Abstract
The NRT2.1 gene of Arabidopsis thaliana encodes a major component of the root high-affinity 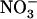 transport system (HATS) that plays a crucial role in
transport system (HATS) that plays a crucial role in 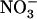 uptake by the plant. Although NRT2.1 was known to be induced by
uptake by the plant. Although NRT2.1 was known to be induced by 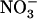 and feedback repressed by reduced nitrogen (N) metabolites, NRT2.1 is surprisingly up-regulated when
and feedback repressed by reduced nitrogen (N) metabolites, NRT2.1 is surprisingly up-regulated when 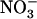 concentration decreases to a low level (<0.5 mm) in media containing a high concentration of
concentration decreases to a low level (<0.5 mm) in media containing a high concentration of 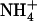 or Gln (≥1 mm). The NRT3.1 gene, encoding another key component of the HATS, displays the same response pattern. This revealed that both NRT2.1 and NRT3.1 are coordinately down-regulated by high external
or Gln (≥1 mm). The NRT3.1 gene, encoding another key component of the HATS, displays the same response pattern. This revealed that both NRT2.1 and NRT3.1 are coordinately down-regulated by high external 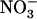 availability through a mechanism independent from that involving N metabolites. We show here that repression of both genes by high
availability through a mechanism independent from that involving N metabolites. We show here that repression of both genes by high 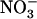 is specifically mediated by the NRT1.1
is specifically mediated by the NRT1.1 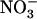 transporter. This mechanism warrants that either NRT1.1 or NRT2.1 is active in taking up
transporter. This mechanism warrants that either NRT1.1 or NRT2.1 is active in taking up 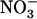 in the presence of a reduced N source. Under low
in the presence of a reduced N source. Under low  provision, NRT1.1-mediated repression of NRT2.1/NRT3.1 is relieved, which allows reactivation of the HATS. Analysis of atnrt2.1 mutants showed that this constitutes a crucial adaptive response against
provision, NRT1.1-mediated repression of NRT2.1/NRT3.1 is relieved, which allows reactivation of the HATS. Analysis of atnrt2.1 mutants showed that this constitutes a crucial adaptive response against 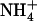 toxicity because
toxicity because 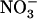 taken up by the HATS in this situation prevents the detrimental effects of pure
taken up by the HATS in this situation prevents the detrimental effects of pure 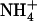 nutrition. It is thus hypothesized that NRT1.1-mediated regulation of NRT2.1/NRT3.1 is a mechanism aiming to satisfy a specific
nutrition. It is thus hypothesized that NRT1.1-mediated regulation of NRT2.1/NRT3.1 is a mechanism aiming to satisfy a specific 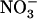 demand of the plant in relation to the various specific roles that
demand of the plant in relation to the various specific roles that 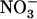 plays, in addition to being a N source. A new model is proposed for regulation of the HATS, involving both feedback repression by N metabolites and NRT1.1-mediated repression by high
plays, in addition to being a N source. A new model is proposed for regulation of the HATS, involving both feedback repression by N metabolites and NRT1.1-mediated repression by high 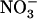 .
.
Higher plants acquire mineral nitrogen (N) from the soil mainly in the form of 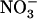 , through the activity of both high-affinity transport systems (HATS) and low-affinity transport systems (LATS), respectively (Crawford and Glass, 1998; von Wirén et al., 2000). Except in agricultural soils after fertilizer application, where
, through the activity of both high-affinity transport systems (HATS) and low-affinity transport systems (LATS), respectively (Crawford and Glass, 1998; von Wirén et al., 2000). Except in agricultural soils after fertilizer application, where 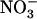 concentration can rise up several millimolar (Crawford and Glass, 1998), it is generally assumed that root
concentration can rise up several millimolar (Crawford and Glass, 1998), it is generally assumed that root 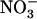 uptake is mostly determined by the activity of the HATS (Crawford and Glass, 1998; von Wirén et al., 2000; Malagoli et al., 2004). The current model of the
uptake is mostly determined by the activity of the HATS (Crawford and Glass, 1998; von Wirén et al., 2000; Malagoli et al., 2004). The current model of the 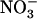 HATS is constituted by at least two genetically separate transport systems: (1) a constitutive HATS present even in the absence of
HATS is constituted by at least two genetically separate transport systems: (1) a constitutive HATS present even in the absence of 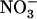 (Wang and Crawford, 1996; Crawford and Glass, 1998); and (2) an inducible HATS (iHATS), quantitatively more important than the constitutive HATS, and activated within hours after
(Wang and Crawford, 1996; Crawford and Glass, 1998); and (2) an inducible HATS (iHATS), quantitatively more important than the constitutive HATS, and activated within hours after 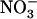 provision to the plant (Behl et al., 1988; Mackown and McClure, 1988; Crawford and Glass, 1998; Daniel-Vedele et al., 1998; Forde, 2000).
provision to the plant (Behl et al., 1988; Mackown and McClure, 1988; Crawford and Glass, 1998; Daniel-Vedele et al., 1998; Forde, 2000).
In Arabidopsis (Arabidopsis thaliana), two gene families have been found to encode transporter proteins involved in root  uptake (Forde, 2000; Williams and Miller, 2001; Orsel et al., 2002; Okamoto et al., 2003): the NRT2 family (seven members) and the NRT1 family, belonging to the large PTR family of transporter genes (53 members). Convincing evidence has accumulated that the
uptake (Forde, 2000; Williams and Miller, 2001; Orsel et al., 2002; Okamoto et al., 2003): the NRT2 family (seven members) and the NRT1 family, belonging to the large PTR family of transporter genes (53 members). Convincing evidence has accumulated that the 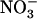 -inducible NRT2.1 gene encodes a major component of the iHATS (Filleur and Daniel-Vedele, 1999; Lejay et al., 1999; Zhuo et al., 1999). First, atnrt2.1 mutants display a strong reduction of root
-inducible NRT2.1 gene encodes a major component of the iHATS (Filleur and Daniel-Vedele, 1999; Lejay et al., 1999; Zhuo et al., 1999). First, atnrt2.1 mutants display a strong reduction of root 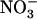 influx in the low external
influx in the low external 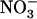 concentration range (i.e. below 1 mm; Filleur et al., 2001; Orsel et al., 2004). Second,
concentration range (i.e. below 1 mm; Filleur et al., 2001; Orsel et al., 2004). Second, 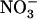 inducibility of high-affinity root
inducibility of high-affinity root 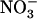 uptake is suppressed by NRT2.1 deletion (Cerezo et al., 2001). Recently, another key component of the
uptake is suppressed by NRT2.1 deletion (Cerezo et al., 2001). Recently, another key component of the 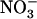 HATS has been identified in the form of the product of the NAR2-like Arabidopsis gene NRT3.1 (Okamoto et al., 2006). It is suspected that interaction between NRT2.1 and NRT3.1 is required for functionality of the iHATS because NRT2 proteins need to be coexpressed with NAR2-like proteins to generate
HATS has been identified in the form of the product of the NAR2-like Arabidopsis gene NRT3.1 (Okamoto et al., 2006). It is suspected that interaction between NRT2.1 and NRT3.1 is required for functionality of the iHATS because NRT2 proteins need to be coexpressed with NAR2-like proteins to generate 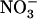 uptake activity in Xenopus oocytes (Quesada and Fernandez, 1994; Zhou et al., 2000; Tong et al., 2005).
uptake activity in Xenopus oocytes (Quesada and Fernandez, 1994; Zhou et al., 2000; Tong et al., 2005).
Besides induction by 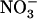 , NRT2.1 expression and
, NRT2.1 expression and 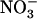 HATS activity are also feedback repressed by reduced N metabolites, such as
HATS activity are also feedback repressed by reduced N metabolites, such as 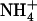 and amino acids (Lejay et al., 1999; Zhuo et al., 1999; Nazoa et al., 2003). This regulation involves systemic signaling (Gansel et al., 2001) and ensures the specific control of root
and amino acids (Lejay et al., 1999; Zhuo et al., 1999; Nazoa et al., 2003). This regulation involves systemic signaling (Gansel et al., 2001) and ensures the specific control of root 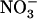 uptake by the N status of the whole plant (Imsande and Touraine, 1994; Forde, 2002). Indeed, N sufficiency triggers the repression exerted by N metabolites and down-regulates the HATS, whereas N deprivation has the opposite effect (Crawford and Glass, 1998; Lejay et al., 1999; Zhuo et al., 1999; Gansel et al., 2001). Again, NRT2.1 plays a key role in these processes because both repression of
uptake by the N status of the whole plant (Imsande and Touraine, 1994; Forde, 2002). Indeed, N sufficiency triggers the repression exerted by N metabolites and down-regulates the HATS, whereas N deprivation has the opposite effect (Crawford and Glass, 1998; Lejay et al., 1999; Zhuo et al., 1999; Gansel et al., 2001). Again, NRT2.1 plays a key role in these processes because both repression of 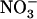 HATS by N metabolites and its stimulation by N starvation are suppressed in the atnrt2.1-1 mutant (Cerezo et al., 2001). Another important aspect of NRT2.1 function relates to the recent reports showing that it is not only involved in high-affinity
HATS by N metabolites and its stimulation by N starvation are suppressed in the atnrt2.1-1 mutant (Cerezo et al., 2001). Another important aspect of NRT2.1 function relates to the recent reports showing that it is not only involved in high-affinity 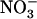 uptake, but also plays a
uptake, but also plays a 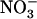 -sensing role in the regulation of lateral root initiation (Little et al., 2005; Remans et al., 2006). This latter role is still unclear because, depending on the conditions, NRT2.1 either represses (Little et al., 2005) or, on the contrary, stimulates (Remans et al., 2006) lateral root development.
-sensing role in the regulation of lateral root initiation (Little et al., 2005; Remans et al., 2006). This latter role is still unclear because, depending on the conditions, NRT2.1 either represses (Little et al., 2005) or, on the contrary, stimulates (Remans et al., 2006) lateral root development.
Recently, investigation of several chl1 mutants of Arabidopsis unexpectedly revealed that disruption of another  transporter gene, NRT1.1 (formerly CHL1), results in a major alteration of the regulation of NRT2.1 expression by the N status of the plant (Muños et al., 2004). First, feedback repression of NRT2.1 by either
transporter gene, NRT1.1 (formerly CHL1), results in a major alteration of the regulation of NRT2.1 expression by the N status of the plant (Muños et al., 2004). First, feedback repression of NRT2.1 by either 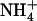 or Gln supply is suppressed or strongly attenuated in chl1 mutants compared with wild type, resulting in its overexpression in normally suppressive conditions (e.g. in NH4NO3-fed chl1 plants). Second, expression of NRT2.1 is no more stimulated by N starvation in chl1 mutants. These data suggest that mutation of NRT1.1 blocks both NRT2.1 expression and
or Gln supply is suppressed or strongly attenuated in chl1 mutants compared with wild type, resulting in its overexpression in normally suppressive conditions (e.g. in NH4NO3-fed chl1 plants). Second, expression of NRT2.1 is no more stimulated by N starvation in chl1 mutants. These data suggest that mutation of NRT1.1 blocks both NRT2.1 expression and 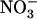 HATS activity in some kind of derepressed state, making chl1 mutants the only known genotypes affected in the regulation of root
HATS activity in some kind of derepressed state, making chl1 mutants the only known genotypes affected in the regulation of root 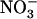 uptake in higher plants.
uptake in higher plants.
NRT1.1 is an unusual dual-affinity 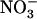 transporter (Tsay et al., 1993; Wang et al., 1998), shifting from low to high affinity in response to phosphorylation of the Thr-101 residue (Liu and Tsay, 2003). Although NRT1.1 also contributes to root
transporter (Tsay et al., 1993; Wang et al., 1998), shifting from low to high affinity in response to phosphorylation of the Thr-101 residue (Liu and Tsay, 2003). Although NRT1.1 also contributes to root 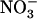 uptake (Tsay et al., 1993; Huang et al., 1996; Touraine and Glass, 1997), its precise role in the regulation of NRT2.1 expression remains unclear. A first hypothesis was that NRT1.1 may be directly involved in the signaling pathway responsible for feedback repression of NRT2.1 by N metabolites. However, this interpretation was questioned by the finding that repression of NRT2.1 by
uptake (Tsay et al., 1993; Huang et al., 1996; Touraine and Glass, 1997), its precise role in the regulation of NRT2.1 expression remains unclear. A first hypothesis was that NRT1.1 may be directly involved in the signaling pathway responsible for feedback repression of NRT2.1 by N metabolites. However, this interpretation was questioned by the finding that repression of NRT2.1 by  could also be alleviated in wild-type plants when the external
could also be alleviated in wild-type plants when the external 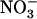 concentration was decreased down to 0.1 mm in the presence of 1 mm
concentration was decreased down to 0.1 mm in the presence of 1 mm 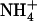 (Muños et al., 2004). This showed that mutation of NRT1.1 is not required to suppress the repressive effect of N metabolites and led to a second alternative hypothesis that NRT2.1 up-regulation in NH4NO3-fed chl1 plants could actually be due to the lowered
(Muños et al., 2004). This showed that mutation of NRT1.1 is not required to suppress the repressive effect of N metabolites and led to a second alternative hypothesis that NRT2.1 up-regulation in NH4NO3-fed chl1 plants could actually be due to the lowered  uptake resulting from NRT1.1 mutation. Because NH4NO3-fed chl1 plants were not N deficient, this in turn suggested that NRT2.1 expression is not only repressed by reduced N metabolites, but also by
uptake resulting from NRT1.1 mutation. Because NH4NO3-fed chl1 plants were not N deficient, this in turn suggested that NRT2.1 expression is not only repressed by reduced N metabolites, but also by 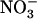 itself (Muños et al., 2004).
itself (Muños et al., 2004).
This work had three aims: (1) to investigate the occurrence of such repression of NRT2.1 by 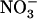 in wild-type plants; (2) to determine whether NRT1.1 plays a direct (i.e. specific) or indirect (through modulation of
in wild-type plants; (2) to determine whether NRT1.1 plays a direct (i.e. specific) or indirect (through modulation of 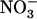 uptake rate) role in triggering this repression; and (3) to determine the physiological role of this regulation. Concerning this last point, we hypothesized that repression of NRT2.1 by
uptake rate) role in triggering this repression; and (3) to determine the physiological role of this regulation. Concerning this last point, we hypothesized that repression of NRT2.1 by 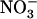 may correspond to a mechanism allowing the HATS to be stimulated by a specific
may correspond to a mechanism allowing the HATS to be stimulated by a specific  demand of the plant independently of its overall N status (Muños et al., 2004). In particular, we anticipated that this may have a crucial function to avoid that the bulk of N acquisition from mixed NH4NO3 medium is made through
demand of the plant independently of its overall N status (Muños et al., 2004). In particular, we anticipated that this may have a crucial function to avoid that the bulk of N acquisition from mixed NH4NO3 medium is made through 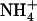 uptake, thus protecting the plant against the toxicity generally associated with
uptake, thus protecting the plant against the toxicity generally associated with 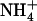 nutrition (Givan, 1979; Hageman, 1984; Salsac et al., 1987; Kronzucker et al., 2001).
nutrition (Givan, 1979; Hageman, 1984; Salsac et al., 1987; Kronzucker et al., 2001).
RESULTS
Low NO3− Availability in the Presence of NH4+ Up-Regulates Both Root NRT2.1 Expression and NO3− HATS Activity
As commonly observed in many experimental conditions, the supply of  together with
together with 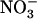 at equimolar concentration (1 mm for both ions) is associated with very faint expression of NRT2.1 in the roots (Fig. 1A, lane T0). However, transfer of plants to fresh nutrient medium with lower
at equimolar concentration (1 mm for both ions) is associated with very faint expression of NRT2.1 in the roots (Fig. 1A, lane T0). However, transfer of plants to fresh nutrient medium with lower 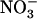 concentration (0.1 mm), but unmodified
concentration (0.1 mm), but unmodified 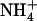 availability (1 mm), rapidly resulted in a marked increase in NRT2.1 mRNA accumulation, which was detectable after 6 h and leveled off between 3 and 4 d (Fig. 1A).
availability (1 mm), rapidly resulted in a marked increase in NRT2.1 mRNA accumulation, which was detectable after 6 h and leveled off between 3 and 4 d (Fig. 1A).
Figure 1.
Changes in NRT2.1 mRNA accumulation in response to various 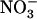 /
/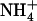 mixtures in the external medium. Plants were grown hydroponically for 6 weeks on complete nutrient solution containing 1 mm NH4NO3 before transfer to the various media indicated in the figure. A, Time-course response in plants transferred to nutrient solution containing 1 mm
mixtures in the external medium. Plants were grown hydroponically for 6 weeks on complete nutrient solution containing 1 mm NH4NO3 before transfer to the various media indicated in the figure. A, Time-course response in plants transferred to nutrient solution containing 1 mm 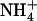 plus 0.1 mm
plus 0.1 mm  . B, Effect of transfer for 4 d to media containing various combinations of
. B, Effect of transfer for 4 d to media containing various combinations of 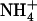 and
and 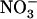 concentrations. Transcript accumulation was monitored in roots both by northern-blot analysis (gel-blot images) and real-time RT-PCR (bar graphs) on the same samples. A 25S rRNA signal is presented as a loading control for northern blots. Quantification by real-time RT-PCR was normalized using actin genes as controls. Errors bars represent se (n = 3). Different letters on top of the bars indicate statistically significant differences (P < 0.05; t test).
concentrations. Transcript accumulation was monitored in roots both by northern-blot analysis (gel-blot images) and real-time RT-PCR (bar graphs) on the same samples. A 25S rRNA signal is presented as a loading control for northern blots. Quantification by real-time RT-PCR was normalized using actin genes as controls. Errors bars represent se (n = 3). Different letters on top of the bars indicate statistically significant differences (P < 0.05; t test).
To identify more precisely which parameter modified by the above treatment ( concentration, total
concentration, total 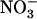 plus
plus 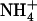 concentration, or
concentration, or  to
to 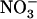 ratio) was responsible for the up-regulation of NRT2.1 expression, we explored the effects of various combinations of
ratio) was responsible for the up-regulation of NRT2.1 expression, we explored the effects of various combinations of 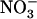 and
and 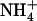 concentrations. Figure 1B displays typical results obtained from this series of experiments. They show that the increase of NRT2.1 transcript accumulation is not due to the lowering of the total external N (
concentrations. Figure 1B displays typical results obtained from this series of experiments. They show that the increase of NRT2.1 transcript accumulation is not due to the lowering of the total external N (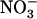 +
+ 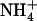 ) concentration because it was also observed after transfer to solutions containing 0.1 mm
) concentration because it was also observed after transfer to solutions containing 0.1 mm 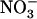 supplemented with 2 mm, or even 5 mm
supplemented with 2 mm, or even 5 mm 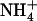 . On the other hand, a high
. On the other hand, a high 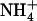 to
to 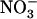 ratio in the external medium is also not responsible per se for up-regulation of NRT2.1 expression because, when compared to 1:0.1 mm
ratio in the external medium is also not responsible per se for up-regulation of NRT2.1 expression because, when compared to 1:0.1 mm 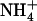 :
: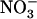 , the 5:0.5 mm
, the 5:0.5 mm 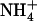 :
: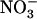 mixture (with the same
mixture (with the same 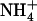 :
: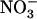 ratio of 10) resulted in much less pronounced NRT2.1 up-regulation (Fig. 1B). Furthermore, NRT2.1 is also overexpressed by low
ratio of 10) resulted in much less pronounced NRT2.1 up-regulation (Fig. 1B). Furthermore, NRT2.1 is also overexpressed by low 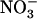 availability in mixed N medium when Gln (another potent repressor of NRT2.1 expression) is provided as the reduced N source (Supplemental Fig. S1). This shows that NRT2.1 up-regulation is not specifically due to excess
availability in mixed N medium when Gln (another potent repressor of NRT2.1 expression) is provided as the reduced N source (Supplemental Fig. S1). This shows that NRT2.1 up-regulation is not specifically due to excess 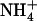 supply. Altogether, the data obtained suggest that NRT2.1 expression is responsive to the absolute
supply. Altogether, the data obtained suggest that NRT2.1 expression is responsive to the absolute  concentration present in the mixed N solution. Indeed, whatever the nature and concentration of the reduced N source (
concentration present in the mixed N solution. Indeed, whatever the nature and concentration of the reduced N source (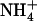 or Gln, 1–5 mm), the NRT2.1 transcript level was always high when the external
or Gln, 1–5 mm), the NRT2.1 transcript level was always high when the external  concentration remained low at 0.1 mm, but strongly decreased when the
concentration remained low at 0.1 mm, but strongly decreased when the  concentration was raised at 0.5 mm.
concentration was raised at 0.5 mm.
One interesting finding related to the increased expression of NRT2.1 under low 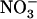 /high
/high 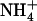 availability is that the effect is local and not systemic. Indeed, split-root experiments showed that when only one side of the root system is provided with 0.1 mm
availability is that the effect is local and not systemic. Indeed, split-root experiments showed that when only one side of the root system is provided with 0.1 mm 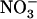 + 1 mm
+ 1 mm 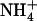 , NRT2.1 is up-regulated in this side only and not in the other one fed with 1 mm NH4NO3 (Fig. 2, compare lanes b and c). Furthermore, the NRT2.1 transcript level in the side of the split-root system supplied with 0.1 mm
, NRT2.1 is up-regulated in this side only and not in the other one fed with 1 mm NH4NO3 (Fig. 2, compare lanes b and c). Furthermore, the NRT2.1 transcript level in the side of the split-root system supplied with 0.1 mm  + 1 mm
+ 1 mm 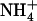 (Fig. 2, lanes c and e) was the same, whatever the
(Fig. 2, lanes c and e) was the same, whatever the 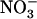 availability on the other side (1 or 0 mm in Fig. 2, lanes b and f, respectively), and equaled that in plants homogeneously supplied with 0.1 mm
availability on the other side (1 or 0 mm in Fig. 2, lanes b and f, respectively), and equaled that in plants homogeneously supplied with 0.1 mm 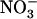 + 1 mm
+ 1 mm 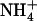 on the whole root system (Fig. 2, lane d).
on the whole root system (Fig. 2, lane d).
Figure 2.
Effect of local changes in the 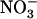 /
/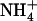 external balance on NRT2.1 mRNA accumulation in split-root plants. A, Representation of the experimental protocol. Plants were grown hydroponically for 5 weeks on complete nutrient solution containing 1 mm NH4NO3. After separation of their root system into two approximately equal sides, plants were transferred to specific containers and left undisturbed during 3 d in the 1 mm NH4NO3 solution before application of localized supplies with different
external balance on NRT2.1 mRNA accumulation in split-root plants. A, Representation of the experimental protocol. Plants were grown hydroponically for 5 weeks on complete nutrient solution containing 1 mm NH4NO3. After separation of their root system into two approximately equal sides, plants were transferred to specific containers and left undisturbed during 3 d in the 1 mm NH4NO3 solution before application of localized supplies with different 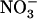 /
/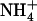 mixtures for 4 d, as indicated in the figure. B, NRT2.1 mRNA accumulation. Transcript accumulation was monitored in various sides of the split-root systems both by northern-blot analysis (gel-blot images) and real-time RT-PCR (bar graphs) on the same samples. The lane labels relate to the various localized treatments as shown in A. A 25S rRNA signal is presented as a loading control for northern blots. Quantification by real-time RT-PCR was normalized using actin genes as controls. Errors bars represent se (n = 3). Different letters on top of the bars indicate statistically significant differences (P < 0.05; t test).
mixtures for 4 d, as indicated in the figure. B, NRT2.1 mRNA accumulation. Transcript accumulation was monitored in various sides of the split-root systems both by northern-blot analysis (gel-blot images) and real-time RT-PCR (bar graphs) on the same samples. The lane labels relate to the various localized treatments as shown in A. A 25S rRNA signal is presented as a loading control for northern blots. Quantification by real-time RT-PCR was normalized using actin genes as controls. Errors bars represent se (n = 3). Different letters on top of the bars indicate statistically significant differences (P < 0.05; t test).
In wild-type plants, up-regulation of NRT2.1 expression by low 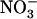 availability in the presence of
availability in the presence of 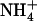 resulted in similar stimulation of
resulted in similar stimulation of 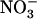 HATS activity (as measured by root 15
HATS activity (as measured by root 15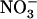 influx at 0.2 mm), which was not observed in the atnrt2.1-1 mutant (Fig. 3A). Interestingly, the
influx at 0.2 mm), which was not observed in the atnrt2.1-1 mutant (Fig. 3A). Interestingly, the 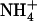 uptake system did not display this stimulation because root 15
uptake system did not display this stimulation because root 15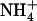 influx measured either at 1 mm (Fig. 3B) or 0.2 mm (data not shown) was only marginally increased upon lowering of the external
influx measured either at 1 mm (Fig. 3B) or 0.2 mm (data not shown) was only marginally increased upon lowering of the external 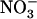 concentration. Total N content of roots and shoots was unaffected between the treatments (data not shown), most probably as a result of both increased
concentration. Total N content of roots and shoots was unaffected between the treatments (data not shown), most probably as a result of both increased 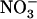 HATS activity and sustained
HATS activity and sustained  uptake.
uptake.
Figure 3.
Effect of various 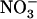 /
/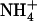 mixtures on NRT2.1 mRNA accumulation and root N uptake in Ws wild-type and atnrt2.1-1 mutant plants. The plants were grown hydroponically for 6 weeks on complete nutrient solution containing 1 mm NH4NO3 before transfer for 4 d to various media containing the same
mixtures on NRT2.1 mRNA accumulation and root N uptake in Ws wild-type and atnrt2.1-1 mutant plants. The plants were grown hydroponically for 6 weeks on complete nutrient solution containing 1 mm NH4NO3 before transfer for 4 d to various media containing the same 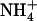 concentration (1 mm), but various concentrations of
concentration (1 mm), but various concentrations of  (0.1, 1, or 10 mm). A, NRT2.1 mRNA accumulation and root 15
(0.1, 1, or 10 mm). A, NRT2.1 mRNA accumulation and root 15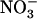 influx. Transcript accumulation was monitored in roots both by northern-blot analysis (gel-blot images) and real-time RT-PCR (bar graphs) on the same samples. A 25S rRNA signal is presented as a loading control for northern blots. Quantification by real-time RT-PCR was normalized using actin genes as controls. Errors bars represent se (n = 3). Root 15
influx. Transcript accumulation was monitored in roots both by northern-blot analysis (gel-blot images) and real-time RT-PCR (bar graphs) on the same samples. A 25S rRNA signal is presented as a loading control for northern blots. Quantification by real-time RT-PCR was normalized using actin genes as controls. Errors bars represent se (n = 3). Root 15 influx was assayed by 5-min labeling at 0.2 mm external 15
influx was assayed by 5-min labeling at 0.2 mm external 15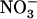 concentration to specifically determine activity of the HATS. Each value is the mean of eight to 12 replicates ± se. B, Root 15
concentration to specifically determine activity of the HATS. Each value is the mean of eight to 12 replicates ± se. B, Root 15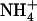 influx. Root 15
influx. Root 15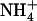 influx was assayed by 5-min labeling at 1 mm external 15
influx was assayed by 5-min labeling at 1 mm external 15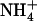 concentration to determine the uptake activity at the same concentration as for growth and treatment of the plants. Each value is the mean of eight to 12 replicates ± se. N.D., Not determined. Different letters on top of the bars indicate statistically significant differences (P < 0.05; t test).
concentration to determine the uptake activity at the same concentration as for growth and treatment of the plants. Each value is the mean of eight to 12 replicates ± se. N.D., Not determined. Different letters on top of the bars indicate statistically significant differences (P < 0.05; t test).
Collectively, the above results suggest that low 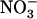 availability alleviates repression of both NRT2.1 expression and
availability alleviates repression of both NRT2.1 expression and 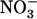 HATS activity triggered by provision of a reduced N source to the plant.
HATS activity triggered by provision of a reduced N source to the plant.
Regulation of NRT2.1 Expression by External NO3− Availability in the Presence of NH4+ Is Specifically Mediated by NRT1.1
In previous experiments with chl1-10 plants, it was observed that increasing 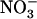 concentration from 0.1 to 10 mm in the presence of 1 mm
concentration from 0.1 to 10 mm in the presence of 1 mm 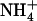 failed to repress NRT2.1 expression (Muños et al., 2004), unlike what is found in wild type (Fig. 3A). To determine whether this is due to a direct or an indirect (through lowered total
failed to repress NRT2.1 expression (Muños et al., 2004), unlike what is found in wild type (Fig. 3A). To determine whether this is due to a direct or an indirect (through lowered total 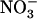 uptake) role of NRT1.1 in regulating NRT2.1 expression, we checked whether the mutation of NRT1.2, encoding another important component of the
uptake) role of NRT1.1 in regulating NRT2.1 expression, we checked whether the mutation of NRT1.2, encoding another important component of the 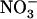 low-affinity transport system (Huang et al., 1999), also resulted in NRT2.1 up-regulation. Therefore, we investigated the quantitative relationship between
low-affinity transport system (Huang et al., 1999), also resulted in NRT2.1 up-regulation. Therefore, we investigated the quantitative relationship between 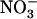 uptake from NH4NO3 medium and NRT2.1 expression in the roots of three genotypes: the Wassilewskija (Ws) wild type, the chl1-10 mutant, and the atnrt1.2-1 mutant, carrying a T-DNA insertion in the NRT1.2 gene.
uptake from NH4NO3 medium and NRT2.1 expression in the roots of three genotypes: the Wassilewskija (Ws) wild type, the chl1-10 mutant, and the atnrt1.2-1 mutant, carrying a T-DNA insertion in the NRT1.2 gene.
Increasing the external 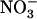 concentration from 0.1 to 5 or 10 mm in the presence of 1 mm
concentration from 0.1 to 5 or 10 mm in the presence of 1 mm 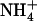 resulted in a strong and almost similar down-regulation of NRT2.1 expression in both Ws and atnr1.2-1 plants, whereas this down-regulation was absent in chl1-10 plants (Fig. 4A). Surprisingly, root 15
resulted in a strong and almost similar down-regulation of NRT2.1 expression in both Ws and atnr1.2-1 plants, whereas this down-regulation was absent in chl1-10 plants (Fig. 4A). Surprisingly, root 15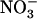 influx (measured at the same concentration as that used for treatment of the plants) was little affected by external
influx (measured at the same concentration as that used for treatment of the plants) was little affected by external 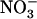 concentration in Ws plants (Fig. 4B), where a 50-fold decrease of this concentration (from 5–0.1 mm) only resulted in a 30% slowing down of root 15
concentration in Ws plants (Fig. 4B), where a 50-fold decrease of this concentration (from 5–0.1 mm) only resulted in a 30% slowing down of root 15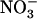 influx. This indicates strong homeostasis of root
influx. This indicates strong homeostasis of root 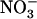 uptake from NH4NO3 medium, which probably explains why root
uptake from NH4NO3 medium, which probably explains why root 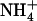 influx was only marginally stimulated by the decrease in external
influx was only marginally stimulated by the decrease in external 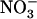 concentration (Fig. 3B). Root 15
concentration (Fig. 3B). Root 15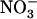 influx was lower in the atnrt1.2-1 mutant than in the wild type, but not specifically in the high external
influx was lower in the atnrt1.2-1 mutant than in the wild type, but not specifically in the high external 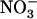 concentration range. Unexpectedly, root 15
concentration range. Unexpectedly, root 15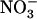 influx was not reduced in chl1-10 plants compared with Ws and was even higher in the middle range of external
influx was not reduced in chl1-10 plants compared with Ws and was even higher in the middle range of external 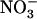 concentration (1–5 mm). These data unambiguously demonstrate that unrepressed NRT2.1 transcript accumulation in chl1-10 plants at high external
concentration (1–5 mm). These data unambiguously demonstrate that unrepressed NRT2.1 transcript accumulation in chl1-10 plants at high external 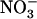 availability cannot be explained by reduced root
availability cannot be explained by reduced root  uptake rate as compared to either Ws or atnrt1.2-1 plants (Fig. 4C). Thus, whatever the signal responsible for down-regulation of NRT2.1 expression by high external
uptake rate as compared to either Ws or atnrt1.2-1 plants (Fig. 4C). Thus, whatever the signal responsible for down-regulation of NRT2.1 expression by high external 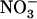 availability, it requires NRT1.1 to trigger the response.
availability, it requires NRT1.1 to trigger the response.
Figure 4.
Relationship between NRT2.1 mRNA accumulation and root 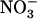 influx as a function of
influx as a function of 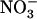 concentration in the presence of
concentration in the presence of 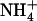 in the external medium. Plants of Ws wild-type, atnrt1.2-1 mutant, and chl1-10 mutant were grown for 6 weeks on complete nutrient solution containing 1 mm NH4NO3, before transfer for 4 d to the nutrient solutions containing 1 mm
in the external medium. Plants of Ws wild-type, atnrt1.2-1 mutant, and chl1-10 mutant were grown for 6 weeks on complete nutrient solution containing 1 mm NH4NO3, before transfer for 4 d to the nutrient solutions containing 1 mm 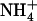 and
and  at various concentrations, as indicated in the figure. A, NRT2.1 mRNA accumulation. Transcript accumulation was monitored in roots both by northern-blot analysis (gel-blot images) and real-time RT-PCR (graph) on the same samples. A 25S rRNA signal is presented as a loading control for northern blots. Quantification by real-time RT-PCR was normalized using actin genes as controls. Errors bars represent se (n = 3). Differences between chl1-10 and the other genotypes are statistically significant at **P < 0.01 and ***P < 0.001 (t test). B, Root 15
at various concentrations, as indicated in the figure. A, NRT2.1 mRNA accumulation. Transcript accumulation was monitored in roots both by northern-blot analysis (gel-blot images) and real-time RT-PCR (graph) on the same samples. A 25S rRNA signal is presented as a loading control for northern blots. Quantification by real-time RT-PCR was normalized using actin genes as controls. Errors bars represent se (n = 3). Differences between chl1-10 and the other genotypes are statistically significant at **P < 0.01 and ***P < 0.001 (t test). B, Root 15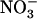 influx. Root 15
influx. Root 15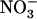 influx was assayed by 5-min labeling at the external 15
influx was assayed by 5-min labeling at the external 15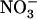 concentration corresponding to that supplied to the plants during the 4-d treatment. Each value is the mean of six to 12 replicates ± se. C, Plot of NRT2.1 mRNA accumulation (A) against root 15
concentration corresponding to that supplied to the plants during the 4-d treatment. Each value is the mean of six to 12 replicates ± se. C, Plot of NRT2.1 mRNA accumulation (A) against root 15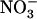 influx (B).
influx (B).
Additional evidence for NRT1.1-dependent control of the 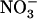 HATS by external
HATS by external 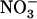 availability was provided by expression analysis of NRT3.1, which shows the same response pattern as NRT2.1, suggesting coregulation of the two genes (Fig. 5). However, the quantitative changes in NRT3.1 transcript abundance were generally less pronounced than those of NRT2.1.
availability was provided by expression analysis of NRT3.1, which shows the same response pattern as NRT2.1, suggesting coregulation of the two genes (Fig. 5). However, the quantitative changes in NRT3.1 transcript abundance were generally less pronounced than those of NRT2.1.
Figure 5.
NRT2.1 and NRT3.1 coregulation. A, Response of NRT3.1 mRNA accumulation in Ws, atnrt1.2-1, and chl1-10 plants to the variation of  concentration in the presence of 1 mm
concentration in the presence of 1 mm 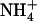 in the external medium. The experiment is the same as in Figure 5. B, Correlation between changes in NRT2.1 and NRT3.1 mRNA accumulation. All RNA samples used to determine changes in NRT2.1 expression presented in Figures 1 to 4 and Supplemental Figure S1 were analyzed for NRT3.1 mRNA accumulation. The figure presents the relative changes in transcript level for both NRT2.1 and NRT3.1 normalized to the control (Ws plants left on 1 mm NO3NH4) of each experiment. Gray squares, Experiment of Figure 1; white diamonds, experiment of Figure 2; gray triangles, experiment of Figure 3; white hexagons, experiment of Supplemental Figure S1; black circles, experiment of Figure 4.
in the external medium. The experiment is the same as in Figure 5. B, Correlation between changes in NRT2.1 and NRT3.1 mRNA accumulation. All RNA samples used to determine changes in NRT2.1 expression presented in Figures 1 to 4 and Supplemental Figure S1 were analyzed for NRT3.1 mRNA accumulation. The figure presents the relative changes in transcript level for both NRT2.1 and NRT3.1 normalized to the control (Ws plants left on 1 mm NO3NH4) of each experiment. Gray squares, Experiment of Figure 1; white diamonds, experiment of Figure 2; gray triangles, experiment of Figure 3; white hexagons, experiment of Supplemental Figure S1; black circles, experiment of Figure 4.
Taken together, the above data strongly support the hypothesis of coordinated regulation of NRT2.1 and NRT3.1, mediated by NRT1.1, which down-regulates the 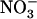 HATS when external
HATS when external 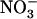 availability in mixed N medium is above 0.2 to 0.5 mm. Below this threshold, this repression is alleviated, which enables the plant to reactivate the
availability in mixed N medium is above 0.2 to 0.5 mm. Below this threshold, this repression is alleviated, which enables the plant to reactivate the  HATS, despite the strongly repressive conditions related to the ample supply of reduced N source.
HATS, despite the strongly repressive conditions related to the ample supply of reduced N source.
Up-Regulation of NRT2.1 by Low NO3− Availability in the Presence of NH4+ Prevents Growth Inhibition Associated with NH4+ Toxicity
To determine the physiological significance of NRT1.1-dependent control of the 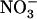 HATS by external
HATS by external 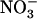 availability, we investigated the hypothesis that up-regulation of NRT2.1 by low
availability, we investigated the hypothesis that up-regulation of NRT2.1 by low  concentration in mixed N medium plays a role in preventing
concentration in mixed N medium plays a role in preventing 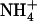 toxicity under situations of excess
toxicity under situations of excess 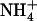 over
over 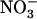 supply. Therefore, we analyzed growth and
supply. Therefore, we analyzed growth and 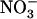 uptake of wild-type plants and of two independent NRT2.1 knockout mutants (atnrt2.1-1 and atnrt2.1-2) under pure
uptake of wild-type plants and of two independent NRT2.1 knockout mutants (atnrt2.1-1 and atnrt2.1-2) under pure  or mixed NH4NO3 nutrition. Seedlings were first grown for 5 weeks on 1 mm NH4NO3 before submitting them for 10 to 14 d to the various N nutrition regimes. During these experiments, wild-type and mutant plants were placed in the same container to make sure that both genotypes experienced the same changes in external pH.
or mixed NH4NO3 nutrition. Seedlings were first grown for 5 weeks on 1 mm NH4NO3 before submitting them for 10 to 14 d to the various N nutrition regimes. During these experiments, wild-type and mutant plants were placed in the same container to make sure that both genotypes experienced the same changes in external pH.
As expected, the supply of 1 mm  as the sole N source led to the appearance of toxicity symptoms in the shoots of both wild-type and atnrt2.1-1 genotypes (Fig. 6). These symptoms became pronounced between 6 and 10 d after transfer to the
as the sole N source led to the appearance of toxicity symptoms in the shoots of both wild-type and atnrt2.1-1 genotypes (Fig. 6). These symptoms became pronounced between 6 and 10 d after transfer to the 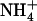 nutrient solution. In particular,
nutrient solution. In particular,  -fed plants started to bolt very early and their leaves wilted and yellowed. Interestingly, addition of 0.1 mm
-fed plants started to bolt very early and their leaves wilted and yellowed. Interestingly, addition of 0.1 mm 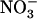 in the 1 mm
in the 1 mm 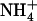 nutrient solution fully prevented the appearance of toxicity symptoms in wild-type plants, but not in the mutant, which seemed to remain as sensitive as when
nutrient solution fully prevented the appearance of toxicity symptoms in wild-type plants, but not in the mutant, which seemed to remain as sensitive as when 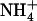 is the sole N source supplied (Fig. 6).
is the sole N source supplied (Fig. 6).
Figure 6.
Effect of NRT2.1 mutation on the protective effect of 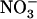 against
against 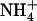 toxicity. Images show the appearance of toxicity symptoms in shoots of Ws and atnrt2.1-1 mutant plants as a function of the N source supplied in the external medium (either 1 mm
toxicity. Images show the appearance of toxicity symptoms in shoots of Ws and atnrt2.1-1 mutant plants as a function of the N source supplied in the external medium (either 1 mm 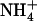 alone or in combination with 0.1 mm
alone or in combination with 0.1 mm 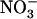 ).
).
These observations were confirmed by shoot growth analysis (Fig. 7A). With 1 mm 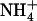 as the sole N source, shoots of atnrt2.1-1 plants grew at the same relative rate as those of wild-type plants, despite slightly lower biomass at the beginning of the experiment. Relative growth rate (RGR) values (determined from the slopes of the linear relationships between ln [fresh weight] and time) were 0.126 g g−1 d−1 (r2 = 0.986) and 0.125 g g−1 d−1 (r2 = 0.975) for wild-type and atnrt2.1-1 shoots, respectively. Supply of 0.1 mm
as the sole N source, shoots of atnrt2.1-1 plants grew at the same relative rate as those of wild-type plants, despite slightly lower biomass at the beginning of the experiment. Relative growth rate (RGR) values (determined from the slopes of the linear relationships between ln [fresh weight] and time) were 0.126 g g−1 d−1 (r2 = 0.986) and 0.125 g g−1 d−1 (r2 = 0.975) for wild-type and atnrt2.1-1 shoots, respectively. Supply of 0.1 mm 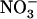 together with 1 mm
together with 1 mm 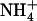 markedly stimulated shoot growth in wild-type plants (RGR = 0.165 g g−1 d−1; r2 = 0.992), but not in atnrt2.1-1 plants (RGR = 0.137 g g−1 d−1; r2 = 0.988). In agreement with the fact that NRT2.1 is up-regulated by low
markedly stimulated shoot growth in wild-type plants (RGR = 0.165 g g−1 d−1; r2 = 0.992), but not in atnrt2.1-1 plants (RGR = 0.137 g g−1 d−1; r2 = 0.988). In agreement with the fact that NRT2.1 is up-regulated by low 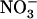 concentration in mixed N medium, and that it encodes a major component of the
concentration in mixed N medium, and that it encodes a major component of the 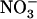 HATS, the cumulative
HATS, the cumulative 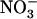 uptake in wild-type plants transferred on 0.1 mm
uptake in wild-type plants transferred on 0.1 mm 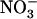 + 1 mm
+ 1 mm 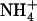 was much higher than that in atnrt2.1-1 plants (Fig. 7B). All the above observations were confirmed with the atnrt2.1-2 mutant allele (Supplemental Fig. S2). On 1 mm NH4NO3, however, a situation where NRT2.1 has a low contribution to total
was much higher than that in atnrt2.1-1 plants (Fig. 7B). All the above observations were confirmed with the atnrt2.1-2 mutant allele (Supplemental Fig. S2). On 1 mm NH4NO3, however, a situation where NRT2.1 has a low contribution to total 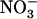 uptake (Cerezo et al., 2001), no significant difference was recorded for both shoot biomass and cumulative
uptake (Cerezo et al., 2001), no significant difference was recorded for both shoot biomass and cumulative 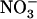 uptake between wild-type and atnrt2.1-1 plants (Supplemental Fig. S3). Thus, the decrease in shoot growth observed in atnrt2 mutants as compared to wild types correlated with the reduction of
uptake between wild-type and atnrt2.1-1 plants (Supplemental Fig. S3). Thus, the decrease in shoot growth observed in atnrt2 mutants as compared to wild types correlated with the reduction of 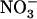 uptake resulting from the NRT2.1 mutation. On the other hand, root biomass was not affected by NRT2.1 mutation in all media investigated (Supplemental Figs. S2 and S3).
uptake resulting from the NRT2.1 mutation. On the other hand, root biomass was not affected by NRT2.1 mutation in all media investigated (Supplemental Figs. S2 and S3).
Figure 7.
Shoot growth and root  uptake of Ws and atnrt2.1-1 mutant plants supplied with 1 mm
uptake of Ws and atnrt2.1-1 mutant plants supplied with 1 mm 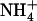 with or without 0.1 mm
with or without 0.1 mm 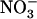 . Plants were grown hydroponically for 5 weeks on complete nutrient solution containing 1 mm NH4NO3 before transfer for 12 d to media containing either 1 mm
. Plants were grown hydroponically for 5 weeks on complete nutrient solution containing 1 mm NH4NO3 before transfer for 12 d to media containing either 1 mm  alone or 1 mm
alone or 1 mm 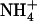 plus 0.1 mm 15
plus 0.1 mm 15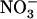 . A, Shoot fresh weight. Values are the means of 10 to 18 replicates ± se. B, Cumulative 15
. A, Shoot fresh weight. Values are the means of 10 to 18 replicates ± se. B, Cumulative 15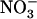 uptake from the 1 mm
uptake from the 1 mm 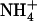 plus 0.1 mm 15
plus 0.1 mm 15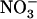 solution during the 12-d period determined by total 15N analysis in both root and shoots at the end of the period. Values are the mean of 10 to 12 replicates ± se. Differences between wild type and mutant are statistically significant at **P < 0.01, ***P < 0.001 (t test). Ns, Not significant (P > 0.05).
solution during the 12-d period determined by total 15N analysis in both root and shoots at the end of the period. Values are the mean of 10 to 12 replicates ± se. Differences between wild type and mutant are statistically significant at **P < 0.01, ***P < 0.001 (t test). Ns, Not significant (P > 0.05).
Taken together, the above data demonstrate that, even at a low concentration of 0.1 mm, the presence of 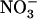 in mixed N solution is able to alleviate the detrimental effects of pure
in mixed N solution is able to alleviate the detrimental effects of pure 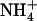 nutrition on shoot growth in wild-type plants, but not in atnrt2.1 mutants. This indicates that the protective action of 0.1 mm
nutrition on shoot growth in wild-type plants, but not in atnrt2.1 mutants. This indicates that the protective action of 0.1 mm 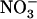 against
against 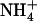 toxicity is dependent on NRT2.1 expression.
toxicity is dependent on NRT2.1 expression.
DISCUSSION
A Novel Regulation of NRT2.1 Expression Involving NRT1.1-Mediated Repression by High NO3−
Knowledge concerning the control of NRT2.1 expression by N is that this gene is under two main regulations, namely (1) induction by 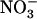 ; and (2) repression by high N status of the whole plant (Filleur and Daniel-Vedele, 1999; Lejay et al., 1999; Zhuo et al., 1999; Forde, 2000; Gansel et al., 2001; Orsel et al., 2002; Nazoa et al., 2003; Okamoto et al., 2003). The molecular mechanisms underlying these regulations are unknown, but there is some consensus about the nature of the signal molecules involved. In particular, the
; and (2) repression by high N status of the whole plant (Filleur and Daniel-Vedele, 1999; Lejay et al., 1999; Zhuo et al., 1999; Forde, 2000; Gansel et al., 2001; Orsel et al., 2002; Nazoa et al., 2003; Okamoto et al., 2003). The molecular mechanisms underlying these regulations are unknown, but there is some consensus about the nature of the signal molecules involved. In particular, the 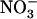 ion itself is believed to be the inducer (Crawford and Glass, 1998; Forde, 2000), and
ion itself is believed to be the inducer (Crawford and Glass, 1998; Forde, 2000), and 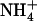 and Gln are thought to be the main signal molecules involved in the feedback repression exerted by the N status of the plant (Lejay et al., 1999; Zhuo et al., 1999; Nazoa et al., 2003).
and Gln are thought to be the main signal molecules involved in the feedback repression exerted by the N status of the plant (Lejay et al., 1999; Zhuo et al., 1999; Nazoa et al., 2003).
This model now appears to be incomplete because NRT2.1 overexpression in wild-type plants under low  /high
/high 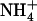 availability (Figs. 1–4) cannot be explained by the above mechanisms (i.e. induction by
availability (Figs. 1–4) cannot be explained by the above mechanisms (i.e. induction by 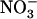 and repression by
and repression by 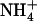 ). A striking illustration of this is the observation that transfer of the plants from 1 mm NH4NO3 to 5 mm
). A striking illustration of this is the observation that transfer of the plants from 1 mm NH4NO3 to 5 mm 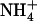 + 0.1 mm
+ 0.1 mm 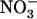 led to 4-fold stimulation of NRT2.1 expression (Fig. 1B), although this corresponded to a 10-fold decrease in the concentration of the inducer (
led to 4-fold stimulation of NRT2.1 expression (Fig. 1B), although this corresponded to a 10-fold decrease in the concentration of the inducer ( ) and a 5-fold increase in the concentration of the repressor (
) and a 5-fold increase in the concentration of the repressor (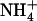 ). Furthermore, as already shown for up-regulation of NRT2.1 in NH4NO3-fed chl1 mutants (Muños et al., 2004), up-regulation of NRT2.1 by low
). Furthermore, as already shown for up-regulation of NRT2.1 in NH4NO3-fed chl1 mutants (Muños et al., 2004), up-regulation of NRT2.1 by low 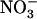 in the presence of
in the presence of 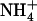 cannot be mistaken with relief of the feedback repression exerted by the overall N status of the plant: (1) plants subjected to low
cannot be mistaken with relief of the feedback repression exerted by the overall N status of the plant: (1) plants subjected to low 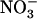 /high
/high 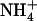 availability have a high total N content of the tissues and thus have high N status; (2) root
availability have a high total N content of the tissues and thus have high N status; (2) root 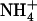 influx is not up-regulated in these plants (Fig. 3B), whereas it is also under negative feedback control by the N status of the plant (Gazzarrini et al., 1999; Rawat et al., 1999; von Wirén et al., 2000); and (3) stimulation of NRT2.1 expression by low
influx is not up-regulated in these plants (Fig. 3B), whereas it is also under negative feedback control by the N status of the plant (Gazzarrini et al., 1999; Rawat et al., 1999; von Wirén et al., 2000); and (3) stimulation of NRT2.1 expression by low 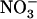 /high
/high 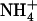 availability is under purely local control (Fig. 2), whereas NRT2.1 regulation by the N status of the plant involves systemic signaling (Imsande and Touraine, 1994; Gansel et al., 2001; Forde, 2002).
availability is under purely local control (Fig. 2), whereas NRT2.1 regulation by the N status of the plant involves systemic signaling (Imsande and Touraine, 1994; Gansel et al., 2001; Forde, 2002).
Altogether, our data provide evidence that NRT2.1 expression is also modulated by a third important regulatory mechanism, triggering repression of this gene by high external  availability, which superimposes on repression exerted by
availability, which superimposes on repression exerted by 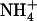 or Gln. Indeed, in the presence of either reduced N source, NRT2.1 expression in wild-type roots was consistently found to be primarily determined by external
or Gln. Indeed, in the presence of either reduced N source, NRT2.1 expression in wild-type roots was consistently found to be primarily determined by external 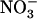 concentration, with strong down-regulation as soon as this concentration exceeded the 0.2 to 0.5 mm range. Although surprising at first glance, the hypothesis that
concentration, with strong down-regulation as soon as this concentration exceeded the 0.2 to 0.5 mm range. Although surprising at first glance, the hypothesis that 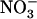 may have opposite regulatory effects (induction and repression) is already well documented for its role in the control of lateral root growth (local stimulation of lateral root elongation and systemic repression of lateral root emergence; Zhang et al., 1999). Furthermore, down-regulation of root
may have opposite regulatory effects (induction and repression) is already well documented for its role in the control of lateral root growth (local stimulation of lateral root elongation and systemic repression of lateral root emergence; Zhang et al., 1999). Furthermore, down-regulation of root  uptake by
uptake by 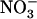 itself has already been postulated, in particular from barley (Hordeum vulgare) experiments where root
itself has already been postulated, in particular from barley (Hordeum vulgare) experiments where root 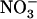 uptake was found to be negatively correlated with root
uptake was found to be negatively correlated with root  concentration, but only when root
concentration, but only when root 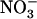 concentration exceeded a certain threshold level (Siddiqi et al., 1989; Crawford and Glass, 1998). Although these physiological studies provided circumstantial evidence for repression of root
concentration exceeded a certain threshold level (Siddiqi et al., 1989; Crawford and Glass, 1998). Although these physiological studies provided circumstantial evidence for repression of root 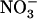 uptake by high
uptake by high 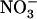 , our results bring insight to these aspects because they highlight NRT2.1 and NRT3.1 as molecular targets of this regulation. Recently, NRT3.1 expression was also shown to be induced by
, our results bring insight to these aspects because they highlight NRT2.1 and NRT3.1 as molecular targets of this regulation. Recently, NRT3.1 expression was also shown to be induced by 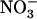 (Okamoto et al., 2006) and repressed by high N status of the plant (Remans et al., 2006). Thus, NRT3.1 appears to be, at least partially, controlled by the same regulatory network as NRT2.1, suggesting coordinated regulation of these two components of the HATS. Most importantly, our data also reveal specific involvement of NRT1.1 in triggering repression by high
(Okamoto et al., 2006) and repressed by high N status of the plant (Remans et al., 2006). Thus, NRT3.1 appears to be, at least partially, controlled by the same regulatory network as NRT2.1, suggesting coordinated regulation of these two components of the HATS. Most importantly, our data also reveal specific involvement of NRT1.1 in triggering repression by high 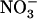 (Figs. 4 and 5). Indeed, down-regulation of NRT2.1/NRT3.1 expression by high
(Figs. 4 and 5). Indeed, down-regulation of NRT2.1/NRT3.1 expression by high 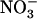 availability in the presence of
availability in the presence of 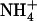 is fully suppressed in the chl1-10 mutant (and not in a nrt1.2 mutant), whereas root
is fully suppressed in the chl1-10 mutant (and not in a nrt1.2 mutant), whereas root 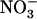 uptake is not reduced in chl1-10 compared to wild type (whereas it is reduced in atnrt1.2-1). This clearly invalidates one of our initial hypotheses that this phenotype of chl1 mutants is simply a compensatory response to a general defect in
uptake is not reduced in chl1-10 compared to wild type (whereas it is reduced in atnrt1.2-1). This clearly invalidates one of our initial hypotheses that this phenotype of chl1 mutants is simply a compensatory response to a general defect in 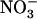 acquisition and thus an indirect consequence of NRT1.1 mutation. Our proposal for the regulatory role of NRT1.1 in wild-type plants is that the increase in external
acquisition and thus an indirect consequence of NRT1.1 mutation. Our proposal for the regulatory role of NRT1.1 in wild-type plants is that the increase in external 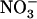 concentration results in an increasing activity of this transporter, which in turn generates an increasing repressive signal for NRT2.1 expression (Fig. 4A). Whether this indicates a direct signaling function for NRT1.1 (in analogy with the role of the
concentration results in an increasing activity of this transporter, which in turn generates an increasing repressive signal for NRT2.1 expression (Fig. 4A). Whether this indicates a direct signaling function for NRT1.1 (in analogy with the role of the 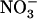 sensor recently proposed for NRT2.1) and calls for the specific involvement of one isoform of NRT1.1 (high or low affinity) are open questions that deserve further investigation.
sensor recently proposed for NRT2.1) and calls for the specific involvement of one isoform of NRT1.1 (high or low affinity) are open questions that deserve further investigation.
To account for our observations, we propose a model for N regulation of NRT2.1 expression (Fig. 8). In addition to the positive regulation corresponding to the induction by 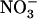 , this model postulates dual negative regulation involving both feedback repression by reduced N metabolites and NRT1.1-mediated repression by high external
, this model postulates dual negative regulation involving both feedback repression by reduced N metabolites and NRT1.1-mediated repression by high external  . An important point is that the absence of NRT1.1-mediated repression (due to mutation of NRT1.1 or to low external
. An important point is that the absence of NRT1.1-mediated repression (due to mutation of NRT1.1 or to low external 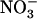 availability) overrides the negative feedback exerted by reduced N metabolites to yield a high NRT2.1 expression level even in the presence of ample
availability) overrides the negative feedback exerted by reduced N metabolites to yield a high NRT2.1 expression level even in the presence of ample 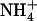 supply to the plant (e.g. 5 mm; see Fig. 1B). Conversely, there is also evidence that lack of negative feedback regulation by reduced N metabolites overrides the repressive effect of high external
supply to the plant (e.g. 5 mm; see Fig. 1B). Conversely, there is also evidence that lack of negative feedback regulation by reduced N metabolites overrides the repressive effect of high external 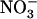 availability. This is shown by the high NRT2.1 expression level in nitrate reductase-deficient plants supplied with
availability. This is shown by the high NRT2.1 expression level in nitrate reductase-deficient plants supplied with 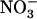 as a sole N source (Lejay et al., 1999; Zhuo et al., 1999). Taken together, these observations suggest that NRT2.1 expression is suppressed only when both negative regulations by reduced N metabolites and by high
as a sole N source (Lejay et al., 1999; Zhuo et al., 1999). Taken together, these observations suggest that NRT2.1 expression is suppressed only when both negative regulations by reduced N metabolites and by high 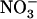 are effective (as illustrated in Fig. 8). Whether this means that the two respective signaling pathways directly interfere at some common crucial node or, alternatively, that they are independently strong enough to overcome each other is not known. However, because NRT1.1 mutation prevents NRT2.1 repression by high
are effective (as illustrated in Fig. 8). Whether this means that the two respective signaling pathways directly interfere at some common crucial node or, alternatively, that they are independently strong enough to overcome each other is not known. However, because NRT1.1 mutation prevents NRT2.1 repression by high 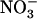 , but does not alter its reinduction by
, but does not alter its reinduction by 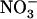 after a period of N starvation (Muños et al., 2004), it is concluded that these opposite actions of
after a period of N starvation (Muños et al., 2004), it is concluded that these opposite actions of  most probably involve independent signaling pathways.
most probably involve independent signaling pathways.
Figure 8.
Model for N regulation of NRT2.1 expression in Arabidopsis roots. The model postulates that, in addition to the induction by  , NRT2.1 is also under dual control by feedback repression by reduced N metabolites and NRT1.1-mediated repression by high external
, NRT2.1 is also under dual control by feedback repression by reduced N metabolites and NRT1.1-mediated repression by high external  availability, which are both required to suppress NRT2.1 expression.
availability, which are both required to suppress NRT2.1 expression.
NRT1.1-Mediated Regulation of NRT2.1 Allows an Adaptive Response of the Plant to NH4+ Toxicity
A key issue concerning NRT1.1-mediated regulation of the HATS by high 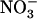 is to determine what physiological role such a mechanism may play. In analogy with the well-accepted postulate that repression of root
is to determine what physiological role such a mechanism may play. In analogy with the well-accepted postulate that repression of root 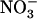 (or
(or 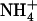 ) uptake systems by reduced N metabolites corresponds to a regulation by the N demand of the plant (Imsande and Touraine, 1994; vonWirén et al., 2000; Forde, 2002), we hypothesize that repression of NRT2.1 by high
) uptake systems by reduced N metabolites corresponds to a regulation by the N demand of the plant (Imsande and Touraine, 1994; vonWirén et al., 2000; Forde, 2002), we hypothesize that repression of NRT2.1 by high 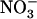 corresponds to regulation by a
corresponds to regulation by a 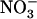 demand of the plant. Accordingly, relief of this NRT1.1-mediated repression due either to decreased
demand of the plant. Accordingly, relief of this NRT1.1-mediated repression due either to decreased 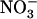 availability in the presence of
availability in the presence of 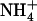 , or to NRT1.1 mutation activated the
, or to NRT1.1 mutation activated the  HATS but not the
HATS but not the 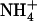 uptake system (Fig. 3; Muños et al., 2004). This shows that root
uptake system (Fig. 3; Muños et al., 2004). This shows that root 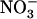 uptake, and not total root N uptake, is the specific target of this mechanism. Clearly, what the plant perceives under these situations is a lack of
uptake, and not total root N uptake, is the specific target of this mechanism. Clearly, what the plant perceives under these situations is a lack of 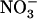 and not an overall nutritional N deficiency.
and not an overall nutritional N deficiency.
In this context, the significance of the model depicted in Figure 8 is that repression of NRT2.1 by reduced N metabolites in N-sufficient plants is allowed only when NRT1.1 is active in transporting 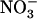 . This warrants that a significant
. This warrants that a significant 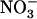 uptake rate is always ensured in any situation, either by NRT1.1 or by NRT2.1, even when
uptake rate is always ensured in any situation, either by NRT1.1 or by NRT2.1, even when 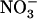 accounts for only a minor fraction of the total N available in the external medium (Fig. 4B). One of the most obvious interests of such a mechanism is to protect the plant against
accounts for only a minor fraction of the total N available in the external medium (Fig. 4B). One of the most obvious interests of such a mechanism is to protect the plant against 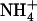 toxicity. It is known for decades that pure
toxicity. It is known for decades that pure  nutrition is toxic for many plant species (Givan, 1979; Hageman, 1984; Salsac et al., 1987; Kronzucker et al., 2001). In particular, growth of herbaceous dicotyledons such as tomato (Lycopersicon esculentum), French bean (Phaseolus vulgaris), spinach (Spinacia oleracea), and here Arabidopsis, is generally strongly hampered by pure
nutrition is toxic for many plant species (Givan, 1979; Hageman, 1984; Salsac et al., 1987; Kronzucker et al., 2001). In particular, growth of herbaceous dicotyledons such as tomato (Lycopersicon esculentum), French bean (Phaseolus vulgaris), spinach (Spinacia oleracea), and here Arabidopsis, is generally strongly hampered by pure  nutrition, with a decrease in yield of up to 60% as compared with supply of
nutrition, with a decrease in yield of up to 60% as compared with supply of  as the sole N source (Salsac et al., 1987). A general observation is, however, that
as the sole N source (Salsac et al., 1987). A general observation is, however, that  toxicity is fully prevented by supply of
toxicity is fully prevented by supply of  as a N source together with
as a N source together with 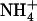 (Cox and Reisenauer, 1973; Kronzucker et al., 1999; Rahayu et al., 2005). Actually, highest growth rates are generally achieved with mixed
(Cox and Reisenauer, 1973; Kronzucker et al., 1999; Rahayu et al., 2005). Actually, highest growth rates are generally achieved with mixed  +
+ 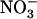 supplies (Cox and Reisenauer, 1973; Heberer and Below, 1989; Adriaanse and Human, 1993; Cao and Tibbitts, 1993).
supplies (Cox and Reisenauer, 1973; Heberer and Below, 1989; Adriaanse and Human, 1993; Cao and Tibbitts, 1993).
Despite this firmly established role of 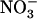 in preventing the detrimental effects of
in preventing the detrimental effects of 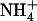 nutrition, a strong paradox remained unresolved. On the one hand,
nutrition, a strong paradox remained unresolved. On the one hand,  uptake by the plant was shown to ensure full protection against
uptake by the plant was shown to ensure full protection against 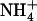 toxicity and, on the other hand,
toxicity and, on the other hand, 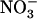 uptake systems were shown to be strongly repressed by the supply of high
uptake systems were shown to be strongly repressed by the supply of high  concentration to the plant. To date, no mechanism was known to stimulate
concentration to the plant. To date, no mechanism was known to stimulate 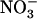 uptake in the presence of potentially toxic concentrations of
uptake in the presence of potentially toxic concentrations of 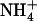 in the external medium. We propose that the NRT1.1-mediated regulation of NRT2.1/NRT3.1 corresponds to such a mechanism because it relieves repression of the HATS under low
in the external medium. We propose that the NRT1.1-mediated regulation of NRT2.1/NRT3.1 corresponds to such a mechanism because it relieves repression of the HATS under low 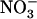 /high
/high 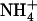 availability (Figs. 3A and 4). Furthermore, the phenotype of both atnrt2.1 mutants demonstrates that up-regulation of the HATS under this condition constitutes an essential adaptive response of the plant to avoid
availability (Figs. 3A and 4). Furthermore, the phenotype of both atnrt2.1 mutants demonstrates that up-regulation of the HATS under this condition constitutes an essential adaptive response of the plant to avoid 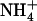 toxicity (actually the only one documented at the molecular level; Figs. 6 and 7; Supplemental Figs. S2 and S3).
toxicity (actually the only one documented at the molecular level; Figs. 6 and 7; Supplemental Figs. S2 and S3).
A surprising aspect of the HATS repression by high 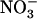 is that it seems to rely on purely local signaling because only the portions of the root system subjected to low
is that it seems to rely on purely local signaling because only the portions of the root system subjected to low 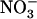 /high
/high 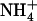 availability react in up-regulating NRT2.1 expression (Fig. 2). Furthermore, high
availability react in up-regulating NRT2.1 expression (Fig. 2). Furthermore, high 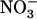 supply on one portion of the root system does not prevent the adaptive response of NRT2.1 in other portions fed with excess
supply on one portion of the root system does not prevent the adaptive response of NRT2.1 in other portions fed with excess 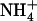 over
over 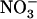 (see Fig. 3, lanes b and c). This suggests that the
(see Fig. 3, lanes b and c). This suggests that the 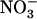 demand governing NRT2.1 expression is not sensed at the whole-plant level and that the adaptive response of NRT2.1 aims at stimulating
demand governing NRT2.1 expression is not sensed at the whole-plant level and that the adaptive response of NRT2.1 aims at stimulating 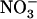 uptake specifically in the root cells experiencing high external
uptake specifically in the root cells experiencing high external 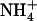 availability. This is in full agreement with the results from split-root experiments on maize (Zea mays) and soybean (Glycine max), indicating that
availability. This is in full agreement with the results from split-root experiments on maize (Zea mays) and soybean (Glycine max), indicating that 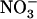 plays its protective role against
plays its protective role against 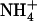 toxicity only when it is locally supplied together with
toxicity only when it is locally supplied together with  , and not when the two N sources are separately provided to only one half on the root sy
stem (Schortemeyer et al., 1993; Saravitz et al., 1994).
, and not when the two N sources are separately provided to only one half on the root sy
stem (Schortemeyer et al., 1993; Saravitz et al., 1994).
Our data thus show that, besides its key role in ensuring the bulk of N acquisition by the plant in many various environmental conditions, NRT2.1 also plays a critical function in maintaining a healthy balance between 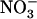 and
and 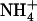 uptake. It is highlighted that, in this latter case, NRT2.1 activity is not required to supply an N source for amino acid synthesis, but to allow the plant to benefit from a specific role of
uptake. It is highlighted that, in this latter case, NRT2.1 activity is not required to supply an N source for amino acid synthesis, but to allow the plant to benefit from a specific role of 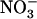 that
that 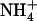 cannot fulfill. This illustrates very well why NRT2.1 cannot be regulated only by feedback repression by N metabolites because this regulation aims at adjusting N uptake to amino acid utilization and is not specific for
cannot fulfill. This illustrates very well why NRT2.1 cannot be regulated only by feedback repression by N metabolites because this regulation aims at adjusting N uptake to amino acid utilization and is not specific for 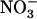 uptake systems (vonWirén et al., 2000). Interestingly, up-regulation of NRT2.1 expression by low
uptake systems (vonWirén et al., 2000). Interestingly, up-regulation of NRT2.1 expression by low  availability was also observed in the presence of Gln (Supplemental Fig. S1), suggesting that
availability was also observed in the presence of Gln (Supplemental Fig. S1), suggesting that 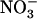 demand signaling may be operative under other circumstances than those associated with
demand signaling may be operative under other circumstances than those associated with 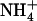 toxicity. It is thus tempting to postulate a more general role for this signaling in regulating
toxicity. It is thus tempting to postulate a more general role for this signaling in regulating 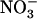 acquisition by the plant. Nitrate is not only a nutrient, but also a key signaling compound governing crucial aspects of plant metabolism and development (Crawford, 1995; Stitt, 1999). In particular,
acquisition by the plant. Nitrate is not only a nutrient, but also a key signaling compound governing crucial aspects of plant metabolism and development (Crawford, 1995; Stitt, 1999). In particular, 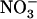 regulates many genes related to N or C metabolism (Crawford, 1995; Stitt, 1999), triggers several adaptive responses of root and shoot growth (Forde, 2002; Walch-Liu et al., 2005), and modulates cytokinin signaling (Sakakibara, 2003). Thus, plants may have evolved specific regulatory mechanisms to tightly control these important signaling effects of
regulates many genes related to N or C metabolism (Crawford, 1995; Stitt, 1999), triggers several adaptive responses of root and shoot growth (Forde, 2002; Walch-Liu et al., 2005), and modulates cytokinin signaling (Sakakibara, 2003). Thus, plants may have evolved specific regulatory mechanisms to tightly control these important signaling effects of 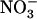 . At the uptake level, this would require a regulatory mechanism specific for
. At the uptake level, this would require a regulatory mechanism specific for  transporters and independent from the feedback regulation by reduced N metabolites, which aims at ensuring efficient use of this ion (as well as of
transporters and independent from the feedback regulation by reduced N metabolites, which aims at ensuring efficient use of this ion (as well as of 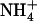 ) as a nutrient. In this context, the model of Figure 8 corresponds to an elegant mechanism for integrating both requirements for
) as a nutrient. In this context, the model of Figure 8 corresponds to an elegant mechanism for integrating both requirements for 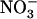 as a nutrient and as a signal in the regulation of root
as a nutrient and as a signal in the regulation of root 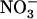 uptake. Therefore, the question of whether NRT1.1-mediated regulation of NRT2.1 reported in this work has additional functions other than just protecting the plant from
uptake. Therefore, the question of whether NRT1.1-mediated regulation of NRT2.1 reported in this work has additional functions other than just protecting the plant from  toxicity deserves further investigation. In particular, given the role of NRT1.1 in regulating NRT2.1 expression, and the role of NRT2.1 in controlling lateral root initiation, it will be of interest to investigate whether putative
toxicity deserves further investigation. In particular, given the role of NRT1.1 in regulating NRT2.1 expression, and the role of NRT2.1 in controlling lateral root initiation, it will be of interest to investigate whether putative 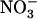 signaling mediated by NRT1.1 is also involved in modulating root branching as a function of external
signaling mediated by NRT1.1 is also involved in modulating root branching as a function of external 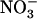 availability.
availability.
MATERIALS AND METHODS
Plant Material and Treatments
The Arabidopsis (Arabidopsis thaliana) genotypes used in this study were the wild-type Ws and Columbia-0 ecotypes; the atnrt2.1-1 mutant in the Ws background (formerly atnrt2a), obtained from the collection of Institut National de la Recherche Agronomique, Versailles, and deleted for the NRT2.1 (At1g08090) and NRT2.2 (At1g08100) genes (Filleur et al., 2001); the atnrt2.1-2 mutant in the Columbia-0 background, obtained from the Salk Institute (SALK_035429), and carrying a T-DNA insertion in the first intron of NRT2.1 (these two mutants were renamed according to the nomenclature proposed by Little et al., 2005); and the atnrt1.2-1 mutant in the Ws background, obtained from the collection of the Institut National de la Recherche Agronomique, and carrying a T-DNA insertion in the third intron of NRT1.2. NRT1.2 mRNA was not detected by reverse transcription (RT)-PCR in the roots of this mutant (data not shown).
Plants were grown for 6 weeks in hydroponics under nonsterile conditions, as previously described by Lejay et al. (1999). The growth chamber was set with the following environmental conditions: 8-h light/16-h dark 22°C/20°C temperature, 250 μmol m−2 s−1 irradiance, and 70% hygrometry. Briefly, seeds were sown directly on sand contained by a cut 1.5-mL Eppendorf tube closed at the bottom by a stainless grid. Tubes were supported by PVC discs (six Eppendorf/disc) placed on a floating polystyrene raft (12 discs/raft). These systems were disposed on top of 10-L tanks filled with tap water for the first week, and then with nutrient solution for 4 to 5 additional weeks (during this period, nutrient solutions were renewed weekly). The basal nutrient solution common to all experiments included 1 mm KH2PO4, 1 mm MgSO4, 0.25 mm K2SO4, 0.25 mm CaCl2, 0.1 mm FeNa-EDTA, 50 μm KCl, 30 μm H3BO3, 5 μm MnSO4, 1 μm ZnSO4, 1 μm CuSO4, and 0.1 μm (NH4)6Mo7O24. For growth of the plants, 1 mm NH4NO3 was added to the basal medium as the N source. Depending on the experiments, 1 mm NH4NO3 was replaced as a N source by either KNO3 or NH4Cl, or various mixtures of these salts, as indicated in the text and figures. The pH of all solutions was adjusted to 5.8, and the solutions were renewed every other day during the experiments to prevent nutrient depletion. For experiments with media at low 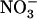 concentration (0.1 mm), nutrient solutions were renewed daily, which allowed maintenance of the external
concentration (0.1 mm), nutrient solutions were renewed daily, which allowed maintenance of the external  concentration above 0.06 to 0.07 mm. For treatments with Gln, 25 mg L−1 chloramphenicol and 50 mg L−1 penicillin were added to solutions to prevent microbial development. For time-course studies, various treatments were initiated at different times to allow harvest of all plants at the same time of the day (7–8 h into the light period) to prevent any diurnal effect on NRT2.1 expression (Lejay et al., 2003).
concentration above 0.06 to 0.07 mm. For treatments with Gln, 25 mg L−1 chloramphenicol and 50 mg L−1 penicillin were added to solutions to prevent microbial development. For time-course studies, various treatments were initiated at different times to allow harvest of all plants at the same time of the day (7–8 h into the light period) to prevent any diurnal effect on NRT2.1 expression (Lejay et al., 2003).
For split-root experiments, the protocol was reported previously (Gansel et al., 2001). At the age of 2 weeks, seedlings were cleared to leave only one plant per tube. After gentle separation of the root system into two approximately equal portions, 5-week-old plants were transferred to specific containers and allowed to adapt 3 d to split-root conditions, with the two parts of the root system supplied with the 1 mm NH4NO3 solution. The various treatments were then initiated at the end of this period.
For growth analysis, roots and shoot were separated after harvest and their fresh weight determined. Fresh weight data are the means of 10 to 18 replicates.
RNA Extraction and RNA Gel-Blot Analysis
RNA extraction was performed as previously described (Lobreaux et al., 1992) from eight to 12 plants per treatment (except for six plants in the split-root experiment). Ten micrograms of total RNA were then separated by electrophoresis on MOPS-formaldehyde agarose gel and blotted on nylon membrane (Hybond N+; Amersham-Pharmacia Biotech). Membranes were prehybridized for 2 h at 60°C in church buffer: 0.5 m NaHPO4, 1% bovine serum albumin, and 7% SDS (pH 7.2 with H3PO4). Hybridization was performed overnight at 60°C after addition of a randomly primed 32P-labeled cDNA probe in the hybridization buffer. Membranes were washed twice at root temperature for 2 min and twice at 60°C with 0.5× SSC, 0.1% SDS. The probe used in this study corresponds to the full-length of AtNRT2.1 cDNA (Filleur and Daniel-Vedele, 1999). A 25S rRNA probe was used to normalize quantifications achieved using a phosphor imager (BAS-5000; Fujifilm).
Quantitative RT-PCR
Ten to 15 μg of total RNA were digested by RQ-DNase (Promega). After phenol-chloroform purification and isopropanol precipitation, RNA was reverse transcribed to one-strand cDNA using Moloney murine leukemia virus reverse transcriptase (Promega) and dT(18) V primers, according to the manufacturer's protocol. Gene expression was determined by quantitative real-time PCR (LightCycler; Roche Diagnostics) using gene-specific primers: NRT2.1 (forward, 5′-aacaagggctaacgtggatg-3′ and reverse, 5′-ctgcttctcctgctcattcc-3′); NRT3.1 (forward, 5′-ggccatgaagttgcctatg-3′ and reverse, 5′-tcttggccttcctcttctca3-′); ACT2/8 (forward, 5′-ggtaacattgtgctcagrggtgg-3′ and reverse, 5′-aacgaccttaatcttcatgctgc-3′), and LightCycler FastStart DNA Master SYBR Green (Roche Diagnostics). Expression levels of tested genes were normalized to expression levels of the ACT2/8 genes (Charrier et al., 2002).
15NO3− and 15NH4+ Uptake
Influx of either 15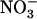 or 15
or 15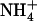 into the roots was assayed as described previously (Lejay et al., 1999) by 5-min labeling in basal nutrient medium (pH 5.8) supplemented with appropriate concentrations of K15NO3 or 15NH4Cl (atom % 15N excess: 99%). For specific determination of the activity of the HATS, 15
into the roots was assayed as described previously (Lejay et al., 1999) by 5-min labeling in basal nutrient medium (pH 5.8) supplemented with appropriate concentrations of K15NO3 or 15NH4Cl (atom % 15N excess: 99%). For specific determination of the activity of the HATS, 15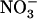 or 15
or 15 were at 0.2 mm in the labeling solution. Cumulative
were at 0.2 mm in the labeling solution. Cumulative 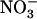 uptake during long-term growth studies (10–12 d) was assayed by supplying the plants with nutrient solution containing 15
uptake during long-term growth studies (10–12 d) was assayed by supplying the plants with nutrient solution containing 15 (atom % 15N excess: 1%) for the whole experimental period and by measuring total 15N accumulation in roots and shoots at the end of this period. Each influx or cumulative
(atom % 15N excess: 1%) for the whole experimental period and by measuring total 15N accumulation in roots and shoots at the end of this period. Each influx or cumulative 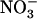 uptake value is the mean of eight to 12 replicates.
uptake value is the mean of eight to 12 replicates.
The total N content and atomic percentage 15N abundance of the samples were determined by continuous-flow mass spectrometry, as described previously (Clarkson et al., 1996), using a Euro-EA Eurovector elemental analyzer coupled with an IsoPrime mass spectrometer (GV Instruments).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Up-regulation of NRT2.1 in the presence of Gln.
Supplemental Figure S2.
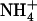 toxicity in the atnrt2.1-2 mutant.
toxicity in the atnrt2.1-2 mutant.Supplemental Figure S3.
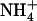 toxicity in the atnrt2.1-1 mutant.
toxicity in the atnrt2.1-1 mutant.
Supplementary Material
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Alain Gojon (gojon@ensam.inra.fr).
The online version of this article contains Web-only data.
References
- Adriaanse FG, Human JJ (1993) Effect of time of application and nitrate: ammonium ratio on maize grain yield, grain N concentration and soil mineral N concentration in semi-arid region. Field Crops Res 34: 57–70 [Google Scholar]
- Behl R, Tishner R, Raschke K (1988) Induction of a high-capacity nitrate-uptake mechanism in barley roots prompted by nitrate uptake through a constitutive low-capacity mechanism. Planta 176: 235–240 [DOI] [PubMed] [Google Scholar]
-
Cao W, Tibbitts TW (1993) Study of various
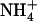 /
/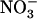 mixtures for enhancing growth of potatoes. J Plant Nutr 16: 1691–1704 [DOI] [PubMed] [Google Scholar]
mixtures for enhancing growth of potatoes. J Plant Nutr 16: 1691–1704 [DOI] [PubMed] [Google Scholar] -
Cerezo M, Tillard P, Filleur S, Munos S, Daniel-Vedele F, Gojon A (2001) Major alterations of the regulation of root
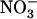 uptake are associated with the mutation of Nrt2.1 and Nrt2.2 genes in Arabidopsis. Plant Physiol 127: 262–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
uptake are associated with the mutation of Nrt2.1 and Nrt2.2 genes in Arabidopsis. Plant Physiol 127: 262–271 [DOI] [PMC free article] [PubMed] [Google Scholar] - Charrier B, Champion A, Henry Y, Kreis M (2002) Expression profiling of the whole Arabidopsis shaggy-like kinase multigene family by real-time reverse transcriptase-polymerase chain reaction. Plant Physiol 130: 577–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson DT, Gojon A, Saker LR, Wiersema PK, Purves JV, Tillard P, Arnold GM, Paams AJM, Waalburg W, Stulen I (1996) Nitrate and ammonium influxes in soybean (Glycine max) roots: direct comparison of 13N and 15N tracing. Plant Cell Environ 19: 859–868 [Google Scholar]
- Cox WJ, Reisenauer HM (1973) Growth and ion uptake by wheat supplied nitrogen as nitrate, or ammonium, or both. Plant Soil 38: 363–380 [Google Scholar]
- Crawford NM (1995) Nitrate—nutrient and signal for plant growth. Plant Cell 7: 859–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford NM, Glass ADM (1998) Molecular and physiological aspects of nitrate uptake in plants. Trends Plant Sci 3: 389–395 [Google Scholar]
- Daniel-Vedele F, Filleur S, Caboche M (1998) Nitrate transport: a key step in nitrate assimilation. Curr Opin Plant Biol 1: 235–239 [DOI] [PubMed] [Google Scholar]
- Filleur S, Daniel-Vedele F (1999) Expression analysis of a high-affinity nitrate transporter isolated from Arabidopsis thaliana by differential display. Planta 207: 461–469 [DOI] [PubMed] [Google Scholar]
- Filleur S, Dorbe MF, Cerezo M, Orsel M, Granier F, Gojon A, Daniel-Vedele F (2001) An Arabidopsis T-DNA mutant affected in Nrt2 genes is impaired in nitrate uptake. FEBS Lett 489: 220–224 [DOI] [PubMed] [Google Scholar]
- Forde BG (2000) Nitrate transporters in plants: structure, function and regulation. Biochim Biophys Acta 1465: 219–235 [DOI] [PubMed] [Google Scholar]
- Forde BG (2002) Local and long-range signaling pathways regulating plant responses to nitrate. Annu Rev Plant Biol 53: 203–224 [DOI] [PubMed] [Google Scholar]
-
Gansel X, Munos S, Tillard P, Gojon A (2001) Differential regulation of the
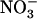 and
and 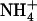 transporter genes AtNrt2.1 and AtAmt1.1 in Arabidopsis: relation with long-distance and local controls by N status of the plant. Plant J 26: 143–155 [DOI] [PubMed] [Google Scholar]
transporter genes AtNrt2.1 and AtAmt1.1 in Arabidopsis: relation with long-distance and local controls by N status of the plant. Plant J 26: 143–155 [DOI] [PubMed] [Google Scholar] - Gazzarrini S, Lejay L, Gojon A, Ninnemann O, Frommer WB, von Wirén N (1999) Three functional transporters for constitutive, diurnally regulated, and starvation-induced uptake of ammonium into Arabidopsis roots. Plant Cell 11: 937–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givan CV (1979) Metabolic detoxification of ammonia in tissues of higher plants. Phytochemistry 18: 375–382 [Google Scholar]
- Hageman RH (1984) Ammonium versus nitrate nutrition of higher plants. In RD Hauck, ed, Nitrogen in Crop Production. ASA-CSSA-SSSA, Madison, WI, pp 67–85
- Heberer JA, Below FE (1989) Mixed nitrogen nutrition and productivity of wheat grown in hydroponics. Ann Bot (Lond) 63: 643–649 [Google Scholar]
- Huang NC, Chiang CS, Crawford NM, Tsay YF (1996) CHL1 encodes a component of the low-affinity nitrate uptake system in Arabidopsis and shows cell type-specific expression in roots. Plant Cell 8: 2183–2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang NC, Liu KH, Lo HJ, Tsay YF (1999) Cloning and functional characterization of an Arabidopsis nitrate transporter gene that encodes a constitutive component of low-affinity uptake. Plant Cell 11: 1381–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imsande J, Touraine B (1994) N demand and the regulation of nitrate uptake. Plant Physiol 105: 3–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronzucker HJ, Britto DT, Davenport RJ, Tester M (2001) Ammonium toxicity and the real cost of transport. Trends Plant Sci 6: 335–337 [DOI] [PubMed] [Google Scholar]
- Kronzucker HJ, Siddiqi MY, Glass AD, Kirk GJ (1999) Nitrate-ammonium synergism in rice: a subcellular flux analysis. Plant Physiol 119: 1041–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejay L, Gansel X, Cerezo M, Tillard P, Muller C, Krapp A, von Wirén N, Daniel-Vedele F, Gojon A (2003) Regulation of root ion transporters by photosynthesis: functional importance and relation with hexokinase. Plant Cell 15: 2218–2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
-
Lejay L, Tillard P, Lepetit M, Olive F, Filleur S, Daniel-Vedele F, Gojon A (1999) Molecular and functional regulation of two
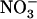 uptake systems by N- and C-status of Arabidopsis plants. Plant J 18: 509–519 [DOI] [PubMed] [Google Scholar]
uptake systems by N- and C-status of Arabidopsis plants. Plant J 18: 509–519 [DOI] [PubMed] [Google Scholar] - Little DY, Rao H, Oliva S, Daniel-Vedele F, Krapp A, Malamy JE (2005) The putative high-affinity nitrate transporter NRT2.1 represses lateral root initiation in response to nutritional cues. Proc Natl Acad Sci USA 102: 13693–13698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KH, Tsay YF (2003) Switching between the two action modes of the dual-affinity nitrate transporter CHL1 by phosphorylation. EMBO J 22: 1005–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobreaux S, Massenet O, Briat J (1992) Iron induces ferritin synthesis in maize plantlets. Plant Mol Biol 19: 563–575 [DOI] [PubMed] [Google Scholar]
- Mackown CT, McClure PR (1988) Development of accelerated net nitrate uptake: effects of nitrate concentration and exposure time. Plant Physiol 87: 162–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malagoli P, Laine P, Le Deunff E, Rossato L, Ney B, Ourry A (2004) Modeling nitrogen uptake in oilseed rape cv Capitol during a growth cycle using influx kinetics of root nitrate transport systems and field experimental data. Plant Physiol 134: 388–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muños S, Cazettes C, Fizames C, Gaymard F, Tillard P, Lepetit M, Lejay L, Gojon A (2004) Transcript profiling in the chl1-5 mutant of Arabidopsis reveals a role of the nitrate transporter NRT1.1 in the regulation of another nitrate transporter, NRT2.1. Plant Cell 16: 2433–2447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazoa P, Vidmar JJ, Tranbarger TJ, Mouline K, Damiani I, Tillard P, Zhuo D, Glass AD, Touraine B (2003) Regulation of the nitrate transporter gene AtNRT2.1 in Arabidopsis thaliana: responses to nitrate, amino acids and developmental stage. Plant Mol Biol 52: 689–703 [DOI] [PubMed] [Google Scholar]
- Okamoto M, Kumar A, Li W, Wang Y, Siddiqi MY, Crawford NM, Glass ADM (2006) High-affinity nitrate transport in roots of Arabidopsis depends on expression of the NAR2-like gene AtNRT3.1. Plant Physiol 140: 1036–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M, Vidmar JJ, Glass AD (2003) Regulation of NRT1 and NRT2 gene families of Arabidopsis thaliana: responses to nitrate provision. Plant Cell Physiol 44: 304–317 [DOI] [PubMed] [Google Scholar]
- Orsel M, Eulenburg K, Krapp A, Daniel-Vedele F (2004) Disruption of the nitrate transporter genes AtNRT2.1 and AtNRT2.2 restricts growth at low external nitrate concentration. Planta 219: 714–721 [DOI] [PubMed] [Google Scholar]
- Orsel M, Krapp A, Daniel-Vedele F (2002) Analysis of the NRT2 nitrate transporter family in Arabidopsis: structure and gene expression. Plant Physiol 129: 886–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada A, Fernandez E (1994) Expression of nitrate assimilation related genes in Chlamydomonas reinhardtii. Plant Mol Biol 24: 185–194 [DOI] [PubMed] [Google Scholar]
-
Rahayu YS, Walch-Liu P, Neumann G, Romheld V, von Wirén N, Bangerth F (2005) Root-derived cytokinins as long-distance signals for
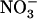 -induced stimulation of leaf growth. J Exp Bot 56: 1143–1152 [DOI] [PubMed] [Google Scholar]
-induced stimulation of leaf growth. J Exp Bot 56: 1143–1152 [DOI] [PubMed] [Google Scholar] -
Rawat SR, Silim SN, Kronzucker HJ, Siddiqi MY, Glass AD (1999) AtAMT1 gene expression and
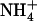 uptake in roots of Arabidopsis thaliana: evidence for regulation by root glutamine levels. Plant J 19: 143–152 [DOI] [PubMed] [Google Scholar]
uptake in roots of Arabidopsis thaliana: evidence for regulation by root glutamine levels. Plant J 19: 143–152 [DOI] [PubMed] [Google Scholar] - Remans T, Nacry P, Pervent M, Girin T, Tillard P, Lepetit M, Gojon A (2006) A central role for the nitrate transporter NRT2.1 in the integrated morphological and physiological responses of the root system to nitrogen limitation in Arabidopsis. Plant Physiol 140: 909–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara H (2003) Nitrate-specific and cytokinin-mediated nitrogen signaling pathways in plants. J Plant Res 116: 253–257 [DOI] [PubMed] [Google Scholar]
- Salsac L, Chaillou S, Morot-Gaudry JF, Lesaint C, Jolivet E (1987) Nitrate and ammonium nutrition in plants. Plant Physiol Biochem 25: 805–812 [Google Scholar]
- Saravitz CH, Chaillou S, Musset J, Raper CD, Morotgaudry JF (1994) Influence of nitrate on uptake of ammonium by nitrogen-depleted soybean—is the effect located in roots or shoots. J Exp Bot 45: 1575–1584 [Google Scholar]
- Schortemeyer M, Feil B, Stamp P (1993) Root morphology and nitrogen uptake of maize simultaneously supplied with ammonium and nitrate in a split-root system. Ann Bot (Lond) 72: 107–115 [Google Scholar]
-
Siddiqi MY, Glass AD, Ruth TJ, Fernando M (1989) Studies of the regulation of nitrate influx by barley seedlings using 13
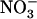 . Plant Physiol 90: 806–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
. Plant Physiol 90: 806–813 [DOI] [PMC free article] [PubMed] [Google Scholar] - Stitt M (1999) Nitrate regulation of metabolism and growth. Curr Opin Plant Biol 2: 178–186 [DOI] [PubMed] [Google Scholar]
- Tong Y, Zhou JJ, Li Z, Miller AJ (2005) A two-component high-affinity nitrate uptake system in barley. Plant J 41: 442–450 [DOI] [PubMed] [Google Scholar]
-
Touraine B, Glass AD (1997)
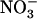 and
and  fluxes in the chl1-5 mutant of Arabidopsis thaliana: does the CHL1-5 gene encode a low-affinity
fluxes in the chl1-5 mutant of Arabidopsis thaliana: does the CHL1-5 gene encode a low-affinity 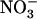 transporter? Plant Physiol 114: 137–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
transporter? Plant Physiol 114: 137–144 [DOI] [PMC free article] [PubMed] [Google Scholar] - Tsay YF, Schroeder JI, Feldmann KA, Crawford NM (1993) The herbicide sensitivity gene CHL1 of Arabidopsis encodes a nitrate-inducible nitrate transporter. Cell 72: 705–713 [DOI] [PubMed] [Google Scholar]
- von Wirén N, Gazzarrini S, Gojon A, Frommer WB (2000) The molecular physiology of ammonium uptake and retrieval. Curr Opin Plant Biol 3: 254–261 [PubMed] [Google Scholar]
- Walch-Liu P, Filleur S, Gan Y, Forde BG (2005) Signaling mechanisms integrating root and shoot responses to changes in the nitrogen supply. Photosynth Res 83: 239–250 [DOI] [PubMed] [Google Scholar]
- Wang R, Crawford NM (1996) Genetic identification of a gene involved in constitutive, high-affinity nitrate transport in higher plants. Proc Natl Acad Sci USA 93: 9297–9301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Liu D, Crawford NM (1998) The Arabidopsis CHL1 protein plays a major role in high-affinity nitrate uptake. Proc Natl Acad Sci USA 95: 15134–15139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LE, Miller AJ (2001) Transporters responsible for the uptake and partitioning of nitrogenous solutes. Annu Rev Plant Physiol Plant Mol Biol 52: 659–688 [DOI] [PubMed] [Google Scholar]
- Zhang H, Jennings A, Barlow PW, Forde BG (1999) Dual pathways for regulation of root branching by nitrate. Proc Natl Acad Sci USA 96: 6529–6534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou JJ, Fernandez E, Galvan A, Miller AJ (2000) A high affinity nitrate transport system from Chlamydomonas requires two gene products. FEBS Lett 466: 225–227 [DOI] [PubMed] [Google Scholar]
- Zhuo D, Okamoto M, Vidmar JJ, Glass AD (1999) Regulation of a putative high-affinity nitrate transporter (Nrt2;1At) in roots of Arabidopsis thaliana. Plant J 17: 563–568 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.










