Abstract
The Cucurbitaceae translocate a significant portion of their photosynthate as raffinose and stachyose, which are galactosyl derivatives of sucrose. These are initially hydrolyzed by α-galactosidase to yield free galactose (Gal) and, accordingly, Gal metabolism is an important pathway in Cucurbitaceae sink tissue. We report here on a novel plant-specific enzyme responsible for the nucleotide activation of phosphorylated Gal and the subsequent entry of Gal into sink metabolism. The enzyme was antibody purified, sequenced, and the gene cloned and functionally expressed in Escherichia coli. The heterologous protein showed the characteristics of a dual substrate UDP-hexose pyrophosphorylase (PPase) with activity toward both Gal-1-P and glucose (Glc)-1-P in the uridinylation direction and their respective UDP-sugars in the reverse direction. The two other enzymes involved in Glc-P and Gal-P uridinylation are UDP-Glc PPase and uridyltransferase, and these were also cloned, heterologously expressed, and characterized. The gene expression and enzyme activities of all three enzymes in melon (Cucumis melo) fruit were measured. The UDP-Glc PPase was expressed in melon fruit to a similar extent as the novel enzyme, but the expressed protein was specific for Glc-1-P in the UDP-Glc synthesis direction and did not catalyze the nucleotide activation of Gal-1-P. The uridyltransferase gene was only weakly expressed in melon fruit, and activity was not observed in crude extracts. The results indicate that this novel enzyme carries out both the synthesis of UDP-Gal from Gal-1-P as well as the subsequent synthesis of Glc-1-P from the epimerase product, UDP-Glc, and thus plays a key role in melon fruit sink metabolism.
The raffinose oligosaccharides (RFOs) raffinose and stachyose are galactosyl derivatives of Suc containing one and two Gal moieties, respectively. They are near ubiquitous in the plant kingdom, found in seeds, leaves, rhizomes, tubers, stems, and translocation pathways (Kandler and Hopf, 1982; Keller and Pharr, 1996). In the Cucurbitaceae family, approximately one-half of the translocated photoassimilate can be raffinose and stachyose (Mitchell et al., 1992; Schaffer et al., 1996). The family therefore can serve as excellent material for elucidating Gal metabolic pathways in plants which, compared to Suc metabolism, is still largely unexplored.
Upon arrival at the sink tissue, the Gal moieties of the translocated RFOs are initially hydrolytically removed by α-galactosidases, which may be of either the acidic or neutral α-galactosidase enzyme families (Gao and Schaffer, 1999; Carmi et al., 2003). Enigmatically, both RFOs and free Gal are practically absent in cucurbit sink tissues (Gross and Pharr, 1982; Schaffer et al., 1996), indicating an efficient direct continuation of free Gal metabolism. The entry of the free Gal into metabolism begins with its phosphorylation by galactokinase (GalK; EC 2.7.1.6) to Gal-1-P. Following phosphorylation, two alternative pathways exist for the fate of the Gal-1-P in plants (for review, see Keller and Pharr, 1996).
One pathway is via the Leloir reaction, carried out by a uridyltransferase (UT; UDP-Glc, hexose-1-P UT, EC 2.7.7.12) utilizing UDP-Glc in a transferase reaction.
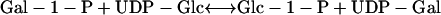 |
The UT pathway is present in both prokaryotes and eukaryotes, including bacteria, yeast (Saccharomyces cerevisiae), and mammals but is observed to a lesser extent in plants. Soybean (Glycine max) plants, whose seeds, like other legumes, accumulate RFOs (Kuo et al., 1988), show significant UT activity in embryonic axis of germinating seeds but not in the germinated RFO-storing cotyledons (Main et al., 1983; Feusi et al., 1999). The enzyme is generally not observed in other plants (Keller and Pharr, 1996).
The alternative pathway can be carried out by a pyrophosphorylase (PPase; Gal-1-P, UTP transferase) utilizing UTP.
 |
Previous studies have shown that the melon (Cucumis melo) fruit, with its active Gal metabolism, shows little UT activity, suggesting that a PPase is responsible for Gal-1-P metabolism (Smart and Pharr, 1981; Feusi et al., 1999). However, there is no known PPase that is specific for the Gal moiety. Rather, there appears to be a PPase in melon fruit that can utilize both Gal-1-P and Glc-1-P. This dual substrate PPase is present in cucurbit fruit in addition to the UDP-Glc PPase (UGPase; E.C. 2.7.7.9), which is specific for the Glc-1-P sugar and inactive with Gal-1-P (Smart and Pharr, 1981; Feusi et al., 1999; Gao et al., 1999). Feusi et al. (1999) purified and characterized an enzyme fraction from melon fruit that catalyzed the nucleotide transfer to both Glc-1-P and Gal-1-P and were unable to further separate the activities, suggesting that the two reactions are catalyzed by the same protein.
The UDP-Gal product of this pathway is further metabolized to UDP-Glc via the epimerase reaction. The UDP-Glc can be directly utilized in cell wall metabolism or in Suc synthesis. However, for Suc synthesis, which characterizes the developing melon fruit (Schaffer et al., 1996), UDP-Glc must be further metabolized to Glc-1-P. This can be carried out either by the UGPase in the reverse direction or by the dual substrate PPase itself operating in the reverse direction. According to the latter possibility, the three-step pathway of Gal-1-P to Glc-1-P could be carried out by a single PPase, functioning sequentially in reverse directions separated by the epimerase reaction. Such a pathway was initially proposed by Smart and Pharr (1981); however, the uncertainty that the dual substrate activity is in fact due to a single protein precluded testing the hypothesis and determining the flux path of RFO Gal.
In this study, we report on the purification, peptide sequencing, and cloning of a novel plant-specific enzyme from melon fruit that carries out the UDP-hexose PPase reaction and can utilize both Gal-1-P and Glc-1-P sugars in the nucleotide-hexose synthesis direction and UDP-Glc and UDP-Gal in the reverse direction. In addition, we cloned the genes for UT and UGPase from melon fruit and compared the developmental expression of these three genes involved in Glc-P and Gal-P metabolism in melon fruit. The results indicate that the flux of Gal metabolism in the RFO-translocating cucurbit fruit, from Gal-1-P to Glc-1-P, is carried out by this novel enzyme.
RESULTS
Purification and Peptide Sequencing and Cloning
Antibodies prepared against the purified protein of Feusi et al. (1999) were used to identify a partially purified protein extract from young melon fruit (Fig. 1). The corresponding 68-kD band from the SDS-PAGE gel was excised and the protein sequenced after partial peptide hydrolysis.
Figure 1.
A, Partial purification of melon fruit UGGPase on HPLC-MonoQ. Black circles indicate activity with UDP-Glc in the pyrophosphorolytic direction and white circles indicate activity with Gal-1-P in the synthesis direction. B, Western blot using UGGPase antibodies (Feusi et al., 1999) of electrophoretic separations of MW markers (first lane), crude melon fruit extracts (second lane), and HPLC MonoQ fractions exhibiting UGGPase activity (third lane). C, Coomassie Blue protein stain of the HPLC MonoQ fractions exhibiting UGGPase activity: MW markers (first lane) and HPLC MonoQ fractions exhibiting UGGPase activity (second lane). The band in the Coomassie stain, indicated by the arrow and corresponding to the band in the immunoblot, was excised and microsequenced.
Based on seven peptide sequences obtained, we carried out a BLAST analysis, identifying At5g52560 as a homologous gene (79%) included in Pfam01704 and containing a UGPase motif (Fig. 2A). Based on the homology with the Arabidopsis (Arabidopsis thaliana) homolog and additional plant homologs reported in The Institute for Genomic Research expressed sequence tag (EST) databases, we synthesized degenerate primers and a 546-bp amplified product was sequenced. The upstream and downstream portions of the gene were sequenced from a young melon fruit cDNA library in pBK-CMV phagemid vector. Figure 2A shows the deduced sequence of the protein and its homologies to similar plant enzymes, as well as the seven peptide sequences obtained. The calculated molecular weight (MW) of the enzyme is 67,787, consistent with the band in Figure 1. We refer to this enzyme as UDP-Gal/Glc PPase (UGGPase; deposited in gene bank as DQ399739).
Figure 2.
Protein sequence homology alignments of plant UGGPase, UGPase, and UT. Sequences in bold indicate the seven peptide sequences obtained from the peptide microsequencing of the purified protein. Underlined sequences indicate conserved sequences used for the preparation of degenerate primers for the PCR cloning of the melon genes. Accession numbers of the sequence presented are UGGPase: melon (DQ399739), Arabidopsis (AF360236), and pea (AB178642); UT: melon (DQ445484), potato (TC28197), and Arabidopsis (NM_121825); UGPase: melon (DQ445483), Arabidopsis (NM_121737), potato (U20345), and barley (Q07131).
The genes for melon fruit UGPase and UT were cloned by PCR, based on homologous and conserved sequences of other plant genes in the database (as indicated in Fig. 2, B and C). Full-length sequences were obtained from the young fruit cDNA library and melon bacterial artificial chromosome (BAC) library for UGPase and UT, respectively. The UGPase gene encodes for a protein of 52 kD and the UT gene encodes for a protein of 38 kD, consistent with the MW of enzymes in these two families.
Functional Expression and Characterization of the Gene Products
Figure 3 shows the heterologously expressed proteins of UGGPase, UGPase, and UT in Escherichia coli extracts. The expressed UGGPase and UGPase enzymes were active and were partially purified by ion-exchange chromatography and characterized with regard to substrate specificity and affinity. The UT enzyme was sequestered in inclusion bodies, and we were not successful in determining functional activity of the heterologously expressed protein. Mass spectrometry analysis of the differentially expressed band in Figure 3C indicated that it is indeed UT (data not shown).
Figure 3.
Expressed proteins in E. coli, separated on SDS-PAGE, 20 μg protein/well. A, Melon UGGPase. B, Melon UGPase. C, Melon UT. For each enzyme, the three lanes represent, respectively, the MW marker, the E. coli extract with the expressed protein (+IPTG), and the E. coli extract without the heterologously expressed protein (−IPTG).
The novel UGGPase can utilize both Glc-1-P and Gal-1-P in the synthesis of the respective nucleotide sugars and also can utilize either UDP-Glc or UDP-Gal in the reverse direction. Substrate affinity is higher for the Glc moiety in both directions, but Vmax is higher for the Gal moiety in each direction. (Table I).
Table I.
Km (mm) and Vmax (μmol mg protein−1min−1) of heterologously expressed and partially purified melon UGGPase and UGPase
ND, Not detected.
| Enzyme | Pyrophosphorolysis
|
UDP Sugar Synthesis
|
||||||
|---|---|---|---|---|---|---|---|---|
| UDP-Gal
|
UDP-Glc
|
Gal-1-P
|
Glc-1-P
|
|||||
| Km | Vmax | Km | Vmax | Km | Vmax | Km | Vmax | |
| UGGPase | 0.44 | 625 | 0.14 | 238 | 0.43 | 714 | 0.27 | 222 |
| UGPase | 0.26 | 714 | 0.11 | 277 | ND | ND | 0.24 | 238 |
The heterologously expressed melon UGPase is specific for the Glc moiety in the direction of nucleotide sugar synthesis (using Glc-1-P as substrate) and shows no observable activity with Gal-1-P. However, in the reverse direction the UGPase did show activity with UDP-Gal, as well as with UDP-Glc. Affinity of the UGPase for the UDP-Gal is slightly lower than for UDP-Glc; however, the Vmax is significantly higher (Table I).
In light of the surprising result that the melon UGPase is active with UDP-Gal, we compared the characteristics of the purified melon UGPase with those from a noncucurbit plant, young tomato (Lycopersicon esculentum) fruit, to determine whether the ability to metabolize UDP-Gal is unique to the melon UGPase. Surprisingly, we observed that a partially purified tomato fruit UGPase did, in fact, metabolize UDP-Gal in addition to UDP-Glc (Table II). The tomato enzyme fraction did not show any UDP-Glc-4′ epimerase activity (data not shown), indicating that the activity measured with UDP-Gal was not artifactually due to UDP-Gal to UDP-Glc conversion. In the reverse direction, the tomato UGPase was specific for Glc-1-P and did not metabolize Gal-1-P, similar to the melon UGPase, indicating that the partially purified fraction did not contain a UGGPase.
Table II.
Comparison of substrate specificity of melon and tomato UGPase and UGGPase
Control noninduced transformed E. coli extracts (−IPTG) showed approximately 5% of the activity with either UDP-Gal or UDP-Glc, as compared to the induced (+IPTG) E. coli extracts. ND, Not detected.
| Substrates | Enzyme Activity
|
||
|---|---|---|---|
| Melon UGPase E. coli | Melon UGGPase E. coli | Tomato UGPase Native | |
| μmol mg protein−1 min−1 | |||
| UDP-Gal + PPi | 460 | 410 | 12 |
| UDP-Glc + PPi | 245 | 233 | 38 |
| Gal-1-P + UTP | ND | 678 | ND |
| Glc-1-P + UTP | 187 | 223 | 8 |
Gal Metabolism Gene Expression and Enzyme Activities in Young Fruit
To determine the potential relative contribution of the three enzymes in Gal-1-P flux in developing melon fruit, we assayed activity in crude extracts from immature and developing ovaries and compared these to the relative quantitative expression of their respective genes. The enzyme activities of the UGPase, UGGPase, and UT in developing ovaries are presented in Table III. The assay of UGGPase was carried out using Gal-1-P as substrate so that the assay was specific for this enzyme. However, because both UGPase and UGGPase are active with the Glc moiety in either direction, the assay does not distinguish between the two enzymes. We therefore separated the two activities using hydrophobic interaction chromatography, and the results show that the two enzymes are of approximate equal activity (Fig. 4). The results indicate that Gal-1-P metabolism is carried out preferentially by the UGGPase enzyme. Although both the UGGPase as well as the Glc-1-P specific UGPase are active in the developing fruit, the latter is inactive on the GalK reaction product Gal-1-P, as described above. UT activity is barely observed in the developing ovaries (Table III).
Table III.
Activity of UGGPase, UGPase, and UT in crude extracts from young melon ovaries
| Substrate | Assay for Enzyme | Activity |
|---|---|---|
| μmol product mg protein−1 min−1 | ||
| Gal-1-P + UTP | UGGPase | 8 |
| UDP-Glc + PPi | UGPase + UGGPase | 14 |
| UDP-Glc + Gal-1-P | UT | 0.007 |
Figure 4.
Hydrophobic interaction chromatography separation (phenyl Sepharose) of UGPase and UGGPase from melon fruit ovaries. Black circles indicate activity with Gal-1-P and white circles indicate activity with Glc-1-P. Peak I is the Glc-1-P specific UGPase and peak II is the UGGPase enzyme.
The gene expression patterns of the three genes paralleled the enzyme activity in crude extracts of developing melon ovaries and fruit. Northern blots showed expression of both UGGPase and UGPase throughout fruit development, while UT expression was not observed at the level of detection of northern blots (Fig. 5A). We further performed quantitative reverse transcription-PCR on mRNA of melon ovaries and developing fruit, and UT expression was very low compared to UGGPase (Fig. 5B).
Figure 5.
Expression patterns of UGGPase, UGPase, and UT in developing melon fruit. A, Northern blots (UT was not detected and is not presented). B, Quantitative reverse transcription-PCR of UT, and UGGPase. mRNA expression is relative to the expression of the melon actin gene. DAA, Days after anthesis; rRNA, ribosomal RNA.
Flux of Gal-1-P
To prove that both PPase reactions in the Gal-1-P to Glc-1-P flux can be carried out in consort by UGGPase in the absence of UGPase, we measured production of Glc-1-P from the substrates Gal-1-P and UTP in the presence of only UGGPase and epimerase. Inorganic pyrophosphate (PPi) was also not added to test whether the second PPase reaction, which is dependent on PPi, can take place dependent on the production of PPi in the initial reaction. The partially purified native melon UGGPase (Table IV), as well as the heterologously expressed melon UGGPase (Table V), were each used in conjunction with a purified epimerase to make certain that UGPase activity was not present. The Glc-1-P product was continuously removed by the linked phosphoglucomutase (PGM) and Glc-6-P dehydrogenase (G6PDH) reactions in an enzyme-linked assay. The results of these experiments (Tables IV and V) show that the UGGPase alone can carry out both the Gal-1-P conversion to UDP-Gal and the subsequent reverse reaction of UDP-Glc to Glc-1-P. Most significantly, the synthesis of Glc-1-P from UDP-Glc took place without the external addition of PPi, indicating that the PPi produced in the Gal-1-P + UTP → UDP-Gal + PPi reaction was cycled into the reverse reaction following the epimerase step.
Table IV.
Dependence of Glc-1-P production from Gal-1-P and UTP on the addition of partially purified melon fruit UGGPase and purified epimerase
Each reaction was carried out with approximately 13 μg protein from fraction 18 of Figure 1A in a 1-mL reaction mix. ND, No activity detected.
| Substrate | Enzyme in Reaction
|
Glc-1-P Produced | |
|---|---|---|---|
| UGGPase | Epimerase | ||
| μmol Glc-1-P mg protein−1 min−1 | |||
| Gal-1-P, UTP | + | + | 1.8 |
| Gal-1-P, UTP | + | − | ND |
| Gal-1-P, UTP | − | − | ND |
Table V.
Glc-1-P production from Gal-1-P and UTP with crude extracts of E. coli expressed protein of either melon UGGPase or melon UGPase, together with purified epimerase
The E. coli extract (−IPTG) did not express the UGGPase protein and served as blank reaction. Each reaction was carried out with approximately 10 μg protein from the crude E. coli extractions shown in Figure 3 in a 1-mL reaction mix. ND, No activity detected.
| Substrate | E. coli Extract | Glc-1-P Produced |
|---|---|---|
| μmol Glc-1-P mg protein−1 min−1 | ||
| Gal-1-P, UTP | UGGPase, −IPTG | ND |
| Gal-1-P, UTP | UGGPase, +IPTG | 14.5 |
| Gal-1-P, UTP | UGPase, +IPTG | ND |
DISCUSSION
The results of our study shed light on the pathway of Gal metabolism in the fruit sink of the RFO translocating melon plant. This pathway is not limited only to melon but is evident in other cucurbits (Smart and Pharr, 1981) and is likely present in other noncucurbitaceous plants as well, as evidenced by the presence of expressed sequences of the homologous genes from other families (see below).
We cloned and expressed the two genes capable of Gal-1-P metabolism, the novel UGGPase and the Leloir pathway UT, and show that, of the two, the gene expression of only the UGGPase gene is evident in the young fruit. Based on both the gene expression and enzyme activity studies, there does not appear to be significant Gal-1-P flux in cucurbits via the Leloir pathway utilizing a UT, as suggested by previous studies (Smart and Pharr, 1981; Feusi et al., 1999). The Glc-1-P specific UGPase is also highly expressed and active; however, its presence is irrelevant to Gal-1-P metabolism because it is inactive with this substrate.
The pathway presented in Figure 6 begins with the hydrolytic removal of Gal from the galactosyl-Suc translocate upon its arrival at the fruit sink. Since free Gal is practically absent in these sink tissues (Gross and Pharr, 1982; Hubbard et al., 1989), the continuation of free Gal metabolism is likely colocalized with the initial α-galactosidase hydrolysis reaction. The free Gal is phosphorylated by GalK to Gal-1-P, which allows its further metabolism. We have partially purified the GalK enzyme from melon fruit and cloned the gene encoding for it. Our results show that the enzyme is specific for both Gal and ATP and also requires Mg for activity, similar to other hexose kinases (Z. Gao and A.A. Schaffer, unpublished data). The metabolism may take place in the fruit pedicel (Smart and Pharr, 1981) or be associated with the fruit vasculature (Burger and Schaffer, 1991; Pharr and Hubbard, 1994; Schaffer et al., 1996).
Figure 6.
Proposed pathway of Gal metabolism in melon fruit, emphasizing the dual role of the UGGPase. The enzymes involved in Gal metabolism in melon fruit are represented in italics.
The resultant Gal-1-P is converted to UDP-Gal via the novel UGGPase enzyme, utilizing UTP and producing PPi. The epimerase reaction can convert the UDP-Gal to UDP-Glc to be used directly for numerous sugar metabolism reactions, particularly cell wall synthesis (Kleczkowski, 1994). Alternatively, and particularly in the case of the Suc accumulating melon fruit, the UDP-Glc is converted to Glc-1-P for the continuation of hexose metabolism leading to the synthesis of Suc, the major stored sugar of the sweet melon (Rosa, 1928; Schaffer et al., 1987; Hubbard et al., 1989).
The cloning and expression of the UGGPase allowed us to prove that the dual activity described previously in purified fractions (Feusi et al., 1999) is in fact due to a single enzyme. It also allowed us to prove that the first and last step of the three-step hexose-P sugar conversion, from Gal-1-P to Glc-1-P, is carried out by the same enzyme, as indicated by Figure 6. First, we show that the UGGPase enzyme itself is capable of carrying out the conversion of the epimerized product UDP-Glc to Glc-1-P, because its dual substrate specificity characterizes both its phosphorolytic and synthesis directions. More significantly, we show that the expressed UGGPase, together with epimerase and in the absence of UGPase, can carry out the three-step flux of Gal-1-P to Glc-1-P conversion. This occurs without the addition of PPi as substrate for the UDP-Glc to Glc-1-P reaction and depending only on the production of PPi from the initial reaction (Tables IV and V). It is therefore tempting to speculate, although there is no actual evidence for it, that such a metabolic pathway might imply a linkage of the UGGPase and epimerase enzymes in a complex, with transfer of products and substrates between the two enzymes. Such a physical complex would also envision a cycling of the UTP and PPi substrate/products within the complex. One of the advantages of such an intracomplex cycling would be that the hydrolysis of PPi by ubiquitous pyrophosphatases present in the cytoplasm, which might otherwise make in vivo UDP-hexose cleavage unlikely (Feingold and Avigad, 1980) would not occur, allowing the enzymatic pyrophosphorolysis of the epimerase product (UDP-Glc → Glc-1-P) to take place.
This mechanism would suggest that the UGPase enzyme that can carry out the pyrophosphorylation step of the epimerase product UDP-Glc to Glc-1-P may be relegated to other pathways of nucleotide sugar metabolism, such as cell wall synthesis, and play a minor, if any, role in the metabolism of galactosyl-sugar translocate. One of the interesting characteristics of the UGPase is that while it is specific for the Glc-P moiety in the direction of UDP-hexose synthesis, it can carry out the reverse reaction with UDP-Gal to synthesize Gal-1-P. Surprisingly, this is not unique for the melon UGPase, and we report here that a partially purified tomato fruit UGPase also can metabolize UDP-Gal in addition to UDP-Glc, although it too is specific for the Glc-1-P in the nucleotide sugar synthesis direction. Previous studies of UGPase from other plants have not reported the relative enzyme activity with UDP-Gal. Studies of purified UGPase from mung bean (Vigna radiata; Ginsburg, 1958), cucumber (Cucumis sativus; Smart and Pharr, 1981) peduncles, and germinated barley (Hordeum vulgare; Elling, 1996) have only compared the hexose-P specificity and have shown that the enzyme is specific for Glc-1-P and is inactive toward Gal-1-P. Similarly, studies in the reverse direction have compared the various nucleotide-Glc substrates and shown that the enzyme is specific for UDP-Glc with little activity toward the other nucleotide-Glc compounds, as in, for example, potato (Solanum tuberosum; Nakano et al., 1989), Jerusalem artichoke (Helianthus tuberosus; Otozai et al., 1973), and calf liver (Hansen et al., 1966). However, we have not found a comparison of the substrate specificity of plant UGPase with respect to UDP-Gal. Calf liver UGPase was characterized with respect to both UDP-Glc and UDP-Gal, and activity toward the latter was less than 4% that of UDP-Glc (Hansen et al., 1966). This report of activity by plant UGPase with UDP-Gal in the synthesis of Gal-1-P suggests the possible involvement of this enzyme in UDP-Gal metabolism, although not in the metabolism of Gal-1-P.
The ability of UGPase to metabolize UDP-Gal is not limited to the melon enzyme described here and, as indicated, the tomato enzyme has similar activity. Furthermore, comparison of amino acid sequences of the melon UGPase with other plant UGPases indicates a very high homology with no indication of a significant change in the sugar binding site of the melon enzyme that would indicate that its substrate specificity would be unique. Recently, Geisler et al. (2004) carefully homology-modeled the three-dimensional (3-D) structure of plant UGPase. The homology comparisons between the melon sequence and the modeled sequences indicate a few otherwise conserved amino acids that are not conserved in the melon sequence (replacements of the following, according to the melon sequence: I154V, Y180F, V234I, Q238K Q293E, and I334V), but based on the 3-D homology modeling these amino acid differences are unlikely to play a role in the reaction that might alter or extend the melon enzyme substrate specificity to uniquely include UDP-Gal. Rather, it appears that UGPases as a class can metabolize UDP-Gal. The ability of plant UGPases to metabolize UDP-Gal presents the possibility of the involvement of UGPase in UDP-Gal metabolism, although not in its synthesis.
The phylogenetic relationship between the three melon enzymes and other members of related nucleotide-sugar metabolism enzymes is presented in Figure 7. The UGGPase enzyme is most closely related to the UDP-N-acetyl Gal/Glc amine PPase enzyme family (approx 25%–30% homology), which is present in both animals and plants, and the two enzymes are listed together in pfam01704. The human UDP-N-acetyl Gal/Glc amine PPase can accommodate either UDP-N-acetyl Glc or UDP-N-acetyl Gal (Peneff et al., 2001) in a manner analogous to the accommodation of both UDP-Glc and UDP-Gal by the melon UGGPase enzyme. The 3-D structure of the human UDP-N-acetyl Gal/Glc amine PPase (AGX1; pdb file, ijv1) has been deciphered, and it accounts for the ability of this enzyme to accommodate both the equatorial and axial conformations of the OH of the C4 carbon of the hexose sugars of Glc and Gal, respectively (Peneff et al., 2001). The C4 equatorial (Glc) conformation is bonded to the G290 and N327 backbone atoms, whereas the C4 axial (Gal) conformation is accommodated by the side chain of the same N327 and the backbone amide of the A329. Therefore, the amino acid of UGGPase that corresponds to the A329 of AGX1 is likely to contribute to the Gal substrate-binding pocket structure of the enzyme. Modeling of the UGGPase against the AGX1, performed by 3DPSSM (www.sbg.bio.ic.ac.uk), indicates that NIN is the corresponding sequence to the NIA in the corresponding β-sheet of the AGX1 (data not shown). Accordingly, this replacement of Ala in the AGX1 by Asn in the plant enzyme (NIA to NIN) would not impede the accommodation of the Gal equatorial 4OH. In contrast, the model of UGPase structure (Geisler et al., 2004) indicates that the respective amino acids in the corresponding β-sheet are NLW in place of NIA of the AGX1, and that the large hydrophobic Trp in the UGPase NLW sequence may indeed impede the equatorial Gal 4OH, explaining the specificity for Glu-P for this enzyme. We attempted to show that this is the case by performing a site-directed mutagenesis of the UGPase W to N. We hypothesized that the modified UGPase would be capable of metabolizing Gal-1-P. However, the mutated enzyme expressed in E. coli was inactive (data not shown), presumably due to improper folding, as described by Chang et al. (1996) who similarly performed site-directed mutagenesis of the human liver UGPase and observed that the Trp was essential for folding and activity of UGPase.
Figure 7.
Phylogenetic tree of nucleotide-sugar metabolism enzymes. The abbreviations and the accession numbers of the sequences used in the preparation of the tree are as follows: UGP, UGPase: Homo sapiens (Hs), Q07131; Arabidopsis (At), NM_121737; melon (Cm), DQ445483; and potato (St), U20345. NAGA, UDP-N-acetyl-Gal/Glc amine PPase: Homo sapiens (Hs), BC009377; Arabidopsis (At), BT020380; rice (Os), AK071409. UGGP, UGGPase: rice (Os), AK064009; Arabidopsis (At), AF360236; pea (Ps), AB178642; melon (Cm), DQ399739. UT: Homo sapiens (Hs), P07902; Arabidopsis (At), NM_121825; melon (Cm), DQ445484; potato (St), TC28197. AGP, ADPglu PPase, small subunit: Arabidopsis (At), NM_124205; Citrus unshiu (Cu), AF184597; melon (Cm), AF030382; pea (Ps), X96764. The tree was prepared using the ClustalX alignment and Treeview programs. Bar represents distance value of 0.1 substitution/site.
As mentioned earlier, the UGGPase enzyme is not limited to the cucurbits. Recently, a homologous enzyme was described in germinating pea (Pisum sativum) seeds (Kotake et al., 2004) and a search of the EST databases (www.tigr.org) shows expressed homologs from other plant families, including Solanaceae (tomato, BF05177), Brassicaceae (Arabidopsis, TC262279), Leguminoseae (soya, TC228175), Compositaceae (sunflower [Helianthus annuus], TC10097), and Graminae (wheat [Triticum aestivum], TC251010), although these are described as unknown proteins. Pea seeds, as well as other seeds of the Leguminosae, store RFOs in their seeds, and therefore Gal metabolism in germinating legume seeds and sprouts is likely analogous to RFO metabolism in cucurbit sink tissue. In fact, germinating legume seeds also have an alkaline α-galactosidase that is present in cucurbit sinks and presumably carries out the initial step of RFO catabolism (Gao and Schaffer, 1999; Carmi et al., 2003). However, the UGGPase is not limited to plants characterized by an active RFO metabolic pathway and is also expressed in numerous other plants. This is again analogous to the presence of the alkaline α-galactosidase in plants other than RFO translocators (Gao and Schaffer, 1999; Carmi et al., 2003). In fact, numerous plants carry out RFO metabolism, although perhaps not to the extent of the cucurbits and legumes. Graminae seeds (Kuo et al., 1988) and Arabidopsis seedlings (Haritatos et al., 2000) accumulate or translocate RFO sugars to some degree and the metabolism of the Gal degradation product presumably utilizes this enzymatic pathway. In addition, this pathway may be ubiquitous in plants unrelated to RFO and it may offer a pathway for UDP-Gal metabolism alternative to the UT pathway. The novel enzyme may play a role in Glc-1-P/UDP-Glc metabolism in general, and it is tempting to see in this alternative pathway an explanation for the lack of striking phenotypes in transgenic antisense UGPase plants (Zrenner et al., 1993; Spychalla et al., 1994). Furthermore, the homologous enzyme from pea seed was characterized in detail for specificity with respect to the sugar moiety of the sugar-phosphate substrate, and it shows activity also toward the pentoses, Ara and Xyl, suggesting possible involvement in pentose salvaging and cell wall metabolism (Kotake et al., 2004).
With respect to the cucurbits, however, the metabolism of translocated RFOs appears to be via the novel UGGPase. The results of this research, together with the previous description of a novel α-galactosidase that is active at neutral to slightly basic pH, and with the similar neutral-basic pH optima of the melon GalK (Z. Gao and A.A. Schaffer, unpublished data), the UGGPase, and the epimerase enzymes, indicate a closely linked, colocalized, presumably cytosolic metabolic pathway of Gal metabolism in these plants.
MATERIALS AND METHODS
Gene Cloning
UGGPase Protein Purification and Peptide Sequencing
Fresh tissue of melon (Cucumis melo subsp. melo Group Reticulatus cv Noy Yizre'el) fruitlets, 3 d after anthesis 3.5 g, was ground in liquid nitrogen and protein was extracted with 20 mL of buffer containing 50 mm HEPES-NaOH, pH 7.5, 1 mm EDTA, 5 mm dithiothreitol (DTT), 1 mm phenylmethylsulfonyl fluoride, and 0.1% polyvinylpyrrolidone. After centrifugation at 10,000g for 30 min, the supernatant was filtered through 0.2 μm cellulose acetate filter (Schleicher and Schuell) and loaded on a MonoQ HR 5/5 (Pharmacia Biotech) column as described in Petreikov et al. (2001). The column was equilibrated and the unbound protein washed out with 20 mm HEPES-NaOH, pH 7.2, 2.5 mm DTT (buffer A), and the bound protein was eluted with the 0 to 0.25 mm KCl gradient in the same buffer. Protein of the fractions exhibiting UDP-Gal/Glu PPase activity (described below) were concentrated by acetone precipitation and subjected to SDS-PAGE (10%), using a Bio-Rad Mini-Electrophoresis system according to the manufacturer's instruction. A total of 40 and 10 μg of protein were loaded for Coomassie Brilliant Blue R-250 and immunoblotting, respectively. Polyclonal UDP-Gal PPase antibodies, developed as described in Feusi et al. (1999), were used for immunoblotting at a dilution of 1:1,000. Following immunoblotting, as described in Schaffer and Petreikov (1997), the UDP-Gal/Glu PPase band was visualized using 5-bromo-4-chloro-3 indolyl phosphate/nitroblue tetrazolium (Promega), according to the manufacturer's instructions.
Peptide Sequencing
The excised 68-kD gel band stained with Coomassie Blue and corresponding to the band of UGGPase as determined by immunoblotting was subjected to MS/MS analysis following protein digestion treatment with trypsin, further mass spectrometry carried out with Otof2 (Micromass) using nanospray attachment, and data analysis (Otof Laboratory Interdepartmental Equipment Unit, The Hebrew University Medical School, Israel). Seven peptide sequences were obtained and a BLAST analysis was carried out, identifying a homologous Arabidopsis (Arabidopsis thaliana) gene At5g52560 of unknown function. Using the Arabidopsis protein sequence as a query, we identified additional homologous genes from the various plant EST databases available at The Institute of Genomic Research (www.tigr.org). The following ESTs were identified and used to perform a ClustalW homology alignment to identify conserved sequences: wheat (Triticum aestivum; TC251010), rice (Oryza sativa; TC277270), and barley (Hordeum vulgare; TC148351).
UGGPase Cloning
The initial DNA fragment of the melon UGGPase gene was cloned from young melon fruit cDNA using two degenerate primers: UGGP-F1 5′GCN GGN YTN AAR TGG GT3′ and UGGP-R1 5′GGC CAN ACY TCN ACY TC3′, based on the sequences AGLKWV and EVEVWP. The 546-bp product was sequenced and cloning of the upstream region of the gene was carried out using the upstream degenerate primer UGGP-F3 5′TCN AGY TAY CCN GGN GG3′, for the SSYPGG, N-terminal sequence together with degenerate primer UGGP-R1. The UGGPase full-length sequence was cloned from a young melon fruit EST library (see below) using: (1) UGGPase internal primers, UGGP-R5′ 5′CCCACCAGCAACAAGAACAAA3′ and UGGP-F3′ 5′CTTCAACCCGATTGGAATGTA3′ and (2) primers of T7 and T3 promoter sequences from the multiple cloning site region of the pBK-CMV phagemid vector.
Young Fruit EST Library
Total RNA was isolated from 10 g fresh weight from a mixture of Noy Yizre'el fruits, collected at 0, 1, 3, 12, and 25 d post anthesis, using a modification of the method of La Claire and Herrin (1997). Poly(A)+ mRNA was purified from 1 mg of total RNA by use of Oligotex mRNA purification kit according to manufacturer's recommendations (Qiagen). Five micrograms of poly(A)+ mRNA were used for preparation of the library. The EST library was constructed using ZAP cDNA synthesis kit and ZAP Express cDNA Gigapack III gold cloning kit according to manufacturer's recommendations (Stratagene). Phage clones were mass excised to pBK-CMV phagemid vector following the manufacturer's instructions (Stratagene).
UGPase Cloning
Melon UGPase was cloned from young melon cDNA using four degenerate primers: UGP-F1 5′ACN ATG GGN TGY CAN GG3′; UGP-F2 5′GAY GGN TGG TAY CCN CC3′; UGP-R1 5′CCN CCY TTN ACR TCN GC3′; and UGP-R2 5′CCR TCN ACY TCY TTN GG3′. The degenerate primers were constructed based on consensus amino acid sequences (TMGCTG, DGWYPP, ADVKGG, and PKEVDG, respectively) identified by multiple alignments of potato (Solanum tuberosum; AAB71613), Arabidopsis (AKK64100), banana (Musa spp.; AAF17422), rice (BAB69069), barley (CAA62689), and Japanese pear (Pyrus serotina Rehd.; BAA25917) published full-length sequences. The initial 705-bp PCR fragment of the melon UGPase gene was cloned and sequenced using the T/A cloning vector pGEM-Teasy (Promega). Melon UGPase full-length sequence was cloned from a young melon (cv Noy Yizre'el) cDNA library constructed in a yeast (Saccharomyces cerevisiae) shuttle vector, pFL61 (Minet et al., 1992). The internal primers UGP-F202 5′ACATTCAACCAGAGCCAATATC3′ and UGP-R603 5′CACCCACAAATTGTTAGTGTTG3′ together with the pFL61 flanking region primers pFL-F and pFL-R were used to clone the UGPase 5′ and 3′ regions and assemble the full gene sequence.
UT Cloning
The cloning of melon Gal-1-P UT gene from melon was also carried out based on conserved amino acid sequences. However, when we started this cloning work, there were only a few partial plant ESTs in the databases, in addition to human, bacterial, and fungal UTs and an Arabidopsis UT. Based on the ClustalW homology alignment from plant UT sequences of tomato (Lycopersicon esculentum; TC103202), potato (TC41313), wheat (TC46973), and Arabidopsis (NM_121825), we selected conserved sequences of E(H/Q)(E/Q) CAPE and QVFKN(Q/H) GA for the preparation of degenerate primers. The first 320 bp of the melon Gal-1-P UT gene was cloned by the degenerate primers UT-F7 5′GAG CAN SAG TGY GCN CCN GAG3′ and UT-R7 5′GCN CCN TGG TTY TTG AAN ACC TG3′. Full sequence of the melon UT was completed by direct sequencing of the BAC clone 121K16 selected from the Clemson University Genomics Institute (www.cugi.edu) melon BAC library MR-1 EcoR1 filters using the UT 320-bp fragment as a probe. Cloning was done from the BAC due to the very low abundance of UT mRNA in melon fruit. The primers (UT-R 5′ACATCCTCGGGGGTCAAATCAGA3′ and UT-F3′ 5′CAGGCTTCGGATTCAGACTTAG3′) were used to sequence the melon UT 5′ end and 3′ end from the BAC DNA, respectively.
Expression of UGPase, UGGPase, and UT mRNA in Escherichia coli
Full-length open reading frames were cloned in the bacterial expression vector pET-28a (Novagen, EMD Biosciences) using the following restriction sites: NdeI site for 5′ end of the three open reading frames; XhoI site for 3′ end of the UGPase and UGGPase; and BamHI site for 3′ end of UT.
Expression plasmids containing UGPase, UGGPase, or UT mRNA were transformed into E. coli BL21 (DE3) LysE cells (Dubendorff and Studier, 1991). Bacterial colonies were grown in a 50-mL flask containing 10 mL of Luria-Bertani medium to an OD600 of 0.5, induced for expression with 0.4 mm of isopropylthio-β-galactoside (IPTG; control bacterial extracts were prepared from noninduced [−IPTG] cultures) and harvested after 6 h or overnight by centrifugation at 4,000g for 10 min. Cells were lysed by resuspension in 2 mL extraction buffer (20 mm phosphate buffer, pH 8, 1 mm EDTA, 500 mm NaCl, 0.1% Triton X-100, 2.5 mm DTT, and 1 mg/mL lysozyme) for 1 h at 4°C and mechanically broken by freezing and thawing three times. The viscous bacterial lysate was sheared using a 21-gauge needle, and crude soluble protein extract was obtained after centrifugation at 15,000g for 30 min at 4°C and collection of the soluble fraction. The bacterial crude proteins extracts were used to assay enzyme activities.
RNA Extraction Northern-Blot Analysis
Total RNA was isolated from 10 g fresh weight of melon flesh using a modification of the method of La Claire and Herrin (1997). Each 10-g sample was pooled from three melon fruit of the same developmental stage. For northern-blot analysis, 20 μg of total RNA from each developmental stage was separated on a denaturing 1% agarose gel using MOPS buffer. Expression of UGPase and UGGPase mRNAs were analyzed by RNA gel blotting using specific probes for UGPase (422 bp) and UGGPase (710 bp). Detection of UGPase and UGGPase mRNAs was analyzed using a PhosphoImager (Molecular Dynamics). To indicate the amount of RNA loaded in each well, the nylon membrane was stained for 5 min with 5% methylene blue.
Quantitative Real-Time PCR
cDNA was synthesized from 1 μg RNA (DNase treated) using the Reverse-iT 1st Strand Synthesis kit (ABgene), according to manufacturer's instructions. A total of 1 μL cDNA product was used as template for real-time PCR reaction based on eurogentec qPCR core kit and SYBRR Green I as a fluorescent substance. The specific primers used for the UGGPase and UT genes were: UGGP-QF, 5′AACCCGATTGGAATGTATGAT3′; UGGP-QR, 5′CCGAAGTAGCACTGTGATAAG3′; UT-QF, 3′TCCTGCTCTCAGTAGGGATAAGG5′; and UT-QR, 5′ACATCCTCGGGGGTCAAATCAGA3′. The melon actin cDNA (AY859055) was quantified with the following primers: forward, 5′GATTCCGTGCCCAGAAGTT3′ and reverse, 5′TTCCTTGCTCATCCTGTCTG3′ and used for normalizing the expression data. The real-time PCR reaction was initiated by heat activation of 10 min at 95°C and continued for 40 cycles of 15 s at 95°C, 30 s at 60°C, and 30 s at 72°C, using the GeneAmp 5700 Sequence Detection system (PE Biosystems). Each specific amplicon: 167 bp for UGGPase, 159 bp for UT, and 187 bp for the melon actin genes had only one dissociation peak (data not shown) and linear calibration curves (for all genes, R2 = 0.96–0.99). The specific gene expression was calculated relative to the actin mRNA level in each sample according to the equation 2−(Ct sample− Ct actin), where Ct is the threshold cycle of the specific gene and actin.
Enzyme Extraction
Assays of native fruit enzyme activities were carried out on the crude extracts as described in the enzyme purification section above. When enzyme fractions were separated by ion-exchange chromatography, conditions were as described above with the exception of the separated tomato fruit extract in which the extraction buffer and elution buffer consisted of BisT propane, pH 9.0, in an attempt to bind the UGPase enzyme to the MonoQ column.
Phenyl Sepharose hydrophobic interaction chromatography (Hi Trap HIC, 1 mL, Pharmacia Biotech) was also used for the separation of the melon UGGPase and UGPase enzymes. The extraction mixture consisted of 50 mm phosphate, pH 7.0, 2 mm EDTA, 5 mm MgCl2, 0.8 mm gal, 5 mm DTT, and 1 mm phenylmethylsulfonyl fluoride. The supernatant after centrifugation at 10,000g for 30 min was adjusted to 1 m ammonium sulfate, incubated in ice for 20 min, centrifuged, filtered, and applied in the column. The unbound protein washed out with 50 mm phosphate, pH 7.0, 1 m ammonium sulfate, and the bound protein was eluted with the 1 to 0 m ammonium sulfate gradient in the phosphate buffer.
The bacterial-expressed enzymes were extracted as described above. For ion-exchange chromatographic separation, the enzyme extracts were diluted in 25 mm HEPES-NaOH, pH 7.5, 1 mm EDTA, 5 mm MgCl2, and 0.5 mm DTT and separated by MonoQ anion-exchange chromatography under conditions identical to the melon fruit enzyme extract conditions described above.
Enzyme Assays
Nucleotide-Sugar Synthesis Direction
In the synthesis direction of UDP-sugars, enzyme activities were assayed as described in detail in Gao et al. (1999), using Glc-1-P or Gal-1-P as substrates. The reaction mixture, in a total volume of 0.2 mL, contained 25 mm HEPES-NaOH, pH 7.5, 1 mm EDTA, 5 mm MgCl2, 0.5 mm DTT, 10 mm Gal-1-P or Glc-1-P, and 2.5 mm UTP. The reaction was initiated by adding 20 μL enzyme preparation at 30°C and terminated after 3 min by 2 min boiling. After cooling to room temperature, 1 mL 50 mm Tricine buffer, pH 8.7, containing 0.5 mm NAD, 0.01 unit of UDP-Glc dehydrogenase (Sigma), and 0.02 unit of UDP-Glc-4′ epimerase (Sigma) was added and the mixture was incubated at 30°C for 1 h prior to measuring 340 nm. Enzyme activity was expressed as μmol UDP-Gal produced per min at 30°C.
For the determination of the kinetic parameters of the partially purified enzymes, the substrates Glc-1-P and Gal-1-P were used in concentrations from 0 to 5 mm. The amount of UDP-sugars produced was quantified from standard curves of 0 to 75 nmol UDP-Glc and UDP-Gal in 0.5 mL of reaction mixture under the same assay conditions, and activity was expressed as the production of μmol UDP-Glc or UDP-Gal per min at 30°C.
Pyrophosphorolytic Direction
In the pyrophosphorolytic direction, the sugar-phosphate production was measured according to Smart and Pharr (1981) with modifications as follows. The reaction buffer contained 25 mm HEPES-NaOH, pH 7.5, 1 mm EDTA, 5 mm MgCl2, 0.5 mm DTT with addition of 1 mm of either UDP-Glc or UDP-Gal, and 10 μL of partially purified enzyme sample in a 100-μL reaction mixture. The reaction was initiated by 1 mm PPi and stopped after 3 min by boiling for 2 min and the mixture was cooled on ice. For the measurement of the respective hexose-1-P product, a single mixture was added: 400 μL of 50 mm HEPES-NaOH, pH 7.8, containing 5 mm MgCl2, 4 mm UDP-Glc, 0.02 units Gal-1-P UT (Sigma), 1 mm NAD, 10 μm gl-1,6 bis P, 2 units PGM, and 1 unit G6PDH (from Leuconostoc). After 40 min incubation at 30°C, absorbance of NADH product was recorded at 340 nm.
For the determination of kinetic parameters, the substrates UDP-Glc or UDP-Gal were used in concentrations from 0 to 1 mm. The amount of hexose-P produced was quantified from a standard curve of 0 to 100 nmol Glc-1-P/Gal-1-P in 0.5 mL of reaction mixture under the same assay condition and expressed as μmol Glc-1-P/Gal-1-P per min at 30°C.
For screening enzyme activities in the HPLC fractions during purification, two separate assays were used. For determining activity with the Glc moiety a coupled continuous assay was used in the pyrophosphorolytic direction and Glc-1-P formation was monitored as in Schaffer and Petreikov (1997). In brief, the PPi-dependent production of Glc-1-P from UDP-Glc was measured in a linked assay containing NADH, PGM, and G6PDH. For the determination of fractions active with the Gal moiety, we used the Gal-1-P specific assay described above in the nucleotide-sugar synthesis direction.
Gal-1-P UT
Two separate methods were used to measure UT activity in light of the near absence of activity in melon fruit. A continuous coupled enzyme assay modified from Elsevier and Fridovich-Keil (1996) was carried out in a 0.5-mL reaction mixture containing 50 mm HEPES-NaOH, pH 7.8, 5 mm MgCl2, 0.5 mm DTT, 10 mm Gal-1-P, 1 mm NAD, 10 μm gl-1,6 bis P, 1 unit G6PDH (from Leuconostoc), 2 units PGM, and enzyme sample. The reaction was initiated by 4 mm UDP-Glc and monitored for 10 min at 37°C. The amount of Glc-1-P produced was expressed as the amount of enzyme necessary to produce 1 μmol Glc-1-P per min at 37°C. Alternatively, a two-step end point assay, modified from Main et al. (1983), was used for determining UT activity. The reaction buffer contained 25 mm HEPES-NaOH, pH 7.5, 1 mm EDTA, 5 mm MgCl2, 0.5 mm DTT with addition of 10 mm Gal-1-P, and 10 μL of partially purified enzyme sample in a 100-μL reaction mixture. The reaction was initiated by 4 mm UDP-Glc and stopped after 10 min by boiling for 2 min and the mixture was cooled on ice. For the measurement of the Glc-1-P product, 400 μL consisting of 50 mm HEPES-NaOH, pH 7.8, 5 mm MgCl2, 1 mm NAD, 10 μm gl-1,6 bis P, 2 units PGM, and 1 unit G6PDH was used. After 40 min incubation at 30°C, absorbance of NADH product was recorded at 340 nm.
Protein Estimation
The Bio-Rad protein assay and bovine serum albumin as a standard were used to estimate the protein concentration according to the method of Bradford (1976).
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers DQ399739, DQ445483, and DQ445484.
Acknowledgments
The authors thank Dr. Yelena Yeselson and Mr. Shmuel Shen for technical assistance.
This work was supported by the U.S.-Israel Binational Agricultural Research and Development Fund (grant no. 2270–94) and by an Israel Ministry of Agriculture Chief Scientist Grant. This work is part of the research of the Center for the Genetic Enhancement of Cucurbit Fruit Quality. This is journal series number 133–05 of the Agricultural Research Organization.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Arthur A. Schaffer (vcaris@volcani.agri.gov.il).
References
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Burger Y, Schaffer AA (1991) Sucrose metabolism in mature fruit peduncles of Cucumis melo and Cucumis sativus. In JL Bonnemain, S Delrot, WJ Lucas, J Dainty, eds, Recent Advances in Phloem Transport and Assimilate Compartmentation. Ouest Editions, Nantes, France, pp 244–247
- Carmi N, Zhang G, Petreikov M, Gao Z, Eyal Y, Granot D, Schaffer AA (2003) Cloning and functional expression of alkaline alpha-galactosidase from melon fruit: similarity to plant SIP proteins uncovers a novel family of plant glycosyl hydrolases. Plant J 33: 97–106 [DOI] [PubMed] [Google Scholar]
- Chang HY, Peng HL, Chao YC, Duggleby RG (1996) The importance of conserved residues in human liver UDPglucose pyrophosphorylase. Eur J Biochem 236: 723–728 [DOI] [PubMed] [Google Scholar]
- Dubendorff JW, Studier FW (1991) Controlling basal expression in an inducible T7 expression system by blocking the target T7 promoter with lac repressor. J Mol Biol 219: 45–59 [DOI] [PubMed] [Google Scholar]
- Elling L (1996) Kinetic characterization of UDP-glucose pyrophosphorylase from germinated barley (Malt). Phytochemistry 42: 955–960 [Google Scholar]
- Elsevier JP, Fridovich-Keil JL (1996) The Q188R mutation in human galactose-1-phosphate uridylyltransferase acts as a partial dominant negative. J Biol Chem 271: 32002–32007 [DOI] [PubMed] [Google Scholar]
- Feingold DS, Avigad G (1980) Sugar nucleotide transformations in plants. In J Preiss, ed, The Biochemistry of Plants, Carbohydrates: Structure and Function, Vol 3. Academic Press, New York, pp 101–170
- Feusi MES, Burton JD, Williamson JD, Pharr DM (1999) Galactosyl-sucrose metabolism and UDP-galactose pyrophosphorylase from Cucumis melo L. fruit. Physiol Plant 106: 9–16 [Google Scholar]
- Gao Z, Petreikov M, Zamski E, Schaffer AA (1999) Carbohydrate metabolism during early fruit development of sweet melon (Cucumis melo). Physiol Plant 106: 1–8 [Google Scholar]
- Gao Z, Schaffer AA (1999) A novel alkaline alpha-galactosidase from melon fruit with a substrate preference for raffinose. Plant Physiol 119: 979–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler M, Wilczynska M, Karpinski S, Kleczkowski LA (2004) Toward a blueprint for UDP-glucose pyrophosphorylase structure/function properties: homology-modeling analyses. Plant Mol Biol 56: 783–794 [DOI] [PubMed] [Google Scholar]
- Ginsburg V (1958) Purification of uridinediphosphate glucose pyrophosphorylase from mung bean seedlings. J Biol Chem 232: 55–61 [PubMed] [Google Scholar]
- Gross KC, Pharr DM (1982) A potential pathway for galactose metabolism in Cucumis sativus L., a stachyose transporting species. Plant Physiol 69: 117–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen RG, Albrecht GJ, Bass ST, Seifert LL (1966) UDP-Glucose pyrophosphorylase (crystalline) from liver. Methods Enzymol 38: 248–253 [Google Scholar]
- Haritatos E, Medville R, Turgeon R (2000) Minor vein structure and sugar transport in Arabidopsis thaliana. Planta 211: 105–111 [DOI] [PubMed] [Google Scholar]
- Hubbard NL, Pharr DM, Huber SC (1989) Sucrose phosphate synthase and acid invertase as determinants of sucrose concentration in developing muskmelon (Cucumis melo L.) fruits. Plant Physiol 91: 1527–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler O, Hopf H (1982) Oligosaccharides based on sucrose (sucrosyl oligosaccharides). In FA Loewus, W Tanner, eds, Encyclopedia of Plant Physiology New Series, Plant Carbohydrates. I. Intracellular Carbohydrates, Vol 13A. Springer-Verlag, Berlin, pp 348–383
- Keller F, Pharr DM (1996) Metabolism of carbohydrates in sink and sources: galactosyl-sucrose oligosaccharide. In E Zamski, AA Schaffer, eds, Photoassimilate Distribution in Plants and Crops. Marcel Dekker, New York, pp 157–183
- Kleczkowski LA (1994) Glucose activation and metabolism through UDP-glucose pyrophosphorylase in plants. Phytochemistry 37: 1507–1515 [Google Scholar]
- Kotake T, Yamaguchi D, Ohzono H, Hojo S, Kaneko S, Ishida HK, Tsumuraya Y (2004) UDP-sugar pyrophosphorylase with broad substrate specificity toward various monosaccharide 1-phosphates from pea sprouts. J Biol Chem 279: 45728–45736 [DOI] [PubMed] [Google Scholar]
- Kuo TM, VanMiddlesworth JF, Wolf WJ (1988) Content of raffinose oligosaccharides and sucrose in various plant seeds. J Agric Food Chem 36: 32–36 [Google Scholar]
- La Claire J, Herrin D (1997) Co-isolation of high-quality DNA and RNA from coenocytic green algae. Plant Mol Biol Report 15: 263–272 [Google Scholar]
- Main EL, Pharr DM, Huber SC, Moreland DE (1983) Control of galactosyl-sugar metabolism in relation to rate of germination. Physiol Plant 59: 387–392 [Google Scholar]
- Minet M, Dufour ME, Lacroute F (1992) Complementation of Saccharomyces cerevisiae auxotrophic mutants by Arabidopsis thaliana cDNAs. Plant J 2: 417–422 [DOI] [PubMed] [Google Scholar]
- Mitchell DE, Gadus MV, Madore MA (1992) Patterns of assimilate production and translocation in musk melon (Cucumis melo L.). I. Diurnal patterns. Plant Physiol 99: 959–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano K, Omura Y, Tagaya M, Fukui T (1989) UDP-glucose pyrophosphorylase from potato tuber: purification and characterization. J Biochem (Tokyo) 106: 528–532 [DOI] [PubMed] [Google Scholar]
- Otozai K, Taniguchi H, Nakamura N (1973) UDP-glucose pyrophosphorylase from tubers of Jerusalem artichoke (Helianthus tuberosus L.). Agric Biol Chem 37: 531–537 [Google Scholar]
- Peneff C, Ferrari P, Charrier V, Taburet Y, Monnier C, Zamboni V, Winter J, Harnois M, Fassy F, Bourne Y (2001) Crystal structures of two human pyrophosphorylase isoforms in complexes with UDPGlc(Gal)NAc: role of the alternatively spliced insert in the enzyme oligomeric assembly and active site architecture. EMBO J 20: 6191–6202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petreikov M, Dai N, Granot D, Schaffer AA (2001) Characterization of native and yeast-expressed tomato fruit fructokinase enzymes. Phytochemistry 58: 841–847 [DOI] [PubMed] [Google Scholar]
- Pharr DM, Hubbard NL (1994) Melons: biochemical and physiological control of sugar accumulation. In CJ Arntzen, ed, Encyclopedia of Agricultural Science. Academic Press, New York, pp 1912–1934
- Rosa JT (1928) Changes in composition during ripening and storage of melons. Hilgardia 3: 421–443 [Google Scholar]
- Schaffer AA, Aloni B, Fogelman M (1987) Sucrose metabolism and accumulation in developing fruit of Cucunis. Phytochemistry 26: 1883–1887 [Google Scholar]
- Schaffer AA, Petreikov M (1997) Sucrose-to-starch metabolism in tomato fruit undergoing transient starch accumulation. Plant Physiol 113: 739–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer AA, Pharr DM, Madore MA (1996) Cucurbits. In E Zamski, AA Schaffer, eds, Photoassimilate Distribution in Plants and Crops. Marcel Dekker, New York, pp 729–757
- Smart EL, Pharr DM (1981) Separation and characteristics of galactose-1-phosphate and glucose-1-P uridyltransferase from fruit peduncles of cucumber. Planta 153: 370–375 [DOI] [PubMed] [Google Scholar]
- Spychalla JP, Scheffler BE, Sowokinos JR, Bevan MW (1994) Cloning, antisense RNA inhibition and the coordinated expression of UDP-glucose pyrophosphorylase with starch biosynthetic genes in potato tubers. J Plant Physiol 144: 444–453 [Google Scholar]
- Zrenner R, Willmitzer L, Sonnewald U (1993) Analysis of the expression of potato uridinediphosphate-glucose pyrophosphorylase and its inhibition by antisense RNA. Planta 190: 247–252 [DOI] [PubMed] [Google Scholar]









