Abstract
Background
This study examined characteristics of adult and adolescent patients with cystic fibrosis (CF) to determine factors associated with an increased risk of pulmonary exacerbations.
Methods
249 patients with CF infected with multidrug resistant bacteria were recruited and prospectively followed for up to 4.5 years until they experienced a pulmonary exacerbation severe enough to require intravenous antibiotics. Multivariable regression analyses were used to compare the characteristics of patients who experienced an exacerbation with those who did not.
Results
124 of the 249 patients (50%) developed a pulmonary exacerbation during the first year and 154 (62%) experienced an exacerbation during the 4.5 year study period. Factors predictive of exacerbations in a multivariable survival model were younger age (OR 0.98, 95% CI 0.96 to 0.99), female sex (OR 1.45, 95% CI 1.07 to 1.95), lower forced expiratory volume in 1 second (FEV1) (OR 0.98, 95% CI 0.97 to 0.99), and a previous history of multiple pulmonary exacerbations (OR 3.16, 95% CI 1.93 to 5.17). Chronic use of inhaled corticosteroids was associated with an increased risk of exacerbation (OR 1.92, 95% CI 1.00 to 3.71) during the first study year.
Conclusions
Patients who experience pulmonary exacerbations are more likely to be younger, female, using inhaled steroids, have a lower FEV1, and a history of multiple previous exacerbations. It is hoped that knowledge of these risk factors will allow better identification and closer monitoring of patients who are at high risk of exacerbations.
Keywords: cystic fibrosis, pulmonary exacerbations, bacterial resistance, lung infections, risk factors
Patients with cystic fibrosis (CF) have viscous pulmonary secretions and decreased mucociliary clearance resulting in chronic airway infection and bronchiectasis.1 Although there have been steady significant improvements in median survival over the past decades, premature mortality in patients with CF is still common due to chronic respiratory infection and subsequent respiratory failure.2
Pulmonary disease in CF is characterised by chronic airway infection. Increasingly, patients are being infected with multi‐resistant bacterial pathogens, including Pseudomonas aeruginosa and Burkholderia cepacia, that are resistant to all antibiotics in at least two of three major anti‐pseudomonal antibiotic drug classes.3
Chronic bacterial airway infections in patients with CF generally follow a smouldering course punctuated by acute unpredictable pulmonary exacerbations characterised by worsening cough, increased sputum production, and increased dyspnoea.3,4 Treatment of severe pulmonary exacerbations often requires hospital admission and intravenous antibiotics which negatively affect health related quality of life5 and contribute to significant healthcare costs.6,7 Furthermore, pulmonary exacerbations are associated with impaired sleep and neurocognitive abilities,8 and with increased patient mortality.9,10
No studies to date have systematically examined patient factors associated with pulmonary exacerbation. In a retrospective study Schechter et al11 found that American CF patients with lower socioeconomic status were more likely to require treatment for pulmonary exacerbation. This study did not examine other characteristics such as age, sex, lung function, previous CF complications, or medication use, all of which may affect the risk of pulmonary exacerbation.
This prospective study examined characteristics of adult CF patients infected with multidrug resistant bacteria to determine patient factors that would predict increased risk for pulmonary exacerbation. Determination of risk factors associated with pulmonary exacerbations could allow for early identification and close monitoring of at‐risk patients.
Methods
Patients
Patients were recruited as part of a clinical trial that evaluated combination antibiotic susceptibility testing for treatment of pulmonary exacerbations in CF.12 We recruited patients from 10 Canadian and two Australian CF clinics with a confirmed diagnosis of CF who were known to be infected with multidrug resistant bacteria. Patients were excluded from the study if they were unable to provide informed consent, were less than 12 years of age, pregnant, on continuous home intravenous antibiotic therapy, or had previously undergone lung transplantation.
Procedures
Recruitment of study patients began in July 2000. At enrolment, research nurses at each study site completed a detailed standardised baseline questionnaire for each patient that included the following: (1) age, sex, height, weight, and body mass index; (2) number of recent hospitalisations, presence of physician‐diagnosed CF associated diabetes mellitus, pancreatic insufficiency, or liver disease; and (3) use of chronic (>2 months) inhaled or oral antibiotics, use of inhaled bronchodilators, inhaled corticosteroids, insulin, DNase or prednisone. In addition, lung function was measured in the pulmonary function laboratory and sputum bacterial cultures and sensitivities were performed.
After enrolment, patients were followed prospectively every 3 months for up to 4.5 years. At each clinic visit patients provided a sputum sample for culture. Patients were followed until their first pulmonary exacerbation requiring intravenous antibiotics or, for those patients who did not exacerbate, until the end of the study period (31 December 2004). Patients who provided no further sputum samples or who were lost to follow up were censored 3 months after their last documented study clinic visit.
A pulmonary exacerbation was defined as an acute exacerbation of pulmonary symptoms that, in the opinion of the patient's CF physician, was severe enough to require intravenous antibiotics. All exacerbations also had to fulfil the criteria published by the 1994 Cystic Fibrosis Foundation Microbiology and Infectious Disease Consensus Conference,3 including the presence of at least three of the following 11 new findings or changes in clinical status when compared with the most recent baseline visit: increased cough; increased amount (or change in appearance of) sputum; decreased exercise tolerance; school or work absenteeism; fever (⩾38°C for 4 hours in a 24 hour period); weight loss (⩾1 kg or 5% of body weight); increased respiratory rate or work of breathing; new wheeze or crackles; decrease in forced expiratory volume in 1 second (FEV1) of ⩾10%; decrease in O2 saturation of ⩾10% (compared with baseline within the past 3 months); or new chest radiograph findings.
Informed written consent was obtained from all patients. The Research Ethics Boards of each hospital approved data collection for the purposes of the randomised clinical trial and further ethical approval was obtained for the analysis of this study from the Ottawa Hospital Research Ethics Board.
Statistical analysis
Baseline characteristics of patients with and without pulmonary exacerbation were compared by univariate analysis. Quantitative variables were summarised as mean (SD) values while qualitative variables were given as frequency and percentages. Odds ratios and 95% confidence intervals (CI) were calculated for each variable separately, and a Bonferroni adjustment was made to account for multiple univariate comparisons so that p values of ⩽0.0056 were taken to be significant.
Multivariable logistic regression models were generated to look at one‐year risk factors for exacerbation. In addition, Cox proportional hazards models were generated to look at survival time to first exacerbation for the entire study duration. SAS version 9.1 (SAS Institute Inc, Cary, NC, USA) was used for all statistical procedures. For the multivariate models, all potentially relevant clinical prognostic risk factors were included. We selected variables that were (1) deemed clinically important and (2) that previous studies had suggested were risk factors for other poor clinical outcomes in CF patients. We did not formally assess model fit as our model was built on clinically rather than statistically meaningful criteria. Multiple models were therefore not developed or compared. Our goal was not to produce a clinical prediction rule but rather to elucidate the associations of postulated risk factors and pulmonary exacerbation.
Appropriate interaction terms such as age×FEV1 were tested in the models and retained if significant. Odds ratios and hazard ratios and their 95% confidence intervals were calculated for variables included in the logistic model and Cox proportional hazards model. p values of <0.05 were considered significant.
Results
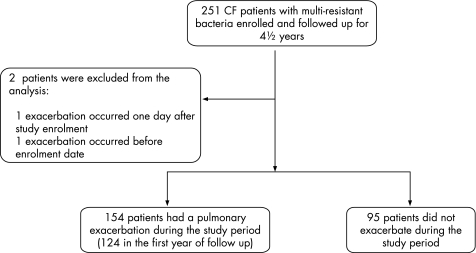
Two hundred and fifty one CF patients were recruited from eight Canadian and two Australian centres but two were excluded from the study analysis, one because pulmonary exacerbation occurred one day after study enrolment and the second because the recorded date of exacerbation was before the collection of baseline data (fig 1).
Figure 1 Participant flow sheet.
Most of the 249 included patients were adults; only seven (3%) were under the age of 18 years at study entry. After 12 months of prospective observation, 124 of the 249 patients (50%) had experienced a pulmonary exacerbation requiring intravenous antibiotics. During the entire 4.5 year study period, 154 patients (62%) developed at least one pulmonary exacerbation (fig 1). The median time to exacerbation in this group was 144 days (range 56–1577). The remaining 95 patients were observed for a median duration of 815 days, and these patients did not experience a pulmonary exacerbation requiring intravenous antibiotics during the study period.
Table 1 compares the baseline data of the 249 patients. Patients with pulmonary exacerbations were more likely to be younger (mean age 30.1 v 33.5 years, p = 0.002), female (OR 3.37, 95% CI 1.92 to 5.90), pancreatic insufficient (OR 4.31, 95% CI 1.47 to 12.65), and to have a lower body mass index (21.2 v. 22.9, p = 0.002) than those who did not exacerbate during the study period. In addition, patients who experienced an exacerbation had a lower baseline FEV1 (48% predicted v 63% predicted, p<0.0001) and a lower baseline FVC (66% predicted v 77% predicted, p = 0.0002), and they had experienced more courses of intravenous antibiotics for pulmonary exacerbations in the 24 month period prior to study enrolment. Patients who experienced pulmonary exacerbations were also more likely to be taking an inhaled corticosteroid (OR 3.12, 95% CI 1.81 to 5.35). Use of DNase (OR 1.85, 95% CI 1.03 to 3.33) or inhaled tobramycin (OR 1.82, 95% CI 1.05 to 3.13) was also somewhat more likely in the group who exacerbated, but these variables did not meet our predetermined level of significance of p<0.0056 for univariate comparisons.
Table 1 Patient characteristics.
| Characteristic | Exacerbated (n = 154) | Did not exacerbate (n = 95) | Odds ratio (95% CI) | p value |
|---|---|---|---|---|
| Mean (SD) age (years) | 30.1 (13.8) | 33.5 (8.8) | 0.95 (0.92 to 0.98) | 0.002 |
| Female sex (%) | 82 (53%) | 24 (25%) | 3.37 (1.92 to 5.90) | <0.0001 |
| Mean (SD) BMI (kg/m2) | 21.2 (4.3) | 22.9 (3.1) | 0.88 (0.82 to 0.96) | 0.002 |
| Mean (SD) lung function | ||||
| FEV1 (% predicted) | 48.3 (18.1) | 63.0 (20.2) | 0.96 (0.95 to 0.98) | <0.0001 |
| FVC (% predicted) | 66.4 (20.3) | 76.9 (19.2) | 0.97 (0.96 to 0.99) | 0.0002 |
| CF related diabetes mellitus | 35 (22.7%) | 18 (18.9%) | 1.26 (0.67 to 2.38) | 0.48 |
| CF related pancreatic insufficiency | 149 (96.8%) | 83 (87.4%) | 4.31 (1.47 to 12.65) | 0.008 |
| CF related liver disease | 18 (11.6%) | 8 (8.4%) | 1.44 (0.60 to 3.45) | 0.41 |
| Courses of IV antibiotics for pulmonary exacerbation in past 24 months | ||||
| 0 | 38 (24.7%) | 61 (64.2%) | – | – |
| 1 | 30 (19.5%) | 19 (20.0%) | 2.54 (1.26 to 5.12) | 0.001 |
| 2–3 | 40 (26.0%) | 8 (8.4%) | 8.03 (3.40 to 18.98) | <0.0001 |
| >3 | 46 (29.9%) | 7 (7.4%) | 10.55 (4.32 to 25.75) | <0.0001 |
| Bacteria in sputum at enrolment | ||||
| Burkholderia cepacia complex (BC) | 55 (35.7%) | 38 (40%) | – | – |
| Pseudomonas aeruginosa (PA) | 78 (50.6%) | 38 (40%) | 1.42 (0.81 to 2.50) | 0.26 |
| Both BC and PA | 6 (3.9%) | 8 (8.4%) | 0.52 (0.17 to 1.62) | 0.26 |
| Other | 15 (9.7%) | 11 (11.6%) | 0.94 (0.39 to 2.27) | 0.89 |
| New bacterial species in sputum during study | 8 (5.2%) | 7 (7.4%) | 0.69 (0.24 to 1.97) | 0.49 |
| Burkholderia cepacia complex | 2 | 0 | ||
| Pseudomonas aeruginosa | 3 | 6 | ||
| Stenotrophomonous maltophilia | 1 | 0 | ||
| Achromobacter xylosoxidans | 2 | 1 | ||
| None | 146 (94.8%) | 88 (92.6%) | ||
| Medications used chronically | ||||
| Inhaled corticosteroid | 89 (57.8%) | 29 (30.5%) | 3.12 (1.81 to 5.35) | <0.0001 |
| DNase | 53 (34.4%) | 21 (22.1%) | 1.85 (1.03 to 3.33) | 0.04 |
| Inhaled antibiotics | ||||
| None | 54 (35.1%) | 49 (51.6%) | – | – |
| Tobramycin | 78 (50.6%) | 39 (41.1%) | 1.82 (1.05 to 3.13) | 0.03 |
| Colimycin | 8 (5.2%) | 2 (2.1%) | 3.63 (0.74 to 17.9) | 0.11 |
| Tobramycin and colimycin | 14 (9.1%) | 5 (5.3%) | 2.54 (0.85 to 7.57) | 0.09 |
| Oral antibiotics | ||||
| None | 101 (65.6%) | 66 (69.5%) | – | – |
| Azithromycin | 21 (13.6%) | 15 (15.8%) | 0.92 (0.44 to 1.90) | 0.81 |
| Other | 19 (12.3%) | 11 (11.6%) | 1.13 (0.51 to 2.52) | 0.77 |
| Azithromycin and other | 13 (8.4%) | 3 (3.2%) | 2.83 (0.78 to 10.32) | 0.11 |
BMI, body mass index; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity.
The prevalence of physician‐diagnosed CF related diabetes mellitus or physician‐diagnosed CF related liver disease did not significantly differ between the two groups. Acquisition of a new bacterial species during the study period was rare (occurring in only 5% and 7% of patients in the exacerbating and non‐exacerbating groups, respectively) and was not associated with an increased risk of exacerbation (p = 0.49).
A multivariable Cox proportional hazards model was used to examine survival time to first exacerbation for the entire study duration (table 2). Factors predictive of exacerbations in the multivariable survival model were younger age (OR 0.98, 95% CI 0.96 to 0.99), female sex (OR 1.45, 95% CI 1.07 to 1.95), lower FEV1 (OR 0.98, 95% CI 0.97 to 0.99), and a previous history of multiple pulmonary exacerbations (OR 3.16, 95% CI 1.93 to 5.17). Patients infected with Stenotrophomonous maltophilia and Achromobacter xylosidans bacteria (that is, bacteria other than Burkholderia cepacia or Pseudomonas aeruginosa) were also more likely to have accelerated times to first exacerbation. Significant interactions between age and lung function were not found.
Table 2 Cox proportional hazards model of time to first exacerbation.
| Patient characteristic | Hazard ratio (95% CI) | p value |
|---|---|---|
| Age | 0.98 (0.96 to 0.998) | 0.03 |
| Female sex | 1.45 (1.07 to 1.95) | 0.02 |
| BMI (kg/m2) | 0.99 (0.95 to 1.05) | 0.94 |
| Lung function | ||
| FEV1 (% predicted) | 0.98 (0.97 to 0.995) | 0.006 |
| FVC (% predicted) | 1.01 (0.995 to 1.02) | 0.23 |
| CF related diabetes mellitus | 0.79 (0.56 to 1.11) | 0.17 |
| Courses of IV antibiotics for pulmonary exacerbation in past 24 months | ||
| 0 | – | – |
| 1 | 1.51 (1.03 to 2.21) | 0.03 |
| 2–3 | 1.64 (1.07 to 2.52) | 0.02 |
| >3 | 3.16 (1.93 to 5.17) | <0.0001 |
| <0.0001 (trend) | ||
| Bacteria in sputum at enrolment | ||
| Burkholderia cepacia complex (BC) | 0.39 (0.24 to 0.64) | 0.0002 |
| Pseudomonas aeruginosa (PA) | 0.68 (0.43 to 1.08) | 0.1 |
| Both BC and PA | 0.29 (0.14 to 0.59) | 0.0008 |
| Other | – | – |
| New bacterial species acquired in sputum during study | 1.03 (0.82 to 1.28) | 0.82 |
| Medications used chronically | ||
| Inhaled corticosteroid | 1.13 (0.86 to 1.48) | 0.39 |
| DNase | 1.28 (0.92 to 1.78) | 0.14 |
| Inhaled antibiotics | 0.92 (0.69 to 1.21) | 0.54 |
| Oral antibiotics | ||
| None | – | – |
| Azithromycin | 1.04 (0.70 to 1.55) | 0.83 |
| Other | 0.89 (0.58 to 1.37) | 0.61 |
FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; BMI, body mass index.
Patients who experienced a pulmonary exacerbation within 1 year of entry to the study were compared with those who remained free of exacerbations over the first year in a logistic regression model that mutually controlled for other patient factors (table 3). Within 1 year of entering the study, 124 patients (50%) experienced a pulmonary exacerbation. Significant risk factors that predicted an increased risk of exacerbation within the first year were younger age (OR 0.93, 95% CI 0.89 to 0.97), lower FEV1 (OR 0.97, 95% CI 0.94 to 1.00), use of inhaled corticosteroids (OR 1.92, 95% CI 1.00 to 3.71), and a history of at least two pulmonary exacerbations requiring intravenous antibiotics within the previous 24 months (OR 7.74, 95% CI 2.94 to 20.39). Patients using chronic oral azithromycin were less likely to develop a pulmonary exacerbation during the first year (OR 0.28, 95% CI 0.11 to 0.72). The infecting organism was not a significant risk factor for exacerbation over 1 year.
Table 3 Multivariable logistic model of factors predicting pulmonary exacerbation during the first year of follow up.
| Patient characteristic | Odds ratio (95% CI) | p value |
|---|---|---|
| Age | 0.93 (0.89 to 0.97) | 0.002 |
| Female sex | 1.37 (0.69 to 2.72) | 0.37 |
| BMI (kg/m2) | 0.92 (0.83 to 1.01) | 0.07 |
| Lung function | ||
| FEV1 (% predicted) | 0.97 (0.94 to 1.00) | 0.05 |
| FVC (% predicted) | 1.02 (0.99 to 1.05) | 0.14 |
| CF related diabetes mellitus | 0.81 (0.36 to 1.81) | 0.61 |
| Courses of IV antibiotics for pulmonary exacerbation in past 24 months | ||
| 0 | – | – |
| 1 | 2.30 (0.99 to 5.31) | 0.05 |
| 2–3 | 7.74 (2.94 to 20.39) | <0.0001 |
| >3 | 20.81 (6.37 to 67.92) | <0.0001 |
| <0.0001 (trend) | ||
| Bacteria in sputum at enrolment | ||
| Burkholderia cepacia complex (BC) | 0.96 (0.30 to 3.09) | 0.94 |
| Pseudomonas aeruginosa (PA) | 0.79 (0.25 to 2.47) | 0.68 |
| Both BC and PA | 0.63 (0.10 to 4.00) | 0.63 |
| Other | – | – |
| New bacterial species acquired in sputum during study | 0.50 (0.13 to 1.93) | 0.32 |
| Medications used chronically | ||
| Inhaled corticosteroid | 1.92 (1.00 to 3.71) | 0.05 |
| DNase | 1.24 (0.56 to 2.76) | 0.59 |
| Inhaled antibiotics | 1.46 (0.74 to 2.89) | 0.28 |
| Oral antibiotics | ||
| None | – | – |
| Azithromycin | 0.28 (0.11 to 0.72) | 0.01 |
| Other | 0.87 (0.31 to 2.46) | 0.79 |
FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; BMI, body mass index.
Discussion
This is the first published study to comprehensively examine patient related risk factors for CF pulmonary exacerbations. One previous retrospective study found that patients with lower socioeconomic status were more likely to require treatment for pulmonary exacerbation;11 however, the authors did not examine other patient characteristics such as age, sex, lung function, previous CF complications, or medication use, all of which may affect the rate of pulmonary exacerbation.
Our study suggests that female patients with CF are at greater risk of pulmonary exacerbation than their male counterparts. Although no other published studies have looked at sex as a predictor of pulmonary exacerbation, many studies in CF patients have identified female sex as a significant risk factor for mortality.9,13,14,15 The fact that female patients with CF may be more predisposed to exacerbations may help explain why they also have shorter median life expectancies, since exacerbations are known to be associated with premature mortality.
The impact of patient age on the risk of death or pulmonary exacerbation has been a matter of debate in the CF literature. Kerem et al14 studied 673 Canadian children and found an increased risk of death in younger patients after controlling for other patient factors. A clinical trial of rhDNase for CF found that patients aged 17–23 years had a higher incidence of pulmonary exacerbations regardless of treatment group assignment.16 The data from our study similarly suggest that younger age is associated with an increased risk of pulmonary exacerbation after controlling for other factors. Patients who are more severely affected by CF may have earlier more frequent pulmonary exacerbations and earlier death, resulting in a survivor effect in which patients with phenotypically milder forms of CF may live longer and be at less risk for severe exacerbations.
Impaired lung function has been repeatedly shown to be the best predictor of death in patients with CF.9,13,14 Our data suggest that CF patients with lower baseline FEV1 (% predicted) are also more likely to experience severe pulmonary exacerbations. This is not unexpected since patients with lower baseline lung function would be less likely to tolerate acute viral or bacterial infections that can precipitate pulmonary exacerbations.
Our prospective study also showed that a history of recent pulmonary exacerbation was associated with future pulmonary exacerbation. This finding, although perhaps intuitive, has not been documented in the literature. A logistic regression model by Liou et al9 showed that a higher annual number of pulmonary exacerbations predicted worse survival. However, a similar study by Ellaffi et al17 showed that pulmonary exacerbation did not affect disease progression in patients who survived to 1 year after severe pulmonary exacerbation. The results of our study clearly suggest that a previous history of exacerbations was highly predictive of future exacerbations. Clinical investigators need to be aware of this finding when they design clinical trials with exacerbation as an end point.
Previous clinical trials have shown that CF patients treated with inhaled tobramycin18 and DNase16 were less likely to require intravenous antibiotics for pulmonary exacerbation. In our study, patients taking inhaled tobramycin or DNase had a slightly higher risk of pulmonary exacerbation in the univariate analysis, but this effect was not significant when other patient factors were controlled for in multivariate models. The apparent association from the univariate analysis may reflect confounding by indication—that is, the use of these inhaled medications may be a marker for patients with more severe baseline disease who are on near maximal treatment and who may be more likely to experience pulmonary exacerbations independent of medication use. Some degree of confounding by indication is likely because the use of DNase and tobramycin was not associated with an increased odds ratio of exacerbation in multivariate models which controlled for other risk factors associated with disease severity, such as lung function and sex.
In contrast, the evidence for use of inhaled corticosteroids in the management of CF is unclear.19 Although inflammation plays a major role in the pathophysiology of CF lung disease, studies have not been done to suggest that inhaled steroids exert any clinically relevant anti‐inflammatory effects in patients with CF. In fact, a recent study suggests that withdrawal of inhaled corticosteroids in patients with CF does no harm, raising further questions about the role of this medication.20 In our 1 year logistic model, inhaled corticosteroid use was associated with a higher risk of pulmonary exacerbation, even when additional factors such as age, sex, and lung function were controlled for. While interesting, this association will require further study to determine if there is a potential causal relationship. Again, it is still possible that this association may simply reflect confounding by indication.
The effect of chronic oral azithromycin in CF patients infected with P aeruginosa was described by Saiman et al21 who found that patients taking this medication were at a lower risk of pulmonary exacerbation. Wolter et al22 also observed fewer pulmonary exacerbations in CF patients taking regular oral azithromycin in a study group in which 83% of patients were infected with P aeruginosa. Our study of patients infected with multidrug resistant bacteria including (but not limited to) P aeruginosa also showed an association between chronic azithromycin use and a lower risk of pulmonary exacerbation at 1 year, after controlling for other patient factors in a logistic regression model. This effect is particularly striking given that the use of other commonly prescribed medications (such as tobramycin, DNase, corticosteroids) was associated with, at best, no effect and, at worst, an increased risk of pulmonary exacerbation in the CF patients studied. The results of this study suggest that further research on the use of azithromycin in CF patients infected with organisms other than P aeruginosa is indicated.
A potential limitation of our study is that we only enrolled CF patients infected with multidrug resistant bacteria. Patients with multi‐resistant organisms may be very different from those infected with pan‐sensitive P aeruginosa and those not infected with P aeruginosa. A recent study by Lechtzin et al has suggested that 25–45% of adults with CF have multi‐resistant infections, and that these patients have accelerated rates of lung function decline compared with patients with non‐resistant strains.23 CF patients with multi‐resistant infections provide a good model for patients with severe disease, but this study would need to be repeated in patients with milder disease to see if the same associations are present.
A second potential limitation of our study is that we enrolled mostly adult CF patients, along with a small number of adolescent patients. Our study is not generalisable to children with CF and would need to be repeated in a paediatric population to see if the same associations hold true.
Pulmonary exacerbations are associated with poor quality of life, declines in cognitive status, increased healthcare costs, and increased patient mortality.8,9 Clearly, studies of factors that either predispose or protect CF patients from pulmonary exacerbation have the potential to significantly affect both patient outcomes and healthcare system outcomes. The results of our prospective study suggest that age, sex, lung function, history of previous exacerbations, and use of inhaled corticosteroids are important factors that are associated with pulmonary exacerbations in CF patients. Knowledge of these risk factors would allow for early identification and closer monitoring of patients with high risk profiles. Close monitoring of high risk patients and early identification of worsening pulmonary symptoms might allow timely therapeutic interventions with oral antibiotics and/or physiotherapy to prevent severe exacerbations.
Acknowledgements
The authors are indebted to Dominique Bleskie for help with the manuscript preparation; study coordinators Pat Hazell, Francis Gosse, Kathleen Devecseri, Lesley Gaskin, Holly Truchan, Vanja Dijak, Patrice Kean, Carol Verwolf, Jennifer Jackson Lee, Josette Salgado, Joan Tabak, Manon Roussin, Nadia Beaudoin, Rosamund Hennessey, Carmel Moriarty and Elizabeth Sheppard; and study microbiologists Wendy Ferris, Frank Chan, Randall Wilson, Melissa St Denis, Veronica Yozghatlian, Barbara Rose and Colin Harbour.
Abbreviations
BMI - body mass index
CF - cystic fibrosis
FEV1 - forced expiratory volume in 1 second
FVC - forced vital capacity
Footnotes
Supported by grants from The Canadian Institutes of Health Research, The Australian Cystic Fibrosis Research Trust, Astra‐Zeneca Canada Inc (unrestricted research grant), and The Canadian Cystic Fibrosis Foundation.
Competing interests: none declared.
References
- 1.Gibson R L, Burns J L, Ramsey B W. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med 2003168918–951. [DOI] [PubMed] [Google Scholar]
- 2.Cystic Fibrosis Foundation Patient Registry 2001 Annual Data Report. Bethesda, MD: Cystic Fibrosis Foundation, 2002
- 3.Cystic Fibrosis Foundation Microbiology and infectious disease in cystic fibrosis. V [Section 1]. Bethesda, MD: Cystic Fibrosis Foundation, 19941–26.
- 4.Rosenfeld M, Emerson J, Williams‐Warren J.et al Defining a pulmonary exacerbation in cystic fibrosis. J Pediatr 2001139359–365. [DOI] [PubMed] [Google Scholar]
- 5.Britto M T, Kotagal U R, Hornung R W.et al Impact of recent pulmonary exacerbations on quality of life in patients with cystic fibrosis. Chest 200212164–72. [DOI] [PubMed] [Google Scholar]
- 6.Johnson J A, Connolly M A, Jacobs P.et al Cost of care for individuals with cystic fibrosis: a regression approach to determining the impact of recombinant human DNase. Pharmacotherapy 1999191159–1166. [DOI] [PubMed] [Google Scholar]
- 7.Lieu T A, Ray G T, Farmer G.et al The cost of medical care for patients with cystic fibrosis in a health maintenance organization. Pediatrics 1999103e72. [DOI] [PubMed] [Google Scholar]
- 8.Dobbin C J, Bartlett D, Melehan K.et al The effect of infective exacerbations on sleep and neurobehavioral function in cystic fibrosis. Am J Respir Crit Care Med 200517299–104. [DOI] [PubMed] [Google Scholar]
- 9.Liou T G, Adler F R, FitzSimmons S C.et al Predictive 5‐year survivorship model of cystic fibrosis. Am J Epidemiol 2001153345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayer‐Hamblett N, Rosenfeld M, Emerson J.et al Developing cystic fibrosis lung transplant referral criteria using predictors of 2‐year mortality. Am J Respir Crit Care Med 20021661550–1555. [DOI] [PubMed] [Google Scholar]
- 11.Schechter M S, Shelton B J, Margolis P A.et al The association of socioeconomic status with outcomes in cystic fibrosis patients in the United States. Am J Respir Crit Care Med 20011631331–1337. [DOI] [PubMed] [Google Scholar]
- 12.Aaron S D, Vandemheen K, Ferris W.et al Combination antibiotic susceptibility testing to treat exacerbations of cystic fibrosis associated with multiresistant bacteria: a randomised, double‐blind, controlled clinical trial. Lancet 2005366463–471. [DOI] [PubMed] [Google Scholar]
- 13.Corey M, Farewell V. Determinants of mortality from cystic fibrosis in Canada, 1970–1989. Am J Epidemiol 19961431007–1017. [DOI] [PubMed] [Google Scholar]
- 14.Kerem E, Reisman J, Corey M.et al Prediction of mortality in patients with cystic fibrosis. N Engl J Med 19923261187–1191. [DOI] [PubMed] [Google Scholar]
- 15.Rosenfeld M, Davis R, FitzSimmons S.et al Gender gap in cystic fibrosis mortality. Am J Epidemiol 1997145794–803. [DOI] [PubMed] [Google Scholar]
- 16.Fuchs H J, Borowitz D S, Christiansen D H.et al Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. The Pulmozyme Study Group. N Engl J Med 1994331637–642. [DOI] [PubMed] [Google Scholar]
- 17.Ellafi M, Vinsonneau C, Coste J.et al One‐year outcome after severe pulmonary exacerbation in adults with cystic fibrosis. Am J Respir Crit Care Med 2005171158–164. [DOI] [PubMed] [Google Scholar]
- 18.Ramsey B W, Pepe M S, Quan J M.et al Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. N Engl J Med 199934023–30. [DOI] [PubMed] [Google Scholar]
- 19.Dezateux C, Walters S, Balfour‐Lynn I. Inhaled corticosteroids for cystic fibrosis. Cochrane Database Syst Rev 20002CD001915. [DOI] [PubMed] [Google Scholar]
- 20.Balfour‐Lynn I M, Lees B, Hall P.et al Multicentre randomized controlled trial of withdrawal of inhaled corticosteroids in cystic fibrosis. Am J Respir Crit Care Med 20061731356–1362. [DOI] [PubMed] [Google Scholar]
- 21.Saiman L, Marshall B C, Mayer‐Hamblett N.et al Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA 20032901749–1756. [DOI] [PubMed] [Google Scholar]
- 22.Wolter J, Seeney S, Bell S.et al Effect of long term treatment with azithromycin on disease parameters in cystic fibrosis: a randomised trial. Thorax 200257212–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lechtzin N, John M, Irizarry R.et al Outcomes of adults with cystic fibrosis infected with antibiotic‐resistant Pseudomonas aeruginosa. Respiration 20067327–33. [DOI] [PubMed] [Google Scholar]