Abstract
Background and purpose:
Transforming growth factor β1 (TGFβ1) is generated in atherosclerotic and injured vessel walls. We examined whether the endothelial-to-mesenchymal transdifferentiation induced by TGFβ1 affects endothelial functions.
Experimental approach:
Bovine aortic endothelial cells (BAECs) were treated with 3 ng ml−1 TGFβ1 for 7 days. Contraction of TGFβ1-treated BAECs was assessed by collagen gel contraction assay. Protein expression and phosphorylation were assessed by Western blotting. Intracellular Ca2+ concentration and NO production were measured using fura2 and DAF-2, respectively.
Key results:
TGFβ1-treated BAECs showed dense actin fibers and expressed smooth muscle marker proteins; they also changed into smooth muscle-like, spindle-shaped cells in collagen gel cultures. ATP (10 μM) induced a gradual contraction of collagen gels containing TGFβ1-treated BAECs but not of gels containing control BAECs. ATP-induced contraction of TGFβ1-treated BAECs was not reversed by the removal of ATP but was partially suppressed by a high concentration of sodium nitroprusside (1 μM). TGFβ1-treated BAECs showed sustained phosphorylation of myosin light chain in response to ATP and low levels of basal MYPT1 expression. ATP-induced Ca2+ transients as well as eNOS protein expression were not affected by TGFβ1 in BAECs. However, ATP-induced NO production was significantly reduced in TGFβ1-treated BAECs. Anti-TGFβ1 antibody abolished all of these TGFβ1-induced changes in BAECs.
Conclusions and Implications:
Mesenchymal transdifferentiation induced by TGFβ1 leads to sustained contraction and reduced NO production in endothelial cells. Such effects, therefore, would not be beneficial for vascular integrity.
Keywords: TGFβ1, endothelium, smooth muscle, contraction, nitric oxide
Introduction
Transforming growth factor β1 (TGFβ1) is a multifunctional cytokine involved in the regulation of cell proliferation, differentiation and survival in many cell types (Roberts and Sporn, 1993). TGFβ1 is secreted from various cell types in the vascular wall such as smooth muscle cells (Yue et al., 1994), endothelial cells (Hannan et al., 1988), platelets (Ross et al., 1986) and macrophages (Assoian et al., 1987), especially in injured vessels, atherosclerotic lesions and post-angioplasty restenotic lesions (McCaffrey, 2000; Grainger, 2004). Vascular endothelial cells are one of the main targets of TGFβ1 (Frater-Schroder et al., 1986; Pollman et al., 1999), and it is well-established that TGFβ1 inhibits endothelial proliferation (Frater-Schroder et al., 1986; Heimark et al., 1986) and migration (Krizbai et al., 2000). It has already been proposed that these effects of TGFβ1 on endothelium would lead to the slowing of re-endothelialization of the injured vascular surface and allow time for recruitment of smooth muscle cells into the site of injury (Heimark et al., 1986).
Another distinct characteristic of TGFβ1 is the induction of mesenchymal transdifferentiation and morphological changes in endothelial cells (Sutton et al., 1991; Arciniegas et al., 1992). TGFβ1 has been reported to induce the expression of smooth muscle markers such as α-smooth muscle actin (α-SM actin), SM22α, calponin and SM myosin in aortic and pulmonary artery endothelial cells (Arciniegas et al., 1992; Frid et al., 2002). These authors speculated that the transformed endothelial cells may serve as a source of contractile smooth muscle cells in the repair of atherosclerotic lesions and/or injured vascular walls (Arciniegas et al., 1992; Frid et al., 2002). TGFβ1-induced endothelial–mesenchymal transdifferentiation was also reported in human and ovine valvular endothelial cells, in which cell migration was markedly increased, thereby suggesting that TGFβ1 may play a significant role in replenishing the interstitial cells of cardiac valves (Paranya et al., 2001). However, none of these previous studies have examined the contractility of TGFβ1-treated endothelial cells.
It is also still controversial whether TGFβ1 is beneficial for endothelial NO production or not. Earlier studies showed that TGFβ1 increased NO production via upregulation of eNOS protein expression, thereby suggesting a vasoprotective role of TGFβ1 in injured vessels (Poppa et al., 1998; Tai et al., 2004). In contrast, a recent report has shown that the binding of TGFβ1 to its receptor expels eNOS from caveolae and reduces its activity in human endothelium (Schwartz et al., 2005). As mechanical stress-induced change in cell shape is quite important for endothelial NO production (Kimura et al., 2000), it would be also possible that TGFβ1-induced morphological change may affect NO production in endothelial cells.
We have focused in this study on the functional significance of TGFβ1-induced endothelial–mesenchymal transdifferentiation. For this purpose, we examined the effects of TGFβ1 on contractility and NO production, as representative features of smooth muscle and endothelial functions, respectively, in bovine aortic endothelial cells (BAECs). Our results revealed, for the first time, that TGFβ1 changes endothelial cells into non-smooth muscle contractile cells.
Materials and methods
Cell culture
Thoracic aortas of 1-year-old calves were obtained from the local slaughterhouse, and BAECs and aortic smooth muscle cells (BASMCs) were cultured in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% fetal bovine serum as previously described (Kimura et al., 2001a, 2002).
Treatments with TGFβ1 and ani-TGFβ1 antibody
Treatment with TGFβ1 consisted of incubating the non-confluent cells with culture medium containing TGFβ1 for various periods. Effects of neutralizing antibody for TGFβ1 were examined by using culture medium that was pre-incubated with 3 ng ml−1 TGFβ1 and 300 ng ml−1 anti-TGFβ1 antibody for 1 h at room temperature.
Immunological staining of fibrous actin
Fibrous actin was stained using rhodamine-conjugated phalloidin according to the previously reported method (Knudsen and Frangos, 1997).
Gel contraction assay
Contractility of cultured BAECs and BASMCs were examined with the gel contraction assay (Kimura et al., 2002). The cells were cultured on plates for 7 days with or without TGFβ1, and were harvested by trypsinization. The harvested cells were re-suspended in DMEM containing 0.2% collagen type IA at a density of 4 × 105 cells ml−1, and 0.5 ml of the cell suspension per well was poured into a 24-well culture plate. The plate was kept at 37°C for 10 min to form a gel, and 1 ml of culture medium per well was added to the gel (see diagrams in Figure 3a). The gels were cultured for 3 days and used for the contraction assay. The lateral surface of the gel was carefully detached from the culture well with a fine needle. The culture plate was then placed on a hotplate (MP-10DM; Kitazato Supply, Shizuoka, Japan) and kept at 37°C. The gel surface images were captured with a digital camera (QV-800SX, Casio, Tokyo, Japan) every 1 min throughout the experiment. Contraction of the gel was then evaluated by measuring its surface area with an image analysis software (Adobe Photoshop, Adobe Systems Inc., San Jose, CA, USA).
Figure 3.
Gel contraction assay. BAECs were cultured on a plate for 7 days in the absence or presence of 3 ng ml−1 TGFβ1, and harvested by trypsinization. Cells were then embedded in collagen gels that were overlaid with the same culture media. BASMCs were also embedded in collagen gels. Cell images in the gels after 3 days are shown in (a). Scales, 50 μm. ATP (10 μM)-induced contractions of these gels were assessed by measuring the surface area of the gels (b, n=6–8). See Materials and methods for a detailed protocol.
Western blot analysis
Expressions of smooth muscle marker proteins, MLC, MYPT1 and eNOS protein, and the amount of p-MLC and p-MYPT1 were assessed with chemiluminescence Western blotting. Cell lysates were prepared after each pretreatment and separated by electrophoresis. Western blot analysis was carried out by using the relevant antibody.
In each experiment, the bands were detected with chemiluminescence system (SuperSignal West Dura, Pierce Co., Rockford, IL, USA) and analysed with a lumino image analyzer (FAS-1000, Toyobo, Osaka, Japan).
Measurement of intracellular calcium concentrations ([Ca2+]i)
[Ca2+]i was measured in non-confluent BAECs with Fura-2 by using an Attofluor digital fluorescence microscopy system (Atto Instruments, Rockville, MD, USA), as previously described (Oike et al., 2000). For the statistical analysis of [Ca2+]i, results from 20 to 30 cells in a coverslip were averaged and treated as one data point.
Measurement of intracellular production of NO
To determine the intracellular NO production in BAECs, an NO-sensitive fluorescent dye diaminofluorescein-2 (DAF-2) (Kojima et al., 1998) was used. Non-confluent cells grown on coverslip were incubated with a diacetylated form of DAF-2 (DAF-2/DA, 10 μM) for 20 min at 37°C. DAF-2 fluorescence was measured and analysed as previously described (Kimura et al., 2001b).
Solutions
Krebs solution used in Ca2+ and NO measurements contained (mM); NaCl 132.4, KCl 5.9, CaCl2 1.5, MgCl2 1.2, glucose 11.5, HEPES 11.5 and pH was adjusted to 7.4 by NaOH.
Statistics
Pooled data were expressed as mean±s.e.m. values. Statistical significance was assessed with Student's unpaired t-test. Values of P<0.05 were considered to show significant differences between means.
Materials
Anti-TGFβ1, anti-troponin (clone hCP), anti-SM myosin (clone HSM-V), anti-myosin light chain (MLC, clone MY-21) and anti β-actin (clone AC-15) antibodies were purchased from Sigma (St Louis, MO, USA). Anti-myosin phosphatase target subunit 1 (MYPT1) and anti-phosphorylated MYPT1 (p-MYPT1, Thr 696) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-α-SM actin (clone 1A4) was purchased from Dako (Glostrup, Denmark). Anti-eNOS antibody was purchased from StressGen Biotechnologies (San Diego, CA, USA). Anti-phosphorylated MLC (p-MLC) antibody (Thr18/Ser19) was purchased from Cell Signaling Technology (Beverly, MA, USA). Rhodamine-conjugated phalloidin was purchased from Molecular Probes (Eugene, OR, USA). Collagen type IA was purchased from Nitta Gelatin (Osaka, Japan). DAF-2/DA was purchased from Daiichi Pure Chemicals (Tokyo, Japan). All other reagents were purchased from Sigma.
Results
TGFβ1-induced inhibition of cell proliferation in BAECs
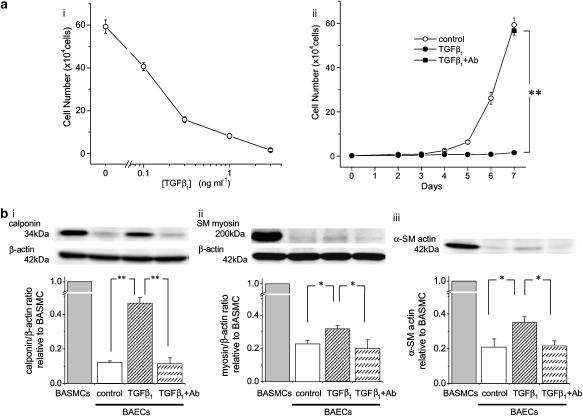
TGFβ1 induced concentration-dependent inhibition of endothelial proliferation, with a concentration of 3 ng ml−1 showing a maximal effect (Figure 1a–i). TGFβ1-induced inhibition of endothelial proliferation was completely abolished by anti-TGFβ1 antibody (Figure 1a–ii). We therefore used the concentration of 3 ng ml−1 in this study and assumed that anti-TGFβ1 antibody totally neutralized the effects of TGFβ1.
Figure 1.
TGFβ1-induced growth inhibition and expression of smooth muscle markers in BAECs. (a) A fixed number of cells (2000 cells) were seeded at day 0, and the cell numbers were manually counted after culturing for 7 days in the absence or presence of various concentrations of TGFβ1 (i), or for 2–7 days in the absence or presence of 3 ng ml−1 TGFβ1 (ii). In some experiments, anti-TGFβ1 antibody (300 ng ml−1) was pre-incubated with 3 ng ml−1 TGFβ1 for 1 h at room temperature and applied to the cells (ii, closed square). **P<0.01. n=5–7. (b) Western blotting of the expressions of calponin (i), SM myosin (ii) and α-SM actin (iii) in BASMCs and control, TGFβ1 (3 ng ml−1)-treated and TGFβ1/antibody-treated BAECs. Expression of β-actin was also measured as an internal control. The cells were cultured in each condition for 7 days. Representative band images are shown in the upper panels. Densitometric analysis of smooth muscle markers/β-actin ratios relative to BASMCs are shown in the lower panels (n=4). *P<0.05, **P<0.01.
Expression of smooth muscle markers in TGFβ1-treated BAECs
Previous reports indicated that TGFβ1 induced an expression of smooth muscle markers in endothelial cells (Arciniegas et al., 1992; Frid et al., 2002). Next we examined the expression of smooth muscle markers in control and TGFβ1-treated BAECs (Figure 1b). Control BAECs showed trace levels of expression of the smooth muscle markers, α-SM actin, calponin and SM myosin. The cells treated with TGFβ1 for 7 days showed a significant increase in the expression of these marker proteins and these changes were abolished by anti-TGFβ1 antibody.
TGFβ1-induced morphological changes in BAECs
Control culture medium did not elicit apparent changes in actin cytoskeleton of BAECs for at least for 7 days (Figure 2a). In contrast, incubation with TGFβ1 (3 ng ml−1) induced actin fibre formation in 1 h in BAECs and these fibres formed by TGFβ1 became denser in a time-dependent manner, so that peripheral thick actin fibres were observed after 7 days (Figure 2b). Anti-TGFβ1 antibody completely inhibited the effects of TGFβ1 on actin fibres (Figure 2c). Dense actin fibres were also observed in BASMCs, but their distribution was not restricted to the peripheral area (Figure 2d).
Figure 2.
Time-dependent change in actin fibres in BAECs. Control culture medium (a) or culture medium containing TGFβ1 (3 ng ml−1, b) or TGFβ1/antibody (c) was applied to subconfluent BAECs. Cytosolic actin cytoskeleton was then stained with rhodamine-phalloidin after 1 h, 24 h or 7 days. Higher magnification images of ‘7 day' cells are taken from the dotted areas of the corresponding lower magnification images. Actin fibres in BASMCs are also shown (d). Scale bar in each panel indicates 50 μm.
Contractile properties of TGFβ1-treated BAECs
The results above indicate that TGFβ1 induces endothelial–mesenchymal transformation in BAECs as previously reported (Arciniegas et al., 1992; Frid et al., 2002). We then examined the functional alterations of the TGFβ1-treated BAECs. Previous reports demonstrated that cultured smooth muscle cells exhibit contractility on being embedded in a collagen gel lattice (Kimura et al., 2002, 2004). So we used this technique to examine whether TGFβ1-treated BAECs exhibited similar contractility.
When BASMCs were embedded in a three-dimensional collagen gel lattice, cells showed a spindle shape (Figure 3a–i). In contrast, control BAECs embedded in a similar collagen gel lattice for 3 days showed a capillary-like appearance (Figure 3a–ii). However, when TGFβ1-treated BAECs were embedded in collagen gel and cultured for 3 days in the presence of TGFβ1, the cells displayed a smooth muscle-like, spindle shape as shown in Figure 3a–iii. Cells treated with TGFβ1 and anti-TGFβ1 antibody showed the capillary-like, and not the spindle shaped, morphology in collagen gels (not shown).
ATP (10 μM) induced rapidly developing contraction of gels containing BASMCs. The contraction was maintained during ATP application and reversed to the basal level after the removal of ATP (Figure 3b, open triangles). ATP (10 μM) did not induce any contraction in gels containing control BAECs (Figure 3b, open circles). In contrast, gels with TGFβ1-treated BAECs showed a slow contraction in response to 10 μM ATP and these gels did not show relaxation for at least 30 min after the removal of ATP (Figure 3b, closed circles). Contraction of TGFβ1-treated BAECs was not observed without ATP application (not shown). When the embedded BAECs were pretreated with TGFβ1 in the presence of its neutralizing antibody, gels did not show contraction in response to ATP (Figure 3b, closed squares).
Relaxation responses of TGFβ1-treated BAECs and BASMCs were further examined with sodium nitroprusside (SNP), an NO donor. In gels containing BASMCs, the sustained contraction induced by ATP was relaxed by SNP in a concentration-dependent manner (Figure 4a, closed triangles). In gels with TGFβ1-treated BAECs, however, 10 nM SNP did not affect the gradual development of ATP-induced gel contraction, and a partial relaxation was obtained only with a higher concentration of SNP (1 μM; Figure 4a, closed circles).
Figure 4.
Effects of SNP and ET-1 on gel contraction. (a) Gels containing BASMCs or TGFβ1-treated BAECs were contracted with 10 μM ATP, and the effects of sequential application of 10 nM and 1 μM SNP were examined (closed symbols, n=4–6). Control data were obtained without SNP application from the same gel preparation (open symbols, n=4–6). Note that 10 nM SNP induced relaxation only in gels containing BASMCs. (b) ET-1 (1 nM) induced contraction in gels with BASMCs, but not in gels containing control or TGFβ1-treated BAECs (n=4–6).
Endothelin-1 (ET-1, 1 nM) induced a contraction in gels containing BASMCs but not in those with TGFβ1-treated BAECs (Figure 4b). It is known that both ETA and ETB receptors are localized in smooth muscle cells, whereas only ETB receptor in endothelium, and ET-1 has a higher affinity to ETA receptor (Schiffrin, 2005). Therefore, we suppose that the absence of ET-1-induced contraction in TGFβ1-treated BAECs was due to the absence of ETA receptor expression in BAECs.
Sustained phosphorylation of MLC and reduced expression of MYPT1 in TGFβ1-treated BAECs
The results above indicate that BAECs acquire contractility by being treated with TGFβ1. To examine whether the contraction of TGFβ1-treated BAECs is due to the phosphorylation of contractile proteins or not, we then evaluated the phosphorylation of MLC in the cells that were grown on culture plates and stimulated with 10 μM ATP. BASMCs showed an increase in phosphorylated MLC (p-MLC) in response to 10 μM ATP, which returned to the basal level after the removal of ATP (Figure 5, open triangles). ATP did not induce MLC phosphorylation in control BAECs (Figure 5, open circles). In contrast, BAECs treated with TGFβ1 for 7 days showed a gradual increase in p-MLC in response to ATP. However, the phosphorylation of MLC was sustained after the removal of ATP in TGFβ1-treated BAECs (Figure 5, closed circles). ATP did not increase p-MLC in BAECs that were pretreated with TGFβ1 and its antibody for 7days (Figure 5, closed squares). These results are compatible with the gel contraction assay, and indicate that the contraction of TGFβ1-treated BAECs was due to the phosphorylation of MLC.
Figure 5.
Effects of ATP on p-MLC in BAECs and BASMCs. BAECs were cultured on a plate with control medium or medium containing TGFβ1 (3 ng ml−1) or TGFβ1/antibody for 7days. BASMCs were also cultured for 7days. Total cellular proteins were collected before (0 min) or during 10 μM ATP application and after removing ATP at the times indicated. Expression levels of p-MLC and total MLC were then assessed with Western blotting. The upper panels show the representative band images of p-MLC, and the lower panel shows the densitometric analysis of p-MLC/total MLC values relative to the 0 min value (n=5).
To elucidate the mechanisms of the sustained phosphorylation of MLC, next we evaluated the expression level of MYPT1, the regulatory subunit of myosin light chain phosphatase (MLCP). As shown in Figure 6a, expression of MYPT1 was more abundant in control BAECs than in BASMCs. TGFβ1 significantly reduced the expression of MYPT1 in BAECs, and anti-TGFβ1 antibody reversed this effect (Figure 6a). However, ATP did not induce any change in the amount of p-MYPT1 both in control and TGFβ1-treated BAECs (Figure 6b). Therefore, although inhibitory phosphorylation of MYPT1 augments the contractility in smooth muscle (Feng et al., 1999), the sustained contraction of TGFβ1-treated BAECs may be mainly due to the reduction of MYPT1 expression but not to its phosphorylation.
Figure 6.
Western blot analysis of the expressions of MYPT1 and p-MYPT1 in BAECs and BASMCs. Total cellular proteins were prepared as described in the legend to Figure 5. Basal expression levels of MYPT1 and β-actin before ATP application are shown in (a) band images show data from a representative experiment and the bars show the densitometric analysis of MYPT1 expression relative to β-actin from six experiments (*P<0.05, **P<0.01). Expression of p-MYPT1 before, during and after ATP (10 μM) application are shown in (b). Band images show a representative result and the graph shows the time-dependent change in p-MYPT1/total MYPT values, relative to the initial (time=0) value (n=5).
Calcium mobilizing properties in TGFβ1-treated BAECs
To further examine the functional alteration of TGFβ1-treated BAECs, we compared the ATP-induced Ca2+ transients in control and TGFβ1-treated BAECs. Basal [Ca2+]i was not different between control and TGFβ1-treated BAECs (control, 106.7±16.9 nM, n=7; TGFβ1-treated, 94.7±16.0 nM, n=6). ATP (10 μM) elicited Ca2+ transients both in control and TGFβ1-treated BAECs (Figure 7a). The degree of Ca2+ elevation in control BAECs was comparable to that observed in BASMCs in our previous report (Kimura et al., 2002). Peak amplitude of Ca2+ transients and the time integral of net [Ca2+]i increment were not different between control and TGFβ1-treated BAECs (Figure 7b).
Figure 7.
ATP (10 μM)-induced Ca2+ transients in BAECs. [Ca2+]i was measured in control and TGFβ1-treated BAECs. Ca2+ traces from representative cells are shown in (a). The bar graphs in (b) show the statistical analysis of the peak value (i) and time-integral (ii) of the ATP-induced net [Ca2+]i increment. Numbers in parenthesis indicate the number of measurements. ns, P>0.05.
ATP-induced NO production in TGFβ1-treated BAECs
Next, we examined production of NO in TGFβ1-treated BAECs to assess their endothelial nature. Expression of eNOS protein was not different between control BAECs and the cells treated with TGFβ1 for 7 days (Figure 8a). Therefore, one can expect that Ca2+-mobilizing stimuli would induce NO production in TGFβ1-treated BAECs. However, a gradual increase in DAF-2 fluorescence, used as an indicator of NO production (Kojima et al., 1998), was induced by 10 μM ATP in control, but not in TGFβ1-treated BAECs (Figure 8b). ATP-induced increase in DAF-2 fluorescence was restored in the cells treated with TGFβ1 in the presence of its neutralizing antibody (Figure 8b).
Figure 8.
NO production in control and TGFβ1-treated BAECs. (a) Expression of eNOS and β-actin proteins were analysed with Western blotting in control and TGFβ1-treated BAECs. Band images show representative data and the bar graph shows the mean eNOS/β-actin values, relative to day 0 control (n=5). ns, P>0.05. (b) ATP (10 μM)-induced NO production was assessed with DAF-2 fluorescence. Cells were excited at 490 nm wavelength every 30 s, and fluorescence intensity at 515 nm was measured. DAF-2 fluorescence relative to its initial value (time 0) was then averaged. Open and closed circles show the data from control (n=22) and TGFβ1-treated (n=20) BAECs, respectively. Anti-TGFβ1 antibody restored NO production (closed square, n=18). **P<0.01, TGFβ1 vs TGFβ1/antibody.
Discussion
We have observed in this study that TGFβ1 induces the formation of dense actin fibres in BAECs that was time dependent and sensitive to anti-TGFβ1 antibody (Figure 2). The time course of TGFβ1-induced actin formation was quite different from that induced by mechanical stress, which is maximally observed in 5 min and converged in 15 min (Koyama et al., 2001). Furthermore, mechanical stress-induced actin reorganization is mediated by the RhoA/Rho-kinase pathway (Koyama et al., 2001), but we failed to observe the TGFβ1-induced membrane translocation of RhoA (Watanebe and Oike; unpublished observation). Therefore, TGFβ1-induced actin fibre formation in BAECs was not due to mechanical stress but to transdifferentiation into smooth muscle-like cells (Arciniegas et al., 1992; Frid et al., 2002). Also TGFβ1 increased the expression of the smooth muscle marker proteins, α-SM actin, calponin and SM myosin, in 7 days in BAECs (Figure 1b). Previous reports had shown that TGFβ1 could induce expression of these smooth muscle markers and had suggested the endothelial–mesenchymal transdifferentiation (Arciniegas et al., 1992; Frid et al., 2002). Thus, it appears that the experimental conditions that we used in the present study were sufficient to differentiate BAECs into the ‘smooth muscle-like cells' previously reported (Arciniegas et al., 1992; Frid et al., 2002).
This is the first report examining the contractility of TGFβ1-treated endothelial cells. When TGFβ1-treated BAECs were embedded in collagen gels, the cells displayed a smooth muscle-like spindle shape (Figure 3a–iii). Also, the gels showed contraction in response to ATP, but the contraction was, in contrast to that observed in gels in which BASMCs were embedded, slow to develop and not reversed by the removal of ATP (Figure 3b). Furthermore, a higher concentration of SNP was required to induce a relaxation in TGFβ1-treated BAECs than in BASMCs (Figure 4a). These observations indicate that the contractile properties and relaxation responses of TGFβ1-treated BAECs are different from those of BASMCs. Furthermore, since ET-1 did not induce the contraction of TGFβ1-treated BAECs (Figure 4b), the ATP-induced contraction of the gels containing TGFβ1-treated BAECs was not due to contamination with BASMCs.
ATP-induced phosphorylation of MLC in BASMCs and TGFβ1-treated BAECs, but not in control or TGFβ1/antibody-treated BAECs (Figure 5). Although the time course of MLC phosphorylation was slower than that of contraction, we consider this discrepancy was to reflect the fact that the phosphorylation study was performed with the cells seeded on culture plates while the contraction assay used those embedded in collagen gels. MLC phosphorylation did not return to control levels after removal of ATP in TGFβ1-treated BAECs, and this finding could explain the sustained contraction of the TGFβ1-treated BAECs in gels. Phosphorylation of MLC is an essential phenomenon for the contraction of smooth muscle cells (Somlyo and Somlyo, 1994) and of non-muscle cells such as fibroblasts (Ehrlich et al., 1991; Kolodney et al., 1999). In previous reports, contraction of fibroblasts embedded in collagen gels was also shown in response to bovine serum (Kolodney et al., 1999), thrombin, ionomycin and lysophosphatidic acid (Emmert et al., 2004), but both contraction and MLC phosphorylation were rapid and reversible (Kolodney et al., 1999; Emmert et al., 2004). Therefore, the present results indicate that the contractile properties of TGFβ1-treated BAECs are not identical to those of the smooth muscle cells or fibroblasts.
Previous reports suggested that endothelial–mesenchymal transdifferentiation may be involved in the repair process of injured mature vessels as the source of luminal cells (Arciniegas et al., 1992; Frid et al., 2002). However, because TGFβ1-treated BAECs showed impairment of relaxation responses, such transdifferentiated cells could not restore all the functions of damaged vessels, in which relaxation is as important as contraction. We have shown that TGFβ1 reduced the expression of MYPT1, a catalytic subunit of MLCP (Pfitzer, 2001), in BAECs (Figure 6a). As ATP did not increase p-MYPT1 in TGFβ1-treated BAECs (Figure 6b), we would postulate that the reduced expression of MLCP but not its inhibitory phosphorylation might be, at least partially, responsible for the sustained contraction of TGFβ1-treated BAECs. We did not expect that the expression level of MYPT1 would be higher in control BAECs than in BASMCs (Figure 6a), but this may be because MLCP plays an important physiological role in controlling barrier function in intact endothelial cells (Verin et al., 2000). Reduction of MYPT1 levels by TGFβ1 could thus lead to the impairment of barrier function, one of the essential functions of endothelium, in injured or atherosclerotic vessels. The expression levels of other MLCP subunits such as PP1c (Ito et al., 2004) have not been examined in this study, so it should be noted that the quantitative and/or functional alteration of any of these subunits may also be involved in the pathogenesis of the impaired relaxation in TGFβ1-treated BAECs.
Previous reports indicated that TGFβ1 acutely increased the expression of eNOS protein and NO production in endothelium (Inoue et al., 1995; Saura et al., 2002; Tai et al., 2004). These authors speculated that TGFβ1 generated in injured vessels or atherosclerotic lesions would increase NO production, thereby relaxing vessels to protect them from increased flow. However, in the present study, TGFβ1-treated BAECs lost their ability to produce NO in 7 days even though expression of eNOS protein and Ca2+ mobilization were intact (Figures 7 and 8). Although TGFβ1 inhibits cell proliferation, this does not explain the reduced NO production in TGFβ1-treated BAECs, because we measured fura-2 and DAF-2 fluorescence from isolated non-confluent cells with similar cell densities both in control and TGFβ1-treated BAECs. Saura et al. (2002) reported that TGFβ1 activated eNOS promoters via Smad2 translocation, but this was observed only during 2–6 h after TGFβ1 treatment. So we suppose that the TGFβ1-induced upregulation of eNOS, if present, is transient, and the reduction of NO production observed in the present study was not due to the alteration of eNOS expression. In human endothelium, TGFβ receptors localize in caveolae, where eNOS protein is also located and generates NO (Minshall et al., 2003), and TGFβ1 rapidly expels eNOS from caveolae thereby inactivating eNOS (Schwartz et al., 2005). This mechanism may explain the present results and we conclude that mesenchymally transformed BAECs would lose another central endothelial function, the generation of NO.
The increased secretion of TGFβ1 in injured vessels was reported to continue for up to 2 weeks (Kanzaki et al., 1995). Therefore, we believe that chronic effects of TGFβ1, as shown in the present study, would have more pathophysiological significance than its acute actions within a few hours. It was reported that anti-TGFβ antibody accelerated atherosclerosis in apoE knockout mice (Mallat et al., 2001), and an atheroprotective role of TGFβ1 has been suggested (Grainger, 2004). However, another group observed that TGFβ1 signalling in T-cells but not in the vascular wall played a role in protecting vessels from arteriosclerosis in apoE knockout mice (Robertson et al., 2003).
The present results firstly suggest that TGFβ1-treated, mesenchymally transdifferentiated endothelial cells would not be capable of substituting fully for injured vascular smooth muscle cells, because the cells would not possess sufficient relaxing ability. Furthermore, the absence of NO production in TGFβ1-treated endothelium would exclude a simple protective role for TGFβ1 through increased NO production in injured and/or atherosclerotic vessels. Previous reports have suggested another possibility for the roles of endothelial–mesenchymal transdifferentiation; that is, non-muscle epithelioid cells are dominant in neointima after injury (Myit et al., 2003), and involved in intimal thickening and pulmonary vascular remodeling in the embryonic arteries (Arciniegas et al., 2005). In conclusion, TGFβ1-induced endothelial–mesenchymal transdifferentiation is not, of itself, beneficial for the maintenance of vascular integrity, and therefore may have other consequences for vascular remodelling that have not been clarified in the present study.
Acknowledgments
This study was supported in part by a Grant-in Aid for Scientific Research (16590197) from the Ministry of Education, Culture, Sports, Sciences and Technology, Japan.
Abbreviations
- α-SM actin
α-smooth muscle actin
- BAECs
bovine aortic endothelial cells
- BASMCs
bovine aortic smooth muscle cells
- DAF-2
diaminofluorescein-2
- DMEM
Dulbecco's Modified Eagle's Medium
- ET-1
endothelin-1
- MLC
myosin light chain
- MLCP
myosin light chain phosphatase
- MYPT1
myosin phosphatase target subunit 1
- p-MLC
phosphorylated myosin light chain
- p-MYPT1
phosphorylated MYPT1
- SNP
sodium nitroprusside
- TGFβ1
transforming growth factor β1
Conflict of interest
The authors state no conflict of interest.
References
- Arciniegas E, Neves CY, Carrillo LM, Zambrano EA, Ramirez R. Endothelial-mesenchymal transition occurs during embryonic pulmonary artery development. Endothelium. 2005;12:193–200. doi: 10.1080/10623320500227283. [DOI] [PubMed] [Google Scholar]
- Arciniegas E, Sutton AB, Allen TD, Schor AM. Transforming growth factor β1 promotes the differentiation of endothelial cells into smooth muscle-like cells in vitro. J Cell Sci. 1992;103:521–529. doi: 10.1242/jcs.103.2.521. [DOI] [PubMed] [Google Scholar]
- Assoian RK, Fleurdelys BE, Stevenson HC, Miller PJ, Madtes DK, Raines EW, et al. Expression and secretion of type β transforming growth factor by activated human macrophages. Proc Natl Acad Sci USA. 1987;84:6020–6024. doi: 10.1073/pnas.84.17.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich HP, Rockwell WB, Cornwell TL, Rajaratnam JB. Demonstration of a direct role for myosin light chain kinase in fibroblast-populated collagen lattice contraction. J Cell Physiol. 1991;146:1–7. doi: 10.1002/jcp.1041460102. [DOI] [PubMed] [Google Scholar]
- Emmert DA, Fee JA, Goeckeler ZM, Grojean JM, Wakatsuki T, Elson EL, et al. Rho-kinase-mediated Ca2+-independent contraction in rat embryo fibroblasts. Am J Physiol. 2004;286:C8–C21. doi: 10.1152/ajpcell.00428.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Ito M, Ichikawa K, Isaka N, Nishikawa M, Hartshorne DJ, et al. Inhibitory phosphorylation site for Rho-associated kinase on smooth muscle myosin phosphatase. J Biol Chem. 1999;274:37385–37390. doi: 10.1074/jbc.274.52.37385. [DOI] [PubMed] [Google Scholar]
- Frater-Schroder M, Muller G, Birchmeier W, Bohlen P. Transforming growth factor-β inhibits endothelial cell proliferation. Biochem Biophys Res Commun. 1986;137:295–302. doi: 10.1016/0006-291x(86)91209-x. [DOI] [PubMed] [Google Scholar]
- Frid MG, Kale VA, Stenmark KR. Mature vascular endothelium can give rise to smooth muscle cells via endothelial-mesenchymal transdifferentiation: in vitro analysis. Circ Res. 2002;90:1189–1196. doi: 10.1161/01.res.0000021432.70309.28. [DOI] [PubMed] [Google Scholar]
- Grainger DJ. Transforming growth factor β and atherosclerosis: so far, so good for the protective cytokine hypothesis. Arterioscler Thromb Vasc Biol. 2004;24:399–404. doi: 10.1161/01.ATV.0000114567.76772.33. [DOI] [PubMed] [Google Scholar]
- Hannan RL, Kourembanas S, Flanders KC, Rogelj SJ, Roberts AB, Faller DV, et al. Endothelial cells synthesize basic fibroblast growth factor and transforming growth factor β. Growth Factors. 1988;1:7–17. doi: 10.3109/08977198809000242. [DOI] [PubMed] [Google Scholar]
- Heimark RL, Twardzik DR, Schwartz SM. Inhibition of endothelial regeneration by type-β transforming growth factor from platelets. Science. 1986;233:1078–1080. doi: 10.1126/science.3461562. [DOI] [PubMed] [Google Scholar]
- Inoue N, Venema RC, Sayegh HS, Ohara Y, Murphy TJ, Harrison DG. Molecular regulation of the bovine endothelial cell nitric oxide synthase by transforming growth factor-β1. Arterioscler Thromb Vasc Biol. 1995;15:1255–1261. doi: 10.1161/01.atv.15.8.1255. [DOI] [PubMed] [Google Scholar]
- Ito M, Nakano T, Erdodi F, Hartshorne DJ. Myosin phosphatase: structure, regulation and function. Mol Cell Biochem. 2004;259:197–209. doi: 10.1023/b:mcbi.0000021373.14288.00. [DOI] [PubMed] [Google Scholar]
- Kanzaki T, Tamura K, Takahashi K, Saito Y, Akikusa B, Oohashi H, et al. In vivo effect of TGF-β1. Enhanced intimal thickening by administration of TGF-β1 in rabbit arteries injured with a balloon catheter. Arterioscler Thromb Vasc Biol. 1995;15:1951–1957. doi: 10.1161/01.atv.15.11.1951. [DOI] [PubMed] [Google Scholar]
- Kimura C, Cheng W, Hisadome K, Wang YP, Koyama T, Karashima Y, et al. Superoxide anion impairs contractility in cultured aortic smooth muscle cells. Am J Physiol. 2002;283:H382–H390. doi: 10.1152/ajpheart.00574.2001. [DOI] [PubMed] [Google Scholar]
- Kimura C, Koyama T, Oike M, Ito Y. Hypotonic stress-induced NO production in endothelium depends on endogenous ATP. Biochem Biophys Res Commun. 2000;274:736–740. doi: 10.1006/bbrc.2000.3205. [DOI] [PubMed] [Google Scholar]
- Kimura C, Oike M, Koyama T, Ito Y. Alterations of Ca2+ mobilizing properties in migrating endothelial cells. Am J Physiol. 2001a;281:H745–H754. doi: 10.1152/ajpheart.2001.281.2.H745. [DOI] [PubMed] [Google Scholar]
- Kimura C, Oike M, Koyama T, Ito Y. Impairment of endothelial nitric oxide production by acute glucose overload. Am J Physiol. 2001b;280:E171–E178. doi: 10.1152/ajpendo.2001.280.1.E171. [DOI] [PubMed] [Google Scholar]
- Kimura C, Oike M, Ohnaka K, Nose Y, Ito Y. Constitutive nitric oxide production in bovine aortic and brain microvascular endothelial cells: a comparative study. J Physiol (London) 2004;554:721–730. doi: 10.1113/jphysiol.2003.057059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen HL, Frangos JA. Role of cytoskeleton in shear stress-induced endothelial nitric oxide production. Am J Physiol. 1997;273:H347–H355. doi: 10.1152/ajpheart.1997.273.1.H347. [DOI] [PubMed] [Google Scholar]
- Kojima H, Nakatsubo N, Kikuchi K, Kawahara S, Kirino Y, Nagoshi H, et al. Detection and imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins. Anal Chem. 1998;70:2446–2453. doi: 10.1021/ac9801723. [DOI] [PubMed] [Google Scholar]
- Kolodney MS, Thimgan MS, Honda HM, Tsai G, Yee HF., Jr Ca2+-independent myosin II phosphorylation and contraction in chicken embryo fibroblasts. J Physiol (London) 1999;515:87–92. doi: 10.1111/j.1469-7793.1999.087ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama T, Oike M, Ito Y. Involvement of Rho-kinase and tyrosine kinase in hypotonic stress-induced ATP release in bovine aortic endothelial cells. J Physiol (London) 2001;532:759–769. doi: 10.1111/j.1469-7793.2001.0759e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizbai IA, Bauer H, Amberger A, Hennig B, Szabo H, Fuchs R, et al. Growth factor-induced morphological, physiological and molecular characteristics in cerebral endothelial cells. Eur J Cell Biol. 2000;79:594–600. doi: 10.1078/0171-9335-00084. [DOI] [PubMed] [Google Scholar]
- Mallat Z, Gojova A, Marchiol-Fournigault C, Esposito B, Kamate C, Merval R, et al. Inhibition of transforming growth factor-β signaling accelerates atherosclerosis and induces an unstable plaque phenotype in mice. Circ Res. 2001;89:930–934. doi: 10.1161/hh2201.099415. [DOI] [PubMed] [Google Scholar]
- McCaffrey TA. TGF-βs and TGF-β receptors in atherosclerosis. Cytokine Growth Factor Rev. 2000;11:103–114. doi: 10.1016/s1359-6101(99)00034-9. [DOI] [PubMed] [Google Scholar]
- Minshall RD, Sessa WC, Stan RV, Anderson RG, Malik AB. Caveolin regulation of endothelial function. Am J Physiol. 2003;285:L1179–L1183. doi: 10.1152/ajplung.00242.2003. [DOI] [PubMed] [Google Scholar]
- Myit S, Delafontaine P, Bochaton-Piallat ML, Giraud S, Gabbiani G, Brink M. Different growth properties of neointimal and medial smooth muscle cells in response to growth factors. J Vasc Res. 2003;40:97–104. doi: 10.1159/000070706. [DOI] [PubMed] [Google Scholar]
- Oike M, Kimura C, Koyama T, Yoshikawa M, Ito Y. Hypotonic stress-induced dual Ca2+ responses in bovine aortic endothelial cells. Am J Physiol. 2000;279:H630–H638. doi: 10.1152/ajpheart.2000.279.2.H630. [DOI] [PubMed] [Google Scholar]
- Paranya G, Vineberg S, Dvorin E, Kaushal S, Roth SJ, Rabkin E, et al. Aortic valve endothelial cells undergo transforming growth factor-β-mediated and non-transforming growth factor-β-mediated transdifferentiation in vitro. Am J Pathol. 2001;159:1335–1343. doi: 10.1016/s0002-9440(10)62520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfitzer G. Invited review: regulation of myosin phosphorylation in smooth muscle. J Appl Physiol. 2001;91:497–503. doi: 10.1152/jappl.2001.91.1.497. [DOI] [PubMed] [Google Scholar]
- Pollman MJ, Naumovski L, Gibbons GH. Vascular cell apoptosis: cell type-specific modulation by transforming growth factor-β1 in endothelial cells versus smooth muscle cells. Circulation. 1999;99:2019–2026. doi: 10.1161/01.cir.99.15.2019. [DOI] [PubMed] [Google Scholar]
- Poppa V, Miyashiro JK, Corson MA, Berk BC. Endothelial NO synthase is increased in regenerating endothelium after denuding injury of the rat aorta. Arterioscler Thromb Vasc Biol. 1998;18:1312–1321. doi: 10.1161/01.atv.18.8.1312. [DOI] [PubMed] [Google Scholar]
- Roberts AB, Sporn MB. Physiological actions and clinical applications of transforming growth factor-β (TGF-β) Growth Factors. 1993;8:1–9. doi: 10.3109/08977199309029129. [DOI] [PubMed] [Google Scholar]
- Robertson AK, Rudling M, Zhou X, Gorelik L, Flavell RA, Hansson GK. Disruption of TGF-β signaling in T cells accelerates atherosclerosis. J Clin Invest. 2003;112:1342–1350. doi: 10.1172/JCI18607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R, Raines EW, Bowen-Pope DF. The biology of platelet-derived growth factor. Cell. 1986;46:155–169. doi: 10.1016/0092-8674(86)90733-6. [DOI] [PubMed] [Google Scholar]
- Saura M, Zaragoza C, Cao W, Bao C, Rodriguez-Puyol M, Rodriguez-Puyol D, et al. Smad2 mediates transforming growth factor-β induction of endothelial nitric oxide synthase expression. Circ Res. 2002;91:806–813. doi: 10.1161/01.res.0000040397.23817.e5. [DOI] [PubMed] [Google Scholar]
- Schiffrin EL. Vascular endothelin in hypertension. Vascul Pharmacol. 2005;43:19–29. doi: 10.1016/j.vph.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Schwartz EA, Reaven E, Topper JN, Tsao PS. Transforming growth factor-β receptors localize to caveolae and regulate endothelial nitric oxide synthase in normal human endothelial cells. Biochem J. 2005;390:199–206. doi: 10.1042/BJ20041182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature. 1994;372:231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- Sutton AB, Canfield AE, Schor SL, Grant ME, Schor AM. The response of endothelial cells to TGF β-1 is dependent upon cell shape, proliferative state and the nature of the substratum. J Cell Sci. 1991;99:777–787. doi: 10.1242/jcs.99.4.777. [DOI] [PubMed] [Google Scholar]
- Tai SC, Robb GB, Marsden PA. Endothelial nitric oxide synthase: a new paradigm for gene regulation in the injured blood vessel. Arterioscler Thromb Vasc Biol. 2004;24:405–412. doi: 10.1161/01.ATV.0000109171.50229.33. [DOI] [PubMed] [Google Scholar]
- Verin AD, Wang P, Garcia JG. Immunochemical characterization of myosin-specific phosphatase 1 regulatory subunits in bovine endothelium. J Cell Biochem. 2000;76:489–498. [PubMed] [Google Scholar]
- Yue TL, Wang XK, Olson B, Feuerstein G. Interleukin-1β (IL-1β) induces transforming growth factor-β (TGF-β1) production by rat aortic smooth muscle cells. Biochem Biophys Res Commun. 1994;204:1186–1192. doi: 10.1006/bbrc.1994.2588. [DOI] [PubMed] [Google Scholar]