Abstract
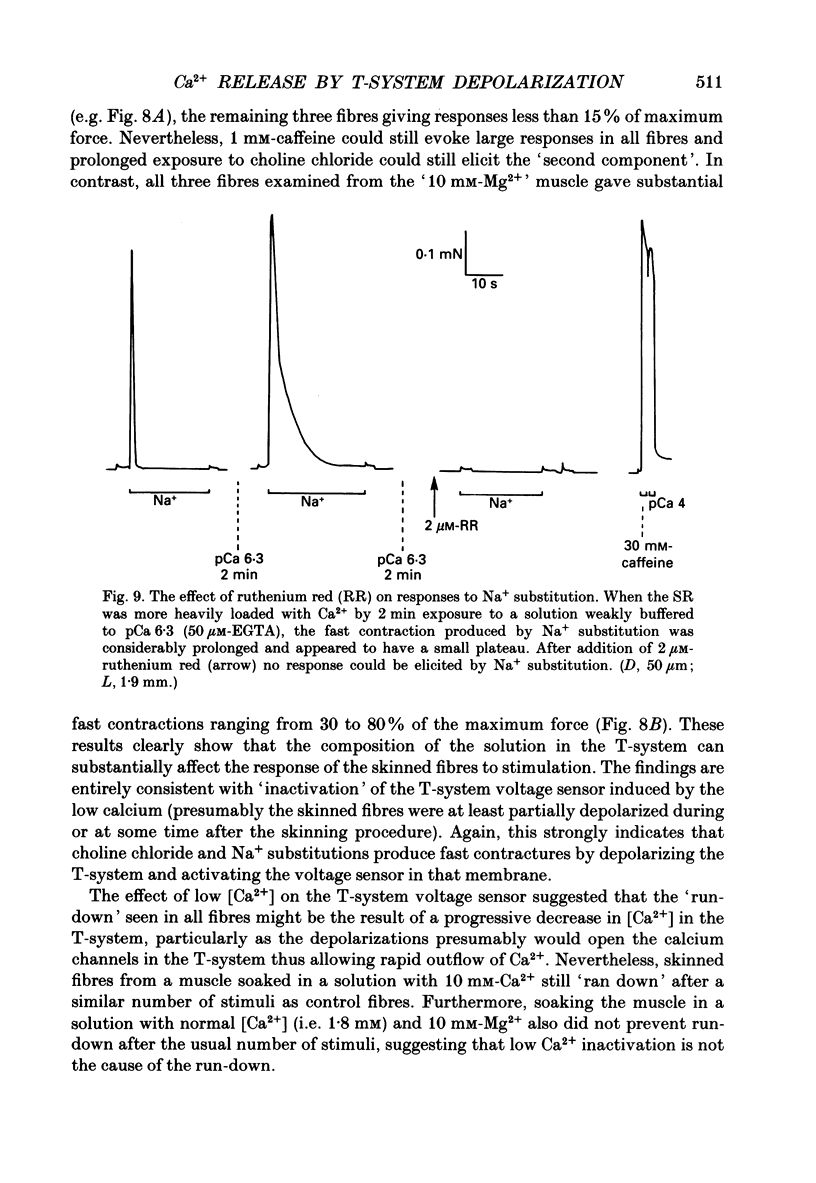
1. Skeletal muscle fibres from the toad were mechanically skinned under paraffin oil and then bathed in a potassium HDTA solution (HDTA: hexamethylenediamine-tetraacetate) which mimicked the ionic composition of the myoplasm. 2. Rapid transient contractions could be triggered by substitution of K+ with Na+ (with no change of anion), which should have virtually no direct effect on the electrical polarization of the sarcoplasmic reticulum (SR) membrane. Up to thirty or more contractions could be evoked by repeated substitutions if there was sufficient 'repriming' time (about 30 s) between them; these rapid contractions were analagous to potassium contractures in intact fibres. 3. When the SR was not heavily loaded, substitution of potassium HDTA with choline chloride also produced a rapid, brief contraction. 4. All treatments designed to 'inactivate' the voltage sensor in the T-system invariably abolished the rapid contractions. Thus, rapid contractions were absent if (i) the T-system was permanently depolarized by pre-soaking the muscle in a high potassium solution with ouabain before skinning, (ii) a fibre was split rather than skinned, (iii) the T-system was temporarily depolarized by Na+ substitution immediately before choline chloride substitution, or vice versa, (iv) a skinned fibre was briefly exposed to saponin (50 micrograms/ml) to selectively disrupt the T-system membrane or (v) the muscle was pre-soaked in a solution with 1 mM-EGTA and no Ca2+ or Mg2+ before skinning. In contrast to (v), if 10 mM-Mg2+ was present in the EGTA solution before skinning, rapid contractions could be elicited, presumably because the presence of Mg2+ prevented the inactivation of the T-system voltage sensor in low [Ca2+]. 5. These results unequivocally demonstrate that (a) the T-system reseals and repolarizes after mechanical skinning under oil and (b) the fast contractions are produced by activation of the voltage sensor in the T-system. 6. When the SR had been heavily loaded, choline chloride substitution (but not Na+ substitution) could also induce an unphysiological, slow contraction ('second component'). In total contrast to the fast contraction, this slow component was unaffected by any of the treatments (i-v) above, indicating that it did not depend on activation of the voltage sensor in the T-system but resulted from a direct action of choline chloride on the SR.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniu B., Kim D. H., Morii M., Ikemoto N. Inhibitors of Ca2+ release from the isolated sarcoplasmic reticulum. I. Ca2+ channel blockers. Biochim Biophys Acta. 1985 Jun 11;816(1):9–17. doi: 10.1016/0005-2736(85)90387-6. [DOI] [PubMed] [Google Scholar]
- Baylor S. M., Hollingworth S., Marshall M. W. Effects of intracellular ruthenium red on excitation-contraction coupling in intact frog skeletal muscle fibres. J Physiol. 1989 Jan;408:617–635. doi: 10.1113/jphysiol.1989.sp017480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brum G., Ríos E., Stéfani E. Effects of extracellular calcium on calcium movements of excitation-contraction coupling in frog skeletal muscle fibres. J Physiol. 1988 Apr;398:441–473. doi: 10.1113/jphysiol.1988.sp017052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo C., Bolaños P., Gonzalez G. F. Effect of membrane polarization on contractile threshold and time course of prolonged contractile responses in skeletal muscle fibers. J Gen Physiol. 1984 Dec;84(6):927–943. doi: 10.1085/jgp.84.6.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo C., Fernandez de Bolaños P. Membrane potential, contractile activation and relaxation rates in voltage clamped short muscle fibres of the frog. J Physiol. 1979 Apr;289:175–189. doi: 10.1113/jphysiol.1979.sp012731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Rakowski R. F., Schneider M. F. Effects of glycerol treatment and maintained depolarization on charge movement in skeletal muscle. J Physiol. 1976 Jan;254(2):285–316. doi: 10.1113/jphysiol.1976.sp011233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua M., Dulhunty A. F. Inactivation of excitation-contraction coupling in rat extensor digitorum longus and soleus muscles. J Gen Physiol. 1988 May;91(5):737–757. doi: 10.1085/jgp.91.5.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson S. K. Peeled mammalian skeletal muscle fibers. Possible stimulation of Ca2+ release via a transverse tubule-sarcoplasmic reticulum mechanism. J Gen Physiol. 1985 Oct;86(4):501–525. doi: 10.1085/jgp.86.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M., Iino M. Specific perforation of muscle cell membranes with preserved SR functions by saponin treatment. J Muscle Res Cell Motil. 1980 Mar;1(1):89–100. doi: 10.1007/BF00711927. [DOI] [PubMed] [Google Scholar]
- Fill M. D., Best P. M. Contractile activation and recovery in skinned frog muscle stimulated by ionic substitution. Am J Physiol. 1988 Jan;254(1 Pt 1):C107–C114. doi: 10.1152/ajpcell.1988.254.1.C107. [DOI] [PubMed] [Google Scholar]
- Fink R. H., Stephenson D. G., Williams D. A. Potassium and ionic strength effects on the isometric force of skinned twitch muscle fibres of the rat and toad. J Physiol. 1986 Jan;370:317–337. doi: 10.1113/jphysiol.1986.sp015937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia A. M., Miller C. Channel-mediated monovalent cation fluxes in isolated sarcoplasmic reticulum vesicles. J Gen Physiol. 1984 Jun;83(6):819–839. doi: 10.1085/jgp.83.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf F., Schatzmann H. J. Some effects of removal of external calcium on pig striated muscle. J Physiol. 1984 Apr;349:1–13. doi: 10.1113/jphysiol.1984.sp015138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. Potassium contractures in single muscle fibres. J Physiol. 1960 Sep;153:386–403. doi: 10.1113/jphysiol.1960.sp006541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kometani T., Kasai M. Ionic permeability of sarcoplasmic reticulum vesicles measured by light scattering method. J Membr Biol. 1978 Jul 18;41(4):295–308. doi: 10.1007/BF01871994. [DOI] [PubMed] [Google Scholar]
- Lamb G. D., Stephenson D. G. Control of calcium release and the effect of ryanodine in skinned muscle fibres of the toad. J Physiol. 1990 Apr;423:519–542. doi: 10.1113/jphysiol.1990.sp018037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattanzio F. A., Jr, Schlatterer R. G., Nicar M., Campbell K. P., Sutko J. L. The effects of ryanodine on passive calcium fluxes across sarcoplasmic reticulum membranes. J Biol Chem. 1987 Feb 25;262(6):2711–2718. [PubMed] [Google Scholar]
- Lüttgau H. C., Oetliker H. The action of caffeine on the activation of the contractile mechanism in straited muscle fibres. J Physiol. 1968 Jan;194(1):51–74. doi: 10.1113/jphysiol.1968.sp008394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüttgau H. C., Spiecker W. The effects of calcium deprivation upon mechanical and electrophysiological parameters in skeletal muscle fibres of the frog. J Physiol. 1979 Nov;296:411–429. doi: 10.1113/jphysiol.1979.sp013013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinley D., Meissner G. Evidence for a K+, Na+ permeable channel in sarcoplasmic reticulum. J Membr Biol. 1978 Dec 15;44(2):159–186. doi: 10.1007/BF01976037. [DOI] [PubMed] [Google Scholar]
- Moisescu D. G. Kinetics of reaction in calcium-activated skinned muscle fibres. Nature. 1976 Aug 12;262(5569):610–613. doi: 10.1038/262610a0. [DOI] [PubMed] [Google Scholar]
- Nakajima Y., Endo M. Release of calcium induced by 'depolarisation' of the sarcoplasmic reticulum membrane. Nat New Biol. 1973 Dec 19;246(155):216–218. doi: 10.1038/newbio246216a0. [DOI] [PubMed] [Google Scholar]
- Rousseau E., Ladine J., Liu Q. Y., Meissner G. Activation of the Ca2+ release channel of skeletal muscle sarcoplasmic reticulum by caffeine and related compounds. Arch Biochem Biophys. 1988 Nov 15;267(1):75–86. doi: 10.1016/0003-9861(88)90010-0. [DOI] [PubMed] [Google Scholar]
- Schneider M. F., Chandler W. K. Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature. 1973 Mar 23;242(5395):244–246. doi: 10.1038/242244a0. [DOI] [PubMed] [Google Scholar]
- Smith J. S., Imagawa T., Ma J., Fill M., Campbell K. P., Coronado R. Purified ryanodine receptor from rabbit skeletal muscle is the calcium-release channel of sarcoplasmic reticulum. J Gen Physiol. 1988 Jul;92(1):1–26. doi: 10.1085/jgp.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson D. G., Williams D. A. Calcium-activated force responses in fast- and slow-twitch skinned muscle fibres of the rat at different temperatures. J Physiol. 1981 Aug;317:281–302. doi: 10.1113/jphysiol.1981.sp013825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson E. W. Activation of fast skeletal muscle: contributions of studies on skinned fibers. Am J Physiol. 1981 Jan;240(1):C1–19. doi: 10.1152/ajpcell.1981.240.1.C1. [DOI] [PubMed] [Google Scholar]
- Stephenson E. W. Excitation of skinned muscle fibers by imposed ion gradients. I. Stimulation of 45Ca efflux at constant [K][Cl] product. J Gen Physiol. 1985 Dec;86(6):813–832. doi: 10.1085/jgp.86.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara J., Tsien R. Y., Delay M. Inositol 1,4,5-trisphosphate: a possible chemical link in excitation-contraction coupling in muscle. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6352–6356. doi: 10.1073/pnas.82.18.6352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe P., Stephenson E. W. Ca2+ dependence of transverse tubule-mediated calcium release in skinned skeletal muscle fibers. J Gen Physiol. 1986 Feb;87(2):271–288. doi: 10.1085/jgp.87.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]


