent the influx of calcium and excitotoxicity (Fujimura et al., 1997). Application of drugs with action as potassium channel openers, such as levo-cromakalin (Herteaux et al., 1995) or diazoxide (Takaba et al., 1997), reduces neuronal damage induced by an experimental ischemia through bilateral occlusion of common carotid arteries (Jiang et al., 1994; Herteaux et al., 1995; Mironov et al., 1998; Nakagawa et al., 2003).
The CA1 area of the hippocampus is a region of the brain that is particularly vulnerable to ischemia/reperfusion-induced damage (Smith et al., 1984), together with the fact that CA1 is the region in which there is a lower density of ATP-sensitive potassium channels (Karschin et al., 1997; Zawar et al., 1999). This further supports the hypothesis that KATP channels play an important role in the cellular response to ischemic damage. Although progress has been made in delineating the mechanism involved in KATP-mediated protection during ischemic damage, not much is known about the biophysical properties of the KATP channels in the hippocampus. Therefore, the aim of the present study is to examine the biophysical properties of glucose-sensitive potassium channels in hippocampal neurons under metabolically stressful conditions, as well as their modulation by intracellular nucleotides and sulphonylureas. According to our results, hippocampal neurons respond to glucose depletion through the activation of at least three different types of potassium channels that are sensitive to ATP. These glucose-sensitive channels could participate as effectors in the biochemical cascades triggered during abnormal metabolic conditions, such as cerebral ischemia.
MATERIALS AND METHODS
Culture of Hippocampal Neurons
The experiments were carried out in cultured neurons from hippocampus of Wistar rats. To culture the hippocampal neurons, we used the method described by Jahr and Stevens (1987) with minor changes. Briefly, hippocampus was removed from 1- to 3-day-old newborn rats, and placed in Ringer solution (see Table I). Ringer solution was supplemented with antibiotic-antimycotic (10,000 unit of penicillin, 10,000 μg of streptomycin and 25 μg of amphotericin B) (5 μL/mL, Sigma Chemical Co. St. Louis, MO, USA), and saturated with oxygen at 4°C. Coronal hippocampal slices were incubated in 0.05% Trypsin-EDTA solution for 35 min at room temperature. The slices were then washed three times with Dulbecco's Modified Eagle Medium (D-MEM), and resuspended in the complete growth medium D-MEM (supplemented with antibiotic, 10% fetal bovine serum and 2 mg/mL of albumin). The slices were dissociated by triturating with a fire-polished Pasteur pipette and centrifuged for 5 min at 800 rpm. The pellet was resuspended in complete D-MEM and plated onto glass coverslips previously coated with poly-l-lysine (2 mg/mL). Arabinosylcytosine (1 μM) was added to cultures on the second and fourth days after plating to suppress the proliferation of non-neuronal cells. The neurons were used after the eighth day in culture.
Table I.
Composition of Solutions (mM)
| Ringer | High-K (pipette) | Internala (bath) | |
|---|---|---|---|
| NaCI | 130 | ||
| KCl | 5.4 | 150 | 150 |
| CaCl2 | 1 | ||
| MgCl2 | 1 | ||
| HEPES | 10 | 10 | 10 |
| D-Glucose | 25 | ||
| EGTA | 5 |
aIn this solution, ATP or glibenclamide were added at different concentrations. The pH of Ringer and high-K solutions were adjusted to 7.4 with NaOH or KOH, respectively, and the pH of internal solution was adjusted to 7.2 with KOH. To study channel sensitivity to glucose, glucose was substituted by mannitol in the Ringer solution.
Electrophysiological Techniques
The single-channel current records were obtained with the cell-attached or inside-out patch-clamp technique (Hamill et al., 1981) by using an Axopatch 200A amplifier (Axon Instruments Inc., Foster City, CA, USA). Pipettes with resistance ranging from 8 to 10 M when filled with a high-K solution (see Table I) were used. Patch pipettes were pulled from 1.5 mm borosilicate capillaries, fire polished and coated with Sylgard (Dow Corning Corp., Midland, MI, USA). Unitary currents were stored with a PCM Data Recorder 200 (A.R. Vetter Co., Rebersburg, PA, USA) for subsequent off-line acquisition and analysis. These signals were filtered (1–2 kHz), amplified and sampled at 10 kHz using a Digidata 1200 A/D converter (Axon Instruments Inc., Foster City, CA, USA). Analysis was made using a Pentium microcomputer with pClamp6 software (Axon Instruments Inc., Foster City, CA, USA). The open-state probability (P o) values were calculated by using the method described by Quayle et al. (1988). We used current recording periods of 30 s at different membrane potentials to measure the open-state probability and to obtain single-channel conductance values; this recording period was representative for that particular membrane potential and several patches were used to calculate mean values and SEM.
Solutions
In all experiments, the pipettes were filled with high-K solution. In the inside-out configuration experiments, the internal face of the membrane was bathed with internal solution. See Table I for the composition of experimental solutions.
Adenosine 5′-triphosphate (ATP) potassium salt, N-[2-hydroxyethyl]pipera- zine-N′-2-ethanesulfonic acid (HEPES), ethyleneglycol-bis[-aminoethylether] N,N,N′,N′-tetraacetic acid] (EGTA), cytosine 1-β-D-arabinofuranoside, poly-l-Lysine hydrobromide, and mannitol were all purchased from Sigma Chemical Co. (St. Louis, MO, USA). Dulbecco's Modified Eagle Medium, Fetal Bovine Serum, antibiotic-antimycotic (10,000 unit of penicillin, 10,000 μg of streptomycin and 25 μg of amphotericin B), and Trypsin-EDTA were all purchased from GIBCO Invitrogen Co (Grand Island, NY, USA).
RESULTS
Glucose Deprivation Activates Several K+ Unitary Conductances in Neuronal Membranes
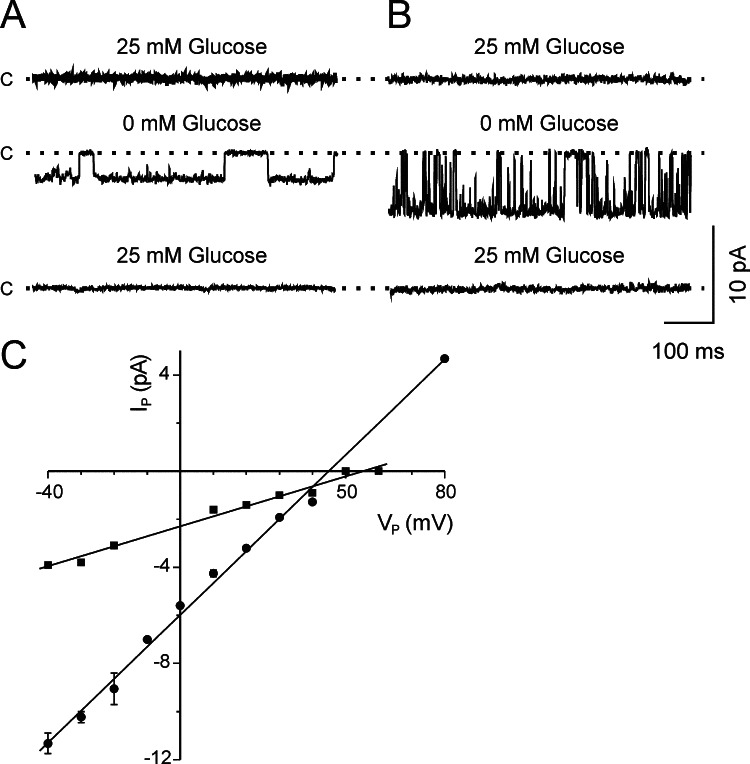
Unitary currents through K+ channels were obtained in the cell-attached patches from cultured hippocampal neurons. It was evident that upon removing the glucose from the extracellular perfusing solution, two different K+ conductances were activated at the resting potential of the cell, and their activity disappeared when glucose was restored (Fig. 1A and B). Conductance values obtained by fitting the experimental data to the linear equation were 41 and 133 pS (Fig. 1C).
Fig. 1.
Effect of glucose on potassium channel activity. (A, B) Unitary activity records obtained in the cell-attached configuration from two different neurons at resting potential (V P=0 mV), in Ringer solution, without glucose, and then perfused again with Ringer solution. Pipette solution high-K (see Table I). Closed (c) current level is indicated to the left of the traces. (C) Current–voltage relationships for the two types of K+ channel in the cell-attached configuration. Straight lines represent the experimental data fitting using the linear equation. Data are presented as means ± SEM (n=2,• 1, ▪)
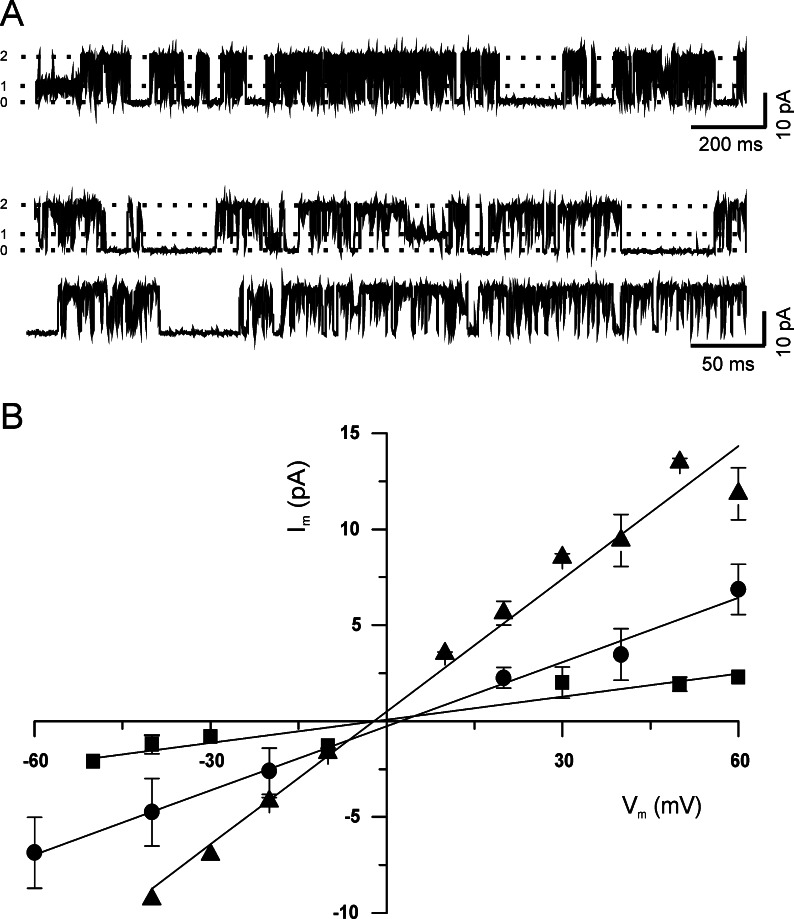
When inside-out membrane patches were excised from the surface of hippocampal neurons in the absence of ATP (internal solution), the activity of K+ channels was observed again (Fig. 2A and B). Under this experimental condition we recorded up to three different types of potassium channels (Fig. 2A) that were classified as small (SK), intermediate (IK) and large (LK), according to their conductance: 37, 147, and 241 pS, respectively (Fig. 2B).
Fig. 2.
Potassium channels in the inside-out patches. (A, B) Single-channel activity in the inside-out configuration obtained in the absence of glucose from one patch of a cultured neuron, displayed at different time scales (V m=40 mV). The three (0–2) current levels are indicated to the left of the traces. Pipettes were filled with high K solution. Membrane patches were perfused with internal solution. (C) Current–voltage relationships for the three types of K+ channel found in the inside-out configuration. Straight lines represent the experimental data fitting using the linear equation. Data are presented as means ± SEM (n=3, ▴; 5, •; 2, ▪).
The application of 1 mM ATP to the intracellular side of the membrane patch blocked the activity of all different types of channels (SK, IK, and LK, Fig. 3) observed at a membrane potential of +40 mV. Furthermore, the activity of glucose-sensitive K+ channels was abolished upon bath application of glibenclamide (100 μM) to the intracellular face of membrane patches.
Fig. 3.
ATP blockade K+ channels. Single-channel activity for SK (A), IK (B), and LK (C) channels obtained under control conditions, in the presence of ATP (1 mM) in the intracellular face of the patch, and after washout (V m=40 mV). Pipettes were filled with high K solution. Membrane patches were perfused with internal solution.
Voltage-Dependence and Kinetic Properties of the Different K+ Channels
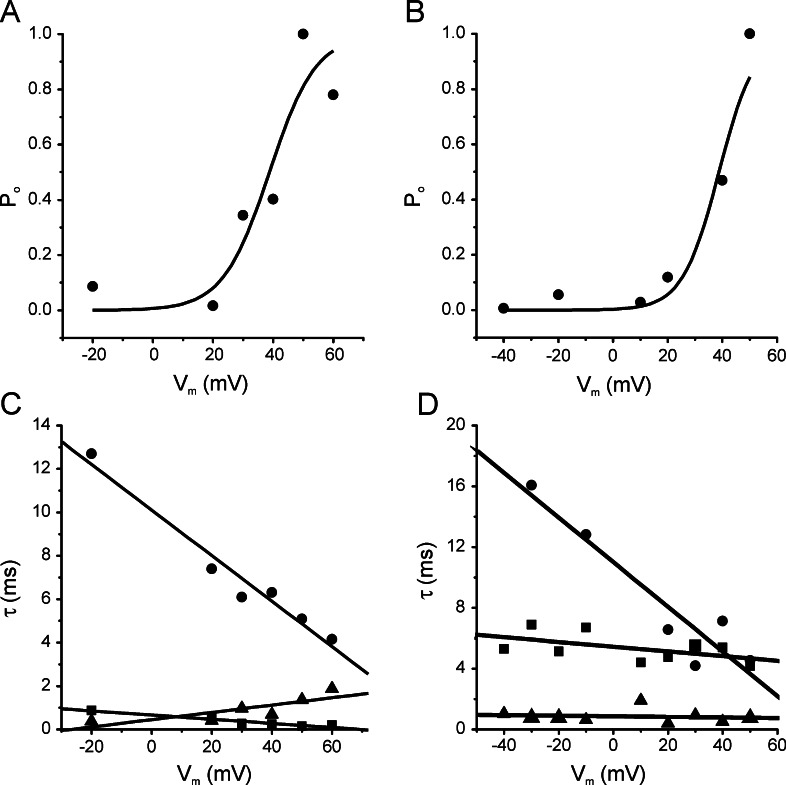
SK and LK channels were voltage-dependent. To analyze this property, we fitted the experimental values of steady state open probability (P o), obtained at different membrane potentials, to the Boltzmann equation:
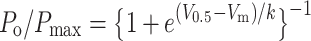 |
where P o is determined experimentally at a specific membrane potential (V m). V 0.5 is the membrane potential at which P o is one-half of its maximum (P max) and k is the factor associated with voltage sensitivity. For SK and LK channels, the midpoint activation (V 0.5) was 38.8 mV (both), and the k values were 7.7 and 6.7 mV, respectively (Fig. 4A and B). The IK channels did not show voltage-dependence.
Fig. 4.
Voltage-dependence and kinetic properties of the SK and LK channels in symmetrical K+ concentration. Steady state open probability (P o) of membrane potential (V m) values for the SK (A) and LK (B) channels. The curve lines correspond to Boltzmann fits for experimental values of P o. Open (τo, ▴) and closed (τc1, ▪; τc2, •) time constants for the SK (C) and LK (D) channels to different membrane potentials. Pipettes were filled with high K solution. Membrane patches were perfused with internal solution.
Open and closed time histograms were obtained from a 30 s recording period in the inside-out configuration at different membrane potentials. The open time distribution was fitted with a simple exponential function, whereas closed time distribution was fit using the sum of two exponentials. For SK as well as LK channels, the open time constant (τ0; ▴) and short closed time constant (τc1; ▪) did not depend on membrane potential, while the longer closed time constant (τc2; •) diminished when the membrane potential was depolarized (Fig. 4C and D).
The kinetics of the SK and LK channels appear to be a classic KATP channel, exhibiting bursting behavior with a burst consisting of a number of fast openings defined by brief closure. The bursts are separated by long closed or silent states. The duration of the bursts determines the time that the channel spends in the open state and this time is delimited by the silent intervals between bursts. The application of ATP without Mg2+ reduces the duration of the bursts and therefore prolongs the silent states of the channel (Kakeim and Noma, 1984; Qin et al., 1989).
In accordance with our results, when the membrane potential depolarizes, the long closed time constant (τc2; •) diminishes (Fig. 4C and D). This means that the time between bursts diminishes when the membrane potential is at its most positive value, therefore increasing the open probability of the channel (Fig. 4A and B).
DISCUSSION
We have recorded inside-out single-channel currents in cultured neurons from rat hippocampus, not having selected one type of cell from one particular region. Thus, only neurons with a pyramidal form were chosen, discarding gial cells and interneurons. In these neurons, we observed the activation of at least three types of K+ channels with conductances of 37 pS (SK), 147 pS (IK), and 241 pS (LK) under hypoglycemic conditions. When experiments were performed in the cell-attached configuration, only SK and IK were observed. Since the activity of the KATP channels is regulated by the metabolic activity of the neurons, it is probable that some cytosolic components involved in cellular metabolism prevent LK channel activation in the cell-attached configuration. Among the cytosolic components, the ATP/ADP ratio is perhaps the most important.
All these K+ conductances were glucose- and ATP-sensitive. Even when the conductance value that we calculated for the LK channels was higher than that previously reported for the KATP channels in basal forebrain neurons and other central neurons (Allen and Brown, 2004), similar conductance for KATP channels has been measured in neocortex (Jiang and Haddad, 1997) and substantia nigra (Jiang et al., 1994). Furthermore, we can discard the identity of these channels as high-conductance calcium-activated potassium channels (Vergara et al., 1998) because our intracellular solution contained enough EGTA (5 mM) to maintain free calcium concentration within a nanomolar range. However, single-channel conductances for SK and IK channels are consistent with the reported values for neuronal tissues (Tromba et al., 1992, 1994; Jiang and Haddad, 1997; Lee et al., 1998;). The heterogeneity of KATP channel conductances has been attributed to different combinations of Kir6.1 or Kir6.2 subunits with SUR1, SUR2A, or SUR2B (Babenko et al., 1998), resulting in different heteromultimers (Kir6.X/SUR)4. Even when it has been established that brain KATP channels are formed by Kir6.2/SUR1 (Inagaki et al., 1995; Aguilar-Bryan et al., 1998), the presence of other isoforms has not been discarded (Zawar et al., 1999).
Furthermore to study the ATP effect, we used the sulphonylurea glibenclamide to abolish activity of these channels, showing that these channels have pharmacological properties similar to a classic KATP channel.
Typically, KATP channels are not voltage-dependent although some authors (Spruce et al., 1985; Jiang et al., 1994) have reported that single-channel kinetics is modified by membrane potential. According to our data, the activity of SK and LK channels from hippocampal neurons is voltage-dependent; in both channels membrane depolarization increases the open-channel probability (P o) by reducing the fraction of time that the channels spend in long interburst closed state (Fig. 4). Voltage-dependence of SK and LK channels is similar to that described in hippocampal neurons by Tromba et al. (1994) for a 100 pS potassium channel; membrane potential does not modulate gating kinetics of hippocampal IK channels.
In the absence of ATP, hippocampal neurons would hyperpolarize; this change in membrane potential might result in a reduction in open probability of SK and LK channels, which in turn would initiate a depolarization. As a whole, the activity of SK and LK channels would modulate action potentials discharge. Since the contribution of potassium current through the SK and LK channels is different due to the difference in the channel conductance, the spike shaping and spontaneous firing pattern regulation would depend on the balance between the levels of activity of both channels.
Our data supports the idea that hippocampal neurons are able to sense and respond to glucose deprivation by changing their membrane conductance; the biochemical cascade seems to involve al least one member of the family of KATP channels, as well as other metabolism-gated K+ channels. Neuronal glucose sensitivity comes into play in critical physiological processes; it has been associated with the maintenance of energy metabolic homeostasis by hypothalamic neurons (Miki et al., 2001; Ibrahim et al., 2003). Also, it has been demonstrated that KATP channels act as mediators of changes in membrane excitability both during physiological conditions and during metabolic insults in forebrain neurons (Allen and Brown, 2004) and Purkinje cerebellar neurons (Ballanyi, 2004).
By studying the effects of KATP channel modulators on the shape and frequency of action potentials in CA1 hippocampal cells, Griesemer et al. (2002) showed that operation of KATP channels suppressed electrical activity only in interneurons with the highest channel density. According to these authors, KATP channels contribute only slightly to the control of pyramidal cell excitability under physiological conditions, but neuroprotective effects may result from their activation during long periods of energy depletion (Griesemer et al., 2002).
We have reported three types of ATP-sensitive potassium channels that are activated by glucose deprivation; at least two different types of these channels coexist in the same pyramidal cell because their activity was observed in the same patch (Fig. 2B), therefore discarding the possibility that the different types of KATP channels correspond to different hippocampus cell types.
Furthermore, we observed for the first time in the hippocampus an ATP-sensitive large conductance potassium channel (LK), described in other cerebral regions; the molecular identity remains to be known.
The presence of several metabolically regulated K+ channels with different conductances in the neurons of the hippocampus may reflect the adaptive ability of these neurons to respond to changes in redox balance. ATP-sensitive potassium channels may also represent a mechanism for neuronal protection during cerebral ischemia or another kind of metabolic stress, but they may also contribute to the modulation of electrical activity as a response to basal levels of glucose.
The subsequent mechanism to a hypoxic has still not been very well understood, but it is known that oxygen depletion in an area of the brain first produces a hypoxic hyperpolarization due to the activation of the channels KATP, and later on, a depolarization of the neighbor cells due to the increment of extracellular potassium. Besides the KATP channels, other factors such as adenosine receptor, glutamate, and calcium jointly participate in neuronal death. Since the channels begin the cascade of events, they have been the target of studies to try to understand the mechanisms of endogenous neuroprotection that neurons suffer when they are subjected to a process called ischemic preconditioning. During ischemic preconditioning, brief ischemia and reperfusion make the brain more resistant to subsequent lethal ischemia. The participation of the KATP channels in the preconditioning has been demonstrated since the channel openers, such as diazoxide and cromakalim, can simulate the ischemic preconditioning (Herteaux et al., 1995; Blondeau et al., 2000).
ACKNOWLEDGMENTS
The authors wish to express their gratitude to Adriana Hernández for technical assistance. This work has been partially supported by grant 229/03 from the “Fondo Ramón Álvarez-Buylla de Aldana,” Universidad de Colima, México to C. G. Onetti.
REFERENCES
- Aguilar-Bryan, L., and Bryan, J. (1999). Molecular biology of adenosine triphosphate-senstive potassium channels. Endocr. Rev.20:101–135. [DOI] [PubMed] [Google Scholar]
- Aguilar-Bryan, L., Clement, J. P. T., Gonzalez, G., Kunjilwar, K., Babenko, A., and Bryan, J. (1998). Toward understanding the assembly and structure of KATP channels. Physiol. Rev.78:227–245. [DOI] [PubMed] [Google Scholar]
- Allen, T. G. J., and Brown, D. A. (2004). Modulation of the excitability of cholinergic basal forebrain neurones by KATP channels. J. Physiol.554(2):353–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft, F. M. (1988). Adenosine 5′-triphosphate-sensitive potassium channels. Annu. Rev. Neurosci.11:97–118. [DOI] [PubMed] [Google Scholar]
- Ashcroft, F. M., and Gribble, F. M. (1998). Correlating structure and function in ATP-sensitive K+ channels. Trends Neurosci.21(7):288–294. [DOI] [PubMed] [Google Scholar]
- Babenko, A. P., Aguilar-Bryan, L., and Bryan, J. (1998). A view of SUR/Kir6.X, KATP channels. Annu. Rev. Physiol.60:667–687. [DOI] [PubMed] [Google Scholar]
- Ballanyi, K. (2004). Protective role of neuronal KATP channels in brain hypoxia. J. Exp. Biol.207(18):3201–3212. [DOI] [PubMed] [Google Scholar]
- Blondeau, N., Plamondon, H., Richelme, C., Heurteaux, C., and Lazdunski, M. (2000). KATP channel openers, adenosine agonists and epileptic preconditioning are stress signals inducing hippocampal neuroprotection. Neuroscience100(3):465–474. [DOI] [PubMed] [Google Scholar]
- Dunn-Meynell, A. A., Routh, V. H., McArdle, J. J., and Levin, B. E. (1997). Low-affinity sulfonylurea binding sites reside on neuronal cell bodies in the brain. Brain Res.745:1–9. [DOI] [PubMed] [Google Scholar]
- Fujimura, N., Tanaka, E., Yamamoto, S., Shigemori, M., and Higashi, H. (1997). Contribution of ATP-sensitive potassium channels to hypoxic hyperpolarization in rat hippocampal CA1 neurons in vitro. J. Neurophysiol.77:378–385. [DOI] [PubMed] [Google Scholar]
- Griesemer, D., Zawar, C., and Neumcke, B. (2002). Cell-type specific depression of neuronal excitability in rat hippocampus by activation of ATP-sensitive potassium channels. Eur. Biophys. J.31:467–477. [DOI] [PubMed] [Google Scholar]
- Hall, A. C., Hoffmaster, R. M., Stern, E. L., Harrington, M. E., and Bickar, D. (1997). Suprachiasmatic nucleus neurons are glucose sensitive. J. Biol. Rhythms12(5):338–400. [DOI] [PubMed] [Google Scholar]
- Hamill, O. P., Marty, A., Neher, E., Sakmann, B., and Sigworth, F. J. (1981). Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch.391:85–100. [DOI] [PubMed] [Google Scholar]
- Herteaux, C., Lauritzen, I., Widmann, C., and Lazdunski, M. (1995). Essential role of adenosine, adenosine A1 receptors, and ATP-sensitive K+ channels in cerebral ischemic preconditioning. Proc. Natl. Acad. Sci. USA92:4666–4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim, N., Bosch, M. A., Smart, J. L., Qiu, J., Rubinstein, M., Ronnekleiv, O. K., Low, M. J., and Kelly, M. J. (2003). Hypothalamic proopiomelanocortin neurons are glucose responsive and express KATP channels. Endocrinology144:1331–1340. [DOI] [PubMed] [Google Scholar]
- Inagaki, N., Tsuura, Y., Namba, N., Masuda, K., Gonoi, T., Horie, M., Seino, Y., Mizuta, M., and Seino, S. (1995). Cloning and functional characterization of a novel ATP-sensitive potassium channel ubiquitously expressed in rat tissues, including pancreatic islets, pituitary, skeletal muscle, and heart. J. Biol. Chem.270:5691–5694. [DOI] [PubMed] [Google Scholar]
- Jahr, C. E., and Stevens, C. F. (1987). Glutamate activates multiple single channel conductances in hippocampal neurons. Nature325:522–525. [DOI] [PubMed] [Google Scholar]
- Jiang, C., and Haddad, G. G. (1997). Modulation of K+ channels by intracellular ATP in human neocortical neurons. J. Neurophysiol.77:93–102. [DOI] [PubMed] [Google Scholar]
- Jiang, C., Sigworth, F. J., and Haddad, G. G. (1994). Oxygen deprivation activates an ATP-inhibitable K+ channel in substantia nigra neurons. J. Neurosci.14(9):5590–5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakeim, M. and Noma, A. (1984). Adenosine-5´-triphosphate-sensitive single potassium channel in the atrioventricular node cell of the rabbit heart. J. Physiol.352:265–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karschin, C., Ecke, C., Ashcroft, F. M., and Karschin, A. (1997). Overlaping distribution of KATP channel-forming Kir 6.2 subunit and the sulfonylurea receptor SUR1 in rodent brain. FEBS Lett.401:59–64. [DOI] [PubMed] [Google Scholar]
- Lee, K., Dixon, K. A., Freeman, C. T., and Richardson, J. P. (1998). Identification of an ATP-sensitive potassium channel current in rat striatal cholinergic interneurones. J. Physiol.510:441–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki, T., Liss, B., Minami, K., Shiuchi, T., Saraya, A., Kashima, Y., Horiuchi, M., Ashcroft, F., Minokoshi, Y., Roeper, J., and Seino, S. (2001). ATP-sensitive K+ channels in the hypothalamus are essential for the maintenance of glucose homeostasis. Nat. Neurosci.4:507–512. [DOI] [PubMed] [Google Scholar]
- Mironov, S. L., Langohr, K., Haller, M., and Richter, D. W. (1998) Hypoxia activates ATP-dependent potassium channels in inspiratory neurons of neonatal mice. J. Physiol.509:755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa, I., Ogawa, Y., Noriyama, Y., Nakase, H., Yamashita, M., and Sakaki, T. (2003). Chemical preconditioning prevents paradoxical increase in glutamate release during ischemia by activating ATP-dependent potassium channels in gerbil hippocampus. Exp. Neurol.183:180–187. [DOI] [PubMed] [Google Scholar]
- Noma, A., and Takano, M. (1991). The ATP-sensitive K+ channel. Jpn. J. Physiol.41:177–187. [DOI] [PubMed] [Google Scholar]
- Qin, D. Y., Tanako, M., and Noma, A. (1989). Kinetics of ATP-sensitive K+ channel revealed with oil-gate concentration jump method. Am. J. Physiol.257:H1624–H1633. [DOI] [PubMed] [Google Scholar]
- Quayle, J. M., Standen, N. B., and Stanfield, P. R. (1988). The voltage-dependent block of ATP-sensitive potassium channels of frog skeletal muscle by cesium and barium ions. J. Physiol.405:677–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routh, V. H., McArdle, J. J., and Levin, B. E. (1997). Phosphorylation modulates the activity of the ATP-sensitive K+ channel in the ventromedial hypothalamic nucleus. Brain Res.778:107–119. [DOI] [PubMed] [Google Scholar]
- Seino, S., and Miki, T. (2003). Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog. Biophys. Mol. Biol.81:133–176. [DOI] [PubMed] [Google Scholar]
- Smith, M. L., Auer, R. N., and Siesjo, B. K. (1984). The density and distribution of ischemic brain injury in the rat following 2-10 min of forebrain ischemia. Acta Neuropathol.64:319–332. [DOI] [PubMed] [Google Scholar]
- Spruce, A. E., Standen, N. B., and Stanfield, P. R. (1985). Voltage-dependent ATP-sensitive potassium channels of skeletal muscle membrane. Nature316:736–738. [DOI] [PubMed] [Google Scholar]
- Stanford, I. M., and Lacey, M. G. (1995). Regulation of a potassium conductance in rat midbrain dopamine neurons by intracellular Adenosine triphosphate (ATP) and the sulfonylurea tolbutamide and glibenclamide. J. Neurosci.15(6):4651–4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford, I. M., and Lacey, M. G. (1996). Electrophysiological investigation of adenosine trisphosphate-sensitive potassium channels in the rat substantia nigra pars reticulata. Neuroscience74:499–509. [DOI] [PubMed] [Google Scholar]
- Takaba, H., Nagao, T., Yao, H., Kitazono, T., Ibayashi, S., and Fujishima, M. (1997). An ATP-sensitive potassium channel activator reduces infarct volume in focal cerebral ischemia in rats. Am. J. Physiol.273:R583–R586. [DOI] [PubMed] [Google Scholar]
- Tang, X. D., Santarelli, L. C., Heinemann, S. H., and Hoshi, T. (2004). Metabolic regulation of potassium channels. Annu. Rev. Physiol.66:131–159. [DOI] [PubMed] [Google Scholar]
- Tromba, C., Salvaggio, A., Racagni, G., and Volterra, A. (1992). Hypoglycaemia-activated K+ channels in hippocampal neurons. Neurosci. Lett.143:185–189. [DOI] [PubMed] [Google Scholar]
- Tromba, C., Salvaggio, A., Racagni, G., and Volterra, A. (1994). Hippocampal hypoglycaemia-activated K+ channels: single-channel analysis of glucose and voltage dependence. Pflugers Arch.429:58–63. [DOI] [PubMed] [Google Scholar]
- Vergara, C., Latorre, R., Marrion, N. V., and Adelman, J. P. (1998). Calcium-activated potassium channels. Curr. Opin. Neurobiol.8:321–329. [DOI] [PubMed] [Google Scholar]
- Zawar, C., Plant, T. D., Schirra, C., Konnerth, A., and Neumcke, B. (1999). Cell-type specific expression of ATP-sensitive potassium channels in the rat hippocampus. J. Physiol.514:327–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zini, S., Tremblay, E., Pollard, H., Moreau, J., and Ben-Ari, Y. (1993). Regional distribution of sulfonylurea receptors in the brain of rodent and primate. Neuroscience55:1085–1091. [DOI] [PubMed] [Google Scholar]