Abstract
Embryonic mouse STO (S, SIM; T, 6-thioguanine resistant; O, ouabain resistant) and 3(8)21-enhanced green fluorescent protein (EGFP) cell lines exhibit long-term survival and hepatic progenitor cell behaviour after xenogeneic engraftment in non-immunosuppressed inbred rats, and were previously designated major histocompatibility complex (MHC) class I- and class II-negative lines. To determine the molecular basis for undetectable MHC determinants, the expression and haplotype of H-2K, H-2D, H-2L and I-A proteins were reassessed by reverse transcriptase–polymerase chain reaction (RT-PCR), cDNA sequencing, RNA hybridization, immunoblotting, quantitative RT-PCR (QPCR), immunocytochemistry and flow cytometry. To detect cell differentiation (CD) surface antigens characteristic of stem cells, apoptotic regulation or adaptive immunity that might facilitate progenitor cell status or immune privilege, flow cytometry was also used to screen untreated and cytokine [interferon (IFN)-γ]-treated cultures. Despite prior PCR genotyping analyses suggestive of H-2q haplotypes in STO, 3(8)21-EGFP and parental 3(8)21 cells, all three lines expressed H-2K cDNA sequences identical to those of d-haplotype BALB/c mice, as well as constitutive and cytokine-inducible H-2Kd determinants. In contrast, apart from H-2Ld[LOW] display in 3(8)21 cells, H-2Dd, H-2Ld and I-Ad determinants were undetectable. All three lines expressed constitutive and cytokine-inducible CD34; however, except for inducible CD117[LOW] expression in 3(8)21 cells, no expression of CD45, CD117, CD62L, CD80, CD86, CD90·1 or CD95L/CD178 was observed. Constitutive and cytokine-inducible CD95[LOW] expression was detected in STO and 3(8)21 cells, but not in 3(8)21-EGFP cells. MHC (class I+[LOW]/class II−) and CD (CD34+/CD80−/CD86−/CD95L−) expression patterns in STO and STO cell-derived progenitor cells resemble patterns reported for human embryonic stem cell lines. Whether these patterns reflect associations with mechanisms that are regulatory of immune privilege or functional tissue-specific plasticity is unknown.
Keywords: immune privilege, progenitor cell phenotypes
Introduction
Successful clinical transplantation depends upon immunosuppression of the host to prevent the rejection of histoincompatible grafts.1,2 Apart from host immune privilege reflected by fetal tolerance to foreign grafts3 or the in utero survival of human bone marrow-derived mesenchymal stem cells (MSCs) grafted xenogeneically into sheep,4 several tissues including the brain,5 eye,6 uterine cervix,7 pregnant uterus,8 testis9 and ovary10 are well-known sites of immune privilege. However, foreign grafts usually do not encounter immune-privileged sites. Thus, immunosuppressive treatments are essential to sustain graft survival, although they are frequently toxic and often lead to significant patient morbidity and death.11,12
To circumvent clinical management problems and to provide competent xenogeneic or allogeneic grafts, various systems of pluripotential stem or progenitor cells that express syngeneic host histocompatibility antigens or display intrinsic immune privilege are under investigation as models for xenogeneic or allogeneic transplantation in non-immunosuppressed hosts.13 For example, human embryonic stem (hES) cell lines, generated either by somatic cell nuclear transfer (SCNT) in enucleated human oocytes or by fusion and reprogramming of adult somatic cells, have been proposed as sources of patient- and organ-specific donor cells.14–17 Although the engraftment and functional properties of cloned and reprogrammed ES and SCNT cells have yet to be demonstrated, transplantation studies with conventional hES and non-human stem, MSC or progenitor cell systems in non-immunosuppressed hosts have been described.18–24 Properties of this group of immune-privileged systems, summarized in Table 1, suggest the following trends: firstly, the incidence of class I[LOW] expression is higher than that of non-expression; secondly, none of the cells expresses MHC/human leucocyte antigen (HLA) class II determinants; thirdly, graft survival times vary markedly, from 2 to 90 days; and, lastly, common patterns and mechanisms of intrinsic immune privilege have not yet emerged.18–25 Furthermore, the biological status of the xenogeneic and allogeneic grafts in several reported situations is questionable:18–22 for example, potential artifacts caused by unwanted donor cell fusion with recipient cells,26–29 by genomically integrated vectors and reporter genes, or by transfection of recipient cells with naked DNA released from dying donor cells have yet to be eliminated; in one investigation, primary data were lacking.22
Table 1.
Experimental models of immune-privileged stem and progenitor cells or cell lines transplanted in non-immunosuppressed histoincompatible hosts
| Sources of donor cells | In vitro MHC/HLA status of donor cells | In vitro IFN-γ inducibility | Recipient/injection site/homing site | Injury model/donor cell phenotype or method of detection | Minimal survival time (days) | Proposed mechanissm of immune privilege | References |
|---|---|---|---|---|---|---|---|
| Adult mouse LacZ+ bone marrow stromalcells | Class I+ | Not reported | Adult rat/penile vein/heart | Coronaryinfarction/cardiac myocytes; angiogenesis | 84 | Immunological danger | 18, 25 |
| Class II− | Not reported | ||||||
| GFP+neonatal mouse neural progenitor cells | Class I− | Yes | Adult mouse/renal subcapsule | Nestin expression (day 7); neuronal-like processes | 30 | Antigenic; non-immunogenic | 19 |
| Class II− | Yes | ||||||
| Nestin+adult human pancreatic islet progenitor cells | Class I− | Not reported | Adult mouse/renal subcapsuleor systemic injection | Insulin, glucagon,and PDX-1expression (day 15) | 60 | Mixed chimerism | 20 |
| Class II− | Not reported | ||||||
| Adult humanbone marrow MSCs | Class I+ | Not reported | 44-week-old SPF mouse/skinand spinal cord | Dermal incision, laminectomy/wound repair, behavioural tests | 15–30 (skin) | Unknown | 22 |
| Class II− | Not reported | 90 (spinal cord) | |||||
| GFP+ human ES cell lines | Class I+[LOW] | Yes | Adult mouse/quadricep muscles | GFP+ expression | 2 | Relatively low expression of class I HLA; costimulator expression absent | 21 |
| Class II− | No | ||||||
| Human EScell lines | Class I+[LOW] | Yes | Adult mouse/ renal subcapsule | H&E staining | 30 | Relatively low expression of class I HLA; costimulator expression absent | 23 |
| Class II− | No | ||||||
| Embryonic Swiss mouse STO and SCD cell lines | Class I+[LOW] | Yes | Adult rat/spleen/liver | Hepatocytes | 90 | Relatively low expression of class I MHC; costimulator expression left | 24; this report |
| Class II− | No |
IFN, interferon; GFP, green fluorescent protein; HLA, human leucocyte antigen; MHC, major histocompatibility complex; ES, embryonic stem; MSC, mesenchymal stem cell; SCD, STO cell-derived; PDX, pancreatic and duodenal homeobox transcription factor.
Recently, these types of artifact were addressed and eliminated in a system of embryonic mouse STO (S, SIM; T, 6-thioguanine resistant; O, ouabain resistant) and STO cell-derived (SCD) progenitor 3(8)21 and 3(8)21-enhanced green fluorescent protein (EGFP) cell lines,30 the cells of which differentiate intrahepatically into hepatocytes following intrasplenic transplantation in non-immunosuppressed adult rats.24 Previously reported haplotype analyses using PCR-coupled D17Mit28 primers31 revealed the presence of d(CA)17–18 and d(CA)17 microsatellite motifs in peripheral blood cells from outbred Swiss Webster mice (used as q-haplotype controls) and in STO and 3(8)21-EGFP progenitor cell lines, respectively.24 These findings and identical gel electrophoretic sizes of the three observed PCR products (∼100 bp) suggested that STO and SCD cell lines, descendants of embryonic ‘fibroblasts’ of Swiss albino Sandoz Inbred Mouse (SIM) origin, expressed MHC determinants of the q-haplotype.24,31–33 Thus, based upon PCR genotyping, STO and SCD cells were designated to be of H-2q haplotype, and subsequent flow cytometric analyses revealed MHC class I (H-2Kq, H-2Dq/Lq)- and class II (I-Aq)-negative phenotypes in untreated and interferon (IFN)-γ treated cells.24 The inability to detect by flow cytometry putative H-2Kq cell surface determinants in either progenitor cell line, with or without in vitro treatment with exogenous IFN-γ, a potent cytokine inducer of H-2 gene expression,34 raised the possibility of intracellular H-2Kq expression defects at various levels.
To ascertain the basis of undetectable MHC expression and its potential role in mechanisms of intrinsic immune privilege displayed by STO and SCD cells, and to determine if these cells express either CD antigens, such as CD34 (haematopoeitic stem cell antigen35), CD45 [leucocyte common antigen (LCA)36] and CD117 (stem cell factor c-kit receptor37), which are associated with normal stem cells, or molecules associated with MHC-independent forms of tolerance such as the CD95 (Fas)-CD95L/CD178 (Fas-ligand) system,38,39 STO and SCD progenitor cell lines were investigated further for prominent sets of MHC-related molecules and CD antigens by direct molecular and flow-cytometric methods. Unexpectedly, based upon the DNA sequences of RT-PCR products and cloned full-length cDNA molecules, these investigations revealed an MHC haplotype identical, not to the q-haplotype expected of cells derived from SIM mice and frequently found in cells of Swiss mouse origin,33 but rather to the d-haplotype of BALB/c mice; also revealed was a combined phenotype, MHC class I+[LOW] (IFN-γ inducible)/class II− (IFN-γ non-inducible), CD34+/CD80−/CD86−/CD95L−, which resembles that of widely studied hES cell lines (Table 1).
Materials and methods
Animals
Male adult DBA/1 (q-haplotype) and BALB/c (d-haplotype) mice (15–20 g) were obtained from Harlan Laboratories (Indianapolis, IN). Mice were fed ad libitum and were housed in conditions of 12-hr cycles of light and darkness. Isoflurane anaesthesia and euthanasia were performed in accordance with National Institutes of Health (NIH) and Institutional Animal Care and Use Committee (IACUC) guidelines at the University of California at San Diego (UCSD).
Splenocyte isolation, cell lines and tissue culture
Splenocytes were isolated by standard procedures.40 STO and SCD lines 3(8)21 and 3(8)21-EGFP were cultured by standard procedures;24,30 passage numbers were recorded and cell counts performed as described elsewhere.24,30 Swiss mouse q-haplotype NIH3T3 cells, d-haplotype BALB/c 3T3 cells, human embryonic kidney 293 cells (American Type Culture Collection #CRL-1573), mouse H-2Kb/d-, H-2Db/d- and H-2Lb/d-positive B6-2 cells41 and primary BALB/c kidney cells prepared from trypsinized tissue were cultured as described previously.24,30 Fetal bovine serum (FBS; Invitrogen, Carlsbad, CA) was heat-inactivated at 56° for 30 min. Unless noted, cells were cultured in 3- or 10-cm-diameter plastic tissue culture dishes (BD Falcon™, Bedford, MA).
Reverse transcriptase–polymerase chain reaction (RT-PCR)
RT-PCR was performed using primers based upon GenBank cDNA sequences for H-2Kq (M14827), H-2Dq (X14090), H-2Lq (X14091), β2-microglobulin (β2m) (X01838 and NM_009735) and mouse β-actin [used as an internal standard (NM_007393)].
Total RNA was isolated from freshly isolated splenocytes or cultured cells using RNeasy Mini Kits (Qiagen, Valencia, CA). RNA quality was assessed by standard procedures of gel electrophoresis as described below. Purified RNA (2 μg) was subjected to reverse transcription in a reaction volume (20 μl) using an ImProm-II reverse transcription system (Promega, Madison, WI). One μl of the reaction mixture was subjected to PCR for amplification of each molecule. PCR was performed using a MiniCycler (MJ Research, Inc., Watertown, MA), in reaction volumes (50 μl) containing 25 pmol of each primer, 0·2 mm dNTPs, 2 mm MgCl2, 1× PCR buffer, and 2·5 U Taq DNA polymerase (Promega). For each reaction, the mouse primer sequences, the expected product sizes, and the annealing temperatures employed are shown in Table 2. Thermal cycles were programmed as follows: 30 seconds at 94°, 30 seconds at 58–60°, and 30 seconds at 72° for 35 cycles, followed by final extension for 3 min at 72°. Solutions of PCR products (25 μl) were separated by electrophoresis on 1·5% agarose gels stained with 0·5 μg ethidium bromide (EtBr)/ml and were transilluminated under ultraviolet (UV) light.
Table 2.
Oligodeoxynucleotide primers used for reverse transcriptase–polymerase chain reaction (RT-PCR)
| Putative target cDNA | Sequence (5′ → 3′) | Annealingtemperature (°) | Product size (bp) |
|---|---|---|---|
| H-2Kq Set 1 | S: CGCCCTGAACGAAGACCTGAAA | 60 | 376 |
| AS: CACCACCACAGATGCCCACTTC | |||
| H-2Kq Set 2 | S: GCTGTCTCCAACACGGTAATCA | 60 | 216 |
| AS: TCACGCTAGAGAATGAGGGTCAT | |||
| H-2Dq/H-2Lq | S: GGCCCACACTCGATGCGGTATT | 60 | 438 |
| AS: ACTTGCGTCGGGTGATCTGCG | |||
| β2m | S: GCTGCTACTCGGCGCTTCA | 60 | 343 |
| AS: GCAGGCGTATGTATCAGTCTCAGT | |||
| β-actin | S: CCCAGAGCAAGAGAGGTATC | 60 | 267 |
| AS: GACCAGAGGCATACAGGGAC |
β2m, β2-microglobulin; S, sense; AS, antisense.
Analysis of RNA by northern blots
Total RNA (10–20 μg) or poly (A)+ RNA (2 μg) was isolated from cultured cells using RNeasy kits (Qiagen). RNA samples were separated by denaturing electrophoresis on formaldehyde (0·65%)-agarose (1·2%) gels. The resulting products were transferred by standard procedures in 20× standard saline citrate (SSC) by capillary flow to Hybond-N+ nitrocellulose membranes (Amersham Pharmacia Biotech, Piscataway, NJ). The membranes were air-dried, and the transferred products were cross-linked by UV irradiation (Stratalinker® 2400; Stratagene, La Jolla, CA). Membranes were incubated at 68° for 2 hr in prehybridization/hybridization buffer [0·1% sodium dodecyl sulphate (SDS), 50% formamide, 5× SSC, 50 mm NaPO4, pH 6·4, 0·1% sodium pyrophosphate, 5× Denhardt's solution, and 50 µg of sheared herring sperm DNA/ml]. α-[32P]-dCTP was incorporated into hybridization probes using Radiprimer™ II kits (Amersham Pharmacia Biotech). Hybridization reactions were performed overnight at 48°; the membranes were washed three times each for 20 min in 2× SSC−0·1% SDS at room temperature, and twice each for 30 min in 0·1× SSC−0·1% SDS at 50°. Membranes were exposed to Hyperfilm™ (Amersham Pharmacia Biotech) for 16–72 hr at −80° using intensifier screens.
Total RNAs from q-haplotype DBA/1 splenocytes and d-haplotype BALB/c mouse kidneys were used as templates for synthesis of β2m and H-2K hybridization probes. Mouse β2m (340-bp) and H-2K (1·4-kb) probes were prepared by RT-PCR using the following primers: β2m, sense 5′-GCTGCTACTCGGCGCTTCA-3′, antisense 5′-GCAGGCGTATGTATCAGTCTCAGT-3′; H-2K, sense 5′-GTGTGGAAGTGAGGAGCTGCA-3′, antisense 5′-AAGTCGCTAATCGCCGACCAG-3′. Prior to radiolabelling, to assess the specificity of the PCR reactions, the expected sizes of the probes were verified by electrophoresis on 1·5% agarose gels. PCR products were gel-purified using Qiagen QIAEX II Gel Extraction Kits, and 0·5–25 ng of each probe was radiolabelled as described above.
PCR cloning, DNA sequencing and DNA sequence analysis
Total RNA from cultured cells was isolated and characterized as described above. First-strand cDNA was synthesized using an ImProm-II reverse transcription kit as described above.
To sequence uncloned RT-PCR products, PCR reactions were performed with primers (sense 5′-CCGGTTCATCTCTGTCGG-3′; antisense 1 5′-TCACCAAGTCCACTCCAG-3′) identical to a published mouse H-2Kq cDNA sequence (GenBank M14827). Reaction mixtures (50 μl) contained 1 μl of first-stage RT-PCR product, 25 pmol H-2Kq primers, 2 mm MgCl2, 0·2 mm Nucleotide Mix and 2·5 units DNA Taq polymerase (Promega). Thermal cycles were programmed as follows: denaturation for 5 min at 95°; 40 cycles of 30 seconds at 95°, 30 seconds at 60° and 1 min at 72°; final extension for 5 min at 72°; hold at 4°. The amplified products were analysed by electrophoresis in 1% agarose minigels containing 0·5 µg EtBr/ml. Gel bands were visualized by UV light, and extracted and purified with Qiagen QIAEX II Gel Extraction Kits. The isolated PCR products were sequenced at the UCSD Cancer Center DNA Sequencing Core Facility, using sequencing primers (sense, 5′-CCGGTTCATCTCTGTCGG-3′; antisense 1, 5′- TCACCAAGTCCACTCCAG-3′; antisense 2, 5′-TCACGCTAGAGAATGAGGGTCAT-3′) and a 3130XL Genetic Analyzer (PE Applied Biosystems, Foster City, CA).
To sequence cloned products, cDNA was employed in a first PCR amplification reaction using primers identical to a published mouse H-2Kd sequence (GenBank J00402): sense 5′-AAGTCGCTAATCGCCGACCAG-3′, antisense 5′-GTGTGGAAGTGAGGAGCTGCA-3′. Thermal cycles were programmed as follows: 5 min at 95°; 35 cycles of 95° for 30 seconds, 58° for 30 seconds and 72° for 90 seconds; followed by 72° for 10 min. PCR products were separated, analysed and visualized as described above. PCR fragments were gel-purified and ligated into pCR®-TOPO® vectors using the TOPO TA Cloning® kit (Invitrogen). Following transformation of competent Escherichia coli Top10F′ cells, recombinants were identified by PCR and validated by restriction digest analysis. Inserts in isolated plasmid DNAs were sequenced as described above using standard T7 (5′-TAATACGACTCACTATAGGG-3′) and T3 (5′-ATTAACCCTCACTAAAGGGA-3′) sequencing primers.
In both sequencing studies, the resulting DNA sequence data were analysed by BLASTn searches at National Center for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov/) and were processed and aligned using vector nti v.8 (Informax, Frederick, MD). GenBank files were created with sequin v.6·0; GenBank (http://info@ncbi.nlm.gov) accession numbers are given in the Results.
Quantitative real-time PCR (QPCR)
Total RNA was isolated from freshly isolated splenocytes, BALB/c mouse kidney tissues, and cultured cell lines, and was characterized qualitatively by gel electrophoresis as described above. Complementary DNAs were synthesized with the ImProm-II reverse transcription kit as described above.
Reverse transcription was performed using 4 μg of RNA in a 20-μl reaction volume. cDNA solutions equivalent to 0·2 μg of total RNA (1 μl) were subjected to 40 cycles of PCR amplification consisting of incubation for 15 seconds at 95°, followed by incubation for 1 min at 60°. Output was monitored using SYBR Green core reagents and an ABI Prism 7700 System (PE Applied Biosystems). For each sample, the quantitative results were normalized to the quantitative levels of mouse glyceraldehyde phosphate dehydrogenase (GAPDH) mRNA.
To create optimal sense (forward) and antisense (reverse) primer pairs, oligonucleotide sequences were designed online (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www_slow.cgi) using the following parameters: product size, min 50 opt 75 max 100; primer size, min 19 opt 20 max 21; primer Tm, min 59 opt 60 max 61 max Tm difference 2; and accept default values for other parameters. Primer sequences were: H-2Kd, first primer set reaction, R1 (sense 5′-AAGTCGCTAATCGCCGACCA-3′, antisense 5′-TACCTCAGCGAATGTGGGCC-3′); H-2Kd, second primer set reaction, R2 (sense 5′-CATGCCATGTGCACCATAAGG-3′, antisense 5′-GGAGACAGTGGATGGAGGAAG-3′); H-2Kd, third primer set reaction, R3 (sense 5′-CCTCTCACCCTGAGATGGAA-3′, antisense 5′-CAAGGACAACCAGAACAGCA-3′); H-2Dd (sense 5′-GTGGTCATCCTTGGAGCTGT-3′, antisense 5′-GCATAGTCCCCTCCTTTTCC-3′); β2m (sense 5′-AGAATGGGAAGCCGAACATA-3′, antisense 5′-CCGTTCTTCAGCATTTGGAT-3′); and GAPDH (sense 5′-ACCCAGAAGACTGTGGATGG-3′, antisense 5′-GGATGCAGGGATGATGTTCT-3′).
Analysis of cellular proteins by western blots
Late log-phase cultures (3 × 106 cells/dish) were washed three times with ice-cold standard phosphate-buffered saline (PBS, pH 7·4) at room temperature. The washed cells were incubated with RIPA buffer (Tris-HCl, pH 7·4; 1% NP-40; 0·25% sodium-deoxycholate; 150 mm NaCl; 1 mm PMSF)42 supplemented with Complete™ protease inhibitors (Roche Applied Sciences, Indianapolis, IN) for 20 min at 4°, and centrifuged at 1000 g for 5 min at 4°, and the supernatants were then collected for further analyses. Cell or control protein extracts (0·01–18·5 μg per lane) were boiled in 2× SDS sample buffer and were separated by electrophoresis on 10% or 12·5% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) gels (see figure legends). Separated proteins were transferred to nitrocellulose membranes (Schleicher & Schuell Inc., Keene, NH), and immunoblotted with p8 (1 : 3000), 216F (1 : 3000), JV2 (1 : 3000) or anti-gp96 (1 : 3000) polyclonal rabbit antisera.43–45 To reduce non-specific binding, membranes were blocked by incubation in solutions of Tris-buffered saline (TBS, pH 7·4) containing 5% skimmed milk and 0·1% Tween 20. After incubation with primary antibodies for 24 hr at 4°, the membranes were washed three times for 30 min at 4° in TBS/0·1% Tween 20 before the addition of anti-rabbit immunoglobulin G (IgG) horseradish peroxidase-conjugated secondary antibody (1 : 10 000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Subsequently, the membranes were incubated for 1 hr at 4° and washed three times for 10 min at 4° in TBS/0·1% Tween 20. Specific proteins were detected by enhanced chemiluminescence using an ECL™ kit (Amersham Pharmacia Biotech).
Commercial antibodies used for immunohistochemistry, immunocytochemistry and flow cytometry
Table 3 lists key properties of commercial anti-MHC class I, anti-MHC class II and anti-CD antibodies (including matched isotype control antibodies for each primary antibody). Except for the group of primary anti-H-2Kd, anti-H-2Dd and anti-I-Ad antibodies, all primary antibodies were covalently labelled with phycoerythrin (R-PE) as described by the vendors (see below). R-PE-labelled anti-mouse IgG (Abcam, Cambridge, MA) was employed as the secondary antibody to detect unlabelled MHC determinants in immunohistochemical, immunocytochemical and flow cytometry studies.
Table 3.
Properties and specificities of commercial anti-major histocompatibility complex (MHC) and anti-cell differentiation (CD) primary antibodies
| Primaryantibody | Reactivity | Form | Isotype |
|---|---|---|---|
| H-2Kd | Mouse | Unlabelled | IgG2a, κ |
| H-2Dd | Mouse | Unlabelled | IgG2a, κ |
| H-2Ld | Mouse | R-PE | IgG2a, κ |
| I-Ad | Mouse | Unlabelled | IgG3, κ |
| CD34 | Mouse | R-PE | Rat IgG2a, κ |
| CD45 | Mouse | R-PE | Rat IgG2b, κ |
| CD62L(l-selectin) | Mouse | R-PE | Rat IgG2a, κ |
| CD80(B7-1) | Mouse | R-PE | Hamster IgG |
| CD86(B7-2) | Mouse | R-PE | Rat IgG2a, κ |
| CD90/CD90·1(Thy-1·1) | Mouse/rat | R-PE | Mouse IgG1, κ |
| CD95(Fas) | Mouse | R-PE | Hamster IgG |
| CD117(c-kit) | Mouse | R-PE | Rat IgG2b, κ |
| CD178(FasL, CD95L) | Mouse | R-PE | Hamster IgG |
CD, cell differentiation; IgG, immunoglobulin G; R-PE, Rhodophyta phycoerythrin.
Immunohistochemistry and immunocytochemistry
Six-week-old BALB/c mice were anaesthetized and perfused transcardially with physiological saline followed by a solution of 4% paraformaldehyde in 0·1 m phosphate buffer (pH 7·3). Subsequently, the spleens were excised and were postfixed in the same fixative for 24 hr at 4°. The samples were stored at 4° in 30% sucrose in 0·1 m phosphate buffer. The tissue was transferred to an embedding mould filled with Tissue Freezing Medium (Triangle Biomedical Sciences, Durham, NC), which was rapidly submerged in ethanol and dry ice. Tissue sections (35 μm) were cut at −20° and stored at −20° in a solution (total volume, 1·05 l) of glycerin (250 ml), ethylene glycol (300 ml) and 0·1 m phosphate buffer, pH 7·4 (500 ml). Free-floating serial sections were washed for 30 min at room temperature in TBS, pH 7·4. The sections were permeabilized on a shaker with 0·25% Triton-X100-TBS (TBSTx) for 10 min at room temperature, preincubated with 3% bovine serum albumin (BSA) in TBSTx for 1 hr at room temperature, and incubated overnight at 4° with primary antibody (antimouse H-2Kd, 1 : 10; BioLegend, San Diego, CA) or anti-mouse isotype control antibody (1 : 10; BioLegend) in TBSTx containing 3% BSA. The antibodies were removed, the tissues were rinsed three times at room temperature with TBSTx, and the sections were incubated with R-PE-labelled anti-mouse IgG secondary antibodies (1 : 50) for 1 hr at room temperature. Negative control experiments included omission of the primary antibody followed by incubation with secondary antibody alone. The tissues were rinsed, mounted on glass slides (Fisher Scientific, Pittsburgh, PA), dehydrated, cover-slipped with Fluoromount-G (Southern Biotech, Birmingham, AL), examined by phase and fluorescence microscopy, and digitally photographed as described elsewhere.24,30
Cultured cells were plated onto glass-bottom 35-mm tissue culture dishes (MatTek Corporation, Ashland, MA) at a cellular density of 1 × 105 cells/dish. At ∼70% confluence (∼2 days), the cells were washed with PBS three times at room temperature, and fixed with 4% paraformaldehyde in 0·1 m phosphate buffer (pH 7·4) for 15 min at room temperature. The fixative was removed, and the attached cells were washed three times at room temperature with TBSTx and incubated with either anti-mouse H-2Kd primary antibody, anti-mouse isotype control antibody, or buffer alone for 10 min at room temperature. Following similar washes with TBSTx, the three groups of dishes were incubated with R-PE-labelled anti-mouse IgG secondary antibody (1 : 50) for 1 hr at room temperature. Secondary antibody solutions were removed, and the dishes were rinsed similarly again with TBSTx and examined by phase contrast and fluorescence microscopy using a dual EGFP/DSRed filter (Chroma Technology, Brattleboro, VT).
Photomicrographs were processed and annotated graphically using paint shop pro v.9·01 (Jasc Software, Eden Prairie, MN).
Flow cytometric analysis
Cultured cells were plated (2 × 105 cells/10-cm dish/10 ml medium) and, following attachment 24 hr later, either vehicle (PBS supplemented with 0·1% BSA) or 33 ng IFN-γ/ml (MP Biomedicals, Irvine, CA) was added to the cultures. Four days later (day 5), trypsinized cells were collected24 and washed twice with 10 ml of PBS at 4°. The washed cultured cells, or similarly washed untreated BALB/c splenocytes (used as positive internal controls to validate the advertised reactivities of commercial antibodies with specific MHC and CD markers) were resuspended in PBS-BSA solutions (1 × 106 cells/100 µl) and distributed into round-bottom Falcon tubes on ice.
For each experiment, cell aliquots in PBS-BSA solutions were incubated for 30 min at room temperature with individual unlabelled or R-PE-labelled primary antibodies (see Table 2) as follows: unlabelled H-2Kd (BioLegend), H-2Dd (BD Pharmingen, San Diego, CA) or I-Ad (BioLegend); or R-PE-labelled H-2Ld (Cedarlane, Hornby, Ontario, Canada), CD34 (BD Pharmingen), CD45 (BD Pharmingen), CD62L (BioLegend), CD80 (BioLegend), CD86 (eBioscience, San Diego, CA), CD90·1 (BD Pharmingen), CD95 (BD Pharmingen), CD117 (BioLegend) or CD95L/CD178 (BioLegend). As a control, parallel cell samples were incubated with identical concentrations of unlabelled non-specific isotype-matched antibody. After two washes with PBS-BSA, the cells incubated with unlabelled antibodies were incubated for an additional 30 min at room temperature with R-PE-labelled secondary anti-mouse IgG against H-2Kd, H-2Dd and I-Ad or isotype antibodies. Subsequently, all cell suspensions were washed twice in PBS-BSA supplemented with 0·1% sodium azide. Flow cytometric analyses were conducted using a standard FACScan (BD Biosciences, Mountain View, CA); 20 000 individual cells were analysed in each sample. Data were analysed with cellquest software (BD Biosciences) and displayed and annotated graphically using paint shop pro v.9·01.
Results
STO and SCD progenitor cells express non-q haplotype MHC class I and two size classes of β2m messenger RNAs
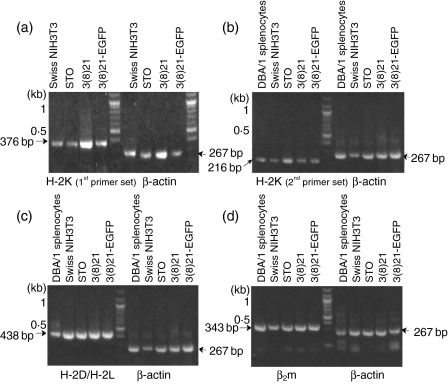
To investigate postulated intracellular H-2Kq expression defects in STO and 3(8)21-EGFP cells, RT-PCR was performed in the absence of IFN-γ on both lines, on 3(8)21 cells [parents of 3(8)21-EGFP cells] and on known q-haplotype cells (Swiss NIH3T3 fibroblasts and fresh DBA/1 splenocytes). Primers were designed to amplify exons 2–3 (set 1) and exons 4–7 (set 2) of the expected MHC class I H-2Kq heavy chain (Table 2). Indeed, as shown in Fig. 1(a) and Fig. 1(b), RT-PCR products of predicted size (376 and 216 bp, respectively) were observed throughout. Putative q-haplotype products (438 bp) of H-2D/H-2L heavy chain exons 2–3 were also observed (Fig. 1c). STO, SCD and Swiss NIH3T3 cells also expressed products (343 bp) of exons 1–2 of the mouse invariant heavy-chain partner, β2m, a molecule required for functional heavy chain expression (Fig. 1d).
Figure 1.
Detection of major histocompatibility complex (MHC) class I and β2-microglobulin (β2m) mRNAs in STO (S, SIM; T, 6-thioguanine resistant; O, ouabain resistant) and STO cell-derived (SCD) [3(8)21 and 3(8)21-enhanced green fluorescent protein (EGFP)] cells. RNA extracts were analysed by reverse transcriptase–polymerase chain reaction (RT-PCR) for exonic expression of putative H-2K and H-2D/H-2L mRNAs using primers chosen from the q-haplotype cDNA sequence; the β2m cDNA sequence was used to design invariant β2m primers. Swiss NIH3T3 cells and DBA/1 splenocytes served as sources of authentic H-2Kq mRNA; β-actin determinations served as loading controls. Electrophoresed RT-PCR products are shown on agarose gels transilluminated under ultraviolet light. (a) H-2K mRNA (first primer set); (b) H-2K mRNA (second primer set); (c) H-2D/H-2L mRNA; and (d) β2m mRNA (invariant mouse-specific primer set). See text for further details.
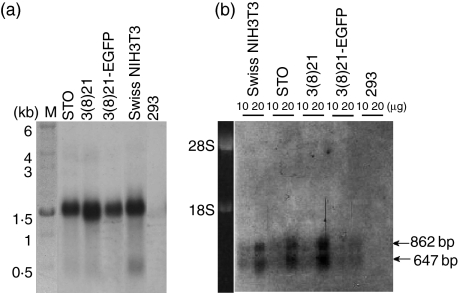
Autoradiograms of northern blots revealed hybridization of a random primer-labelled 1·5-kb H-2K-selective cDNA probe to molecules of ∼1·5 kb size in all three cell lines (Fig. 2a). RNA isolated from Swiss NIH3T3 cells (Fig. 2a) and from BALB/c mouse kidney and BALB/c NIH3T3 cells displayed similarly sized hybridization bands (not shown), whereas no such bands were observed in poly (A)+ RNA isolated from human 293 cells, used as a negative control (Fig. 2a). Similar analyses (Fig. 2b) using a β2m probe revealed two mRNA size classes of ∼647 and ∼862 bp, as expected from published GenBank sequences (X01838 and NM_009735, respectively); β2m-probe specificity was suggested by the absence of specific hybridization products in human 293 cells.
Figure 2.
Size analyses of H-2K and β2 microglobulin (β2m) mRNAs in STO (S, SIM; T, 6-thioguanine resistant; O, ouabain resistant) and STO cell-derived (SCD) cells. RNA extracts were analysed by northern blotting using labelled hybridization probes specific for invariant β2m mRNAs and non-specific for H-2K mRNAs. Swiss NIH3T3 cells were used as a positive control for β2m mRNA expression; human 293 cells were used as negative controls for both types of mRNAs. (a) H-2K mRNA. The track at the left gives kb size standards; 2 µg of each poly (A)+ RNA sample was analysed. (b) β2m mRNAs. The track at the left gives rRNA size standards; 10 and 20 µg of each total RNA sample were analysed. The relative positions of hybridization complexes were visualized on autoradiograms of northern blots; hybridization signals in (b) were heightened uniformly by image processing. See text for further details.
Because overlaps existed between the sequences of the primers used to generate RT-PCR products and the published cDNA sequences of other non-q haplotype H-2K targets (Fig. 3a), the nucleotide (nt) sequences of the q-haplotype primer-driven RT-PCR reactions were investigated by sequencing such DNA products. The sequencing strategy and the annotated primer sequences are shown diagrammatically in Fig. 3(a). The resulting ‘experimental’ consensus sequence, obtained from a set of 18 strands (specifically, with each cell line, duplicate sequencing reactions with each of three primers), and its alignment with published H-2K q-, b-, k-, and d-haplotype sequences are shown in Fig. 3(b). Unexpectedly, the experimental consensus sequence did not match published sequences of H-2Kq, or sequences of H-2Kb, H-2Kk or DBA/2 H-2Kd haplotypes; instead, a perfect match to BALB/c H-2Kd was observed (Fig. 3b). These findings suggested that STO and SCD cells expressed an H-2Kd-like mRNA. However, as these results were based upon the sequences of uncloned and truncated cDNA products (for example, it appeared that the partial consensus BALB/c coding sequence lacked 147 and 78 nt of upstream and downstream sequence, respectively), further experiments were needed to substantiate the d-haplotype and MHC class I and class II expression status of these cells.
Figure 3.
Partial cDNA sequence of H-2K heavy chains in STO (S, SIM; T, 6-thioguanine resistant; O, ouabain resistant) and STO cell-derived (SCD) cells. Messenger RNAs from STO, 3(8)21 and 3(8)21-enhanced green fluorescent protein (EGFP) cell extracts were amplified by reverse transcriptase–polymerase chain reaction (RT-PCR) using non-specific sense (S) and antisense (AS1) primers designed against the H-2K q-haplotype sequence [GenBank M14827]. Purified PCR products were subjected to sequencing using primers S, AS1 and AS2 [see (a)]. Sequencing reactions were performed twice, and the resulting sequences were aligned by standard procedures. (a) Sequencing strategy diagram. DNA sequences, homologous but not identical to the primers employed, were obtained from published H-2K molecules of b-, k-, DBA/2 d-, and BALB/c d-haplotypes, and are shown for comparison. S and AS1 were used as RT-PCR primers, and S, AS1 and AS2 as sequencing primers. (b) Experimental consensus sequence and comparative sequence alignments. The ‘experimental’ sequence (top row) reflects the consensus determined from 18 separate sequence reactions. GenBank accession numbers of published H-2K molecules of q-, b-, k-, DBA/2 d-, and BALB/c d-haplotypes are shown in the figure. See text for further details.
STO and SCD cells express full-length H-2Kd mRNA identical to that of BALB/c mice
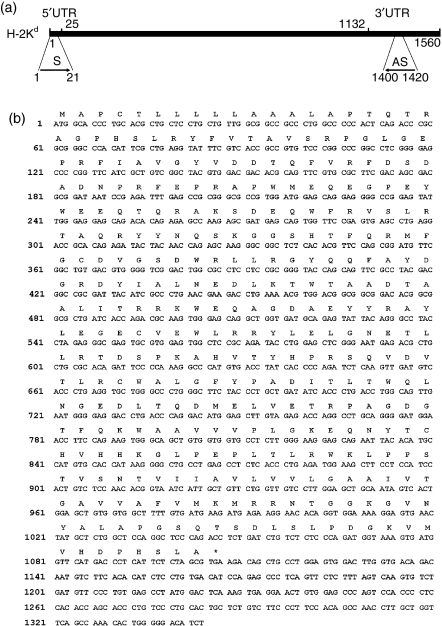
To obtain unequivocal sequence information, the sequencing of full-length cDNAs was performed on endogenous H-2K mRNAs obtained from STO and SCD cells using cloned RT-PCR products generated with primers flanking a published d-haplotype open reading frame (GenBank J00402). The sequencing strategy is shown diagrammatically in Fig. 4(a). Under these conditions, a single invariant sequence was obtained from a set of six 0·8–1·5-kb cDNA strands [one forward (+) and one reverse (−) strand per clone per cell line]. In agreement with partial cDNA sequencing results (Fig. 3b), the full-length cDNA sequence from each cell line (STO, GenBank DQ346646; 3(8)21, GenBank DQ346647; 3(8)21-EGFP, GenBank DQ346648) and its predicted translation product, with ATG start and TGA termination codons beginning at nt positions 1 and 1105, respectively (Fig. 4b), perfectly matched the published d-haplotype nucleotide and amino acid sequences of the 1·1-kb open reading frame of the class I mRNA encoded by the H-2Kd locus in BALB/c mice (reference 46; GenBank U47329). Throughout the remaining 236-bp 3′ untranslated region (UTR) sequence (starting and ending at nt 1108–1344) only one nucleotide mismatch was observed (G, instead of A, at nt 1234; see Fig. 4b) with previously reported H-2Kd sequences (GenBank X01815 and U47329).
Figure 4.
Full-length cDNA sequence of BALB/c-like H-2Kd heavy chains in STO (S, SIM; T, 6-thioguanine resistant; O, ouabain resistant) and STO cell-derived (SCD) cells. (a) Sequencing strategy diagram. To obtain full-length open reading frame cDNA sequences, mRNAs from STO, 3(8)21 and 3(8)21-enhanced green fluorescent protein (EGFP) cell extracts were amplified by reverse transcriptase–polymerase chain reaction (RT-PCR) and PCR-cloning using sense (S) and antisense (AS) primers designed from a published H-2Kd-haplotype sequence (GenBank J00402). Purified products were subjected to sequence and alignment analyses using standard procedures. 5′UTR and 3′UTR, 5′ and 3′ untranslated regions, respectively. (b) Experimental sequence. The experimental sequence (top row) reflects the consensus determined from six separate sequencing reactions (one S and AS strand per cell line). The predicted amino acid sequence (single letter code) is given above each codon. See text for further details.
Thus, the combined observations indicated that STO and SCD cells of SIM origin32 do not express the most prevalent H-2K specificity associated with Swiss mice, namely the q-haplotype,33 but instead express the d-haplotype reported for BALB/c mice.
STO and SCD cells express H-2Kd and β2m mRNAs at relatively low levels compared with BALB/c splenocytes
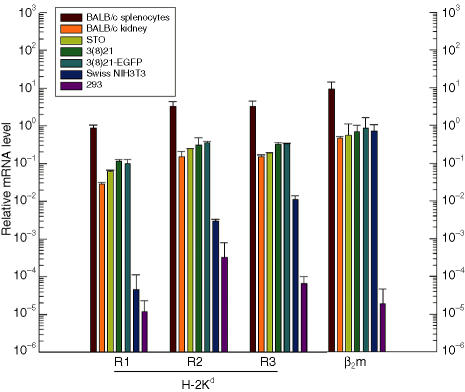
To quantify specific H-2Kd, H-2Dd and β2m mRNA expression, QPCR was performed using poly (A)+ RNA samples isolated from STO and SCD cell lines. RNA samples from d-haplotype BALB/c splenocytes and kidney, from q-haplotype Swiss NIH3T3 cells, and from embryonic human 293 cells served as positive, negative, and non-specific controls, respectively. Primer reaction pairs (R) were chosen to span the following mouse exons (E): H-2Kd R1 (E1–E2), R2 (E4–E6) and R3 (E4–E6); H-2Dd R1 (E5–E6); and β2m (E2).
As shown in Fig. 5, STO and SCD lines expressed equivalent levels of H-2Kd mRNAs. Although these H-2Kd mRNA levels were 2–3 times higher than normal BALB/c mouse kidney mRNA levels, they were only ∼10% of the level of H-2Kd mRNA expressed by BALB/c mouse splenocytes. Primer and d-haplotype specificity for the H-2Kd sequence motifs was suggested by the markedly reduced QPCR signals (3–5 logarithms) in samples from Swiss NIH3T3 and human 293 cell lines. Attempts to quantify H-2Dd expression revealed mRNA levels similar to H-2Kd mRNA levels among all murine tissues and cell lines; these levels were significantly higher than the levels for 293 cells (data not shown). However, because of equivalent signals obtained from q-haplotype NIH3T3 cells, and near identities in sequences between murine H-2D and H-2L loci,47 these results were judged to be non-specific for haplotype. β2m expression was observed in all murine tissues and lines tested, although generally at ∼10% of the expression level in BALB/c splenocytes. Specificity of measurement for murine β2m expression was suggested by QPCR signals in human 293 cells that were 5–6 orders of magnitude lower than any murine equivalent.
Figure 5.
Quantification of H-2Kd and β2 microglobulin (β2m) mRNAs in STO (S, SIM; T, 6-thioguanine resistant; O, ouabain resistant) and STO cell-derived (SCD) cells. Relative mRNA levels in poly (A)+ RNA extracts were analysed by quantitative reverse transcriptase-mediated polymerase chain reaction (QPCR) using three different exon-specific H-2Kd mRNA primer sets (R1, R2 and R3) and one exon-specific primer set for invariant β2m mRNA. Histogram bar colours (see key) represent the following cell sources: positive-control BALB/c splenocytes (brown) and kidney tissue (orange), STO (olive), 3(8)21 (green), 3(8)21-enhanced green fluorescent protein (EGFP) (turquoise), negative-control Swiss NIH3T3 (blue) and non-specific control embryonic human 293 cells (purple). The results, normalized to GAPDH mRNA levels, are the averages of two experiments; error bars reflect deviations from the mean value. See text for further details.
H-2Kd proteins are expressed and transported to cell surfaces in STO and SCD cells
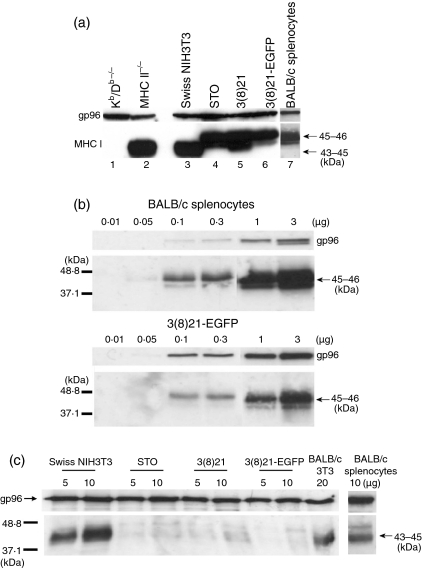
Consistent with the results of cDNA sequencing, northern blotting, QPCR, and previous determinations of relative molecular mass (Mr) values of mature glycosylated H-2K heavy chains,44 45–46-kDa proteins were detected in STO, SCD and BALB/c splenocyte extracts on western blots (Fig. 6a), using anti-MHC class I antiserum-p8 directed against the cytoplasmic tails of glycosylated or non-glycosylated class I heavy chains derived from the H-2K locus.43,44 Extracts from MHC class I (Kb/Db–/–) and class II knockout mice48 served as negative and positive anti-MHC controls, respectively; purified gp96 served as a gel loading control. Serial dilutions of extracts of 3(8)21-EGFP cells suggested that, as expected from QPCR results, the levels of H-2Kd proteins in SCD cells were at least 2- to 3-fold lower than the levels of H-2Kd proteins in splenocytes (Fig. 6b).
Figure 6.
Relative sizes and cellular levels of H-2Kd heavy chain proteins in STO (S, SIM; T, 6-thioguanine resistant; O, ouabain resistant) and STO cell-derived (SCD) cells. Proteins were separated by 12·5% (a), 10% (b) and 10% (c) sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) under denaturing conditions and subjected to western blots. For gel loading controls, commercially supplied anti-gp96 antisera were used; immunoblotted proteins were revealed by chemiluminescence (film exposure times were 30 seconds). Arrows indicate relative molecular mass (Mr) values in kDa as determined from electrophoresed protein standards (not shown). (a) Qualitative detection with major histocompatibility complex (MHC) class I antiserum p8. The following extracts (unless noted, 18·5 µg protein/lane) were analysed (left to right): MHC class I (Kb/Db–/–) and class II knockout mice (lanes 1 and 2), which served as negative and positive anti-MHC class I controls, respectively; Swiss NIH3T3, STO, 3(8)21 and 3(8)21-enhanced green fluorescent protein (EGFP) cells, and 5 µg protein/lane of positive-control BALB/c splenocytes (lanes 3–7, respectively). (b) Semiquantitative detection by serial dilution with antiserum p8. Extracts (0·01, 0·05, 0·1, 0·3, 1 or 3 µg protein/lane, as indicated) from BALB/c splenocytes (top panel) and 3(8)21-EGFP cells (bottom panel) were analysed. The absolute staining intensities and the ratios of the band intensities (H2:gp96) were estimated visually, with serial dilution. No 3(8)21-EGFP band is detectable at the 0·05 µg level, whereas a band at that level is detectable in the splenocyte fraction. (c) Qualitative detection with pan-MHC class I antiserum 216F. Extracts (5, 10 or 20 µg protein/lane, as indicated) of q-haplotype Swiss NIH3T3 cells, d-haplotype STO, 3(8)21 and 3(8)21-EGFP cells, and d-haplotype BALB/c 3T3 cells and BALB/c splenocytes were analysed. See text for further details.
Notably, H-2Kq proteins from extracts of cultured Swiss NIH3T3 cells migrated faster than H-2Kd proteins from STO and SCD cells and BALB/c splenocytes, namely to gel positions ∼43–45 kDa (Fig. 6a), whereas no 43–46-kDa proteins were detected in extracts of BALB/c 3T3 cells (data not shown). To investigate these results further, pan-MHC specificity was studied in parallel western blots of STO and SCD cells, using anticlass I antiserum-216F which recognizes free heavy chains of b-, d-, k- and u-haplotypes not associated with β2m molecules.44 Under these conditions, 43–45-kDa proteins were observed in control extracts from Swiss NIH3T3 and BALB/c 3T3 cells, as well as BALB/c splenocytes, whereas only faint or non-specific bands were observed in extracts from STO and SCD cells (Fig. 6c). Clearly, both antibodies are individually specific for differences between Swiss NIH3T3 q-, BALB/c d-, and STO and SCD H-2K proteins. These antibody studies are suggestive of some similarity of STO and SCD cells with BALB/c splenocytes, and also of some difference, or of a possible anomaly.
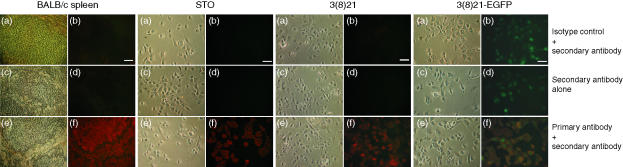
To ascertain if these anomalous observations might reflect defective class I protein expression in intact cells, STO and SCD lines were investigated by immunocytochemistry and by flow cytometry using a commercially available anti-mouse H-2Kd monoclonal antibody. As shown in Fig. 7, using BALB/c spleen tissues as a positive control, virtually all STO, 3(8)21 and 3(8)21-EGFP cells were found to express cytoplasmic H-2Kd proteins. Cytoplasmic fluorescence signals in immunocytochemically stained 3(8)21-EGFP cells appeared yellow-orange as a result of the concomitant expression of green EGFP epifluorescence and H-2Kd-specific red immunofluorescence in single cells. Specificity of H-2Kd expression was indicated by the absence of fluorescence signals in cell samples incubated with either isotype control antibodies (as primary antibody) or R-PE-labelled secondary antibodies alone.
Figure 7.
Visualization of H-2Kd heavy chain expression in cultured STO (S, SIM; T, 6-thioguanine resistant; O, ouabain resistant) and STO cell-derived (SCD) cells. Immunofluorescence analyses were performed on four sets of samples (left to right): positive-control d-haplotype BALB/c spleen tissue, and cultured STO, 3(8)21 and 3(8)21-EGFP cells. Three treatment groups are shown: isotype control + R-PE-labelled secondary antibody (a, b); R-PE-labelled secondary antibody alone (c, d); and primary anti-H-2Kd antibody plus R-PE-labelled secondary antibody (e, f). Identical microscopic fields are shown (bar, 100 µm): phase (a, c, e) or immunofluorescence (b, d, f). See text for further details.
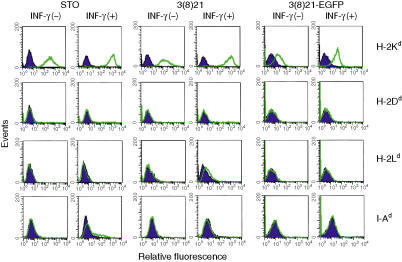
To determine whether H-2Kd expression occurred on cell surfaces, flow cytometry was performed. As shown in Fig. 8 (top row), all three untreated cell lines constitutively expressed cell surface H-2Kd as indicated by significant rightward shifts in fluorescence signals compared with cells incubated with control isotype antibodies. The shifted positions of the relative peak signals suggest quantitative differences [STO > 3(8)21 ≫ 3(8)21-EGFP] but direct biochemical measurements are needed to support this interpretation. Similar considerations apply to STO and SCD cultures treated with IFN-γ, in which H-2Kd expression was variably augmented between 4- and 10-fold. Relatively low levels of H-2Kd expression were also observed in untreated primary cultured BALB/c kidney cells (data not shown) consistent with results of northern blotting, QPCR and western blotting (data not shown).
Figure 8.
Analysis of major histocompatibility complex (MHC) class I and class II expression on cell surfaces in STO (S, SIM; T, 6-thioguanine resistant; O, ouabain resistant) and STO cell-derived (SCD) cells. Untreated (−) or interferon (IFN)-γ-treated (+) cultured cells were incubated with either isotype control antibodies (shaded dark blue curves) or antigen-specific antibodies (unshaded green curves), and the cell suspensions were subjected to flow cytometry [H-2Ld antigen expression was analysed with R-PE-labelled primary antibody; R-PE-labelled secondary anti-mouse immunoglobulin G (IgG) antibodies were used for specific antigen detection and for isotype monitoring in the remaining groups]. Untreated BALB/c splenocytes were used as positive controls to validate MHC marker reactivities. EGFP, enhanced green fluorescent protein. See text and Table 2 for further details.
H-2Dd and H-2Ld cell-surface determinants are not constitutively expressed in STO and SCD cells, whereas low levels of H-2Ld are expressed in IFN-γ-treated 3(8)21 cells
In contrast to constitutive and IFN-γ-augmented expression of H-2Kd determinants, H-2Dd and H-2Ld determinants were not observed on the surfaces of untreated STO and SCD cells subjected to flow cytometry (Fig. 8, second and third rows, respectively). H-2Ld determinants were also not observed on the cell surfaces of untreated or IFN-γ-treated STO and 3(8)21-EGFP cells; however, in response to IFN-γ, 3(8)21 cells displayed relatively low but reproducible levels of cell surface H-2Ld. Positive signals were obtained with the same primary antibodies using untreated BALB/c splenocytes as internal controls.
I-Aβd proteins are not expressed in STO or SCD cells
Using well-characterized anti-I-A polyclonal antiserum-JV2, broadly specific for MHC class II proteins,45 no evidence of 29-kDa MHC class II I-Aβ proteins was detected in extracts of STO, 3(8)21 and 3(8)21-EGFP cells on western blots (data not shown). These results were consistent with the undetectable mRNA expression in RT-PCR analyses using I-Aα- and I-Aβ-specific primers (data not shown), and with the undetectable cell surface expression of I-Ad determinants in both untreated and IFN-γ-treated cells subjected to flow cytometry (Fig. 8, bottom row). Positive signals were obtained again with the same primary antibody using untreated BALB/c splenocytes as internal controls.
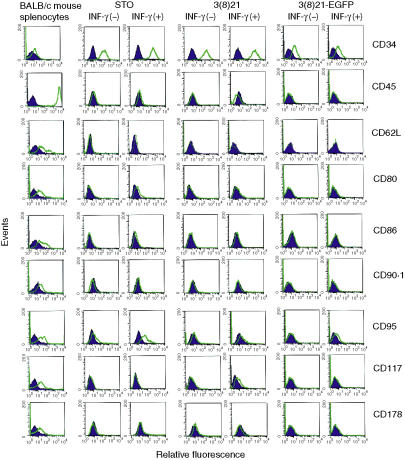
CD marker analyses of untreated and IFN-γ-treated STO and SCD cells
Cell surface expression of sets of CD markers in STO, 3(8)21 and 3(8)21-EGFP cells was analysed by flow cytometry (Fig. 9). With respect to markers associated with stem and progenitor cells, all three lines expressed CD34 (gp105–120), the expression of which was augmented up to 3·5-fold by IFN-γ. In contrast, the expression of CD45 (LCA36), CD90·1 (Thy-1·149) and CD117 (stem cell factor c-kit receptor37) was undetectable. Similar results were observed with 3(8)21 cells, except for the expression of CD117 which, like H-2Ld, was constitutively absent but was slightly inducible by IFN-γ. No expression was observed for CD62L (matrix receptor l-selectin50), CD178/CD95L (Fas ligand39), or, in particular, CD80 (costimulator B7-1) or CD86 (costimulator B7-251).
Figure 9.
Analysis of cell differentiation (CD) marker expression on cell surfaces in STO (S, SIM; T, 6-thioguanine resistant; O, ouabain resistant) and STO cell-derived (SCD) cells. Untreated (−) or interferon (IFN)-γ-treated (+) cultured cells were incubated with either isotype control antibodies (shaded dark blue curves) or antigen-specific antibodies (unshaded green curves), and the cell suspensions were subjected to flow cytometry [primary R-PE-labelled antibody was used for specific antigen detection; R-PE-labelled anti-mouse immunoglobulin (IgG) antibodies were used to monitor isotype controls]. Untreated BALB/c splenocytes were used as positive controls to validate individual CD marker reactivities. See text and Table 2 for further details.
STO and 3(8)21 cells both displayed detectable but low constitutive levels of CD95 (Fas38); however, CD95 expression was augmented by IFN-γ only in STO cells. In contrast, neither constitutive nor IFN-γ-inducible expression of CD95 was observed in 3(8)21-EGFP cells.
No other instances of clonal variation in CD expression were observed among the three lines; and, except for IFN-γ stimulation of low levels of CD95 and CD117 expression in STO and 3(8)21 cells, respectively, no other CD markers examined, apart from CD34, showed augmented responses to IFN-γ. As above, with respect to each CD marker, positive signals were obtained with the same primary antibodies, using untreated BALB/c splenocytes as internal controls.
Discussion
STO fibroblasts (the term ‘fibroblast’ refers to the initial morphology of the cells at the time the cell lines were isolated) have been used widely as feeder layers to grow authentic mouse ES cells.52 STO cells were derived from SIM embryo cell lines32 by Alan Bernstein, who selected for cells resistant to both ouabain and 6-thioguanine, hence the STO acronym [S, SIM; T, 6-thioguanine resistant; O, ouabain resistant (Gail Martin, University of California, San Francisco, CA, USA, personal communication)]. Recent investigations have indicated that STO and 3(8)21-EGFP cells (the latter is one of several SCD lines30) exhibit properties of immune-privileged progenitor cells, with the potential to form differentiated hepatocytes or neuroglia following xenogeneic transplantation into either the livers or the lesioned spinal cords of non-immunosuppressed adult Fisher rats, respectively (Zhang et al.24 and K. S. Koch, P. Lu, K.-H. Son, M. Tuszynski, Y. Deng, S. Sell and H. L. Leffert, manuscript in preparation). Limited proteomic information53 and microarray information (M. Zhang, K.-H. Son, K. S. Koch, S. Chien, S. Sell and H. L. Leffert, unpublished observations) are available, but the germ layer origins and the biological properties of STO cells remain poorly understood. In this report, to obtain a better understanding of the molecular basis for immune privilege and progenitor cell status of STO and SCD cells, we have characterized sets of MHC and CD expression markers in these cells. The results reveal haplotype and MHC class I status as H-2Kd-positive; they confirm previous conclusions of MHC class II (I-Ad) negative status; and, with respect to cell surfaces, they show that STO and two SCD lines [3(8)21 and 3(8)21-EGFP] express CD34, but not CD80, CD86 or CD95L. These observations suggest that STO and SCD cells share phenotypic markers and the property of immune-privilege with other murine and human progenitor/stem cell lines (Table 1).
Contrary to prior conclusions,24 previously genotyped STO and SCD cells are not phenotypically H-2K-negative, but such cells do not manifest q-haplotype-encoded H-2 antigens, as would have been expected from the reported Swiss mouse origin. Instead, MHC class I H-2Kd heavy chains produced by these cells share identical amino acid sequence and d-haplotype specificity with BALB/c, as opposed to the d-haplotype of DBA/2, mice. DNA sequencing of the entire H-2K open reading frame in all three lines supports this conclusion.
The H-2K sequences in d-haplotype DBA/2 (J00402) and BALB/c (U47329) mice differ at codon 135, which specifies histidine (CAT) or glutamine (CAG) residues, respectively, in the α2 variable regions of the two different heavy chains. The finding of an identical BALB/c d-haplotype in STO and SCD cells is unexpected as most Swiss albino mice from which these cells were derived purportedly display predominantly pure q-haplotypes, although some, like Swiss CTS and NOD mice, display mixed d/dx- or d/b-haplotypes, respectively.33 Flow cytometry suggests that other major class I cell surface molecules, including H-2Dd and H-2Ld, are neither constitutively expressed nor inducible by IFN-γ in STO or SCD cells; the one exception, namely low levels of IFN-γ-inducible H-2Ld in 3(8)21 cells, may be a result of clonal variability which is often observed in cultured cell lines. Further gene expression and cytokine response studies are needed, however, to exclude possibilities of mixed haplotypes other than b-, d-, k- or q-origin, or abnormal and cryptic expression of H-2L and/or H-2D molecules.
The current observations provide insight into the mechanisms by which engrafted STO and 3(8)21-EGFP cells evade rejection in non-immunosuppressed xenogeneic hosts: surprisingly, the data accumulated to date suggest that immune privilege is not hindered by the expression of MHC class I molecules. Although the presence or formation of endogenous peptide antigen or β2m was not demonstrated directly, evidence of normal H-2Kd expression is supported by results of RNA and protein blotting, immunocytochemistry, flow cytometry and IFN-γ responsiveness. These results suggest that trimeric H-2Kd complexes (i.e. glycosylated heavy chain, non-covalently associated β2m and peptide) are synthesized, post-translationally processed, and transported to cell surfaces in the cultured cells. This conclusion is consistent with biochemical studies of cell-surface expression of MHC class I molecules which suggest that the detection of such molecules on cell surfaces requires transport and assembly of complexes containing all three proteins, including post-translationally processed heavy chains.54 [Although expression of H-2Kd in differentiated hepatocytes derived from 3(8)21-EGFP donor cells has not yet been examined in xenogeneic grafts,24 H-2Kd expression has been observed in neuroglia-like donor cells 1 month after xenoengraftment into lesioned rat spinal cords (K. S. Koch, P. Lu, K.-H. Son, M. Tuszynski, Y. Deng, S. Sell and H. L. Leffert, manuscript in preparation).
Host avoidance of foreign H-2Kd complexes might be brought about in at least three ways. First, H-2Kd complexes might be displayed properly but insufficiently on the surfaces of STO and SCD cells. Indeed, as judged by protein blotting with serially diluted anti-p8 antiserum, RNA blotting and QPCR, the levels of protein (H-2Kd) and mRNA (H-2Kd and β2m) expression, although equivalent among the different cell lines, were relatively low compared with fresh BALB/c splenocytes; hence the phenotypic designation of H-2Kd[LOW] in STO and SCD cells (Table 1). However, insufficient expression seems an unlikely explanation: previous experiments have shown that one trimeric H-2 complex on cell surfaces is sufficient for T-cell recognition.55 In addition, despite robust cell surface expression of H-2Kd molecules revealed by flow cytometry, such findings alone do not provide sufficient quantitative information.
Secondly, unusual conformational display or weak affinities of H-2Kd complexes for T-cell receptors are not rigorously excluded. These possibilities are implicated by paradoxical findings on protein blots, on which H-2K heavy chains in donor cell STO or SCD extracts were detected by one well-characterized antiserum (p8), but not by another (216F). Unexpectedly, p8 recognized blotted proteins of similarly sized Mr ∼45–46 kDa in extracts of BALB/c splenocytes, whereas 216F recognized bands of Mr ∼43–45 kDa, which were largely, if not completely, absent in STO and SCD cells. Antiserum 216F might have been raised against H-2Kd epitopes missing in STO and SCD cells, or against class I heavy chains with altered conformation or post-translational glycosylation, both modifications that might interfere with 216F binding, and perhaps with functionality.
Thirdly, soluble STO and SCD H-2Kd antigens might block cytotoxic T cells.56 Previous findings indicate that mammalian liver, as well as other immune-privileged organ sites,5–10 can exert tolerogenic effects on foreign organ grafts; in fact, intrahepatic tolerance can be conferred by generation of blood-borne class I antigens.57,58 Consequently, this situation might apply to several models in which STO and 3(8)21-EGFP cells have been investigated: for example, the rat liver model, in which intrasplenically injected 3(8)31-EGFP and STO cells migrate rapidly into the organ;24 and the xenogeneic model of injured rat dorsal spinal cord, wherein donor cell survivors in lesion cavities are exposed to cellular and molecular constituents from plasma (K. S. Koch, P. Lu, K.-H. Son, M. Tuszynski, Y. Deng, S. Sell and H. L. Leffert, manuscript in preparation). Further work is needed to determine if there is a unique predisposition for release of immunologically active H-2Kd antigens from STO and SCD cells in vitro and in vivo.
The absence of constitutive and IFN-γ inducible expression of MHC class II proteins in STO and SCD cells, as indicated by results of RT-PCR, protein blotting and flow cytometry, is consistent with findings in human MSCs and ES cells (see Table 1), and is also expected from observations that class II molecules are predominantly expressed by lymphocytes, macrophages and dendritic cells.59 However, potential interactions between donor cells and host antigen-presenting cells (APCs) via indirect pathways or graft chimerism60 are not excluded.
Independently of MHC class I+[LOW]/class II− molecular status and of possible shifts in MHC expression post-engraftment, it is also possible that other molecules might facilitate immune privilege. Support for this conclusion was obtained from the failure of flow cytometry to detect two major cell surface costimulatory molecules, B7-1 (CD80) and B7-2 (CD86), in all three untreated or IFN-γ-treated cell lines. Although B7-1 and B7-2 operate through different regulatory pathways, both are required by T cells for Signal 2 generation and antigen recognition in combination with class I molecules.51,59 Thus, lack of expression of B7-1 and B7-2 might facilitate immune privilege in transplanted cells through abrogation of direct pathways of adaptive immunity, via the failure of Signal 1 transmission as a result of the absence of expression of Signal 2 costimulators. However, the expression of other costimulatory family members on cultured or transplanted cells is not excluded, as structurally related, but so far unstudied, non-lymphocytic B7 homologues (ICOS-L, PD-L1, PD-L2, B7-H3 and B7-H4) are expressed and are cytokine-inducible in fibroblasts, endothelial and epithelial cells, dendritic cells, and cells from human epithelial tumours.51,61–63 STO and SCD cells also variably expressed FAS molecules (CD95), as revealed by flow cytometry. Notably, the expression of CD95L on grafts is also well known to facilitate Fas-induced tolerance through T-cell killing.64,65 However, the involvement of CD95L-related pathways seems unlikely, as CD95L expression was undetectable in vitro even following IFN-γ treatment in all three lines.
Studies on the MHC expression and transplantability of embryonic STO and SCD cells, which suggest that these progenitor cells are not rejected across H2 barriers partly because of low class I MHC and absent class II MHC, are consistent with similar studies on several prominent murine and human progenitor/stem cell systems. This conclusion is supported by two sets of observations. First, donor cell immune privilege is associated with class I+[LOW] status in ∼70% of immune-privileged murine and human MSC and ES cell systems described thus far [references 18–25; see Table 1]. Secondly, in particular, MHC and CD phenotypes observed in STO and SCD cells, class I+ [LOW] (IFN-γ inducible)/class II− (IFN-γ non-inducible), CD34+/CD80−/CD86−/CD95L−, resemble the phenotypes of prominent hES cell lines reported by two different laboratories.21,23 Notably, STO and SCD cells partially resemble human MSCs, which also display class I+[LOW] (HLA-A)/class II− (HLA-DR)/CD80−/CD86−/CD95L−/CD117− phenotypes (Table 1); the latter, however, tend to be CD34-negative and CD90-positive.66
STO and SCD cells originated from day 15 to day 17 embryos;32 the low levels of MHC class I expression in these cell lines are thus also consistent with previous findings in embryonic mouse cell lines and normal mouse embryos.67,68 The expression of cell-surface CD34 in STO and SCD cells is also similar to surface expression of CD34 in activated haematopoietic stem cells35 and in mouse NIH3T3 fibroblasts with properties of mesodermal stem cells.69,70 Comparisons among hES, STO and SCD lines with respect to patterns of growth rates, genomic polymorphisms, methylation and karyotypic stability have not yet been made.71 However, some prominent stem cell markers were not detected in STO and SCD cells: CD45 (LCA), a differentiation antigen associated with haematopoietic precursors36 but absent in putative hepatic stem cells from mice;72,73 CD90·1 (CD90/Thy1·1), a marker associated with putative liver stem cells in some73 but not all instances;72 and, with the exception of very low inducible expression in 3(8)21 cells, CD117, the stem cell factor (SCF) c-kit receptor.37 Expression of these markers may occur in vitro in response to soluble factors not yet tested, or in vivo, after transplantation, or, may depend upon specific niches such as the liver24 or spinal cord (K. S. Koch, P. Lu, K.-H. Son, M. Tuszynski, Y. Deng, S. Sell and H. L. Leffert, manuscript in preparation).
Other ways to obtain immune-privileged stem cells may eventually involve patient-specific SCNT into human eggs or directed fusion of human fibroblasts and ES cell lines.14–17 However, these systems do not currently provide patient-specific, unlimited, replenishable or universal sources of stem or progenitor cells. These problems might be circumvented with non-infectious, non-malignant stem cell lines with properties of ‘universal’ immune privilege. The extent to which STO and SCD cells can meet these requirements, and avoid hyperacute rejection caused by xenoantibodies against α-GAL or related xenogeneic sugars,74 remains to be determined.
Acknowledgments
This work was supported by grants from the NIH (to HP; to MZ, RO1-CA077427; to SS, R01-DK05761905 and R01-ES09495; to HLL, P42-ES10337), the ACS (to KSK) and the UCSD Academic Senate (to HLL). We thank M. Kagnoff (UCSD School of Medicine) in whose laboratory QPCR was performed, B. Ju and D. Spector (UCSD Departments of Medicine and Biology) for gifts of Swiss NIH3T3 and BALB/c 3T3 cells, R. Germain (Laboratory of Immunology, NIAID) for gifts of anti-MHC class II antisera, and F. Villinger (Emory University School of Medicine) for helpful comments.
Abbreviations
- β2m
β2-microglobulin
- BSA
bovine serum albumin, CD, cell differentiation
- E
exon
- EGFP
enhanced green fluorescent protein
- EtBr
ethidium bromide
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde phosphate dehydrogenase
- hES
human embryonic stem
- IFN
interferon
- LCA
leucocyte common antigen
- MSCs
mesenchymal stem cells
- nt
nucleotide
- PBS
phosphate-buffered saline
- R
primer reaction pairs
- R-PE
Rhodophyta phycoerythrin
- QPCR
quantitative reverse transcriptase-mediated polymerase chain reaction
- RT-PCR
reverse transcriptase–polymerase chain reaction
- SCD
STO cell-derived
- SCNT
somatic cell nuclear transfer
- SIM
Sandoz inbred mouse
- SSC
standard saline citrate
- STO (S
SIM
- T
6-thioguanine resistant
- O
ouabain resistant)
- TBS
Tris-buffered saline
- TBSTx
Triton-X100-TBS
References
- 1.Auchincloss H., Jr In search of the elusive Holy Grail: the mechanisms and prospects for achieving clinical transplantation tolerance. Am J Transplant. 2001;1:6–12. doi: 10.1034/j.1600-6143.2001.010103.x. [DOI] [PubMed] [Google Scholar]
- 2.Kamradt T, Mitchison NA. Tolerance and autoimmunity. N Engl J Med. 2001;344:655–64. doi: 10.1056/NEJM200103013440907. [DOI] [PubMed] [Google Scholar]
- 3.Bellingham RE, Brent L, Medawar PB. ‘Actively acquired tolerance’ of foreign cells. Nature. 1953;172:603–6. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 4.Liechty KW, MacKenzie TC, Shaaban AF, Radu A, Mosely AM, Deans R, Marshak DR, Flake AW. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nature Med. 2000;6:1282–6. doi: 10.1038/81395. [DOI] [PubMed] [Google Scholar]
- 5.Taylor AW, Streilein JW. Inhibition of antigen-stimulated effector T cells by human cerebrospinal fluid. Neuroimmunomodulation. 1996;3:112–8. doi: 10.1159/000097235. [DOI] [PubMed] [Google Scholar]
- 6.Niederkorn JY. Mechanisms of immune privilege in the eye and hair follicle. J Invest Dermatol Symp Proc. 2003;8:168–72. doi: 10.1046/j.1087-0024.2003.00803.x. [DOI] [PubMed] [Google Scholar]
- 7.Hoglund P, Karre K, Klein G. The uterine cervix – a new member of the family of immunologically exceptional sites? Cancer Immunol. 2003;3:6. [PubMed] [Google Scholar]
- 8.Guller S, LaChapelle L. The role of placental Fas ligand in maintaining immune privilege at maternal–fetal interfaces. Semin Reprod Endocrinol. 1999;17:39–44. doi: 10.1055/s-2007-1016210. [DOI] [PubMed] [Google Scholar]
- 9.Ferguson TA, Griffith TS. A vision of cell death: insights into immune privilege. Immunol Rev. 1997;156:167–84. doi: 10.1111/j.1600-065x.1997.tb00967.x. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson TA, Green DR, Griffith TS. Cell death and immune privilege. Int Rev Immunol. 2002;21:153–72. doi: 10.1080/08830180212058. [DOI] [PubMed] [Google Scholar]
- 11.Hong JC, Kahan BD. Immunosuppressive agents in organ transplantation. Past, present and future. Semin Nephrol. 2000;20:108–25. [PubMed] [Google Scholar]
- 12.Krensky AM, Strom TB, Bluestone JA. Immunomodulators: immunosuppressive agents, tolerogens, and immunostimulants. In: Hardman JG, Limbird LE, editors. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10. New York: McGraw-Hill; 2001. pp. 1463–84. [Google Scholar]
- 13.Rhind SM, Taylor JE, De Sousa PA, King TJ, McGarry M, Wilmut I. Human cloning: can it be made safe? Nat Rev Genet. 2003;4:855–64. doi: 10.1038/nrg1205. [DOI] [PubMed] [Google Scholar]
- 14.Fairchild PJ, Cartland S, Nolan KF, Waldmann H. Embryonic stem cells and the challenge of transplantation tolerance. Trends Immunol. 2004;25:465–70. doi: 10.1016/j.it.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Surani MA. Nuclear reprogramming by human embryonic stem cells. Cell. 2005;122:653–4. doi: 10.1016/j.cell.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 16.Chung Y, Klimanskaya I, Becker S, Marh J, Lu S-J, Johnson J, Meisner L, Lanza R. Embryonic and extraembryonic stem cell lines derived from single mouse blastomeres. Nature. 2006;439:216–19. doi: 10.1038/nature04277. [DOI] [PubMed] [Google Scholar]
- 17.Meissner A, Jaenisch R. Generation of nuclear-transfer derived pluripotent ES cells from cloned Cdx2-deficient blastocysts. Nature. 2006;439:212–15. doi: 10.1038/nature04257. [DOI] [PubMed] [Google Scholar]
- 18.Saito T, Kuang JQ, Bittera B, Al-Khaldi A, Chiu RC. Xenotransplant cardiac chimera: immune tolerance of adult stem cells. Ann Thorac Surg. 2002;74:19–24. doi: 10.1016/s0003-4975(02)03591-9. [DOI] [PubMed] [Google Scholar]
- 19.Hori J, Ng TF, Shatos M, Klassen H, Streilen JW, Young MJ. Neural progenitor cells lack immunogenicity and resist destruction as allografts. Stem Cells. 2003;21:405–16. doi: 10.1634/stemcells.21-4-405. [DOI] [PubMed] [Google Scholar]
- 20.Abraham EJ, Kodama S, Lin JC, Ubeda M, Faustman DL, Habener JF. Human pancreatic islet-derived progenitor cell engraftment in immunocompetent mice. Am J Pathol. 2004;164:817–30. doi: 10.1016/S0002-9440(10)63170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li L, Baroja ML, Majumdar A, et al. Human embryonic stem cells possess immune-privileged properties. Stem Cells. 2004;22:448–56. doi: 10.1634/stemcells.22-4-448. [DOI] [PubMed] [Google Scholar]
- 22.Mansilla E, Marin GH, Sturla F, et al. Human mesenchymal stem cells are tolerized by mice and improve skin and spinal cord injuries. Transplantation Proc. 2005;37:292–4. doi: 10.1016/j.transproceed.2005.01.070. [DOI] [PubMed] [Google Scholar]
- 23.Drukker M, Katchman H, Katz G, et al. Human embryonic stem cells and their differentiated derivatives are less susceptible for immune rejection than adult cells. Stem Cells. 2006;24:221–29. doi: 10.1634/stemcells.2005-0188. [DOI] [PubMed] [Google Scholar]
- 24.Zhang M, Joseph B, Gupta S, et al. Embryonic mouse STO cell-derived xenografts express hepatocytic functions in the livers of non-immunosuppressed adult rats. Stem Cells. 2005;23:186–99. doi: 10.1634/stemcells.2004-0129. [DOI] [PubMed] [Google Scholar]
- 25.Anderson CC, Matzinger P. Immunity or tolerance: opposite outcomes of microchimerism from skin grafts. Nat Med. 2001;7:80–7. doi: 10.1038/83393. [DOI] [PubMed] [Google Scholar]
- 26.Ying Q-L, Nichols J, Evans EP, Smith AG. Changing potency by spontaneous fusion. Nature. 2002;416:545–8. doi: 10.1038/nature729. [DOI] [PubMed] [Google Scholar]
- 27.Terada N, Hamazaki T, Oka M, et al. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:542–5. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Willenbring H, Akkari Y, et al. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422:897–901. doi: 10.1038/nature01531. [DOI] [PubMed] [Google Scholar]
- 29.Vassilopoulos G, Wang P-R, Russell DW. Transplanted bone marrow regenerates liver by cell fusion. Nature. 2003;422:901–4. doi: 10.1038/nature01539. [DOI] [PubMed] [Google Scholar]
- 30.Zhang M, Sell S, Leffert HL. Hepatic progenitor cell lines from allyl alcohol-treated adult rats are derived from γ-irradiated mouse STO cells. Stem Cells. 2003;21:449–58. doi: 10.1634/stemcells.21-4-449. [DOI] [PubMed] [Google Scholar]
- 31.Meagher S, Potts WK. A microsatellite-based MHC genotyping system for house mice (Mus domesticus) Hereditas. 1997;127:75–82. doi: 10.1111/j.1601-5223.1997.00075.x. [DOI] [PubMed] [Google Scholar]
- 32.Ware LM, Axelrad AA. Inherited resistance to N- and B tropic murine leukemia viruses in vitro: evidence that congenic mouse strains SIM and SIM.R differ at the Fv-1 locus. Virology. 1972;50:339–48. doi: 10.1016/0042-6822(72)90385-6. [DOI] [PubMed] [Google Scholar]
- 33.Beck JA, Lloyd S, Hafezparast M, Lennon-Pierce M, Eppig JT, Festing MFW, Fisher EMC. Genealogies of mouse inbred strains. Nature Gen. 2000;24:23–5. doi: 10.1038/71641. [DOI] [PubMed] [Google Scholar]
- 34.Warner CM, Almquist CD, Toulimat MH, Xu Y. Induction of embryonic major histocompatibility complex antigen expression by gamma-IFN. J Reprod Immunol. 1993;24:111–21. doi: 10.1016/0165-0378(93)90014-9. [DOI] [PubMed] [Google Scholar]
- 35.Krause DS, Fackler MJ, Civin CI, May WS. CD34: Structure, biology, and clinical utility. Blood. 1996;87:1–13. [PubMed] [Google Scholar]
- 36.Huntington ND, Tarlinton DM. CD45: direct and indirect government of immune regulation. Immunol Lett. 2004;94:167–74. doi: 10.1016/j.imlet.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 37.Roskoski R., Jr Signaling by Kit protein-tyrosine kinase – the stem cell factor receptor. Biochem Biophys Res Commun. 2005;337:1–13. doi: 10.1016/j.bbrc.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 38.Maher S, Toomey D, Condron C, Bouchier-Hayes D. Activation-induced cell death. The controversial role of Fas and Fas ligand in immune privilege and tumor counterattack. Immunol Cell Biol. 2002;80:131–7. doi: 10.1046/j.1440-1711.2002.01068.x. [DOI] [PubMed] [Google Scholar]
- 39.Askenasy N, Yolcu ES, Yaniv I, Shirwan H. Induction of tolerance using Fas ligand: a double-edged immunomodulator. Blood. 2005;105:1396–404. doi: 10.1182/blood-2004-06-2364. [DOI] [PubMed] [Google Scholar]
- 40.Zellweger R, Ayala A, DeMaso CM, Chaudry IH. Trauma-hemorrhage causes prolonged depression in cellular immunity. Shock. 1995;4:149–53. doi: 10.1097/00024382-199508000-00012. [DOI] [PubMed] [Google Scholar]
- 41.Gerloni M, Castiglioni P, Zanetti M. The cooperation between two CD4 T cells induces tumor protective immunity in MUC.1 transgenic mice. J Immunol. 2005;175:6551–9. doi: 10.4049/jimmunol.175.10.6551. [DOI] [PubMed] [Google Scholar]
- 42.Lu XP, Koch KS, Lew DJ, Dulic V, Pines J, Reed SI, Hunter T, Leffert HL. Induction of cyclin mRNA and histone H1-kinase during liver regeneration. J Biol Chem. 1992;267:2841–4. [PubMed] [Google Scholar]
- 43.Smith MJ, Parker JMR, Hodges RS, Barber BH. The preparation and characterization of anti-peptide heteroantisera recognizing subregions of the intracytoplasmic domain of class I H-2 antigens. Mol Immunol. 1986;23:1077–92. doi: 10.1016/0161-5890(86)90006-4. [DOI] [PubMed] [Google Scholar]
- 44.Machold RP, Ploegh HL. Intermediates in the assembly and degradation of class I major histocompatibility complex (MHC) molecules probed with free heavy chain-specific monoclonal antibodies. J Exp Med. 1996;184:2251–9. doi: 10.1084/jem.184.6.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bryant PW, Roos P, Ploegh HL, Sant AJ. Deviant trafficking of I-Ad mutant molecules is reflected in their peptide binding properties. Eur J Immunol. 1999;29:2729–39. doi: 10.1002/(SICI)1521-4141(199909)29:09<2729::AID-IMMU2729>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 46.Wang M, Stepkowski SM, Hebert JS, Tian LYuJ, Kahan BD. Nucleotide sequences of three H-2K and three H-2D complementary DNA clones coding mouse class I MHC heavy chain proteins. Ann Transplant. 1996;1:26–31. [PubMed] [Google Scholar]
- 47.Rubocki RJ, Lee DR, Lie WR, Myers NB, Hansen TH. Molecular evidence that the H-2D and H-2L genes arose by duplication. Differences between the evolution of the class I genes in mice and humans. J Exp Med. 1990;171:2043–61. doi: 10.1084/jem.171.6.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vugmeyster Y, Glas R, Perarnau B, Lemonnier FA, Eisen H, Ploegh H. Major histocompatibility complex (MHC) class I KbDb-/- deficient mice possess functional CD8+ T cells and natural killer cells. Proc Natl Acad Sci USA. 1988;95:12492–7. doi: 10.1073/pnas.95.21.12492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haeryfar SM, Hoskin DW. Thy-1: more than a mouse pan-T cell marker. J Immunol. 2004;173:3581–8. doi: 10.4049/jimmunol.173.6.3581. [DOI] [PubMed] [Google Scholar]
- 50.Rosen SD. Ligands for 1-selectin. Homing, inflammation, and beyond. Annu Rev Immunol. 2004;22:129–56. doi: 10.1146/annurev.immunol.21.090501.080131. [DOI] [PubMed] [Google Scholar]
- 51.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–48. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 52.Martin GR, Evans MJ. Differentiation of clonal lines of teratocarcinoma cells: formation of embryoid bodies in vitro. Proc Natl Acad Sci USA. 1975;72:1441–5. doi: 10.1073/pnas.72.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lim JWE, Bodnar A. Proteome analysis of conditioned medium from mouse embryonic fibroblast feeder layers which support the growth of human embryonic stem cells. Proteomics. 2002;2:1187–203. doi: 10.1002/1615-9861(200209)2:9<1187::AID-PROT1187>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 54.Cresswell P, Bangia N, Dick T, Diedrich G. The nature of the MHC class I peptide loading complex. Immunol Rev. 1999;172:21–8. doi: 10.1111/j.1600-065x.1999.tb01353.x. [DOI] [PubMed] [Google Scholar]
- 55.Eisen Sykulev Y, Joo M, Vturina I, Tsomides TJ, Eisen HN. Evidence that a single peptide–MHC complex on a target cell can elicit a cytolytic T cell response. Immunity. 1996;4:565–71. doi: 10.1016/s1074-7613(00)80483-5. [DOI] [PubMed] [Google Scholar]
- 56.Gotze D, Reisfeld R. Immune response to soluble H-2Kd antigens. Z Immun-Forsch Bd. 1974;148:45–61. [PubMed] [Google Scholar]
- 57.Starzl TE. Chimerism and tolerance in transplantation. Proc Natl Acad Sci USA. 2004;101(Suppl. 2):14607–14. doi: 10.1073/pnas.0404829101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sriwatanawongsa V, Davies HS, Calne RW. The essential roles of parenchymal cells and passenger leukocytes in the tolerance induced by liver grafting in rats. Nature Med. 1995;1:428–32. doi: 10.1038/nm0595-428. [DOI] [PubMed] [Google Scholar]
- 59.Abbas AK, Lichtman AH. Basic Immunology Functions and Disorders of the Immune System. Philadelphia: W. B. Saunders; 2001. [Google Scholar]
- 60.Rao SO, Starzl TE, Demetris AJ, Trucco M, Thomson A, Qian S, Murase N, Fung JJ. The two-way paradigm of transplantation immunology. Clin Immunol Immunopath. 1996;80:S46–S51. doi: 10.1006/clin.1996.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Collins M, Ling V, Carreno BM. The B7 family of immune-regulatory ligands. Genome Biol. 2005;6:223.1–223.7. doi: 10.1186/gb-2005-6-6-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ding H, Wu X, Gao W. PD-L1 is expressed by human renal tubular epithelial cells and suppresses T cell cytokine synthesis. Clin Immunol. 2005;115:184–91. doi: 10.1016/j.clim.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 63.Khayyamian S, Hutloff A, Buchner K, Grafe M, Henn V, Kroczek RA, Mages HW. ICOS-ligand, expressed on human endothelial cells, costimulates Th1 and Th2 cytokine secretion by memory CD4+ T cells. Proc Natl Acad Sci USA. 2002;99:6198–203. doi: 10.1073/pnas.092576699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pan TL, Goto S, Lin YC, et al. The Fas and Fas ligand pathways in liver allograft tolerance. Clin Exp Immunol. 1999;118:180–7. doi: 10.1046/j.1365-2249.1999.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee H, Ferguson TA. Biology of FasL. Cytokine Growth Factor Rev. 2003;14:325–35. doi: 10.1016/s1359-6101(03)00028-5. [DOI] [PubMed] [Google Scholar]
- 66.Barry FP, Murphy JM, English K, Mahon BP. Immunogenicity of adult mesenchymal stem cells: lessons from the fetal allograft. Stem Cells Dev. 2005;14:252–65. doi: 10.1089/scd.2005.14.252. [DOI] [PubMed] [Google Scholar]
- 67.Silverman T, Rein A, Orrison B, Langloss J, Bratthauer G, Miyazaki J, Ozato K. Establishment of cell lines from somite stage mouse embryos and expression of major histocompatibility class I genes in these cells. J Immunol. 1988;140:4378–87. [PubMed] [Google Scholar]
- 68.Ozato K, Wan YJ, Orrison BM. Mouse major histocompatibility class I gene expression begins at midsomite stage and is inducible in earlier-stage embryos by interferon. Proc Natl Acad Sci USA. 1985;82:2427–31. doi: 10.1073/pnas.82.8.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lin G, Finger E, Gutierrez-Ramos JC. Expression of CD34 in endothelial cells, hematopoietic progenitors and nervous cells in fetal and adult mouse tissues. Eur J Immunol. 1995;25:508–16. doi: 10.1002/eji.1830250606. [DOI] [PubMed] [Google Scholar]
- 70.Ntambi JN, Kim YC. Adipocyte differentiation and gene expression. J Nutr. 2000;130:3122S–26S. doi: 10.1093/jn/130.12.3122S. [DOI] [PubMed] [Google Scholar]
- 71.Rao MS, Civin CI. Translational Research: Toward better characterization of human embryonic stem cell lines. Stem Cells. 2005;23:1453. doi: 10.1634/stemcells.2005-ed.4. [DOI] [PubMed] [Google Scholar]
- 72.Tsuchiya A, Heike T, Fujino H, et al. Long-term extensive expansion of mouse hepatic stem/progenitor cells in a novel serum-free culture system. Gastroenterology. 2005;128:2089–104. doi: 10.1053/j.gastro.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 73.Li WL, Su J, Yao YC, et al. Isolation and characterization of bipotent liver progenitor cells from adult mouse. Stem Cells. 2006;24:322–32. doi: 10.1634/stemcells.2005-0108. [DOI] [PubMed] [Google Scholar]
- 74.Galili U, Shohet SB, Kobrin E, Stults CL, Macher BA. Man, apes, and Old World monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells. J Biol Chem. 1988;263:17755–62. [PubMed] [Google Scholar]