Abstract
We have found that the cyclic AMP (cAMP) receptor protein (CRP)-cAMP regulatory complex in Escherichia coli is subject to osmoregulation at the level of crp gene expression. This osmoregulation was lost in a cya mutant strain but could be restored by external addition of cAMP, suggesting that the intracellular level of cAMP is a key factor in the osmoregulation of CRP. The ability of the cell to maintain optimal CRP activity was essential for the growth and survival of the bacteria under low-osmolarity conditions as shown by studies with different crp mutant alleles. A suppressor mutant with a novel amino acid substitution (L124R) in CRP showed restored growth at low osmolarity. CRP(L124R) was not activated by cAMP and was shown to be dominant negative over the wild type. Our findings suggest that the fine-tuning of the CRP activity may be critical for bacterial viability and adaptability to changing osmotic conditions.
Bacterial cells may be subject to drastic changes of the extracellular osmolarity in their natural environments. The adaptation to osmotic alterations involves osmoregulatory responses with both short- and long-term effects, and during this adaptive process the bacteria undergo physiological and structural changes. Such changes can affect the composition of the cell surface, the cytoplasmic composition, and the nucleoid organization (49). As shown in Escherichia coli, there is a specific induction of expression of several genes during the course of the osmotic response (15). The osmoregulatory response presumably involves a complex network of mechanisms, which encompasses several regulons. The cyclic AMP (cAMP) receptor protein (CRP) is one of the best-known global regulatory proteins in E. coli. cAMP, acting through CRP, has been proposed to be a sensory signal in global gene control in E. coli (29). cAMP was initially described in bacteria as a positive effector in the catabolite-repressible operons (44). The regulatory role of the CRP-cAMP complex has been extensively studied in the catabolite repression system, in which the presence of glucose represses the expression of enzymes involved in the metabolism of other carbon sources. The uptake of glucose decreases the levels of both CRP and its effector cAMP (21). Variations in the level of CRP-cAMP contribute to the fine regulation of several operons whose control is necessary for the metabolism of non-phosphotransferase system carbon sources (22). The CRP-cAMP complex regulates several hundred genes, whereas only a small proportion of them are involved in catabolism (9, 29). This complex acts as a transcriptional activator or repressor by binding to specific DNA sites within or near the promoter of different genes. Therefore, it is likely that CRP-cAMP is involved in multiple regulatory networks.
In a previous study of the effect of osmolarity on the protein expression in E. coli, we showed that the expression of some proteins regulated by CRP genes was also dependent on the osmolarity of the medium (5). This prompted us to further investigate the relation between the CRP regulatory system and gene regulation in response to the osmolarity of the medium. Here, we demonstrate that the level of CRP is influenced by the external osmolarity and that both genetic and physiological analyses show that cAMP acts as a key regulatory component in the osmoregulation of crp expression.
MATERIALS AND METHODS
Bacterial strains.
The E. coli strain MC4100 (12) and its cya derivative RH76 (20), MG1655 (19), MC1000 (39) and its fis derivative RJ1617 (26), BSN26 and its hns derivative BSN27 (24), M182 (13) and its crp derivative M182Δcrp (Δcrp39) (11), the crp mutant Tcr strain BRE2055 (Δcrp96 zhd-732::Tn10) (10), the lacI mutant strain CSH140 (32), the relA spoT mutant strain CF1693 (50), the strain RLG4994 (MG1655 lacZ lacI pyrE+ containing lacUV5 [−59 to +36]-lacZ) (6), and the strain 5K (17) have been described elsewhere.
Growth conditions.
LB (7) or the synthetic medium rich morpholinepropanesulfonic acid (MOPS) (35) was used for the growth of strains. When rich MOPS was used, the carbon source was glucose (0.4%) unless otherwise noted. As described by Neidhardt et al. (35), the rich MOPS medium contains 50 mM of NaCl. In this report, for our purposes, rich MOPS was prepared with no NaCl (designated 0 M NaCl rich MOPS or low-osmolarity conditions). NaCl was added as indicated in the text. When present, isopropyl-β-d-thiogalactopyranoside (IPTG) and cAMP were added at 0.5 mM and 5 mM, respectively. Cultures were incubated at 37°C with vigorous shaking. Growth was monitored by measuring Klett units on a Klett-Summerson colorimeter, where 50 Klett units correspond approximately to an A600 of 0.4 (mid-log phase).
Genetic techniques.
Generalized transduction mediated by P1 was performed as described previously (48). The strain BEU742 (Δcrp39 Tcr) was derived from M182Δcrp (Δcrp39), which was transduced with P1 grown on BRE2055 (Δcrp96 zhd-732::Tn10), and the transductants were selected for the ability to grow on tetracycline-containing plates. The clones carrying the Δcrp39 allele were identified using PCR amplification with the primers CRP1 and CRP3 (see below). Strain CBP2 (Δcrp39 Tcr) was derived from RH76, which was transduced with P1 grown on BEU742. Strains CBP3 and CBP4 were derived from RH76 and CBP1, respectively, by the introduction of an Flac+ plasmid using strain BEU227 as a donor in conjugation. CBP5 (Δcrp39 Tcr) and CBP6 (crpL124R Tcr) were derived from MG1655 that was transduced with P1 grown on BEU742 and CBP10, respectively. The strain CBP10 (crpL124R Tcr) was derived from CBP1 that was transduced with P1 grown on BEU742. The genotype of the strain CBP10 was confirmed by sequencing of the crp gene. The crp genes from the strains RH76 and CBP1 were PCR amplified and sequenced using the primers CRP1 (5′-CAGTCGCGCTTGCATTTTTGC-3′) and CRP3 (5′-CCAGGTAACGCGCCACTCCG-3′). Both PCR-amplified crp alleles were cloned using the cloning vector pGEM-T (Promega), resulting in the plasmids pCBP75 and pCB76. Both SalI-SphI fragments, containing the crp alleles, were cloned in the vector pLG338 (40), resulting in the plasmids pCBP68 and pCBP69. Plasmid pHA7* (41), carrying the cAMP-independent crp allele (G141D) in pBR322, was used. By using a gene replacement method based on the pKO3 vector (31), the MG1655Δcya strain was obtained (MG1655 carrying a cya::kan mutation [C. Balsalobre et al., unpublished data]). By P1 transduction, the cya allele of MG1655Δcya was transferred to the strain MC4100, resulting in the strain CBP9.
Measurement of β-galactosidase activity.
The β-galactosidase assay was performed as previously described (32).
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblot analysis.
For determination of the CRP content in cells, samples were subjected to immunoblot analysis as described previously (23). Polyclonal rabbit antisera raised against CRP (22) or enzyme IIA (28) were used as the primary antibodies, followed by a horseradish peroxidase-conjugated antibody. The membrane was developed with ECL+Plus reagent (Amersham Pharmacia Biotech) according to the method specified by the manufacturer and scanned for quantification using the STORM system and the ImageQuant program (Molecular Dynamics). The identity of the CRP-specific band was confirmed by tests with extracts from otherwise isogenic crp+ and Δcrp strains.
In vivo protein stability experiment.
Protein stability was monitored by immunoblot analysis after the protein synthesis was inhibited by the addition of spectinomycin (100 μg · ml−1) to bacterial cultures grown to an optical density at 600 nm (OD600) of 0.4. Samples to be analyzed by Western blotting were removed at indicated times.
RNA analysis.
At mid-log phase, RNA was extracted from bacterial cultures by the hot-phenol method (47) and 20 μg of each RNA sample was loaded onto the gel. RNA blotting, hybridization, and washing of the membrane were performed essentially as described previously (4). The probe used for analyzing the expression of the crp mRNA by Northern hybridization was the [γ-32P]ATP kinase-labeled CAP-1 oligonucleotide: 5′-GGCCATGAGACAAGAACCATTCGAGAGTCGGGTCTGTTTGCGGTTTGCCAAGCACC-3′. For the detection of the crp transcription start points by primer extension, the [γ-32P]ATP kinase-labeled CAP-3 oligonucleotide (5′-CTGTCTCTGGATTGCCGAAATATG-3′) was used. The primer extension reactions were performed as previously described (38).
Determination of cAMP concentration.
Intracellular cAMP levels were determined using the cAMP enzyme immunoassay system (Amersham Pharmacia Biotech). One-milliliter culture samples were taken at 50 Klett units and then centrifuged at 12,000 × g for 5 min at 4°C. The pellets were resuspended in 270 μl assay buffer. For total cAMP determination 20-μl samples of the same culture samples were taken and 250 μl of assay buffer was added. In both cases, the samples were heated for 5 min at 100°C. Thirty microliters assay buffer containing 2.5% dodecyltrimethylammonium bromide was added, and the samples were centrifuged at 12,000 × g for 5 min at 4°C. The cAMP level in the supernatant was determined in duplicate using the method specified by the manufacturer. Average values of three independent experiments were calculated. Estimations of molar concentrations of intracellular cAMP have been calculated using the following assumptions earlier described (22): an OD600 of 1.4 corresponds to 109 cells/ml, and the volume of the cell is 2 × 10−12 ml.
Protein cross-linking in vivo.
To determine the formation of CRP dimers in vivo, protein cross-linking was performed basically as described elsewhere (14). Briefly, glutaraldehyde at the final concentrations of 2.5, 5, and 10 mM was added to a stationary-phase culture of the strains MC4100/pCBP68, MC4100/pCBP69, MC4100(Δcrp39)/pCBP68, and MC4100(Δcrp39)/pCBP69 in LB broth. The cultures were incubated for another 10 min at 37°C in a shaking water bath. The treated cells were collected by centrifugation at 12,000 rpm for 10 min at 4°C, resuspended in 20 μl of sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer, and analyzed by immunoblotting.
RESULTS
Osmolarity modulates the expression of CRP.
In previous studies, we observed that the expression of some proteins in E. coli was influenced by the osmolarity of the medium (5). Since the effect included some proteins involved in the catabolite repression system, we decided to investigate if the osmolarity also would influence the levels of CRP. E. coli strain MC4100 was grown in rich MOPS medium to mid-log phase, either in the absence of NaCl (low-osmolarity condition) or in the presence of 400 mM NaCl (high-osmolarity condition). The amount of CRP was detected by Western blot analysis, and the results suggest that the level of CRP indeed was affected by the osmolarity of the medium (Fig. 1A). In the high-osmolarity growth condition, the level of CRP was clearly lower than in the low-osmolarity condition. To determine if the change in external osmolarity might alter the protein turnover, the CRP protein stability was studied at low and high osmolarity. The de novo protein synthesis was inhibited by the addition of spectinomycin (25), and samples were taken for analysis of CRP levels over 4 hours. The levels of CRP remained stable in both osmotic conditions (data not shown). To confirm that the effect observed on the expression of CRP was truly caused by altered osmolarity, the level of CRP was monitored in the strain MC4100 growing in rich MOPS medium either in the presence or in the absence of 30% sucrose (Fig. 1B). Similarly to the above-mentioned results, a lower level of CRP (2.5-fold reduction) was observed when osmolarity was increased by the presence of sucrose. This result supported the conclusion that the modulation of the levels of CRP was due to osmolarity and not to NaCl per se. When osmolarity was increased using a membrane-permeant compound, rich MOPS 0 M NaCl containing 0.7 M of glycerol or not, no clear difference was observed in the levels of CRP (Fig. 1B). Throughout the rest of this study, alterations of NaCl concentrations were used to modify the osmolarity of the medium.
FIG. 1.
Effect of osmolarity on CRP expression and crp gene transcription. A. Immunodetection of CRP in cultures of E. coli strain MC4100 growing in the absence or presence of 400 mM NaCl. As a control, immunodetection of EIIA is shown. B. The level of CRP was monitored by Western blotting in strain MC4100 growing in rich MOPS without NaCl in the presence (+) and in the absence (−) of either 30% sucrose (upper panel) or 0.7 M glycerol (lower panel). C. RNA samples from cultures of E. coli strain MC4100 grown in the absence or presence of 400 mM NaCl were analyzed by Northern blotting. As a control, ethidium bromide-stained bands corresponding to the 16S and 23S rRNAs are shown.
In order to determine if the difference in the levels of CRP was due to regulation at the transcriptional level, RNA samples were prepared from strain MC4100 grown in either low- or high-osmolarity conditions. The Northern blot analysis indicated that the crp gene was transcribed at higher levels under the low-osmolarity condition (Fig. 1C), in agreement with the differences detected at the protein level (Fig. 1A). The level of crp mRNA was two to three times lower under the high-osmolarity condition. Primer extension analysis of the RNA samples confirmed the results of the Northern blot analysis (data not shown). Two extension products were detected: one corresponding to the major transcriptional initiation point determined by Aiba (1) and one corresponding to the additional mRNA start point detected during in vitro transcriptional analysis of crp (18). Both transcriptional starts were affected by the alteration in osmolarity.
The crp osmoregulation was also examined in the lac-positive E. coli strain MG1655. This strain was grown in rich MOPS medium, supplemented with either glucose or glycerol, as well as different concentrations of NaCl (0, 50, 150, or 400 mM NaCl). The amount of CRP was monitored, and as shown in Fig. 2A, the level of CRP decreased progressively with increasing osmolarity. Within the tested range of NaCl concentrations, the levels of CRP fell about fivefold in the presence of glucose and about twofold in the presence of glycerol. Similar results were obtained when the CRP content was analyzed in other E. coli strains growing under the same osmolarity conditions, e.g., strains MC1000, M182, and 5K (data not shown).
FIG. 2.
A. Quantitative determinations of CRP content by Western blotting analysis of strain MG1655. The cells were grown in rich MOPS supplemented with either 0.5% glycerol or 0.4% glucose and the indicated concentrations of NaCl. The relative amount of CRP was obtained by dividing each value by the value obtained from the culture growing in the presence of glycerol in the absence of NaCl (arbitrarily set to 1). Data shown are from one of two separate experiments that gave similar results. B. Effect of the external osmolarity on lacZ expression. The cells were grown in rich MOPS supplemented with either 0.5% glycerol or 0.4% glucose and the indicated concentrations of NaCl. IPTG was added to a final concentration of 0.5 mM in the cultures of strain MG1655. At mid-log phase, samples were taken for β-galactosidase activity measurements. C. Generation times and total protein content of strains MG1655 and MC4100 during exponential phase. The strains were grown in rich MOPS supplemented with 0.4% glucose and containing either 0 or 400 mM of NaCl. Total protein content was determined at mid-log phase.
In rich MOPS medium containing 50 mM NaCl, the growth rate was found to be optimal, and consequently, this medium was considered equivalent to normal-osmolarity conditions (36). In 50 mM NaCl rich MOPS cultures growing with glycerol, the level of CRP was about twofold higher than the level of CRP in cultures growing in the presence of glucose (Fig. 2A). This effect of the presence of glucose on the level of CRP was as expected and is quantitatively equivalent to the previously described effect from studies with E. coli in LB and M9 media and is a central feature of the catabolite repression (22).
The growth behavior of both strains used (MC4100 and MG1655) in cultures containing glucose at low and high osmolarity is summarized in Fig. 2C. The results clearly indicate that the change in the levels of CRP was not simply due to alterations in the growth rate under the experimental conditions used. Furthermore, to assess if the optical density faithfully reflected the cell mass under the different growth conditions, the protein content was determined in cultures of both strains. There was no significant difference in the ratio of μg protein/OD600 between cultures grown at low and high osmolarity (Fig. 2C).
Two proteins that have been reported to be involved in the transcriptional regulation of crp are FIS (18) and H-NS (23). Moreover, we have shown that the expression of crp is stringently regulated (23). To determine whether the osmoregulation of crp was mediated by FIS, H-NS, or (p)ppGpp, the levels of CRP were examined at low and high osmolarity in a fis mutant strain (RJ1617), an hns mutant strain (BSN27), and a ppGpp0 strain (CF1693) and their otherwise isogenic wild-type (wt) strains (MC1000, BSN26, and MG1655, respectively). For the growth of CF1693 (relA251::Km spoT207::Cm) and the MG1655 control, we used the medium containing 50 mM of NaCl as the low-osmolarity condition since the strain CF1693 had an impaired ability to grow in rich MOPS without salt. We found that the osmotically induced change in the CRP content occurred also in the different mutant strains to the same extent as in their isogenic wild-type strains (data not shown). Altogether, we concluded that the osmoregulation of CRP was independent of H-NS, FIS, and (p)ppGpp.
Osmoregulation of in vivo CRP activity.
Bearing in mind the importance of CRP for gene regulation, we were interested to determine if the difference in CRP levels at different osmolarities might affect the expression of CRP-regulated genes, and the expression of the lacZ gene was used here as a model system. The effect of osmolarity on expression of the lacZ gene was measured using the lac-positive strain MG1655 growing in the presence of IPTG (0.5 mM). Cultures were grown in rich MOPS supplemented with either glucose or glycerol, as well as different concentrations of NaCl, and β-galactosidase activity was determined at mid-log phase. The results obtained (Fig. 2B) indicated that there is an unexpected inverse relation between in vivo CRP activity and the CRP level (compare Fig. 2A and 2B). In other words, a higher level of lacZ expression was detected in conditions when the concentration of CRP was lower. Hence, the level of β-galactosidase activity rose with increasing osmolarity. The pattern of lacZ expression versus osmolarity was observed when using both glycerol-containing and glucose-containing rich MOPS (Fig. 2B). The results were consistent with the earlier observed effect of the external osmolarity on the expression of the lac operon in cultures growing in LB (5).
Considering that the β-galactosidase results gave an estimation of the level of functional CRP-cAMP complex, the seemingly paradoxical inverse relation could be explained if the levels of cAMP were lower under the low-osmolarity conditions and higher in the high-osmolarity conditions. If that was the case, addition of cAMP to the cultures should increase the β-galactosidase activity and result in higher values under conditions where the level of CRP is higher (i.e., low-osmolarity conditions). To test this hypothesis, we determined the β-galactosidase activity in cultures of the strain MG1655 grown in rich MOPS containing glucose and IPTG at different osmolarities after addition of 5 mM cAMP, an amount known to be sufficient to restore the lacZ expression in an adenylate cyclase-deficient strain (22). As seen in Fig. 3A, the results confirmed our hypothesis. Although the addition of cAMP increased lacZ levels at both osmolarities, the largest increase was observed at the lowest osmolarity (a condition where the level of CRP is higher). The β-galactosidase activity remained constant throughout growth in cultures grown in the absence of cAMP. To rule out the possibility that there is a differential uptake of IPTG under different osmolarity conditions, similar experiments were performed using the lacI mutant strain CSH140. Similar results as when strain MG1655 was used in the presence of IPTG were obtained (Fig. 3B). Furthermore, the expression of malT and lamB, representing two other CRP-dependent operons, was monitored by using chromosomal lacZ gene fusion constructs. Similar results as for the lac operon were found when changing the osmolarity of the media as well as when cAMP was added (data not shown). In order to ensure that the above effect observed was directly due to a different level of functional CRP-cAMP, experiments with a CRP-independent lac promoter (lacUV5) were performed. Strain RLG4998 carrying a lambda lysogen containing lacUV5 was used. The strain was grown in the same culture medium as in Fig. 3A (see above), but the cultures were maintained at 30°C since the strain RLG4998 is temperature sensitive (6). As seen in Fig. 3C, the expression from the lacUV5 promoter did not respond to the addition of cAMP. Control experiments were performed to show that the lac operon of MG1655 responded to the osmolarity and to the addition of cAMP in the same way at 30°C as it did at 37°C (Fig. 3D).
FIG. 3.
A. Effect of the addition of cAMP on lacZ expression. The MG1655 strain was grown in rich MOPS supplemented with glucose (0.4%) and containing 0 (squares) or 400 (triangles) mM NaCl. IPTG was added to a final concentration of 0.5 mM in the cultures of strain MG1655. At an OD600 of about 0.15, cAMP (5 mM) was added and samples were taken for the determination of the β-galactosidase activity at different time points. The different values were divided by the values before the addition of cAMP (arbitrarily set to 1.0). The relative value of 1 corresponds to 471 Miller units in the absence of NaCl and to 1,011 Miller units in cultures with 400 mM of NaCl. B. Effect of addition of cAMP on lacZ expression of the lacI strain CSH140. The experiment was performed as described for panel A. The relative value of 1 corresponds to 1,050 Miller units in the absence of NaCl and 3,233 Miller units in cultures with 400 mM of NaCl. C and D. Effect of the addition of cAMP on lacUV5 and lacZ expression. Strains RLG4998 (C) and MG1655 (D) were grown in rich MOPS supplemented with glucose (0.4%) and containing 0 (squares) or 400 (triangles) mM NaCl at 30°C. IPTG was added to a final concentration of 0.5 mM in the cultures of strain MG1655. At an OD600 of about 0.15, cAMP (5 mM) was added and samples were taken for the determination of the β-galactosidase activity at different time points. The different values were divided by the values before the addition of cAMP (arbitrarily set to 1.0). For strain RLG4998, the relative value of 1 corresponds to 1,559 Miller units in the absence of NaCl and to 1,278 Miller units in cultures with 400 mM of NaCl. For strain MG1655, the relative value of 1 corresponds to 773 Miller units in the absence of NaCl and to 995 Miller units in cultures with 400 mM of NaCl. E. Effect of the osmolarity on the cAMP levels in E. coli cultures growing in rich MOPS supplemented with glucose in the absence or presence of 400 mM NaCl at 37°C. The results represent the averages ± standard errors of three independent experiments.
Taken together, these results suggested that the levels of cAMP differed in cells grown at different osmolarities. Therefore, the levels of total and intracellular cAMP were measured in cultures of the strain MG1655 growing at low and high osmolarity (Fig. 3E). There was a significant variation in the levels of total cAMP when comparing cultures grown at high and those at low osmolarity. The level of total cAMP was about 2.5-fold higher at high osmolarity. An even larger difference was found when the levels of intracellular cAMP were estimated, with more than an eightfold increase in the level of cAMP at high osmolarity compared to low-osmolarity conditions.
The osmoregulation of CRP is cAMP dependent.
The results described above led us to ask whether the level of cAMP could be directly responsible for the crp osmoregulation. If so, we would expect that the osmoregulation of crp would be abolished in a cya mutant strain and that the osmoregulation should be restored by external addition of cAMP. To test these predictions, the levels of CRP in the strains MC4100 (cya+) and RH76 (cya) were monitored at low and high osmolarity in the presence or absence of cAMP. The results confirmed the above hypothesis (Fig. 4). The crp osmoregulation was lost in the RH76 strain since the level of CRP in strain RH76 (cya) grown at high osmolarity in the absence of cAMP was similar to the level of CRP in the same strain at low osmolarity. Furthermore, the level of CRP in the cya strain grown at high osmolarity decreased, after the addition of external cAMP, to a level similar to that of the wild type (MC4100) grown at high osmolarity. To rule out that the variations in the intracellular level of CRP observed (Fig. 4) were due to a growth effect, the generation time of the strains MC4100 and RH76 growing under the conditions used was calculated (Fig. 4, bottom panel). We concluded that the growth behavior per se could not explain the above-described variations. Moreover, the growth kinetics of the strains MC4100 and RH76 in the different conditions are consistent with the differences in the CRP-cAMP activity at high and low osmolarity (Fig. 2). Hence, at low osmolarity (when a low CRP-cAMP acivity was detected) the lack of cAMP (strain RH76) did not cause a severe effect in growth (generation times of 35 and 41 min for MC4100 and RH76, respectively). However, the addition of cAMP in low-osmolarity conditions affected negatively the growth rate of MC4100 and had a very severe effect in the RH76 strain (no growth). On the other hand, the growth of RH76 (CRP-cAMP defective) at high osmolarity was severely affected and partially restored by addition of cAMP, consistent with the fact that under such conditions higher CRP-cAMP activity was detected. Furthermore, no detrimental effect was observed either from the osmolarity or from the addition of cAMP at high osmolarity for the proficient CRP strain MC4100. At an intermediate salt concentration (rich MOPS containing 50 mM of NaCl), strain RH76 showed an intermediate growth rate (generation time of 45.6 min), but the addition of cAMP caused a significant drop in the growth rate (generation time of 82.3 min).
FIG. 4.
Osmoregulation of CRP is lost in a strain lacking cya. Strains MC4100 (wt) and RH76 (cya) were grown in rich MOPS medium containing either 0 or 400 mM NaCl without or with cAMP (5 mM). At mid-log phase, the cells were harvested and CRP was detected by Western blotting analysis (bottom panel). Quantitative determination of CRP was performed (top panel); the results shown are the averages of three experiments. The relative amount of CRP was obtained by dividing each value with the value for the MC4100 strain growing at low osmolarity without cAMP (arbitrarily set to 1.0). The asterisk shows that no CRP quantification was performed for strain RH76 cultured at low osmolarity with added cAMP since no growth was detected (see text for further details).
Under the conditions that we used, we could not detect an induction of crp in the cya strain as has been described previously in cultures grown in LB (21). However, control experiments performed in LB medium showed that higher expression of CRP in the cya mutant strain was also detected in RH76 compared to MC4100 (data not shown). These results rule out possible abnormalities with the strains used.
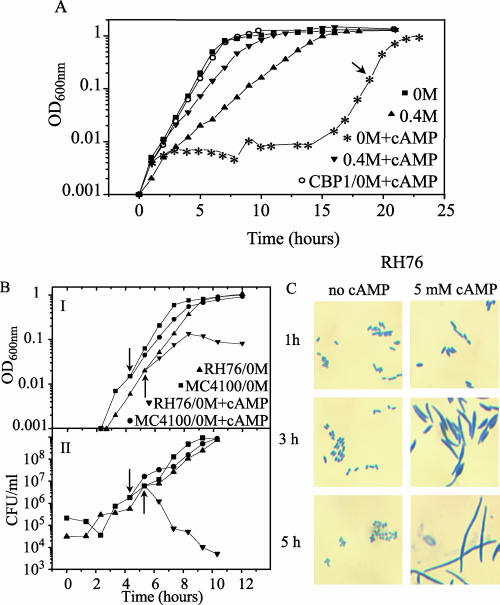
Interestingly, the strain RH76 did not show any growth initially at low osmolarity after the addition of external cAMP. It was therefore not possible to obtain comparable data on the level of CRP expression in that case. Only after very prolonged incubation (15 h) was growth detected and did the culture reach normal cell density (Fig. 5A). This result suggested the selection of some spontaneous compensatory mutation(s). Therefore, the growing cells were isolated and, after single-cell purification, a randomly selected isolate designated CBP1 was used for further studies. The CBP1 isolate was able to grow without any lag period in the low-osmolarity medium containing cAMP (Fig. 5A), demonstrating that it indeed was a spontaneous mutant with restored growth capabilities.
FIG. 5.
Growth-inhibitory effect of the addition of external cAMP and isolation of a suppressor mutant with restored growth ability. A. Growth curves of strain RH76 (cya) growing in rich MOPS supplemented with glucose either in the absence or in the presence of 0.4 M of NaCl and/or 5 mM cAMP. The arrow indicates the time point from which CBP1 was isolated. B. Strains MC4100 and RH76 (cya) were grown in rich MOPS supplemented with glucose in the absence of NaCl. At a cell density of about an OD600 of 0.15 (indicated by arrows), cAMP was added to a final concentration of 5 mM. The growth was monitored by determination of the OD600 (I) as well as the number of viable bacteria (CFU/ml) (II). C. Cell morphology of strain RH76. The samples were taken 1, 3, and 5 h after the cultures were divided into a portion without cAMP and a portion with cAMP.
To confirm that the growth deficiency shown by strain RH76 at low osmolarity was due to the externally added cAMP, the growth of strains MC4100 and RH76 at low osmolarity was followed before and after addition of cAMP (Fig. 5B, subpanel I). The addition of cAMP to the medium caused an immediate and irreversible decrease of the viability of the cya mutant strain RH76. Two hours after the addition of cAMP, the number of viable cells was reduced to about 1% (Fig. 5B, subpanel II). The morphology of the bacterial cells was observed, and within 3 hours of exposure to cAMP, the cells of strain RH76 appeared as filaments and fusiform cells (Fig. 5C). These results suggest that both cell division and viability were affected. The cell morphology of the parental strain, MC4100, did not suffer any of those changes after addition of cAMP (data not shown). To ensure that no compensatory mutations in the cya strain gave the above phenotype, a new cya deletion mutant strain derived from MC4100 (CBP9; see Materials and Methods) was constructed. As for RH76, CBP9 did not grow at low osmolarity when cAMP was added, clearly suggesting that the above phenotype is caused by the lack of the cya gene.
Effect of mutant crp alleles on growth and osmoregulation.
We reasoned that characterization of the suppressor mutant isolated from strain RH76, displaying an ability to grow at low osmolarity in the presence of additional cAMP, would provide genetic evidence towards further understanding of the key role of cAMP in the osmoadaptation of the bacteria. The entire crp locus was sequenced, and a single point mutation was found in the suppressor strain CBP1, compared with the sequence from the parental strain RH76. This point mutation changed the leucine codon (CTG) at position 124 to an arginine codon (CGG) (Fig. 6A).
FIG. 6.
Characterization of the mutant strain CBP1. A. CRP is shown as a horizontal bar representing amino acids 1 to 209. The cAMP binding domain and the DNA binding domain are indicated by arrows, and the α-helix domains (A to F) are shown by open boxes (3). The amino acid sequence of the region where the substitution was detected in the mutant crp(L124R) is shown at the bottom. B. Effect of the presence of different crp alleles on the growth in rich MOPS under low-osmolarity conditions in the presence or absence of cAMP (5 mM). Cultures were monitored after 7 h of incubation. +, growth; −, no growth; n.d., not determined. C. Generation time in minutes of strains RH76, CBP1, and CBP2 growing in rich MOPS under the conditions indicated. D. Western immunoblot analyses of the CRP levels in the different strains. E. β-Galactosidase activity in strains CBP3 and CBP4 growing either in LB or in rich MOPS medium in the absence of NaCl, containing IPTG, up to an OD600 of 0.25. Importantly, cAMP was then added at the indicated concentrations and β-galactosidase activity was measured after 1 hour. F. Effect of the presence of different crp alleles on the expression of the maltose regulon. The different strains were grown on maltose-MacConkey agar plates. Bacterial phenotype was scored after overnight growth on plates at 37°C.
CRP has been extensively analyzed at both the structural and the functional level, and amino acid 124 is located in the α-helix C of the cAMP binding domain (Fig. 6A). Interestingly, the adjacent Arg 123 residue is directly involved in the establishment of the interaction between cAMP and CRP (33). To determine if the amino acid substitution L124R present in crp from CBP1 was solely responsible for the restored growth phenotype observed, the crp genes from RH76 and CBP1 were cloned in the low-copy-number vector pLG338 (resulting in the plasmids pCBP68 and pCBP69, respectively) and tested in derivatives of both RH76 (cya) and CBP2 (a Δcrp derivative of RH76). As shown in Fig. 6B, the derivatives that contained the crp+ allele were not able to grow at low osmolarity in the presence of cAMP (i.e., RH76, RH76/pCBP68, RH76/pCBP69, and CBP2/pCBP68). However, the strains containing only the mutant allele (i.e., CBP1 and CBP2/pCBP69) were able to grow equally well in both the presence and the absence of cAMP. Interestingly, the strain RH76/pCBP69, containing both the wild-type and the mutant crp alleles, was not able to grow in the presence of cAMP, suggesting that the presence of wild-type CRP causes the deleterious effect on the bacterial growth. As suggested by the previous results, the inhibition of bacterial growth and survival for a cya strain under low osmolarity in the presence of cAMP might be due to a high in vivo CRP activity. To substantiate this hypothesis, the strain CBP2 (a Δcrp derivative of RH76) grew in the presence of cAMP at low osmolarity, indicating that the effects of cAMP on growth and survival were strictly dependent on CRP (Fig. 6B). The growth behavior of both crp mutant strains, CBP1 and CBP2, which carried crpL124R and Δcrp, respectively, was studied (Fig. 6C). Interestingly, compared to the RH76 strain the two mutant strains showed similar responses to the external osmolarity and to the addition of cAMP. Moreover, the effect on the bacterial growth of one cAMP-independent crp allele (crpG141D) was studied. When such a cAMP-independent crp allele was expressed in RH76, the growth at low osmolarity was strongly inhibited even in the absence of cAMP (Fig. 6B). These finding are consistent with the conclusion that the inhibition of growth was due to a high in vivo CRP activity. Interesting results, further supporting the above conclusions, were obtained when plasmid pHA7* was introduced in strains CBP1 and CBP2 (crpL124R and Δcrp, respectively). In contrast to what we observed in strain RH76, the presence of plasmid pHA7* in strains CBP1 and CBP2 did not cause any growth inhibition at low osmolarity (Fig. 6B). When such strains were tested on maltose-MacConkey agar plates (Fig. 6F), they showed a CRP-deficient phenotype, corroborating the correlation between CRP activity and growth inhibition. The results shown suggest that the crpG141D allele requires the coexpression of crp wt for a CRP-proficient phenotype independent of cAMP.
The effect of the L124R substitution on the CRP activity was also studied using the lacZ gene as a reporter system. Strains CBP3 and CBP4 are derivatives of RH76 and CBP1, respectively, carrying the lac operon on an F factor. The β-galactosidase activity of strains CBP3 and CBP4, growing in either LB medium or rich MOPS in the absence of NaCl, was measured after addition of increasing amounts of cAMP. In strains containing the crp+ allele the synthesis of high levels of β-galactosidase was strictly dependent upon the levels of cAMP (Fig. 6E). The CBP4 strain with the CRP(L124R) mutation failed to promote the synthesis of β-galactosidase with increasing levels of cAMP. The level of expression of both CRP and CRP(L124R) was monitored by Western blot analysis, and the expression of the CRP derivatives was similar (Fig. 6D), indicating that the turnover of CRP is not changed by the amino acid substitution. Furthermore, when both a crp deletion and the crpL124R mutations were introduced into MG1655, resulting in strains CBP5 and CBP6, respectively, both strains had a white (Mal−) phenotype on maltose-MacConkey agar plates (Fig. 6F). Taken together, these results suggested that the mutant protein CRP(L124R) could not generate a functional CRP-cAMP complex, supporting the computer prediction using the SIFT software (37) that the amino acid substitution L124R would abolish the function of CRP (data not shown). Such a phenotype could also be explained if the L124R substitution causes loss of function. However, our results from in vivo protein cross-linking experiments (see Materials and Methods) showed that CRP(L124R) has the ability to form dimers to the same extent as CRP (wt) (data not shown). Interestingly, when the crpL124R allele was overexpressed in the high-copy-number plasmid pCBP76, the strain (MG1655/pCBP76) showed a CRP-deficient phenotype (Fig. 6F). Such a phenotype suggests that CRP(L124R) can heterodimerize with CRP (wt) and make the dimer unable to activate transcription. The CRP(L124R) protein can therefore be regarded as a dominant-negative form of CRP.
DISCUSSION
In this study, we show that the level of CRP varies with changes in the external osmolarity. This osmoregulation occurs at the level of crp gene expression and requires the cAMP molecule. The comparison of the results obtained at the transcriptional and the protein level showed that there is approximately 2.5 times more crp transcript in low osmolarity than in high osmolarity and that the difference observed in the protein samples is greater (five times), indicating that the major regulatory effect is exerted at the transcriptional level although we cannot exclude control at the posttranscriptional level (Fig. 1A and C). We found that the total cAMP level was significantly increased at high osmolarity. In particular, the intracellular cAMP level was greatly increased, suggesting that osmolarity directly affected synthesis and/or turnover of cAMP. cAMP is a ubiquitous signaling molecule with important roles in both eukaryotic and prokaryotic cells. In bacteria it is mostly known for its role in catabolite repression in E. coli (43). The new role, defined by the present findings, points out that cAMP is also an important component of the regulatory network by which bacterial cells adapt to altered osmotic environments.
We found that the levels of CRP and cAMP in E. coli cells vary depending on the external osmolarity in steady-state conditions and that there was an inverse relation between the levels of CRP and cAMP at low and high osmolarity. This is consistent with a regulatory loop involving adenylate cyclase, cAMP, CRP, and the crp gene. The architecture of this regulatory loop is summarized in Fig. 7. It is well established that CRP down-regulates the activity of adenylate cyclase, both at the level of the transcription of the cya gene by CRP-cAMP-mediated repression (2) and at the level of enzymatic activity by reducing the levels of the phosphorylated IIAGlc enzyme (42). It has also been shown that the level of cAMP increases in the absence of CRP and that the level of cAMP decreases when CRP is overexpressed in E. coli (8, 22). On the other hand, the regulation of the crp promoter is sensitive to the cAMP concentration, and it has been shown that the level of CRP is increased in cya mutants (21). Under physiological conditions it was previously reported that the presence of glucose reduces the levels of CRP-cAMP by about threefold with important consequences for the gene expression and physiology of the cell (22). The effect of the glucose in this regulatory loop is mediated by reducing the level of phosphorylated IIAGlc enzyme. Reduced levels of phosphorylated IIAGlc enzyme lead to a decrease of the adenylate cyclase activity (42). Our present work raises the question at what step the osmolarity affects this regulatory loop. We have reasons to suggest that it is not at the same step where catabolite repression (i.e., at the IIAGlc enzyme regulation) is mediated. We have found that in the presence of glucose, neither the level of total IIAGlc enzyme nor the level of phosphorylated IIAGlc enzyme was affected by the osmolarity of the media (Fig. 1A and unpublished data). Although the level of phosphorylated IIAGlc enzyme was not affected by the osmolarity in the presence of glucose, significant differences in the levels of cAMP were detected under the same conditions (Fig. 3E). Furthermore, there was osmoregulation of CRP seen both when glucose was used as a carbon source and when glycerol was used (Fig. 2A). We therefore favor the hypothesis that the observed osmoregulation of CRP is occurring via a regulatory pathway independent of phosphorylated IIAGlc enzyme and that the external osmolarity affects directly either the adenylate cyclase enzymatic activity, the level of cya gene expression, or both (Fig. 7). Examples of such regulatory mechanisms have been demonstrated for other bacterial osmosensors (34). For instance, the activity of the inner membrane protein KdpD is altered in response to changes in the external osmolarity (27). On the other hand, the proU system responds to osmotic stress mainly on the level of gene expression (16). At present, it is not known at which level the osmoregulation of the adenylate cyclase occurs. Further studies will be performed to address this question.
FIG. 7.
Model of osmoregulation of crp. Green arrows designate activation whereas red arrows designate repression. See Discussion for further details.
The finding that osmoregulation of the CRP protein was lost in a cya mutant strain but could be restored by adding external cAMP (Fig. 4) suggests that the level of cAMP is essential for the osmoregulation of CRP. The inhibitory effect of low osmolarity on the bacterial growth and survival of a cya mutant when cAMP was externally supplied (5 mM) led to the discovery of a suppressor mutation (crpL124R) affecting CRP function. The crpL124R mutant per se also provided strong genetic evidence for the key role of cAMP as the mediator of the osmoregulation of crp. We concluded that the mutant CRP(L124R) was insensitive to externally provided cAMP and thereby became unable to form a functional CRP-cAMP complex. This suggests that CRP activity under certain conditions, such as low osmolarity, may be deleterious for bacterial viability and adaptability. In agreement with this hypothesis, a cAMP-independent crp allele (crpG141D) caused loss of growth at low osmolarity also in the absence of externally added cAMP. The fact that a Δcrp derivative could grow in the presence of cAMP furthermore supported our conclusion (Fig. 6B). Altogether, these results strongly indicated that the CRP activity per se causes the deleterious phenotype observed.
It was striking how the addition of cAMP to the cya mutant strain under low-osmolarity conditions caused such a drastic decrease in the number of viable cells. Microscopic observation of the cells exposed to cAMP suggested that the cell division was affected (Fig. 5C). The observed inhibition of the cell division when cAMP was added in the present case is consistent with previous findings that the CRP-cAMP complex is involved in regulation of the bacterial cell elongation and division. It was earlier reported that cell growth is inhibited by increasing cAMP levels in some genetic backgrounds of E. coli (45, 46). It is at present not known why the growth inhibition was detected only in the cya mutant strain. One possibility is that a cya mutant strain has an impaired ability to regulate the concentration of intracellular cAMP (either at the metabolic or at the transport level) and hence the relative concentration of CRP-cAMP. Supporting this, when a cAMP-independent crp allele was coexpressed in MC4100, a severe growth effect was detected at low osmolarity (generation times of 35.2 and 79 min for MC4100/pBR322 and MC4100/pHA7*, respectively). This result indicates that the loss of regulation of CRP activity is deleterious for growth at low osmolarity if an elevated cAMP-CRP activity is maintained. Further studies will be required to clarify this intriguing observation.
The present findings demonstrate that cAMP acts as an osmoregulatory molecule through its receptor protein, CRP. The results highlighted the importance of maintaining the levels of cAMP and CRP within a controlled range. The osmoregulatory effects monitored led to altered steady-state expression of CRP. Landis et al. (30) have shown that the regulation of the proP gene during a hyperosmotic shift included a transient change in the action of CRP. There is a release of CRP from the promoter P1 of the proP operon induced by the osmotic shock that allows the transient derepression of its transcription. This mechanism is apparently cAMP independent since the same effect on the regulation was found when a cAMP-independent crp allele was used. Evidently there are different mechanisms involving CRP that allow either a transient effect in the case of some particular gene(s) or a more long-term global response in the bacteria to changes in the external osmolarity.
Osmoregulation of gene expression is an important feature in the control and maintenance of bacterial physiology, allowing adaptation to new growth environments. It is presumably integrated in the complex network of mechanisms maintaining, e.g., proper turgor pressure and transport of solutes during osmotic transitions (15). The present data demonstrating osmoregulation through cAMP and CRP should be considered when trying to understand how bacteria adapt to different environmental conditions.
Acknowledgments
We thank H. Aiba for the generous gift of antisera against CRP and against EIIA and R. L. Gourse for providing the strain RLG4994. V. Shingler is acknowledged for critical reading of the manuscript.
This work was supported by grants from the Swedish Research Council, the Göran Gustafsson Foundation for Research in Natural Sciences and Medicine, the Medical Faculty of Umeå University, and the program Ramón y Cajal of the Spanish Ministry of Education and Sciences. J.J. was also supported by the Wenner-Gren foundations.
REFERENCES
- 1.Aiba, H. 1983. Autoregulation of the Escherichia coli crp gene: CRP is a transcriptional repressor for its own gene. Cell 32:141-149. [DOI] [PubMed] [Google Scholar]
- 2.Aiba, H. 1985. Transcription of the Escherichia coli adenylate cyclase gene is negatively regulated by cAMP-cAMP receptor protein. J. Biol. Chem. 260:3063-3070. [PubMed] [Google Scholar]
- 3.Aiba, H., T. Nakamura, H. Mitani, and H. Mori. 1985. Mutations that alter the allosteric nature of cAMP receptor protein of Escherichia coli. EMBO J. 4:3329-3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baga, M., M. Goransson, S. Normark, and B. E. Uhlin. 1988. Processed mRNA with differential stability in the regulation of E. coli pilin gene expression. Cell 52:197-206. [DOI] [PubMed] [Google Scholar]
- 5.Balsalobre, C., J. Johansson, B. E. Uhlin, A. Juarez, and F. J. Munoa. 1999. Alterations in protein expression caused by the hha mutation in Escherichia coli: influence of growth medium osmolarity. J. Bacteriol. 181:3018-3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker, M. M., T. Gaal, C. A. Josaitis, and R. L. Gourse. 2001. Mechanism of regulation of transcription initiation by ppGpp. I. Effects of ppGpp on transcription initiation in vivo and in vitro. J. Mol. Biol. 305:673-688. [DOI] [PubMed] [Google Scholar]
- 7.Bertani, G. 1951. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 62:293-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Botsford, J. L., and M. Drexler. 1978. The cyclic 3′,5′-adenosine monophosphate receptor protein and regulation of cyclic 3′,5′-adenosine monophosphate synthesis in Escherichia coli. Mol. Gen. Genet. 165:47-56. [DOI] [PubMed] [Google Scholar]
- 9.Botsford, J. L., and J. G. Harman. 1992. Cyclic AMP in prokaryotes. Microbiol. Rev. 56:100-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bremer, E., P. Gerlach, and A. Middendorf. 1988. Double negative and positive control of tsx expression in Escherichia coli. J. Bacteriol. 170:108-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Busby, S., D. Kotlarz, and H. Buc. 1983. Deletion mutagenesis of the Escherichia coli galactose operon promoter region. J. Mol. Biol. 167:259-274. [DOI] [PubMed] [Google Scholar]
- 12.Casadaban, M. J. 1976. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J. Mol. Biol. 104:541-555. [DOI] [PubMed] [Google Scholar]
- 13.Casadaban, M. J., and S. N. Cohen. 1980. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J. Mol. Biol. 138:179-207. [DOI] [PubMed] [Google Scholar]
- 14.Chen, L. Y., D. Y. Chen, J. Miaw, and N. T. Hu. 1996. XpsD, an outer membrane protein required for protein secretion by Xanthomonas campestris pv. campestris, forms a multimer. J. Biol. Chem. 271:2703-2708. [DOI] [PubMed] [Google Scholar]
- 15.Csonka, L. N., and A. D. Epstein. 1996. Osmoregulation, p. 1210-1223. In F. C. Neidhardt, R. Curtis III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 16.Csonka, L. N., and A. D. Hanson. 1991. Prokaryotic osmoregulation: genetics and physiology. Annu. Rev. Microbiol. 45:569-606. [DOI] [PubMed] [Google Scholar]
- 17.Godessart, N., F. J. Munoa, M. Regue, and A. Juarez. 1988. Chromosomal mutations that increase the production of a plasmid-encoded haemolysin in Escherichia coli. J. Gen. Microbiol. 134:2779-2787. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez-Gil, G., R. Kahmann, and G. Muskhelishvili. 1998. Regulation of crp transcription by oscillation between distinct nucleoprotein complexes. EMBO J. 17:2877-2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guyer, M. S., R. R. Reed, J. A. Steitz, and K. B. Low. 1981. Identification of a sex-factor-affinity site in E. coli as gamma delta. Cold Spring Harbor Symp. Quant. Biol. 45:135-140. [DOI] [PubMed] [Google Scholar]
- 20.Hengge-Aronis, R., and D. Fischer. 1992. Identification and molecular analysis of glgS, a novel growth-phase-regulated and rpoS-dependent gene involved in glycogen synthesis in Escherichia coli. Mol. Microbiol. 6:1877-1886. [DOI] [PubMed] [Google Scholar]
- 21.Ishizuka, H., A. Hanamura, T. Inada, and H. Aiba. 1994. Mechanism of the down-regulation of cAMP receptor protein by glucose in Escherichia coli: role of autoregulation of the crp gene. EMBO J. 13:3077-3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishizuka, H., A. Hanamura, T. Kunimura, and H. Aiba. 1993. A lowered concentration of cAMP receptor protein caused by glucose is an important determinant for catabolite repression in Escherichia coli. Mol. Microbiol. 10:341-350. [DOI] [PubMed] [Google Scholar]
- 23.Johansson, J., C. Balsalobre, S. Y. Wang, J. Urbonaviciene, D. J. Jin, B. Sonden, and B. E. Uhlin. 2000. Nucleoid proteins stimulate stringently controlled bacterial promoters: a link between the cAMP-CRP and the (p) ppGpp regulons in Escherichia coli. Cell 102:475-485. [DOI] [PubMed] [Google Scholar]
- 24.Johansson, J., B. Dagberg, E. Richet, and B. E. Uhlin. 1998. H-NS and StpA proteins stimulate expression of the maltose regulon in Escherichia coli. J. Bacteriol. 180:6117-6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johansson, J., and B. E. Uhlin. 1999. Differential protease-mediated turnover of H-NS and StpA revealed by a mutation altering protein stability and stationary-phase survival of Escherichia coli. Proc. Natl. Acad. Sci. USA 96:10776-10781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson, R. C., C. A. Ball, D. Pfeffer, and M. I. Simon. 1988. Isolation of the gene encoding the Hin recombinational enhancer binding protein. Proc. Natl. Acad. Sci. USA 85:3484-3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung, K., M. Veen, and K. Altendorf. 2000. K+ and ionic strength directly influence the autophosphorylation activity of the putative turgor sensor KdpD of Escherichia coli. J. Biol. Chem. 275:40142-40147. [DOI] [PubMed] [Google Scholar]
- 28.Kimata, K., Y. Tanaka, T. Inada, and H. Aiba. 2001. Expression of the glucose transporter gene, ptsG, is regulated at the mRNA degradation step in response to glycolytic flux in Escherichia coli. EMBO J. 20:3587-3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolb, A., S. Busby, H. Buc, S. Garges, and S. Adhya. 1993. Transcriptional regulation by cAMP and its receptor protein. Annu. Rev. Biochem. 62:749-795. [DOI] [PubMed] [Google Scholar]
- 30.Landis, L., J. Xu, and R. C. Johnson. 1999. The cAMP receptor protein CRP can function as an osmoregulator of transcription in Escherichia coli. Genes Dev. 13:3081-3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Link, A. J., D. Phillips, and G. M. Church. 1997. Methods for generating precise deletions and insertions in the genome of wild-type Escherichia coli: application to open reading frame characterization. J. Bacteriol. 179:6228-6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller, J. H. 1992. A short course in bacterial genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 33.Moore, J., M. Kantorow, D. Vanderzwaag, and K. McKenney. 1992. Escherichia coli cyclic AMP receptor protein mutants provide evidence for ligand contacts important in activation. J. Bacteriol. 174:8030-8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morbach, S., and R. Kramer. 2002. Body shaping under water stress: osmosensing and osmoregulation of solute transport in bacteria. Chembiochem 3:384-397. [DOI] [PubMed] [Google Scholar]
- 35.Neidhardt, F. C., P. L. Bloch, S. Pedersen, and S. Reeh. 1977. Chemical measurement of steady-state levels of ten aminoacyl-transfer ribonucleic acid synthetases in Escherichia coli. J. Bacteriol. 129:378-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neidhardt, F. C., P. L. Bloch, and D. F. Smith. 1974. Culture medium for enterobacteria. J. Bacteriol. 119:736-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ng, P. C., and S. Henikoff. 2002. Accounting for human polymorphisms predicted to affect protein function. Genome Res. 12:436-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nilsson, P., and B. E. Uhlin. 1991. Differential decay of a polycistronic Escherichia coli transcript is initiated by RNaseE-dependent endonucleolytic processing. Mol. Microbiol. 5:1791-1799. [DOI] [PubMed] [Google Scholar]
- 39.Silhavy, T. J., M. L. Berman, and L. W. Enquist. 1984. Experiments with gene fusions. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 40.Stoker, N. G., N. F. Fairweather, and B. G. Spratt. 1982. Versatile low-copy-number plasmid vectors for cloning in Escherichia coli. Gene 18:335-341. [DOI] [PubMed] [Google Scholar]
- 41.Tagami, H., T. Inada, T. Kunimura, and H. Aiba. 1995. Glucose lowers CRP* levels resulting in repression of the lac operon in cells lacking cAMP. Mol. Microbiol. 17:251-258. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi, H., T. Inada, P. Postma, and H. Aiba. 1998. CRP down-regulates adenylate cyclase activity by reducing the level of phosphorylated IIA(Glc), the glucose-specific phosphotransferase protein, in Escherichia coli. Mol. Gen. Genet. 259:317-326. [DOI] [PubMed] [Google Scholar]
- 43.Ullmann, A., and A. Danchin. 1983. Role of cyclic AMP in bacteria. Adv. Cyclic Nucleotide Res. 15:1-53. [Google Scholar]
- 44.Ullmann, A., and J. Monod. 1968. Cyclic AMP as an antagonist of catabolite repression in Escherichia coli. FEBS Lett. 2:57-60. [DOI] [PubMed] [Google Scholar]
- 45.Utsumi, R., M. Kawamukai, H. Aiba, M. Himeno, and T. Komano. 1986. Expression of the adenylate cyclase gene during cell elongation in Escherichia coli K-12. J. Bacteriol. 168:1408-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Utsumi, R., Y. Nakamoto, M. Kawamukai, M. Himeno, and T. Komano. 1982. Involvement of cyclic AMP and its receptor protein in filamentation of an Escherichia coli fic mutant. J. Bacteriol. 151:807-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.von Gabain, A., J. G. Belasco, J. L. Schottel, A. C. Chang, and S. N. Cohen. 1983. Decay of mRNA in Escherichia coli: investigation of the fate of specific segments of transcripts. Proc. Natl. Acad. Sci. USA 80:653-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Willetts, N. S., A. J. Clark, and B. Low. 1969. Genetic location of certain mutations conferring recombination deficiency in Escherichia coli. J. Bacteriol. 97:244-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wood, J. M. 1999. Osmosensing by bacteria: signals and membrane-based sensors. Microbiol. Mol. Biol. Rev. 63:230-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiao, H., M. Kalman, K. Ikehara, S. Zemel, G. Glaser, and M. Cashel. 1991. Residual guanosine 3′,5′-bispyrophosphate synthetic activity of relA null mutants can be eliminated by spoT null mutations. J. Biol. Chem. 266:5980-5990. [PubMed] [Google Scholar]