Abstract
The gram-positive bacterium Staphylococcus aureus is a frequent component of the human microbial flora that can turn into a dangerous pathogen. As such, this organism is capable of infecting almost every tissue and organ system in the human body. It does so by actively exporting a variety of virulence factors to the cell surface and extracellular milieu. Upon reaching their respective destinations, these virulence factors have pivotal roles in the colonization and subversion of the human host. It is therefore of major importance to obtain a clear understanding of the protein transport pathways that are active in S. aureus. The present review aims to provide a state-of-the-art roadmap of staphylococcal secretomes, which include both protein transport pathways and the extracytoplasmic proteins of these organisms. Specifically, an overview is presented of the exported virulence factors, pathways for protein transport, signals for cellular protein retention or secretion, and the exoproteomes of different S. aureus isolates. The focus is on S. aureus, but comparisons with Staphylococcus epidermidis and other gram-positive bacteria, such as Bacillus subtilis, are included where appropriate. Importantly, the results of genomic and proteomic studies on S. aureus secretomes are integrated through a comparative “secretomics” approach, resulting in the first definition of the core and variant secretomes of this bacterium. While the core secretome seems to be largely employed for general housekeeping functions which are necessary to thrive in particular niches provided by the human host, the variant secretome seems to contain the “gadgets” that S. aureus needs to conquer these well-protected niches.
INTRODUCTION
The gram-positive bacterium Staphylococcus aureus is a frequent component of the human microbial flora that can turn into a dangerous pathogen. As such, this organism is capable of infecting almost every tissue and organ system in the human body. It does so by exporting a variety of virulence factors to the cell surface and extracellular milieu of the human host. Like all living organisms (201), S. aureus contains several protein transport pathways, among which the general secretory (Sec) pathway is the most well known and best described. Proteins that need to be transported to an extracytoplasmic location generally contain an N-terminal signal peptide that is needed to target the newly synthesized protein from the ribosome to the translocation machinery in the cytoplasmic membrane. Next, the protein is threaded through the Sec translocon in an unfolded state. During this translocation step, or shortly thereafter, the signal peptide is removed by a so-called signal peptidase (SPase). Upon complete membrane translocation, the protein has to fold into its correct conformation and will then be retained in an extracytoplasmic compartment of the cell or secreted into the extracellular milieu. In the case of gram-positive cocci, such as S. aureus (Fig. 1), we distinguish three extracytoplasmic subcellular compartments, namely, the membrane, the membrane-cell wall interface, and the cell wall. Since surface-exposed and secreted proteins of S. aureus play pivotal roles in the colonization and subversion of the human host, it is of major importance to obtain a clear understanding of the protein transport pathways that are active in this organism (103). Knowledge about the protein sorting mechanism has become all the more relevant with the emergence of staphylococcal resistance against last-defense antibiotics, such as vancomycin. The scope of this review is to provide a state-of-the-art roadmap of staphylococcal secretomes, which include both protein transport pathways and the extracytoplasmic proteins of staphylococcal organisms. The focus is on S. aureus, but comparisons with Staphylococcus epidermidis and the best-characterized gram-positive bacterium, Bacillus subtilis, are included where appropriate. Importantly, the present review aims to integrate the results of genomic and proteomic studies on S. aureus secretomes, representing the first documented “comparative secretomics” study. Specifically, this review deals with known and predicted exported virulence factors, pathways for protein transport, signals for subcellular protein sorting or secretion, and the exoproteomes of different S. aureus isolates, as defined by two-dimensional polyacrylamide gel electrophoresis (2D-PAGE) and mass spectrometry (Fig. 2 and 3). The exoproteome is defined by all S. aureus proteins that can be identified in the extracellular milieu of this organism and thus includes proteins actively secreted by living cells and the remains of dead cells. For a clear appreciation of the present review, it is important to bear in mind that the proteins exported from the cytoplasm could be directly involved in staphylococcal virulence, whereas the respective protein export systems represent the “pathways to pathogenesis.”
FIG. 1.
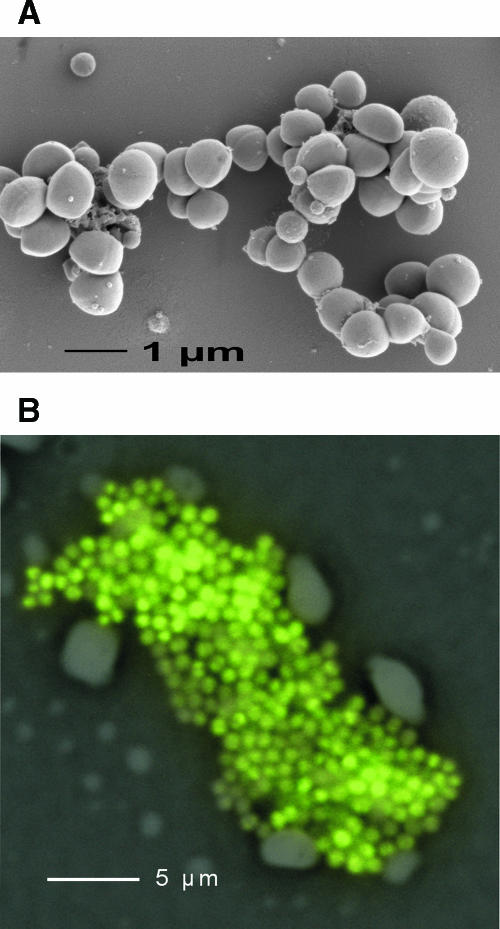
Imaging of S. aureus RN6390. (A) For scanning electron microscopy, a drop of washed culture of bacteria was fixated for 30 min with 2% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.38. Next, the fixated bacteria were placed on a piece (1 cm2) of cleaved 0.1% poly-l-lysine-coated mica sheet and washed in 0.1 M cacodylate buffer. This specimen was dehydrated in an ethanol series consisting of 30%, 50%, 70%, 96%, and anhydrous 100% (3×) solutions for 10 min each, critical point dried with CO2, and sputter coated with 2 to 3 nm Au/Pd (Balzers coater). The specimen was fixed on a scanning electron microscope stub holder and observed in a JEOL FE-SEM 6301F microscope. (B) Micrograph of a cluster of S. aureus cells grown in blood culture medium. The cells were fixed with ethanol and hybridized with the fluorescein-labeled peptide nucleic acid (PNA) probe PNA-Stau. The image was generated by merging an epifluorescence image with the negative of a phase-contrast image.
FIG. 2.
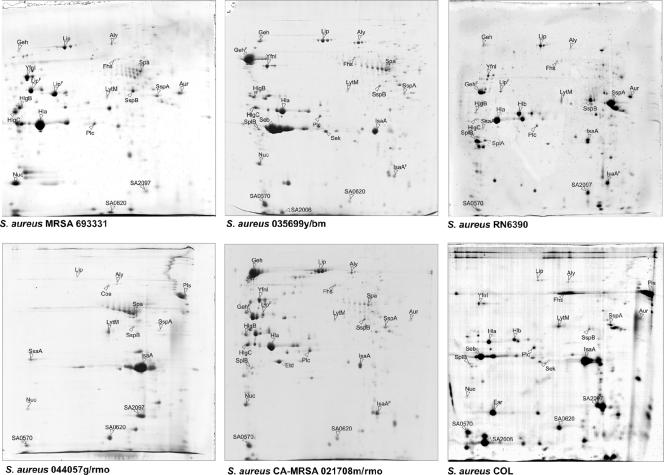
Extracellular proteomes of different S. aureus strains. Proteins in the growth medium fractions of different staphylococcal isolates, grown in TSB medium (37°C) to an optical density at 540 nm (OD540) of 10, were separated by 2D-PAGE using immobilized pH gradient strips in the pH range of 3 to 10 (Amersham Pharmacia Biotech, Piscataway, N.J.). Each gel was loaded with 350 μg protein extracts and, after electrophoresis, stained with colloidal Coomassie blue. Proteins were identified by matrix-assisted laser desorption ionization-time of flight mass spectrometry. The corresponding protein spots are labeled with protein names according to the S. aureus N315 database or NCBI entries for proteins not present in N315. The S. aureus strains that were used in these experiments are RN6390 and COL and four clinical isolates from the University Medical Center Groningen, named MRSA693331, 035699y/bm, 0440579/rmo, and CA-MRSA021708m/rmo.
FIG. 3.
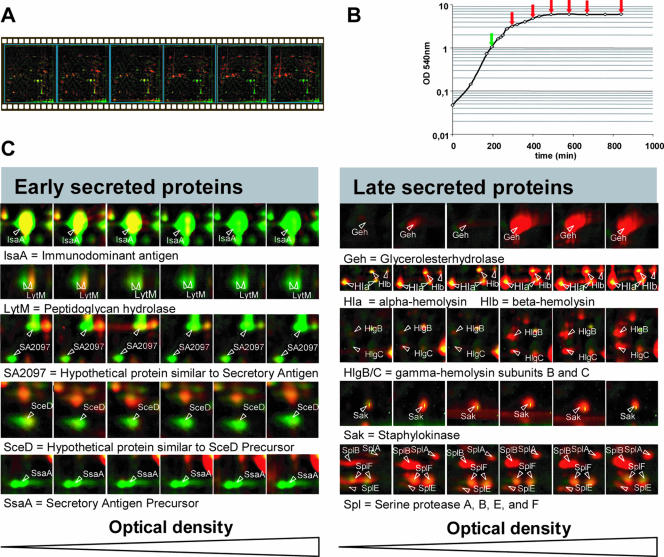
Dynamics of the amount of extracellular proteins during growth of S. aureus RN6390 in TSB medium. (A) Individual dual-channel 2D patterns of extracellular proteins during the different phases of the growth curve for cells grown in TSB medium were assembled into a movie. The protein pattern at an OD540 of 1 (labeled in green) was compared with the protein patterns at higher optical densities (labeled in red). As a consequence of dual channel labeling, spots where the intensities do not differ between the compared gels are yellow, and spots with different intensities are either green or red (15). (B) Growth curve for S. aureus RN6390 grown in TSB medium, as determined by OD540 readings. The sampling points for proteomics analyses are indicated by arrows. (C) Proteomic signatures of selected proteins representing different regulatory groups, as revealed by dual-channel imaging. The amounts of the respective proteins at an OD540 of 1 (spots labeled in green) for cells grown in TSB medium were compared with the relative amounts of these proteins at higher optical densities (spots labeled in red). Proteins were stained with Sypro ruby.
EXPORTED STAPHYLOCOCCAL VIRULENCE FACTORS
S. aureus and S. epidermidis are organisms that occur naturally in and on the human body. While S. epidermidis is mostly present on human skin, S. aureus can be found on mucosal surfaces. S. aureus is carried by 30 to 40% of the population (143) and can be identified readily in the nose, but the organism can also be detected in other moist regions of the human body, such as the axillae, perineum, vagina, and rectum, which thereby form a major reservoir for infections. Although most staphylococcal infections are nosocomial (i.e., hospital acquired), an increase in the number of cases of community-acquired, antibiotic (methicillin)-resistant infections is currently being observed worldwide (27, 67, 185). The risk of intravascular and systemic infection by S. aureus rises when the epithelial barrier is disrupted by intravascular catheters, implants, mucosal damage, or trauma. Interestingly, after infection, cells of S. aureus can persist unnoticed in the human body for a long time (years), after which they can suddenly cause another infection. S. aureus is primarily an extracellular pathogen whose colonization and invasion of human tissues and organs can lead to severe cytotoxic effects. Nevertheless, S. aureus can also be internalized by various cells, including nonphagocytic cells, which seems to induce apoptosis (43, 72, 120). Although S. aureus has the potential to form biofilms (64), S. epidermidis infections are particularly notorious for the formation of thick multilayered biofilms on indwelling catheters and other implanted devices. The formation of such a biofilm takes place in several steps, during which the bacteria first adhere rapidly to the surface of a polymer material that has been coated with a film of proteinaceous and nonproteinaceous organic host molecules (56). Bacteria that adhere to this film produce extracellular polymeric substances, mostly polysaccharides and proteins, in turn resulting in a strong attachment to the polymer surface and other bacteria in the growing biofilm. Ultimately, the biofilm is composed of multiple layers of cells, cellular debris, polysaccharides, and proteins. S. epidermidis factors that are essential for biofilm formation include the polysaccharide intercellular adhesin (107), the accumulation-associated protein (157), and the biofilm-associated protein (184). Polysaccharide intercellular adhesin is most likely identical to the polysaccharide adhesion protein.
Virulence of S. aureus
The pathogenicity of S. aureus is caused by the expression of an arsenal of virulence factors (Table 1), which can lead to superficial skin lesions, such as styes, furunculosis, and paronychia, or to more serious infections, such as pneumonia, mastitis, urinary tract infections, osteomyelitis, endocarditis, and even sepsis. In very rare cases, S. aureus causes meningitis. The virulence factors that S. aureus employs to cause these diseases are displayed at the surface of the staphylococcal cell or secreted into the host milieu (57). Specifically, these virulence factors include (i) surface proteins that promote adhesion to and colonization of host tissues, (ii) invasins that are exported to an extracytoplasmic location and promote bacterial spread in tissues (leukocidin, kinases, and hyaluronidase), (iii) surface factors that inhibit phagocytic engulfment (capsule and protein A), (iv) biochemical properties that enhance staphylococcal survival in phagocytes (carotenoid and catalase production), (v) immunological disguises (protein A, coagulase, and clotting factor), (vi) membrane-damaging toxins that disrupt eukaryotic cell membranes (hemolysins and leukotoxin), (vii) superantigens that contribute to the symptoms of septic shock (SEA-G, toxic shock syndrome toxin [TSST], and ET), and (viii) determinants for inherent and acquired resistance to antimicrobial agents.
TABLE 1.
Virulence factors of S. aureus
| Pathogenic action | Virulence factors | Proteins or other compounds | Functions | References |
|---|---|---|---|---|
| Colonization of host tissues | Surface proteins | ClfA, ClfB, FnbA, FnbB, IsdA, SdrC, SdrD, SdrE, | Adhesins, fibronectin- and fibrinogen-binding proteins | 35, 68, 93, 111, 114, 149, 155,198 |
| Lysis of eukaryotic cell membranes and bacterial spread | Membrane-damaging toxins, invasins | Geh, Hla, Hld, HlgA-C, HysA, Lip, LukD, LukE, LukF, LukS, Nuc | Hemolysins, hyaluronidase, leukocidin, leukotoxin, lipases, nucleases | 97, 108,158 |
| Inhibition of phagocytic engulfment | Surface factors | CapA-P, Efb, Spa | Capsule, protein A | 102,196 |
| Survival in phagocytes | Biochemical compounds | KatA, staphyloxanthin | Carotenoids, catalase production | 96, 109 |
| Immunological disguise and modulation | Surface proteins | ClfA, ClfB, Coa, Spa | Clumping factor, coagulase, protein A | 141, 142, 191 |
| Contribution to symptoms of septic shock | Exotoxins | Eta, Etb, SEA-G, TSST-1 | Enterotoxins SEA to SEG, exfoliative toxin, TSST | 48, 80, 203 |
| Acquired resistance to antimicrobial agents | Resistance proteins | BlaZ, MecA, VanA | Methicillin and vancomycin resistance | 28, 85,199 |
Most virulence factors are expressed in a coordinated fashion during the growth cycle of S. aureus. The best-characterized regulators of virulence factors are the accessory gene regulator (agr) (124, 144, 152) and the staphylococcal accessory regulator (SarA) (29, 30). Ziebandt et al. (208) showed that extracellular proteins can be divided into two groups based on the timing of their expression in cells grown in tryptic soy broth (TSB), i.e., proteins that are expressed only at low cell densities and proteins exclusively expressed at high cell densities. agr seems to be an important positive regulator of proteins that are expressed at higher optical densities (e.g., proteases, hemolysins, and lipases) and a negative regulator of proteins that are expressed during the exponential growth phase (e.g., immunodominant antigen A, secretory antigen precursor, and several proteins with unknown functions). In addition, Gill et al. (63) identified 15 other two-component regulatory systems in the genomes of S. aureus and S. epidermidis that are potentially involved in staphylococcal virulence. In this respect, it is interesting that the antibiotic cerulenin, which is known to inhibit protein secretion by S. aureus at sub-MIC levels, was recently reported to block the transcriptional activation of at least two regulatory determinants, agr and sae. Thus, it seems that cerulenin inhibits the transcription of genes for secretory proteins rather than the secretion process of these proteins (1). In contrast, it was previously believed that cerulenin would interfere with membrane function through an inhibition of normal fatty acid synthesis.
Notably, to date, relatively little information is available on the molecular nature of the stimuli that are perceived by the major regulators of the expression of virulence factors. Overall, it should be clear that strain-specific differences in gene regulation by agr, sae, sarA, or other regulators may dramatically influence the repertoire of produced virulence factors, thereby having a profound impact on the disease-causing potential of different strains. Since the interplay of different regulators and cell-to-cell communication can impact differently on the expression of different virulence factors, even the disease-causing potential of individual S. aureus cells within a genetically identical population may vary.
Resistance of S. aureus to Antibiotics
Resistance of S. aureus to antibiotics was observed very soon after the introduction of penicillin about 60 years ago. In the following years, the amazing ability of staphylococci to develop resistance to antibiotics has resulted in the emergence of methicillin-resistant S. aureus (MRSA) and S. epidermidis strains. In fact, methicillin resistance was observed already in 1961 in nosocomial isolates of S. aureus, 1 year after the introduction of methicillin (85). Resistance towards methicillin is a result of the production of an altered penicillin binding protein, PBP2a (or PBP2′), which has less affinity for most β-lactam antibiotics. The PBP2a protein, which is located at the membrane-cell wall interface, is of major importance for cell wall biogenesis by mediating the cross-linking of peptidoglycans. PBP2a is encoded by the mecA gene, which is located on a mobile genetic element known as the staphylococcal cassette chromosome mec element (SCCmec) (28, 84). SCCmec is a basic mobile genetic element that serves as a vehicle for gene exchange among staphylococcal species (49). In addition to the mecA gene, SCCmec carries the mecA regulatory genes mecI and mecR, an insertion sequence element (IS431mec), and a unique cassette of recombinase genes (ccr), which are responsible for SCCmec chromosomal integration and excision. Five different types of SCCmec elements, types I to V, have been identified so far, based on the classes of mecA gene and ccr gene complexes (84). The type I SCCmec contains the mecA gene as the only resistance element, while the type II and III elements contain, besides mecA, multiple determinants for resistance against non-β-lactam antibiotics. Accordingly, type II and III SCCmec elements are responsible for multidrug resistance in nosocomial MRSA isolates. Type IV SCCmec elements, like type I elements, contain no resistance genes other than mecA, and they are significantly smaller than the type II and III elements. This might serve as an evolutionary advantage, making it easier for these mobile genetic elements to spread across bacterial populations. Type V SCCmec elements are also small compared to the other elements and differ in their set of recombinase genes (84). Whereas the type I to IV SSCmec elements contain the two recombinase genes ccrA and ccrB, the type V elements contain a single copy of a gene, ccrC, homologous to a cassette chromosome recombinase gene. In addition, two open reading frames, hsdS and hsdM, which encode a restriction-modification system, are unique to these elements. Phylogenetic analyses of these genes showed a distant relationship with their homologues in other S. aureus genomes and suggested a foreign origin for these genes.
Vancomycin resistance was first reported for Enterococcus faecium (101), and transfer of vancomycin resistance from enterococci, such as Enterococcus faecalis, to S. aureus has been shown to occur (137). Vancomycin has long been a last-resort antibiotic for multiple-drug-resistant S. aureus strains, but already in 1996 a strain was isolated which showed reduced sensitivity towards vancomycin (78). Shortly afterwards, additional strains were isolated in different countries and were designated vancomycin intermediately resistant S. aureus (VISA). These strains show a significantly thickened cell wall, which allows them to sequester more vancomycin than non-VISA strains, thereby preventing the detrimental effects of this antibiotic (42). A search for the genetic basis of the lowered vancomycin sensitivity of the S. aureus Mu50 strain revealed that important genes for cell wall biosynthesis and intermediary metabolism have mutations compared to those in MRSA strains, which might lead to altered expression of genes involved in cell wall metabolism and a thickened cell wall (4). The first highly-vancomycin-resistant strain was isolated in 2002 (199). This strain was shown to carry a plasmid which contains, among other resistance genes, the vanA gene plus several additional genes required for vancomycin resistance. The proteins encoded by these genes are responsible for replacing the C-terminal d-alanyl-d-alanine (d-Ala-d-Ala) of the disaccharide pentapeptide cell wall precursor with a depsipeptide, d-alanyl-d-lactate (d-Ala-d-Lac), thereby lowering the cell wall affinity for vancomycin (24).
Export of Virulence Factors from the Cytoplasm
Since most proteinaceous virulence factors are displayed at the surface of the staphylococcal cell or released into the medium, it is important for our understanding of the pathogenic potential of these organisms to map their pathways for protein transport. While specific questions relating to surface display or secretion of particular virulence factors have been addressed for several years, more holistic studies on the genomics and proteomics of these processes have been documented in the scientific literature only very recently. Moreover, no systematic analysis of pathways and cellular machinery for protein transport has thus far been performed for staphylococci. This review is aimed at filling this knowledge gap. To do so, we have taken full advantage of the availability of six completely sequenced and annotated S. aureus genomes and one of the two sequenced S. epidermidis strains as well as recently published data on the analysis of staphylococcal cell wall proteomes and exoproteomes. Additionally, we have combined published information with bioinformatics-derived data on all potential signals for protein export from the cytoplasm and secretion into the extracellular milieu or retention in the membrane or cell wall. Since polytopic membrane proteins do not appear to have major direct roles in virulence other than causing drug resistance, such membrane proteins remain beyond the scope of this review. Furthermore, since the secretome of B. subtilis has been characterized extensively, at the level of both the protein export machinery and the exoproteome, we have compared the staphylococcal secretomes with that of B. subtilis. To our knowledge, this has resulted in the first “comparative secretomics” study.
S. AUREUS STRAINS SUITABLE FOR COMPARATIVE SECRETOMICS
Nine sequenced and fully annotated genomes of S. aureus are available in public databases (Table 2) (http://www.ncbi.nlm.nih.gov/genomes/lproks.cgi; http://www.tigr.org), and six of these genomes were used in the present study. These include the genome of one of the first hospital-acquired MRSA isolates, S. aureus COL (63), which has been used widely in research on staphylococcal methicillin and vancomycin resistance. The sequenced MRSA252 strain (79) is a hospital-acquired epidemic strain, which was isolated from a patient who died as a consequence of septicemia. The sequenced MSSA476 strain (79) is a community-acquired invasive strain that is penicillin and fusidic acid resistant but susceptible to most commonly used antibiotics. S. aureus Mu50 and N315 (100) are hospital-acquired MRSA strains isolated from Japanese patients. In addition, the Mu50 strain displays intermediate vancomycin sensitivity. Finally, the community-acquired isolate S. aureus MW2 (7) is a highly virulent MRSA strain isolated from a 16-month-old girl from the United States. Notably, the most widely used laboratory strain, NCTC8325, has been sequenced, but the nucleotide sequence and corresponding annotation were not available for the present analyses. This was also the case for the community-acquired MRSA strain USA300 (46). Furthermore, the sequence of S. aureus RF122, a strain associated with mastitis in cattle, is now also available in the NCBI database (unpublished), but it was not included in the present review, which is primarily focused on staphylococcal pathogenicity towards humans. Using multilocus sequence typing with seven housekeeping genes of the different S. aureus strains, Holden et al. (79) showed that the MRSA252 strain is phylogenetically most distantly related to the other sequenced strains, while the Mu50 and N315 strains are indistinguishable by multilocus sequence typing, as are the MSSA476 and MW2 strains. The COL and NCTC8325 strains are relatively closely related to each other.
TABLE 2.
Sequenced and annotated genomes of S. aureus strains
| Strain | Origina | Genome size (kbp)
|
No. of protein-encoding genes
|
||
|---|---|---|---|---|---|
| Chromosome | Plasmid | Chromosome | Plasmid | ||
| COL | Hospital-acquired MRSA | 2,809 | 4 | 2,615 | 3 |
| MRSA252 | Hospital-acquired MRSA | 2,903 | 2,656 | ||
| MSSA476 | Community-acquired MSSA | 2,800 | 21 | 2,579 | 19 |
| Mu50 | Hospital-acquired VISA | 2,879 | 25 | 2,697 | 34 |
| MW2 | Community-acquired MRSA | 2,820 | 2,632 | ||
| N315 | Hospital-acquired MRSA | 2,815 | 25 | 2,588 | 31 |
| NCTC8325 | Hospital-acquired MSSA | 2,821 | 2,892 | ||
| USA300 | Community-acquired MRSA | 2,873 | 45 | 2,560 | 44 |
| RF122 | Bovine mastitis isolate | 2,743 | 2,515 | ||
MSSA, methicillin-sensitive S. aureus.
Sequenced and annotated genomes of other staphylococcal species, such as S. epidermidis, Staphylococcus haemolyticus,and Staphylococcus carnosus, are also publicly available. However, with the exception of S. epidermidis strain ATCC 12228 (207), these are not included in the present review, which focuses primarily on S. aureus. A comparative genomic analysis of S. aureus COL, Mu50, MW2, and N315 and the sequenced S. epidermidis strains RP62a and ATCC 12228 revealed that these species and strains have a set of 1,681 genes in common (63). In contrast, 454 genes are present in the S. aureus strains but not in S. epidermidis, whereas 286 genes are present in S. epidermidis but not in S. aureus. Most of the strain-specific and species-specific genes can be related to the presence or absence of particular prophages and genomic islands.
PATHWAYS FOR STAPHYLOCOCCAL PROTEIN TRANSPORT
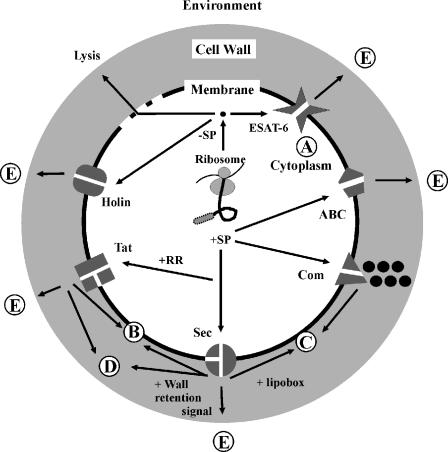
The bacterial machinery for protein transport is currently best described for Escherichia coli (gram negative) and B. subtilis (gram positive) (for reviews, see references 44, 174, and 175). Many of the known components that are involved in the different routes for protein export from the cytoplasm and in posttranslocational modification of exported proteins in these organisms are also conserved in S. aureus and S. epidermidis (Table 3). In general, proteins that are exported are synthesized with an N-terminal signal peptide, which directs them to a particular transport pathway. Consequently, the presently known signal peptides are classified according to the export pathway into which they direct the corresponding proteins or the type of signal peptidase that is responsible for their removal (processing) upon membrane translocation. The staphylococcal protein export pathways that have been characterized experimentally or that can be deduced from sequenced genomes are shown schematically in Fig. 4 and are discussed below. Since these pathways are likely used for the export of virulence factors to the cell surface and the milieu of the host, Fig. 4 can be regarded as a subcellular road map to staphylococcal pathogenesis.
TABLE 3.
Secretion machinery of S. aureus, S. epidermidis, and B. subtilisa
| Pathway and component | Protein(s) | Presence of protein
|
||
|---|---|---|---|---|
| S. aureus | S. epidermidis | B. subtilis | ||
| Sec pathway | ||||
| Chaperone | Ffh | + | + | + |
| FtsY | + | + | + | |
| FlhF | − | − | + | |
| CsaA | − | − | + | |
| Translocation motor | SecA1 | + | + | + |
| SecA2 | + | + | − | |
| Translocation channel | SecY1 | + | + | + |
| SecY2 | + | + | − | |
| SecE | + | + | + | |
| SecG | + | + | + | |
| SecDF | + | + | + | |
| YajC (YrbF) | + | + | + | |
| Lipid modification | Lgt | + | + | + |
| Signal peptidase | SpsA (inactive) | + | + | − |
| SpsB (SipSTUV) | + | +b | + | |
| SipW (ER-type) | − | − | + | |
| LspA | + | +c | + | |
| Folding catalyst | PrsA | + | + | + |
| BdbC | − | − | + | |
| DsbA (BdbD) | + | + | + | |
| Tat pathway | ||||
| Translocase | TatA | + | − | + |
| TatC | + | − | + | |
| Pseudopilin pathway | ComGA | + | + | + |
| ComGB | + | + | + | |
| ComC | + | + | + | |
| Bacteriocins | Bacteriocin-specific ABC transporters | Unknown | Unknown | + |
| Holins | CidA (holin) | + | + | + |
| LrgA (anitholin) | + | + | + | |
| ESAT-6 pathway | EsaA | + | − | + |
| EsaB | + | − | + | |
| EsaCd | + | − | − | |
| EssA | + | − | − | |
| EssB | + | − | + | |
| EssC | + | − | + | |
Based on BLAST searches with the corresponding proteins of B. subtilis in the finished genome database (http://www.ncbi.nlm.nih.gov/sutils/genom_table.cgi).
Two potentially active type I SPases are present in this strain and share homology with B. subtilis SipS and SipU.
Two LspA proteins are present in this strain.
This protein is missing in the S. aureus MRSA252 strain.
FIG. 4.
Staphylococcal pathways to pathogenesis. The figure shows a schematic representation of a staphylococcal cell with potential pathways for protein sorting and secretion. (A) Proteins without signal peptides reside in the cytoplasm. (B) Proteins with one or more transmembrane-spanning domains can be inserted into the membrane via the Sec, Tat, or Com pathway. (C) Lipoproteins are exported via the Sec pathway and are anchored to the membrane after lipid modification. (D) Proteins with cell wall retention signals are exported via the Sec, Tat, or Com pathway and retained in the cell wall via covalent or high-affinity binding to cell wall components. (E) Exported proteins with a signal peptide and without a membrane or cell wall retention signal can be secreted into the extracellular milieu via the various indicated pathways.
Components of the Sec Pathway
The most commonly used pathway for bacterial protein transport is the general secretory (Sec) pathway. Specifically, this pathway is responsible for the secretion of the majority of the proteins found in the exoproteome of B. subtilis, which is probably also the case for most other gram-positive bacteria, including S. aureus (174). Unfortunately, there are very few published data available concerning the Sec pathway of S. aureus, and therefore we have filled in the current knowledge gaps with data obtained from studies of B. subtilis or E. coli. Proteins that are exported via the Sec pathway contain signal peptides with recognition sites for so-called type I or type II SPases. Notably, type II SPase recognition sites overlap with the recognition sites for the diacylglyceryl transferase Lgt. Precursor proteins with a type II SPase recognition sequence are lipid modified prior to being processed, and the resulting mature proteins are retained as lipopoteins in the cytoplasmic membrane via their diacylglyceryl moieties. Furthermore, the Sec-dependent export of proteins can be divided into the following three stages: (i) targeting to the membrane translocation machinery by export-specific or general chaperones, (ii) translocation across the membrane by the Sec machinery, and (iii) posttranslocational folding and modification. If the translocated proteins of gram-positive bacteria lack specific retention signals for the membrane or cell wall, they are secreted into the growth medium.
Preprotein targeting to the membrane.
In B. subtilis, the only known secretion-specific chaperone is the signal recognition particle (SRP), which consists of small cytoplasmic RNA (scRNA), the histone-like protein HBsU, and the Ffh protein. Ffh and HBsU bind to different moieties of the scRNA. Studies with E. coli have shown that upon emergence from the ribosome, the signal peptide of a nascent secretory protein can be recognized by several cytoplasmic chaperones and/or targeting factors, such as Ffh or trigger factor (TF) (55). In contrast to Ffh, which is required for cotranslational protein export in E. coli, the cytoplasmic chaperone SecB has mainly been implicated in posttranslational protein targeting. Notably, however, SecB is absent from the sequenced gram-positive bacteria, including S. aureus and B. subtilis. Most likely, ribosome-nascent chain complexes of S. aureus are thus targeted to the membrane by SRP, which, by analogy to the case in B. subtilis and E. coli, will probably involve the SRP receptor FtsY. At the membrane, the nascent preprotein will be directed to the translocation machinery. This process is likely stimulated by negatively charged phospholipids (45), the Sec translocon (17, 45), and/or the SecA protein (25). In this respect, SecA may function not only as the translocation motor (see below) but also as a chaperone for preprotein targeting (75). While it has been shown that Ffh is essential for growth and viability in E. coli and B. subtilis, this does not seem to be the case for all bacteria. For example, Ffh, FtsY, and scRNA are not essential in Streptococcus mutans. In this organism, the SRP is merely required for growth under stressful conditions, such as low pH (<pH 5), high salt (3.5% NaCl), or the presence of H2O2 (0.3 mM). This suggests that SRP has an important role in the export of proteins to the membrane or cell wall to protect S. mutans against environmental insults (71).
For B. subtilis, it has been proposed that the general chaperone CsaA may have a role in preprotein targeting to the membrane, similar to SecB of E. coli. This view is supported by the observation that the B. subtilis CsaA protein has binding affinity for SecA and preproteins (126). However, CsaA is not conserved in S. aureus. Therefore, it remains to be investigated whether other chaperones with a preprotein targeting function are present in S. aureus.
Translocation across the membrane.
As deduced from known genome sequences, the translocation machinery of S. aureus consists of several Sec proteins. The mode of action of these proteins has been studied in great detail in E. coli (44, 197). After binding of a preprotein to a SecA dimer, the SecA molecules will bind ATP, resulting in conformational changes that promote their insertion together with the preprotein into the membrane-embedded translocation channel. Subsequent hydrolysis of ATP causes SecA to release the preprotein, return to its original conformation, and deinsert from the translocation channel. Repeated cycles of ATP binding and hydrolysis by SecA, together with the proton motive force, drive further translocation of the preprotein across the membrane. The translocation channel is essentially formed by the SecE and SecY proteins, which are conserved in all bacteria (189). An additional nonessential channel component is SecG, which serves to increase the translocation efficiency. While the SecY proteins of different bacteria show a relatively high degree of sequence similarity, the SecE and SecG proteins, though present in all bacteria, are less well conserved. Specifically, the SecE and SecG proteins in B. subtilis, S. aureus, and S. epidermidis are considerably shorter than the equivalent proteins of E. coli. Although SecA and SecY of S. aureus (referred to here as SecA1 and SecY1) have not yet been characterized functionally, they are of major importance for the growth of S. aureus. This was demonstrated with the help of specific antisense RNAs (86). Upon secA antisense induction, a strong growth defect was observed, and secY antisense induction turned out to be lethal.
Remarkably, the genome of S. aureus contains a second set of secA and secY genes, referred to as secA2 and secY2, respectively. In contrast to the SecA1 and SecY1 proteins, their homologues are not essential for growth and viability. It is presently unknown whether SecA2 and SecY2 transport specific proteins across the membrane of S. aureus. However, it has been shown for other pathogenic gram-positive bacteria which also possess a second set of SecA and SecY proteins that these proteins are required for the transport of certain proteins related to virulence. In Streptococcus gordonii, the export of GspB, a large cell surface glycoprotein that contributes to platelet binding, seems to be dependent on the presence of SecA2 and SecY2 (13). This protein contains large serine-rich repeats, an LPXTG motif for cell wall anchoring (see below), and a very large signal peptide of 90 amino acids. In Streptococcus parasanguis, two other proteins, FimA and Fap1, are known to be secreted via SecA2-dependent membrane translocation. FimA is a (predicted) lipoprotein which is a major virulence factor implicated in streptococcal endocarditis. The FimA homologue in S. aureus is a manganese-binding lipoprotein (MntA) associated with an ATP-binding cassette (ABC) transporter. Fap1 of S. parasanguis is involved in adhesion to the surfaces of teeth. Like FimA, Fap1 has a long signal peptide of 50 amino acids, serine-rich repeats, and an LPXTG motif for cell wall anchoring. To date, it is not known what determines the difference in specificity of the SecA1/SecY1 and SecA2/SecY2 translocases. It is also not known whether the SecA2/SecY2 translocase shares SecE and/or SecG with the SecA1/SecY1 translocase, whether these translocases function completely independently from each other, or whether mixed translocases can occur. Clearly, the secE and secG genes are not duplicated in S. aureus. The SecE and SecG functions in the SecA2/SecY2 translocase may, however, be performed by the S. aureus homologues of the Asp4 and Asp5 proteins of S. gordonii, for which SecE- and SecG-like functions have been proposed (172).
In E. coli, the heterotrimeric SecYEG complex is associated with another heterotrimeric complex composed of the SecD, SecF, and YajC proteins (138). This complex has been shown to be involved in the cycling of SecA (51) and the release of the translocated protein from the translocation channel (113). SecD and SecF are separate but structurally related proteins in most bacteria, including E. coli. Interestingly, in B. subtilis and S. aureus, natural gene fusions between the secD and secF genes are observed. Accordingly, the corresponding SecDF proteins can be regarded as molecular “Siamese twins” (20). Unlike SecA, SecY, and SecE, the SecDF protein of B. subtilis is not essential for growth and viability, and its role in protein secretion is presently poorly understood (20). B. subtilis secDF mutants showed only a mild secretion defect under conditions of high-level synthesis of secretory proteins. The known SecDF proteins have 12 (predicted) transmembrane domains with two large extracytoplasmic loops, between the first and second transmembrane segments and between the seventh and eighth transmembrane segments. For E. coli SecD, it has been shown that small deletions in the large extracytoplasmic loop result in malfunctioning of the protein, while the stability of the SecDF-YajC complex is not affected (138). It has therefore been proposed that this loop in SecD might provide a protective structure in which translocated proteins can fold more efficiently. The large extracytoplasmic loop in SecF has been proposed to interact with SecY, thereby stabilizing the translocation channel formed by SecYEG. Homologues of the E. coli YajC protein are present in many bacteria, including S. aureus and B. subtilis (YrbF), but their role in protein secretion has not yet been established. It is presently not known whether the S. aureus SecDF-YajC complex associates specifically with the SecA1/SecY1 translocase, the SecA2/SecY2 translocase, or both.
Type I signal peptidases.
Signal peptides of preproteins are cleaved during or shortly after translocation by an SPase I or SPase II, depending on the nature of the signal peptide (180, 187). The B. subtilis chromosome encodes five type I SPases, named SipS, SipT, SipU, SipV, and SipW (176, 178, 186). Two of these, SipS and SipT, are of major importance for the processing of secretory preproteins, growth, and viability. In S. aureus, only two SPase I homologues are present, namely, SpsA and SpsB. The catalytically active SPase I in S. aureus is SpsB, which is probably essential for growth and viability (38). This SPase can be used to complement an E. coli strain that is temperature sensitive for preprotein processing. In general, type I SPases recognize residues at the −1 and −3 positions relative to the cleavage site (187). For B. subtilis, it has been shown that all secretory proteins identified by proteomics have Ala at the −1 position and that 71% of these secretory proteins have Ala at the −3 position (174). In contrast, various residues are tolerated at the −2 position, including Ser, Lys, Glu, His, Tyr, Gln, Gly, Phe, Leu, Ala, Asp, Asn, Trp, and Pro. Interestingly, Bruton et al. (23) studied the cleavage sites in substrates of S. aureus SpsB and showed that this enzyme has a preference for basic residues at the −2 position and tolerance for hydrophobic residues at this position. However, an acidic residue at the −2 position resulted in a significantly reduced rate of processing. The second SPase I homologue of S. aureus (SpsA) appears to be inactive, since it lacks the catalytic Ser and Lys residues, which are replaced with Asp and Ser residues, respectively. The presence of an apparently catalytically inactive SpsA homologue is a conserved feature of all staphylococci with sequenced genomes. Notably, in addition to an inactive SpsA homologue, S. epidermidis contains two SpsB homologues, which show the greatest similarity to SipS and SipU of B. subtilis. To date, it is not known whether the inactive SpsA homologues contribute somehow to protein secretion in these organisms.
Lipid modification of lipoproteins.
In E. coli, lipid modification of prolipoproteins involves three sequential steps that are catalyzed by cytoplasmic membrane-bound proteins. The first step involves the transfer of a diacylglyceryl group from phosphatidylglycerol to the sulfhydryl group of the invariant Cys residue present at the +1 position of the signal peptide cleavage site in lipoprotein precursors. This step is catalyzed by a phosphatidyl glycerol diacylglyceryl transferase (Lgt), as shown for E. coli by Sankaran and Wu (161). The recognition sequence for Lgt, which includes the Cys residue that becomes modified with diacylglyceryl, is known as the lipobox. Lipid modification of the lipobox Cys residue is necessary for the lipoprotein-specific type II signal peptidase (LspA) to recognize and cleave the signal peptide of a prolipoprotein, which represents the second step in lipoprotein modification. The third step involves the transfer of an N-acyl group by an N-acyl transferase (Lnt), resulting in the formation of N-acyl diacylglycerylcysteine at the N terminus of the mature lipoprotein. Although Lgt and LspA are present in most, if not all, bacteria, Lnt is present only in gram-negative bacteria (180). Like the case for other gram-positive bacteria, no homologue of Lnt could be detected in the genomes of S. aureus or S. epidermidis (169), which suggests that the lipoproteins of these organisms are not N acylated.
S. aureus Lgt is a protein of 279 amino acids that contains a highly conserved HGGLIG motif (residues 97 to 102). Although the His residue in this motif was shown to be essential for the catalytic activity of E. coli Lgt (160), it is not strictly conserved in all known Lgt proteins. On the other hand, the strictly conserved Gly at position 103 of E. coli Lgt, which is equivalent to Gly98 of S. aureus Lgt, is required for the activity of this protein. Stoll et al. (169) showed that an S. aureus lgt mutation has no effect on growth in broth, as also observed for B. subtilis (104). Nevertheless, the absence of Lgt has a considerable effect on the induction of an inflammatory response. Importantly, lipid modification serves to retain exported proteins at the membrane-cell wall interface. This is particularly relevant for gram-positive bacteria, which lack an outer membrane that represents a retention barrier for exported proteins. In the absence of Lgt, B. subtilis cells release a variety of lipoproteins into the extracellular milieu, in the form of both unmodified precursor proteins and alternatively processed mature proteins that lack the N-terminal Cys residue (3). Similarly, the S. aureus lgt mutation resulted in the shedding of certain abundant lipoproteins, such as OppA, PrsA, and SitC, into the broth. These lipoproteins are normally retained in the membrane or cell wall of S. aureus.
Type II signal peptidase.
As described above, lipoprotein signal peptides of prolipoproteins are cleaved by type II SPases after the Cys residue in the lipobox is modified by Lgt. Although B. subtilis and many other bacteria contain only one copy of the lspA gene, some organisms, such as S. epidermidis, Bacillus licheniformis, and Listeria monocytogenes, contain a second copy. LspA is a membrane protein that spans the membrane four times, and both its N and C termini face the cytoplasmic side of the membrane (178, 188). Six amino acid residues are important for SPase II activity, of which two Asp residues form the active site (178). While processing of lipoproteins by LspA is essential for growth and viability for E. coli and other gram-negative bacteria (202), it is not essential for B. subtilis (177) and other gram-positive bacteria, such as Lactococcus lactis (190). This suggests that processing of prolipoproteins is not essential for their functionality. This view is supported by the fact that PrsA, a lipoprotein required for correct folding of translocated proteins, is essential for viability of B. subtilis (99). In the absence of LspA, some of the lipoproteins of B. subtilis are processed in an alternative way by unidentified proteases, and the activity of unprocessed lipoproteins in lspA mutants is reduced. Also, in these B. subtilis mutants, secretion of the nonlipoprotein AmyQ was severely reduced (177). This reduction might be the consequence of a malfunction of unmodified PrsA in AmyQ folding. Although most lspA mutants have been studied in gram-negative bacteria and a few nonpathogenic gram-positive bacteria (177, 190), Sander et al. (159) showed a severely attenuated phenotype of lspA mutants of the pathogen Mycobacterium tuberculosis, which implies an important role for lipoprotein processing by LspA during infection with M. tuberculosis. In S. aureus, both the lspA and lgt genes are present as single copies in the genomes of all six sequenced strains. Interestingly, one of the two LspA homologues in S. epidermidis (125 amino acids) is considerably shorter than other known LspA proteins, including its large paralogue (177 amino acids). This is mainly the result of an additional N-terminal transmembrane domain in the large LspA proteins. As a result, the short S. epidermidis LspA protein is predicted to have three membrane-spanning domains, with the N terminus located on the outside of the cell, the C terminus located on the inside of the cell, and the (putative) active-site Asp residues located on the outer surface of the cytoplasmic membrane.
Signal peptide peptidase.
After translocation and processing of the preproteins by signal peptidases, the signal peptides are rapidly degraded by signal peptide peptidases (SPPases). In B. subtilis, two SPPases, TepA and SppA, are known to be involved in translocation and processing of preproteins (21). While TepA is required for translocation and processing of preproteins, SppA is required only for efficient processing of preproteins. Remarkably, no homologues of SppA or TepA were detectable by BLAST searches in the sequenced genomes of S. aureus and S. epidermidis. As reported by Meima and van Dijl (119), L. lactis contains a protein that shows limited similarity to TepA of B. subtilis and ClpP of Caenorhabditis elegans, suggesting that this protein might be an SPPase analog in L. lactis. In S. aureus and S. epidermidis, this protein homologue also seems to be present and is predicted to be a cytoplasmic membrane protein (our unpublished observations).
Folding catalysts (PrsA and BdbD).
Proteins that are transported across the membrane in a Sec-dependent manner emerge at the extracytoplasmic membrane surface in an unfolded state. These proteins need to be rapidly and correctly folded into their native and protease-resistant conformation before they are degraded by proteases in the cell wall or extracellular environment (162). An important folding catalyst in B. subtilis is PrsA, which shows homology to peptidyl-prolyl cis/trans-isomerases. PrsA is a lipoprotein (see “Lipoproteins” below) that is essential for efficient protein secretion and cell viability in B. subtilis (99, 162). Studies on the effects of PrsA depletion showed that the relative amounts of extracellular proteins from PrsA-depleted cells were significantly reduced (192). No data have been published on S. aureus mutants lacking PrsA, and it will be interesting to investigate whether PrsA is also essential for the viability and virulence of this organism. It has already been shown that S. aureus lacking Lgt releases an increased amount of PrsA into the extracellular milieu (169), which might indicate that (most) pre-PrsA is not fully functional but is sufficient for viability. The observation by Stoll et al. (169) also shows that, like the case in B. subtilis (3), unmodified pre-PrsA is not effectively retained in the cytoplasmic membrane.
Other proteins that are involved in proper folding of extracellular proteins in B. subtilis are the membrane proteins BdbC and BdbD, which are involved in the formation of disulfide bonds. Both proteins have been shown to be necessary for stabilization of the membrane- and cell wall-associated pseudopilin ComGC (118). This protein, which is required for DNA binding and uptake during natural competence, contains an intramolecular disulfide bond (31). Both BdbC and BdbD are also important for the folding of heterologously produced E. coli PhoA, which contains two disulfide bonds, into an active and protease-resistant conformation (21, 118). Although a homologue of BdbD (named DsbA) is present in S. aureus, there is no homologue of BdbC in this organism. The same appears to be true for S. epidermidis. Nevertheless, measurements of the redox potential of purified DsbA indicated that this protein can act as an oxidase, and this view was confirmed by complementation studies with a dsbA mutant strain of E. coli (53). The absence of a BdbC homologue in the staphylococci is remarkable, since B. subtilis BdbC and BdbD are jointly required for the folding of ComGC and E. coli PhoA. Notably, all sequenced S. aureus genomes encode homologues of ComGC, including the Cys residues that form the disulfide bond in B. subtilis ComGC. This raises the question of whether ComGC of S. aureus does indeed contain a disulfide bond and, if so, which protein(s) is involved in the formation of this disulfide bond. Notably, S. aureus DsbA was recently shown to be a lipoprotein that does not seem to contribute to the virulence of this organism, as tested in mouse and Caenorhabditis elegans models (53). Furthermore, DsbA was shown to be dispensable for β-hemolysin activity, despite the fact that this protein contains a disulfide bond, which is required for activity (54). Therefore, the biological function of DsbA in staphylococci remains to be elucidated.
Tat Pathway
The twin-arginine translocation (Tat) pathway exists in many bacteria, archaea, and chloroplasts. This pathway was named after the consensus double (twin) Arg residues that are present in the signal peptide. The twin Arg residues are part of a motif that directs proteins specifically into the Tat pathway. In contrast to the Sec machinery, where only unfolded proteins are translocated across the membrane, the Tat machinery is capable of translocating folded proteins. In gram-negative bacteria, streptomycetes, mycobacteria, and chloroplasts, an active Tat pathway seems to require three core components, named TatA, TatB, and TatC (14, 47, 125, 154, 204). In all gram-positive bacteria except streptomycetes and Mycobacterium smegmatis, the Tat pathway involves only TatA and TatC (47, 204). Recent studies with E. coli and chloroplasts have resulted in a model that proposes key roles for TatB-TatC complexes in signal peptide reception and for TatA-TatB-TatC complexes in preprotein translocation (2, 36). Interestingly, certain mutations in E. coli TatA have been shown to allow this protein to compensate for the absence of TatB (18). This demonstrates that TatA is intrinsically bifunctional, which is consistent with the fact that most gram-positive bacteria lack TatB but have TatA (90). In B. subtilis, two minimal TatA-TatC translocases with distinct specificities are active (88). While the constitutively expressed TatAy-TatCy translocase of B. subtilis is required for secretion of a protein with unknown function, YwbN, the TatAd-TatCd translocase seems to be expressed only under conditions of phosphate starvation for secretion of the phosphodiesterase PhoD (175, 188). Most other gram-positive bacteria that have tatA and tatC genes, including S. aureus, appear to have only one TatA-TatC translocase. The functionality of the S. aureus Tat translocase remains to be demonstrated. In contrast to S. aureus, S. epidermidis seems to lack a Tat pathway.
Pseudopilin Export (Com) Pathway
In B. subtilis, four proteins, ComGC, ComGD, ComGE, and ComGG, have been identified as having an N-terminal pseudopilin-like signal peptide (174, 175). All four of these proteins are involved in DNA binding and uptake and are localized in the membrane and cell wall. It is thought that these proteins form a pilus-like structure in the cell wall or modify the cell wall to provide a passage for DNA uptake. Translocation to the extracytoplasmic membrane surface is possible only when these proteins are processed by the pseudopilin-specific SPase ComC in B. subtilis (52). SPases of this type are bifunctional and catalyze not only signal peptide cleavage but also methylation of the N terminus of the mature protein (170). Furthermore, export and functionality of the four ComG proteins depend on the integral membrane protein ComGB and the traffic ATPase ComGA, which is located at the cytoplasmic side of the membrane (32, 69). Homologues of ComC, ComGA, ComGB, and ComGC, but not ComGD, ComGE, and ComGG, are present in the six sequenced S. aureus strains. This suggests that the Com system of S. aureus is not involved in DNA uptake but is part of another solute transport process.
ABC Transporters
Bacteriocins are peptides or proteins that inhibit the growth of other bacteria. Most of the characterized bacteriocins can be divided into several classes, depending on specific posttranslational modifications, the presence and processing of particular leader peptides, and the machinery for export from the cytoplasm. A well-described class of bacteriocins is formed by the lantibiotics. Members of this class are composed of short peptides that contain posttranslationally modified amino acids, such as lanthionine and β-methyllanthionine (117). The production of bacteriocins in S. aureus has been described for various strains. S. aureus C55 produces the two lantibiotics C55α and C55β (129). These lantibiotics are both encoded by a 32-kb plasmid, which is readily lost upon growth at elevated temperatures. C55α and C55β showed antimicrobial activity towards other S. aureus strains and Micrococcus luteus but not towards S. epidermidis. Furthermore, the nonlantibiotics BacR1 (40), aureocin A53 (134), and aureocin A70 (132, 133) have been identified as bacteriocins with activity against a broad range of bacteria. The genes for both aureocins are located on a plasmid that is present in S. aureus strains isolated from milk. By analogy with the well-described bacteriocin export machineries of other organisms (73, 145), it can be anticipated that all of the aforementioned bacteriocins are exported to the external staphylococcal milieu by dedicated ABC transporters. However, no experimental evidence for this assumption has been published for S. aureus. Notably, it has been demonstrated that secretion of the lantibiotics epidermin and gallidermin of S. epidermidis Tü3298 and Staphylococcus gallinarum, respectively, is facilitated by so-called one-component ABC transporters. Specifically, the ABC transporter GdmT has been implicated in the transport of these lantibiotics (145).
Holins
Holins are dedicated export systems for peptidoglycan-degrading endolysins that have been implicated in the programmed cell death of bacteria. These exporters, which are composed of homo-oligomeric complexes, can be subdivided into two classes, depending on the number of transmembrane segments. While class I holin subunits have three transmembrane segments, class II holin subunits have two transmembrane segments (206). In S. aureus, the lrg and cid operons are involved in murein hydrolase activity and antibiotic tolerance (66, 153). A disrupted lrg operon leads to an increase in murein hydrolase activity and a decrease in penicillin tolerance, and a disrupted cid operon leads to a decrease in murein hydrolase activity and an increase in penicillin tolerance. It is still unclear how the CidA and LrgA proteins are involved in these mechanisms, but these proteins display significant similarity to the bacteriaphage holin protein family, suggesting that they have a role in protein export. It has therefore been proposed that the CidA and LrgA proteins act on murein hydrolase activity and antibiotic tolerance in a manner analogous to that of holins and antiholins, respectively (11, 153). Sequence similarity searches showed that the genes for LrgA and CidA are conserved in the six sequenced S. aureus strains as well as in S. epidermidis and B. subtilis. Notably, none of the three holins of B. subtilis were shown to be involved in the secretion of proteins to the extracellular milieu (174, 200).
ESAT-6 Pathway
The ESAT-6 secretion pathway was first described for M. tuberculosis. It has been proposed that at least two virulence factors, ESAT-6 (early secreted antigen target, 6 kDa) and CFP-10 (culture filtrate protein, 10 kDa), are secreted via this pathway in a Sec-independent manner (16, 166). Since this pathway was discovered in mycobacteria, it is also known as the Snm pathway (secretion in mycobacteria) (37). The genes for ESAT-6 and CFP-10 are located in conserved gene clusters, which also encode proteins with domains that are conserved in FtsK- and SpoIIIE-like transporters. These conserved FtsK/SpoIIIE domains have been termed FSDs (26). In other gram-positive bacteria, including S. aureus, B. subtilis, Bacillus anthracis, Clostridium acetobutylicum, and L. monocytogenes, homologues of ESAT-6 have been identified (140). The genes for these ESAT-6 homologues are also found in gene clusters that contain at least one gene for a membrane protein with an FSD. In S. aureus, two proteins, named EsxA and EsxB, have been identified that seem to be secreted via the ESAT-6 pathway (26). The esxA and esxB genes are part of a cluster containing six other genes for proteins that have been implicated in the translocation of EsxA and EsxB. These include the EsaB and EsaC proteins, with a predicted cytoplasmic location, as well as the predicted membrane proteins EsaA, EssA, EssB, and EssC, among which EssC contains an FSD. Mutations in essA, essB, or essC result in a loss of EsxA and EsxB production, which may be related to inhibition of the synthesis of these proteins or their folding into a protease-resistant conformation. All sequenced S. aureus strains contain this cluster of esa, ess, and esx genes, but it seems to be absent from S. epidermidis. Interestingly, the genes for EsxB and EsaC appear to be absent from the S. aureus MRSA252 strain. This implies that the ESAT-6 machinery of this strain may be required for the transport of only EsxA and perhaps a few other unidentified proteins. If so, EsaC would be dispensable for an active ESAT-6 pathway and might be specifically involved in the export of EsxB. Alternatively, the ESAT-6 pathway could be inactive in the S. aureus MRSA252 strain due to the absence of EsaC.
Lysis
Various studies have shown that certain proteins with typical cytoplasmic functions and without known signals for protein secretion can nevertheless be detected in the extracellular proteomes of different bacteria (174). Notably, many of these proteins, such as catalase, elongation factor G, enolase, glyceraldehyde-3-phosphate dehydrogenase, GroEL, and superoxide dismutase, are among the most highly abundant cytoplasmic proteins. This makes it likely that they are detectable in the extracellular proteome due to cell lysis. Perhaps such proteins are more resistant to extracytoplasmic degradation than are other proteins that are simultaneously released by lysis. However, the possibility that the extracellular localization of typical cytoplasmic proteins is due to the activity of as yet unidentified export pathways cannot be excluded. Clearly, until recently this possibility did still apply for the EsxA and EsxB proteins, which are now known to be exported via the ESAT-6 pathway. A clear indication that the presence of certain “cytoplasmic” proteins in the extracytoplasmic milieu of bacteria may relate to specific export processes was provided by Boël and coworkers (19), who showed that 2-phosphoglycerate-dependent automodification of enolase is necessary for its export from the cytoplasm.
PROPERTIES OF STAPHYLOCOCCAL SIGNAL PEPTIDES AND CELL RETENTION SIGNALS
Signal Peptides
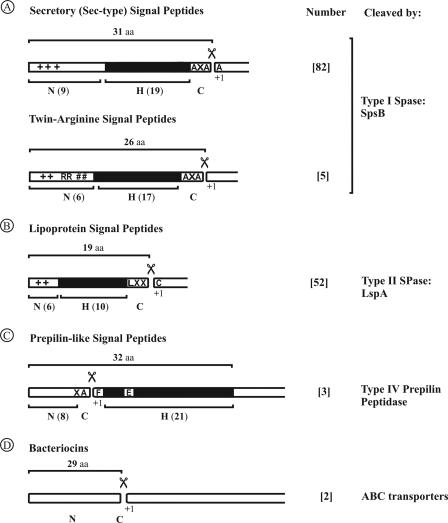
All proteins that have to be transported from the cytoplasm across the membrane to the extracytoplasmic compartments of the cell, or the extracellular milieu, need to contain a specific sorting signal for their distinction from resident proteins of the cytoplasm. The known bacterial sorting signals for protein export from the cytoplasm are signal peptides (195). These signal peptides can be classified by the transport and modification pathways into which they direct proteins. Presently, four different bacterial signal peptides are recognized that share a common architecture but differ in the details (Fig. 5). Two of these direct proteins into the widely used Sec pathway, including the secretory (Sec-type) signal peptides and the lipoprotein signal peptides. Proteins with Sec-type or lipoprotein signal peptides are processed by different SPases (type I and type II SPases, respectively) and are targeted to different destinations. In S. aureus, proteins with Sec-type signal peptides are processed by the type I SPase SpsB and are targeted to the cell wall or extracellular milieu. Proteins with a lipoprotein signal peptide are lipid modified by Lgt prior to being processed by the type II SPase LspA. In principle, these lipoproteins are retained at the membrane-cell wall interface, but they can be liberated from this compartment by proteolytic removal of the N-terminal Cys that contains the diacylglyceryl moiety (3). Proteins with twin-arginine (RR) signal peptides appear to be processed by type I SPases, at least in B. subtilis, and are targeted to the cell wall or extracellular milieu (174). Proteins with a pseudopilin signal peptide are processed by the pseudopilin signal peptidase ComC and most likely are localized to the cytoplasmic membrane and the cell wall. Finally, bacteriocins contain a completely different sorting and modification signal that is usually called the leader peptide. The known leader peptides show no resemblance to the aforementioned signal peptides. The export of bacteriocins via ABC transporters results in their secretion into the extracellular milieu (121, 164).
FIG. 5.
General properties and classification of S. aureus signal peptides. Signal peptide properties are based on SPase cleavage sites and the export pathways by which the preproteins are exported. Predicted signal peptides (144) were divided into the following five distinct classes: secretory (Sec-type) signal peptides, twin-arginine (RR/KR) signal peptides, lipoprotein signal peptides, pseudopilin-like signal peptides, and bacteriocin leader peptides. Most of these signal peptides have a tripartite structure, with a positively charged N domain (N) containing lysine and/or arginine residues (indicated by plus signs), a hydrophobic H domain (H, indicated by a black box), and a C domain (C) that specifies the cleavage site for a specific SPase. Where appropriate, the most frequently occurring amino acid residues at particular positions in the signal peptide or mature protein are indicated. Also, the numbers of signal peptides identified for each class and the respective SPase are indicated.
Sec-type, lipoprotein, and RR signal peptides contain three distinguishable domains, the N, H, and C domains. The N-terminal domain contains positively charged amino acids, which are thought to interact with the secretion machinery and/or negatively charged phospholipids in the membrane. The H domain is formed by a stretch of hydrophobic amino acids which facilitate membrane insertion. Helix-breaking residues in the middle of the H domain may facilitate H domain looping during membrane insertion and translocation of the precursor protein. The subsequent unlooping of the H domain would display the SPase recognition and cleavage site at the extracytoplasmic membrane surface, where the catalytic domains of type I and type II SPases are localized (187). Helix-breaking residues just before the SPase recognition and cleavage site would facilitate precursor processing by SPase I or II. In fact, these helix-breaking residues and the SPase cleavage site, respectively, define the beginning and the end of the C domain. Notably, the C domains of pseudopilin signal peptides are located between the N and H domains (32, 33, 106, 148). Accordingly, processing by pseudopilin-specific SPases, such as ComC, takes place at the cytoplasmic side of the membrane and leaves the H domain attached to the translocated protein.
While many proteins that end up in the extracellular milieu or the cell walls of gram-positive bacteria have signal peptides, proteins without known export signals can also be found at these locations. The relative number of proteins without known signal peptides seems to vary for each organism. While these numbers are relatively low for B. subtilis and S. aureus, they are high for group A streptococcus and M. tuberculosis (174). As indicated above, some of the proteins without known export signals appear to be liberated from the cell by lysis, while others are actively exported, for example, via the ESAT-6 pathway. Although the precise export signal in proteins secreted via the ESAT-6 pathway has not yet been defined, a WXG motif is shared by these proteins and may serve a function in protein targeting (140). Furthermore, the signal for specific release of lysins via holins is presently not known.
Signal Peptide Predictions
Several prediction programs that are accessible through the World Wide Web are useful tools for predicting whether a given protein contains some type of sorting signal or SPase cleavage site. The programs that we used in this and other studies were SignalP-NN and SignalP-HMM, version 2.0 (136), LipoP, version 1.0 (94), PrediSi (76), and Phobius (95). These programs were designed to identify Sec-type signal peptides, N-terminal membrane anchors (Phobius), or lipoprotein signal peptides in gram-negative bacteria (LipoP). The TMHMM program, version 2.0 (41), was used to exclude proteins with (predicted) multiple membrane-spanning domains. Predictions for proteins containing a signal peptide were performed with the SignalP program, using the neural network and hidden Markov model algorithms. Version 2.0 of the SignalP program was preferred above version 3.0 (12) for our signal peptide predictions for S. aureus and S. epidermidis because the best overall prediction accuracy was obtained with version 2.0 in a recent proteomics-based verification of predicted export and retention signals in B. subtilis (179). Specifically, the hidden Markov model in SignalP 2.0 assigns a probability score to each amino acid of a potential signal peptide and indicates whether it is likely to belong to the N, H, or C domain. Proteins with no detectable N, H, or C domain were excluded from the set. Searching for transmembrane domains was performed with the TMHMM program, and proteins with more than one (predicted) transmembrane domain were excluded from the set because they most likely are integral membrane proteins. All proteins with a predicted C-terminal transmembrane segment in addition to a signal peptide were screened for the presence of a conserved motif for covalent cell wall binding. It should be noted that this approach does not automatically result in the exclusion of potential membrane proteins with one N-terminal transmembrane domain. The LipoP program was used to predict lipoproteins. The combined results of all these programs resulted in a list of proteins which have (i) signal peptides with distinctive N, H, and C domains, (ii) no additional transmembrane domains, and (iii) predicted extracytoplasmic localizations. These proteins were scanned for the presence of proteomics-based consensus motifs for type I, type II, or pseudopilin-specific SPase recognition and cleavage sites, twin-arginine motifs, and known leader peptides of bacteriocins by BLAST searches and by use of the PATTINPROT program (http://npsa-pbil.ibcp.fr), as previously described (179). To define the core exoproteome and variant exoproteome of the S. aureus strains, the sets of proteins with predicted signal peptides were used in multiple BLAST searches with the freeware BLASTall from the NCBI. The output was then filtered using Genome2D (10).
Secretory (Sec-type) signal peptides.
Proteomics-based data sets of membrane, cell wall, and extracellular proteins were extremely valuable for a recent verification of signal peptide predictions for B. subtilis (179). Such data sets are now becoming available for S. aureus, as exemplified by studies on the membrane and cell wall proteomes of S. aureus Phillips (127) and the extracellular proteomes of S. aureus strains derived from the recently sequenced NCTC8325 and COL strains (208, 209) (Fig. 2). Additionally, the extracellular proteomes of several clinical S. aureus isolates have been analyzed (Fig. 2). The membrane, cell wall, and extracellular proteins of S. aureus that have been identified by proteomics, involving 2D-PAGE and subsequent mass spectrometry, are listed in Tables 4 and 5.These tables also show the −3-to-+1 amino acid sequences of the respective signal peptidase cleavage sites, if present.
TABLE 4.
Identified proteins in extracellular proteomes of various S. aureus strains with known signal peptidesa
| Identification no. | Protein | Function | Amino acid at position:
|
Localization | Motifi | |||
|---|---|---|---|---|---|---|---|---|
| −3 | −2 | −1 | +1 | |||||
| 15925728 | SA0022 | Hypothetical protein | S | N | A | A | Extracellular | LPKTG |
| 15925799 | Plc | 1-Phosphatidylinositol phosphodiesterase precursor | A | H | A | S | Extracellular | |
| 15925800 | SA0092 | Hypothetical protein | T | A | G | C | Extracellular | Lipo |
| 15925815b | Spa | Immunoglobulin G binding protein A precursor | A | N | A | A | Membrane/cell wall/extracellular | LPETG |
| 15925838c | SasD | Hypothetical protein | A | H | A | D | Extracellular | LPAAG |
| 15925933 | Coa | Staphylocoagulase precursor | A | D | A | I | Extracellular | |
| 15925978 | LytM | Peptidoglycan hydrolase | A | D | A | A | Extracellular | |
| 15925983d | SA0270 | Hypothetical protein | A | Q | A | Y | Extracellular | |
| 15926008 | SA0295 | Hypothetical protein | A | F | A | K | Extracellular | |
| 15926022 | Geh | Glycerol ester hydrolase | A | Q | A | S | Extracellular | |
| 15926073 | SA0359 | Hypothetical protein | L | T | A | C | Extracellular | Lipo |
| 15926099 | Set6 | Exotoxin 6 | V | Q | A | K | Extracellular | |
| 15926111 | Set15 | Exotoxin 15 | V | K | A | S | Extracellular | |
| 15926112 | SA0394 | Hypothetical protein | A | E | A | S | Extracellular | |
| 15926142 | SA0423 | Hypothetical protein | A | N | A | A | Extracellular | LysM |
| 15926239e | SdrC | Ser-Asp-rich, fibrinogen-binding bone sialoprotein-binding protein | A | K | A | A | Membrane/cell wall | LPETG |
| 15926241 | SdrE | Ser-Asp-rich, fibrinogen-binding bone sialoprotein-binding protein | A | K | A | A | Extracellular | LPETG |
| 15926291 | SA0570 | Hypothetical protein | A | E | A | A | Extracellular | |
| 15926342 | SA0620 | Hypothetical protein | A | Q | A | S | Extracellular | |
| 15926373 | SA0651 | Hypothetical protein | A | L | A | K | Extracellular | |
| 15926417 | SA0695 | Hypothetical protein | I | S | A | C | Extracellular | Lipo |
| 15926548 | GlpQ | Glycerophosphoryl diester phosphodiesterase | A | G | A | E | Extracellular | |
| 15926570 | SA0841 | Hypothetical protein | V | S | A | A | Extracellular | |
| 15926634 | SspB | Cysteine protease precursor | A | K | A | D | Extracellular | |
| 15926635 | SspA | Serine protease, V8 protease, glutamyl endopeptidase | A | N | A | L | Extracellular | |
| 15926639 | Atl | Autolysin, N-acetylmuramyl-l-alanine amidase and endo-β-N-acetylglucosaminidase | V | Q | A | A | Extracellular | GW |
| 15926739 | SA1001 | Hypothetical protein | A | K | A | F | Extracellular | |
| 15926746 | SA1007 | α-Hemolysin precursor | A | N | A | A | Extracellular | |
| 15926969c | SA1221 | Thioredoxin reductase | L | G | A | C | Extracellular | Lipo |
| 15927068 | SA1318 | Hypothetical protein | L | S | G | C | Extracellular | Lipo |
| 15927383 | SplF | Serine protease SplF | A | K | A | E | Extracellular | 2 TMD |
| 15927384e | SplD | Serine protease SplD | A | K | A | E | Membrane/cell wall | 2 TMD |
| 15927385 | SplC | Serine protease SplC | A | N | A | E | Extracellular | |
| 15927386 | SplB | Serine protease SplB | A | K | A | E | Extracellular | |
| 15927387b | SplA | Serine protease SplA | A | K | A | E | Membrane/cell wall | |
| 15927389 | SA1633 | Probable β-lactamase | A | K | A | E | Extracellular | |
| 15927393 | LukD | Leukotoxin, LukD | V | D | A | A | Extracellular | |
| 15927394f | LukE | Leukotoxin, LukE | S | R | A | N | Extracellular | |
| 15927483 | SA1725 | Staphopain, cysteine proteinase | A | N | A | E | Extracellular | |
| 15927517 | SA1755 | Hypothetical protein | A | K | A | F | Extracellular | |
| 15927520b | Sak | Staphylokinase precursor | V | S | A | S | Membrane/cell wall | |
| 15927580 | SA1812 | Hypothetical protein | S | Y | A | K | Extracellular | |
| 15927581 | SA1813 | Hypothetical protein | A | N | S | A | Extracellular | |
| 15927670 | SA1898 | Hypothetical protein | A | H | A | S | Extracellular | |
| 15927785 | SA2006 | Hypothetical protein | A | S | A | D | Extracellular | |
| 15927879 | SsaA | Hypothetical protein | A | H | A | S | Extracellular | |
| 15927884 | SA2097 | Hypothetical protein | A | D | A | A | Extracellular | |
| 15927988e | SA2198 | Hypothetical protein | L | T | A | C | Membrane/cell wall | Lipo |
| 15927996 | Sbi | IgG-binding protein SBI | A | K | A | S | Extracellular | |
| 15927998 | HlgC | γ-Hemolysin component C | A | K | A | A | Extracellular | |
| 15927999 | HlgB | γ-Hemolysin component B | A | N | A | E | Extracellular | |
| 15928148 | IsaA | Immunodominant antigen A | A | H | A | A | Extracellular | |
| 15928216 | ClfB | Clumping factor B | A | Q | A | S | Extracellular | LPETG |
| 15928223b | Aur | Zinc metalloproteinase aureolysin | A | L | A | I | Membrane/cell wall | |
| 15928230 | SA2437 | Hypothetical protein | A | Y | A | D | Extracellular | |
| 15928254e | IcaB | Intercellular adhesion protein B | A | N | A | D | Membrane/cell wall | |
| 15928257 | Lip | Triacylglycerol lipase precursor | A | Q | A | A | Extracellular | |
| 57652419g | Pls | Methicillin-resistant surface protein | A | E | A | A | Extracellular | LPDTG |
| 57651319g | SACOL0478 | Exotoxin 3, putative | V | K | A | S | Extracellular | |
| 57651320g | SACOL0479 | Surface protein, putative | A | E | A | S | Extracellular | |
| 57650159g | Sek | Staphylococcal enterotoxin | A | S | A | Q | Extracellular | |
| 57650160g | Sei | Staphylococcal enterotoxin type I | A | Y | A | D | Extracellular | |
| 57651597g | Seb | Staphylococcal enterotoxin B | V | L | A | E | Extracellular | |
| 57651598g | SACOL0908 | Hypothetical protein | A | K | A | S | Extracellular | |
| 57650600g | SACOL1865 | Serine protease SplE, putative | A | K | A | E | Extracellular | |
| 57650605g | SACOL1870 | Hypothetical protein | A | K | A | E | Extracellular | |
| 57650692g | Hlb | β-Hemolysin/phospholipase C | A | K | A | E | Extracellular | |
| 57651004g | SACOL2505 | Cell wall surface anchor family protein | A | E | A | A | Extracellular | LPKTG |
| 24636603h | Etd | Exfoliative toxin D | S | H | A | E | Extracellular | |
| 24636604h | Probable glutamyl-endopeptidase | V | S | A | S | Extracellular | ||
The annotation of proteins is based on that of S. aureus N315, except for those proteins that are not encoded by the N315 genome.
Also found in membrane/cell wall fraction by Nandakumar et al. (127).
Found only in extracellular proteome described by Ziebandt et al. (208). Note that SA1221 is probably not a thioredoxin reductase, but a phosphate binding protein.
Tyr is not present at the +1 position of the optimized S. aureus SpsB search pattern.
Found only in membrane/cell wall fraction by Nandakumar et al. (127).
Arg is not present at the −2 position of the optimized S. aureus SpsB search pattern.
Encoded by S. aureus COL and absent from N315.
Note that the corresponding gene is not present in the sequenced S. aureus genomes.
TMD, transmembrane domain.
TABLE 5.
Identified proteins in extracellular proteomes of various S. aureus strains without known signal peptides
| Identification no. | Protein | Function | Identification no. | Protein | Function | |
|---|---|---|---|---|---|---|
| 15925748a | XylR | Hypothetical protein | 15926930 | CitB | Aconitate hydratase | |
| 15926081a | AhpF | Alkyl hydroperoxide reductase subunit F | 15926982c | CspA | Major cold shock protein CspA | |
| 15926082b | AhpC | Alkyl hydroperoxide reductase subunit C | 15926993 | OdhA | 2-Oxoglutarate dehydrogenase E1 | |
| 15926091b | GuaB | Inositol-monophosphate dehydrogenase | 15927003 | SA1255 | PTS system, glucose-specific enzyme II, A component | |
| 15926092 | GuaA | GMP synthase | ||||
| 15926202 | ClpC | HSP100/Clp ATPase | 15927005 | SA1257 | Peptide methionine sulfoxide reductase | |
| 15926205 | GltX | Glutamyl-tRNA synthetase | 15927057 | SA1308 | 30S ribosomal protein S1 | |
| 15926225b | Fus | Translational elongation factor G | 15927062 | EbpS | Elastin binding protein | |
| 15926226b | TufA | Translational elongation factor TU | 15927092 | Gnd | Phosphogluconate dehydrogenase | |
| 15926266 | Pta | Phosphotransacetylase | 15927109 | SA1359 | Translation elongation factor EF-P | |
| 15926385 | SA0663 | Hypothetical protein | 15927132 | Pbp3 | Penicillin binding protein 3 | |
| 15926396b | YfnI | Hypothetical protein with five transmembrane segments and a potential SPase I cleavage site | 15927133 | SodA | Superoxide dismutase SodA | |
| 15927160b | DnaK | DnaK protein | ||||
| 15926429 | SA0707 | Hypothetical protein | 15927161 | GrpE | GrpE protein | |
| 15926441 | TrxB | Thioredoxin reductase | 15927190 | GreA | Transcription elongation factor | |
| 15926445 | ClpP | Peptidase | 15927229 | SA1475 | Hypothetical protein | |
| 15926449b | Gap | Glyceraldehyde-3-phosphate dehydrogenase | 15927254 | Tig | Trigger factor (prolyl isomerase) | |
| 15926450 | Pgk | Phosphoglycerate kinase | 15927273 | CitZ | Citrate synthase II | |
| 15926451 | Tpi | Triosephosphate isomerase | 15927286 | Ald | Alanine dehydrogenase | |
| 15926452 | Pgm | 2,3-Diphosphoglycerate-independent phosphoglycerate mutase | 15927287 | SA1532 | Hypothetical protein | |
| 15927309b | Fhs | Formyltetrahydrofolate synthetase | ||||
| 15926453b | Eno | Enolase | 15927328 | SA1572 | Hypothetical protein | |
| 15926468 | SA0746 | Staphylococcal nuclease with two transmembrane segments and a potential SPase I cleavage site | 15927409 | TRAP | Signal transduction protein TRAP | |
| 15927495 | SA1737 | Hypothetical protein | ||||
| 15926503 | SA0775 | Hypothetical protein | 15927539 | SA1774 | Hypothetical protein | |
| 15926543 | SA0806 | Hypothetical protein | 15927604b | GroEL | GroEL protein | |
| 15926551 | Pgi | Glucose-6-phosphate isomerase A | 15927605 | GroES | GroES protein | |
| 15926589 | SA0859 | Hypothetical protein | 15927640 | RsbW | Anti-σB factor | |
| 15926642 | SA0908 | Hypothetical protein predicted to have an uncleaved signal peptide | 15927687 | GlyA | Serine hydroxymethyl transferase | |
| 15927699 | FbaA | Fructose-bisphosphate aldolase | ||||
| 15926669 | PtsH | Phosphocarrier protein Hpr, phosphohistidine-containing protein | 15927712 | DeoD | Purine nucleoside phosphorylase | |
| 15927753 | SAS074 | Hypothetical protein | ||||
| 15926670 | PtsI | Phosphoenolpyruvate-protein phosphatase | 15927762 | Asp23 | Alkaline shock protein 23, Asp23 | |
| 15926679 | PdhB | Pyruvate dehydrogenase E1 component beta subunit | 15927994 | SA2204 | Hypothetical protein | |
| 15926680 | PdhC | Dihydrolipoamide S-acetyltransferase component of pyruvate dehydrogenase complex E2 | 15927798 | RplM | 50S ribosomal protein L13 | |
| 15928070 | SA2279 | Hypothetical protein | ||||
| 15926681b | PdhD | Dihydrolipoamide dehydrogenase component of pyruvate dehydrogenase E3 | 15928081 | FnbB | Fibronectin binding protein B | |
| 15928082 | FnbA | Fibronectin binding protein A | ||||
| 15926710 | SA0974 | Hypothetical protein | 15928133 | RocA | 1-Pyrroline-5-carboxylate dehydrogenase | |
| 15926723 | PheT | tRNAPhe synthetase β chain | 15928185 | PanB | 3-Methyl-2-oxobutanoate hydroxymethyltransferase | |
| 15926735 | SA0998 | Hypothetical protein | 15928192 | SA2399 | Fructose-bisphosphate aldolase | |
| 15926776 | IleS | tRNAIle synthetase | 15928284 | SA2490 | Hypothetical protein | |
| 15926829 | SucD | Succinyl-coenzyme A synthetase, α subunit | 57651372 | MetS | Methionyl-tRNA synthetase | |
| 15926840 | Tsf | Elongation factor TS | 57651442 | SACOL0613 | Hypothetical protein | |
| 15926857 | PnpA | Polyribonucleotide nucleotidyltransferase | 57651702 | PdhA | Pyruvate dehydrogenase complex E1 component, α subunit | |
| 15926892 | GlnA | Glutamine-ammonia ligase | ||||
| 15926915 | KatA | Catalase | 24636605d | Edin-B | Epidermal cell differentiation inhibitor B | |
| 15926923b | Tkt | Transketolase |
Found only in the extracellular proteome described by Ziebandt et al. (208).
Also found in the extracellular proteome described by Ziebandt et al. (208).
Contains a pseudopilin SPase recognition and cleavage site.
Note that the corresponding gene is not present in the sequenced S. aureus genomes.
Based on the proteomics data for membrane and extracellular proteins of B. subtilis, the optimized −3-to-+1 pattern (AVSTI)-(SEKYHQFLDGPW)-A-(AQVEKDFHLNS) for signal peptide recognition and cleavage by type I SPases of this organism was identified (179). SPase cleavage occurs C-terminal of the invariant Ala residue at the −1 position. The residues in parentheses in the pattern are listed in the order of frequency, with the most frequently identified residue at each position being placed in the first position. By comparing the predicted SPase recognition and cleavage sites for signal peptides of proteomically identified extracellular proteins of S. aureus (Table 4), we defined the −3-to-+1 pattern (AVS)-(KHNDQSYEGLR)-A-(AESDIKL) for productive recognition and cleavage by the type I SPase SpsB. Compared to the equivalent pattern in B. subtilis, it is interesting that the frequencies of certain residues at the −3, −2, and +1 positions differ, as reflected by the order of frequency with which they are listed in the pattern. Moreover, Asn can be present at the −2 position, while Ile is accepted at the +1 position. These residues are found at the −2 and +1 positions of certain serine proteases, hemolysins, immunoglobulin G (IgG) binding protein A, and aureolysin (Table 4). It should also be noted that compared to the optimized SPase recognition pattern in B. subtilis, several residues are not found at the −3, −2, and +1 positions of potential SpsB recognition and cleavage sites in identified extracellular proteins of S. aureus. Since such residues may be present in SPase recognition and cleavage sites of proteins that have escaped identification through proteomics, we included them in the −3-to-+1 search pattern (shown in lowercase) for the identification of potential secretory proteins of staphylococci, as follows: (AVSit)-(KHNDQSYEGLRfpw)-A-(AESDIKLfhnqv). This optimized S. aureus search pattern was used as an indicator of the quality of signal peptide predictions based on the SignalP-NN, SignalP-HMM, LipoP, PrediSi, Phobius, and TMHMM programs. Proteins with potential signal peptides containing this pattern were assigned to have a high probability of extracytoplasmic localization and a low probability of membrane retention (Tables 6 and 7).Proteins with potential signal peptides that do not contain this pattern were assigned to have a high probability of being retained in the membrane (data not shown). In this case, the uncleaved signal peptide could serve as an N-terminal membrane anchor. Following this approach, sets of 186 to 211 proteins (depending on the S. aureus strain) were identified that contain a potential signal peptide or N-terminal transmembrane segment. Scanning for the presence of the SpsB recognition and cleavage motif [(AVSit)-(KHNDQSYEGLRfpw)-A-(AESDIKLfhnqv)] revealed that, depending on the S. aureus strain investigated, 78 to 93 proteins carry this motif. These proteins are most likely processed by SPase, liberated from the membrane, and secreted into the extracellular milieu, unless they contain a cell wall retention signal (see below). Most of the other proteins with signal peptides that do not conform to the SpsB recognition and cleavage motif lack the invariant Ala at the −1 position. Also, some of these preproteins contain different residues at the −3, −2, or +1 position. For example, Asp, Glu, Phe, and Lys are highly unlikely residues at the −3 position (187). On the other hand, some preproteins have a Gly (e.g., exotoxins 4 and 5 from S. aureus COL) or a Leu at the −3 position (Tables 6 and 7). Since Gly and Leu residues at the −3 position of signal peptides are accepted by the E. coli SPase, it seems likely that they are also accepted at this position by SpsB. However, we did not include these residues in the current SpsB recognition and cleavage motif, since we could neither identify these proteins among the secreted proteins of S. aureus COL (Fig. 2) nor find published evidence that these proteins are indeed secreted. Among the proteins with predicted cleavable Sec-type signal peptides, there are many known extracellular staphylococcal virulence factors, such as exotoxins, enterotoxins (SEM, SEN, and SEO), hemolysins, TSST-1, leukotoxins (LukD and LukE), a secretory antigen SsaA homologue, and immunodominant antigen A (IsaA). Remarkably, the lists of identified extracellular proteins of S. aureus COL and RN6390 (208) (Tables 4 and 5) reveal that about 48% of these proteins lack known signal peptides. This percentage is substantially higher than the initial estimate of 10%, which was based on a limited proteomics-derived data set (174). It is also interesting that the list of identified extracellular proteins without a signal peptide includes enolase, which may be actively exported by an unknown mechanism (19), but lacks EsxA and EsxB, which are exported by the ESAT-6 system (26).
TABLE 6.
The core exoproteome of S. aureus, defined as proteins with predicted Sec-type signal peptides present in all sequenced strainsl
| Identification no. | Protein | Amino acid at position:
|
Function | |||
|---|---|---|---|---|---|---|
| −3 | −2 | −1 | +1 | |||
| 15925728a,b | SA0022 | S | N | A | A | Hypothetical protein |
| 15925815a | Spa | A | N | A | A | Immunoglobulin G binding protein A precursor |
| 15925838c | SA0129 | A | H | A | D | Hypothetical protein |
| 15925848d | SA0139 | S | L | A | I | Hypothetical protein |
| 15925933 | Coa | A | D | A | I | Staphylocoagulase precursor |
| 15925978 | LytM | A | D | A | A | Peptidoglycan hydrolase |
| 15926008 | SA0295 | A | F | A | K | Hypothetical protein |
| 15926022d | Geh | A | Q | A | S | Glycerol ester hydrolase |
| 15926106e | Set13 | V | H | A | K | Exotoxin 13 |
| 15926112 | SA0394 | A | E | A | S | Hypothetical protein |
| 15926142d,f | SA0423 | A | N | A | A | Hypothetical protein |
| 15926239a,d | SdrC | A | K | A | A | Ser-Asp-rich fibrinogen-binding protein |
| 15926291d | SA0570 | A | E | A | A | Hypothetical protein |
| 15926319d,g,h | Pbp4 | A | Q | A | T | Penicillin binding protein 4 |
| 15926342f,d | SA0620 | A | Q | A | S | Secretory antigen SsaA homologue |
| 15926373d | SA0651 | A | L | A | K | Hypothetical protein |
| 15926432f,d | SA0710 | A | H | A | Q | Hypothetical protein |
| 15926464a | ClfA | A | D | A | S | Fibrinogen-binding protein A |
| 15926466 | Ssp | A | K | A | A | Extracellular matrix and plasma binding protein |
| 15926467 | SA0745 | A | N | A | L | Hypothetical protein |
| 15926548h | GlpQ | A | G | A | E | Glycerophosphoryl diester phosphodiesterase |
| 15926570 | SA0841 | V | S | A | A | Hypothetical protein |
| 15926634 | SspB | A | K | A | D | Cysteine protease precursor |
| 15926635d | SspA | A | N | A | L | Serine protease |
| 15926639f,d | Atl | V | Q | A | A | Autolysin |
| 15926713a | IsdB | A | Q | A | A | Hypothetical protein |
| 15926714a | IsdA | V | N | A | A | Cell surface protein |
| 15926715i | IsdC | A | N | A | A | Hypothetical protein |
| 15926738 | SA1000 | S | H | A | Q | Hypothetical protein |
| 15926741 | Efb | A | D | A | S | Hypothetical protein |
| 15926742 | SA1004 | A | D | A | S | Hypothetical protein |
| 15926750 | SA1010 | S | E | A | K | Hypothetical protein |
| 15926830f | LytN | A | Y | A | D | LytN protein |
| 15927055d | GpsA | V | L | A | E | Glycerol-3-phosphate dehydrogenase |
| 15927385 | SplC | A | N | A | E | Serine protease SplC |
| 15927456d | SA1698 | S | L | A | D | Hypothetical protein |
| 15927483d | SspB2 | A | N | A | E | Staphopain, cysteine proteinase |
| 15927580 | SA1812 | S | Y | A | K | Hypothetical protein |
| 15927607d | SA1839† | V | E | A | K | Hypothetical protein |
| 15927670d | SA1898 | A | H | A | S | Hypothetical protein |
| 15927785 | SA2006 | A | S | A | D | Hypothetical protein |
| 15927879d | SsaA | A | H | A | S | Hypothetical protein |
| 15927884d | SA2097 | A | D | A | A | Hypothetical protein |
| 15927996 | Sbi | A | K | A | S | IgG-binding protein Sbi |
| 15927997 | HlgA | S | K | A | E | γ-Hemolysin chain II precursor |
| 15927998 | HlgC | A | K | A | A | γ-Hemolysin component C |
| 15927999 | HlgB | A | N | A | E | γ-Hemolysin component B |
| 15928114 | SA2323 | A | Y | A | H | Hypothetical protein |
| 15928123d | SA2332 | S | H | A | A | Hypothetical protein |
| 15928145d | SA2353 | A | Q | A | A | Hypothetical protein |
| 15928148d | IsaA | A | H | A | A | Immunodominant antigen A |
| 15928216a | ClfB | A | Q | A | S | Clumping factor B |
| 15928223d,j | Aur | A | L | A | I | Zinc metalloproteinase aureolysin |
| 15928224d,k | IsaB | A | Q | A | A | Immunodominant antigen B |
| 15928230d | SA2437 | A | Y | A | D | Hypothetical protein |
| 15928232c,d | SasF | A | Q | A | A | Conserved hypothetical protein |
| 15928254 | IcaB | A | N | A | D | Intercellular adhesion protein B |
| 15928257d | Lip | A | Q | A | A | Triacylglycerol lipase precursor |
Contains an LPXTG motif.
Protein homologue of B. subtilis is found in extracellular proteome (174).
Contains an LPXAG motif.
Also present in S. epidermidis.
Only the S. aureus COL protein has a Thr at the +1 position, which is not included in the recognition and cleavage pattern. All other S. aureus strains conform to the proposed pattern.
Contains a LysM or GW domain motif.
Only the S. aureus N315 protein has a Thr at the +1 position, which is not included in the recognition and cleavage pattern. All other S. aureus strains conform to the proposed pattern.
Protein homologues of B. subtilis are classified as Sec-attached membrane proteins (179).
Contains an NPQTN motif.
Protein homologue of B. subtilis classified as a secretory protein (179).
Only for the S. aureus MRSA252 protein; protein homologues in other strains are predicted to have two transmembrane domains and were excluded from the list.
Signal peptide predictions were performed with SignalP-NN and SignalP-HMM, version 2.0 (136; http://www.cbs.dtu.dk/services/SignalP-2.0/), PrediSi (76; http://www.predisi.de/), Phobius (95; http://phobius.cgb.ki.se/), and LipoP, version 1.0 (94; http://www.cbs.dtu.dk/services/LipoP/). These programs are designed to identify Sec-type signal peptides, amino-terminal membrane anchors (Phobius), or lipoprotein signal peptides in gram-negative bacteria (LipoP). The TMHMM program, version 2.0 (41; http://www.cbs.dtu.dk/services/TMHMM/), was used to identify transmembrane segments in proteins.
TABLE 7.
Variant exoproteome of S. aureus, defined as proteins with predicted Sec-type signal peptides present in at least one sequenced strain
| Identification no. | Protein | Amino acid at position:
|
Function | |||
|---|---|---|---|---|---|---|
| −3 | −2 | −1 | +1 | |||
| 15925799 | Plc | A | H | A | S | 1-Phosphatidylinositol phosphodiesterase precursor |
| 15926099a | Set6 | V | Q | A | K | Exotoxin 6 |
| 15926100a | Set7 | V | H | A | E | Exotoxin 7 |
| 15926101a | Set8 | V | K | A | E | Exotoxin 8 |
| 15926103a | Set10 | V | N | A | S | Exotoxin 10 |
| 15926104a | Set11 | V | H | A | K | Exotoxin 11 |
| 15926105a | Set12 | V | N | A | K | Exotoxin 12 |
| 15926106a | Set13 | V | H | A | K | Exotoxin 13 |
| 15926111a | Set15 | V | K | A | S | Exotoxin 15 |
| 15926113 | SA0395 | A | D | A | K | Hypothetical protein |
| 15926465 | SA0743 | A | S | A | V | Hypothetical protein |
| 15926739 | SA1001 | A | K | A | F | Hypothetical protein |
| 15926746 | HlY | A | N | A | A | α-Hemolysin precursor |
| 15927016b | EbhB | A | H | A | A | Hypothetical protein |
| 15927308c | Fhs | A | Q | A | A | Hypothetical protein |
| 15927386 | SplB | A | K | A | E | Serine protease SplB |
| 15927387 | SplA | A | K | A | E | Serine protease SplA |
| 15927389 | SA1633 | A | K | A | E | Probable β-lactamase |
| 15927393 | LukD | V | D | A | A | Leukotoxin, LukD |
| 15927398 | SEG | V | N | A | Q | Extracellular enterotoxin type G precursor |
| 15927399 | SEN | V | N | A | E | Enterotoxin SeN |
| 15927402 | SEI | T | Y | A | Q | Extracellular enterotoxin type I precursor |
| 15927404 | SEO | A | Y | A | N | Enterotoxin SeO |
| 15927512 | Map | A | S | A | A | Truncated MapW protein |
| 15927513 | Hlb | A | K | A | E | Truncated β-hemolysin |
| 15927516 | SA1754 | A | Q | A | S | Hypothetical protein |
| 15927517 | SA1755 | A | K | A | F | Hypothetical protein |
| 15927520 | Sak | V | S | A | S | Staphylokinase precursor |
| 15927522 | SA1760 | A | K | A | I | Hypothetical protein |
| 15927585 | SEC3 | V | L | A | E | Enterotoxin type C3 |
| 15927586 | SA1818 | A | K | A | E | Hypothetical protein |
| 15927587 | TSST-1 | A | K | A | S | Toxic shock syndrome toxin 1 |
| 15927741c | FmtB | A | S | A | A | FmtB protein |
| 15928076b,c | SA2285 | A | E | A | A | Hypothetical protein |
| 15928174c | SA2381 | A | N | A | E | Hypothetical protein |
| 15928182 | SA2389 | V | L | A | D | Hypothetical protein |
| 16119203 | SAP003 | A | Y | A | N | Hypothetical protein |
| 16119219 | SAP019 | A | E | A | A | Hypothetical protein |
| 14141830 | SAVP008 | A | N | A | E | Hypothetical protein |
| 21282111 | Set16 | V | Q | A | K | Hypothetical protein |
| 21282116 | Set21 | V | K | A | A | Hypothetical protein |
| 21282123 | Set26 | V | K | A | I | Hypothetical protein |
| 21283107 | LukF | V | D | A | A | Panton-Valentine leukocidin chain F precursor |
| 21283108 | LukS | S | K | A | D | Panton-Valentine leukocidin chain S precursor |
| 21284341c | Cna | A | L | A | A | Collagen adhesin precursor |
| 49482651 | SAR0423 | V | H | A | E | Exotoxin |
| 49482652 | SAR0424 | A | N | A | E | Exotoxin |
| 49482653 | SAR0425 | A | N | A | E | Exotoxin |
| 49482925b | SAR0721 | T | F | A | E | Multicopper oxidase protein |
| 49484047 | SAR1886 | A | Y | A | F | Putative exported protein |
| 49484059b | SAR1905 | A | K | A | E | Serine protease |
| 49484898 | SAR2788 | A | E | A | S | Putative exported protein |
| 49484957 | SEH | A | K | A | E | Enterotoxin H |
| 49485293 | SAS0389 | V | K | A | A | Exotoxin |
| 57650600 | SACOL1865 | A | K | A | E | Serine protease SplE, putative |
| 57650605 | SACOL1870 | A | K | A | E | Hypothetical protein |
| 57650609 | EpiP | A | S | A | S | Epidermin leader peptide processing serine protease EpiP |
| 57651309 | SACOL0468 | V | Q | A | K | Exotoxin 3, putative |
| 57651311 | SACOL0470 | V | K | A | E | Exotoxin, putative |
| 57651319 | SACOL0478 | V | K | A | S | Exotoxin 3, putative |
| 57652419c | Pls | A | E | A | A | Methicillin-resistant surface protein |
Although these proteins share homology with exotoxins from other S. aureus strains, they are highly variable (80).
Also present in S. epidermidis.
Contains an LPXTG motif.
Twin-arginine (RR) signal peptides.
The consensus RR motif that directs proteins into the Tat pathway has previously been defined as (KR)-R-X-#-#, where # is a hydrophobic residue (39, 89). Dilks et al. (47) used a genomic approach to identify possible Tat substrates for 84 diverse prokaryotes, using the TATFIND 1.2 program. Their study included S. aureus Mu50, MW2, and N315. Two potential Tat substrates of unknown function were predicted for S. aureus Mu50 and MW2, and one of these was also predicted for S. aureus N315 (47). However, both proteins are conserved in all sequenced S. aureus strains, including the N315 strain. One of the two predicted Tat substrates has no known function, whereas the other was annotated as a hypothetical protein similar to a ferrichrome ABC transporter (permease). These proteins, however, are not in our list of proteins that have a predicted RR signal peptide. Although they have signal peptides according to the SignalP program, these proteins are localized in the cytoplasm or membrane according to the LipoP, PrediSi, and Phobius programs. It is therefore unlikely that these proteins are destined for secretion. Specifically, the hypothetical permease has eight predicted transmembrane helices.
Our own pattern searches for proteins with a possible RR motif resulted in 24 to 32 positive hits, depending on the S. aureus strain investigated. However, most of these proteins have no detectable N, H, or C domain and were therefore discarded from our data set. Also, some other proteins with a possible RR motif are predicted to contain a lipoprotein signal peptide. These predicted lipoproteins were also discarded from the list of potential S. aureus Tat substrates, firstly because none of the identified lipoproteins of B. subtilis that have an RR motif were shown to be secreted via the Tat pathway (87, 89) and secondly because there is no published evidence for any other bacterium that lipoproteins can be exported Tat dependently. Thus, it appears that only four or five proteins, depending on the S. aureus strain investigated, are potentially exported by the Tat pathway and cleaved by SpsB. However, it is noteworthy that none of the B. subtilis proteins with a KR motif were secreted Tat dependently, even though KR motifs are capable of directing proteins into the Tat pathways of chloroplasts and gram-negative bacteria, such as E. coli and Salmonella enterica (77, 82, 123, 167). If KR motifs are also rejected by the S. aureus Tat pathway, there would not be a single protein in any sequenced S. aureus strain that is secreted Tat dependently. This would be highly remarkable in view of the presence of tatA and tatC genes in all of these strains. Notably, the only known strictly Tat-dependent extracellular proteins of B. subtilis are the phosphodiesterase PhoD (175) and a protein of unknown function, YwbN (88). While a homologue of PhoD is not present in any of the six sequenced S. aureus strains, homologues of YwbN are present in all of these strains. Close inspection of the YwbN homologues of S. aureus COL, MRSA252, and MSSA476 revealed the presence of an N-terminal RR motif, but a potential signal peptide was not identified as such by the SignalP program. In contrast, the YwbN homologues of S. aureus Mu50, MW2, and N315 appear to lack this RR motif. According to comparisons of the deduced amino acid sequences, these three YwbN homologues should be missing the first 40 residues of B. subtilis YwbN. This is most likely not the case, since the sequences upstream of the annotated S. aureus Mu50, MW2, and N315 ywbN genes encode a peptide with an RR motif in the same open reading frame as the ywbN structural gene (Table 6). Thus, the RR motifs of the S. aureus Mu30, MW2, and N315 YwbN proteins have so far escaped identification due to a systematic difference in sequence annotation. It remains to be investigated whether these sequences with RR motifs serve as signal peptides in the Tat-dependent export of S. aureus YwbN homologues.
Pseudopilin signal peptides.
The signal peptides of pseudopilins differ from the Sec-type signal peptides in the location of SPase cleavage sites. In pseudopilin signal peptides, the cleavage site is located between the N and H domains (105). The consensus recognition and cleavage motif for pseudopilin SPases, such as ComC, is K-G-F-X-X-X-E. Cleavage by pseudopilinSPases occurs within this motif, between the Gly and Phe residues. Upon cleavage, the Phe residue is methylated. For all sequenced S. aureus strains, three proteins were found to have the canonical pseudopilin SPase recognition and cleavage motif. These proteins are homologues of the cold shock proteins CspB, CspC, and CspD of B. subtilis. However, even though these proteins do contain the pseudopilin SPase recognition and cleavage pattern, they lack the H domain. Since the active sites of pseudopilin SPases are located in the cytoplasm, cleavage of the CspBCD homologues of S. aureus would be possible, but their export via the Com pathway is unlikely. Nevertheless, it should be noted that one of the CspBCD homologues of S. aureus, known as CspA, was found in the extracellular proteome of a clinical isolate (Fig. 2 and Table 5). To verify the absence or presence of pseudopilins in S. aureus, BLAST searches with the known ComGC, ComGD, ComGE, and ComGG proteins of B. subtilis were performed. This revealed the presence of only one potential pseudopilin, which is a homologue of B. subtilis ComGC. Although the consensus pseudopilin SPase recognition and cleavage site is absent from S. aureus ComGC, a putative cleavage pattern (Q-A-F-T-L-I-E) is present at the position in the ComGC signal peptide where a pseudopilin SPase recognition and cleavage site would be expected. Further analyses revealed that similar observations can be made for ComGC homologues in other gram-positive bacteria, such as Bacillus cereus, B. anthracis, L. monocytogenes, S. haemolyticus, and Oceanobacillus iheyensis. By comparing the ComGC homologues of these organisms, an expanded search pattern for gram-positive bacterial pseudopilin SPase recognition and cleavage sites was defined as follows: (KEQRS)-(GA)-F-X-X-X-E. Interestingly, using this expanded search pattern, two additional potential pseudopilins of S. aureus were identified. These potential pseudopilins show similarity to the ComGD proteins of B. cereus and B. anthracis and the ComGF proteins of Bacillus halodurans and L. lactis. It remains to be shown whether the three identified potential pseudopilins of S. aureus are indeed able to assemble into pilin-like structures after being processed by the ComC homologue. If so, it will be even more interesting to identify their biological function, for example, in adhesion to surfaces, motility, or export of proteins. Such functions could play a role in virulence and have been attributed to type IV pili and pseudopilins of gram-negative bacteria (106).
Bacteriocin leader peptides.
Bacteriocins form a distinct group of proteins with cleavable N-terminal signal peptides, which are often called leader peptides. These leader peptides only have N and C domains and completely lack the hydrophobic H domain. The bacteriocin leader peptides are invoked in posttranslational modification and the prevention of premature antimicrobial activity, which would be deleterious to the producing organism. Of the sequenced S. aureus bacteriocins, C55α and C55β contain a leader peptide (130), whereas leader peptides are absent from aureocin A53 (134) and aureocin A70 (134). Two potential lantibiotics with leader peptides were identified by sequencing the genomes of S. aureus MW2 (7) (GI numbers 49486642 and 49486641) and MSSA476. In both strains, the corresponding genes are located on the genomic island νSAβ. Both S. aureus proteins show similarity to the lantibiotic gallidermin precursor GdmA of Staphylococcus gallinarum and to the lantibiotic epidermin precursor EpiA of S. epidermidis. Notably, the S. aureus COL strain contains only one of these two potential lantibiotics, which is most similar to the potential MW2 lantibiotic under GI number 49486641. Two additional putative bacteriocins that were identified by genome sequencing seem to be homologous to L. lactis lactococcin 972. The hypothetical protein SAP019 (N315 annotation) is plasmid encoded in S. aureus N315 and MSSA476 and chromosomally encoded in S. aureus MRSA252. The other hypothetical bacteriocin, SAS029, is chromosomally encoded in all sequenced S. aureus strains. No published data are presently available on the characteristics of these proteins, so it remains to be seen whether they are genuine bacteriocins.
Potential ESAT-6 export signal.
As described above, the EsxA and EsxB proteins are secreted by S. aureus via the ESAT-6 route (26). Both proteins lack a known signal peptide but are specifically transported across the membrane nonetheless. This implies that these two proteins must contain an export signal that is recognized by one or more ESAT-6 pathway components. The nature of this signal is presently unknown. The only common feature of proteins that are known (or predicted) to be translocated across the membrane via the ESAT-6 pathway is a WXG motif, which is located ∼100 amino acids from the N terminus of the protein (140). The involvement of the WXG motif in ESAT-6 targeting remains to be demonstrated.
Retention Signals
Lipoproteins.
Lipoproteins appear to be exported via the Sec pathway. During or shortly after translocation, the invariant Cys in the lipobox is diacylglyceryl modified by Lgt. This results in signal peptide cleavage by SPase II and retention of the mature lipoprotein in the membrane. Based on the cleavage sites of lipoproteins that have been identified in various gram-positive bacteria, Sutcliffe and Harrington (171) reported the −4-to-+2 lipobox pattern (LIVMFESTAG)-(LVIAMGT)-(IVMSTAFG)-(AG)-C-(SGANERQTL). Furthermore, they reported that neither the charged residues Asp, Glu, Arg, and Lys nor Gln is present in the region between 6 and 20 residues N-terminal of the lipobox. A search of the translated proteins encoded by the six S. aureus genomes with the pattern shown above, using the PATTINPROT program, revealed about 50 proteins with this motif (Tables 8 and 9). A comparison of the PATTINPROT results to the results obtained with the LipoP program showed that 10 to 16 more potential lipoproteins may be present in S. aureus. Most of these extra predicted lipoproteins contain an amino acid at the −1 position (mostly Ser) or the +2 position (mostly Asp) that differs from the lipobox pattern reported by Sutcliffe and Harrington (171). Recently, Tjalsma and van Dijl (179) proposed the lipobox search pattern (LITAGMV)-(ASGTIMVF)-(AG)-C-(SGENTAQR) for potential lipoproteins of B. subtilis on the basis of published proteomics data. The only difference compared to the pattern by Sutcliffe and Harrington (171) is that Leu is absent from the +2 position, which is due to the fact that no potential B. subtilis lipoprotein with Leu at this position was identified by proteomics. Consistently, none of the predicted S. aureus lipoproteins has a Leu at the +2 position (Tables 8 and 9). It is also noteworthy that some lipoproteins contain a (KR)-R-X-#-# motif in their signal peptides, although it has not been shown yet that lipoproteins can be transported via the Tat pathway. Finally, the hypothetical protein Lpl2 of S. aureus N315 and Mu50 was excluded from our lipoprotein predictions because Asp does not seem to occur at the +2 position of lipoproteins from gram-positive bacteria (94, 179). Nevertheless, the homologues of Lpl2 of the other sequenced S. aureus strains are classified as lipoproteins because they have residues at the +2 position that conform to the lipobox consensus.
TABLE 8.
Core lipoproteome of S. aureus, defined as proteins with predicted lipoprotein signal peptides present in all sequenced strains
| Identification no. | Protein | Amino acid at position:
|
Function | ||||
|---|---|---|---|---|---|---|---|
| −3 | −2 | −1 | +1 | +2 | |||
| 15925800 | SA0092 | T | A | G | C | G | Hypothetical protein |
| 15925819 | SirA | L | A | G | C | S | Lipoprotein |
| 15925847a | SA0138 | A | A | A | C | G | Hypothetical protein, similar to alkylphosphonate ABC transporter |
| 15925912 | Slp | L | S | G | C | G | RGD-containing lipoprotein |
| 15925918 | SA0207 | V | T | A | C | G | Hypothetical protein, similar to maltose/maltodextrin-binding protein |
| 15925928a,b | SA0217 | L | S | S | C | A | Hypothetical protein, similar to periplasmic-iron-binding protein BitC |
| 15925940c | SA0229 | L | S | G | C | G | Hypothetical protein, similar to nickel ABC transporter nickel-binding protein |
| 15926044 | SA0331 | I | A | A | C | G | Conserved hypothetical protein |
| 15926073 | SA0359 | L | T | A | C | G | Conserved hypothetical protein |
| 15926079a | SA0363 | L | T | G | C | A | Hypothetical protein |
| 15926141a,d | SA0422 | L | A | A | C | G | Hypothetical protein, similar to lactococcal lipoprotein |
| 15926287a | SA0566 | L | S | G | C | G | Hypothetical protein, similar to iron-binding protein |
| 15926308a | SA0587 | V | A | A | C | G | Lipoprotein, streptococcal adhesin PsaA homologue |
| 15926354a | SA0632 | L | T | G | C | G | Conserved hypothetical protein |
| 15926385a,e | SA0663 | L | G | A | C | G | Hypothetical protein |
| 15926413a,f | SA0691 | L | A | A | C | G | Lipoprotein, similar to ferrichrome ABC transporter |
| 15926417a | SA0695 | I | S | A | C | G | Hypothetical protein |
| 15926461 | SA0739 | L | G | A | C | G | Conserved hypothetical protein |
| 15926499a,g | SA0771 | L | A | A | C | G | Conserved hypothetical protein |
| 15926579a | SA0849 | L | S | G | C | A | Hypothetical protein, similar to peptide binding protein OppA |
| 15926625 | SA0891 | V | A | G | C | G | Hypothetical protein, similar to ferrichrome ABC transporter |
| 15926678a | SA0943 | L | A | G | C | T | Conserved hypothetical protein |
| 15926717h | IsdE | L | T | S | C | Q | Hypothetical protein |
| 15926796a | SA1056 | V | A | G | C | S | Hypothetical protein |
| 15926969h | SA1221 | L | G | A | C | G | Thioredoxin reductase |
| 15927111a,e | SA1361 | L | A | G | C | G | Hypothetical protein |
| 15927372h | SA1616 | L | S | S | C | G | Hypothetical protein |
| 15927375a | SA1619 | L | T | A | C | G | Hypothetical protein |
| 15927415a | PrsA | L | G | A | C | G | Peptidyl-prolyl cis/trans isomerase homolog |
| 15927477a | SA1719 | L | A | A | C | G | Conserved hypothetical protein |
| 15927757a,i | SA1979 | V | A | A | C | G | Hypothetical protein, similar to ferrichrome ABC transporter (binding protein) |
| 15927864g | SA2079 | L | A | A | C | G | Hypothetical protein, similar to ferrichrome ABC transporter FhuD precursor |
| 15927948a | SA2158 | L | A | A | C | G | Hypothetical protein, similar to TpgX protein |
| 15927961a,h | SA2171 | L | I | V | C | I | Hypothetical protein |
| 15927984a | DsbA | L | A | A | C | G | DsbA; hypothetical protein similar to Zn-binding lipoprotein AdcA |
| 15927987c | SA2197 | L | T | A | C | G | Conserved hypothetical protein |
| 15927988c | SA2198 | I | S | G | C | G | Hypothetical protein |
| 15927992a,g | SA2202 | L | A | A | C | G | Hypothetical protein, similar to ABC transporter, periplasmic amino acid-binding protein |
| 15928025a | OpuCC | L | S | G | C | S | Glycine betaine/carnitine/choline ABC transporter OpuC |
| 15928037a | SA2247 | L | S | A | C | G | Conserved hypothetical protein |
| 15928046a | Opp-1A | L | T | G | C | G | Oligopeptide transporter putative substrate binding domain |
| 15928066a,i | SA2275 | I | G | A | C | G | Hypothetical protein |
| 15928267h | SA2473 | L | Y | S | C | S | Hypothetical protein |
Also present in S. epidermidis.
Excluded for S. aureus Mu50 and N315 because of the motif proposed by Sutcliffe and Harrington (171).
Excluded for S. epidermidis because of the motif proposed by Sutcliffe and Harrington (171).
Contains the lipoprotein release motif (179) with one amino acid change, except for S. aureus COL and S. epidermidis.
Contains the lipoprotein release motif (179) with one amino acid change for all staphylococcal strains.
Contains the exact lipoprotein release motif (179), except in S. epidermidis.
Contains the lipoprotein release motif (179) with one amino acid change, except in S. epidermidis.
Excluded for all S. aureus strains because of the motif proposed by Sutcliffe and Harrington (171).
Contains the lipoprotein release motif (179) with one amino acid change, only for S. epidermidis.
TABLE 9.
Variant lipoproteome of S. aureus, defined as proteins with predicted lipoprotein signal peptides present in at least one sequenced strain
| Identification no. | Protein | Amino acid at position:
|
Function | ||||
|---|---|---|---|---|---|---|---|
| −3 | −2 | −1 | +1 | +2 | |||
| 15925801a | SA0093 | F | A | G | C | G | Hypothetical protein |
| 15925803 | SA0095 | T | A | G | C | G | Hypothetical protein |
| 15925804 | SA0096 | T | A | G | C | G | Hypothetical protein |
| 15925877a | SA0167 | I | T | G | C | D | Hypothetical protein, similar to membrane lipoprotein SrpL |
| 15926004 | SA0291 | L | A | G | C | S | Hypothetical protein |
| 15926114b | Lpl1 | I | A | G | C | G | Hypothetical protein |
| 15926115a | Lpl2 | I | I | G | C | D | Hypothetical protein |
| 15926116 | Lpl3 | I | A | G | C | G | Hypothetical protein |
| 15926118 | Lpl4 | I | I | G | C | G | Hypothetical protein |
| 15926119 | Lpl5 | V | A | G | C | G | Hypothetical protein |
| 15926120a | Lpl6 | I | I | G | C | D | Hypothetical protein |
| 15926122a | Lpl8 | A | T | S | C | G | Hypothetical protein |
| 15926123 | Lpl9 | I | G | G | C | G | Hypothetical protein |
| 15926465a | SA0743 | G | A | L | C | V | Hypothetical protein |
| 15926580c | SA0850 | L | S | A | C | G | Hypothetical protein, similar to oligopeptide ABC transporter oligopeptide-binding protein |
| 15927067 | SA1317 | L | S | G | C | S | Hypothetical protein |
| 15927068d | SA1318 | L | S | G | C | S | Hypothetical protein |
| 15927069 | SA1319 | L | S | G | C | S | Hypothetical protein |
| 15927373b | SA1617 | L | S | A | C | S | Hypothetical protein |
| 15927396c | SA1640 | L | V | A | C | G | Conserved hypothetical protein |
| 15927859d | ModA | L | A | G | C | S | Probable molybdate-binding protein |
| 19528064a | SA2273 | I | G | G | C | I | Hypothetical protein |
| 21281801 | Mw0072 | T | A | G | C | G | Staphylococcus tandem lipoprotein |
| 21282126 | Lpl10 | I | A | G | C | G | Hypothetical protein |
| 21282127a | Lpl11 | V | T | S | C | G | Hypothetical protein |
| 21282129a | Lpl13 | I | I | G | C | D | Hypothetical protein |
| 21283103 | MW1374 | L | S | G | C | S | Conserved hypothetical protein |
| 21283167 | MW1438 | L | T | A | C | G | Hypothetical protein |
| 21284135 | MW2406 | I | G | A | C | G | Hypothetical protein |
| 21284306 | MW2577 | V | S | G | C | S | Hypothetical protein |
| 49482670 | SAR0445 | I | G | G | C | G | Putative lipoprotein |
| 49483993d | BlaZ | L | S | A | C | N | β-Lactamase precursor |
| 49484246d | SAR2104 | L | S | A | C | G | Putative lipoprotein |
| 49484287a | SAR2149 | L | I | V | C | G | Hypothetical protein |
| 49484977d,e | SAS0074 | I | G | G | C | G | Putative lipoprotein |
| 57650161 | SACOL0888 | L | G | A | C | G | Pathogenicity island, putative lipoprotein |
| 57650441 | SACOL1528 | L | S | G | C | S | Hypothetical protein |
| 57650444 | SACOL1531 | L | S | G | C | S | Hypothetical protein |
| 57650485 | SACOL1574 | L | S | A | C | G | Hypothetical protein |
| 57650696 | SACOL2010 | L | T | A | C | S | Iron compound ABC transporter, iron compound-binding protein |
| 57651323a | SACOL0482 | I | M | G | C | D | Staphylococcus tandem lipoprotein |
| 57652445 | SACOL0081 | T | A | G | C | G | Hypothetical protein |
| 14141829a | SAVP006 | L | V | S | C | N | Hypothetical protein |
Excluded for all S. aureus strains because of the motif proposed by Sutcliffe and Harrington (171).
Excluded for S. aureus COL, MSSA476, Mu50, MW2, and N315 because of the motif proposed by Sutcliffe and Harrington (171).
Contains the lipoprotein release motif (179) with one amino acid change.
Also present in S. epidermidis.
Contains the lipoprotein release motif for S. epidermidis (179), with one amino acid change.
Lipoprotein release determinant.
Although lipoproteins were generally believed to be retained at the membrane-cell wall interface, the presence of lipoproteins in the growth medium of B. subtilis was documented by Antelmann et al. (3). This unexpected finding was correlated with the proteolytic removal of the amino-terminal, lipid-modified Cys, which suggests that the observed lipoprotein release into the growth medium was caused by proteolytic “shaving” after processing by LspA. In most of these lipoproteins, Tjalsma and van Dijl (179) identified the +1-to-+10 consensus sequence C-G-(NSTF)-X-(SGN)-X-(SGKAE)-X-X-(SGA), which might represent the recognition site for an as yet unidentified shaving protease. Most probably, a Gly at the +2 position is of major importance for lipoprotein release into the growth medium, while a Ser at this position seems to inhibit the process. In the sequenced genomes of S. aureus, a gene for only one lipoprotein with the exact motif described above could be found. By searching for patterns with 80% similarity to the consensus sequence (i.e., one different residue), five to seven additional lipoproteins could be found, depending on the S. aureus strain. Thr was not found at the +3 position in any of these proteins. Instead, a Lys was identified at this position in one or two predicted lipoproteins with a potential release motif, depending on the S. aureus strain. In other predicted lipoproteins with a potential release motif, no Gly or Ala residue was found at the +7 position. However, one of these lipoproteins contains a predicted release motif with a Gln at the +7 position. To date, a total of four potential lipoproteins have been identified in the extracellular milieu of S. aureus. The first one was identified by Ziebandt et al. (208). This protein was annotated as a thioredoxin reductase (Table 4), but it shows no similarity to known thioredoxin reductases. Instead, it is highly similar to phosphate-binding lipoproteins, such as PstS of B. subtilis. It should be noted that this protein was not predicted to be a lipoprotein because the signal peptide contains a Gln residue in the N domain. According to the search profile of Sutcliffe and Harrington(171), lipoproteins do not contain a Gln residue at this position. On the other hand, PstS of B. subtilis is a lipoprotein, and it would seem quite likely that this is also true for its S. aureus homologue. The other three potential lipoproteins were identified in the growth media of clinical isolates (Table 4). Remarkably, none of the four lipoproteins with extracellular localization contain the complete lipoprotein release motif that was identified in extracellular lipoproteins of B. subtilis. However, they do contain a Gly residue at the +2 position, which strengthens the idea that this amino acid residue is probably important for lipoprotein release. It is interesting that in lipoproteins of gram-negative bacteria, an Asp, Gly, Phe, or Trp residue at the +2 position prevents transport of the mature lipoprotein to the outer membrane (128, 181). In gram-positive bacteria, no outer membrane is present, and it is currently not known whether the residue at the +2 position has a role in subcellular protein sorting. However, a Gly at this position does seem to promote lipoprotein release into the extracellular milieu, not only in B. subtilis but also in S. aureus.
Cell wall binding domains.
Proteins that have to be displayed on the bacterial surface must be retained by noncovalent or covalent binding to the peptidoglycan moiety of the cell wall. In B. subtilis, several proteins involved in cell wall turnover contain repeated domains in the C-terminal part of the protein which have affinity for cell wall components (62, 110, 150). Specifically, the B. subtilis proteins LytD, WapA, YocH, YvcE, and YwtD have been reported to bind to the cell wall (62, 110, 150). While WapA is not conserved in staphylococci, various S. aureus proteins with regions that show amino acid sequence similarity to LytD, YocH, YvcE, and YwtD of B. subtilis can be found by BLAST searches. Accordingly, these S. aureus proteins may be cell wall bound, but this remains to be shown.
One of the domains that have affinity for cell wall components is the “lysin motif,” or LysM domain, which was first described for bacterial lysins (146). The number of LysM domains can differ for wall-bound proteins from different gram-positive bacterial species (168). For example, XlyA of B. subtilis contains only one LysM domain, whereas three domains can be detected in AcmA of L. lactis and five or six domains can be detected in muramidases from Enterococcus species (92). Using the LysM domain of AcmA from L. lactis in BLAST searches against the six sequenced and annotated S. aureus genomes, four proteins with one or more LysM domains were detected. These proteins include a hypothetical protein similar to autolysins (SA0423), a secretory antigen SsaA homologue (SA0620), a conserved hypothetical protein (SA0710), and the LytN protein.
A different domain that can facilitate protein binding to the cell wall is the GW domain. In L. monocytogenes, the surface-exposed InlB protein contains three C-terminal GW domains. Each domain consists of ∼80 amino acids and starts with a Gly and a Trp residue (22). This domain specifically binds to lipoteichoic acids in the cell wall (91), thereby facilitating the interaction of L. monocytogenes with components of human host cells. The only protein with GW domains found in the sequenced S. aureus strains is the autolysin protein Atl (5, 6). This bifunctional autolysin contains three GW repeats of ∼97 amino acids. The protein is exported as a prepro-Atl precursor of 1,256 amino acids. Subsequent processing steps result in the removal of the signal peptide and the propeptide and the separation of the mature region into an amidase and a glucosaminidase (139). A similar separation of the mature region into an amidase and glucosaminidase has been reported for the AtlE protein of S. epidermidis (74). The GW repeats are both necessary and sufficient to direct reporter proteins to the equatorial surface rings of S. aureus cells, where cell division starts.
Other S. aureus wall proteins that contain repeated domains with potential wall binding properties have been described. These include the clumping factors A and B (ClfAB) (70, 135), several serine-aspartate repeat proteins (SdrCDE) (93), the homologue of S. gordonii GspB (SasA) (165) (see “Covalent attachment to the cell wall” below), and an extracellular matrix-binding protein homologue (34). Although not documented in the literature, additional proteins with Sec-type signal peptides and potential cell wall binding repeats can be recognized readily. These are the cell wall surface anchor family protein SACOL2505 and the methicillin-resistant surface protein SACOL0050, which both contain C-terminal repeat regions of ∼130 amino acids. The latter protein, which shows a high degree of sequence similarity to the SACOL2505 protein, is found only in S. aureus COL, not in the five other sequenced strains. This is due to the fact that the gene for SACOL0050 is localized on mec cassette 1 and therefore not present in the other strains. Notably, the SACOL2505 homologues in S. aureus Mu50 and N315 seem to lack the C-terminal part of the protein with the repeats. A close inspection of the sequences of the corresponding genes in these strains revealed that there is a frameshift mutation or sequencing error in these genes, resulting in an apparent or real C-terminal truncation of the corresponding proteins. Thus, the C-terminal cell wall binding repeats are absent or appear to be absent. Interestingly, most of the aforementioned proteins with cell wall binding motifs also contain the motif LPXTG for covalent attachment to the cell wall by sortase A or sortase B (see below).
It should be noted that a variety of known cell wall binding domains, such as the choline binding domain (205), the Cpl-7 cell wall binding domain (59), and the fructosyltransferase cell wall binding domain (81, 122, 151), appear to be absent from staphylococcal proteins.
Covalent attachment to the cell wall.
Cell wall sorting proteins, known as sortases, exist in many gram-positive bacteria and serve to anchor proteins that are destined for cell surface display to the cell wall (50, 182). In almost all gram-positive bacteria, there is at least one sortase present, and often genes for more than one sortase-like protein can be detected in a single genome. These transpeptidases catalyze the formation of an amide bond between the carboxyl group of a Thr and the free amino end of pentaglycine cross bridges in peptidoglycan precursors. Subsequently, the peptidoglycan precursors with covalently bound proteins are incorporated into the cell wall. More recently, it was shown that sortases can also be involved in protein polymerization, leading to the assembly of pili on the surfaces of gram-positive bacteria, such as Corynebacterium diphtheriae (61, 182). The three-dimensional structure of sortase A (SrtA) of S. aureus revealed that this protein has a unique β-barrel structure in which a catalytic Cys residue is positioned close to a His residue. This suggests that sortase A forms a thiolate-imidazolium ion pair for catalysis (83, 183). Furthermore, it has been shown that a conserved Arg residue is needed for efficient catalysis (112). The catalytic cysteine is part of a conserved motif, TLXTC, which can be found in the C-terminal part of the protein (X is usually Val, Thr, or Ile). Recently, a classification of sortases was proposed by Dramsi et al. (50), based on phylogenetic analyses of 61 sortases that are encoded by the genomes of 22 gram-positive bacteria. These analyses showed that sortases can be grouped into four different classes (A to D). Class A consists of sortases from many low-GC% gram-positive bacteria, including L. monocytogenes, Streptococcus pyogenes, and S. aureus. The second class (class B) is present in only a few low-GC% gram-positive bacteria, including L. monocytogenes, B. anthracis, and S. aureus. Sortases of this class contain three amino acid segments that are not present in the sortases of class A. These sortases recognize a different motif (NPQTN in S. aureus). The genes for substrates of class B sortases are often found at the same locus as the sortase gene. The largest class (class C) consists of sortases from high-GC% and low-GC% gram-positive bacteria. The genes for class C sortases are often present in multiple copies per genome. The characteristics of this class of sortases include a C-terminal hydrophobic domain that might serve as a membrane anchor and a conserved proline residue behind the catalytic site. Finally, class D sortases are present in high- and low-GC% gram-positive bacteria. This class can be divided into three subclusters, depending on whether the sortases are present in bacilli, clostridia, or actinomycetes. Since class C and D sortases are absent from S. aureus, the (potential) substrates of these enzymes are not reviewed here.
(i) Sortase A recognition signal.
For interaction with host cells during infection, many proteins are anchored to the cell walls of staphylococcal cells, thereby enabling the cells to adhere to and invade the host cells or to evade the immune system. Many of these proteins contain an LPXTG motif in their C-terminal part, which is recognized by the cell wall sorting protein sortase A. In each of the six sequenced S. aureus strains, there is only one sortase gene present, which encodes a class A sortase. The LPXTG motif of sortase A substrates is followed by a stretch of hydrophobic amino acids and at least one positively charged amino acid (Lys or Arg) at the C terminus. After protein translocation across the membrane, the LPXTG motif is recognized by SrtA and subsequently cleaved between the Thr and Gly residues (115, 131). Transpeptidation is then mediated by SrtA through amide linkage of the C-terminal Thr of the protein to pentaglycine cross bridges. It has been suggested that SrtA actually uses lipid II as a peptidoglycan substrate and that the proteins linked to lipid II are subsequently incorporated into the cell wall. In addition to the canonical LPXTG motif, an LPXAG motif can also be recognized and cleaved by SrtA (156).
It has been reported that S. aureus has 19 proteins that carry the LPXTG motif and 2 proteins that carry the LPXAG motif at their C termini (156). Many of these proteins have been shown to be expressed. These include protein A (Spa), two clumping factors (ClfA and ClfB; also contain potential wall binding repeats), a collagen-binding protein (Cna), three serine-aspartate repeat proteins (SdrC, SdrD, and SdrE; also contain potential wall binding repeats), two fibronectin-binding proteins (FnbpA and FnbpB) (reviewed by Foster and Hook [58]), a plasmin-sensitive protein (Pls) (163), FmtB (98), and several S. aureus surface (Sas) proteins (156). A recent study on the cell wall and membrane proteome by Nandakumar et al. (127) resulted in the identification of two proteins with an LPXTG cell wall sorting signal. Many of the LPXTG-containing proteins contain a conserved motif, (YF)-SIRK (with some variance), in their N-terminal signal peptides, which has also been observed for other proteins that are substrates for SrtA in several gram-positive bacteria (9). However, this sequence is not found in all SrtA substrates and can also be found in non-cell-wall proteins. This suggests that (YF)-SIRK is not a specific SrtA targeting sequence. One of the sas genes, sasA, is situated in the secA2/secY2 cluster and has an unusually long signal peptide (90 residues), which might indicate that the accessory SecA2/SecY2 system is needed for the transport of SasA across the membrane. If so, this would be similar to the case reported for the cell wall-bound GspB protein of S. gordonii (13).
Depending on the sequenced S. aureus strain, 10 to 13 proteins with an LPXTG cell wall sorting signal followed by a hydrophobic stretch of residues and a positively charged C terminus can be found (Table 10). Among these proteins are fibrinogen-binding protein A (ClfA), the immunoglobulin G binding protein A precursor (Spa), and the Ser-Asp-rich, fibrinogen-binding bone sialoprotein-binding protein (SdrC). Five additional proteins (SdrD, SdrE, SasC, FnbA, and FnbB) with an LPXTG motif can be found among the S. aureus strains (Table 10). These five proteins were excluded from our initial list because the corresponding SignalP scores were lower than our (high) score criterion. However, since some of the domains present in these proteins (besides the LPXTG motif) are conserved in well-described cell wall proteins, they were included in Table 10. The remaining proteins with a cell wall sorting signal either are missing in one or more S. aureus strains or have been annotated wrongly. Interestingly, S. epidermidis ATCC 12228 contains a gene for a class C sortase (srtC) which seems to be absent from other staphylococci. This SrtC protein is most closely related to sortases of L. lactis and Streptococcus suis. Two proteins with LPXTG motifs which are encoded by the same genomic island as SrtC also seem to be strain specific (63).
TABLE 10.
Staphylococcal proteins with (potential) Sec-type signal peptides and (potential) signals for covalent cell wall binding
| Identification no. | Protein | Function | Signal |
|---|---|---|---|
| 15925728 | SA0022 | Hypothetical protein, similar to 5′-nucleotidase | LPKTG |
| 15925815 | Spa | Immunoglobulin G binding protein A precursor | LPETG |
| 15925838 | SasD | Hypothetical protein | LPAAG |
| 15926239a | SdrC | Ser-Asp-rich, fibrinogen-binding bone sialoprotein-binding protein | LPETG |
| 15926240b,c | SdrD | Ser-Asp-rich, fibrinogen-binding bone sialoprotein-binding protein | LPETG |
| 15926241a,c | SdrE | Ser-Asp-rich, fibrinogen-binding bone sialoprotein-binding protein | LPETG |
| 15926464 | ClfA | Fibrinogen-binding protein A, clumping factor | LPDTG |
| 15926713 | IsdB | Conserved hypothetical protein | LPKTG |
| 15926714 | IsdA | Cell surface protein | LPKTG |
| 15926715 | IsdC | Conserved hypothetical protein | NPQTN |
| 15927308b | HarA | Hypothetical protein | LPKTG |
| 15927333c,d | SasC | Hypothetical protein, similar to FmtB protein | LPNTG |
| 15927741a,e | SasB | FmtB protein | LPDTG |
| 15928076a,b,f | Aap | Hypothetical protein, similar to accumulation-associated protein | LPKTG |
| 15928081b,c | FnbB | Fibronectin-binding protein homolog | LPETG |
| 15928082c | FnbA | Fibronectin-binding protein homolog | LPETG |
| 15928174g | SasK | Hypothetical protein | LPKTG |
| 15928216 | ClfB | Clumping factor B | LPETG |
| 15928232a | SasF | Conserved hypothetical protein | LPKAG |
| 15928240h | SasA | Hypothetical protein, similar to streptococcal hemagglutinin protein | LPDTG |
| 57652419i | Pls | Methicillin-resistant surface protein | LPDTG |
| 21284341j | Cna | Collagen adhesin precursor | LPKTG |
| 27467746k | SE0828 | Lipoprotein VsaC | LPETG |
| 27468418k | SE1500 | Hypothetical protein | LPKTG |
| 27468419k | SE1501 | Hypothetical protein | LPNTG |
| 27468546k | SE1628 | Hypothetical protein | LPETG |
| 27469070k | SE2152 | Hypothetical protein | LPNTG |
Also present in S. epidermidis.
The genes encoding SdrC, HarA, and FnbB are not present in S. aureus MRSA252.
Has a lower SignalP score than our threshold score.
Truncated in S. aureus Mu50, thereby missing the C-terminal part containing the LPXTG motif.
The gene encoding SasB is not present in S. aureus MRSA252 and MSSA476 and is truncated in S. aureus MW2.
Truncated in S. aureus Mu50 and N315, thereby missing the C-terminal part containing the LPXTG motif.
The gene encoding SasK is not present in S. aureus COL, MRSA252, and MSSA476 and is truncated in S. aureus MW2, thereby missing the N-terminal signal peptide.
Contains an unusually long signal peptide (90 amino acids).
The gene encoding Pls is present only in S. aureus COL.
The gene encoding Cna is not present in S. aureus COL, Mu50, and N315.
Present only in S. epidermidis.
(ii) Sortase B recognition signal.
All sequenced S. aureus strains contain sortase B (SrtB) in addition to SrtA. The gene for SrtB is situated at a locus which is involved in the uptake of heme iron (116). This locus also contains the gene for the cell wall protein IsdC, which contains the SrtB recognition sequence NPQTN. In addition, this locus contains the genes for the SrtA substrates IsdA and IsdB, which both contain LPXTG motifs. Notably, IsdC is so far the only protein known to be anchored to the cell wall by sortase B. IsdC is cleaved by SrtB between the Thr and Asn residues of the NPQTN motif. The only other S. aureus protein with a motif that resembles NPQTN is the DNA-binding protein II, but this protein is probably not cell wall bound because it lacks a signal peptide for export from the cytoplasm.
COMPARATIVE SECRETOME ANALYSIS
Comparison of the predicted secretomes of S. aureus and S. epidermidis with those of B. subtilis and other gram-positive bacteria revealed that most of the known components of the translocation machinery are present in S. aureus. The most notable differences are the second set of secA and secY genes in S. aureus, the absence of known signal peptide peptidases from S. aureus and S. epidermidis, the absence of a BdbC homologue from S. aureus and S. epidermidis, the presence of a second lspA gene in S. epidermidis, the absence of a Tat system from S. epidermidis, and the absence of two potential components in the ESAT-6 pathway from S. aureus MRSA252 (Table 3). So far, no evidence has been published on whether SecA2 and SecY2 are involved in the export of important virulence factors in S. aureus. However, it has been shown that the second secA/secY set is involved in the export of virulence factors in other pathogens (172). Although most known determinants for protein export, processing, and posttranslocational modification in other gram-positive bacteria are also present in S. aureus, in many cases it remains to be investigated to what extent they are necessary for protein export in general and the export of virulence factors in particular.
As shown by multiple BLAST comparisons, the core exoproteome of the sequenced S. aureus strains consists of 58 proteins (Table 6). All of these proteins have a signal peptide with a potential SpsB recognition and cleavage site. Thirty-three of these core exoproteins have already been identified in the extracellular milieus and/or membrane/cell wall proteomes of different S. aureus isolates (127, 208). Interestingly, 26 core exoproteins of S. aureus are also conserved in S. epidermidis, suggesting that they belong to a core staphylococcal exoproteome, which is presently still poorly defined. Interestingly, the core exoproteome of S. aureus seems to be largely composed of enzymes, such as proteases, and other factors, such as fibrinogen- and IgG-binding proteins, that are required for life in the ecological niches provided by the human host (Table 6). This is particularly true for the proteins that have the potential to be covalently bound to the cell wall (Table 10). In contrast, the variant exoproteome of S. aureus contains most of the known staphylococcal toxins and immunomodulating factors (Tables 7 and 11). This suggests that the components of the variant exoproteome should be regarded as specific gadgets of S. aureus that help this organism to conquer certain protected niches of the human host, thereby causing disease. If this idea is correct, then proteins of unknown function that belong to the variant exoproteome should be regarded as potentially important virulence factors.
TABLE 11.
Composition of the variant exoproteome of sequenced S. aureus strains
| Identification no. | Protein | Function | Presence of protein in straina
|
|||||
|---|---|---|---|---|---|---|---|---|
| COL | MRSA252 | MSSA476 | Mu50 | MW2 | N315 | |||
| 15925799 | Plc | 1-Phosphatidylinositol phosphodiesterase precursor | Y | Y | N | Y | Y | Y |
| 15926099 | Set6 | Exotoxin 6 | N | N | N | Y | N | Y |
| 15926100 | Set7 | Exotoxin 7 | Y | N | Y | Y | Y | Y |
| 15926101 | Set8 | Exotoxin 8 | N | N | Y | Y | Y | Y |
| 15926103 | Set10 | Exotoxin 10 | N | Y | Y | Y | Y | Y |
| 15926104 | Set11 | Exotoxin 11 | N | Y | Y | Y | Y | Y |
| 15926105 | Set12 | Exotoxin 12 | N | N | Y | Y | Y | Y |
| 15926106 | Set13 | Exotoxin 13 | Yb | Y | Y | Y | Y | Y |
| 15926111 | Set15 | Exotoxin 15 | N | N | N | Y | N | Y |
| 15926113 | SA0395 | Hypothetical protein | Y | Y | Y | N | Y | Y |
| 15926465 | SA0743 | Hypothetical protein | Y | N | Y | Y | Y | Y |
| 15926739 | SA1001 | Hypothetical protein | Y | N | Y | Y | Y | Y |
| 15926746 | HlY | α-Hemolysin precursor | Y | N | Y | Y | Y | Y |
| 15927016 | EbhB | Hypothetical protein | Y | Y | N | Y | Y | Y |
| 15927308 | Fhs | Hypothetical protein | Y | N | Y | Y | Y | Y |
| 15927386 | SplB | Serine protease SplB | Y | N | Y | Y | Y | Y |
| 15927387 | SplA | Serine protease SplA | Y | N | Y | Y | Y | Y |
| 15927389 | SA1633 | Probable β-lactamase | N | N | N | Y | N | Y |
| 15927393 | LukD | Leukotoxin, LukD | Y | N | Y | Y | Y | Y |
| 15927398 | SEG | Extracellular enterotoxin type G precursor | N | Y | N | Y | N | Y |
| 15927399 | SEN | Enterotoxin SEN | N | Y | N | Y | N | Y |
| 15927402 | SEI | Extracellular enterotoxin type I precursor | N | Y | N | Y | N | Y |
| 15927404 | SEO | Enterotoxin SEO | N | Y | N | Y | N | Y |
| 15927512 | Map | Truncated MapW protein | Y | Y | N | Y | Y | Y |
| 15927513 | Hlb | β-Hemolysin/phospholipase C | Y | N | N | Y | Y | Y |
| 15927516 | SA1754 | Hypothetical protein | N | Y | Y | Y | Y | Y |
| 15927517 | SA1755 | Hypothetical protein | N | Y | N | N | N | Y |
| 15927520 | Sak | Staphylokinase precursor | N | Y | Y | Y | Y | Y |
| 15927522 | SA1760 | Hypothetical protein | N | Y | Y | Y | Y | Y |
| 15927585 | SEC3 | Enterotoxin type C3 | Y | Y | N | Y | Y | Y |
| 15927586 | SA1818 | Hypothetical protein | Y | N | N | Y | Y | Y |
| 15927587 | TSST-1 | Toxic shock syndrome toxin 1 | N | N | N | Y | N | Y |
| 15927741 | FmtB | FmtB protein | Y | N | N | Y | Y | Y |
| 15928076 | SA228 | Hypothetical protein | Y | N | Y | Y | Y | Y |
| 15928174 | SA2381 | Hypothetical protein | N | N | Y | Y | Y | Y |
| 15928182 | SA2389 | Hypothetical protein | N | N | N | Y | N | Y |
| 16119219 | SAP019 | Hypothetical protein | N | Y | Y | N | N | Y |
| 14141830 | SAVP008 | Hypothetical protein | N | N | N | Y | N | N |
| 21282111 | Set16 | Hypothetical protein | N | Y | Y | N | Y | N |
| 21282116 | Set21 | Hypothetical protein | N | N | Y | N | Y | N |
| 21282123 | Set26 | Hypothetical protein | N | Y | Y | N | Y | N |
| 21283107 | LukF | Panton-Valentine leukocidin chain F precursor | N | N | N | N | Y | N |
| 21283108 | LukS | Panton-Valentine leukocidin chain S precursor | N | N | N | N | Y | N |
| 21284341 | Cna | Collagen adhesin precursor | N | Y | Y | N | Y | N |
| 49482651 | SAR0423 | Exotoxin | N | Y | N | N | N | N |
| 49482652 | SAR0424 | Exotoxin | N | Y | N | N | N | N |
| 49482653 | SAR0425 | Exotoxin | N | Y | N | N | N | N |
| 49482925 | SAR0721 | Multicopper oxidase protein | N | Y | N | N | N | N |
| 49484047 | SAR1886 | Putative exported protein | N | Y | N | N | N | N |
| 49484059 | SAR1905 | Serine protease | N | Y | N | N | N | N |
| 49484898 | SAR2788 | Putative exported protein | N | Y | N | N | N | N |
| 49484957 | SEH | Enterotoxin H | N | N | Y | N | Y | N |
| 49485293 | SAS0389 | Exotoxin | N | N | Y | N | Y | N |
| 57650600 | SACOL1865 | Serine protease SplE, putative | Y | Y | N | N | N | N |
| 57650605 | SACOL1870 | Hypothetical protein | Y | N | Y | N | Y | N |
| 57650609 | EpiP | Epidermin leader peptide processing serine protease EpiP | Y | N | Y | N | Y | N |
| 57651309 | SACOL0468 | Exotoxin 3, putative | Y | N | N | N | N | N |
| 57651311 | SACOL0470 | Exotoxin, putative | Y | N | N | N | N | N |
| 57651319 | SACOL0478 | Exotoxin 3, putative | Y | N | N | N | N | N |
| 57652419 | Pls | Methicillin-resistant surface protein | Y | N | N | N | N | N |
Y, yes; N, no.
This protein has a Thr at the +1 position, which is not included in the SpsB recognition and cleavage pattern. All other S. aureus strains conform to the proposed pattern.
The (predicted) extracellular toxins of S. aureus are not present in S. epidermidis. This is mainly due to the fact that these toxins are encoded by pathogenicity islands in the genomes of S. aureus strains that have thus far not been observed in S. epidermidis genomes. Proteins with predicted signal peptides that are specific for S. epidermidis are listed in Table 12. Notably, the majority (i.e., 26 of 30) of predicted S. epidermidis exoproteins that have homologues in S. aureus share this homology with components of the core exoproteome of S. aureus (Tables 6 and 7). This suggests that in S. epidermidis, the core exoproteome is also involved in housekeeping functions. In contrast to the case for the exoproteome, it is presently difficult to speculate about housekeeping and disease-causing roles of the constant and variant lipoproteomes of S. aureus. This is due to the fact that the functions of only a few S. aureus lipoproteins are known (Tables 8, 9, and 13). In general terms, it is presently not clear why S. epidermidis seems to export a smaller number of different proteins (101 in total) than does S. aureus (∼135 in total). This difference is all the more remarkable since the total numbers of proteins encoded by the genomes of S. aureus (∼2,600) and S. epidermidis (∼2,500) are comparable.
TABLE 12.
Specific S. epidermidis proteins with predicted signal peptides
| Signal peptide type | Identification no. | Protein | Amino acid at position:
|
Function | ||||
|---|---|---|---|---|---|---|---|---|
| −3 | −2 | −1 | +1 | +2 | ||||
| Sec | 27466926a | SE0008 | T | Y | A | S | Truncated β-hemolysin | |
| 27467163 | SE0245 | V | H | A | A | Triacylglycerol lipase precursor | ||
| 27467176 | SE0258 | A | K | A | Q | Immunodominant antigen B | ||
| 27467249b,c | SE0331 | A | K | A | E | Ser-Asp-rich, fibrinogen-binding bone sialoprotein-binding protein | ||
| 27467501a | SE0583 | S | F | A | N | Hypothetical protein | ||
| 27467545a | SE0627 | T | L | A | D | Poly-d-alanine transfer protein | ||
| 27467746b | SE0828 | A | H | A | E | Lipoprotein VsaC | ||
| 27467938a | SE1020 | I | K | A | Q | Hypothetical protein | ||
| 27468418b | SE1500 | S | Y | A | Q | Hypothetical protein | ||
| 27468493 | SE1575 | A | Q | A | H | Immunodominant antigen B | ||
| 27468546b | SE1628 | V | Y | A | D | Hypothetical protein | ||
| 27468801a | SE1883 | V | S | A | K | Lyt divergon expression attenuator LytR | ||
| 27468838 | SE1920 | V | Y | A | Q | Hypothetical protein | ||
| 27469060a | SE2142 | T | L | A | F | 2-Dehydropantoate 2-reductase | ||
| 27469070b | SE2152 | T | H | A | A | Hypothetical protein | ||
| 27469115a | SE2197 | S | Y | A | S | Alkaline phosphatase III precursor | ||
| 27469119 | SE2201 | A | D | A | Z | Phage-related protein | ||
| 27469291 | SE2373 | A | Q | A | S | 1,4-β-N-Acetylmuramidase | ||
| 27469316 | SE2398 | S | S | A | S | Hypothetical protein | ||
| 32470521 | P601 | A | S | A | S | Hypothetical protein | ||
| 32470527 | P607 | I | N | A | D | Hypothetical protein | ||
| 32470549b | P517 | A | K | A | E | Hypothetical protein | ||
| 32470583 | P202 | T | F | A | L | Hypothetical protein | ||
| Lipoprotein | 27466952 | SE0034 | L | S | A | C | S | Hypothetical protein |
| 27467000 | SE0082 | L | T | A | C | G | Hypothetical protein | |
| 27467062 | SE0144 | V | S | G | C | G | Hypothetical protein | |
| 27467063 | SE0145 | V | S | G | C | G | Hypothetical protein | |
| 27467067d | SE0149 | L | A | G | C | D | Hypothetical protein | |
| 27467309d | SE0391 | L | T | T | C | S | Hypothetical protein | |
| 27468024 | SE1106 | V | T | A | C | S | ABC transporter | |
| 27468425d | SE1507 | L | Y | G | C | G | Hypothetical protein | |
| 27469012d | SE2094 | L | I | I | C | S | Hypothetical protein | |
| 27469069d | SE2151 | L | A | G | C | G | Hypothetical protein | |
| 27469130 | SE2212 | V | S | G | C | S | Hypothetical protein | |
| 27469141d | SE2223 | L | G | S | C | S | Hypothetical protein | |
| 27469317 | SE2399 | L | S | A | C | G | Hypothetical protein | |
All S. aureus strains have one or more residues in the cleavage site which are not included in the search pattern.
Contains an LPXTG motif.
Has a lower SignalP score than our threshold score for all S. aureus strains.
Excluded for S. epidermidis because of the motif proposed by Sutcliffe and Harrington (171).
TABLE 13.
Composition of the variant lipoproteome of sequenced S. aureus strains
| Identification no. | Protein | Function | Presence of protein in straina
|
|||||
|---|---|---|---|---|---|---|---|---|
| COL | MRSA252 | MSSA476 | Mu50 | MW2 | N315 | |||
| 15925801 | SA0093 | Hypothetical protein | N | N | N | Y | N | Y |
| 15925803 | SA0095 | Hypothetical protein | N | N | N | Y | N | Y |
| 15925804 | SA0096 | Hypothetical protein | Y | N | N | Y | N | Y |
| 15925877 | SA0167 | Hypothetical protein | N | Y | Y | Y | Y | Y |
| 15926004 | SA0291 | Hypothetical protein | Y | N | Y | Y | Y | Y |
| 15926114 | Lpl1 | Hypothetical protein | Y | Y | N | Y | N | Y |
| 19526115 | Lpl2 | Hypothetical protein | N | N | N | Y | N | Y |
| 15926116 | Lpl3 | Hypothetical protein | Y | N | N | Y | N | Y |
| 15926118 | Lpl4 | Hypothetical protein | N | N | N | Y | N | Y |
| 15926119 | Lpl5 | Hypothetical protein | N | N | N | Y | N | Y |
| 15926120 | Lpl6 | Hypothetical protein | N | N | Y | Y | Y | Y |
| 15926122 | Lpl8 | Hypothetical protein | Y | Y | N | Y | N | Y |
| 15926123 | Lpl9 | Hypothetical protein | Y | Y | Y | Y | N | Y |
| 15926465 | SA0743 | Hypothetical protein, similar to staphylocoagulase precursor | Y | N | Y | Y | Y | Y |
| 15926580 | SA0850 | Hypothetical protein, similar to oligopeptide ABC transporter oligopeptide-binding protein | Y | N | Y | Y | Y | Y |
| 15927067 | SA1317 | Hypothetical protein | N | N | N | Y | N | Y |
| 15927068 | SA1318 | Hypothetical protein | N | N | Y | Y | Y | Y |
| 15927069 | SA1319 | Hypothetical protein | N | Y | Y | Y | Y | Y |
| 15927373 | SA1617 | Hypothetical protein, similar to latent nuclear antigen | Y | Y | Y | Y | Y | Y |
| 15927396 | SA1640 | Conserved hypothetical protein | N | N | N | Y | N | Y |
| 15927859 | ModA | Probable molybdate-binding protein | Y | Y | Y | N | Y | Y |
| 15928064 | SA2273 | Hypothetical protein | Y | N | N | Y | N | Y |
| 21281801 | MW0072 | Hypothetical protein | Y | N | Y | N | Y | N |
| 21282126 | Lpl10 | Hypothetical protein | Y | Y | Y | N | Y | N |
| 21282127 | Lpl11 | Hypothetical protein | N | Y | Y | N | Y | N |
| 21282129 | Lpl13 | Hypothetical protein | N | N | Y | N | Y | N |
| 21283103 | MW1374 | Conserved hypothetical protein | N | N | Y | N | Y | N |
| 21283167 | MW1438 | Hypothetical protein | N | Y | Y | N | Y | N |
| 21284135 | MW2406 | Hypothetical protein | N | N | Y | N | Y | N |
| 21284306 | MW2577 | Hypothetical protein | N | N | Y | N | Y | N |
| 49482670 | SAR0445 | Putative lipoprotein | N | Y | N | N | N | N |
| 49483993 | BlaZ | β-Lactamase precursor | N | Y | N | N | N | N |
| 49484246 | SAR2104 | Putative lipoprotein | N | Y | N | Y | N | N |
| 49484287 | SAR2149 | Putative exported protein | N | Y | N | N | N | N |
| 49484977 | SAS0074 | Putative lipoprotein | N | N | Y | N | N | N |
| 57650161 | SACOL0888 | Pathogenicity island, putative lipoprotein | Y | N | N | N | N | N |
| 57650441 | SACOL1528 | Hypothetical protein SA1528 | Y | Y | N | N | N | N |
| 57650444 | SACOL1531 | Hypothetical protein SA1531 | Y | N | N | N | N | N |
| 57650485 | SACOL1574 | Hypothetical protein SA1574 | Y | Y | N | N | N | N |
| 57650696 | SACOL2010 | Iron compound ABC transporter, iron compound-binding protein | Y | N | Y | Y | Y | N |
| 57651323 | SACOL0482 | Staphylococcus tandem lipoprotein | Y | N | N | N | N | N |
| 57652445 | SACOL0081 | Hypothetical protein SA0081 | Y | N | N | N | N | N |
| 14141829 | SAVP006 | Hypothetical protein | N | N | N | Y | N | N |
Y, yes; N, no.
Compared to B. subtilis and B. licheniformis (193), S. aureus is also predicted to export a relatively large number of proteins from the cytoplasm to the membrane-cell wall interface, the cell wall, and the extracellular milieu. The genomes of B. subtilis and B. licheniformis contain ∼4,100 protein-encoding genes, while S. aureus genomes contain significantly fewer genes (∼2,600). Using the most recent prediction protocols (179), B. subtilis is predicted to export 190 proteins to an extracytoplasmic location, whereas, depending on the strain investigated, S. aureus is predicted to export 130 to 145 proteins (this review). Accordingly, as judged by the relative numbers of protein-encoding genes, S. aureus strains appear to export 6 to 20% more proteins to an extracytoplasmic location than do the aforementioned bacilli. Most probably, this is related to the fact that S. aureus needs an arsenal of virulence factors, such as toxins and surface proteins, for colonization of and survival in its preferred niches in the human host. Such proteins are of less importance for soil bacteria, such as B. subtilis and B. licheniformis, which thrive predominantly on dead organic matter.
PERSPECTIVES
The present review provides a survey of possible protein transport pathways in staphylococcal pathogenesis. In many cases, the knowledge gathered from protein secretion studies in other organisms has been projected on S. aureus, assuming that similar pathways or pathway components have similar functions in different organisms. Clearly, this leaves room for surprises when such pathways are investigated thoroughly for S. aureus. The same was true for studies on protein secretion in B. subtilis. These studies showed, for example, that the absence of SecDF has barely any consequences for protein secretion by B. subtilis, whereas SecD and SecF are of key importance for protein translocation in E. coli, the organism in which SecD/F was first discovered (20). Likewise, LspA was shown to be dispensable in B. subtilis but not in E. coli (147, 177). Thus, the relative importance of different secretion machinery components of S. aureus needs to be assessed in a systematic manner, preferably in an isogenic background. Such studies would need to address the importance of secretion machinery components for in vitro growth on different substrates (e.g., broth or blood) and for virulence in vivo in model systems (e.g., C. elegans, Drosophila melanogaster, mice, or rats) (8, 60, 173). These studies should be complemented with proteomic verification of our present lipoproteome, wall proteome, and exoproteome predictions. Such a verification could involve both gel-based proteomics approaches, as outlined in this review, and more sophisticated gel-free proteomics approaches (194). This would lead to an improved understanding of the contribution of each protein transport pathway and its substrate proteins to staphylococcal cell physiology and virulence. Since the virulence of different S. aureus strains will depend not only on the presence (or absence) of particular genes for virulence factors but also on their expression, such proteomic studies should also include experiments on the impacts of major regulatory systems, such as agr, sae, and sarA. On this basis, it should be possible to identify the most promising candidate drug targets in the staphylococcal secretome. Alternatively, secretome components thus identified could represent promising candidates for novel vaccines. For all of these efforts, comparative secretomics approaches will provide essential information on those potential drug targets that are most important for both bacterial housekeeping functions and virulence. Novel drugs and vaccines designed against these targets are likely to have the highest impact (65).
Acknowledgments
We thank Harold Tjalsma and members of the Groningen and European Bacillus Secretion Groups and the BACELL Health, Tat Machine, and StaphDynamics consortia for stimulating discussions.
M.J.J.B.S., A.K.Z., S.E., M.H., J.Y.F.D., and J.M.V.D. were supported by grants LSHG-CT-2004-503468, LSHG-CT-2004-005257, and LSHM-CT-2006-019064 from the European Union. M.H. was supported by grants from the Deutsche Forschungsgemeinschaft, the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie, and the Fonds der Chemischen Industrie.
REFERENCES
- 1.Adhikari, R. P., and R. P. Novick. 2005. Subinhibitory cerulenin inhibits staphylococcal exoprotein production by blocking transcription rather than by blocking secretion. Microbiology 151:3059-3069. [DOI] [PubMed] [Google Scholar]
- 2.Alami, M., I. Lüke, S. Deitermann, G. Eisner, H. G. Koch, J. Brunner, and M. Müller. 2003. Differential interactions between a twin-arginine signal peptide and its translocase in Escherichia coli. Mol. Cell 12:937-946. [DOI] [PubMed] [Google Scholar]
- 3.Antelmann, H., H. Tjalsma, B. Voigt, S. Ohlmeier, S. Bron, J. M. van Dijl, and M. Hecker. 2001. A proteomic view on genome-based signal peptide predictions. Genome Res. 11:1484-1502. [DOI] [PubMed] [Google Scholar]
- 4.Avison, M. B., P. M. Bennett, R. A. Howe, and T. R. Walsh. 2002. Preliminary analysis of the genetic basis for vancomycin resistance in Staphylococcus aureus strain Mu50. J. Antimicrob. Chemother. 49:255-260. [DOI] [PubMed] [Google Scholar]
- 5.Baba, T., and O. Schneewind. 1996. Target cell specificity of a bacteriocin molecule: a C-terminal signal directs lysostaphin to the cell wall of Staphylococcus aureus. EMBO J. 15:4789-4797. [PMC free article] [PubMed] [Google Scholar]
- 6.Baba, T., and O. Schneewind. 1998. Targeting of muralytic enzymes to the cell division site of gram-positive bacteria: repeat domains direct autolysin to the equatorial surface ring of Staphylococcus aureus. EMBO J. 17:4639-4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baba, T., F. Takeuchi, M. Kuroda, H. Yuzawa, K. Aoki, A. Oguchi, Y. Nagai, N. Iwama, K. Asano, T. Naimi, H. Kuroda, L. Cui, K. Yamamoto, and K. Hiramatsu. 2002. Genome and virulence determinants of high virulence community-acquired MRSA. Lancet 359:1819-1827. [DOI] [PubMed] [Google Scholar]
- 8.Bae, T., A. K. Banger, A. Wallace, E. M. Glass, F. Åslund, O. Schneewind, and D. M. Missiakas. 2004. Staphylococcus aureus virulence genes identified by bursa aurealis mutagenesis and nematode killing. Proc. Natl. Acad. Sci. USA 101:12312-12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bae, T., and O. Schneewind. 2003. The YSIRK-G/S motif of staphylococcal protein A and its role in efficiency of signal peptide processing. J. Bacteriol. 185:2910-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baerends, R. J., W. K. Smits, A. de Jong, L. W. Hamoen, J. Kok, and O. P. Kuipers. 2004. Genome2D: a visualization tool for the rapid analysis of bacterial transcriptome data. Genome Biol. 5:R37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bayles, K. W. 2000. The bactericidal action of penicillin: new clues to an unsolved mystery. Trends Microbiol. 8:274-278. [DOI] [PubMed] [Google Scholar]
- 12.Bendtsen, J. D., H. Nielsen, G. von Heijne, and S. Brunak. 2004. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340:783-795. [DOI] [PubMed] [Google Scholar]
- 13.Bensing, B. A., and P. M. Sullam. 2002. An accessory sec locus of Streptococcus gordonii is required for export of the surface protein GspB and for normal levels of binding to human platelets. Mol. Microbiol. 44:1081-1094. [DOI] [PubMed] [Google Scholar]
- 14.Berks, B. C., T. Palmer, and F. Sargent. 2005. Protein targeting by the bacterial twin-arginine translocation (Tat) pathway. Curr. Opin. Microbiol. 8:174-181. [DOI] [PubMed] [Google Scholar]
- 15.Bernhardt, J., K. Büttner, C. Scharf, and M. Hecker. 1999. Dual channel imaging of two-dimensional electropherograms in Bacillus subtilis. Electrophoresis 20:2225-2240. [DOI] [PubMed] [Google Scholar]
- 16.Berthet, F. X., P. B. Rasmussen, I. Rosenkrands, P. Andersen, and B. Gicquel. 1998. A Mycobacterium tuberculosis operon encoding ESAT-6 and a novel low-molecular-mass culture filtrate protein (CFP-10). Microbiology 144:3195-3203. [DOI] [PubMed] [Google Scholar]
- 17.Bibi, E. 1998. The role of the ribosome-translocon complex in translation and assembly of polytopic membrane proteins. Trends Biochem. Sci. 23:51-55. [DOI] [PubMed] [Google Scholar]
- 18.Blaudeck, N., P. Kreutzenbeck, M. Müller, G. A. Sprenger, and R. Freudl. 2005. Isolation and characterization of bifunctional Escherichia coli TatA mutant proteins that allow efficient tat-dependent protein translocation in the absence of TatB. J. Biol. Chem. 280:3426-3432. [DOI] [PubMed] [Google Scholar]
- 19.Boël, G., V. Pichereau, I. Mijakovic, A. Mazé, S. Poncet, S. Gillet, J. C. Giard, A. Hartke, Y. Auffray, and J. Deutscher. 2004. Is 2-phosphoglycerate-dependent automodification of bacterial enolases implicated in their export? J. Mol. Biol. 337:485-496. [DOI] [PubMed] [Google Scholar]
- 20.Bolhuis, A., C. P. Broekhuizen, A. Sorokin, M. L. van Roosmalen, G. Venema, S. Bron, W. J. Quax, and J. M. van Dijl. 1998. SecDF of Bacillus subtilis, a molecular Siamese twin required for the efficient secretion of proteins. J. Biol. Chem. 273:21217-21224. [DOI] [PubMed] [Google Scholar]
- 21.Bolhuis, A., A. Matzen, H. L. Hyyryläinen, V. P. Kontinen, R. Meima, J. Chapuis, G. Venema, S. Bron, R. Freudl, and J. M. van Dijl. 1999. Signal peptide peptidase- and ClpP-like proteins of Bacillus subtilis required for efficient translocation and processing of secretory proteins. J. Biol. Chem. 274:24585-24592. [DOI] [PubMed] [Google Scholar]
- 22.Braun, L., S. Dramsi, P. Dehoux, H. Bierne, G. Lindahl, and P. Cossart. 1997. InlB: an invasion protein of Listeria monocytogenes with a novel type of surface association. Mol. Microbiol. 25:285-294. [DOI] [PubMed] [Google Scholar]
- 23.Bruton, G., A. Huxley, P. O'Hanlon, B. Orlek, D. Eggleston, J. Humphries, S. Readshaw, A. West, S. Ashman, M. Brown, K. Moore, A. Pope, K. O'Dwyer, and L. Wang. 2003. Lipopeptide substrates for SpsB, the Staphylococcus aureus type I signal peptidase: design, conformation and conversion to alpha-ketoamide inhibitors. Eur. J. Med. Chem. 38:351-356. [DOI] [PubMed] [Google Scholar]
- 24.Bugg, T. D., G. D. Wright, S. Dutka-Malen, M. Arthur, P. Courvalin, and C. T. Walsh. 1991. Molecular basis for vancomycin resistance in Enterococcus faecium BM4147: biosynthesis of a depsipeptide peptidoglycan precursor by vancomycin resistance proteins VanH and VanA. Biochemistry 30:10408-10415. [DOI] [PubMed] [Google Scholar]
- 25.Bunai, K., K. Yamada, K. Hayashi, K. Nakamura, and K. Yamane. 1999. Enhancing effect of Bacillus subtilis Ffh, a homologue of the SRP54 subunit of the mammalian signal recognition particle, on the binding of SecA to precursors of secretory proteins in vitro. J. Biochem. (Tokyo) 125:151-159. [DOI] [PubMed] [Google Scholar]
- 26.Burts, M. L., W. A. Williams, K. DeBord, and D. M. Missiakas. 2005. EsxA and EsxB are secreted by an ESAT-6-like system that is required for the pathogenesis of Staphylococcus aureus infections. Proc. Natl. Acad. Sci. USA 102:1169-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. 2003. Methicillin-resistant Staphylococcus aureus infections in correctional facilities—Georgia, California, and Texas, 2001-2003. Morb. Mortal. Wkly. Rep. 52:992-995. [PubMed] [Google Scholar]
- 28.Chambers, H. F. 1997. Methicillin resistance in staphylococci: molecular and biochemical basis and clinical implications. Clin. Microbiol. Rev. 10:781-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheung, A. L., J. M. Koomey, C. A. Butler, S. J. Projan, and V. A. Fischetti. 1992. Regulation of exoprotein expression in Staphylococcus aureus by a locus (sar) distinct from agr. Proc. Natl. Acad. Sci. USA 89:6462-6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheung, A. L., and S. J. Projan. 1994. Cloning and sequencing of sarA of Staphylococcus aureus, a gene required for the expression of agr. J. Bacteriol. 176:4168-4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung, Y. S., F. Breidt, and D. Dubnau. 1998. Cell surface localization and processing of the ComG proteins, required for DNA binding during transformation of Bacillus subtilis. Mol. Microbiol. 29:905-913. [DOI] [PubMed] [Google Scholar]
- 32.Chung, Y. S., and D. Dubnau. 1998. All seven comG open reading frames are required for DNA binding during transformation of competent Bacillus subtilis. J. Bacteriol. 180:41-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chung, Y. S., and D. Dubnau. 1995. ComC is required for the processing and translocation of comGC, a pilin-like competence protein of Bacillus subtilis. Mol. Microbiol. 15:543-551. [DOI] [PubMed] [Google Scholar]
- 34.Clarke, S. R., L. G. Harris, R. G. Richards, and S. J. Foster. 2002. Analysis of Ebh, a 1.1-megadalton cell wall-associated fibronectin-binding protein of Staphylococcus aureus. Infect. Immun. 70:6680-6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clarke, S. R., M. D. Wiltshire, and S. J. Foster. 2004. IsdA of Staphylococcus aureus is a broad spectrum, iron-regulated adhesin. Mol. Microbiol. 51:1509-1519. [DOI] [PubMed] [Google Scholar]
- 36.Cline, K., and H. Mori. 2001. Thylakoid DeltapH-dependent precursor proteins bind to a cpTatC-Hcf106 complex before Tha4-dependent transport. J. Cell Biol. 154:719-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Converse, S. E., and J. S. Cox. 2005. A protein secretion pathway critical for Mycobacterium tuberculosis virulence is conserved and functional in Mycobacterium smegmatis. J. Bacteriol. 187:1238-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cregg, K. M., I. Wilding, and M. T. Black. 1996. Molecular cloning and expression of the spsB gene encoding an essential type I signal peptidase from Staphylococcus aureus. J. Bacteriol. 178:5712-5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cristóbal, S., J. W. de Gier, H. Nielsen, and G. von Heijne. 1999. Competition between Sec- and Tat-dependent protein translocation in Escherichia coli. EMBO J. 18:2982-2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crupper, S. S., A. J. Gies, and J. J. Iandolo. 1997. Purification and characterization of staphylococcin BacR1, a broad-spectrum bacteriocin. Appl. Environ. Microbiol. 63:4185-4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cserzö, M., E. Wallin, I. Simon, G. von Heijne, and A. Elofsson. 1997. Prediction of transmembrane alpha-helices in prokaryotic membrane proteins: the dense alignment surface method. Protein Eng. 10:673-676. [DOI] [PubMed] [Google Scholar]
- 42.Cui, L., X. Ma, K. Sato, K. Okuma, F. C. Tenover, E. M. Mamizuka, C. G. Gemmell, M. N. Kim, M. C. Ploy, N. El Solh, V. Ferraz, and K. Hiramatsu. 2003. Cell wall thickening is a common feature of vancomycin resistance in Staphylococcus aureus. J. Clin. Microbiol. 41:5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.da Silva, M. C., J. M. Zahm, D. Gras, O. Bajolet, M. Abely, J. Hinnrasky, M. Milliot, M. C. de Assis, C. Hologne, N. Bonnet, M. Merten, M. C. Plotkowski, and E. Puchelle. 2004. Dynamic interaction between airway epithelial cells and Staphylococcus aureus. Am. J. Physiol. Lung Cell Mol. Physiol. 287:L543-L551. [DOI] [PubMed] [Google Scholar]
- 44.de Keyzer, J., C. van der Does, and A. J. Driessen. 2003. The bacterial translocase: a dynamic protein channel complex. Cell. Mol. Life Sci. 60:2034-2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Leeuw, E., K. te Kaat, C. Moser, G. Menestrina, R. Demel, B. de Kruijff, B. Oudega, J. Luirink, and I. Sinning. 2000. Anionic phospholipids are involved in membrane association of FtsY and stimulate its GTPase activity. EMBO J. 19:531-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diep, B. A., S. R. Gill, R. F. Chang, T. H. Phan, J. H. Chen, M. G. Davidson, F. Lin, J. Lin, H. A. Carleton, E. F. Mongodin, G. F. Sensabaugh, and F. Perdreau-Remington. 2006. Complete genome sequence of USA300, an epidemic clone of community-acquired methicillin-resistant Staphylococcus aureus. Lancet 367:731-739. [DOI] [PubMed] [Google Scholar]
- 47.Dilks, K., R. W. Rose, E. Hartmann, and M. Pohlschröder. 2003. Prokaryotic utilization of the twin-arginine translocation pathway: a genomic survey. J. Bacteriol. 185:1478-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dinges, M. M., P. M. Orwin, and P. M. Schlievert. 2000. Exotoxins of Staphylococcus aureus. Clin. Microbiol. Rev. 13:16-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dobrindt, U., B. Hochhut, U. Hentschel, and J. Hacker. 2004. Genomic islands in pathogenic and environmental microorganisms. Nat. Rev. Microbiol. 2:414-424. [DOI] [PubMed] [Google Scholar]
- 50.Dramsi, S., P. Trieu-Cuot, and H. Bierne. 2005. Sorting sortases: a nomenclature proposal for the various sortases of gram-positive bacteria. Res. Microbiol. 156:289-297. [DOI] [PubMed] [Google Scholar]
- 51.Driessen, A. J., P. Fekkes, and J. P. van der Wolk. 1998. The Sec system. Curr. Opin. Microbiol. 1:216-222. [DOI] [PubMed] [Google Scholar]
- 52.Dubnau, D. 1999. DNA uptake in bacteria. Annu. Rev. Microbiol. 53:217-244. [DOI] [PubMed] [Google Scholar]
- 53.Dumoulin, A., U. Grauschopf, M. Bischoff, L. Thöny-Meyer, and B. Berger-Bächi. 2005. Staphylococcus aureus DsbA is a membrane-bound lipoprotein with thiol-disulfide oxidoreductase activity. Arch. Microbiol. 184:117-128. [DOI] [PubMed] [Google Scholar]
- 54.Dziewanowska, K., V. M. Edwards, J. R. Deringer, G. A. Bohach, and D. J. Guerra. 1996. Comparison of the beta-toxins from Staphylococcus aureus and Staphylococcus intermedius. Arch. Biochem. Biophys. 335:102-108. [DOI] [PubMed] [Google Scholar]
- 55.Eisner, G., H. G. Koch, K. Beck, J. Brunner, and M. Müller. 2003. Ligand crowding at a nascent signal sequence. J. Cell Biol. 163:35-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Escher, A., and W. G. Characklis. 1990. Modeling the initial events in biofilm accumulation, p. 445-486. In W. G. Characklis (ed.), Biofilms. Wiley Press, New York, N.Y.
- 57.Fedtke, I., F. Gotz, and A. Peschel. 2004. Bacterial evasion of innate host defenses—the Staphylococcus aureus lesson. Int. J. Med. Microbiol. 294:189-194. [DOI] [PubMed] [Google Scholar]
- 58.Foster, T. J., and M. Hook. 1998. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 6:484-488. [DOI] [PubMed] [Google Scholar]
- 59.Garcia, P., J. L. Garcia, E. Garcia, J. M. Sanchez-Puelles, and R. Lopez. 1990. Modular organization of the lytic enzymes of Streptococcus pneumoniae and its bacteriophages. Gene 86:81-88. [DOI] [PubMed] [Google Scholar]
- 60.Garcia-Lara, J., A. J. Needham, and S. J. Foster. 2005. Invertebrates as animal models for Staphylococcus aureus pathogenesis: a window into host-pathogen interaction. FEMS Immunol. Med. Microbiol. 43:311-323. [DOI] [PubMed] [Google Scholar]
- 61.Gaspar, A. H., and H. Ton-That. 2006. Assembly of distinct pilus structures on the surface of Corynebacterium diphtheriae. J. Bacteriol. 188:1526-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ghuysen, J. M., J. Lamotte-Brasseur, B. Joris, and G. D. Shockman. 1994. Binding site-shaped repeated sequences of bacterial wall peptidoglycan hydrolases. FEBS Lett. 342:23-28. [DOI] [PubMed] [Google Scholar]
- 63.Gill, S. R., D. E. Fouts, G. L. Archer, E. F. Mongodin, R. T. Deboy, J. Ravel, I. T. Paulsen, J. F. Kolonay, L. Brinkac, M. Beanan, R. J. Dodson, S. C. Daugherty, R. Madupu, S. V. Angiuoli, A. S. Durkin, D. H. Haft, J. Vamathevan, H. Khouri, T. Utterback, C. Lee, G. Dimitrov, L. Jiang, H. Qin, J. Weidman, K. Tran, K. Kang, I. R. Hance, K. E. Nelson, and C. M. Fraser. 2005. Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J. Bacteriol. 187:2426-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Götz, F. 2002. Staphylococcus and biofilms. Mol. Microbiol. 43:1367-1378. [DOI] [PubMed] [Google Scholar]
- 65.Götz, F. 2004. Staphylococci in colonization and disease: prospective targets for drugs and vaccines. Curr. Opin. Microbiol. 7:477-487. [DOI] [PubMed] [Google Scholar]
- 66.Groicher, K. H., B. A. Firek, D. F. Fujimoto, and K. W. Bayles. 2000. The Staphylococcus aureus lrgAB operon modulates murein hydrolase activity and penicillin tolerance. J. Bacteriol. 182:1794-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grundmann, H., A. Tami, S. Hori, M. Halwani, and R. Slack. 2002. Nottingham Staphylococcus aureus population study: prevalence of MRSA among elderly people in the community. BMJ 324:1365-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grundmeier, M., M. Hussain, P. Becker, C. Heilmann, G. Peters, and B. Sinha. 2004. Truncation of fibronectin-binding proteins in Staphylococcus aureus strain Newman leads to deficient adherence and host cell invasion due to loss of the cell wall anchor function. Infect. Immun. 72:7155-7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hahn, J., B. Maier, B. J. Haijema, M. Sheetz, and D. Dubnau. 2005. Transformation proteins and DNA uptake localize to the cell poles in Bacillus subtilis. Cell 122:59-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hartford, O., P. Francois, P. Vaudaux, and T. J. Foster. 1997. The dipeptide repeat region of the fibrinogen-binding protein (clumping factor) is required for functional expression of the fibrinogen-binding domain on the Staphylococcus aureus cell surface. Mol. Microbiol. 25:1065-1076. [DOI] [PubMed] [Google Scholar]
- 71.Hasona, A., P. J. Crowley, C. M. Levesque, R. W. Mair, D. G. Cvitkovitch, A. S. Bleiweis, and L. J. Brady. 2005. Streptococcal viability and diminished stress tolerance in mutants lacking the signal recognition particle pathway or YidC2. Proc. Natl. Acad. Sci. USA 102:17466-17471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hauck, C. R., and K. Ohlsen. 2006. Sticky connections: extracellular matrix protein recognition and integrin-mediated cellular invasion by Staphylococcus aureus. Curr. Opin. Microbiol. 9:5-11. [DOI] [PubMed] [Google Scholar]
- 73.Håvarstein, L. S., D. B. Diep, and I. F. Nes. 1995. A family of bacteriocin ABC transporters carry out proteolytic processing of their substrates concomitant with export. Mol. Microbiol. 16:229-240. [DOI] [PubMed] [Google Scholar]
- 74.Heilmann, C., M. Hussain, G. Peters, and F. Götz. 1997. Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface. Mol. Microbiol. 24:1013-1024. [DOI] [PubMed] [Google Scholar]
- 75.Herbort, M., M. Klein, E. H. Manting, A. J. Driessen, and R. Freudl. 1999. Temporal expression of the Bacillus subtilis secA gene, encoding a central component of the preprotein translocase. J. Bacteriol. 181:493-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hiller, K., A. Grote, M. Scheer, R. Münch, and D. Jahn. 2004. PrediSi: prediction of signal peptides and their cleavage positions. Nucleic Acids Res. 32:W375-W379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hinsley, A. P., N. R. Stanley, T. Palmer, and B. C. Berks. 2001. A naturally occurring bacterial Tat signal peptide lacking one of the ‘invariant’ arginine residues of the consensus targeting motif. FEBS Lett. 497:45-49. [DOI] [PubMed] [Google Scholar]
- 78.Hiramatsu, K., H. Hanaki, T. Ino, K. Yabuta, T. Oguri, and F. C. Tenover. 1997. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J. Antimicrob. Chemother. 40:135-136. [DOI] [PubMed] [Google Scholar]
- 79.Holden, M. T., E. J. Feil, J. A. Lindsay, S. J. Peacock, N. P. Day, M. C. Enright, T. J. Foster, C. E. Moore, L. Hurst, R. Atkin, A. Barron, N. Bason, S. D. Bentley, C. Chillingworth, T. Chillingworth, C. Churcher, L. Clark, C. Corton, A. Cronin, J. Doggett, L. Dowd, T. Feltwell, Z. Hance, B. Harris, H. Hauser, S. Holroyd, K. Jagels, K. D. James, N. Lennard, A. Line, R. Mayes, S. Moule, K. Mungall, D. Ormond, M. A. Quail, E. Rabbinowitsch, K. Rutherford, M. Sanders, S. Sharp, M. Simmonds, K. Stevens, S. Whitehead, B. G. Barrell, B. G. Spratt, and J. Parkhill. 2004. Complete genomes of two clinical Staphylococcus aureus strains: evidence for the rapid evolution of virulence and drug resistance. Proc. Natl. Acad. Sci. USA 101:9786-9791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Holtfreter, S., K. Bauer, D. Thomas, C. Feig, V. Lorenz, K. Roschack, E. Friebe, K. Selleng, S. Lovenich, T. Greve, A. Greinacher, B. Panzig, S. Engelmann, G. Lina, and B. M. Broker. 2004. egc-encoded superantigens from Staphylococcus aureus are neutralized by human sera much less efficiently than are classical staphylococcal enterotoxins or toxic shock syndrome toxin. Infect. Immun. 72:4061-4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huard, C., G. Miranda, F. Wessner, A. Bolotin, J. Hansen, S. J. Foster, and M. P. Chapot-Chartier. 2003. Characterization of AcmB, an N-acetylglucosaminidase autolysin from Lactococcus lactis. Microbiology 149:695-705. [DOI] [PubMed] [Google Scholar]
- 82.Ignatova, Z., C. Hornle, A. Nurk, and V. Kasche. 2002. Unusual signal peptide directs penicillin amidase from Escherichia coli to the Tat translocation machinery. Biochem. Biophys. Res. Commun. 291:146-149. [DOI] [PubMed] [Google Scholar]
- 83.Ilangovan, U., H. Ton-That, J. Iwahara, O. Schneewind, and R. T. Clubb. 2001. Structure of sortase, the transpeptidase that anchors proteins to the cell wall of Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 98:6056-6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ito, T., X. X. Ma, F. Takeuchi, K. Okuma, H. Yuzawa, and K. Hiramatsu. 2004. Novel type V staphylococcal cassette chromosome mec driven by a novel cassette chromosome recombinase, ccrC. Antimicrob. Agents Chemother. 48:2637-2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jevons, M. P. 1961. “Celbenin”-resistant staphylococci. BMJ 1:124-126. [Google Scholar]
- 86.Ji, Y., B. Zhang, S. F. Van Horn, P. Warren, G. Woodnutt, M. K. Burnham, and M. Rosenberg. 2001. Identification of critical staphylococcal genes using conditional phenotypes generated by antisense RNA. Science 293:2266-2269. [DOI] [PubMed] [Google Scholar]
- 87.Jongbloed, J. D., H. Antelmann, M. Hecker, R. Nijland, S. Bron, U. Airaksinen, F. Pries, W. J. Quax, J. M. van Dijl, and P. G. Braun. 2002. Selective contribution of the twin-arginine translocation pathway to protein secretion in Bacillus subtilis. J. Biol. Chem. 277:44068-44078. [DOI] [PubMed] [Google Scholar]
- 88.Jongbloed, J. D., U. Grieger, H. Antelmann, M. Hecker, R. Nijland, S. Bron, and J. M. van Dijl. 2004. Two minimal Tat translocases in Bacillus. Mol. Microbiol. 54:1319-1325. [DOI] [PubMed] [Google Scholar]
- 89.Jongbloed, J. D., U. Martin, H. Antelmann, M. Hecker, H. Tjalsma, G. Venema, S. Bron, J. M. van Dijl, and J. Müller. 2000. TatC is a specificity determinant for protein secretion via the twin-arginine translocation pathway. J. Biol. Chem. 275:41350-41357. [DOI] [PubMed] [Google Scholar]
- 90.Jongbloed, J. D., R. van der Ploeg, and J. M. van Dijl. 2005. Bifunctional TatA subunits in minimal Tat protein translocases. Trends Microbiol. 14:2-4. [DOI] [PubMed] [Google Scholar]
- 91.Jonquieres, R., H. Bierne, F. Fiedler, P. Gounon, and P. Cossart. 1999. Interaction between the protein InlB of Listeria monocytogenes and lipoteichoicacid: a novel mechanism of protein association at the surface of gram-positive bacteria. Mol. Microbiol. 34:902-914. [DOI] [PubMed] [Google Scholar]
- 92.Joris, B., S. Englebert, C. P. Chu, R. Kariyama, L. Daneo-Moore, G. D. Shockman, and J. M. Ghuysen. 1992. Modular design of the Enterococcus hirae muramidase-2 and Streptococcus faecalis autolysin. FEMS Microbiol. Lett. 70:257-264. [DOI] [PubMed] [Google Scholar]
- 93.Josefsson, E., K. W. McCrea, D. Ni Eidhin, D. O'Connell, J. Cox, M. Hook, and T. J. Foster. 1998. Three new members of the serine-aspartate repeat protein multigene family of Staphylococcus aureus. Microbiology 144:3387-3395. [DOI] [PubMed] [Google Scholar]
- 94.Juncker, A. S., H. Willenbrock, G. von Heijne, S. Brunak, H. Nielsen, and A. Krogh. 2003. Prediction of lipoprotein signal peptides in gram-negative bacteria. Protein Sci. 12:1652-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kall, L., A. Krogh, and E. L. Sonnhammer. 2004. A combined transmembrane topology and signal peptide prediction method. J. Mol. Biol. 338:1027-1036. [DOI] [PubMed] [Google Scholar]
- 96.Kanafani, H., and S. E. Martin. 1985. Catalase and superoxide dismutase activities in virulent and nonvirulent Staphylococcus aureus isolates. J. Clin. Microbiol. 21:607-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kaneko, J., and Y. Kamio. 2004. Bacterial two-component and hetero-heptameric pore-forming cytolytic toxins: structures, pore-forming mechanism, and organization of the genes. Biosci. Biotechnol. Biochem. 68:981-1003. [DOI] [PubMed] [Google Scholar]
- 98.Komatsuzawa, H., K. Ohta, M. Sugai, T. Fujiwara, P. Glanzmann, B. Berger, and H. Suginaka. 2000. Tn551-mediated insertional inactivation of the fmtB gene encoding a cell wall-associated protein abolishes methicillin resistance in Staphylococcus aureus. J. Antimicrob. Chemother. 45:421-431. [DOI] [PubMed] [Google Scholar]
- 99.Kontinen, V. P., and M. Sarvas. 1993. The PrsA lipoprotein is essential for protein secretion in Bacillus subtilis and sets a limit for high-level secretion. Mol. Microbiol. 8:727-737. [DOI] [PubMed] [Google Scholar]
- 100.Kuroda, M., T. Ohta, I. Uchiyama, T. Baba, H. Yuzawa, I. Kobayashi, L. Cui, A. Oguchi, K. Aoki, Y. Nagai, J. Lian, T. Ito, M. Kanamori, H. Matsumaru, A. Maruyama, H. Murakami, A. Hosoyama, Y. Mizutani-Ui, N. K. Takahashi, T. Sawano, R. Inoue, C. Kaito, K. Sekimizu, H. Hirakawa, S. Kuhara, S. Goto, J. Yabuzaki, M. Kanehisa, A. Yamashita, K. Oshima, K. Furuya, C. Yoshino, T. Shiba, M. Hattori, N. Ogasawara, H. Hayashi, and K. Hiramatsu. 2001. Whole genome sequencing of methicillin-resistant Staphylococcus aureus. Lancet 357:1225-1240. [DOI] [PubMed] [Google Scholar]
- 101.Leclercq, R., E. Derlot, M. Weber, J. Duval, and P. Courvalin. 1989. Transferable vancomycin and teicoplanin resistance in Enterococcus faecium. Antimicrob. Agents Chemother. 33:10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee, L. Y., X. Liang, M. Hook, and E. L. Brown. 2004. Identification and characterization of the C3 binding domain of the Staphylococcus aureus extracellular fibrinogen-binding protein (Efb). J. Biol. Chem. 279:50710-50716. [DOI] [PubMed] [Google Scholar]
- 103.Lee, V. T., and O. Schneewind. 2001. Protein secretion and the pathogenesis of bacterial infections. Genes Dev. 15:1725-1752. [DOI] [PubMed] [Google Scholar]
- 104.Leskelä, S., E. Wahlström, V. P. Kontinen, and M. Sarvas. 1999. Lipid modification of prelipoproteins is dispensable for growth but essential for efficient protein secretion in Bacillus subtilis: characterization of the lgt gene. Mol. Microbiol. 31:1075-1085. [DOI] [PubMed] [Google Scholar]
- 105.Lory, S. 1994. Leader peptidase of type IV prepilins and related proteins, p. 17-29. In G. von Heijne (ed.), Signal peptidases. R. G. Landes Company, Austin, Tex.
- 106.Lory, S. 1998. Secretion of proteins and assembly of bacterial surface organelles: shared pathways of extracellular protein targeting. Curr. Opin. Microbiol. 1:27-35. [DOI] [PubMed] [Google Scholar]
- 107.Mack, D., W. Fischer, A. Krokotsch, K. Leopold, R. Hartmann, H. Egge, and R. Laufs. 1996. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear beta-1,6-linked glucosaminoglycan: purification and structural analysis. J. Bacteriol. 178:175-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Makris, G., J. D. Wright, E. Ingham, and K. T. Holland. 2004. The hyaluronate lyase of Staphylococcus aureus—a virulence factor? Microbiology 150:2005-2013. [DOI] [PubMed] [Google Scholar]
- 109.Mandell, G. L. 1975. Catalase, superoxide dismutase, and virulence of Staphylococcus aureus. In vitro and in vivo studies with emphasis on staphylococcal-leukocyte interaction. J. Clin. Investig. 55:561-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Margot, P., and D. Karamata. 1996. The wprA gene of Bacillus subtilis 168, expressed during exponential growth, encodes a cell-wall-associated protease. Microbiology 142:3437-3444. [DOI] [PubMed] [Google Scholar]
- 111.Marraffini, L. A., and O. Schneewind. 2005. Anchor structure of staphylococcal surface proteins. V. Anchor structure of the sortase B substrate IsdC. J. Biol. Chem. 280:16263-16271. [DOI] [PubMed] [Google Scholar]
- 112.Marraffini, L. A., H. Ton-That, Y. Zong, S. V. Narayana, and O. Schneewind. 2004. Anchoring of surface proteins to the cell wall of Staphylococcus aureus. A conserved arginine residue is required for efficient catalysis of sortase A. J. Biol. Chem. 279:37763-37770. [DOI] [PubMed] [Google Scholar]
- 113.Matsuyama, S., Y. Fujita, and S. Mizushima. 1993. SecD is involved in the release of translocated secretory proteins from the cytoplasmic membrane of Escherichia coli. EMBO J. 12:265-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mazmanian, S. K., E. P. Skaar, A. H. Gaspar, M. Humayun, P. Gornicki, J. Jelenska, A. Joachmiak, D. M. Missiakas, and O. Schneewind. 2003. Passage of heme-iron across the envelope of Staphylococcus aureus. Science 299:906-909. [DOI] [PubMed] [Google Scholar]
- 115.Mazmanian, S. K., H. Ton-That, and O. Schneewind. 2001. Sortase-catalysed anchoring of surface proteins to the cell wall of Staphylococcus aureus. Mol. Microbiol. 40:1049-1057. [DOI] [PubMed] [Google Scholar]
- 116.Mazmanian, S. K., H. Ton-That, K. Su, and O. Schneewind. 2002. An iron-regulated sortase anchors a class of surface protein during Staphylococcus aureus pathogenesis. Proc. Natl. Acad. Sci. USA 99:2293-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McAuliffe, O., R. P. Ross, and C. Hill. 2001. Lantibiotics: structure, biosynthesis and mode of action. FEMS Microbiol. Rev. 25:285-308. [DOI] [PubMed] [Google Scholar]
- 118.Meima, R., C. Eschevins, S. Fillinger, A. Bolhuis, L. W. Hamoen, R. Dorenbos, W. J. Quax, J. M. van Dijl, R. Provvedi, I. Chen, D. Dubnau, and S. Bron. 2002. The bdbDC operon of Bacillus subtilis encodes thiol-disulfide oxidoreductases required for competence development. J. Biol. Chem. 277:6994-7001. [DOI] [PubMed] [Google Scholar]
- 119.Meima, R., and J. M. van Dijl. 2003. Protein secretion in gram-positive bacteria, p. 271-296. In B. Oudega (ed.), Protein secretion pathways in bacteria. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 120.Mempel, M., C. Schnopp, M. Hojka, H. Fesq, S. Weidinger, M. Schaller, H. C. Korting, J. Ring, and D. Abeck. 2002. Invasion of human keratinocytes by Staphylococcus aureus and intracellular bacterial persistence represent haemolysin-independent virulence mechanisms that are followed by features of necrotic and apoptotic keratinocyte cell death. Br. J. Dermatol. 146:943-951. [DOI] [PubMed] [Google Scholar]
- 121.Michiels, J., G. Dirix, J. Vanderleyden, and C. Xi. 2001. Processing and export of peptide pheromones and bacteriocins in gram-negative bacteria. Trends Microbiol. 9:164-168. [DOI] [PubMed] [Google Scholar]
- 122.Milward, C. P., and N. A. Jacques. 1990. Secretion of fructosyltransferase by Streptococcus salivarius involves the sucrose-dependent release of the cell-bound form. J. Gen. Microbiol. 136:165-169. [DOI] [PubMed] [Google Scholar]
- 123.Molik, S., I. Karnauchov, C. Weidlich, R. G. Herrmann, and R. B. Klösgen. 2001. The Rieske Fe/S protein of the cytochrome b6/f complex in chloroplasts: missing link in the evolution of protein transport pathways in chloroplasts? J. Biol. Chem. 276:42761-42766. [DOI] [PubMed] [Google Scholar]
- 124.Morfeldt, E., L. Janzon, S. Arvidson, and S. Löfdahl. 1988. Cloning of a chromosomal locus (exp) which regulates the expression of several exoprotein genes in Staphylococcus aureus. Mol. Gen. Genet. 211:435-440. [DOI] [PubMed] [Google Scholar]
- 125.Mori, H., and K. Cline. 2001. Post-translational protein translocation into thylakoids by the Sec and DeltapH-dependent pathways. Biochim. Biophys. Acta 1541:80-90. [DOI] [PubMed] [Google Scholar]
- 126.Müller, J. P., J. Ozegowski, S. Vettermann, J. Swaving, K. H. Van Wely, and A. J. Driessen. 2000. Interaction of Bacillus subtilis CsaA with SecA and precursor proteins. Biochem. J. 348:367-373. [PMC free article] [PubMed] [Google Scholar]
- 127.Nandakumar, R., M. P. Nandakumar, M. R. Marten, and J. M. Ross. 2005. Proteome analysis of membrane and cell wall associated proteins from Staphylococcus aureus. J. Proteome Res. 4:250-257. [DOI] [PubMed] [Google Scholar]
- 128.Narita, S., and H. Tokuda. 2006. An ABC transporter mediating the membrane detachment of bacterial lipoproteins depending on their sorting signals. FEBS Lett. 580:1164-1170. [DOI] [PubMed] [Google Scholar]
- 129.Navaratna, M. A., H. G. Sahl, and J. R. Tagg. 1998. Two-component anti-Staphylococcus aureus lantibiotic activity produced by Staphylococcus aureus C55. Appl. Environ. Microbiol. 64:4803-4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Navaratna, M. A., H. G. Sahl, and J. R. Tagg. 1999. Identification of genes encoding two-component lantibiotic production in Staphylococcus aureus C55 and other phage group II S. aureus strains and demonstration of an association with the exfoliative toxin B gene. Infect. Immun. 67:4268-4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Navarre, W. W., and O. Schneewind. 1994. Proteolytic cleavage and cell wall anchoring at the LPXTG motif of surface proteins in gram-positive bacteria. Mol. Microbiol. 14:115-121. [DOI] [PubMed] [Google Scholar]
- 132.Netz, D. J., M. do Carmo de Freire Bastos, and H. G. Sahl. 2002. Mode of action of the antimicrobial peptide aureocin A53 from Staphylococcus aureus. Appl. Environ. Microbiol. 68:5274-5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Netz, D. J., R. Pohl, A. G. Beck-Sickinger, T. Selmer, A. J. Pierik, M. do Carmo de Freire Bastos, and H. G. Sahl. 2002. Biochemical characterisation and genetic analysis of aureocin A53, a new, atypical bacteriocin from Staphylococcus aureus. J. Mol. Biol. 319:745-756. [DOI] [PubMed] [Google Scholar]
- 134.Netz, D. J., H. G. Sahl, R. Marcelino, J. dos Santos Nascimento, S. S. de Oliveira, M. B. Soares, and M. do Carmo de Freire Bastos. 2001. Molecular characterisation of aureocin A70, a multi-peptide bacteriocin isolated from Staphylococcus aureus. J. Mol. Biol. 311:939-949. [DOI] [PubMed] [Google Scholar]
- 135.Ní Eidhin, D., S. Perkins, P. Francois, P. Vaudaux, M. Höök, and T. J. Foster. 1998. Clumping factor B (ClfB), a new surface-located fibrinogen-binding adhesin of Staphylococcus aureus. Mol. Microbiol. 30:245-257. [DOI] [PubMed] [Google Scholar]
- 136.Nielsen, H., J. Engelbrecht, S. Brunak, and G. von Heijne. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10:1-6. [DOI] [PubMed] [Google Scholar]
- 137.Noble, W. C., Z. Virani, and R. G. Cree. 1992. Co-transfer of vancomycin and other resistance genes from Enterococcus faecalis NCTC 12201 to Staphylococcus aureus. FEMS Microbiol. Lett. 72:195-198. [DOI] [PubMed] [Google Scholar]
- 138.Nouwen, N., M. Piwowarek, G. Berrelkamp, and A. J. Driessen. 2005. The large first periplasmic loop of SecD and SecF plays an important role in SecDF functioning. J. Bacteriol. 187:5857-5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Oshida, T., M. Sugai, H. Komatsuzawa, Y. M. Hong, H. Suginaka, and A. Tomasz. 1995. A Staphylococcus aureus autolysin that has an N-acetylmuramoyl-l-alanine amidase domain and an endo-beta-N-acetylglucosaminidase domain: cloning, sequence analysis, and characterization. Proc. Natl. Acad. Sci. USA 92:285-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pallen, M. J. 2002. The ESAT-6/WXG100 superfamily—and a new gram-positive secretion system? Trends Microbiol. 10:209-212. [DOI] [PubMed] [Google Scholar]
- 141.Palmqvist, N., J. M. Patti, A. Tarkowski, and E. Josefsson. 2004. Expression of staphylococcal clumping factor A impedes macrophage phagocytosis. Microbes Infect. 6:188-195. [DOI] [PubMed] [Google Scholar]
- 142.Palmqvist, N., G. J. Silverman, E. Josefsson, and A. Tarkowski. 2005. Bacterial cell wall-expressed protein A triggers supraclonal B-cell responses upon in vivo infection with Staphylococcus aureus. Microbes Infect. 7:1501-1511. [DOI] [PubMed] [Google Scholar]
- 143.Peacock, S. J., I. de Silva, and F. D. Lowy. 2001. What determines nasal carriage of Staphylococcus aureus? Trends Microbiol. 9:605-610. [DOI] [PubMed] [Google Scholar]
- 144.Peng, H. L., R. P. Novick, B. Kreiswirth, J. Kornblum, and P. Schlievert. 1988. Cloning, characterization, and sequencing of an accessory gene regulator (agr) in Staphylococcus aureus. J. Bacteriol. 170:4365-4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Peschel, A., N. Schnell, M. Hille, K. D. Entian, and F. Gotz. 1997. Secretion of the lantibiotics epidermin and gallidermin: sequence analysis of the genes gdmT and gdmH, their influence on epidermin production and their regulation by EpiQ. Mol. Gen. Genet. 254:312-318. [DOI] [PubMed] [Google Scholar]
- 146.Ponting, C. P., L. Aravind, J. Schultz, P. Bork, and E. V. Koonin. 1999. Eukaryotic signalling domain homologues in archaea and bacteria. Ancient ancestry and horizontal gene transfer. J. Mol. Biol. 289:729-745. [DOI] [PubMed] [Google Scholar]
- 147.Prágai, Z., H. Tjalsma, A. Bolhuis, J. M. van Dijl, G. Venema, and S. Bron. 1997. The signal peptidase II (Isp) gene of Bacillus subtilis. Microbiology 143:1327-1333. [DOI] [PubMed] [Google Scholar]
- 148.Pugsley, A. P. 1993. Processing and methylation of PuIG, a pilin-like component of the general secretory pathway of Klebsiella oxytoca. Mol. Microbiol. 9:295-308. [DOI] [PubMed] [Google Scholar]
- 149.Que, Y. A., J. A. Haefliger, L. Piroth, P. Francois, E. Widmer, J. M. Entenza, B. Sinha, M. Herrmann, P. Francioli, P. Vaudaux, and P. Moreillon. 2005. Fibrinogen and fibronectin binding cooperate for valve infection and invasion in Staphylococcus aureus experimental endocarditis. J. Exp. Med. 201:1627-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rashid, M. H., M. Mori, and J. Sekiguchi. 1995. Glucosaminidase of Bacillus subtilis: cloning, regulation, primary structure and biochemical characterization. Microbiology 141:2391-2404. [DOI] [PubMed] [Google Scholar]
- 151.Rathsam, C., P. M. Giffard, and N. A. Jacques. 1993. The cell-bound fructosyltransferase of Streptococcus salivarius: the carboxyl terminus specifies attachment in a Streptococcus gordonii model system. J. Bacteriol. 175:4520-4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Recsei, P., B. Kreiswirth, M. O'Reilly, P. Schlievert, A. Gruss, and R. P. Novick. 1986. Regulation of exoprotein gene expression in Staphylococcus aureus by agar. Mol. Gen. Genet. 202:58-61. [DOI] [PubMed] [Google Scholar]
- 153.Rice, K. C., B. A. Firek, J. B. Nelson, S. J. Yang, T. G. Patton, and K. W. Bayles. 2003. The Staphylococcus aureus cidAB operon: evaluation of its role in regulation of murein hydrolase activity and penicillin tolerance. J. Bacteriol. 185:2635-2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Robinson, C., and A. Bolhuis. 2001. Protein targeting by the twin-arginine translocation pathway. Nat. Rev. Mol. Cell Biol. 2:350-356. [DOI] [PubMed] [Google Scholar]
- 155.Roche, F. M., R. Downer, F. Keane, P. Speziale, P. W. Park, and T. J. Foster. 2004. The N-terminal A domain of fibronectin-binding proteins A and B promotes adhesion of Staphylococcus aureus to elastin. J. Biol. Chem. 279:38433-38440. [DOI] [PubMed] [Google Scholar]
- 156.Roche, F. M., R. Massey, S. J. Peacock, N. P. Day, L. Visai, P. Speziale, A. Lam, M. Pallen, and T. J. Foster. 2003. Characterization of novel LPXTG-containing proteins of Staphylococcus aureus identified from genome sequences. Microbiology 149:643-654. [DOI] [PubMed] [Google Scholar]
- 157.Rohde, H., C. Burdelski, K. Bartscht, M. Hussain, F. Buck, M. A. Horstkotte, J. K. Knobloch, C. Heilmann, M. Herrmann, and D. Mack. 2005. Induction of Staphylococcus epidermidis biofilm formation via proteolytic processing of the accumulation-associated protein by staphylococcal and host proteases. Mol. Microbiol. 55:1883-1895. [DOI] [PubMed] [Google Scholar]
- 158.Ruzin, A., and R. P. Novick. 2000. Equivalence of lauric acid and glycerol monolaurate as inhibitors of signal transduction in Staphylococcus aureus. J. Bacteriol. 182:2668-2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sander, P., M. Rezwan, B. Walker, S. K. Rampini, R. M. Kroppenstedt, S. Ehlers, C. Keller, J. R. Keeble, M. Hagemeier, M. J. Colston, B. Springer, and E. C. Böttger. 2004. Lipoprotein processing is required for virulence of Mycobacterium tuberculosis. Mol. Microbiol. 52:1543-1552. [DOI] [PubMed] [Google Scholar]
- 160.Sankaran, K., K. Gan, B. Rash, H. Y. Qi, H. C. Wu, and P. D. Rick. 1997. Roles of histidine-103 and tyrosine-235 in the function of the prolipoprotein diacylglyceryl transferase of Escherichia coli. J. Bacteriol. 179:2944-2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sankaran, K., and H. C. Wu. 1994. Lipid modification of bacterial prolipoprotein. Transfer of diacylglyceryl moiety from phosphatidylglycerol. J. Biol. Chem. 269:19701-19706. [PubMed] [Google Scholar]
- 162.Sarvas, M., C. R. Harwood, S. Bron, and J. M. van Dijl. 2004. Post-translocational folding of secretory proteins in gram-positive bacteria. Biochim. Biophys. Acta 1694:311-327. [DOI] [PubMed] [Google Scholar]
- 163.Savolainen, K., L. Paulin, B. Westerlund-Wikström, T. J. Foster, T. K. Korhonen, and P. Kuusela. 2001. Expression of pls, a gene closely associated with the mecA gene of methicillin-resistant Staphylococcus aureus, prevents bacterial adhesion in vitro. Infect. Immun. 69:3013-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Schnell, N., K. D. Entian, U. Schneider, F. Götz, H. Zähner, R. Kellner, and G. Jung. 1988. Prepeptide sequence of epidermin, a ribosomally synthesized antibiotic with four sulphide-rings. Nature 333:276-278. [DOI] [PubMed] [Google Scholar]
- 165.Siboo, I. R., H. F. Chambers, and P. M. Sullam. 2005. Role of SraP, a serine-rich surface protein of Staphylococcus aureus, in binding to human platelets. Infect. Immun. 73:2273-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sørensen, A. L., S. Nagai, G. Houen, P. Andersen, and A. B. Andersen. 1995. Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect. Immun. 63:1710-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Stanley, N. R., T. Palmer, and B. C. Berks. 2000. The twin arginine consensus motif of Tat signal peptides is involved in Sec-independent protein targeting in Escherichia coli. J. Biol. Chem. 275:11591-11596. [DOI] [PubMed] [Google Scholar]
- 168.Steen, A. 2005. Functional characterization and cell wall interactions of peptidoglycan hydrolases of Lactococcus lactis. University of Groningen, Groningen, The Netherlands.
- 169.Stoll, H., J. Dengjel, C. Nerz, and F. Götz. 2005. Staphylococcus aureus deficient in lipidation of prelipoproteins is attenuated in growth and immune activation. Infect. Immun. 73:2411-2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Strom, M. S., D. N. Nunn, and S. Lory. 1993. A single bifunctional enzyme, PilD, catalyzes cleavage and N-methylation of proteins belonging to the type IV pilin family. Proc. Natl. Acad. Sci. USA 90:2404-2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sutcliffe, I. C., and D. J. Harrington. 2002. Pattern searches for the identification of putative lipoprotein genes in gram-positive bacterial genomes. Microbiology 148:2065-2077. [DOI] [PubMed] [Google Scholar]
- 172.Takamatsu, D., B. A. Bensing, and P. M. Sullam. 2005. Two additional components of the accessory sec system mediating export of the Streptococcus gordonii platelet-binding protein GspB. J. Bacteriol. 187:3878-3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tarkowski, A., L. V. Collins, I. Gjertsson, O. H. Hultgren, I. M. Jonsson, E. Sakiniene, and M. Verdrengh. 2001. Model systems: modeling human staphylococcal arthritis and sepsis in the mouse. Trends Microbiol. 9:321-326. [DOI] [PubMed] [Google Scholar]
- 174.Tjalsma, H., H. Antelmann, J. D. Jongbloed, P. G. Braun, E. Darmon, R. Dorenbos, J. Y. Dubois, H. Westers, G. Zanen, W. J. Quax, O. P. Kuipers, S. Bron, M. Hecker, and J. M. van Dijl. 2004. Proteomics of protein secretion by Bacillus subtilis: separating the “secrets” of the secretome. Microbiol. Mol. Biol. Rev. 68:207-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tjalsma, H., A. Bolhuis, J. D. Jongbloed, S. Bron, and J. M. van Dijl. 2000. Signal peptide-dependent protein transport in Bacillus subtilis: a genome-based survey of the secretome. Microbiol. Mol. Biol. Rev. 64:515-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tjalsma, H., A. Bolhuis, M. L. van Roosmalen, T. Wiegert, W. Schumann, C. P. Broekhuizen, W. J. Quax, G. Venema, S. Bron, and J. M. van Dijl. 1998. Functional analysis of the secretory precursor processing machinery of Bacillus subtilis: identification of a eubacterial homolog of archaeal and eukaryotic signal peptidases. Genes Dev. 12:2318-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tjalsma, H., V. P. Kontinen, Z. Prágai, H. Wu, R. Meima, G. Venema, S. Bron, M. Sarvas, and J. M. van Dijl. 1999. The role of lipoprotein processing by signal peptidase II in the gram-positive eubacterium Bacillus subtilis. Signal peptidase II is required for the efficient secretion of alpha-amylase, a non-lipoprotein. J. Biol. Chem. 274:1698-1707. [DOI] [PubMed] [Google Scholar]
- 178.Tjalsma, H., M. A. Noback, S. Bron, G. Venema, K. Yamane, and J. M. van Dijl. 1997. Bacillus subtilis contains four closely related type I signal peptidases with overlapping substrate specificities. Constitutive and temporally controlled expression of different sip genes. J. Biol. Chem. 272:25983-25992. [DOI] [PubMed] [Google Scholar]
- 179.Tjalsma, H., and J. M. van Dijl. 2005. Proteomics-based consensus prediction of protein retention in a bacterial membrane. Proteomics 5:4472-4482. [DOI] [PubMed] [Google Scholar]
- 180.Tjalsma, H., G. Zanen, S. Bron, and J. M. van Dijl. 2001. The eubacterial lipoprotein-specific (type II) signal peptidase, p. 3-23. In R. E. Dalbey and D. S. Sigman (ed.), The enzymes, vol. XXII. Co- and post translational proteolysis of proteins. Academic Press, San Diego, Calif. [Google Scholar]
- 181.Tokuda, H., and S. Matsuyama. 2004. Sorting of lipoproteins to the outer membrane in E. coli. Biochim. Biophys. Acta 1694:IN1-IN9. [PubMed] [Google Scholar]
- 182.Ton-That, H., L. A. Marraffini, and O. Schneewind. 2004. Protein sorting to the cell wall envelope of gram-positive bacteria. Biochim. Biophys. Acta 1694:269-278. [DOI] [PubMed] [Google Scholar]
- 183.Ton-That, H., S. K. Mazmanian, L. Alksne, and O. Schneewind. 2002. Anchoring of surface proteins to the cell wall of Staphylococcus aureus. Cysteine 184 and histidine 120 of sortase form a thiolate-imidazolium ion pair for catalysis. J. Biol. Chem. 277:7447-7452. [DOI] [PubMed] [Google Scholar]
- 184.Tormo, M. A., E. Knecht, F. Götz, I. Lasa, and J. R. Penadés. 2005. Bap-dependent biofilm formation by pathogenic species of Staphylococcus: evidence of horizontal gene transfer? Microbiology 151:2465-2475. [DOI] [PubMed] [Google Scholar]
- 185.Vandenesch, F., T. Naimi, M. C. Enright, G. Lina, G. R. Nimmo, H. Heffernan, N. Liassine, M. Bes, T. Greenland, M. E. Reverdy, and J. Etienne. 2003. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg. Infect. Dis. 9:978-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.van Dijl, J. M., A. de Jong, J. Vehmaanperä, G. Venema, and S. Bron. 1992. Signal peptidase I of Bacillus subtilis: patterns of conserved amino acids in prokaryotic and eukaryotic type I signal peptidases. EMBO J. 11:2819-2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.van Roosmalen, M. L., N. Geukens, J. D. Jongbloed, H. Tjalsma, J. Y. Dubois, S. Bron, J. M. van Dijl, and J. Anné. 2004. Type I signal peptidases of gram-positive bacteria. Biochim. Biophys. Acta 1694:279-297. [DOI] [PubMed] [Google Scholar]
- 188.van Roosmalen, M. L., J. D. Jongbloed, J. Y. Dubois, G. Venema, S. Bron, and J. M. van Dijl. 2001. Distinction between major and minor Bacillus signal peptidases based on phylogenetic and structural criteria. J. Biol. Chem. 276:25230-25235. [DOI] [PubMed] [Google Scholar]
- 189.Veenendaal, A. K., C. van der Does, and A. J. Driessen. 2004. The protein-conducting channel SecYEG. Biochim. Biophys. Acta 1694:81-95. [DOI] [PubMed] [Google Scholar]
- 190.Venema, R., H. Tjalsma, J. M. van Dijl, A. de Jong, K. Leenhouts, G. Buist, and G. Venema. 2003. Active lipoprotein precursors in the gram-positive eubacterium Lactococcus lactis. J. Biol. Chem. 278:14739-14746. [DOI] [PubMed] [Google Scholar]
- 191.Viau, M., N. S. Longo, P. E. Lipsky, and M. Zouali. 2005. Staphylococcal protein A deletes B-1a and marginal zone B lymphocytes expressing human immunoglobulins: an immune evasion mechanism. J. Immunol. 175:7719-7727. [DOI] [PubMed] [Google Scholar]
- 192.Vitikainen, M., I. Lappalainen, R. Seppala, H. Antelmann, H. Boer, S. Taira, H. Savilahti, M. Hecker, M. Vihinen, M. Sarvas, and V. P. Kontinen. 2004. Structure-function analysis of PrsA reveals roles for the parvulin-like and flanking N- and C-terminal domains in protein folding and secretion in Bacillus subtilis. J. Biol. Chem. 279:19302-19314. [DOI] [PubMed] [Google Scholar]
- 193.Voigt, B., T. Schweder, M. J. Sibbald, D. Albrecht, A. Ehrenreich, J. Bernhardt, J. Feesche, K. H. Maurer, G. Gottschalk, J. M. van Dijl, and M. Hecker. 2006. The extracellular proteome of Bacillus licheniformis grown in different media and under different nutrient starvation conditions. Proteomics 6:268-281. [DOI] [PubMed] [Google Scholar]
- 194.Völker, U., and M. Hecker. 2005. From genomics via proteomics to cellular physiology of the gram-positive model organism Bacillus subtilis. Cell. Microbiol. 7:1077-1085. [DOI] [PubMed] [Google Scholar]
- 195.von Heijne, G. 1990. The signal peptide. J. Membr. Biol. 115:195-201. [DOI] [PubMed] [Google Scholar]
- 196.Voyich, J. M., K. R. Braughton, D. E. Sturdevant, A. R. Whitney, B. Said-Salim, S. F. Porcella, R. D. Long, D. W. Dorward, D. J. Gardner, B. N. Kreiswirth, J. M. Musser, and F. R. DeLeo. 2005. Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J. Immunol. 175:3907-3919. [DOI] [PubMed] [Google Scholar]
- 197.Vrontou, E., and A. Economou. 2004. Structure and function of SecA, the preprotein translocase nanomotor. Biochim. Biophys. Acta 1694:67-80. [DOI] [PubMed] [Google Scholar]
- 198.Walsh, E. J., L. M. O'Brien, X. Liang, M. Hook, and T. J. Foster. 2004. Clumping factor B, a fibrinogen-binding MSCRAMM (microbial surface components recognizing adhesive matrix molecules) adhesin of Staphylococcus aureus, also binds to the tail region of type I cytokeratin 10. J. Biol. Chem. 279:50691-50699. [DOI] [PubMed] [Google Scholar]
- 199.Weigel, L. M., D. B. Clewell, S. R. Gill, N. C. Clark, L. K. McDougal, S. E. Flannagan, J. F. Kolonay, J. Shetty, G. E. Killgore, and F. C. Tenover. 2003. Genetic analysis of a high-level vancomycin-resistant isolate of Staphylococcus aureus. Science 302:1569-1571. [DOI] [PubMed] [Google Scholar]
- 200.Westers, H., R. Dorenbos, J. M. van Dijl, J. Kabel, T. Flanagan, K. M. Devine, F. Jude, S. J. Seror, A. C. Beekman, E. Darmon, C. Eschevins, A. de Jong, S. Bron, O. P. Kuipers, A. M. Albertini, H. Antelmann, M. Hecker, N. Zamboni, U. Sauer, C. Bruand, D. S. Ehrlich, J. C. Alonso, M. Salas, and W. J. Quax. 2003. Genome engineering reveals large dispensable regions in Bacillus subtilis. Mol. Biol. Evol. 20:2076-2090. [DOI] [PubMed] [Google Scholar]
- 201.Wickner, W., and R. Schekman. 2005. Protein translocation across biological membranes. Science 310:1452-1456. [DOI] [PubMed] [Google Scholar]
- 202.Wu, H. C. 1996. Biosynthesis of lipoproteins, p. 1005-1014. In F. C. Neidhardt, R. Curtis III, J. L. Ingraham, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology. American Society for Microbiology, Washington, D.C.
- 203.Yamasaki, O., T. Yamaguchi, M. Sugai, C. Chapuis-Cellier, F. Arnaud, F. Vandenesch, J. Etienne, and G. Lina. 2005. Clinical manifestations of staphylococcal scalded-skin syndrome depend on serotypes of exfoliative toxins. J. Clin. Microbiol. 43:1890-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yen, M. R., Y. H. Tseng, E. H. Nguyen, L. F. Wu, and M. H. Saier, Jr. 2002. Sequence and phylogenetic analyses of the twin-arginine targeting (Tat) protein export system. Arch. Microbiol. 177:441-450. [DOI] [PubMed] [Google Scholar]
- 205.Yother, J., and J. M. White. 1994. Novel surface attachment mechanism of the Streptococcus pneumoniae protein PspA. J. Bacteriol. 176:2976-2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Young, R., and U. Bläsi. 1995. Holins: form and function in bacteriophage lysis. FEMS Microbiol. Rev. 17:191-205. [DOI] [PubMed] [Google Scholar]
- 207.Zhang, Y. Q., S. X. Ren, H. L. Li, Y. X. Wang, G. Fu, J. Yang, Z. Q. Qin, Y. G. Miao, W. Y. Wang, R. S. Chen, Y. Shen, Z. Chen, Z. H. Yuan, G. P. Zhao, D. Qu, A. Danchin, and Y. M. Wen. 2003. Genome-based analysis of virulence genes in a non-biofilm-forming Staphylococcus epidermidis strain (ATCC 12228). Mol. Microbiol. 49:1577-1593. [DOI] [PubMed] [Google Scholar]
- 208.Ziebandt, A. K., D. Becher, K. Ohlsen, J. Hacker, M. Hecker, and S. Engelmann. 2004. The influence of agr and sigma(B) in growth phase dependent regulation of virulence factors in Staphylococcus aureus. Proteomics 4:3034-3047. [DOI] [PubMed] [Google Scholar]
- 209.Ziebandt, A. K., H. Weber, J. Rudolph, R. Schmid, D. Höper, S. Engelmann, and M. Hecker. 2001. Extracellular proteins of Staphylococcus aureus and the role of SarA and sigma B. Proteomics 1:480-493. [DOI] [PubMed] [Google Scholar]