Abstract
The present report describes the clinical signs, magnetic resonance imaging (MRI) findings, surgical procedure, pathological findings and follow-up in four cats with multiple meningiomas; three castrated male and one spayed female domestic shorthair indoor cats, ranging in age from 11 to 14 years. In three of four cats, clinical signs at presentation were suggestive of a focal lesion. Three cats had two meningiomas and one had four meningiomas. Most of the tumours were supratentorial, one arose from the tentorium and one was infratentorial. The duration of presenting signs before surgery ranged from 10 days to 11 months. Postoperative MRI revealed complete gross tumour removal in three cases. In one cat with two cranial fossa meningiomas, subtotal excision with a small basal remnant (2×2 mm) of the ventral part of one meningioma lying on the floor of the skull, was observed. Based on histopathological architecture, six tumours revealed features of a transitional subtype meningioma, and four of a meningotheliomatous meningioma. In each cat, the multiple meningiomas were all assigned to the same histopathological group. The preoperative presenting signs had resolved by the follow-up examinations 4 weeks after surgery in two cats. Long-term follow-up evaluation revealed that surgically-induced or exacerbated neurological deficits in two cats had completely or almost completely resolved within 8 weeks of surgery. All patients are still alive 12 to 21 months after surgery and no clinical signs of recurrence could be detected at that time.
Meningiomas are extra-axial mesenchymal tumours that arise from neoplastic arachnoid cap cells, which are themselves a unique population of mesothelial-like cells. These display morphology similar to the embryological cells found at the interface of the developing arachnoid and dura within the cranial and spinal cavities. The primitive meningeal cells originate from cells of the neural crest and from mesodermal cells that migrate into the area of the developing neural tube. In adults, the arachnoid cap cells are found at the apices of arachnoid granulations and are morphologically similar, in many respects, to the cells of the arachnoid barrier (Nafe 1979, Yasargil 1994). The majority of meningiomas develop from these cells and grow as unilateral hemispherical lesions with a flat dural base. They initially grow outward into the arachnoidal spaces and onto the brain.
Meningiomas are the most common primary intracranial tumour in cats (Summers et al 1995, Troxel et al 2003, Vite 2005). They are slow growing tumours, and about 50% of affected cats do not manifest any clinical signs. The diagnosis is, therefore, often an incidental finding during pathological examination (Summers et al 1995, Pocknell et al 2003). In addition to single meningioma, there is also a high incidence of multiple meningiomas in cats (Summers et al 1995, Vite 2005). Multiple meningiomas are described in human medicine and they are usually associated with neurofibromatosis 2 (NF2), an autosomal dominant disorder caused by inactivating mutations of the NF2 tumour suppressor gene (Perry et al 2004, Salvati et al 2004, Evans et al 2006). To the authors' knowledge, only a few clinico-histopathological reports of feline multiple meningiomas have been published (Pocknell et al 2003, Troxel et al 2003). Furthermore, there are no publications, thus far, focusing on the clinical sign, therapy and prognosis of multiple meningiomas in cats.
The present report describes the clinical signs, MRI findings, surgical procedure, pathological examination, and follow-up in four cats with multiple meningiomas.
Materials and Methods
Four cats with multiple meningiomas were treated surgically at the Small Animal Clinic of the University of Berne between 2004 and 2006. Only cats with neuro-radiologically and surgically documented multiple meningiomas were included in this report. Multiple meningiomas were defined as two and more meningiomas without gross contact between each other. The records of all four cats were reviewed for clinical presentation, neuro-radiological evaluation, surgical management, histopathological examination and outcome. All cats were clinically and neurologically examined and the lesion localised. A complete blood count, serum biochemistry panel and thoracic radiographs were performed in all cats. Cerebrospinal fluid (CSF) puncture from the cisterna magna was performed directly following MRI examination in all cases. The CSF analysis consisted in a Pandy reaction, cell count and differential cell count.
MRI examination
The cats were anaesthetised using induction with a combination of midazolam (Dormicum; Roche 0.2 mg/kg IV), methadone (Methadon; Streuli, 0.2 mg/kg IV) and propofol (Diprivan; Astra Zeneca, 4 mg/kg IV), followed by isoflurane (Isoflurane; Abbott) and oxygen inhalation. MRI was performed with a 0.3 T permanent magnet MRI unit (Hitachi AIRIS II; Hitachi Medical Systems; Düsseldorf, Germany). Preoperative sequences included a transverse FSE T2 and an FE 3D MPR T1-weighted (high resolution gradient echo) sequence in each cat.
After the intravenous injection of 0.15 mmol Gadodiamide (Omniscan; GE Health Care) the T1-weighted sequences were repeated. As the diagnoses were not clear before the MRI was performed, a dorsal CSF suppressing FLAIR, and an FE 3D T1 (plain and contrast enhanced) were also performed.
Criteria for the MRI diagnosis of meningioma have been described (Pocknell et al 2003, Troxel et al 2003) and were used as inclusion criteria in this report. These are extra-axial space-occupying lesions, iso- to hyperintense signal in T2, iso- to slightly hypointense signal in T1, contrast uptake and occasionally a dural tail sign.
A second MRI was performed immediately after surgery; sequences included FSE T2 in transverse and FE 3D MPR T1 (plain and contrast enhanced) in dorsal orientation.
Surgical treatment
All cats were intravenously premedicated with methylprednisolone sodium succinate (Medrate solubile; Pfizer, 30 mg/kg) and mannitol (Braun-melsungen; Melsungen, 0.5 g/kg over 20 min) to minimise brain oedema and inflammation during surgery. Mild mechanical hyperventilation (end-tidal CO2 3–3.5%) was applied and anaesthesia was maintained with 1.5–2% isoflurane (Abbott; Wiesbaden) with oxygen. Lactated Ringer's solution (3 ml/kg/h IV) was administered throughout surgery. Prophylactic antimicrobial therapy with cefazolin (Kefzol; Medica, 25 mg/kg IV) was administered immediately before induction and again at the end of surgery.
Cats were positioned in sternal recumbency with the head slightly rotated and elevated, taking care to maintain airway patency. The head was shaved from the eyebrows to the level of C2, scrubbed with chlorhexidine and disinfected with alcohol and povidone-iodine.
A straight or T-shaped incision was performed, followed by multiple craniotomies, centred over the meningiomas. One of two techniques was used to remove the tumours: en-bloc resection for small superficial meningiomas, and a single-window technique for large meningiomas with a broad-based deep contact area with brain parenchyma. In the latter cases, the visible surface of the tumour was fenestrated and the centre of the tumour was enucleated using tumour forceps through this window.
Postoperative management
The cats were kept in an oxygen box for 12 h after the operation. Postoperative analgesia was provided with fentanyl (Janssen, Neuss, Germany, 0.02 mg/kg CRI) for the first 12 h, followed by buprenorphine (Temgesic; Essex, München, Germany, 0.01 mg/kg SCq8h) for the next 24–48 h. Lactated Ringer's solution (2 ml/kg/h CRI) was administered for 24–36 h.
The cats received cefalexine (Cefaseptin mite; Chassot, 25 mg/kgPOq12h) for 1 week following surgery and phenobarbital (Aphenylbarbit; Streuli, 2 mg/kgPOq12h) for 4 weeks postoperatively. In addition, all patients received hydroxyurea (Litalir; Bristol-Myers Squibb, 20 mg/kg PO daily).
Pathological examination
Tumours were immersed in 10% neutral buffered formalin immediately following excision. After proper fixation, samples were processed routinely in an automatic tissue processor, embedded in paraffin, sectioned at 5 μm and stained with haematoxylin and eosin and Masson trichrome. Further slices underwent immunohistochemical staining for S-100, vimentin, cytokeratin, and MIB-1 (Dakocytomation, Glostrup, Denmark). Histological typing was based on the WHO classification of tumours of the nervous system of domestic animals (Koestner et al 1999). Moreover, histological growth pattern, mitotic index, MIB-labelling index and presence or absence of anaplastic features were considered indicative for tumour biology and gave rise to further classification into WHO grades I to IV as has been proposed for human tumours (Kleinhues and Cavenee 2000).
Outcome
A complete physical and neurological examination was performed on the first few days, as well as 6 weeks and 3 months after surgery. A complete blood count was performed twice a month during the first 2 months after surgery to detect adverse effects associated with the use of hydroxyurea. Subsequent follow-up information for 6 months to 1 year after surgery was, in part, gathered by the referring veterinarians.
Results
Patient population
There were three castrated male and one spayed female domestic shorthair indoor cats, ranging in age from 11 to 14 years in this study. Three cats had two meningiomas and one had four meningiomas. Most of the tumours were supratentorial; one arose from the tentorium, and one was infratentorial (Table 1).
Table 1.
MRI sequence parameters
| Sequence | TR/TE (TI) (ms) | FA (°) | FOV (mm) | Matrix | Slth/Slsep (mm) |
|---|---|---|---|---|---|
| FSE T2 tra | 3540/125 | 90 | 120×120 | 160×160 | 3/0.5 |
| FLAIR dor | 8031/125 (1900) | 90 | 190×174 | 192×110 | 3.5/0.5 |
| FE 3D T1 tra | 30/15 | 30 | 120×120 | 200×180 | 3/0 |
| FE 3D MPR dor | 30/12 | 30 | 180×108 | 180×108 | 1/1 |
TR=repetition time; TE=echo time; TI=inversion time; FA=flip angle; FOV=field of view; Slth=slice thickness; Slsep=slice separation; FSE=fast spin echo; FLAIR=fluid attenuation inversion recovery (CSF suppression technique); FE=field echo; MPR=multiplanar reconstruction; tra=transverse; dor=dorsal.
Clinical signs
The duration of presenting signs ranged from 10 days to 11 months before surgery. Behavioural disturbances (four cats), pacing to one side (three cats), gait ataxia (two cats) and reduced menace reaction (four cats) were the most common presenting signs. Clinical signs were more pronounced on one side in three cats (cases 1, 3 and 4) and lead to a suspicion of a neuroanatomical single forebrain lesion, whereas in cat (case 2) with four meningiomas, a multifocal lesion was suspected. All cats were premedicated with prednisolone or dexamethasone and a short-lived amelioration followed by a rapid worsening of clinical signs was observed, despite continued treatment. The median time between the first clinical signs and presentation was 36 days. All cats were tested by ELISA and were FeLV and FIV negative.
Mild anaemia (haematocrit 30%) and a mildly elevated serum creatinine (182 μmol/l; reference interval 53–141 μmol/l) were detected in one cat (case 3). The remaining results of laboratory analyses and radiographs were unremarkable.
MRI
Nine of 10 tumours were extra-axial with a broad meningeal base and lead to compression of cortical tissue. One meningioma, growing between the two hemispheres, showed a limited contact with the ventromedial edge of the tentorium. Nine meningiomas were within the cranial fossa, and one was detected in the caudal fossa. Two cats (cases 1 and 4) had two temporal meningiomas, one (case 3) a tentorial and temporal meningioma, and the fourth cat (case 2) had two temporal, one retrobulbar and one supracerebellar tumour. In all cases, bilateral tumour localisation (one left- and one right-sided tumour) was observed. In each patient, there was a large discrepancy between the size of the tumours, the diameter of the largest being at least four times that of the smallest. Only in the cat (case 2) with four tumours were some meningiomas of similar size. However, even in this animal, there were two similar large masses (temporal left, supracerebellar left) and two smaller ones.
The lesions were iso- to hyperintense to the grey matter on T2-weighted sequences. In T1 sequences, they were slightly hypointense and in FLAIR images, slightly hyperintense. Inhomogeneous, slight contrast enhancement after gadolinium application was seen in six tumours, particularly at the periphery of the mass. In four tumours, a homogeneous, moderate contrast enhancement could be seen. The surrounding meninges were strongly contrast enhancing with discrete dural tail signs. In two cases (cases 2 and 4) the signal properties of the multiple meningiomas differed between the different tumours (isointense or slightly hyperintense in T2, homogeneous or mainly peripheral contrast uptake).
CSF
Cerebrospinal fluid (CSF) analysis was performed after MRI examination in order to exclude other differentials leading to multifocal lesions (lymphoma, metastasis, abscess). In all cases a slight elevation of protein concentration (Pandy 1+ positive) with normal (cases 2, 3 and 4) and/or slightly elevated cell count (6 cells/μl) was detected (case 1). Blood contamination was excluded because no erythrocytes were detected. The differential cell count in case 1 was consistent with a reaction from a compressive space-occupying lesion (64% neutrophils, 20% lymphocytes, 14% monocytes/macrophages and 2% eosinophils).
Postoperative MRI
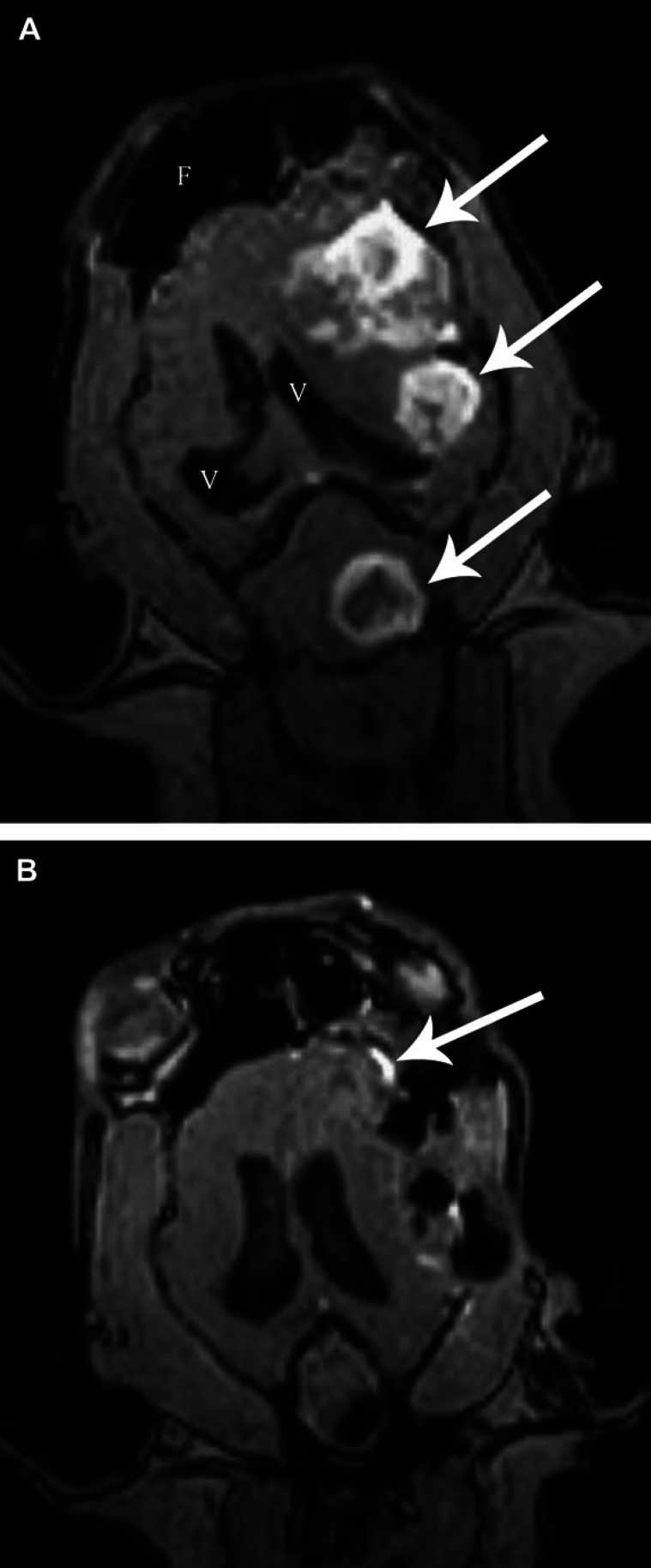
Postoperative MRI revealed complete gross tumour removal in three cases. In case 1 with two cranial fossa meningiomas, subtotal excision with a small basal remnant (2×2 mm) of the ventral part of one meningioma lying on the floor of the skull, was observed. In three cases (cases 1, 3, and 4), diffuse superficial signal intensity on T2 sequences suggested postoperative peritumoural oedema as a consequence of iatrogenic parenchymal manipulation and removal of the space-occupying lesions. At the tumour site, pneumocephalus was observed in all cases. In two cases (cases 2 and 4), strong contrast enhancement and broad meninges around the operative site suggested either tumour remnants infiltrating the meningeal layers or a secondary meningeal reaction (Fig. 1).
Fig 1.
A: Case 2, contrast enhanced FE 3D MPR, T1-weighted, dorsal plane, before surgery. Three enhancing meningiomas (arrows) can be seen on the patient's left side. The lateral ventricles (V) are displaced to the right side. (F: frontal sinus). The fourth tumour lying more ventrally on the right side is not visible. B: Case 2, postoperatively. Hypointense (air) spaces can be seen instead of the meningiomas. There is enhancement of the meninges in the region of the most rostral tumour (arrow), either due to reactive inflammation or focal infiltration.
Pathology
In all cases, histomorphology and immunohistochemistry confirmed the diagnosis of a meningioma. Based on the histopathological architecture, six tumours revealed features of a transitional subtype meningioma (cases 2 and 4), and four of a meningotheliomatous meningioma (cases 1 and 3). In each cat, the multiple meningiomas all were of the same histopathological group. None of the tumours exhibited histological signs of anaplasia. The mitotic index was 0.1±0.08, and a mean of 3.1% of tumour cells stained MIB-1 positive. All tumours were Vimentin positive and cytokeratin negative and in two cases they were S-100 positive (cases 1 and 3). If analysed in accordance with human neuro-oncology standards, all of these features are compatible with benign WHO grade-I tumours (Kleinhues and Cavenee 2000).
Outcome
Patients were discharged from the clinic 2 to 3 days after surgery. Most of the preoperative presenting signs had resolved by the follow-up examinations after 4 weeks. Follow-up evaluation revealed that surgically-induced or exacerbated neurological deficits in two cats had completely (case 4) or almost completely (case 2) resolved within 8 weeks of surgery. No abnormalities were observed in any of the complete blood count examinations. All patients are still alive and no clinical signs of recurrence could be detected at this time (12–24 months following surgery) (Table 2).
Table 2.
Overview of the medical reports of the four cats with multiple meningiomas
| Case 1 | Case 2 | Case 3 | Case 4 | |
|---|---|---|---|---|
| Breed | Domestic shorthair | Domestic shorthair | Domestic shorthair | Domestic shorthair |
| Age | 11 years | 13 years | 13 years | 14 years |
| Gender | Male castrated | Female neutered | Male castrated | Male castrated |
| Activity | Indoor cat | Indoor cat | Indoor cat | Indoor cat |
| Presenting signs | Inappetence, lethargy, pacing to the right | Lethargy, uncoordinated gait | Aggression, pacing to the left, slight ataxia | Lethargy, pacing to the right |
| Time between first signs and presentation | 10 days | 11 months | 4 weeks | 2 weeks |
| Preoperative therapy | Prednisolone 1 mg/kgq24h | Dexamethasone 0.2 mg/kgq24h | Dexamethasone 0.2 mg/kgq24h | Prednisolone 1 mg/kgq24h |
| Effect | Worsening after 5 days | Stabilisation then worsening | Worsening after a few days | Worsening after a few days |
| Abnormal neurological findings | Circling to the right, menace reaction left, proprioception left | Menace reaction both sides, proprioception both sides | Menace reaction right, proprioception right | Menace reaction left, proprioception left |
| Neuroanatomical localisation | Right forebrain | Bilateral forebrain | Left forebrain | Right forebrain |
| MRI location and sizes of the masses | Dorsal temporal right (2 cm3), ventral temporal left (0.5 cm3) | Two dorsal temporal left (0.6 and 1.8 cm3), retrobulbar right (0.5 cm3), supracerebellar left (1.2 cm3) | Dorsal temporal left (0.8 cm3), median tentorial (2 cm3) | Dorsal temporal right (2.2 cm3), dorsal temporal left (0.6 cm3) |
| Histological classification of meningiomas | Meningotheliomatous | Transitional | Meningotheliomatous | Transitional |
| Neurological deficits (4 weeks) | None | Tetraparesis, ataxia | Slight ataxia | None |
| Neurological deficits (8 weeks) | None | Slight left-sided proprioceptive deficits | None | None |
| Follow-up | 6 months | 6 months | 13 months | 15 months |
Discussion
Several characteristics, including a median age of 13 years and a male to female ratio of 3:1, are similar to previous reports of feline meningiomas (Troxel et al 2003). The domestic shorthair was the only breed in this report, most likely because of the relative frequency of this breed in the general cat population (Nafe 1979, Gordon et al 1994, Troxel et al 2003).
There are two theories regarding the pathogenesis of multiple meningiomas. According to non-clonal theory, wide neoplastic fields in the dura mater give rise to multiple synchronous or metachronous meningiomas (Borovich et al 1988). Other authors (Von Deimling et al 1999) suggest that multiple meningiomas are due to dissemination of tumour cells either by subarachnoidal spread via the cerebrospinal fluid or by surgical manipulation (iatrogenic). Pathological examination confirmed an identical type of meningioma in each cat. However, histological variations of meningiomas in the same patient are also described (Salvati et al 2004). This phenomenon could be interpreted as evidence that the meningiomas are unrelated in origin, supporting the non-clonal theory.
Clinical signs in our patients suggested a focal lesion in three cats. However, slight deficits on the contralateral side in cases 1, 3 and 4 were observed, and possible lesion- or tumour-related signs (compression, peritumoural oedema) were suspected. At the time of neurological examination, cats with two meningiomas were not suspected as having a multifocal lesion, and MRI examination was necessary to reveal the multiple characters of the lesions. This finding could be explained by the disparity in diameter between the two tumours. In each cat, one large main lesion and a secondary smaller tumour were found. The histological examination demonstrated that the multiple meningiomas belong to the same histopathological group, indirectly indicating a similar growth pattern of the tumours. On the other hand, the MRI findings in two cats (cases 2 and 4) showing a different contrast enhancement pattern between the meningiomas may suggest the contrary. It seems that even belonging to the same group, meningiomas may have different biological properties. To the authors' knowledge no references exist about correlation between different types of meningioma and MRI findings in veterinary medicine.
Corroborating previous reports, eight of the 10 meningiomas in our four cats were convexity meningiomas located in the cranial fossa. The temporal region appeared to be a favoured site (seven tumours). Moreover, at least one temporal meningioma was diagnosed in each cat. This confirms a previous report, in which the third ventricle, parietal and temporal lobes were found to be the most common sites of meningioma in 93 cats (Troxel et al 2003).
Surgical removal was elected because worsening of neurological deficits was observed despite cortisone therapy, and because of the relatively large size of the tumours in our patients. Interestingly, the therapeutic effect of steroid administration was only transient in our patients. This is in contrary with previous studies describing complete remission of signs in cats with meningioma for up to 3 months after cessation of daily corticosteroid treatment (Adamo et al 2004). The clinical effects of corticosteroids appear to be the result of directly decreasing the permeability of tumour capillaries. Steroid administration was found to decrease the blood supply to a tumour by 29% within 6 h of administration and further decreases tumour blood volume by 21% within 24 h. These changes can result in reduced intracranial pressure, decreased brain oedema and an attenuation of clinical signs (Jarden et al 1989, Adamo et al 2004). The limited effect of steroids in our cats may be due to the very large volume occupied by the lesions and a reduced responsiveness of the peritumoural compressed brain tissue.
In human neurosurgery, leaving a small tumour residue during surgical excision is favoured over complete resection when the meningioma is located at a critical site (Salvati et al 2004). Surgery is the first option of treatment in human patient with multiple meningiomas. However, in these patients, each tumour is evaluated separately, and the mere presence of multiple tumours does not justify their removal (Salvati et al 2004). Indeed, these authors did not remove meningiomas if they remained asymptomatic and smaller than 3 cm at a mean follow-up of 12 years in their case series.
The surgical prognosis of single intracranial meningiomas in cats is good and survival is significantly prolonged with surgical intervention (Lawson et al 1984, Gallagher et al 1993). Median postoperative survival time is 685 days (23 months) compared to 18 days for cats that are treated medically (Gordon et al 1994). Similar survival would be obtained with radiotherapy. Median survival time for dogs after irradiation of meningiomas ranged from 4.9 to 16 months. Axlund et al (2002) compared the outcome in canine patients with intracranial meningiomas treated with surgical resection alone or followed by radiation therapy and found median survival times of 7 and 16 months, respectively. The same results could be expected in cats, but to our knowledge no concluding comparative studies available at this time (Rohrer Bley et al 2005). Our results suggest that the postoperative outcome seems not to be influenced by the number of meningiomas present. The clinico-neurological status of our patients was excellent at the time of writing this report. Follow-up MRI examination was not performed, and recurrence of these, typically slow growing, tumours in our patients cannot be excluded. All the more so because asymptomatic meningiomas have been described both in people and in cats (Summers et al 1995, Karatsu et al 2000, Pocknell et al 2003). Owners of the patients in the current study declined additional radiation treatment.
Hydroxyurea was administered to cats in this study in an attempt to diminish tumour re-growth. The rationale for this was based on in vitro studies that showed that multiplication of feline meningioma cells was slowed or completely arrested by the application of hydroxyurea, and on subsequent clinical studies, corroborating this in vitro effect (Forterre et al 2000).
Surgical treatment of multiple meningiomas in cats is possible and has a good outcome. It seems that the number of tumours present does not influence, in any significant manner, the prognosis and, that the clinical outcome is similar to that in cats with a solitary meningioma treated surgically. However, a bigger case series would be necessary to definitely confirm this theory. In addition, further clinical studies are necessary to establish the extent to which hydroxyurea may increase survival in cats treated surgically for meningioma.
References
- Adamo P.F., Forrest L., Dubielzig R. Canine and feline meningiomas: diagnosis, treatment, and prognosis, Compendium on Continuing Education for the Practicing Veterinarian 26, 2004, 951–957. [Google Scholar]
- Axlund T.W., Mc Glasson M.L., Smith A.N. Surgery alone or in combination with radiotherapy for treatment of intracranial meningiomas in dogs: 31 cases (1989–2002), Journal of American Veterinary Medical Association 221, 2002, 1597–1600. [DOI] [PubMed] [Google Scholar]
- Borovich B., Doron Y., Braun J., Feinsod M., Goldsher D., Gruszkiewicz J., Guilburd J.N., Zaaroor M., Levi L., Soustiel J.F., Lemberger A. The incidence of multiple meningiomas – do solitary meningiomas exist?, Acta Neurochirurgia 90, 1988, 15–22. [DOI] [PubMed] [Google Scholar]
- Evans D.G.R., Watson C., King A., Wallace A.J., Baser M.E. Multiple meningiomas: differential involvement of the NF2 gene in children and adults, Journal of Medical Genetics 42, 2006, 45–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forterre F., Matis U., Schrell U., Geier M., Gutmannsbauer B., Schmahl W. Intracranial meningiomas – findings, therapy and results in nine cats and one dog, Tierärztliche Praxis 28, 2000, 170–177. [Google Scholar]
- Gallagher J.G., Berg J., Knowles K.E., Williams L.L., Bronson R.T. Prognosis after surgical excision of cerebral meningiomas in cats: 17 cases (1986–1992), Journal of American Veterinary Medical Association 203 (10), 1993, 1437–1440. [PubMed] [Google Scholar]
- Gordon L.E., Thacher C., Matthiesen D.T., Joseph R.J. Results of craniotomy for the treatment of cerebral meningioma in 42 cats, Veterinary Surgery 23, 1994, 94–100. [DOI] [PubMed] [Google Scholar]
- Jarden J., Dhawan V., Moeller J. The time course of steroid action on blood-to-brain and blood-to-tumor transport of 82 Rb: a positron emission study, Annals of Neurology 25, 1989, 239–245. [DOI] [PubMed] [Google Scholar]
- Karatsu J., Kochi M., Ushio Y. Incidence and clinical futures of asymptomatic meningiomas, Journal of Neurosurgery 92, 2000, 766–770. [DOI] [PubMed] [Google Scholar]
- Kleinhues P., Cavenee W.K. WHO Classification of Tumors. Pathology and Genetics – Tumours of the Nervous System, 2000, IARC Press: Lyon, pp. 176–184 [Google Scholar]
- Koestner A., Bilzer T., Fatzer R., Schulman F.Y., Summers B.A., van Winkle T.J. Histological Classification of the Tumors of the Nervous System of Domestic Animals, 2nd Series Vol. V, 1999, AFIP: Washington, p. 23. [Google Scholar]
- Lawson D.C., Burk R.L., Prata R.G. Cerebral meningioma in the cat: diagnosis and surgical treatment of ten cases, Journal of the American Animal Hospital Association 20, 1984, 333–342. [Google Scholar]
- Nafe L.A. Meningiomas in cats: a retrospective clinical study of 36 cases, Journal of the American Veterinary Medical Association 174, 1979, 1224–1227. [PubMed] [Google Scholar]
- Perry A., Gutmann D.H., Reifenberger G. Molecular pathogenesis of meningiomas, Journal of Neuro-Oncology 70, 2004, 183–202. [DOI] [PubMed] [Google Scholar]
- Pocknell D.L., Lamb R.L., Targett M.P. Concurrent benign and malignant multiple meningiomas in a cat: clinical, MRI and pathological findings, Veterinary Record 152, 2003, 780–782. [DOI] [PubMed] [Google Scholar]
- Bley C. Rohrer, Surnova A., Roos M., Kaser-Hotz B. Irradiation of brain tumors in dogs with neurologic disease, Journal of Veterinary Internal Medicine 19, 2005, 849–854. [DOI] [PubMed] [Google Scholar]
- Salvati M., Caroli E., Ferrante L., Rocchi G., D'Andrea G., Piccirilli M., Delfini R. Spontaneous, multiple meningiomas, Zentralblatt Neurochirurgie 65, 2004, 180–184. [DOI] [PubMed] [Google Scholar]
- Summers B.A., Cummings J.F., de Lahunta A. Tumors of the Central Nervous System, Veterinary Neuropathology, 1995, Mosby: St. Louis, pp. 351–401 [Google Scholar]
- Troxel M.T., Vite C.H., Van Winkle T.J., Newton A.L., Tiches D., Dayrell-Hart B., Kapatkin A.S., Shofer F.S., Steinberg S.A. Feline intracranial neoplasia: retrospective review of 160 cases (1985–2001), Journal of Veterinary Internal Medicine 17, 2003, 850–859. [DOI] [PubMed] [Google Scholar]
- Von Deimling A., Larson J., Wellenreuther R., Stangl A.P., van Velthoven V., Warnick R., Tew J., Balko G., Menon A.G. Clonal origin of recurrent meningiomas, Brain Pathology 9, 1999, 645–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vite C.H. Neoplasia of the nervous system. Vite C.H. Braund's Clinical Neurology in Small Animals – Localisation, Diagnosis and Treatment, 2005, IVIS: Ithaca, New York. [Google Scholar]
- Yasargil M.G. 1st edn, Microneurosurgery Vols IVa and IVb, 1994, Georg Thieme Verlag: Stuttgart, New York. [Google Scholar]