Abstract
The neutralizing activities of anti-V3 antibodies for HIV-1 isolates is affected both by sequence variation within V3 and by epitope masking by the V1/V2 domain. To analyze the relative contribution of V3 sequence variation, chimeric Env genes that contained consensus V3 sequences from seven HIV-1 subtypes in the neutralization-sensitive SF162 Env backbone were constructed. Resulting viral pseudotypes were tested for neutralization by 15 anti-V3 MAbs isolated from humans infected with viruses of either subtype B (anti-V3B MAbs) or subtype A (anti-V3A MAbs). Pseudovirions with the subtype B consensus V3 sequence were potently neutralized (IC50 < 0.006 μg/ml) by all but one of these MAbs, while pseudovirions with V3 subtypes A, C, F, H, AG, and AE were generally neutralized more effectively by anti-V3A MAbs than by anti-V3B MAbs. A V1/V2-masked Env version of SF162 Env with the consensus B V3 sequence was also neutralized by these MAbs, although with considerably lower potency, while similarly masked chimeras with V3 sequences of subtype A, C, or AG were weakly neutralized by anti-V3A MAbs but not by anti-V3B MAbs. Mutations in the V1/V2 domain of YU-2 Env increased the sensitivity of this highly resistant Env to a pool of anti-V3B MAbs several thousand-fold. These results demonstrated (i) the exceptional sensitivity of representative V3 domains of multiple subtypes to neutralization in the absence of epitope masking, (ii) the broader neutralizing activity of anti-V3A MAbs for viruses containing diverse V3 sequences, and (iii) the generality and dominant effect of V1/V2 masking on restriction of V3-mediated neutralization.
Developing immunogens that elicit potent, cross-reactive neutralizing antibody responses against primary virus isolates remains an elusive goal of HIV-1 vaccine research. While early studies performed with T-cell-line adapted (TCLA) HIV-1 isolates identified the V3 domain of gp120 as the “principal neutralization domain” of HIV-1 (16), subsequent data resulted in more pessimistic attitudes about the importance of V3 as a target of the protective immune response and its suitability as a vaccine target (15, 20). Much of this pessimism was based on data obtained with monoclonal antibodies (MAbs) derived against TCLA viruses that possess atypical V3 sequences and consequently display limited cross-reactivity with more representative isolates present in infected people (1, 2, 5, 9, 10). Recent evidence obtained with a newer panel of V3-specific MAbs and polyclonal antibodies derived from HIV-infected patients indicated the existence of conserved V3 epitopes that can in some cases act as potent neutralization targets (12-14, 18). However, the breadth of neutralizing activity of these V3-specific antibodies for typical primary isolates is limited compared to that of broadly neutralizing MAbs, such as b12, 2F5, and 2G12.
A number of studies have documented roles for N-linked glycans at various positions in gp120 (7, 35) and in the V2 domain in particular (6, 11, 24, 31, 32) in limiting the neutralizing activities of antibodies specific for multiple domains of Env. Epitope masking by the V1/V2 domain was shown to account for the great difference in neutralization sensitivity of the SF162 and JR-FL Envs; exchanging the V1/V2 domains of these two Envs switched the sensitivities of the corresponding viral pseudotypes to neutralization by many polyclonal human sera and MAbs directed towards the V3 domain, the CD4-binding site, and CD4-induced epitopes, often by more than 3 orders of magnitude (31). The occurrence of such indirect epitope-masking activities can obscure the effects of epitope variability and complicate the determination of the relative importance of these two effects in the limited neutralizing activity of particular antibodies for primary isolates.
In order to facilitate the analysis of the specificity, cross-reactivity, and neutralization potential of V3-specific MAbs in the absence of epitope masking, a series of plasmids was prepared containing chimeric envelopes with V3 domains corresponding to the consensus sequences of multiple Env subtypes in the context of SF162 Env, an unmasked, neutralization-sensitive envelope. Similar constructs were prepared in the epitope-masked version of this Env in which the V1/V2 domain was replaced by that of JR-FL Env. Viruses pseudotyped with these chimeric envelopes were tested against a panel of 15 anti-V3 human MAbs, eight isolated from B cells of U.S. subjects infected with subtype B viruses (anti-V3B MAbs) and seven from cells of West African subjects infected with viruses carrying envelopes from subtype A (anti-V3A MAbs) (12). These studies provided a quantitative measure of the relative contributions of V3 sequence variability and epitope masking to neutralization sensitivity to V3-specific MAbs derived in response to infection by both subtype A and subtype B strains of HIV-1.
MATERIALS AND METHODS
Monoclonal antibodies.
The panel of V3-specific MAbs used in this study is described in Table 1. MAb 447-52D was isolated by screening against V3MN peptide (12a), and MAbs 4117 and 4148 were isolated by screening against gp120MN (32a). The rest of the MAbs were isolated as previously described from Epstein-Barr virus-transformed B-cell cultures derived from HIV-1 subtype B-infected patients living in New York (anti-V3B MAbs) and from subtype A-infected subjects from West Africa (anti-V3A MAbs) by screening for binding against fusion glycoproteins that expressed correctly glycosylated and disulfide-bonded V3 domains (17). The affinities of MAbs isolated with this approach for native V3 structures was usually greater than that of MAbs screened with linear V3 peptides, and such MAbs possessed greater functional activities than previously available anti-V3 MAbs (13, 14). Individual MAbs were screened as indicated in Table 1, against either the JR-CSF V3 sequence (identical to the consensus subtype B except for an N-to-S change at the fifth position of the loop), the CRF02_AG consensus V3 sequence, or a mixture of the two proteins (12-14). The isolated B-cell lines were stabilized by fusions to form heterohybridoma cell lines, and the MAbs were purified by protein A chromatography. The following control MAbs were obtained from the NIH AIDS Research and Reference Reagent Program: IgG1-b12 (also referred to as b12) (4), directed against an epitope that overlaps the CD4-binding site, was contributed by Dennis Burton and Paul Parren, and 2G12 (34), directed against a conformational epitope involving high-mannose glycans, and 2F5 (28), directed against an epitope in the ectodomain of gp41, were contributed by Hermann Katinger.
TABLE 1.
Human anti-V3 MAbs used in this study
| MAb | Antigen used for selection | Patient origin | Reference |
|---|---|---|---|
| Anti-V3B | |||
| 447-52D | V3 peptide, MN | United States | 12a |
| 4117C | gp120, MN | United States | 32a |
| 4148 | gp120, MN | United States | 32a |
| 2191 | V3JR-CSF FP | United States | 14 |
| 2219 | V3JR-CSF FP | United States | 14 |
| 2412 | V3JR-CSF FP | United States | 14 |
| 2442 | V3JR-CSF FP | United States | 14 |
| 2456 | V3JR-CSF FP | United States | 14 |
| Anti-V3A | |||
| 2182 | V3JR-CSF FP | Côte d'Ivoire | 14 |
| 2557 | V3JR-CSF FP | Cameroon | 13 |
| 2558 | V3JR-CSF FP | Cameroon | 13 |
| 2601 | V3consAG FP | Cameroon | 18 |
| 3019 | V3JR-CSF FP + V3consAG FP | Cameroon | 12 |
| 3074 | V3JR-CSF FP + V3consAG FP | Cameroon | 12 |
| 3224 | V3JR-CSF FP + V3consAG FP | Cameroon | 12 |
Chimeric Envs and V3 variants.
The expression plasmid for SF162 Env and the chimeric form of this Env in which the V1/V2 domain was exchanged with that of JR-FL have been described (31). An expression plasmid for YU-2 Env was constructed by cloning a 3.9-kb EcoRI-HindIII fragment from the pYU-2 provirus (23) into pcDNA, and mutations were introduced into the V1 and V2 domains of this Env by QuikChange site-directed mutagenesis (Stratagene, Inc.). pYU-2 was obtained from Beatrice Hahn and George Shaw through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health. The various chimeric Envs with consensus V3 sequences were generated by introducing the necessary modifications into SF162 Env sequentially either by PCR overlap or by QuikChange mutagenesis. The corresponding epitope-masked versions of the V3 chimeric Envs were generated by exchanging DraIII/StuI fragments (these restriction sites are located in the conserved stem of the V1/V2 loops) between JR-FL and SF162 env-carrying plasmids.
Neutralization assays.
Neutralization activity was determined as previously described (19) with a single-cycle infectivity assay using virions generated from the Env-defective luciferase-expressing pNL4-3.Luc.R−E− genome (8) pseudotyped with molecularly cloned HIV Envs. Briefly, pseudotyped virions in culture supernatants from transfected 293T cells were incubated with serial dilutions of MAbs for 1 h at 37°C and then added to U87-T4-CCR5 target cells plated out in 96-well plates in the presence of Polybrene (10 μg/ml). After 24 h, cells were refed with RPMI medium containing 10% fetal bovine serum and Polybrene (Sigma-Aldrich Co.) and incubated for an additional 24 to 48 h. Luciferase activity was determined 48 to 72 h postinfection with a microtiter plate luminometer (HARTA, Inc.), using assay reagents from Promega. The 50% inhibitory concentrations (IC50s) reported were determined by interpolation from neutralization curves and are averages of at least three independent assays.
RESULTS
Relative effects of variation in the V1/V2 and V3 regions on neutralization sensitivity.
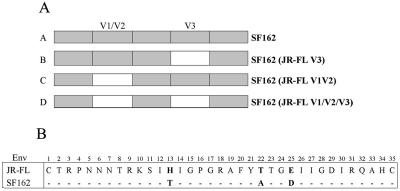
In order to assess the contributions of variations in the V1/V2 region versus variations in the V3 domain on the large difference in sensitivity of SF162 and JR-FL Envs to neutralization by V3-specific MAbs, a set of chimeric SF162 Envs were generated in which the V1/V2 and/or V3 domains were replaced with the corresponding regions of JR-FL Env (Fig. 1A). In contrast to the SF162 V1/V2 domain, the JR-FL V1/V2 domain strongly masks epitopes in the V3 domain and other regions of gp120 (31). The JR-FL V3 sequence corresponds to the subtype B consensus sequence, while the SF162 V3 differs from the consensus at three positions, residues 13, 22, and 25 (Fig. 1B). Position 13 (corresponding to position 308 in the standard Env numbering system based on the IIIB sequence) is relatively polymorphic for subtype B sequences, with H being the most common amino acid (present in 46% of sequences) and T the second most common residue (present in 11% of sequences) at this position within 176 subtype B sequences listed in the 2003 HIV Sequence Compendium database (21). Position 22 of subtype B Envs contains only two common variants, with T as the major residue at this position (61%) and A occurring less frequently (37%). Position 25 is also polymorphic in subtype B sequences, with E being the most common residue (41%) and D the second most common (19%).
FIG. 1.
(A) Structures of chimeric envelopes used for examining the roles of the V1/V2 domains on neutralization sensitivity of V3 epitopes. Chimeric envelopes were constructed in the SF162 backbone (gray bars) by exchanging the V1/V2 and/or V3 domains with the corresponding domains of JR-FL Env (white bars). (B) Comparison of V3 loop sequences of JR-FL and SF162 Env proteins.
Virus pseudotyped with the SF162 Env was strongly neutralized by most of the V3-specific MAbs tested; seven of eight anti-V3B MAbs and four of seven anti-V3A MAbs neutralized with IC50s below ∼0.07 μg/ml (Table 2, column A). Converting the SF162 V3 sequence to the JR-FL sequence resulted in an increase in neutralization sensitivity to all of these MAbs, with IC50s of ≤0.006 μg/ml in all but one case (Table 2, column B). With the one exception, these activities exceeded that of IgG-b12, one of the most potent broadly reactive MAbs available, and were ∼100- to 1,000-fold more potent than that of another broadly neutralizing control MAb, 2G12. These results highlight the exceptional intrinsic sensitivity of the V3 domain to neutralization.
TABLE 2.
IC50s and IC50 ratios of anti-V3 MAbs against virions pseudotyped with SF162/JR-FL chimeric Envs with exchanged V1/V2 and/or V3 domainsa
| MAb | IC50 (μg/ml)
|
IC50 ratiob for:
|
||||||
|---|---|---|---|---|---|---|---|---|
| V3 sequence variation
|
V1/V2 sequence variation
|
|||||||
| SF162 (A) | SF162 (JR-FL V3) (B) | SF162 (JR-FL V1/V2) (C) | SF162 (JR-FLV1/V2/V3) (D) | A/B | C/D | C/A | D/B | |
| Anti-V3B | ||||||||
| 2219 | 0.0044 | 0.0011 | 25 | 0.62 | 4.0 | 40 | 5,700 | 560 |
| 2456 | 0.047 | 0.0033 | 78 | 2.9 | 14 | 27 | 1,700 | 880 |
| 4148 | 0.019 | 0.0050 | 100 | 7.7 | 3.8 | 12a | 5,300 | 1,500 |
| 447-52D | 0.067 | 0.00061 | 92 | 0.57 | 11 | 160 | 1,400 | 930 |
| 2191 | 0.0029 | 0.0021 | 17 | 1.0 | 1.4 | 17 | 5,500 | 480 |
| 2442 | 0.031 | 0.0025 | 36 | 0.56 | 12 | 64 | 1,200 | 220 |
| 4117 | 0.036 | 0.0044 | 46 | 5.3 | 8.2 | 8.7 | 1,300 | 1,200 |
| 2412 | 0.6 | 0.0012 | >100 | 12 | 500 | >8 | >170 | 10,000 |
| Anti-V3A | ||||||||
| 3074 | 0.041 | 0.0059 | >100 | 11 | 6.9 | >9 | >2,400 | 1,900 |
| 2557 | 0.021 | 0.0031 | 56 | 1.8 | 6.8 | 31 | 2,600 | 580 |
| 2558 | 0.027 | 0.0033 | >100 | 3.5 | 8.2 | >29 | >3,700 | 1,100 |
| 3019 | 0.020 | 0.0028 | 69 | 6.3 | 7.1 | 11 | 3,400 | 2,300 |
| 3224 | 1.08 | 0.0051 | >20 | 6.7 | 210 | >3 | >18 | 1,300 |
| 2601 | >20 | 0.11 | >50 | >50 | >180 | >450 | ||
| 2182 | 1.1 | 0.0013 | >100 | 0.88 | 850 | >10 | >90 | 680 |
| Control | ||||||||
| b12 | 0.026 | 0.009 | 0.011 | 0.014 | 2.9 | 0.79 | 0.42 | 1.6 |
| 2G12 | 0.78 | 0.59 | 1.3 | 1.6 | 1.3 | 0.81 | 1.7 | 2.7 |
Viruses were pseudotyped with either unmasked (A and B) or masked (C and D) Envs which contained the V3 sequence of either SF162 (A and C) or JR-FL (B and D) in the SF162 backbone.
Ratios of IC50s obtained for each MAb against virus pseudotyped with Envs with the indicated V1/V2 domain and the SF162 or JR-FL V3 domains. Ratios for samples for which IC50s could not be obtained at the highest concentration tested are given as minimum values.
As reported previously for a subset of these MAbs (31), replacement of the V1/V2 domain of SF162 Env by that of JR-FL resulted in a great reduction in sensitivity to neutralization. The masked chimeric SF162 Env with the SF162 V3 sequence was partially or completely refractory to neutralization by all of the anti-V3 MAbs; IC50s could not be obtained for six of the fifteen MAbs at the highest concentrations tested, and those MAbs that did neutralize required at least 17 μg/ml to achieve 50% neutralization (Table 2, column C). However, the similarly masked Env with the subtype B consensus V3 domain (SF162 [JR-FL V1/V2/V3]) was considerably more sensitive to neutralization by the V3-specific MAbs. IC50s for all but one of the MAbs were at or below 12 μg/ml, and three of the anti-V3B and one of the anti-V3A MAbs neutralized with IC50s below 1 μg/ml (Table 2, column D).
The relative effects due to variation within either the V3 or the V1/V2 domain are tabulated in the last four columns of Table 2 as ratios of IC50 for pseudoviruses with chimeric Envs that differ in one of these domains. This analysis shows that except for MAb 2182, which was extremely type specific for the consensus subtype B V3 sequence, the reduction in neutralization efficiency due to substitution of the V1/V2 domains was considerably greater than that due to sequence variation in V3 itself. Whereas varying the V3 region resulted in changes in IC50 ratios ranging from 1.4- to 850-fold, with a median value of 12 (calculated for samples for which both endpoints were obtained), exchanging the V1/V2 regions resulted in much higher IC50 ratios, which ranged from 220 to 10,000, with a median value of 1,300. In contrast to these effects, the IC50s for IgG-b12 and 2G12, control MAbs directed against epitopes that are independent of either V1/V2 or V3 domains, varied less than threefold for all of these variants. Thus, for this pair of Env sequences, variation in the V1/V2 domain played a much greater role in restricting neutralization sensitivity to V3-specific MAbs than variation within the V3 sequence itself.
The neutralizing activity of the majority of anti-V3 MAbs tested extends across multiple V3 subtypes.
The SF162 and JR-FL V3 domains vary at three positions, and thus the difference between these sequences is a relatively limited example of the possible diversity in V3, especially when sequences are being compared across subtypes. In order to examine the effect of more extensive variation in V3, additional chimeric Envs were prepared in both the neutralization-sensitive SF162 and neutralization-resistant SF162 (JR-FL V1/V2) backbones (Fig. 2A). The V3 sequences expressed in these chimeras corresponded to consensus sequences of subtypes A1, C, F, H, CRF01_AE, and CRF02_AG and differed from the subtype B consensus sequence at four to nine positions (Fig. 2B).
FIG. 2.
Structures of chimeric envelopes used for preparing HIV-1 pseudotypes. (A) Model of chimeric Envs used in these studies. The Envs utilized the SF162 backbone (gray bars) with modified V3 sequences (white bars). The V1/V2 domains of several of the V3 chimeric Envs were replaced by that of JR-FL, resulting in epitope-masked Envs which were relatively resistant to V3-mediated neutralization. (B) Sequences of substituted V3 domains. Residues that vary from the subtype B consensus sequence residues are indicated.
While the subtype B consensus V3 sequence was the most sensitive target for neutralization by both the anti-V3B and anti-V3A MAbs, significant levels of neutralization were frequently obtained for the other V3 subtypes as well. Each of the chimeric viruses was neutralized by the majority of the anti-V3 MAbs, and, except for 2182, all of the MAbs possessed cross-neutralizing activity for at least three different consensus V3 sequences (Table 3). The neutralizing activities were often quite potent, with IC50s below 0.1 μg/ml for 43% (45/105) of the MAb-virus combinations.
TABLE 3.
Neutralization sensitivity of SF162 Env chimeras containing different V3 domains to a panel of V3-specific MAbs
| V3 sequencea | IC50 (μg/ml)b
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anti-V3B MAbs
|
Anti-V3A MAbs
|
Control MAbs
|
|||||||||||||||
| 2219 | 2456 | 4148 | 447-52D | 2191 | 2442 | 4117 | 2412 | 3074 | 2557 | 2558 | 3019 | 3224 | 2601 | 2182 | b12 | 2G12 | |
| Subtype B (JR-FL) | 0.0011 | 0.0033 | 0.0050 | 0.00061 | 0.0021 | 0.0025 | 0.0044 | 0.0012 | 0.0059 | 0.0031 | 0.0033 | 0.0028 | 0.0051 | 0.11 | 0.0013 | 0.0090 | 0.59 |
| Subtype F | 0.0034 | 0.015 | 0.046 | 0.27 | 0.044 | 0.17 | 0.019 | 1.3 | 0.0023 | 0.026 | 0.018 | 0.0059 | 0.035 | 0.014 | >20 | 0.0078 | 1.1 |
| Subtype A1 | 0.029 | 0.083 | 0.068 | 0.58 | 0.043 | 0.027 | 17 | 2.8 | 0.015 | 0.025 | 0.038 | 0.032 | 0.044 | 0.029 | >20 | 0.0075 | 1.4 |
| Subtype C | 0.15 | 0.44 | 2.5 | >20 | 0.59 | >20 | >20 | >20 | 0.20 | 0.16 | 0.20 | 0.25 | 1.8 | 0.17 | >20 | 0.0096 | 0.42 |
| CRF02_AG | 0.054 | 1.2 | 2.3 | 0.76 | 0.32 | 9.8 | >20 | >20 | 0.0094 | 0.024 | 0.079 | 0.56 | 0.041 | 0.035 | >20 | 0.0095 | 0.34 |
| Subtype H | 0.21 | 16 | 7.3 | 16 | >20 | 6.7 | 9.4 | >20 | 0.092 | 0.12 | 0.29 | 4.3 | 0.13 | 9.6 | >20 | 0.0068 | 0.73 |
| CRF01_AE | 0.23 | 4.4 | >20 | 1.2 | >20 | >20 | >20 | >20 | 0.0038 | 0.076 | 0.43 | 0.75 | 0.65 | >20 | >20 | 0.011 | 1.5 |
The chimeric Envs tested contain the indicated subtype consensus sequence.
Values for the most sensitive antibody-virus combinations (IC50s < 0.01 μg/ml) are highlighted in bold, and combinations for which no neutralization was obtained at 20 μg/ml are underlined.
With the exception of 2182, the neutralizing activity of the anti-V3A MAbs for viruses with the six non-B V3 sequences was broader and often more potent than that of the anti-V3B MAbs. Excluding 2182, 50% neutralization was obtained for 35/36 (97%) anti-V3A MAb-virus combinations versus 35/48 (73%) anti-V3B MAb-virus combinations. Furthermore, again excluding 2182, the anti-V3A MAbs neutralized at <1 μg/ml in 32/36 (89%) combinations, whereas anti-V3B MAbs neutralized at this level in only 21/48 (44%) cases. The broadest and most potent of the MAbs, 3074, strongly neutralized all seven viruses tested, with a mean IC50 of 0.047 μg/ml and a median IC50 of 0.009 μg/ml.
Contribution of residues at the crown of the loop to immunoreactivity.
One of the most consistent differences between the subtype B V3 sequences and those of the other subtypes is the sequence at the crown of the loop (V3 residue 18): whereas for subtype B, the dominant residue at this position is arginine (R, usually as GPGR), an R at this position is relatively rare for the other subtypes, and the dominant residue is the less basic glutamine (Q, usually as GPGQ). For ∼11% of subtype B sequences, a basic lysine residue (K) occurs in place of R at this position. It was previously shown that MAb 447-52D strongly prefers the presence of R versus Q at this position (36). In order to quantitate the effect of the amino acid at this position on the specificity of the MAbs in the panel, the R at the tip of the subtype B consensus V3 loop in SF162 Env was mutated to either Q or K, and the IC50 was determined for each of the anti-V3 MAbs.
These assays showed that the residue at the crown of the V3 loop is also a major determinant for MAbs 2412 and 2812 but that substitutions at this position had much smaller effects on the other anti-V3B MAbs (Table 4). MAb 2182 was unique in its absolute dependence on R at position 18; this MAb possessed no neutralizing activity at all for either the Q or K variant. The dependence of MAbs 2412 and 447-52D on R18 was less stringent than that of 2182, since these MAbs did neutralize the Q18 variant at ∼600- to 700-fold-higher antibody concentrations than that required for the wild-type sequence. Converting R18 to K also increased the resistance of the virus to these two MAbs to a smaller extent (13- to 26-fold). For the six remaining anti-V3B MAbs, the Q18 variant was one- to ninefold more resistant than the parental sequence, while the K18 substitution usually resulted in a small increase in neutralization sensitivity. Anti-V3A MAb 2601 was the only MAb that preferentially neutralized the Q18 variant, with a ninefold-lower IC50 than for the R variant. The other anti-V3A MAbs were affected to a lesser extent than the anti-V3B MAbs by substitutions at the tip of the loop, exhibiting one- to threefold-reduced potency for the Q variant and less-than-twofold changes for the K18 variant.
TABLE 4.
Effect of the residue at the crown of the loop (position 18) on neutralization by anti-V3 MAb
| MAb | IC50 (μg/ml) for variant with consensus sequencea
|
IC50 ratiob
|
|||
|---|---|---|---|---|---|
| B—R18 | B—Q18 | B—K18 | Q18/R18 | K18/R18 | |
| Anti-V3B | |||||
| 2219 | 0.0012 | 0.0012 | 0.00076 | 1.0 | 0.63 |
| 2456 | 0.0033 | 0.025 | 0.0035 | 7.6 | 1.1 |
| 4148 | 0.0053 | 0.031 | 0.0031 | 5.8 | 0.58 |
| 447-52D | 0.00054 | 0.4 | 0.0068 | 740 | 13 |
| 2191 | 0.0023 | 0.0052 | 0.00081 | 2.3 | 0.35 |
| 2442 | 0.0018 | 0.017 | 0.0019 | 9.4 | 1.1 |
| 4117 | 0.0055 | 0.019 | 0.00068 | 3.5 | 0.12 |
| 2412 | 0.00091 | 0.58 | 0.024 | 637 | 26 |
| Anti-V3A | |||||
| 3074 | 0.0055 | 0.0072 | 0.0032 | 1.3 | 0.58 |
| 2557 | 0.0037 | 0.01 | 0.0017 | 2.7 | 1.6 |
| 2558 | 0.0049 | 0.0047 | 0.0028 | 1.0 | 0.57 |
| 3019 | 0.0031 | 0.0079 | 0.0039 | 2.9 | 1.4 |
| 2601 | 0.12 | 0.013 | 0.14 | 0.11 | 1.2 |
| 2182 | 0.0013 | >20 | >20 | >16,000 | >16,000 |
IC50s for MAbs against SF162 Env with the subtype B consensus V3 loop or variants in which residue 18 at the crown of the loop was converted to either Q or K.
Ratios of the IC50s for the Q18 versus R18 chimeras or K18 versus R18 chimeras. Bold indicates samples with IC50 ratios of >10.
Extent of the V1/V2 masking effect varies for different V3 MAbs and sequences.
The potent neutralizing activity of the anti-V3 MAbs for viruses containing subtype B V3 sequences in the unmasked but not the masked Env suggests that the common resistance of subtype B primary isolates to neutralization by V3-specific antibodies may be due less to the absence of recognizable epitopes than to masking of those epitopes. However, the relative level of sensitivity of V3 sequences of other subtypes to epitope masking is unknown. To examine this, the masking activity of the JR-FL V1/V2 sequence was examined for the consensus V3 sequences from four subtypes, A1, B, C, and CRF02_AG, expressed as chimeras in the SF162 Env backbone (Fig. 2A).
Similar to the effect previously seen for the subtype B V3 chimera, all of the masked viruses were considerably more resistant to neutralization than the corresponding unmasked Envs (Table 5). In contrast to their modest neutralizing activities for the masked Env with the subtype B consensus V3 region, with only one exception, the anti-V3B MAbs did not neutralize the masked chimeras containing V3 sequences of other subtypes with IC50s below 100 μg/ml. However, five of the six anti-V3A MAbs tested in this assay were able to neutralize one or more of the masked Envs with the non-B V3 subtypes. With the exception of 2182, all of the anti-V3A MAbs had low but detectable neutralizing activities for the masked Envs with the subtype A1 V3 sequence; two of these MAbs also neutralized the masked Envs with the subtype C V3 sequence, and 3074 neutralized all four masked viruses. As expected from its specificity for the subtype B sequence, MAb 2182 neutralized only the virus with the consensus B V3 loop.
TABLE 5.
Neutralization sensitivity of epitope-masked Env chimeras containing different V3 domains
| V3 sequencea | IC50 (μg/ml)b
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anti-V3B MAbs
|
Anti-V3A MAbs
|
|||||||||||||
| 2219 | 2456 | 4148 | 447-D | 2191 | 2442 | 4117 | 2412 | 3074 | 2557 | 2558 | 3019 | 2601 | 2182 | |
| Subtype B cons | 0.62 | 2.9 | 7.7 | 0.57 | 1.0 | 0.56 | 5.3 | 12 | 11 | 1.8 | 3.5 | 6.3 | >50 | 0.88 |
| Subtype A1 cons | 100 | >100 | >100 | >100 | >100 | 37 | >100 | >100 | 22 | 91 | 53 | 74 | 42 | >100 |
| Subtype C cons | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | 67 | >100 | 71 | >100 | >50 | >100 |
| CRF02_AG cons | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | 41 | >100 | >100 | >100 | >50 | >100 |
cons, consensus.
IC50s for anti-V3 MAbs against viruses pseudotyped with SF162 Env in which the V1/V2 domain was replaced with that of JR-FL V1/V2 and the V3 domain was replaced with the indicated V3 consensus sequences. Samples with no discernable neutralization at the highest MAb concentration tested (50 or 100 μg/ml) are in bold. Data for the masked Env with the subtype B V3 sequence are from Table 2.
These results indicate that V1/V2-mediated masking is also effective at blocking the neutralization of Envs with non-B V3 sequences by both anti-V3A and anti-V3B MAbs. The greater neutralizing activity of the anti-V3A MAbs for the masked Envs with the non-B V3 subtypes presumably correlates with a greater affinity of these MAbs for these V3 sequences, as does the greater activity of the anti-V3B MAbs for the masked Env with the subtype B V3 sequence.
The resistance of YU-2 Env to neutralization by anti-V3 MAbs is also determined by V1/V2 domain masking.
The data described above and in a previous report (31) demonstrate a critical role for the V1/V2 domain in determining the differential neutralization sensitivities of V3 epitopes in the SF162 and JR-FL Env backbones. However, these studies did not address the question of how dominant V1/V2 masking was in limiting the neutralization sensitivity of other Envs, particularly Envs derived from primary HIV-1 isolates more resistant to neutralization than even JR-FL (the so-called “tier 2” and “tier 3” isolates [25]). One such Env currently being used as a target immunogen in many studies is YU-2, a highly resistant Env derived from a primary strain isolated directly from the brain of an infected patient (23). The importance of epitope masking by the V1/V2 domain in resistance of YU-2 to V3-mediated neutralization was evaluated in the following experiments.
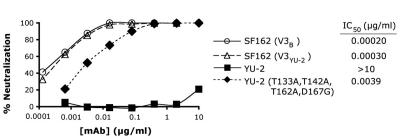
Studies using a pool of the anti-V3B MAbs in Table 1 showed that virus pseudotyped with YU-2 Env was not neutralized at an antibody concentration of 10 μg/ml (Fig. 3). This contrasted to an IC50 of 0.76 μg/ml for the antibody pool against virus pseudotyped with the masked SF162 variant containing the JR-FL V1/V2 and V3 domains (data not shown), consistent with the greater resistance of YU-2 Env to neutralization. The YU-2 V3 sequence contains two substitutions relative to the consensus subtype B sequence, H308N and F317L at positions 13 and 20 of the V3 loop. In order to determine the extent to which these changes contributed to the resistance to neutralization by anti-V3 MAbs, these two modifications were introduced into the SF162 (JR-FL V3) Env by mutagenesis. The modified Env remained extremely sensitive to neutralization by the MAb pool, with an IC50 of 0.00020 μg/ml, a 1.5-fold increase over that for the consensus sequence (Fig. 3). This indicated that the substitutions in the YU-2 V3 sequence were not major factors in the neutralization resistance of this virus.
FIG. 3.
Neutralization curves obtained for pooled anti-V3B MAbs against SF162 Env with the consensus B V3 sequence (circles), SF162 Env with the YU-2 V2 sequence (triangles), parental YU-2 Env (squares), and YU-2 Env in which the indicated four positions in the V1/V2 domain were mutated (diamonds).
Since attempts to replace the YU-2 V1/V2 domain with that of SF162 did not result in a functional Env, site-specific mutagenesis was used instead to study the contribution of the V1/V2 region to the neutralization-resistant phenotype of YU-2 Env. Three N-linked glycosylation sites present in YU-2 but absent at the corresponding positions of SF162 V1 and V2 regions (positions 131, 140, and 160 based on the HXB2 numbering convention) were mutated by Thr-to-Ala mutations, and an additional mutation previously found to affect neutralization sensitivity, an Asp-to-Gly mutation at position 167 (30), was also introduced. The resulting YU-2 Env mutant was found to be highly sensitive to neutralization by the anti-V3 MAb pool, with an IC50 of 0.0039 μg/ml (Fig. 3). This represented an increased sensitivity of over 2,500-fold relative to the parental YU-2 Env, and this mutant was only 13-fold less sensitive than the SF162 Env bearing the YU-2 V3 sequence. This result indicated that V1/V2 masking was the predominant factor for the resistance of YU-2 Env to neutralization by V3-specific MAbs and that sequence differences elsewhere in Env played only a minor role.
DISCUSSION
Extensive cross-reactivity of anti-V3 antibodies.
Much of the recent focus of HIV immunology has been on the so-called “broadly conserved” epitopes, such as those in gp120 recognized by MAbs IgG1-b12 and 2G12, and epitopes in the membrane-proximal external region of gp41 recognized by MAbs 2F5 and 4E10 (3, 29). The potential of the V3 loop as a useful vaccine target is believed to be limited in view of evidence indicating a relatively narrow neutralization breadth for V3 antibodies against primary isolates (1, 15). One recent example of such data is found in a comprehensive analysis of the neutralizing activity of HIV-specific MAbs, which concludes that the neutralizing activity of two anti-V3B MAbs is restricted to subtype B primary isolates (1). A second example is contained in a recent description of a panel of primary Env clones proposed for use in standardized assessments of vaccine-elicited neutralizing antibodies (22). This study includes neutralization data for six of the anti-V3B MAbs described in the present study and reports that while 3 of the 19 isolates tested were strongly neutralized by some of these MAbs (with IC50s as low as 0.04 μg/ml), only three of the remaining Envs were neutralized by one or more of the anti-V3 MAbs at 25 μg/ml. The basis for the limited activity of the V3 MAbs was not addressed in either of these studies.
The data presented in the present paper highlight the extensive reactivity of the majority of V3-specific MAbs isolated from infected patients for V3 sequences representative of multiple subtypes, the greater cross-reactivity of MAbs generated from patients infected with viruses carrying subtype A than subtype B Env, and the exceptional neutralizing potency of many of these antibodies for viruses with consensus V3 sequences in the absence of epitope masking. In contrast to the view that the limited neutralization breadth of V3-specific antibodies is due to the highly restricted specificities of such antibodies, 14 of the 15 human MAbs tested neutralized viruses with V3 regions that represented three to seven different subtype consensus sequences. In many cases, these MAbs neutralized with potencies greater than that of 2G12, an antibody that is believed to possess clinically relevant neutralizing activity and one that has been shown to have some ability to inhibit HIV replication in vivo in both macaques (26) and humans (33).
Only one MAb, 2182, was strictly subtype B specific. This MAb was also unique in its absolute dependency on the presence of R at position 18 in the crown of the loop (Table 4). Although 2182 was isolated from an African patient infected with a virus bearing a subtype A Env, the V3 sequence of this virus contained R at position 18 (12), a feature that occurs in ∼4% of subtype A V3 structures (21). The induction of 2182 by a subtype A isolate bearing the GPGR motif in a V3 loop that is otherwise quite distant from the consensus subtype B sequence may account for the highly restricted specificity of this MAb.
Two additional anti-V3B MAbs, 447-52D and 2412, also displayed a strong preference for R over Q at position 18, and these possessed preferential neutralizing activity for Envs with V3B sequences. These two MAbs differed from 2182 in that they possessed residual neutralizing activity for the Q18 variant, which correlated with a lower level of neutralizing activity for viruses with V3 sequences from other subtypes (Table 3). The remaining six anti-V3B MAbs and five anti-V3A MAbs tested were affected to a much lesser extent by the residue at position 18, and these also neutralized viruses with V3 sequences corresponding to multiple subtypes (Table 3). It is interesting that 447-52D and one other GPGR-specific mouse MAb were the only V3-specific MAbs included in the comprehensive analysis of the cross-clade neutralizing activity of MAbs described in reference 1, suggesting that the atypically narrow specificities of these MAbs may have contributed to the finding that V3-specific neutralization was highly restricted to subtype B isolates.
A broader cross-reactivity for the anti-V3A MAbs than for the anti-V3B MAbs was also found in a study of the neutralizing activities of these MAbs against a panel of primary viruses from multiple subtypes (12). The correlation of these results with the greater cross-reactivity and neutralizing potency previously reported for V3-specific polyclonal antibodies present in the sera of subjects infected with viruses carrying subtype A Envs than in sera of subtype B-infected patients (18) suggests that induction of broadly cross-reactive V3-specific antibodies is a general characteristic of the humoral response to subtype A Envs. This may reflect the lower dependence of these antibodies on the sequence at the tip of the loop, and a consequent redirection of binding specificity towards other positions in the V3 loop that are more conserved across subtypes.
The previous study showing the frequent cross-reactivity of African sera with both subtype A and subtype B V3s also showed that the V3-reactive activity of the majority of the cross-reactive sera was competed more efficiently by a subtype A V3 peptide than by a subtype B peptide, and that a significant fraction of such sera possessed only subtype A-specific V3 antibodies (18). These results suggested that V3-specific antibodies with preferential or exclusive affinities for subtype A V3 sequences are common in such sera. In view of this, it was surprising that, with the exceptions of 3074 and 2601, the anti-V3A MAbs neutralized the chimeric virus with the subtype B V3 consensus sequence more potently than any of the other V3 sequences (Table 3), and that only one of the anti-V3A MAbs (2601) preferentially neutralized the Q18 variant of SF162-V3B consensus sequence (Table 4). The preferential neutralizing activity of the anti-V3A MAbs for the subtype B sequence might reflect a greater sensitivity of the subtype B V3 domain to neutralization. Alternatively, it could suggest a possible bias in the selection of these MAbs towards the subtype B sequence, due to use of the V3B fusion protein as a screening antigen. The latter possibility would suggest that screening specifically with fusion proteins expressing native V3 regions of other subtypes may allow the isolation of antibodies with increased specificities for the non-subtype B sequences that may possess greater affinities and neutralization potencies for those viruses. Since the overwhelming majority of the worldwide HIV-1 epidemic is fueled by infection with non-subtype B viruses, such MAbs could find important applications, and their characterization could facilitate the design of immunogens that effectively induce such activities.
Dominant role of V1/V2 epitope masking in modulating neutralization activity of antibodies to V3.
These studies also show that in several different Env backbones, epitope masking by the V1/V2 region is the predominant factor restricting V3-directed neutralization. The >2,500-fold increase in neutralization sensitivity of YU-2 Env to a pool of V3-specific MAbs upon mutation of sites in the V1 and V2 domains indicated that V1/V2 masking was the dominant factor for the antibody-resistant phenotype of this virus as well. Epitope masking by the JR-FL V1/V2 loop effectively limited the neutralizing potency of both subtype A- and subtype B-derived anti-V3 MAbs for V3 subtypes of multiple subtypes, further suggesting that restriction of V3-mediated neutralization by V1/V2 masking is not limited to clade B isolates but may also occur for primary viruses across clades. Efficient inhibition of V3-mediated neutralization by V1/V2 masking may be a factor permitting the relative conservation in V3 sequences across subtypes that accounts for the high level of cross-reactivity of many V3-specific MAbs generated in response to infection. Inhibiting neutralization by anti-V3 MAbs via introducing masking sequences into V1/V2 may result in a lower cost to viral fitness and replicative ability than would mutating the V3 domain itself to the extent needed to reduce antibody affinity below that point required for neutralization.
V1/V2 masking activity described in this study was generally defined as ratios in neutralization potencies for V1/V2-masked Env with a given V3 sequence compared to that for the same V3 sequence in the SF162 backbone. It is important to note that previous studies have shown that the sensitivity of SF162 Env itself to neutralization by several anti-V3 MAbs is further increased upon removal of N-linked glycans present at sites adjacent to the base of both the V2 and V3 loops and within the V3 region itself (24, 27), indicating that masking of V3 epitopes occurs even in the context of the highly sensitive SF162 Env backbone. The V2 glycosylation sites identified in the earlier study are distinct from those positions shown in Fig. 3 to mask V3 epitopes of YU-2 Env (Fig. 3). Thus, it is likely that the remarkable sensitivity of the chimeric SF162 and YU-2 mutant Envs described in the present study would be increased even further by removal of the additional masking sites. Four of the five glycosylation sites shown in the earlier studies to possess masking activity are highly conserved in primary viruses (the exception is the site N terminal to the V3 loop, which is generally lacking in clade C isolates [21]), suggesting that these may be required for some structural or functional property of Env and thus are less amenable to modification.
Finally, the differences observed in the extent of masking for various V3 antibodies and epitopes, and the fact that neutralization was frequently observed with sufficiently high concentrations of many of the anti-V3 MAbs even for V1/V2-masked Envs, provide an encouraging indication that V3 epitopes do remain accessible to an extent even in such neutralization-resistant Envs. The greater sensitivity of the subtype B consensus sequence to neutralization by almost all of the MAbs and the more potent neutralization of the non-subtype B V3 sequences by most of the anti-V3A MAbs presumably reflect differences in affinities of the MAbs for the various V3 sequences, suggesting that increased affinity may compensate for epitope masking. This further suggests that generation of sufficient titers of antibodies with high affinities for representative V3 sequences could lead to significant neutralization of many masked Envs, which in turn might translate to broader neutralization of primary isolates. Additional information about the structure of V3-specific antibodies and their epitopes and the development of efficient methods of presenting these epitopes in highly immunogenic forms may facilitate the design of V3-based immunogens that could elicit such desirable antibody responses.
Acknowledgments
These studies were supported by U.S. Public Health Service grants AI46283 and AI50452 to A.P. and grants HL59725, AI36085, and AI47053 to S.Z.-P., by the Immunology Core of the NYU Center for AIDS Research (NIH grant AI27742), and by research funds from the Department of Veterans Affairs.
We thank Karl Drlica for helpful comments on the manuscript.
REFERENCES
- 1.Binley, J. M., T. Wrin, B. Korber, M. B. Zwick, M. Wang, C. Chappey, G. Stiegler, R. Kunert, S. Zolla-Pazner, H. Katinger, C. J. Petropoulos, and D. R. Burton. 2004. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J. Virol. 78:13232-13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bou-Habib, D. C., G. Roderiquez, T. Oravecz, P. W. Berman, P. Lusso, and M. A. Norcross. 1994. Cryptic nature of envelope V3 region epitopes protects primary monocytotropic human immunodeficiency virus type 1 from antibody neutralization. J. Virol. 68:6006-6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burton, D. R., R. C. Desrosiers, R. W. Doms, W. C. Koff, P. D. Kwong, J. P. Moore, G. J. Nabel, J. Sodroski, I. A. Wilson, and R. T. Wyatt. 2004. HIV vaccine design and the neutralizing antibody problem. Nat. Immunol. 5:233-236. [DOI] [PubMed] [Google Scholar]
- 4.Burton, D. R., J. Pyati, R. Koduri, S. J. Sharp, G. B. Thornton, P. W. Parren, L. S. Sawyer, R. M. Hendry, N. Dunlop, P. L. Nara, et al. 1994. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science 266:1024-1027. [DOI] [PubMed] [Google Scholar]
- 5.Candotti, D., M. Rosenheim, J. M. Huraux, and H. Agut. 1997. Two PBMC-based neutralization assays depict low reactivity of both anti-V3 monoclonal antibodies and immune sera against HIV-1 primary isolates. J. Virol. Methods 64:81-93. [DOI] [PubMed] [Google Scholar]
- 6.Cao, J., N. Sullivan, E. Desjardin, C. Parolin, J. Robinson, R. Wyatt, and J. Sodroski. 1997. Replication and neutralization of human immunodeficiency virus type 1 lacking the V1 and V2 variable loops of the gp120 envelope glycoprotein. J. Virol. 71:9808-9812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng-Mayer, C., A. Brown, J. Harouse, P. A. Luciw, and A. J. Mayer. 1999. Selection for neutralization resistance of the simian/human immunodeficiency virus SHIVSF33A variant in vivo by virtue of sequence changes in the extracellular envelope glycoprotein that modify N-linked glycosylation. J. Virol. 73:5294-5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connor, R., B. K. Chen, S. Choe, and N. R. Landau. 1995. Vpr is required for efficient replication of human immunodeficiency virus type 1 in mononuclear phagocytes. Virology 206:935-944. [DOI] [PubMed] [Google Scholar]
- 9.D'Souza, M. P., D. Livnat, J. A. Bradac, S. H. Bridges, et al. 1997. Evaluation of monoclonal antibodies to human immunodeficiency virus type 1 primary isolates by neutralization assays: performance criteria for selecting candidate antibodies for clinical trials. J. Infect. Dis. 175:1056-1062. [DOI] [PubMed] [Google Scholar]
- 10.D'Souza, M. P., G. Milman, J. A. Bradac, D. McPhee, C. V. Hanson, and R. M. Hendry. 1995. Neutralization of primary HIV-1 isolates by anti-envelope monoclonal antibodies. AIDS 9:867-874. [DOI] [PubMed] [Google Scholar]
- 11.Fox, D. G., P. Balfe, C. P. Palmer, J. C. May, C. Arnold, and J. A. McKeating. 1997. Length polymorphism within the second variable region of the human immunodeficiency virus type 1 envelope glycoprotein affects accessibility of the receptor binding site. J. Virol. 71:759-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorny, M., C. Williams, B. Volsky, K. Revesz, X.-H. Wang, S. Burda, T. Kimura, F. A. J. Konings, C. A. Anyangwe, P. Nyambi, C. Krachmarov, A. Pinter, and S. Zolla-Pazner. 2006. Cross-clade neutralizing activity of human anti-V3 monoclonal antibodies derived from the cells of individuals infected with non-B clades of human immunodeficiency virus type 1. J. Virol. 80:6865-6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12a.Gorny, M. K., A. J. Conley, S. Karwowska, A. Buchbinder, J. Y. Xu, E. A. Emini, S. Koenig, and S. Zolla-Pazner. 1992. Neutralization of diverse human immunodeficiency virus type 1 variants by an anti-V3 human monoclonal antibody. J. Virol. 66:7538-7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorny, M. K., K. Revesz, C. Williams, B. Volsky, M. K. Louder, C. A. Anyangwe, C. Krachmarov, S. C. Kayman, A. Pinter, A. Nadas, P. N. Nyambi, J. R. Mascola, and S. Zolla-Pazner. 2004. The v3 loop is accessible on the surface of most human immunodeficiency virus type 1 primary isolates and serves as a neutralization epitope. J. Virol. 78:2394-2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorny, M. K., C. Williams, B. Volsky, K. Revesz, S. Cohen, V. R. Polonis, W. J. Honnen, S. C. Kayman, C. Krachmarov, A. Pinter, and S. Zolla-Pazner. 2002. Human monoclonal antibodies specific for conformation-sensitive epitopes of V3 neutralize human immunodeficiency virus type 1 primary isolates from various clades. J. Virol. 76:9035-9045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartley, O., P. J. Klasse, Q. J. Sattentau, and J. P. Moore. 2005. V3: HIV's switch-hitter. AIDS Res. Hum. Retrovir. 21:171-189. [DOI] [PubMed] [Google Scholar]
- 16.Javaherian, K., A. J. Langlois, C. McDanal, K. L. Ross, L. I. Eckler, C. L. Jellis, A. T. Profy, J. R. Rusche, D. P. Bolognesi, S. D. Putney, and T. J. Matthews. 1989. Principal neutralizing domain of the human immunodeficiency virus type 1 envelope protein. Proc. Natl. Acad. Sci. USA 86:6768-6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kayman S. C., Z. Wu, K. Revesz, H. Chen, R. Kopelman, and A. Pinter. 1994. Presentation of native epitopes in the V1/V2 and V3 regions of human immunodeficiency virus type 1 gp120 by fusion glycoproteins containing isolated gp120 domains. J. Virol. 68:400-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krachmarov, C., A. Pinter, W. J. Honnen, M. K. Gorny, P. N. Nyambi, S. Zolla-Pazner, and S. C. Kayman. 2005. Antibodies that are cross-reactive for human immunodeficiency virus type 1 clade A and clade B V3 domains are common in patient sera from Cameroon, but their neutralization activity is usually restricted by epitope masking. J. Virol. 79:780-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krachmarov, C. P., S. C. Kayman, W. J. Honnen, O. Trochev, and A. Pinter. 2001. V3-specific polyclonal antibodies affinity purified from sera of infected humans effectively neutralize primary isolates of human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 17:1737-1748. [DOI] [PubMed] [Google Scholar]
- 20.Labrijn, A. F., and W. H. I. P. Parren. 1999. Neutralizing epitopes of HIV-1, p. IV-18-IV-34. In B. Korber, C. Brander, B. F. Haynes, J. P. Moore, R. Koup, B. D. Walker, and D. I. Watkins (ed.), HIV Molecular Immunology Database 1999. Los Alamos National Laboratory, Theoretical Biology and Biophysics, Los Alamos, N.Mex.
- 21.Leitner, T., B. Foley, B. H. Hahn, P. A. Marx, F. MCutchan, J. Mellors, S. Wolinsky, and B. Korber. 2003. 2003 HIV and SIV alignments. Los Alamos National Laboratory [Online.] http://hiv-web.lanl.gov/content/hiv-db/ALIGN_04/ALIGN-INDEX.html.
- 22.Li, M., F. Gao, J. R. Mascola, L. Stamatatos, V. R. Polonis, M. Koutsoukos, G. Voss, P. Goepfert, P. Gilbert, K. M. Greene, M. Bilska, D. L. Kothe, J. F. Salazar-Gonzalez, X. Wei, J. M. Decker, B. H. Hahn, and D. C. Montefiori. 2005. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J. Virol. 79:10108-10125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li, Y., H. Hui, C. J. Burgess, R. W. Price, P. M. Sharp, B. H. Hahn, and G. M. Shaw. 1992. Complete nucleotide sequence, genome organization, and biological properties of human immunodeficiency virus type 1 in vivo: evidence for limited defectiveness and complementation. J. Virol. 66:6587-6600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ly, A., and L. Stamatatos. 2000. V2 loop glycosylation of the human immunodeficiency virus type 1 SF162 envelope facilitates interaction of this protein with CD4 and CCR5 receptors and protects the virus from neutralization by anti-V3 loop and anti-CD4 binding site antibodies. J. Virol. 74:6769-6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mascola, J. R., P. D'Souza, P. Gilbert, B. H. Hahn, N. L. Haigwood, L. Morris, C. J. Petropoulos, V. R. Polonis, M. Sarzotti, and D. C. Montefiori. 2005. Recommendations for the design and use of standard virus panels to assess neutralizing antibody responses elicited by candidate human immunodeficiency virus type 1 vaccines. J. Virol. 79:10103-10107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mascola, J. R., G. Stiegler, T. C. VanCott, H. Katinger, C. B. Carpenter, C. E. Hanson, H. Beary, D. Hayes, S. S. Frankel, D. L. Birx, and M. G. Lewis. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6:207-210. [DOI] [PubMed] [Google Scholar]
- 27.McCaffrey, R. A., C. Saunders, M. Hensel, and L. Stamatatos. 2004. N-linked glycosylation of the V3 loop and the immunologically silent face of gp120 protects human immunodeficiency virus type 1 SF162 from neutralization by anti-gp120 and anti-gp41 antibodies. J. Virol. 78:3279-3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muster, T., F. Steindl, M. Purtscher, A. Trkola, A. Klima, G. Himmler, F. Ruker, and H. Katinger. 1993. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J. Virol. 67:6642-6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pantophlet, R., and D. R. Burton. 2006. GP120: target for neutralizing HIV-1 antibodies. Annu. Rev. Immunol. 24:739-769. [DOI] [PubMed] [Google Scholar]
- 30.Pinter, A., W. J. Honnen, P. D'Agostino, M. K. Gorny, S. Zolla-Pazner, and S. C. Kayman. 2005. The C108g epitope in the V2 domain of gp120 functions as a potent neutralization target when engineered into envelope proteins derived from neutralization-resistant human immunodeficiency virus type 1 primary isolates. J. Virol. 79:6909-6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pinter, A., W. J. Honnen, Y. He, M. K. Gorny, S. Zolla-Pazner, and S. C. Kayman. 2004. The V1/V2 domain of gp120 is a global regulator of the sensitivity of primary human immunodeficiency virus type 1 isolates to neutralization by antibodies commonly induced upon infection. J. Virol. 78:5205-5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stamatatos, L., and C. Cheng-Mayer. 1998. An envelope modification that renders a primary, neutralization-resistant clade B human immunodeficiency virus type 1 isolate highly susceptible to neutralization by sera from other clades. J. Virol. 72:7840-7845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32a.Tilley, S. A., W. J. Honnen, M. E. Racho, T. C. Chou, and A. Pinter. 1992. Synergistic neutralization of HIV-1 by human monoclonal antibodies against the V3 loop and the CD4-binding site of gp120. AIDS Res. Hum. Retrovir. 8:461-467. [DOI] [PubMed] [Google Scholar]
- 33.Trkola, A., H. Kuster, P. Rusert, B. Joos, M. Fischer, C. Leemann, A. Manrique, M. Huber, M. Rehr, A. Oxenius, R. Weber, G. Stiegler, B. Vcelar, H. Katinger, L. Aceto, and H. F. Gunthard. 2005. Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat. Med. 11:615-622. [DOI] [PubMed] [Google Scholar]
- 34.Trkola, A., M. Purtscher, T. Muster, C. Ballaun, A. Buchacher, N. Sullivan, K. Srinivasan, J. Sodroski, J. P. Moore, and H. Katinger. 1996. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J. Virol. 70:1100-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei, X., J. M. Decker, S. Wang, H. Hui, J. C. Kappes, X. Wu, J. F. Salazar-Gonzalez, M. G. Salazar, J. M. Kilby, M. S. Saag, N. L. Komarova, M. A. Nowak, B. H. Hahn, P. D. Kwong, and G. M. Shaw. 2003. Antibody neutralization and escape by HIV-1. Nature 422:307-312. [DOI] [PubMed] [Google Scholar]
- 36.Zolla-Pazner, S., P. Zhong, K. Revesz, B. Volsky, C. Williams, P. Nyambi, and M. K. Gorny. 2004. The cross-clade neutralizing activity of a human monoclonal antibody is determined by the GPGR V3 motif of HIV type 1. AIDS Res. Hum. Retrovir. 20:1254-1258. [DOI] [PubMed] [Google Scholar]