Abstract
We have recently identified a population of mechanosensory myenteric S-interneurons in the distal colon of guinea-pigs. However, the role of the longitudinal (LM) and circular muscle (CM) in transducing these mechanosensory signals into enteric reflexes is unclear. In this study, we have investigated whether the LM or CM layer is necessary for activation of ascending excitatory and descending inhibitory neuronal pathways by static stretch of the paralysed isolated guinea-pig distal colon. Simultaneous intracellular recordings were made from pairs of CM cells at either end of isolated sheet preparations of distal colon that were devoid of mucosa and submucous plexus; and were maintained under circumferential stretch. In the presence of nifedipine (1 μm), an ongoing discharge of excitatory junction potentials (EJPs) and inhibitory junction potentials (IJPs) were recorded simultaneously at the oral and anal ends of the preparation. When the LM was sharp dissected off the myenteric plexus, the synchronized discharge of ascending EJPs and descending IJPs in the CM layer was unaffected. In contrast, when the majority of CM was sharp dissected off the myenteric plexus, ongoing neural activity was absent, or substantially decreased in both the LM and CM. In these preparations, immunohistochemical staining, together with transmural electrical stimuli confirmed that the myenteric plexus was always present and intact in these preparations. When full-thickness strips of CM were removed from progressively longer lengths of myenteric plexus, a graded reduction in the correlation of coordinated oral EJPs and anal IJPs occurred. However, removing ∼40% of the thickness of CM layer from the entire preparation did not significantly disrupt, nor reduce the degree of correlation between oral EJPs and anal IJPs, suggesting that critical sensory elements did not lie adjacent to the submucosal plexus. It is concluded that mechanosensory transmission that underlies repetitive firing of ascending excitatory and descending inhibitory neuronal pathways is critically dependent upon sensory elements within the CM layer. These elements are likely to activate stretch-sensitive interneurons in the myenteric plexus. No evidence was found to suggest that the connectivity between the LM and the myenteric plexus was required for mechanotransduction.
In the large intestine, local stimulation (distension or mucosal stimulation) produces peristaltic reflex responses consisting of a simultaneous contraction orally (ascending excitation) and a simultaneous relaxation anally (descending inhibition) of the longitudinal and circular muscles (Smith et al. 1992b; Smith & McCarron, 1998; Stevens et al. 1999; Spencer et al. 1999; Spencer & Smith, 2001). Activation of these reflex pathways is essential for propelling luminal contents along the colon (Bayliss & Starling, 1900; Frigo & Lecchini, 1970; Costa & Furness, 1976; Smith & Robertson, 1998; D'Antona et al. 2001; Smith et al. 2003).
Reflex responses activated by stretch or mucosal stimulation appear to be mediated by two different intrinsic sensory neurons that converge upon common interneurons and motor neurons (Smith et al. 1991, 1992a; Furness et al. 1995; Spencer & Smith, 2004). Myenteric after-hyperpolarizing (AH) neurons project into the intestinal villi (Furness et al. 1990; Neunlist et al. 1999; reviewed in Kunze & Furness, 1999 and Sanders & Smith, 2003) and are directly activated by chemicals applied to the mucosa (Smith, 1994, 1996; Kunze et al. 1995). AH neurons are therefore likely to be the sensory neurons mediating mucosal reflexes. The enteric sensory neurons mediating stretch reflexes, on the other hand, are more controversial and appear to be mediated by both AH neurons (Kunze et al. 1998) and mechanosensitive S (fast synaptic input)-interneurons (Spencer & Smith, 2004; reviewed in Smith et al. 2005).
Myenteric AH neurons, in addition to responding to chemical stimulation of the mucosa, also respond to both stretch and contraction with a discharge of action potentials (Kunze et al. 1998; Kunze & Furness, 1999). The response of AH neurons to stretch, like their response to contraction, results from an increase in active muscle tension (tone), since their activity is abolished by drugs that abolish muscle tension (isoproterenol or nicardipine) despite maintained stretch (Kunze et al. 1998). Thus the stretch-activated firing in AH neurons is generated by the muscle's resistance to stretch, rather than by passive lengthening of the muscle fibres. In both the small and large intestine of the guinea-pig, anally propagating peristaltic waves activated by balloon distension are blocked by drugs that reduce muscle tension when applied around the site of stimulation, suggesting that AH neurons are likely to be important in the initiation of these waves (Spencer et al. 2001a; Smith et al. 2003).
In addition to the low-frequency ongoing peristaltic waves (see Smith et al. 2003), we have recently shown that maintained stretch applied to the distal colon also activates another motor pattern that is independent of muscle tone (Spencer et al. 2002a, 2003). This more rapid motor pattern activated by circumferential stretch consists of a repetitive firing of peristaltic reflex pathways, i.e. an ongoing discharge of oral excitatory junction potentials (EJPs) that are temporally synchronized with an ongoing discharge of anal inhibitory junction potentials (IJPs). This ongoing discharge of oral EJPs and anal IJPs, which occurs at the same time in both the longitudinal muscle (LM) and circular muscle (CM), is unaffected by muscle paralysis with nifedipine and by removal of the mucosa and submucous plexus (Spencer et al. 2002a, 2003). When the oral EJPs reach threshold to elicit action potentials they elicit a vigorous contraction of the muscle that is likely to also contribute to moving a pellet anally (Spencer et al. 2002a; Smith et al. 2003). AH neurons were found to be electrically silent in these stretched colonic preparations, which is perhaps not surprising since both smooth muscle layers lacked tone because most of these experiments were carried out in the presence of nifedipine (Spencer & Smith, 2004). This activity appears to be mediated by a population of myenteric, stretch-sensitive mechano-sensory ascending and descending S-interneurons that exhibit an ongoing discharge of action potentials and proximal process potentials that is unaffected by synaptic blockade or muscle paralysis (Spencer & Smith, 2004). Surprisingly some of the fine dendrites of these filamentous mechano-sensitive interneurons projected down through the ganglia and ran within and parallel to the CM fibres (Spencer & Smith, 2004). By analogy with stretch-sensitive muscle spindles in skeletal muscle, this, in parallel arrangement with the CM, may be ideal for transducing muscle stretch rather than muscle tension (see Smith et al. 2005).
Given the dendritic projections of these sensory interneurons, we were therefore particularly interested in this study in determining whether either the LM or CM was necessary for mechanically transducing the stretch-sensitive input required for maintaining the ongoing discharge of reflex pathways in the guinea-pig distal colon, that were independent of muscle tone.
Methods
Preparation of tissues
Guinea-pigs weighing 200–350 g were killed by CO2 inhalation overdose, in accordance with the animal ethics committee of The University of Nevada School of Medicine. The abdominal cavity was opened and the terminal 10 cm of distal colon was removed, flushed clean with modified Krebs solution (∼25°C: see composition below), and placed immediately into a Petri dish containing Krebs solution.
Dissection procedure
The preparation was incised along the mesenteric border and pinned flat with the mucosa uppermost in a Sylgard-lined Petri dish. Using sharp dissection, the mucosa and submucosa were peeled off to expose the underlying circular muscle layer.
Longitudinal muscle myenteric plexus preparations (LMMP)
In these preparations the CM was removed by peeling off strips of CM from the myenteric plexus, using standard sharp dissection. At both the oral and anal cut ends of the preparation, full-width strips of CM (<1 mm wide) were left intact to allow simultaneous microelectrode recordings into two CM cells situated at either end of the tissue. In other studies, simultaneous recordings were made from a CM cell in the strip and an adjacent LM cell situated ∼100 μm apart at the same end of the LMMP preparation (see Spencer & Smith, 2001).
Circular muscle myenteric plexus preparations (CMMP)
CMMP preparations were prepared by removing the LM. The LM was removed by teasing up muscle fibres at the oral end, pinching these fibres with a pair of curved forceps and peeling the LM off the preparation, in an oral to anal direction. This easily removed the LM, which often came off as wide strips of muscle, without damaging the myenteric plexus that remained intact upon the CM.
After dissection, both the LMMP and the CMMP preparations were cut to 20 mm in length and transferred to and pinned serosa side down to the base of a recording chamber (∼8 ml capacity). The base of the recording chamber, which was mounted on the stage of an inverted microscope (Olympus, CK2; Napa, CA, USA), consisted of a microscope coverslip that was laminated with a fine layer (∼2–3 mm deep) of Sylgard (Dow Corning Corp. Midland, MI, USA). The preparations were stretched circumferentially, the distance between either circumferential edge was ∼11–14 mm, which represents approximately twice the circumference of the unstretched colon (∼6 mm). In both CMMP and LMMP preparations, the entire preparation was stretched uniformly in the circumferential axis. with no longitudinal stretch imposed.
Simultaneous intracellular recordings from pairs of circular muscle cells
In both types of preparations (i.e. LMMP or CMMP), simultaneous intracellular recordings were routinely made from the CM at either end of the colon, using two independently mounted micromanipulators (model M3301R; WPI Inc., Sarasota, FL, USA; see Spencer et al. 2002a). In other studies, simultaneous microelectrode recordings were made from both the CM strip and the adjacent LM at either the oral or anal end of the LMMP preparation (see Spencer et al. 2003). Microelectrodes (i.d. 0.5 mm) were filled with 1.5 m KCl solution and had tip resistances of about 100 MΩ. Electrical signals were amplified using a dual input Axoprobe 1A amplifier, and digitized at between 660 Hz and 1.5 kHz on a PC using Axoscope software (version 8.0; Axon Instruments, Foster City, CA, USA).
To ensure that the myenteric plexus was undamaged in these preparations, transmural stimulating electrodes were mounted in the middle of the preparation. If the myenteric plexus was undamaged, then single-shot stimuli 20–40 V, duration of 0.5 ms applied with a Grass stimulator (S44) evoked an oral EJP and an anal IJP in both muscles (see Spencer et al. 2003).
NADPH-diaphorase histochemistry
As a further test for the integrity of the myenteric plexus in preparations from which the LM or CM had been removed, histochemical staining for NADPH diaphorase was routinely performed following each experiment. Whole-mount colonic preparations were fixed for 4 h in a 4% paraformaldehyde solution, then washed four times (30 min each wash) using a phosphate-buffered saline solution. Then reduced NADP (NADPH)-diaphorase staining was applied, following the same protocol as that used by Song et al. (1993).
Drugs and solutions
Preparations were perfused with Krebs solution at 35–36°C. Also, nifedipine (1–2 μm) was present in the Krebs perfusion solution in order to facilitate intracellular recording from the CM or LM, since it reduces spontaneous and evoked contractions by abolishing muscle action potentials (Spencer et al. 2002b).
The composition of the modified Krebs solution was (mm): NaCl, 120.35; KCl, 5.9; NaHCO3, 15.5; NaH2PO4, 1.2; MgSO4, 1.2; CaCl2, 2.5; and glucose, 11.5. Nifedipine and tetrodotoxin (TTX) were obtained from Sigma Chemical Co. (St Louis, MO, USA).
Measurements and statistics
Student's paired t tests were used where appropriate. A minimum significance level of P < 0.05 was used throughout. The use of n in the Results section refers to the number of animals on which observations were made, and data are presented as means ± s.e.m. Measurements of amplitude and half width, and time to peak response were made using Axoscope 8.0 (Axon Instruments, Foster City, CA, USA).
Analysis of data
The methods used to analyse and compare electrical recordings have been previously described (Spencer et al. 2001b). Briefly, voltage data were exported as a text file and imported into a custom-written program (OpenGL-based) in which the traces were resampled to 250–300 Hz and smoothed (36 ms moving average; 5 iterations). An average baseline (5–10 s) was calculated to follow slow undulations in voltage while ignoring faster events (e.g. junction potentials). Inflexions in the voltage traces were detected, and the peaks were subtracted from the average baseline to calculate junction potential amplitudes. Once peaks had been located in both traces, the peak in the second trace which was closest in time to a reference peak in the first trace was identified. Plots were then constructed of the changes in junction potential amplitudes versus the time difference between the closest oral EJP and anal IJP peaks that occurred at opposite ends of the tissue. To determine the degree of temporal correlation between synchronized oral EJPs and anal IJPs activated by stretch, plots of correlation coefficient (R) were calculated. These plots show how the degree of temporal correlation (synchronization) between oral EJPs and anal IJPs changes when an imposed time shift is applied only to the oral recording of up to 5 s positive or negative of real time. We refer to ‘real time’ as the time at which both intracellular recordings were made simultaneously from the oral and anal regions of CM, without imposing any time shifting of oral or anal recordings. At the anal recording electrode, many depolarizing events were detected. These events could represent small EJPs or myogenic ‘rebound’ depolarizations that immediately follow some IJPs. Rebound depolarizations can be identified over EJPs by their resistance to atropine, and were not temporally synchronized with any oral EJPs at the oral recording electrode.
Results
Recording from intact sheet preparations
We have previously reported that in the guinea-pig distal colon, if circumferential stretch is applied to the intact colon, or circumferential stretch is applied to sheet preparations of colon with both the LM and CM intact, an ongoing discharge of coordinated oral EJPs and anal IJPs occurs synchronously in both the LM and CM (Spencer et al. 2002a, 2003). This ongoing reflex activity is activated by stretch alone; and is independent of muscle tone, since it occurs even after the muscle has been paralysed with nifedipine (see Fig. 1A). In the experiments described in this study, we applied maintained circumferential stretch to both intact preparations, CMMP preparations and LMMP preparations, to the same degree. This varied from a slack diameter of ∼6 mm, to a diameter of 11–14 mm under stretch, which represents an increase in circumferential slack diameter of between 85% and 130%. In these preparations, like those described below, the mucosa and submucous plexus were removed and the preparation pinned serosal surface down, so as to facilitate simultaneous microelectrode impalements into the underlying CM at both ends of the preparation. The fact that this ongoing motor pattern occurred following removal of the mucosa and submucous plexus implies that the sensory elements underlying this activity must be in either the myenteric plexus, the LM or the CM.
Figure 1. Effects of removing longitudinal (LM) and circular muscle (CM) on stretch-activated ascending excitatory and descending inhibitory neural pathways.
A, schematic of the intact preparation with both muscle layers present, the CM is uppermost and the mucosa and submucosa have been removed. An example of normal synchronized stretch-activated firing of ascending excitatory (oral EJPs) and descending inhibitory (anal IJPs) pathways is shown. B, CMMP preparation, effect of removing LM muscle. Schematic illustration of simultaneous recordings made from the circular muscle (CM) at either end (within 100 μm of the cut ends) of the circumferentially stretched ‘sheet’ preparation of distal colon devoid of LM. An example of activity in CM of a CMMP preparation pinned to twice its resting circumferential slack diameter from which the LM had been removed. An ongoing discharge of oral EJPs synchronized with anal IJPs was found that was similar to that observed in an intact preparation. Note that the oral EJPs and the anal IJPs were usually temporally locked and matched in amplitude, having simultaneous onsets. C, schematic of the LMMP preparation, that was devoid of the majority of CM but the LM was left intact. In this preparation, microelectrode recordings were made from full-width strips of CM (<1 mm wide) at either end of the preparation (represented as grey strips at either end of the preparation). Only small irregular uncoordinated activity (<5 mV) was observed in both CM cells.
Recording from CMMP preparations
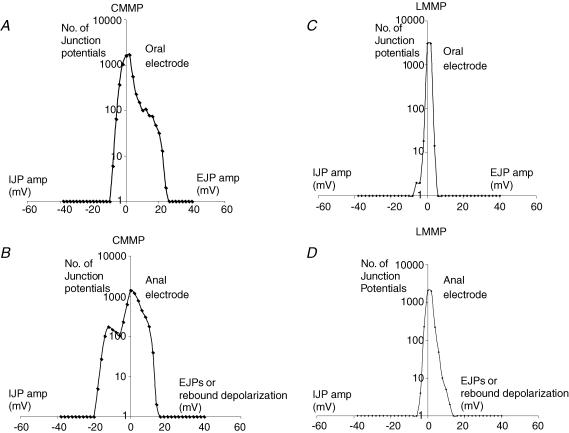
In order to investigate the possible role of the LM in transducing this ongoing peristaltic reflex activity, we removed the LM, without affecting the myenteric plexus, which normally adheres to the CM in the colon (Furukawa et al. 1986; Spencer & Smith, 2004). When circumferential stretch was applied to these sheet preparations of colon (from which the LM had been removed), interestingly, an ongoing discharge of synchronized oral EJPs and anal IJPs still occurred in the CM layer, whose characteristics were indistinguishable from those when both the muscle layers remained intact with the myenteric plexus (Spencer et al. 2002a, 2003). In total, 34 pairs of simultaneous recordings were made from two CM cells (from n = 15 animals), at the oral and anal ends of the stretched tissue, that was devoid of LM. Figure 1B shows the recording configuration and a typical example of this ongoing reflex activity. Oral EJPs and anal IJPs greater than 5 mV in amplitude were considered to occur synchronously if their peaks occurred within 80 ms of each other (Fig. 2A and B). When the peaks of oral EJPs were cross-correlated with the peaks of IJPs recorded from the anal end of the colon, it was found that there was a high degree of temporal correlation, where R2 values reached 0.26 (n = 15) (Fig. 2B). When the two electrical traces occurring at either end of the preparation were phase shifted up to +5 or −5 s from t = 0, it was found the R2 values decreased rapidly and became uncoordinated when traces were shifted by >±1 s from t = 0 (Fig. 2B). The distribution of the amplitudes of oral EJPs and anal IJPs that occur in these CMMP preparations is shown in Fig. 3A and B. The most frequently occurring EJPs in the CM were between 0 and 2 mV in amplitude, and these had mean interval of 3.0 ± 0.5 s (n = 15). In general, it was found that the larger the amplitude of synchronized oral EJPs or anal IJPs, the less frequently occurred (Fig. 3). The largest-amplitude oral EJPs reached 22 mV, where the mean interval between successive synchronized oral EJPs and anal IJPs in the range of 20–22 mV was 46.4 ± 36.5 s (n = 15) (Fig. 3A). At the oral and anal electrodes, a clear polarity in the nature of junction potentials was identified. That is, although both IJPs and EJPs could be recorded at both the oral and anal recording sites in CMMP preparations, the largest-amplitude and most frequently occurring events were always EJPs at the oral electrode and IJPs at the anal electrode (see distribution graph in Fig. 3A and B).
Figure 2. Temporal coordination between stretch-activated EJPs and IJPs in preparations with the CM attached to (A and B) and removed from (C and D) the myenteric plexus.
A, in CMMP preparations, there was a tight synchrony between the peaks of large-amplitude IJPs in CM at the anal end of the preparation, when compared to the peaks of corresponding oral EJPs in CM. That is, the increasing-amplitude IJPs show smaller time differences (higher temporal correlation) between their peak amplitude and the corresponding peak amplitude of oral EJPs, such that the delay between an oral EJP and its corresponding anal IJP was never more than 100 ms. The data presented reflect the temporal position of all anal events to their nearest correlating oral EJP. The positive values on the ordinate represent depolarizing events occurring at the anal electrode that can be either small EJPs or rebound depolarizations that follow some IJPs (data from 10 animals). Rebound depolarizations are not considered EJPs, due to their resistance to atropine. Negative t (−t) values represent the peaks of anal IJPs occurring slightly prior to the peaks of oral EJPs, whereas positive t (+t) values represent the peaks of anal IJPs occurring slightly after the peaks of oral EJPs. It is apparent that regardless of amplitude, the t values for depolarizing events at the anal electrode are poorly correlated with any oral EJPs. B, a plot of the degree of temporal correlation (correlation coefficient =R2) between the peak amplitudes of oral EJPs, compared to anal IJPs plotted against the amount of time shift (s) imposed on the oral recording trace. It is apparent that there is a high degree of temporal correlation between peaks without any time shifting of traces (i.e. at Δt = 0). Any imposed time shift of up to ±5 s did not improve the degree of correlation. C, in LMMP preparations, the amplitudes of EJPs and IJPs in CM at the oral and anal ends was usually small (<5 mV) and uncoordinated. The peaks of small junction potentials at the oral and anal ends of colon showed no temporal correlation, giving rise to noise. D, the correlation coefficient between events in CM at the oral end and those at the anal end of the colonic preparation was zero in LMMP preparations.
Figure 3. Frequency plots of junction potentials occurring in the CM layer, when the LM or bulk CM was removed from the myenteric plexus.
A, in CMMP preparations, predominantly EJPs are recorded at the oral recording electrode that are <10 mV in amplitude. The maximum-amplitude EJPs reached 22 mV. Some IJPs were also recorded at the oral recording site <10 mV in amplitude. B, IJPs occurred predominantly at the anal electrode. However, many depolarizing events were detected at this recording site that probably represent small EJPs and/or myogenic ‘rebound’ depolarizations that immediately follow some IJPs. Rebound depolarizations can be identified over EJPs by their resistance to atropine, and were not temporally synchronized with any oral EJPs at the oral recording electrode. C and D, LMMP preparations, where the CM was removed from the myenteric plexus. There was no obvious consistent polarity to the nature of the electrical events occurring at the oral or anal electrodes. Note: positive- and negative-going potentials can occur at both the oral and anal electrode that are typically <10 mV in amplitude and temporally unsynchronized between the two recording sites.
The mean resting membrane potential (RMP) of CM cells was −43.0 ± 1.3 mV (34 cells; n = 15), while the mean RMP in the LM was −35.9 ± 1.8 (22 cells, n = 10). These values were similar to those previously reported for this preparation (Spencer et al. 2003). These RMP values represented the number of CM and LM cells in which dislodgement of the electrode was recorded, and an accurate measurement of membrane potential could be ascertained.
Recording from LMMP preparations
To test the role of neural elements in the CM as a site for mechanotransduction of ongoing stretch-activated junction potentials, we now left the LM intact, but sharp dissected off as much CM as possible from the entire preparation, except for two full-width strips of CM (<1 mm wide) at either end of the preparation (see Fig. 1C). These two small strips of CM allowed us to make simultaneous recordings from two CM cells, while the majority of the CM was removed. In these classic LMMP-type preparations, surprisingly, no coordinated oral EJPs or anal IJPs were ever observed (Fig. 1C). In these preparations, an irregular baseline activity <5 mV in amplitude was recorded (Figs 1C and 2C and D). The activities recorded by the two electrodes at either end of the tissue were temporally uncoordinated (see Fig. 2C and D). A frequency distribution of the amplitudes of residual junction potentials recorded from these LMMP preparations (from n = 10 animals) is shown in Fig. 3C and D. In contrast to the CMMP, cross-correlation of the small uncoordinated junction potentials in the CM cells revealed a poor correlation coefficient of <0.01 (see Fig. 2D), and furthermore, phase shifting the two traces by up to +5 s or −5 s from t = 0, failed to modify the poorly correlated events.
This lack of ongoing coordinated reflex activity was not due to damage of the myenteric plexus during dissection, since when intracellular recordings were made simultaneously from adjacent cells in both the LM and CM, transmural stimulation applied to the centre of the preparation consistently evoked EJPs that occurred at the same time in both the LM and CM at the oral end of the tissue, and IJPs that occurred at the same time in both muscles at the anal end of the tissue (Fig. 4).
Figure 4. Effect of transmural stimulation in LMMP preparations.
To confirm the myenteric plexus was intact in preparations devoid of CM (i.e. LMMP preparations), single-shot transmural stimuli were applied to all preparations once few or no stretch-activated junction potentials were observed. A, schematic of the preparation where recordings were made from LM and CM cells simultaneously at the oral or anal cut ends of the preparation. B, under maintained circumferential stretch, no junction potentials were recorded in LM or CM cells. C and D, despite the relative lack of electrical activity in both muscles, single-shot stimuli (40 V, 0.5 ms) evoked an EJP in both the LM and CM at the oral end, and an IJP in both the LM and CM at the anal end of the tissue; responses in both muscles occurred at the same time. E and F, following fixation of the LMMP preparations NADPH-diaphorase histochemistry also revealed that our dissection procedures had left the myenteric plexus intact.
Effects of papaverine after the application of nifedipine on mechanotransduction
Since nifedipine does not completely block all smooth muscle contraction, it might be argued that some residual level of muscle tension still exists in the musculature of the guinea-pig colon that could possibly activate tension-sensitive, rather than purely stretch-sensitive myenteric neurons. Therefore, to test this, we applied the smooth muscle relaxant papavarine (10 μm) to the colon after stretch-activated junction potentials had been activated in the presence of nifedipine. In tissue samples from three animals, we found no detectable, nor significant effect of papavarine on the degree of correlation between synchronized oral EJPs and anal IJPs in the CM layer (nifedipine: R2 = 0.4 ± 0.06; papavarine: R2 = 0.37 ± 0.05; n = 3; P > 0.05) (see Fig. 5B and C). Interestingly, the RMP of CM cells did not significantly change from the presence of nifedipine, to a solution containing nifedipine and papavarine (10 μm) (control: 48.1 ± 0.96 mV; papavarine: 46.2 ± 0.6 mV; n = 3; P = 0.17). Under these conditions in the combined presence of nifedpine and papavarine, it is reasonable to assume there is negligible active muscle tension in the musculature, yet stretch-activated junction potentials persisted.
Figure 5. Removal of circular muscle from myenteric ganglia does not affect radial muscle tension.
A, graded increases in circumferential stretch were applied using weights and a cantilever to preparations with the CM attached to the myenteric plexus (•) and removed from the myenteric plexus (▪), as in LMMP-preparations. At 36°C and in the presence of nifedipine, there was no significant change in the length of the preparation evoked by graded increases in stretch when the CM was removed from the myenteric plexus. B and C, the further addition of the smooth muscle relaxant, papavarine (10 μm) after nifedipine to the colon did not affect stretch-activated firing of oral EJPs and anal IJPs, suggesting that muscle tension is not required in mechanotransduction. Error bars represent s.e.m.
In light of our observation that removal of the CM, but not LM abolished stretch-activated junction potentials, it might have been argued that this response was due to different degrees of muscle tension in the two different preparations. That is, in preparations with the CM removed from the myenteric plexus, the degree of radially applied muscle tension or tone may be vastly different from preparations with only the LM removed from the myenteric plexus, and this difference in muscle tension may account for the differences observed in sensory neural activation and motor output. Although this scenario seemed unlikely based on our findings above that junction potentials persisted in the combined presence of nifedipine and papavarine, we further tested this hypothesis. This was performed using a cantilever system to apply increasing degrees of stretch to the colon (1–7 g circumferentially) and then measure the corresponding length of the preparation. These increasing degrees of stretch were applied to both CMMP preparations and control preparations where both the LM and CM remained intact with the myenteric plexus, while in the maintained presence of nifedipine (1–2 μm) at 36°C. Overall, we found no significant difference between the degree of radially applied muscle tension in LMMP preparations and control preparations that had both the LM and CM intact with the myenteric ganglia (n = 4; see Fig. 5A; P > 0.05).
Effects of removing increasing length segments of circular muscle from the myenteric plexus
Since removal of the majority of the CM from the myenteric plexus abolished stretch-activated junction potentials in both the LM and CM, it was of particular interest to determine whether continuity of the CM along the entire length of the preparation was important for sustained activation of the underlying mechanosensory neural elements. To test this, we first sharp dissected a 7-mm-wide full-thickness strip of CM from the middle region of the 20-mm-long preparation of colon (see Fig. 6C). Then in the same preparation, we increased the width of the dissected strip of CM to 14 mm (Fig. 6E). In these preparations, under these conditions, a significant and graded decrease in the correlation coefficient was detected (Fig. 7E). In preparations with 7 mm of width of CM removed from the myenteric plexus, synchronized oral EJPs and anal IJPs were still recorded, but the degree of correlation was significantly smaller than in the same preparations when all the CM remained intact with the plexus (n = 4; Fig. 6 and Fig. 7E). When a 14-mm-wide segment of CM was sharp dissected from the myenteric plexus, no synchronized oral EJPs and anal IJPs were ever recorded, and R2 values approached zero (i.e. no coordination in either amplitude or temporal onset) (Figs 6F and 7E).
Figure 6. Effects of removing increasingly larger strips of CM on ongoing peristaltic reflex activity activated by circumferential stretch.
A, a diagrammatic representation of the control preparation with a complete CM layer attached to the myenteric plexus. B, normal stretch-activated junction potentials in CM. C, diagram to show removal of a 7-mm-wide strip of CM from the middle region of colon. D, stretch-activated junction potentials persist following removal of 7 mm of CM. E, diagram to show removal of 14 mm of CM from the anal and middle region of colon taken from the same preparation where the 7-mm-wide strip of CM was taken from. E, when 14 mm of CM was removed from the myenteric plexus, stretch-activated junction potentials were substantially reduced in amplitude (<5 mV) to within the recording noise.
Figure 7. Effects of removing half the depth of CM on ongoing peristaltic reflex activity activated by circumferential stretch.
To test whether mechanosensory neural elements lay adjacent to the submucosal plexus, we removed approximately half the depth of CM from the entire preparation. A, a control preparation with all CM preserved and normal stretch-activated junction potentials. C and D, when 40% of the depth of CM was removed, no detectable change was noted in stretch-activated junction potential in the CM layer (D). E, the change in correlation (R2) of coordinated junction potentials in the CM when increasingly larger strips of CM were removed from the myenteric plexus. There was no significant effect of removing 40% of the depth of CM, however, when a 7-mm or 14-mm-wide full-thickness strip of CM was removed, a significant and graded reduction in correlation of junction potentials was observed when compared to control preparations with CM present (*P < 0.05).
Effects of removing the inner layers of CM
In three animals, we investigated whether the mechanosensory neural elements lay close to the submucous plexus. To test this we sharp dissected off approximately 40% of the thickness of the CM layer from the entire 20-mm-long preparation (see Fig. 7D). In these preparations, we found no detectable, or significant difference in the R2 value (degree of correlation between oral EJPs and anal IJPs) in the remaining CM layer (Fig. 7C–E; n = 3). When these preparations were fixed and visualized under bright field microscope it was found that the thickness of the CM was 52.5 ± 2.5 μm once sharp dissected, and was 82.5 ± 2.5 μm in undissected preparations CM (n = 3). This means that removal of approximately 37% of the depth of the CM failed to affect mechanotransduction or neuromuscular output to the CM.
Discussion
Recently, we showed that under maintained circumferential stretch, isolated segments of guinea-pig distal colon generated simultaneous oral EJPs and anal IJPs in the CM over large regions of colon. This activity was found to be dependent upon muscle stretch, but insensitive to muscle tone (Spencer et al. 2002a, 2003). It was also found that the oral EJPs and anal IJPs discharged simultaneously in both the LM and CM (see Spencer et al. 2003; Smith et al. 2005). We concluded that many ascending interneurons simultaneously activated separate populations of excitatory motor neurons that projected to the LM and CM orally, at the same time as many descending interneurons simultaneously activated separate populations of inhibitory motor neurons that projected to the LM and CM anally (see Spencer et al. 2003; Smith et al. 2005). However, the nature or location of the mechanosensory elements that generate this ongoing discharge of intrinsic neural activity has not been identified.
The major finding of the current study is that removal of the CM layer from the myenteric plexus abolishes the stretch-induced ongoing discharge of ascending excitatory and descending inhibitory neuronal pathways to both the LM and CM layers. The loss of this neural activity was not due to damage of the myenteric plexus, since NADPH diaphorase staining after each experiment revealed that the myenteric plexus remained intact. Furthermore, transmural electrical nerve stimuli consistently evoked robust oral EJPs and anal IJPs in these preparations, where their amplitudes could be graded in amplitude according to stimulus intensity. In direct contrast, in preparations devoid of most of the CM, junction potentials were usually <5 mV, and no synchronized oral EJPs or anal IJPs ever occurred. Taken together, these observations suggest that mechanosensory transduction underlying stretch-induced firing of ascending excitatory and descending inhibitory pathways is critically dependent upon connectivity between the myenteric plexus and the CM layer. Importantly, removal of approximately 37% of the depth of the CM failed to affect the ongoing EJPs and IJPs, suggesting that the sensory elements transducing this ongoing peristaltic reflex activity lie within the CM close to the myenteric plexus.
In contrast, removal of the LM from the myenteric plexus did not affect stretch-activated ascending excitatory and descending inhibitory neuronal pathways. These preparations that were devoid of LM exhibited ongoing oral EJPs and anal IJPs that occurred at a similar frequency and exhibited the same degree of temporal coordination as observed in preparations in which both muscle layers were intact (see Spencer et al. 2002a, 2003).
We tested whether continuity of the CM layer along the length of the preparation was a critical element underlying the mechanosensory activation of repetitive oral EJPs and anal IJPs. Interestingly, it was found that stretch-activated junction potentials were not abolished when a 7-mm-wide strip of CM was removed from the myenteric plexus (Fig. 7D and E), although there was a significant reduction in the degree of correlation between oral EJPs and anal IJPs (Fig. 7E). When 14 mm of CM was sharp dissected off the myenteric plexus from either the oral or anal cut ends, a further graded significant decrease in the correlation of junction potentials occurred, such that synchronized oral EJPs and anal IJPs were no longer recorded (Fig. 6F). These observations suggest that although continuity of the CM layer along the length of colon is not critical for the activation of synchronized oral EJPs and anal IJPs, it is clear that a graded increase in the removal of CM from the myenteric plexus results in a graded decrease in the level of synchronized oral EJPs and anal IJPs.
Spontaneous neuronal activity is weak in classic LMMP preparations of colon
Both Wade & Wood (1988a, b) and Lomax et al. (1999) studied the electrical characteristics of myenteric neurons in the guinea-pig distal colon, using the classic longitudinal muscle, myenteric plexus (LMMP) preparation, i.e. where the CM was removed from the myenteric plexus, but the LM layer was preserved. Despite the fact that the LM in these preparations was presumably stretched both longitudinally and circumferentially, both groups reported a relative lack of spontaneous activity in myenteric neurons. Lomax et al. (1999) found that only 3 out of 145 myenteric S-neurons showed ongoing spontaneous fast EPSPs. In contrast, when we recorded from the same length preparations, but preserved the CM intact with the myenteric plexus, essentially all S-neurons exhibited ongoing spontaneous fast EPSPs and action potentials (Spencer & Smith, 2004). The only difference between the two studies was that we preserved much of the CM intact with the myenteric plexus. These findings absolutely support our conclusions in this study, in that stretch-activated neuronal activities that underlie ascending excitatory and descending inhibitory neuronal pathways are critically dependent upon the CM, but not the LM. Since the only difference between the studies of Lomax et al. (1999) and our study was the presence of the CM in our preparations, this led us to believe that the CM is critical for mechanosensory transmission. In preparations with the CM removed from the myenteric plexus where there was an absence of any stretch-activated junction potentials in either muscle layer, our observation that in these same preparations transmural stimulation evoked robust oral EJPs and anal IJPs in both muscle layers showed that interneuronal communication along the myenteric plexus was intact.
Previous studies strongly suggest that there are at least two distinct classes of intrinsic sensory neuron in the intestine (Smith et al. 1991, 1992a; Spencer & Smith, 2004): AH neurons (Holman et al. 1972; Hirst et al. 1974, 1975; Bornstein et al. 1991; Smith et al. 1992a; Kunze et al. 1998, 1999, 2000) and myenteric S-interneurons (Spencer & Smith, 2004).
The location of the mechanosensory transduction sites that generate stretch-activated oral EJPs and anal IJPs in the colon was previously unclear. In the canine jejunum it was suggested that the stretch receptors mediating the inhibitory reflex were in the LM, because removal of this layer, but not the CM, abolished this reflex activity (Hukuhara et al. 1960). However, others have suggested that in guinea-pig colon ‘… the longitudinal muscle seems not essential for propulsion’ (Crema et al. 1970). Interestingly, Ohkawa & Prosser (1972) reported that extracellularly recorded spike discharges in myenteric neurons could be evoked by pressing on ganglia with a fine glass probe, whereas pressing on the connective tissues or the neighbouring muscle was ineffective. Importantly, probing around a ganglion initiates generator potentials or action potentials in AH neurons, suggesting that some processes of AH neurons within the myenteric plexus are mechanosensitive (Kunze et al. 2000). These ‘receptive sites’ were located in the ganglion from which recordings were made, and ‘… did not extend over the interganglionic connectives or adjacent longitudinal muscle’ (Kunze et al. 2000). Presumably the tension-sensitive processes of AH neurons should be in series with the muscle, as tension-sensitive Golgi tendon organs are associated with skeletal muscle. To date, the evidence suggests that the mechanosensory endings of AH neurons, at least in the small intestine, have no specialized ending in either the LM or CM, but probably lie within the myenteric plexus.
In contrast, our data are strongly consistent with the idea that the dendrites of filamentous stretch-sensitive ascending and descending interneurons that project through myenteric ganglia and run within and parallel to the CM fibres are indeed mechanosensory. We speculate that when circumferential stretch is applied, the mechanosensitive dendrites of these interneurons become activated, which initiates the ongoing coordinated reflex activity, providing stretch is maintained (Fig. 8). Therefore, these mechanosensory S-interneurons are critical for transducing stretch-sensitive, but muscle tone-independent ongoing peristaltic reflex activity (Spencer & Smith, 2004). When we recorded from interneurons in preparations where the CM was intact but the LM removed, they exhibited spontaneous action potentials, proximal process potentials that were insensitive to synaptic blockade with low-Ca2+ solution, and spontaneous fast excitatory post synaptic potentials (FEPSPs) that were blocked by low-Ca2+ solution (Spencer et al. 2004). In contrast, in preparations where the CM was removed but the LM was intact, interneurons were electrically quiescent until current was injected into their soma, or FEPSPs were evoked by focal electrical stimulation of fibre tracts (Lomax et al. 1999). The difference in these studies can be attributed to the fact that spontaneous action potentials in the soma of interneurons are probably initiated by proximal process potentials, which result from action potentials in the stretch-sensitive sensory processes of these cells that project to the underlying CM (see Spencer et al. 2004). Presumably, the excited interneurons in our preparations synapse with each other, giving rise to FEPSPs in other interneurons (see Fig. 8; Smith et al. 2005; Spencer et al. 2005). Removal of the CM probably destroys these mechanosensitive dendrites and prevents the activation of mechanosensitive interneurons. It is, at present, unknown whether the dendrites of interneurons within the CM are themselves stretch sensitive or part of a more complex sensory apparatus analogous to muscle spindles in skeletal muscle. Such sensory elements in the CM that lie in parallel with CM could be the intramuscular interstitial cells of Cajal (ICC-IM) (see Smith et al. 2005). In the stomach, ICC-IM run parallel to and within the CM, and are stretch sensitive and also mediate neurotransmission to the CM (see Beckett et al. 2004; Won et al. 2005).
Figure 8. Circuitry underlying stretch-activated ongoing peristaltic reflex activity.
To maintain temporal coordination of oral EJPs with anal IJPs, stretch-sensitive ascending interneurons (AI) and descending interneurons (DI) synapse with each another in a self-reinforcing circuit. Both AI and DI have stretch-sensitive dendritic processes in the CM but not in the LM. Maintained stretch causes an ongoing discharge of action potentials in these processes that activates AI and DI. AI activate excitatory motor neurons (EMN) orally to both the LM and CM, that in turn initiate excitatory junction potentials (EJPs) in both the LM and CM. DI activate inhibitory motor neurons (IMN) anally to both the LM and CM, that in turn produce inhibitory junction potentials (IJPs) in both the LM and CM.
In addition, it is possible that in the colon the myenteric plexus adheres more strongly to the CM (see Furukawa et al. 1986; Spencer & Smith, 2004), because the mechanosensitive dendrites of sensory interneurons project into the muscle and anchor the ganglia more firmly to this layer (Spencer & Smith, 2004). In contrast, the myenteric plexus adheres more firmly to the LM in the ileum, suggesting that the sites of sensory transduction may differ in these different regions of the gut. This is supported by the fact that spontaneously active neurons can be recorded within the corners of ganglia within LMMP preparations in the small bowel (see Shuttleworth & Smith, 1999; Smith et al. 1999).
Acknowledgments
This study was supported by a grant from the National Institutes of Health (USA) (no. RO1 NIDDK 45713 to T.K.S. and N.S.).
References
- Bayliss WM, Starling EH. The movements and innervation of the large intestine. J Physiol. 1900;26:107–118. doi: 10.1113/jphysiol.1900.sp000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckett EA, Bayguinov YR, Sanders KM, Ward SM, Hirst GD. Properties of unitary potentials generated by intramuscular interstitial cells of Cajal in the murine and guinea-pig gastric fundus. J Physiol. 2004;559:259–269. doi: 10.1113/jphysiol.2004.063016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein JC, Furness JB, Smith TK, Trussell DC. Synaptic responses evoked by mechanical stimulation of the mucosa in morphologically characterized myenteric neurons of the guinea-pig ileum. J Neurosci. 1991;1:505–518. doi: 10.1523/JNEUROSCI.11-02-00505.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M, Furness JB. The peristaltic reflex: an analysis of the nerve pathways and their pharmacology. Naunyn Schmiedebergs Arch Pharmacol. 1976;294:47–60. doi: 10.1007/BF00692784. [DOI] [PubMed] [Google Scholar]
- Crema A, Frigo GM, Lecchini S. A pharmacological analysis of the peristaltic reflex in the isolated colon of the guinea-pig or cat. Br J Pharmacol. 1970;39:334–345. doi: 10.1111/j.1476-5381.1970.tb12897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Antona G, Hennig GW, Costa M, Humphreys CM, Brookes SJ. Analysis of motor patterns in the isolated guinea-pig large intestine by spatio-temporal maps. Neurogastroenterol Mot. 2001;13:483–492. doi: 10.1046/j.1365-2982.2001.00282.x. [DOI] [PubMed] [Google Scholar]
- Frigo GM, Lecchini S. An improved method for studying the peristaltic reflex in isolated colon. Br J Pharmacol. 1970;39:346–356. doi: 10.1111/j.1476-5381.1970.tb12898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness JB, Johnson PJ, Pompolo S, Bornstein JC. Evidence that enteric motility reflexes can be initiated through entirely intrinsic mechanisms in the guinea-pig small intestine. Neurogastroenterol Motil. 1995;7:89–96. doi: 10.1111/j.1365-2982.1995.tb00213.x. [DOI] [PubMed] [Google Scholar]
- Furness JB, Trussell DC, Pompolo S, Bornstein JC, Smith TK. Calbindin neurons of the guinea-pig small intestine: quantitative analysis of their numbers and projections. Cell Tissue Res. 1990;260:261–272. doi: 10.1007/BF00318629. [DOI] [PubMed] [Google Scholar]
- Furukawa K, Taylor GS, Bywater RAR. An intracellular study of myenteric neurons in the mouse colon. J Neurophysiol. 1986;55:1395–1406. doi: 10.1152/jn.1986.55.6.1395. [DOI] [PubMed] [Google Scholar]
- Hirst GDS, Holman ME, McKirdy HC. Two descending nerve pathways activated by distension of guinea-pig small intestine. J Physiol. 1975;244:113–127. doi: 10.1113/jphysiol.1975.sp010786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GDS, Holman ME, Spence I. Two types of neurons in the myenteric plexus of duodenum in the guinea-pig. J Physiol. 1974;236:303–326. doi: 10.1113/jphysiol.1974.sp010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman ME, Hirst GDS, Spence I. Preliminary studies of the neurons of Auerbach's plexus using intracellular microelectrodes. Aust J Exp Biol. 1972;7:795–801. doi: 10.1038/icb.1972.76. [DOI] [PubMed] [Google Scholar]
- Hukuhara T, Nakayama S, Nanba R. Locality of receptors concerned with the intestine-intestinal extrinsic and intestinal muscular intrinsic reflexes. Jap J Physiol. 1960;10:414–419. doi: 10.2170/jjphysiol.10.414. [DOI] [PubMed] [Google Scholar]
- Kunze WAA, Bornstein JC, Furness JB. Identification of sensory nerve cells in a peripheral organ (the intestine) of a mammal. Neuroscience. 1995;66:1–4. doi: 10.1016/0306-4522(95)00067-s. [DOI] [PubMed] [Google Scholar]
- Kunze WA, Clerc N, Furness JB, Gola M. The soma and neurites of primary afferent neurons in the guinea-pig intestine respond differentially to deformation. J Physiol. 2000;526:375–385. doi: 10.1111/j.1469-7793.2000.00375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze WAA, Furness JB. The enteric nervous system and regulation of intestinal motility. Ann Rev Physiol. 1999;61:117–142. doi: 10.1146/annurev.physiol.61.1.117. [DOI] [PubMed] [Google Scholar]
- Kunze WAA, Furness JB, Bertrand PP, Bornstein JC. Intracellular recording from myenteric neurons of the guinea-pig ileum that respond to stretch. J Physiol. 1998;506:827–842. doi: 10.1111/j.1469-7793.1998.827bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax AEG, Sharkey KA, Bertrand PP, Low AM, Bornstein JC, Furness JB. Correlation of morphology, electrophysiology and chemistry of neurons in the myenteric plexus of the guinea-pig distal colon. J Auton Nerv System. 1999;76:45–61. doi: 10.1016/s0165-1838(99)00008-9. [DOI] [PubMed] [Google Scholar]
- Neunlist M, Dobreva G, Schemann M. Characteristics of mucosally projecting myenteric neurons in the guinea-pig proximal colon. J Physiol. 1999;517:533–546. doi: 10.1111/j.1469-7793.1999.0533t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa H, Prosser CL. Functions of neurons in enteric plexuses of cat intestine. Am J Physiol. 1972;222:1420–1426. doi: 10.1152/ajplegacy.1972.222.6.1420. [DOI] [PubMed] [Google Scholar]
- Sanders KM, Smith TK. Neural regulation of colonic motor function. In: Koch T, editor. Colonic Diseases. Totowa, New Jersey: Humana Press; 2003. pp. 35–52. chap 3. [Google Scholar]
- Shuttleworth CW, Smith TK. Action potential-dependent calcium transients in myenteric S neurons of the guinea-pig ileum. Neuroscience. 1999;92:751–762. doi: 10.1016/s0306-4522(99)00012-3. [DOI] [PubMed] [Google Scholar]
- Smith TK. Myenteric AH neurons are sensory neurons in the guinea-pig proximal colon: an electrophysiological analysis in intact preparations. Gastroenterology. 1994;862:A216. [Google Scholar]
- Smith TK. An electrophysiological identification of intrinsic sensory neurons responsive to 5-HT applied to the mucosa that underlie peristalsis in the guinea-pig proximal colon. J Physiol. 1996;495.P:102P. [Google Scholar]
- Smith TK, Bornstein JC, Furness JB. Interactions between reflexes evoked by distension and mucosal stimulation: electrophysiological studies of guinea-pig ileum. J Auton Nerv Syst. 1991;34:69–75. doi: 10.1016/0165-1838(91)90009-r. [DOI] [PubMed] [Google Scholar]
- Smith TK, Bornstein JC, Furness JB. Convergence of reflex pathways excited by distension and mechanical stimulation of the mucosa onto the same myenteric neurons of the guinea-pig small intestine. J Neurosci. 1992a;12:1502–1510. doi: 10.1523/JNEUROSCI.12-04-01502.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TK, Burke EP, Shuttleworth CWR. Topographical and electrophysiological characteristics of highly excitable S neurons in the myenteric plexus of the guinea-pig ileum. J Physiol. 1999;517:817–830. doi: 10.1111/j.1469-7793.1999.0817s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TK, Bywater RAR, Taylor GS, Holman ME. Electrical responses of the muscularis externa to distension of the isolated guinea-pig distal colon. J Gastrointest Mot. 1992b;4:145–156. [Google Scholar]
- Smith TK, McCarron SL. Nitric oxide modulates cholinergic reflex pathways to the longitudinal and circular muscle in the isolated guinea-pig distal colon. J Physiol. 1998;512:893–906. doi: 10.1111/j.1469-7793.1998.893bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TK, Oliver GR, Hennig GW, O'Shea D, Vanden Berghe P, Kang SK, Spencer NJ. A smooth muscle tone-dependent migrating motor pattern in guinea-pig distal colon. J Physiol. 2003;551:955–969. doi: 10.1113/jphysiol.2003.049163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TK, Robertson W. Synchronous movements of the longitudinal and circular muscle during peristalsis in the isolated guinea-pig distal colon. J Physiol. 1998;506:563–577. doi: 10.1111/j.1469-7793.1998.563bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TK, Spencer NJ, Hennig GW. Mechanosensory transduction in the enteric nervous system. Physiology News. 2005;58:23–25. http://www.physoc.org/publications. [Google Scholar]
- Song ZM, Brookes SJ, Costa M. NADPH-diaphorase reactivity in nerves supplying the rat anococcygeus muscle. Neurosci Lett. 1993;158:221–224. doi: 10.1016/0304-3940(93)90269-q. [DOI] [PubMed] [Google Scholar]
- Spencer NJ, Hennig GW, Dickson E, Smith TK. Synchronization of enteric neuronal firing during the murine colonic MMC. J Physiol. 2005;564:829–847. doi: 10.1113/jphysiol.2005.083600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer NJ, Hennig GW, Smith TK. Spatial and temporal coordination of junction potentials in circular muscle of guinea-pig distal colon. J Physiol. 2001b;535:565–578. doi: 10.1111/j.1469-7793.2001.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer NJ, Hennig GW, Smith TK. A rhythmic motor pattern activated by circumferential stretch in guinea-pig distal colon. J Physiol. 2002b;545:629–648. doi: 10.1113/jphysiol.2002.028647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer NJ, Hennig GW, Smith TK. Electrical rhythmicity and spread of action potentials in longitudinal muscle of guinea-pig distal colon. Am J Physiol Gastrointest Liver Physiol. 2002a;282:G904–G917. doi: 10.1152/ajpgi.00345.2001. [DOI] [PubMed] [Google Scholar]
- Spencer NJ, Hennig GW, Smith TK. Stretch activated neuronal pathways in longitudinal and circular muscle of guinea-pig distal colon. Am J Physiol. 2003;284:G231–G241. doi: 10.1152/ajpgi.00291.2002. [DOI] [PubMed] [Google Scholar]
- Spencer N, McCarron SL, Smith TK. Sympathetic inhibition of ascending and descending interneurons during the peristaltic reflex in the guinea-pig distal colon. J Physiol. 1999;519:539–550. doi: 10.1111/j.1469-7793.1999.0539m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer N, Smith CB, Smith TK. Role of muscle tone in peristalsis in guinea-pig small intestine. J. Physiol. 2001a;530:295–306. doi: 10.1111/j.1469-7793.2001.0295l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer N, Smith TK. Simultaneous intracellular recordings from longitudinal and circular muscle during the peristaltic reflex in guinea-pig colon. J Physiol. 2001;533:787–799. doi: 10.1111/j.1469-7793.2001.00787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer NJ, Smith TK. Mechanosensory S-neurons rather than AH neurons appear to generate a rhythmic motor pattern in guinea-pig distal colon. J Physiol. 2004;558:577–596. doi: 10.1113/jphysiol.2004.063586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens RJ, Publicover NG, Smith TK. Induction and regulation of Ca2+ waves by enteric neural reflexes. Nature. 1999;399:62–66. doi: 10.1038/19973. [DOI] [PubMed] [Google Scholar]
- Wade PR, Wood JD. Synaptic behaviour of myenteric neurons in guinea-pig distal colon. Am J Physiol. 1988a;225:G184–G190. doi: 10.1152/ajpgi.1988.255.2.G184. [DOI] [PubMed] [Google Scholar]
- Wade PR, Wood JD. Electrical behavior of myenteric neurons in guinea pig distal colon. Am J Physiol. 1988b;254:G522–G530. doi: 10.1152/ajpgi.1988.254.4.G522. [DOI] [PubMed] [Google Scholar]
- Won KJ, Sanders KM, Ward SM. Interstitial cells of Cajal mediate mechanosensitive responses in the stomach. Proc Natl Acad Sci U S A. 2005;102:14913–14918. doi: 10.1073/pnas.0503628102. [DOI] [PMC free article] [PubMed] [Google Scholar]