Abstract
An axiom of life-history theory, and fundamental to our understanding of ageing, is that animals must trade-off their allocation of resources since energy and nutrients are limited. Therefore, animals cannot ‘have it all’—combine high rates of fecundity with extended lifespans. The idea of life-history trade-offs was recently challenged by the discovery that ageing may be governed by a small subset of molecular processes independent of fitness. We tested the ‘trade-off’ and ‘having it all’ theories by examining the fecundities of C57BL/6J mice placed onto four different dietary treatments that generated caloric intakes from −21 to +8.6% of controls. We predicted body fat would be deposited in relation to caloric intake. Excessive body fat is known to cause co-morbidities that shorten lifespan, while caloric restriction enhances somatic protection and increases longevity. The trade-off model predicts that increased fat would be tolerated because reproductive gain offsets shortened longevity, while animals on a restricted intake would sacrifice reproduction for lifespan extension. The responses of body fat to treatments followed our expectations, however, there was a negative relationship between reproductive performance (fecundity, litter mass) and historical intake/body fat. Our dietary restricted animals had lower protein oxidative damage and appeared able to combine life-history traits in a manner contrary to traditional expectations by having increased fecundity with the potential to have extended lifespans.
Keywords: life history, trade-off, resource allocation, oxidative stress
1. Introduction
A fundamental axiom of our understanding of life-history evolution and ageing in animals is that they work with a limited resource base and are, therefore, forced to trade-off certain life-history parameters against each other. Resultant life histories then reflect optimal resource allocation to these competing demands under a variety of external constraints (Stearns 1992). This approach dates back to Fisher's seminal contributions concerning trade-offs in resource allocation and their consequences for survival and fecundity (Fisher 1930), and has formed the basis of the entire field of life-history evolution (Stearns 1992). Our understanding of the evolutionary basis of ageing, in the disposable soma hypothesis (Kirkwood 1992; Austad 1997), is derived from this same framework. Animals cannot ‘have it all’, i.e. combine long lifespans with high reproductive output.
The responses of animals to caloric restriction (McCay et al. 1935; Walford et al. 1987), have been previously interpreted within in this framework. When animals are calorically restricted to at least 40% of baseline intake they experience an increase in longevity roughly proportional to the extent of restriction (Merry 2002). The effect is reproducible in a wide variety of organisms from Caenorhabditis elegans to Drosophila and mice (Vaupel et al. 2003; Weindruch 2004). The increase in longevity when calorically restricted is classically interpreted as a reallocation of resources away from reproduction capacity into somatic protection (Weindruch & Walford 1982; Shanley & Kirkwood 2000). Molecular work supports the notion that mechanisms are switched on during caloric restriction that reduce oxidative damage (Merry 2002).
Conversely, when mice are fed diets that are high in fat many strains increase in body mass (BM) due to fat storage (West et al. 1992). Excess body fatness, however, leads to a variety of inflammatory states and co-morbidities such as heart disease and diabetes that generally shorten lifespan (Lyon et al. 2003). The animals may tolerate this reduction in longevity because the stored fat may be utilized to support high levels of reproductive output. A trade-off between somatic protection and reproduction is therefore presumed to underpin the responses to historical levels of calorie intake. This hypothesis suggests that calorically restricted animals would have lowered fecundity relative to non-restricted animals but animals historically fed on high-fat diets would be able to divert their stored fat to support elevated reproductive outputs.
In a recent review (Partridge et al. 2005), the fundamental trade-off axiom was challenged. Partridge et al. (2005) reviewed molecular studies on the physiology underlying differential rates of ageing in mutant organisms (yeast (Lin et al. 2000), C. elegans (Kenyon et al. 1993) and Drosophila (Clancy et al. 2001; Tatar et al. 2001)) and indicated that ageing appears to be governed by a relatively small set of key molecular processes (Hsin & Kenyon 1999; Guarente & Kenyon 2000; Lithgow & Gill 2003; Nemoto & Finkel 2004; Huang et al. 2004). This has also been suggested by recent studies in mice (Holzenberger et al. 2003). The regulation of molecular processes may be independent of the presumed trade-offs between survival and reproduction. By this interpretation ‘having it all’ may not be an impossible scenario. If this were the case the implications for our understanding of life histories and ageing would be profound. However, Partridge et al. (2005) point out a failure in the literature to refute a trade-off between reproduction and lifespan extension in dietary restricted mice. In the current study, we aimed to test the ‘trade-off’ and ‘having it all’ hypotheses by examining the fecundities of mice that had been historically placed on caloric restriction, fed a normal diet or diets that are high in fat content.
2. Material and methods
Female C57BL/6J mice (n=152; Harlan UK Ltd., Oxon, UK) at six weeks of age were kept in a 12 : 12 h light : dark photoperiod and held in accordance with Home Office regulations. Animals received an ad libitum supply of laboratory chow (Rat and Mouse Breeder Grower diet CRM; Special Diet Services, Essex, UK; 12.5 kJ g−1 metabolizable energy) and were mated for 16 days when they were seven weeks of age to ensure all animals were fertile before receiving treatment. After weaning (i.e. 21 days after birth) and a 10 day recovery period, all animals were placed on a 30 day measurement period, during which the animals were measured daily for BM and food intake (FI). Body composition was measured once at the end of this 30 day period using a PixiMus2 DXA (GE Medical Systems Ultrasound and BMD, Bedford, UK). Fat mass (FM) and lean mass (LM) were estimated by DXA then corrected using an equation developed specifically for our machine (Johnston et al. 2005).
Following the measurement period animals received either an ad libitum supply of control (10% kcal fat; n=35), medium fat (45% kcal fat; n=40) or high fat (60% kcal fat, n=40) diet (Research Diets Inc., NJ, USA; D12450B, D12451 and D12492, respectively) or a daily ration of 75% matched control intake (n=40). Measurements were repeated at week nine of treatment when the animals were six months old. Ten animals from each group received treatment for 16 weeks and then culled. Liver and gastrocnemius muscle were harvested and snap frozen in liquid nitrogen. Homogenates in a buffer containing 250 mM sucrose, 25 mM HEPES, 10 mM MgCl2 and 1 mM EDTA were diluted to 1 mg protein ml−1 and analysed for protein carbonyl content by ELISA as described by Sitte et al. (1998). The remaining animals in each group (n=30 except for controls n=25) were removed from treatment after 11 weeks and placed on ad libitum laboratory chow for 10 days prior to pairing for 6 days with proven breeder male C57BL/6J mice (Harlan UK Ltd., Oxon, UK). Both the females and their pups were weighed at birth and weaning (i.e. 21 days after birth).
Statistical analysis was carried out using GenStat seventh edition (VSN International Ltd., UK). Chi-squared tests were used to compare the number of animals that were successful or failed to give birth to live pups in each treatment group. General ANOVA using dietary treatment group and/or stage (pre-treatment versus post-treatment or birth versus weaning) with interactions in the model and blocked for animal, and post hoc t-tests were used to assess differences between diets.
3. Results
Pre-treatment intake of laboratory chow averaged 50.3±s.e.m. 0.48 kJ day−1. At the end of nine weeks of treatment with purified diets, FI for animals that received the high-fat diet (48.2±1.28 kJ day−1) was not different from animals that received the medium fat diet (46.5±0.89 kJ day−1) but control animal (43.0±0.62 kJ day−1) and restricted animal FI (38.6±0.51 kJ day−1) were both different from all other treatments (p<0.001). The FI at the end of the nine week treatment was lower than the intake prior to the animals being placed on the treatments in all the ad libitum fed mice, however, the apparent energy assimilation efficiency of animals on the purified diets at post-treatment (93.8%) was greater than on the cereal-based chow pre-treatment (82.6%; p<0.001). Assuming the decline in metabolizable intake in the ad libitum groups was linear, the estimated total energy intakes over the nine weeks of treatment plus the 30 days of measurement were 4212, 4083 and 3880, for the high fat, medium fat and control animals, respectively, while in the restricted animals total intake was 3042 kJ. These represented +8.6, +5.2, 0 and −21.6% of the intake of the control mice, respectively (% values calculated as ((value/control)−1)×100).
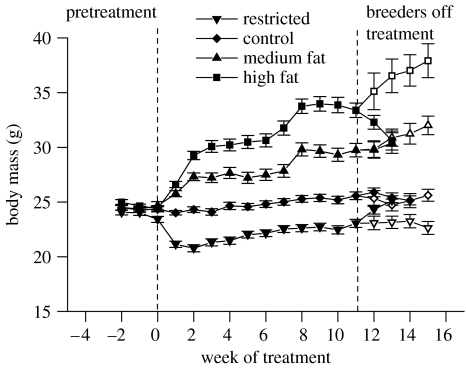
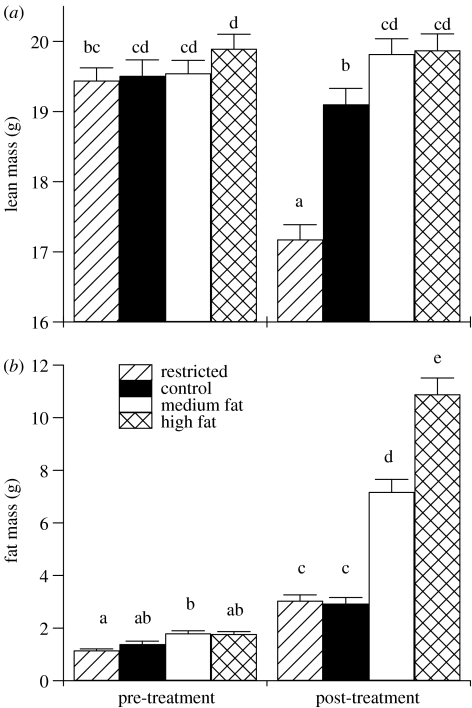
Body masses reflected the different levels of energy intake (figure 1). Body masses at week nine were significantly different from those one week before treatment for each group (p<0.05). By week nine, the animals receiving the control diet had an increase in BM of on an average 1.1±s.e.m. 0.25 g (4.5%), while the animals receiving the medium and high-fat diets increased their body masses by an average of 5.3±0.46 (22%) and 9.4±0.62 g (38%), respectively. The change in BM was due predominately to change in FM, as control, medium and high-fat diet fed animals did not experience a significant change in LM, but significantly increased FM (figure 2). Animals receiving the restricted diet lost on an average 1.3±0.32 g (−5.5%) of BM, which was attributed to a decrease in LM while FM increased (figure 2). Large errors in small animal DXA estimates of FM have been shown for animals with less than 5 g of fat (Johnston et al. 2005) and since LM is calculated by subtraction of FM from total tissue mass, both LM and FM for restricted animals may be incorrect. This may also explain why FM of restricted animals was not different from controls post-treatment.
Figure 1.
Body mass of female C57BL/6J mice that received standard laboratory chow then after week zero either an ad libitum supply of control (10% kcal fat; n=35), medium fat (45% kcal fat; n=40) or high-fat diet (60% kcal fat, n=40) or a daily ration of 75% of ad libitum intake of control diet (n=37). Ten animals from each group (open symbols) were culled at week 16 and tissues were analysed for protein carbonyl content. Breeding animals were removed from treatment at week 11 so breeding would not be directly affected. Some animals were maintained on the original diet (open symbols). Error bars represent standard error of the means.
Figure 2.
Body composition of female C57BL/6J mice was measured once during a 30 day period ca 10 days after pups from the pre-treatment test mating had been weaned (21 days after birth) and again when females were six months of age after nine weeks of treatment (restricted, n=37; control, n=35; medium fat, n=40; high fat, n=40). (a) LM and (b) fat mass were estimated by DXA then corrected using an equation developed specifically for our machine. Error bars represent standard error of the means and columns without a common letter are significantly different (p<0.05, ANOVA with post hoc t-tests).
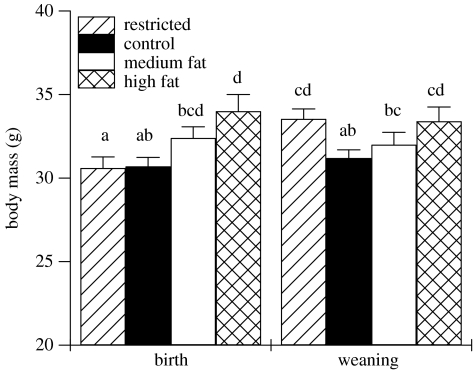
The protocol of removing breeding animals from treatment and providing them with ad libitum supply of standard laboratory chow for 10 days prior to mating removed any immediate impact of the dietary treatments on fecundity. Control animals maintained their BM once they were removed from treatment and animals that received the medium fat diet appeared to defend their heavier BM (figure 1). However, the high fat fed animals rapidly lost BM when they were returned to chow diet. Both medium and high fat fed animals remained significantly heavier than controls at birth, but only the high fat fed animals were heavier than controls at weaning (figure 3). Animals previously on the restricted diet recovered BM within the 10 days prior to mating and were not significantly different in BM from the controls at birth, and continued to gain weight during lactation so that they were heavier than controls at weaning (figure 3).
Figure 3.
Post-treatment body mass of females that gave birth to live pups and weaned litters 21 days after birth (restricted, n=27; control, n=18; medium fat, n=16; high fat, n=12). Error bars represent standard error of the means and columns without a common letter differ (p<0.05, ANOVA with post hoc t-tests).
All of the animals tested for breeding success post-treatment were successful at giving birth before treatment. Holinka & Finch (1981) showed that C57BL/6J females with one previous pregnancy were 77% successful when mated again at eight months of age. Consistent with this report, we found that 72% of control animals successfully gave birth when they were 7.5 months of age (table 1). However, only 53% of animals fed medium fat, and 40% of animals fed high-fat diet were successful at giving birth after treatment. In contrast to these treatment groups, animals receiving the restricted diet were 90% successful at giving birth.
Table 1.
The number of females that successfully gave birth and weaned litters post-treatment. Females were removed from treatment diet and placed on ad libitum CRM diet 10 days prior to pairing with proven breeder male C57BL/6J mice for 6 days. Animals were 100% successful in pre-treatment test mating.
| diet | mated | gave birth | % success |
|---|---|---|---|
| high fat | 30 | 12a | 40 |
| medium fat | 30 | 16ab | 53 |
| control | 25 | 18bc | 72 |
| restricted | 30 | 27c | 90 |
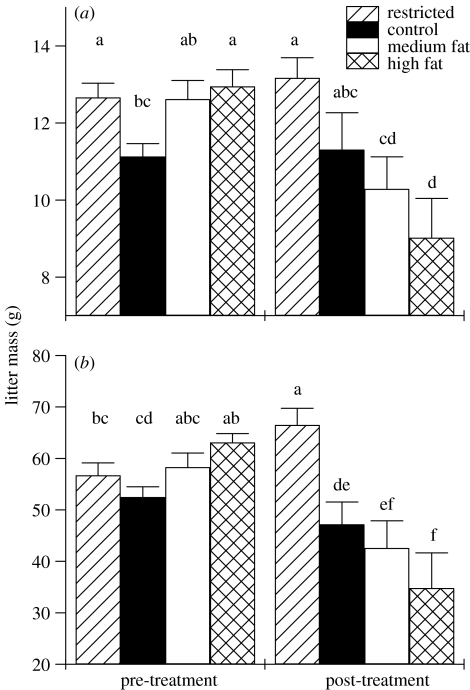
The pre-treatment mating of the animals tested for breeding success post-treatment resulted in the birth and weaning of, on average, 6.8±0.13 pups per litter, with an average litter mass of 12.5±0.18 g at birth and of 58.3±1.04 g at weaning. For the animals that went on to become controls, treatment did not affect birth litter mass (pre-treatment: 11.2±0.33 g, post-treatment: 11.3±0.96 g; p=0.8), or number of pups born per litter (pre: 6.2±0.25 pups, post: 5.2±0.69 pups; p=0.3). Animals fed the medium fat diet had lighter litters (pre: 12.6±0.48 g, post: 10.3±0.83 g; p<0.005) and fewer pups at birth post-treatment (pre: 6.9±0.33 pups, post: 4.6±0.60 pups; p<0.001) but were not different from controls for litter mass or size post-treatment.
Animals fed the high-fat diet gave birth to litters that were significantly smaller in mass (pre: 13.0±0.43 g, post: 9.0±1.02 g; p<0.001) and size (pre: 7.3±0.22 pups, post: 3.8±0.53 pups; p<0.001) than pre-treatment litters. Treatment with high-fat diet significantly decreased litter mass and size relative to controls at birth (figures 4 and 5).
Figure 4.
The differences between litter mass from the pre-treatment and post-treatment matings at the time of (a) birth and (b) at weaning 21 days after birth (restricted, n=27; control, n=18; medium fat, n=16; high fat, n=12). Error bars represent standard error of the means and columns without a common letter differ (p<0.05, ANOVA with post hoc t-tests).
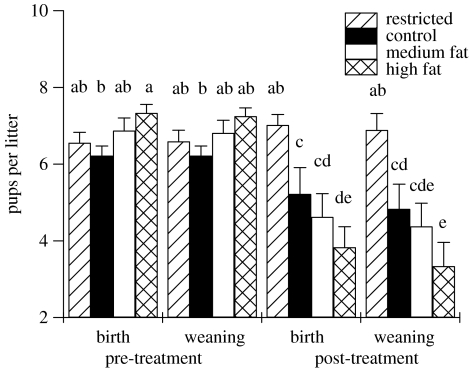
Figure 5.
The differences between the number of pups per litter from the pre-treatment and post-treatment matings at the time of birth and weaning 21 days after birth (restricted, n=27; control, n=18; medium fat, n=16; high fat, n=12). Error bars represent standard error of the means and columns without a common letter differ (p<0.05, ANOVA with post hoc t-tests).
Litters at birth post-treatment were not different from pre-treatment for dietary restricted animals in mass (pre: 12.7±0.37 g, post: 13.2±0.52 g; p=0.5) or size (pre: 6.6±0.27 pups, post: 7.0±0.39 pups; p=0.4). While the post-treatment litter masses at birth for restricted animals were not different from controls, restricted animals gave birth to litters with significantly more pups per litter than animals receiving the other treatments including the controls post-treatment (figure 5).
At post-treatment weaning, the animals that received the high-fat diet weaned significantly lighter litters and fewer pups than control animals (figures 4 and 5). Animals that had received the medium fat diet weaned litters that were not different in mass or size from control litters. However, dietary restricted animals weaned bigger, heavier litters than controls post-treatment and litters were also heavier than at pre-treatment for the same animals.
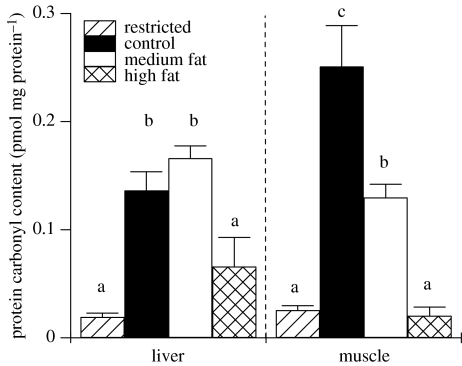
BM of control animals that were not removed from treatment for post-treatment breeding was the same at nine weeks and 15 weeks of treatment (figure 1). Dietary restricted animals also maintained a stable BM, but medium fat and high-fat diet fed animals both continued to gain weight to 15 weeks of treatment (figure 1). Of the animals that were not selected for post-treatment breeding, 35% failed to give birth pre-treatment. Levels of protein carbonyls in the tissues of non-parous animals were not different from single parity animals (liver: non-parous 0.094±0.0187 nmol mg−1 protein; single parity 0.098±0.0159; p=0.8 and muscle: non-parous 0.094±0.0259; single parity 0.11±0.024; p=0.4) and there was no interaction between parity and dietary treatment (liver: p=0.8; muscle p=0.3). Dietary treatment significantly affected the protein carbonyl content of liver and muscle (figure 6). Dietary restricted animals had lower levels of protein carbonyls than controls in both liver and muscle. Protein carbonyl levels of medium fat fed animals were the same as controls for liver but lower than controls for muscle. Protein carbonyl content of liver and muscle in high-fat fed animals was lower than controls and not different from dietary restricted animals.
Figure 6.
Protein carbonyl content of liver and gastrocnemius muscle of animals post-treatment (n=10 per treatment). Error bars represent standard error of the means and columns without a common letter differ by p<0.05 (ANOVA with post hoc t-tests).
4. Discussion
A priori, from the classical trade-off model of life-history evolution, we predicted that mice under caloric restriction would show reduced fecundity because they would have spent the previous three months devoting their reduced resources to somatic protection rather than reproductive capacity and, as a consequence, would have lowered energy reserves to support a reproductive event. In contrast, we expected those mice fed high-fat diets would have large deposited reserves available, on which they could draw to support elevated levels of fecundity and reproductive output. Indeed, the changes in BM under the different dietary treatments followed our predictions. Unexpectedly, however, animals that received a restricted diet appeared no different in FM from controls after treatment, although this result may be confounded by errors in estimating FM using small animal DXA. Therefore, with the exception of the restricted diet animals, stored fat was directly proportional to the levels of caloric intake. However, when we examined levels of fecundity the pattern was completely opposite to that predicted from the trade-off model. The mice that had the greatest reserves also had levels of fecundity that were only about half those of the mice that had been previously in caloric restriction.
Mechanistically, the reason why the mice with the highest levels of fat storage had the poorest fecundity may reflect endocrine consequences that attend high levels of fatness. Tortoriello et al. (2004) showed that dietary induced obese female DBA/2J mice had increased neuropeptide-Y expression leading to suppressed GnRH and reduced fertility, but they failed to bring about dietary-induced obesity in C57BL/6J females that were fed the same diet as medium fat fed animals in the present study. Our medium fat fed animals had greater FM than controls post-treatment but similar fecundity while our high-fat fed animals had reduced fecundity perhaps resulting from suppressed GnRH as shown in the DBA/2J mice (Tortoriello et al. 2004).
Alternatively, oxidative stress, which is generally presumed to be the mechanism that leads to reduced survival (Weindruch 2004), may have parallel effects on reproductive performance in animals with high energy intake. Reduced free radical production and delayed ageing may explain why animals on the restricted diet were the most capable breeders. Consistent with this dietary restricted animals in the present study had low levels of protein damage in both liver and muscle compared with controls and may have been at a younger physiological age than animals fed ad libitum diets because of their historically restricted energy intakes. However, high-fat diet fed animals had levels of protein damage comparable with dietary restricted animals. A combination of both endocrine factors and oxidative damage cannot be ruled out. Parity did not affect oxidative damage and dietary restricted animals had reduced damage, therefore, this signifies that restricted animals had the potential to have extended lifespans irrespective of fecundity.
Yet another alternative to explain these results is a phenomenon known as ‘flushing’ in sheep, where animals on a rising plane of nutrition have higher reproductive success than those on a decreasing plane of nutrition. Sheep, in low body condition, on a high plane of nutrition for 17 days prior to mating resulted in higher ovulation and conception rates than in sheep in high condition placed on a low plane of nutrition (Gunn et al. 1984; Gunn et al. 1991). This may be analogous to events of the present study where mice fed the high-fat diet may have been on a decreasing plane of nutrition when returned to chow diet and mice that had their dietary restriction lifted may have been on a rising plane of nutrition. It is unknown what effect flushing has on future survival in sheep.
In mice, caloric restriction implemented as late as 12 months of age extends lifespan (Weindruch & Walford 1982). Thus, in the present study, restricted intake imposed after the post-treatment reproduction may lead to increased longevity when compared to non-restricted mice. Whatever the mechanisms invoked, in contrast to the rodent studies outlined by Partridge et al. (2005), in the present study the effect of dietary restriction was that the mice have the potential to live longer and thus were capable of ‘having it all’.
These data have important implications. If animals are capable of having both high levels of somatic protection and therefore enhanced longevity at the same time as high fecundity, then why do they not routinely combine these options in nature? Perhaps observing trade-offs in these parameters is critically dependent on the ecological context in which the traits are expressed. Mice in the wild may be differentially exposed to predation, e.g. which obviously was not a feature of the laboratory environment in which we studied them. However, resolving our data in this framework would be difficult because it would be expected that risk of predation might increase as a function of body weight and fat storage (Witter & Cuthill 1993; Rands & Cuthill 2001; Covas et al. 2002) and so might exacerbate rather than annul the observations we have made. If such an ecological context explanation proves not to be the case then a fundamental rethink of our whole conceptualization of the evolutionary processes involved in life-history evolution and ageing may be indicated.
Acknowledgments
We wish to thank Dr Bert Tolkamp for his contribution to the experimental design and for his useful comments on this manuscript. We would also like to acknowledge members of BioSS, especially Dr Grietje Holtrop, for help with experimental design and statistical analysis. This study was funded by the SEERAD grant RRI/RO723. Portions of this work were presented at the Nutrition Society summer meeting in Dublin 2004 and the International Gerontological Congress in Brazil 2005.
References
- Austad S.N. Comparative ageing and life histories in mammals. Exp. Gerontol. 1997;32:23–38. doi: 10.1016/s0531-5565(96)00059-9. 10.1016/S0531-5565(96)00059-9 [DOI] [PubMed] [Google Scholar]
- Clancy D.J, Gems D, Harshman L.G, Oldham S, Stocker H, Hafen E, Leevers S.J, Partridge L. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292:104–106. doi: 10.1126/science.1057991. 10.1126/science.1057991 [DOI] [PubMed] [Google Scholar]
- Covas R, Brown C.R, Anderson M.D, Bomberger Brown M. Stabilizing selection on body mass in the sociable weaver Philetairus socius. Proc. R. Soc. B. 2002;269:1905–1909. doi: 10.1098/rspb.2002.2106. 10.1098/rspb.2002.2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R.A. Clarendon Press; Oxford, UK: 1930. The genetical theory of natural selection. [Google Scholar]
- Guarente L, Kenyon C. Genetic pathways that regulate ageing in model organisms. Nature. 2000;408:255–262. doi: 10.1038/35041700. 10.1038/35041700 [DOI] [PubMed] [Google Scholar]
- Gunn R.G, Doney J.M, Smith W.F. The effect of a level of pre-mating nutrition on ovulation rate in Scottish blackface ewes in different body condition at mating. Anim. Prod. 1984;39:235–239. [Google Scholar]
- Gunn R.G, Maxwell T.J, Sun D.A, Jones J.R, James M.E. The effect of level of nutrition prior to mating on the reproductive performance of ewes of two Welsh breeds in different levels of body condition. Anim. Prod. 1991;52:157–163. [Google Scholar]
- Holinka C.F, Finch C.E. Efficiency of mating in C57BL/6J female mice as a function of age and previous parity. Exp. Gerontol. 1981;16:393–398. doi: 10.1016/0531-5565(81)90060-7. 10.1016/0531-5565(81)90060-7 [DOI] [PubMed] [Google Scholar]
- Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even P.C, Cervera P, Le Bouc Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. 10.1038/nature01298 [DOI] [PubMed] [Google Scholar]
- Hsin H, Kenyon C. Signals from the reproductive system regulate the lifespan of C. elegans. Nature. 1999;399:362–366. doi: 10.1038/20694. 10.1038/20694 [DOI] [PubMed] [Google Scholar]
- Huang C, Xiong C, Kornfeld K. Measurements of age-related changes of physiological processes that predict lifespan of Caenorhabditis elegans. Proc. Natl Acad. Sci. USA. 2004;101:8084–8089. doi: 10.1073/pnas.0400848101. 10.1073/pnas.0400848101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston S.L, Peacock W.L, Bell L.M, Lonchampt M, Speakman J.R. PIXImus DXA with different software needs individual calibration to accurately predict fat mass. Obes. Res. 2005;13:1558–1565. doi: 10.1038/oby.2005.191. [DOI] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. 10.1038/366461a0 [DOI] [PubMed] [Google Scholar]
- Kirkwood T.B. Comparative life spans of species: why do species have the life spans they do? Am. J. Clin. Nutr. 1992;55:1191S–1195S. doi: 10.1093/ajcn/55.6.1191Sa. [DOI] [PubMed] [Google Scholar]
- Lin S.J, Defossez P.A, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. 10.1126/science.289.5487.2126 [DOI] [PubMed] [Google Scholar]
- Lithgow G.J, Gill M.S. Physiology: cost-free longevity in mice? Nature. 2003;421:125–126. doi: 10.1038/421125a. 10.1038/421125a [DOI] [PubMed] [Google Scholar]
- Lyon C.J, Law R.E, Hsueh W.A. Minireview: adiposity, inflammation and atherogenesis. Endocrinology. 2003;144:2195–2200. doi: 10.1210/en.2003-0285. 10.1210/en.2003-0285 [DOI] [PubMed] [Google Scholar]
- McCay C.M, Crowell M.F, Maynard L.A. The effects of retarded growth upon the length of life span and upon the ultimate body size. J. Nutr. 1935;10:63–79. [PubMed] [Google Scholar]
- Merry B.J. Molecular mechanisms linking calorie restriction and longevity. Int. J. Biochem. Cell Biol. 2002;34:1340–1354. doi: 10.1016/s1357-2725(02)00038-9. 10.1016/S1357-2725(02)00038-9 [DOI] [PubMed] [Google Scholar]
- Nemoto S, Finkel T. Ageing and the mystery at Arles. Nature. 2004;429:149–152. doi: 10.1038/429149a. 10.1038/429149a [DOI] [PubMed] [Google Scholar]
- Partridge L, Gems D, Withers D.J. Sex and death: what is the connection? Cell. 2005;120:461–472. doi: 10.1016/j.cell.2005.01.026. 10.1016/j.cell.2005.01.026 [DOI] [PubMed] [Google Scholar]
- Rands S.A, Cuthill I.C. Separating the effects of predation risk and interrupted foraging upon mass changes in the blue tit Parus caeruleus. Proc. R. Soc. B. 2001;268:1783–1790. doi: 10.1098/rspb.2001.1653. 10.1098/rspb.2001.1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanley D.P, Kirkwood T.B. Calorie restriction and ageing: a life-history analysis. Evol. Int. J. Org. Evol. 2000;54:740–750. doi: 10.1111/j.0014-3820.2000.tb00076.x. [DOI] [PubMed] [Google Scholar]
- Sitte N, Merker K, Grune T. Proteasome-dependent degradation of oxidized proteins in MRC-5 fibroblasts. FEBS Lett. 1998;440:399–402. doi: 10.1016/s0014-5793(98)01495-1. 10.1016/S0014-5793(98)01495-1 [DOI] [PubMed] [Google Scholar]
- Stearns S.C. Oxford University Press; Oxford, UK: 1992. The evolution of life histories. [Google Scholar]
- Tatar M, Kopelman A, Epstein D, Tu M.P, Yin C.M, Garofalo R.S. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001;292:107–110. doi: 10.1126/science.1057987. 10.1126/science.1057987 [DOI] [PubMed] [Google Scholar]
- Tortoriello D.V, McMinn J, Chua S.C. Dietary-induced obesity and hypothalamic infertility in female DBA/2J mice. Endocrinology. 2004;145:1238–1247. doi: 10.1210/en.2003-1406. 10.1210/en.2003-1406 [DOI] [PubMed] [Google Scholar]
- Vaupel J.W, Carey J.R, Christensen K. Ageing. It's never too late. Science. 2003;301:1679–1681. doi: 10.1126/science.1090529. 10.1126/science.1090529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walford R.L, Harris S.B, Weindruch R. Dietary restriction and ageing: historical phases, mechanisms and current directions. J. Nutr. 1987;117:1650–1654. doi: 10.1093/jn/117.10.1650. [DOI] [PubMed] [Google Scholar]
- Weindruch R. Caloric restriction, gene expression and ageing. Int. Congr. Ser. 2004;1260:13–20. 10.1016/S0531-5131(03)01560-7 [Google Scholar]
- Weindruch R, Walford R.L. Dietary restriction in mice beginning at 1 year of age: effect on life-span and spontaneous cancer incidence. Science. 1982;215:1415–1418. doi: 10.1126/science.7063854. [DOI] [PubMed] [Google Scholar]
- West D.B, Boozer C.N, Moody D.L, Atkinson R.L. Dietary obesity in nine inbred mouse strains. Am. J. Physiol. 1992;262:R1025–R1032. doi: 10.1152/ajpregu.1992.262.6.R1025. [DOI] [PubMed] [Google Scholar]
- Witter M.S, Cuthill I.C. The ecological costs of avian fat storage. Phil. Trans. R. Soc. B. 1993;340:73–92. doi: 10.1098/rstb.1993.0050. [DOI] [PubMed] [Google Scholar]