Abstract
Pollen tube adhesion and guidance on extracellular matrices within the pistil are essential processes that convey the pollen tube cell and the sperm cells to the ovule. In this study, we purified an additional molecule from the pistil that enhances pollen tube adhesion when combined with the SCA (stigma/stylar cysteine-rich adhesin)/pectin matrix in our in vitro assay. The enhancer of adhesion was identified as free ubiquitin (Ub). This was confirmed by use of bovine Ub as a substitute for lily (Lilium longiflorum Thunb.) stigma Ub. To study the interaction of SCA and Ub with the lily pollen tube, we labeled both proteins with biotin. We observed uptake of biotin-labeled SCA and Ub into the pollen tube cells in vitro using confocal microscopy. For SCA, a strong signal occurred first at the tip of the pollen tube, suggestive of an endocytosis event, and then progressively throughout the tube cytoplasm. SCA was also localized inside the in vivo pollen tube using immunogold electron microscopy and found to be present in endosomes, multivesicular bodies, and vacuoles, all known to be endocytic compartments. It was also confirmed that SCA is endocytosed in the in vitro adhesion assay. Internalization of SCA was increased in pollen tubes treated with exogenous Ub compared to those without Ub, suggesting that Ub may facilitate SCA endocytosis. These results show that Ub can act as an enhancer of pollen tube adhesion in vitro and that it is taken up into the pollen tube as is SCA. The Ub machinery may play a role in pollen tube adhesion and guidance in lily.
Pollination in flowering plants is mediated by a series of events that regulate cell-cell communication (signaling) between the pollen tube and the pistil. These events begin with pollen adhesion to the stigma surface, followed by pollen tube growth through the style to the ovules (Sanchez et al., 2004; Swanson et al., 2004). In lily (Lilium longiflorum Thunb.), with a wet and hollow stigma/style, pollen tube adhesion is a mechanism for guidance of the pollen tube toward the ovary (Lord et al., 1996; Jauh et al., 1997; Lord, 2000, 2001; Lord and Russell, 2002; Kim et al., 2003).
So far, two molecules involved in pollen tube adhesion have been isolated from lily pistils. One is SCA (stigma/stylar Cys-rich adhesin), a protein with sequence similarity to lipid transfer proteins (LTPs), and the other is a pectin. SCA was localized in the extracellular matrix (ECM) of the stigma and style, where pollen tubes adhere and are guided to the ovule (Park et al., 2000; Kim et al., 2003). The pectin is a low-esterified pectic polysaccharide, also localized to the ECM of the stigma/style (Mollet et al., 2000). An in vitro adhesion assay was developed (Jauh et al., 1997) and recombinant SCA was found to be active when combined with pectin in the in vitro assay (Park and Lord, 2003). The activity levels were decreased in these experiments compared to those found using a SCA preparation referred to as stigma proteins (SPs), which is enriched in SCA protein, indicating that there might be an additional factor that can enhance adhesion.
Previously, immunolocalization experiments demonstrated that SCA binds to pollen tubes grown in vivo, suggesting that SCA acts as an adhesin binding the pollen tubes to the stylar transmitting tract (Park et al., 2000). However, confocal microscopy on cryo-sections of in vivo-grown pollen tubes showed an antibody signal inside the pollen tube cytoplasm, and quantum dot-labeled SCA was also found to be internalized, indicating that SCA is taken up into the pollen tubes in vivo (Ravindran et al., 2005). These data do not support the idea of SCA as an adhesin protein linking the pectin matrices between the stylar transmitting tract epidermis (TTE) and pollen tube, but rather suggest a more complex mechanism of action.
In this article, we provide a detailed study of the endocytosis of SCA into pollen tubes using a biotin labeling system and immuno transmission electron microscopy (TEM). We found that SCA is endocytosed into the pollen tube starting at the tip and subsequently moves through an endocytic route. We discovered the additional component that enhances adhesion activity by using a biochemical/proteomics approach. Analysis using electrospray ionization (ESI)-tandem mass spectrometry (MS/MS) revealed this unknown component as free ubiquitin (Ub). In addition, we found that exogenous Ub facilitates the endocytosis of SCA into pollen tubes. Taken together, these data suggest a more complex mechanism of action of SCA in pollen tube adhesion, one that may involve both endocytosis and the ubiquitination machinery of the cell.
RESULTS
Efficient Purification of SCA Protein Using Methanol Precipitation and a Bio-Gel P-10 Size-Exclusion Column
We report various new methods to increase the efficiency of purification of SPs enriched in SCA. In this study, methanol precipitation was used for preparing SPs after extraction of total stigma exudates with phosphate buffered saline (PBS) buffer. In general, it is well known that methanol precipitates proteins as does acetone. In the lily extract, however, SPs are not completely precipitated by methanol, with most remaining in the methanol supernatant. PBS extracts of lily stigma were separated as two fractions, pellet and supernatant, using methanol precipitation with different concentrations (50%–80%). In the 70% to 80% supernatant, one major band (10 kD) and three minor bands were more clearly separated on SDS-PAGE gels stained with Coomassie Brilliant Blue (Fig. 1A, top). SCA was detected not only in the supernatant fractions but also in the pellet fractions after methanol treatment. Chemocyanin, however, which is another small basic protein that copurifies with SCA and is involved in pollen tube chemotropism (Kim et al., 2003), was only detected in the supernatant fractions (Fig. 1A, bottom). The 70% supernatant was directly applied to a CM Sephadex column to better separate proteins in SPs. SCA was eluted in the 0.25 m NaCl fraction (fractions 1–4; Fig. 1B). However, three minor bands were detected in the unbound fraction. Bio-Gel P-10 (Bio-Rad) filtration (Fig. 1C) was used to further separate the preparation into seven fractions, resulting in a separation of chemocyanin from some SCA fractions. However, SCA was evenly distributed in all of the fractions. As a result, we obtained enough protein for assays (approximately 25 mg/65 g stigma) and especially chemocyanin-free SCA (fractions 26–30) with this new purification method. The following studies were performed with SCA or SPs purified by this method.
Figure 1.
SP purification. A, Total PBS extracts (T) were precipitated with various concentrations (50%, 60%, 70%, and 80%) of methanol and then separated as two fractions, pellet and supernatant. B, Among them, the 70% methanol supernatant was subjected to the CM-Sephadex C-25 cation exchange column and eluted stepwise with 0.1 and 0.25 m NaCl in 20 mm MES buffer. C, To further separate elutes 1 and 2 in 0.25 m NaCl (B), they were fractionated on Bio-Gel P-10 gel (Bio-Rad). Fraction size in B and C: 20 mL and 1.5 mL, respectively. Five micrograms of proteins was loaded into the SDS-PAGE gel. Western-blot analysis of gels using purified polyclonal antibodies raised from HPLC-purified SCA (Anti-SCA) and Escherichia coli-expressed chemocyanin (Anti-Chemo) were performed. S, Methanol supernatant fraction. UB, Unbound proteins. SP, Starting material as a positive control. Molecular mass standards (in kD) are shown on the left. Blot (C) was stained with Ponceau S. The secondary antibody was an affinity-purified goat anti-rabbit IgG alkaline phosphatase and blots were detected with NBT/BCIP.
Fraction 30 Contains SCA and an Enhancer of Pollen Tube Adhesion Activity
Fractions (27–30) were evaluated for their activities in the pollen tube adhesion assay in combination with stylar pectin. Each fraction revealed different activities in the adhesion assay (Fig. 2A). Among them, fraction 30 showed the strongest adhesion activity when the same amount of protein (5 μg/lane) was used for the assay (Fig. 2A, insert). To determine their composition, fractions 27 and 30 were analyzed by ESI-MS. As seen in Figure 2B, the major component has a mass of 9,369 D, which corresponds to SCA (Kim et al., 2003), indicating that an additional component in fraction 30 is involved in enhancing lily pollen tube adhesion.
Figure 2.
Fraction 30 contains SCA and the unknown enhancer of pollen tube adhesion activity. Adhesion assays were done using fractions 27 to 30. A, For each assay, 5 μg of fractionated SCA and 25 μg of pectin were used. Inserted box in A indicates a loading control. Proteins were separated on a 15% acrylamide gel and stained with Coomassie Brilliant Blue. Bars indicate the sd from the mean for three replicates. B, ESI-MS spectra of fractions 27 and 30. Peaks at m/z 9,369.4 and 9,369.6 correspond to the SCA1 molecular mass, which predominated in both fractions 27 and 30.
Adhesion Activity of SCA (Peak 2) Increases When It Is Combined with Peak 3
Fraction 30 was separated by reverse-phase HPLC into five peaks, including one major peak and four other proteins (peaks 1, 3, 4, and 5; Fig. 3A). A western-blot analysis using SCA antibodies revealed that the major peak (2) corresponds to SCA (Fig. 3B, right). The amounts of protein in fraction 30, HPLC-purified peak 2, and peaks 2 and 3 were estimated by SDS-PAGE prior to use in the assay (Fig. 3B, left). Peak 2 (5 μg) alone showed a weak adhesion activity compared to fraction 30 (Fig. 3C). However, full activity in the adhesion assay was recovered when peak 3 (125 ng) was combined with peak 2 (5 μg; Fig. 3, C and D). These results indicated that peak 3 was the enhancer of pollen tube adhesion in fraction 30. Peaks 4 and 5 showed no enhancing activity when combined with peak 2 (data not shown).
Figure 3.
Further separation of fraction 30 reveals the enhancer of adhesion as peak 3. A and B, HPLC profile of fraction 30 (A), SDS gel stained with Coomassie Brilliant Blue, and western blot (WB; B) of peak 2 against anti-SCA antibody. The secondary antibody was used with an affinity-purified goat anti-rabbit IgG alkaline phosphatase and blots were detected with NBT/BCIP. B, Proteins were separated on a 15% acrylamide gel and stained with Coomassie Brilliant Blue. Lane 1 is fraction 30 prior to HPLC (5 μg), lane 2 is HPLC-purified SCA (peak 2 in A, 5 μg), and lane 3 is a mixture of HPLC-purified SCA (peak 2 in A, 5 μg) and peak 3 (50 ng). Molecular mass standards (in kD) are shown at left. C, Adhesion assays. For each assay, 5 μg of HPLC-purified SCA and 25 μg of pectin were used. Bars indicate the sd from the mean for three replicates. D shows adhesion assay using 25 μg of pectin and, for the SCA source, fraction 30, peak 2 alone, and peaks 2 and 3 combined. Assays were stained with Coomassie Brilliant Blue to detect adhered pollen tubes.
Peak 3 Protein Is Identified as Ub
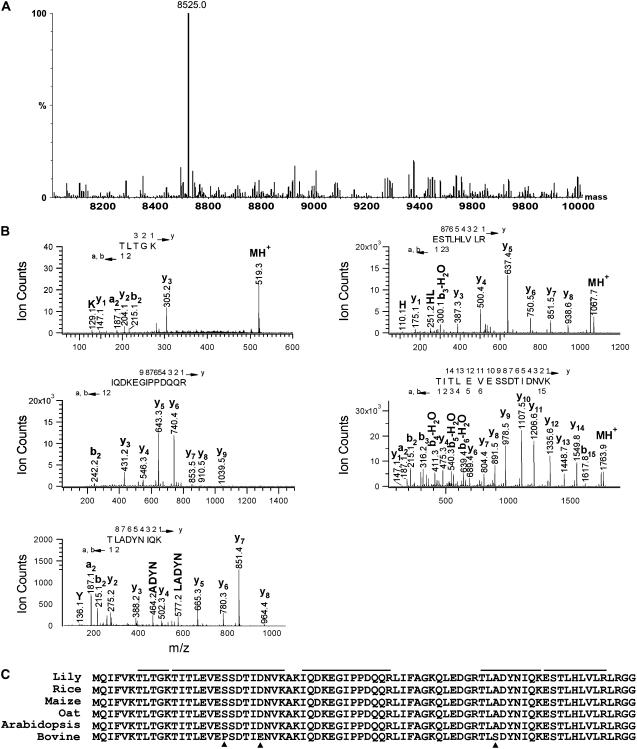
Peak 3 was collected and analyzed by ESI-MS to determine the accurate molecular mass and its purity (Fig. 4A). One protein in peak 3 (8,525 D) was prominently present but not clearly visualized, probably due to its very low amount (Fig. 4A). The amount of peak 3 in fraction 30 was determined by MASSLYNX chromatogram integration. The amount of peak 3 was approximately 2.5% of the total SP fraction (50 μg/mL; Kim et al., 2003) and found to be approximately 40:1. Peak 3 was subjected to nano ESI-MS/MS, following tryptic digestion, and five peptides were analyzed (Fig. 4B). All peptides analyzed were readily identified as free Ub (Fig. 4C). The Ub amino acid sequences from various plants (rice [Oryza sativa], maize [Zea mays], oats [Avena sativa], and Arabidopsis [Arabidopsis thaliana]) showed 100% amino acid identity with lily Ub. Indeed, only three amino acid differences were seen when lily Ub was compared to bovine Ub (Fig. 4C).
Figure 4.
Peak 3 is Ub. A, Spectrum of ESI-MS of HPLC-purified peak 3. B, The major peak was eluted at m/z 8,525 D. Shown is nano LC-MS/MS sequence analysis of trypsin-digested peptides from peak 3. The analyzed peptide sequences (TITLEVESSDTIDNVK, ESTLHLVLR, TLADYNIQK, IQDKEGIPPDQQR, and TLTGK) correspond to Ub. C, This is indicated along with the b (N-terminal) and y (C-terminal) fragment ions. Full-length amino acid sequence of lily Ub was compared with plant and bovine Ub. The peptides identified by nano LC-MS/MS in Ub are underlined. Arrowheads indicate the different amino acids compared to bovine Ub.
Bovine Ub Can Substitute for Peak 3 in the Adhesion Assay
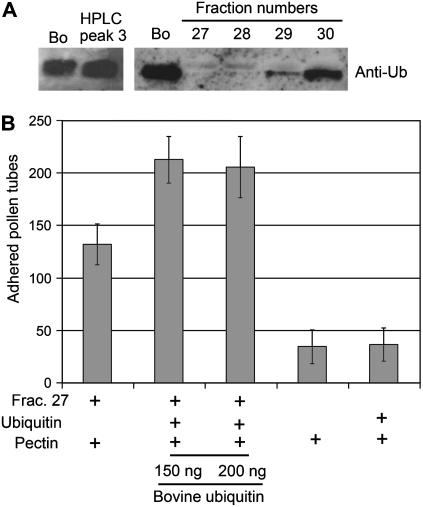
To confirm peak 3 as free Ub, we used anti-human Ub western-blot analysis on HPLC-purified peak 3 and each fraction (fractions 27–30). Peak 3 showed a clear positive signal in the western blot (Fig. 5A, left). It was also shown that Ub is present in fractions 29 and 30, both of which exhibited the strongest adhesion activity, but it was not detected in fractions 27 and 28, both of which showed weak activity (Fig. 5A, right). To confirm that Ub was working as an enhancer in pollen tube adhesion, we tested commercially made bovine Ub in the adhesion assay (Fig. 5B). Fraction 27 showed a weak adhesion activity by itself but, when combined with bovine Ub, at the concentration of 150 and 200 ng per matrix, which corresponds to the amount of peak 3 in fraction 30, it showed increased activity. In addition, a dosage-dependent experiment confirmed that bovine Ub increases adhesion activity when combined with SCA (Supplemental Fig. S1). Both bovine Ub plus pectin and pectin alone were used as negative controls (Fig. 5B). Taken together, bovine Ub enhanced the adhesion activity of SCA, as did lily stigma Ub.
Figure 5.
Bovine Ub can substitute for peak 3 in the pollen tube adhesion assay. A, Western-blot analysis of HPLC-purified peak 3 and fractions 27 to 30 was carried out using anti-human Ub. Bovine Ub (Bo; 200 ng) was used as a positive control. Ten micrograms of protein from each fraction was loaded into the SDS-PAGE gel. After treatment with primary antibody (dilution 1/200), the blot was incubated with an affinity-purified goat anti-rabbit IgG horseradish peroxidase-conjugated secondary antibody (Bio-Rad), which was detected by the enhanced chemiluminescence system. B, Adhesion assays were conducted with lily pollen tubes on a pectin matrix coated with SP fraction 27 (SCA) and bovine Ub. For each assay, 5 μg of fraction 27 (SCA) and 25 μg of pectin were used. Bovine Ub (150 ng and 200 ng) was also used. As negative controls, pectin alone and a pectin/Ub mixture without SCA were used. Bars indicate the sd from the mean for three replicates.
SCA Binds First to the Pollen Tube Tip and Then Is Endocytosed
We used Sulfo-SBED (Pierce) containing a disulfide bridge and biotin molecules to label SCA. To test whether SCA is successfully labeled with Sulfo-SBED, labeled (LSCA; 5 μg) and unlabeled SCA (5 μg) were applied on both reducing (Fig. 6A, left) and nonreducing (Fig. 6A, right) SDS-PAGE gels. This was done because Sulfo-SBED contains a disulfide bridge. Biotin was clearly detected in the nonreducing gel at 10-kD size, which corresponds to the unlabeled SCA, as shown in Figure 6A (right). This implies that biotin-labeled SCA (LSCA) was successfully generated without changing its size. LSCA can still function in the adhesion assay along with pectin (50 μg), demonstrating that this system is useful as a tool to see localization of SCA on the pollen tube (Fig. 6B). As expected, LSCA is endocytosed into the pollen tube through its tip (Fig. 6C), where it is then routed through endosomes to the vacuoles (Blackbourn and Jackson, 1996; Hepler et al., 2001). Confocal images of pollen tubes treated with biotin LSCA showed specific pollen tube tip localization at an early stage (2.5 min after treatment of the pollen tubes with LSCA). When incubation duration was increased (5–10 min after treatment), LSCA appeared inside the pollen tube starting at the tip (Fig. 6C). Scanned confocal images of pollen tubes at 2.5 and 10 min are seen in Supplemental Figure S1. To confirm whether SCA also is endocytosed in in vitro adhered pollen tubes, we used the adhesion assay. Pollen tubes were adhered to a matrix containing pectin and SCA bound to a cellophane sheet. Immunolocalization using purified SCA antibody results showed that adhered pollen tubes contained SCA (Fig. 6D). In addition, a colocalization experiment confirmed that LSCA and SCA colocalized on the emerging tip of a young pollen tube (Supplemental Fig. S3A). An antibody to SCA was used to confirm the Sulfo-SBED data. LSCA was localized on the pollen tube tip and also detected within the pollen tube. On the other hand, SCA detected with antibody appeared only at the pollen tube tip due to the difficulty of penetration of the antibody into the pollen tube (Supplemental Fig. S3B). The endocytosis of LSCA and pollen tube adhesion were both inhibited in the presence of sodium azide, a known inhibitor of endocytosis (data not shown). Taken together, these results indicate that SCA binds the pollen tube tip and then is taken up into the pollen tube in the manner expected for an endocytosis event.
Figure 6.
SCA binds to the pollen tube tip and is endocytosed. A, Labeled (LSCA) and unlabeled (SCA) SCA were run in the SDS-PAGE gels. Left and right gels are reduced and nonreduced gels, respectively. Blots were stained with Ponceau S. The signal was detected with a streptavidin conjugated with horseradish peroxidase. Molecular mass standards (in kD) are shown at left in lane 1. B, Comparison of activities of LSCA and unlabeled SCA in the adhesion assay. For this assay, 10 μg of SCA or LSCA and 50 μg of pectin were used to make the matrix. Bars indicate the sd from the mean for three replicates. C, Localization of LSCA using Sulfo-SBED. Pollen tubes were treated with 100 μg/mL LSCA, Sulfo-SBED alone, and LBSA (100 μg/mL). The signal was detected with streptavidin conjugated with FITC (1/200). D, Immunolocalization of SCA in adhered pollen tubes using purified SCA antibody. Lily pollen tube adhesion assay was performed on cellulose sheets coated with pectin and SCA. Adhered and in vitro-grown pollen tubes were fixed, sectioned, and analyzed with antibodies against SCA (dilution 1/50). FITC-conjugated secondary antibody (dilution 1/100) was used to detect signals. Pollen tubes were examined under a confocal microscope (TCS SP2; Leica) at 488 nm excitation. Bars represent 10 μm.
Localization of SCA in the Lily Style and in in Vivo-Grown Pollen Tubes
To confirm whether SCA is indeed localized within the endocytic compartments of the endosome, the multivesicular bodies (MVBs), and the vacuole in in vivo-grown pollen tubes, we performed immunogold localization using TEM. Figure 7B shows signal in the cytoplasm, wall, and secretory matrix in the TTE cells of the style where SCA is produced and secreted into the ECM. There was no signal in the negative control in both the TTE and the pollen tubes (Fig. 7, A and C). This result is consistent with previous immunolocalization data at the light microscope level (Park and Lord, 2003). Immunogold labeling of SCA in in vivo-grown pollen tubes confirms the LSCA localization data, showing that SCA is endocytosed. Gold particles were observed in the MVBs (Fig. 7, F and G) and vacuoles (Fig. 7, D and E), both endocytic compartments (Murphy et al., 2005; Samaj et al., 2004, 2005). Gold label was also found in the cytoplasm in the form of clusters overlying uniform structures that may be early endosomes (Fig. 7, H–J). These results show that SCA enters into the pollen tube via an endocytic route.
Figure 7.
Immunogold localization of SCA in the TTE of the style and in vivo pollen tubes. Thin sections were prepared from chemically fixed specimens and immunostained with affinity-purified antibodies against HPLC-purified SCA (dilution 1/5). A (secondary antibody, control) and B (anti-SCA), Localization of SCA on lily TTE. C (secondary antibody, control) to H, Localization of SCA in in vivo-grown pollen tubes (D to J: anti-SCA). Arrows indicate internal vesicles. cyt, Cytoplasm of TTE; C, stylar canal; g, Golgi; mvb, multivesicular body; OW, outer wall of TTE; V, vacuole. Bars represent 200 nm.
Localization of Ub in the Lily Style and in in Vivo-Grown Pollen Tubes
We used N-terminal, biotin-labeled Ub (10 μg/mL LUb) to see whether exogenous Ub is taken up into the pollen tubes. LUb is detected throughout the pollen tubes at 5 and 10 min after treatment (Fig. 8).
Figure 8.
Uptake of LUb into the pollen tube. The N-terminal LUb was purchased from BostonBiochem. Complied confocal images are displayed. Pollen tubes were harvested at 10 min after treatment of LUb (10 μg/mL). As a negative control, Sulfo-SBED alone (Biotin alone) was used. The signal was detected with streptavidin conjugated with FITC. The pollen tubes were examined under a confocal microscope (TCS SP2; Leica) at 488 nm excitation. Bars represent 10 μm.
Using immuno TEM, localization of Ub was surveyed in both the TTE cell and the pollen tube. Ub was detected in the cytoplasm of the TTE, but not in the wall or ECM part of this cell (Fig. 9A). Figure 9B shows that most of the label, corresponding to both free Ub and conjugated Ub, was in the cytoplasm in the pollen tubes. This is where the enzymes of the Ub protein ligase system and the proteasome reside. Our results are in agreement with others for the subcellular localization of free Ub or ubiquitinated proteins (Beers et al., 1992). To determine whether Ub is secreted out of the TTE cells of the stigma, we harvested exudates from wet stigmas and then used them for western-blot analysis, but no Ub signal was detected (data not shown). These data show that Ub is not secreted from the TTE cells at detectable levels. Therefore, we may have inadvertently discovered an effect of Ub on adhesion by releasing it from its intracellular stores in the process of SCA protein purification. Nevertheless, the addition of Ub to the adhesion assay enhanced the adhesion effect.
Figure 9.
Immunogold localization of Ub in the stylar TTE and in vivo pollen tubes. Thin sections were prepared from chemically fixed specimens and immunostained with anti-Ub from Santa Cruz Biotechnology (dilution 1/10). A, Localization of Ub on lily TTE. B, Localization of Ub in in vivo-grown pollen tubes. cyt, Cytoplasm of TTE; C, stylar canal; OW, outer wall of TTE; V, vacuole. Bars represent 200 nm.
Ub Facilitates the Endocytosis of SCA into the Pollen Tubes
To investigate whether Ub facilitates SCA endocytosis, we assessed the effects of cotreatment of in vitro-grown pollen tubes with and without added bovine Ub (Fig. 10). When pollen tubes were then tested for the presence of internalized SCA, using western-blot analysis (Fig. 10), the lowest levels were seen in the pollen tube samples treated with SCA alone and the highest levels were in those treated with SCA plus bovine Ub. However, when we used FM (membrane-associated lipophilic styryl) dye to monitor endocytosis in living pollen tubes in vitro, with added SCA and Ub, we did not see an effect on the total level of endocytosis (data not shown). This suggests that the increase in internalized SCA, when pollen tubes are grown in the presence of Ub, may be due to a specific increase in SCA endocytosis.
Figure 10.
Western-blot analysis of SCA in in vitro-grown pollen tubes exposed to Ub and SCA in the medium. Pollen tubes grown in vitro were harvested at 30 min after treatment of SCA and bovine Ub (bUb). Left, The first lane is total pollen tube proteins without any treatment of SCA or BUb (C). The second lane is 10 μg/mL SP fraction without lily Ub (Fraction). The third lane is 10 μg/mL SP fraction plus 1 μg/mL bovine Ub (Fraction + bUb). Right, The first lane is 10 μg/mL HPLC-purified SCA (HPLC SCA). The second lane is HPLC SCA plus 1 μg/mL bovine Ub (HPLC SCA + bUb). The third lane is fraction 30 (SCA + peak 3) as a positive control. Pollen tubes were washed to remove nonspecific SCA from the pollen tube wall. Proteins were extracted from the pollen tubes with SDS-PAGE buffer. Ten micrograms of total protein extracted was loaded in each lane. Blot (Anti-SCA) was immunostained with affinity-purified antibodies against HPLC-purified SCA (dilution 1/5). P, Ponceau S.
DISCUSSION
Endocytosis of SCA
We report here for the first time, to our knowledge, the use of a biotin label to trace the uptake of proteins into the pollen tube. The small basic protein SCA is involved in adhesion and guidance in lily. The pistil produces SCA in the ECM of the transmitting tract, and, together with pectin, SCA makes an adhesive matrix. The model we reported previously was that SCA, a 9.4-kD peptide, binds the pollen tube, providing a link between the pectins on the pollen tube wall and those on the stylar TTE (Park et al., 2000). Localization data, though, using SCA antibodies, showed that SCA was found inside in vivo-grown pollen tubes, even though it is not produced by the pollen tube (Ravindran et al., 2005). We utilized quantum dot-labeled SCA and detected signals inside the pollen tube as well as at the tip, but the labeling method was difficult and hard to reproduce. This result suggested that SCA is endocytosed. In this study, we sought to further investigate the apparent endocytosis of SCA into pollen tubes and to revise the model we suggested previously for pollen tube adhesion (Park et al., 2000). In general, fluorescein isothiocyanate (FITC) has been used to label proteins. However, it was reported that FITC itself inhibited pollen tube growth at low concentrations (O'Driscoll et al., 1993). So, we utilized LSCA as a detection system using Sulfo-SBED containing a disulfide bridge and a biotin molecule (Fig. 7A).
Biotin has been used frequently as an ideal adaptor due to its small size and low toxicity in vivo (Suzuki and Dale, 1987). One difficulty with using biotin, though, as reported, is that any protein labeled with biotin is endocytosed. Insulin, RNase, and bovine serum albumin (BSA) have been used to study endocytosis in cultured soybean (Glycine max) and rice cells (Horn et al., 1990, 1992; Bahaji et al., 2001). In these studies, the proposal was that receptor-mediated endocytosis occurred in plant cells via biotin receptors located on the plasma membrane. However, there is no evidence for a biotin receptor in plants. When this literature is critically examined, the data conflict. In one study using biotin-labeled BSA (LBSA), the uptake of the BSA was drastically decreased in rice cells when they were in the exponential phase of growth (Bahaji et al., 2001). In soybean cell cultures, however, the results were opposite to those using rice cells (Horn et al., 1992). In vitro culture of cell suspensions can produce many artifacts. We used LBSA and biotin alone as negative controls and neither was internalized into the lily pollen tube, but LSCA was shown to be taken up at the pollen tube tip. We have independent localization data showing that SCA does enter the pollen tube from the style in vivo, so the biotin labeling experiment with SCA in vitro is credible. We suggest that the pollen tube is a more optimal system for examination of protein endocytosis than use of the in vitro-cultured suspension cells.
Endocytosis is an essential process occurring in all eukaryotic cells from mammals to plants. It regulates many processes, including cell-to-cell communication and nutrient uptake (Samaj et al., 2004, 2005; Murphy et al., 2005). In animal cells and yeast, receptor-mediated endocytosis involves the uptake of specific macromolecules bound to plasma membrane receptors and occurs via clathrin-coated vesicles. Therefore, major functions of clathrin are the internalization of membrane receptors and the correct targeting of clathrin-coated vesicles to the early endosome, MVB, or vacuole. We found evidence that SCA is endocytosed into pollen tubes and localized to compartments using TEM. A polarized cell like the pollen tube must recycle a huge amount of membrane, resulting in massive vesicle delivery to the tip growing region, the site of endocytosis and exocytosis. The pollen tube tip is also where external signals are perceived in vivo (Hepler et al., 2001). In elongating tobacco (Nicotiana tabacum) pollen tubes, clathrin-coated pits and vesicles have been visualized in the apical regions and clathrin was immunolocalized to an area just back from the tip of the pollen tube (Blackbourn and Jackson, 1996), suggesting that clathrin-dependent endocytosis occurs in the pollen tube (Holstein, 2002). This has been confirmed using the amphiphilic styryl FM dyes as membrane-selective markers to monitor endocytosis (Emans et al., 2002). These dyes were taken up into the lily pollen tube through its tip (Parton et al., 2001).
Plasma membrane receptors (LePRK1 and 2) identified in tomato (Lycopersicon esculentum) pollen tubes (Muschietti et al., 1998) were proposed to interact with signal molecules like LAT52 and LeSTIG that play key roles in pollen tube growth (Tang et al., 2002, 2004). Many stylar-secreted proteins, such as S-RNase, 120-kD glycoprotein, and a small Asn-rich protein (HT-B) required for S-specific pollen rejection in incompatible interactions, have been reported to be taken up into the pollen tube (Luu et al., 2000; Goldraij et al., 2006). However, there is no direct evidence that factors required for pollen-pistil interaction are internalized through the pollen tube tip. To our knowledge, our data are the first evidence for endocytosis of a pistil factor through the pollen tube tip.
Recently, plant cell surface receptors that are internalized by endocytosis were discovered (Shah et al., 2002; Ron and Avni, 2004; Russinova et al., 2004; Gifford et al., 2005). In animals and yeast, many studies have also shown that ubiquitination, like phosphorylation, can provide a signal coordinating a network of interactions required for endocytosis. Monoubiquitin, attached to cargo proteins, serves as a signal for the endocytosis of some transmembrane proteins, such as growth factor receptors, plasma membrane channels, and transporters (Hicke, 2001; Horak, 2003). More recently, the flagellin receptor FLS2, tagged with green fluorescent protein, was seen to be internalized into intracellular mobile vesicles by stimulation with the ligand protein flagellin flg22. In addition, internalization of FLS2 was strongly inhibited by MG132, a proteasome inhibitor, suggesting that the Ub-proteasome activity influences FLS2 endocytosis (Robatzek et al., 2006).
Effect of Exogenous Ub on Pollen Tube Adhesion
We found a pollen tube adhesion-enhancing component from the lily stigma to be monoubiquitin. Though we have no evidence that monoubiquitin is secreted into the ECM of the stigma or style, there is a possibility that it is there in low amounts, sufficient to enhance adhesion. Ub is a heat-stable, 8.5-kD protein composed of 76 amino acids and showing a highly conserved sequence from protozoans to animals and plants. The ubiquitinated proteins are then targeted for degradation by the 26S proteasome in an ATP-dependent manner. In plants, the ubiquitination pathway has also been tightly involved in a variety of cellular processes, including pollen germination (Speranza et al., 2001), self-incompatibility (Stone et al., 2003; Ushijima et al., 2003; Qiao et al., 2004), hypersensitive and defense responses (Zeng et al., 2004), and hormone and light responses (Xie et al., 1998; Gagne et al., 2004; Seo et al., 2004; Dharmasiri et al., 2005; Kepinski and Leyser, 2005). Ub is a cytoplasmic protein but, in some mammalian systems, Ub has also been detected in the extracellular space (Daino et al., 2000; Kieffer et al., 2003; Majetschak et al., 2003). In addition, in vitro studies have revealed the effects of exogenous Ub on induction of apoptosis, growth regulation, microbial defense, and cytokine production (Huet et al., 1994; Daino et al., 2000; Sawada et al., 2002; Kieffer et al., 2003; Majetschak et al., 2003, 2006; Kutty et al., 2005). In the case of lily, we have no direct evidence that Ub exists in the ECM of the stigma/style or that conjugation to intracellular proteins occurs after it is endocytosed.
We have shown that exogenous Ub applied at a very low concentration (approximately 100–200 ng) enhances lily pollen tube adhesion. The physiological free Ub concentration in other systems is approximately 60 ng/mL in serum and 10 fg/mL in monocytic cells, suggesting that secreted Ub does function at low concentrations (Majetschak et al., 2003, 2006). In addition, we confirmed that bovine LUb was taken up into the lily pollen tube, a result in agreement with experiments using radiolabeled (Kutty et al., 2005), biotinylated (Daino et al., 2000), and FITC-labeled Ub (Majetschak et al., 2006) in yeast and human cells.
Putative Function of SCA as a Signal Molecule
In this study, we have shown that SCA is endocytosed into the lily pollen tube through its tip and then passed through an endocytic route. We have also shown that exogenous free Ub, coextracted with SPs from the lily stigma, enhances pollen tube adhesion when combined with a SCA/pectin matrix. However, many questions remain unanswered. What is the receptor of SCA in the pollen tube tip? Is Ub secreted into the ECM of the stigma/style? What is the mechanism of pollen tube adhesion and how is SCA endocytosis a factor in adhesion? SCA is related to the LTPs (Kader, 1997). The function for these proteins is not in intracellular lipid transfer as first thought. In fact, the function of LTPs is mainly unknown. A wheat (Triticum aestivum) LTP is thought to bind to high-affinity sites on tobacco cells identified as putative elicitin receptors (Buhot et al., 2001, 2004). Elicitins are able to transfer sterols and share some structural properties with LTPs (Blein et al., 2002). In these experiments with elicitins, it was noticed that Ub was also secreted into the medium during cell culture along with the elicitins (Huet et al., 1994). These results support the idea that secreted LTP-like molecules and Ub may be interacting with a receptor in the plasma membrane. This model makes SCA a signal molecule for pollen tube adhesion in the manner of receptor-ligand interactions rather than an active adhesin. Based on our results and previous knowledge of receptor-mediated endocytosis, we propose a model for the activity of SCA in adhesion (Fig. 11).
Figure 11.
A model for endocytosis of SCA in pollen tube adhesion. A, Ub-dependent internalization of GPCR in Saccharomyces cerevisiae. Interaction of mating factor with a GPCR activates a signal transduction pathway and subsequently stimulates endocytosis of the receptor-ligand complex by ubiquitination. Modification of the receptor with monoubiquitination in the cytoplasmic side of the GPCR promotes rapid internalization, followed by degradation in the vacuole. B, SCA binds to its putative receptor (SCAR), which may be present at the membrane in the pollen tube tip. This binding would activate the signals for pollen tube adhesion, which are currently unknown. Subsequently, SCA is endocytosed through the endocytic route (early endosome, MVB, and vacuole). PM, Plasma membrane. The yeast model is from Hicke (1999) with some modification.
It has been shown that ubiquitination of membrane receptors (on their cytoplasmic side) can be triggered in response to extracellular stimuli, such as ligand binding (Hicke, 1999; Robatzek et al., 2006). For example, when the peptide pheromones or mating factors bind to yeast receptors, which are expressed on yeast cells of opposite mating types, signals are activated and transmitted through G protein-coupled receptors (GPCRs). The receptors with their ligands are subsequently ubiquitinated and down-regulated rapidly (Hicke, 1999). In yeast and animal systems, degradation of most plasma membrane proteins is known to be mediated by endocytosis in an Ub-dependent manner and the proteins are subsequently sorted into the MVBs, which are likely to represent a branch point in the endocytic pathway that leads to either the vacuole or the cell surface (Murphy et al., 2005). We found that SCA was targeted to MVBs and the vacuole in the in vivo pollen tubes. In vitro-grown pollen tubes show no detectable SCA mRNA or protein as demonstrated by northern blots, western blots (Park and Lord, 2003), and immunolocalization of in vitro-grown pollen tubes (Fig. 6D). In our data, it is unclear whether the SCA label that appears in clusters within the cytoplasm is in early endosomes, the first station and branch point in the endocytic pathway. The endosome is believed to participate in the rapid cycling of plasma membrane molecules in other systems, but this process is not well defined structurally in plants (Murphy et al., 2005). This pattern implies that SCA is subject to protein degradation after endocytosis and that the pollen tube adhesion mechanism is somehow coupled with the binding of SCA to a receptor. This process may also involve ubiquitination for a down-regulation signaling event. So, SCA could be analogous to the yeast mating factor, acting as a signal to the pollen tube by binding at the tip (Fig. 11). Our results suggest that SCA-receptor complexes enter the cell by receptor-mediated endocytosis. However, how extracellular Ub could facilitate this process is unclear. In addition, the manner in which these events are coupled to adhesion activity of the pollen tube on the stylar matrix is still a mystery.
MATERIALS AND METHODS
Plant Materials
Lily (Lilium longiflorum Thunb. cv Nellie White) plants were grown in a greenhouse in Riverside, CA. Adhesion assays were done with pollen from Nellie White. Stigmas were collected within 5 d and anthers within 2 to 3 d of anthesis.
Antibodies
The antibodies used were generated from rabbits and raised against HPLC-purified SCA and Escherichia coli-expressed recombinant chemocyanin (Dong et al., 2005). Anti-Ub was from Santa Cruz Biotechnology. To purify the SCA antibody, SPs containing SCA (2 mg) were blotted on nitrocellulose membrane. The blot stained with Ponceau S was cross-linked with 1% glutaraldehyde for 30 min at room temperature. The cross-linked blot was blocked for 2 h in TTBS (50 mm Tris-HCl, pH 7.5, 0.1% [v/v] Tween 20, and 50 mm NaCl) containing 5% (w/v) nonfat dry milk. After blocking, the membrane was incubated with the polyclonal SCA antiserum in TTBS. The antigen-antibody interaction was carried out overnight at 4°C. The blot was washed (3 × 10 min) in TTBS, and SCA antibody was eluted with 100 mm Gly, pH 2.5, for 5 min and neutralized with 1 m Tris-HCl, pH 9.0.
SP Purification
The SP protein purification method was described previously (Kim et al., 2003) and used with some modifications. Sixty-four grams of stigmas were ground in 150 mL of PBS (0.14 m NaCl, 2.7 mm KCl, 1.5 mm KH2PO4, 8.1 mm Na2HPO4, pH 7.4). The PBS soluble fraction was further separated using a methanol precipitation method. For this separation, methanol (final concentration 70%) was added into the PBS soluble fraction. Following incubation at −20°C for 2 h, methanol supernatants were collected by centrifugation at 3,000 rpm for 5 min. To verify whether SCA and chemocyanin were present in the supernatant, we performed western blots using SCA and chemocyanin antibodies (Dong et al., 2005). The methanol supernatant was directly applied to a CM-Sephadex C-25 cation exchange column equilibrated with 20 mm MES buffer, pH 5.6, without evaporating the large volume of methanol, and eluted stepwise with 0.1 and 0.25 NaCl in 20 mm MES buffer. The fractions containing SCA and chemocyanin were pooled, concentrated with Speed Vac, and further fractionated on a Bio-Gel P 10 gel column (Bio-Rad; 120 × 1 cm). Active fractions were dried and dissolved in water (MilliQ; Millipore). Proteins were quantified by the Modified Lowry Protein Assay (Pierce).
HPLC Analysis
SP fraction 30, which exhibited the strongest adhesion activity among the fractions, was further purified by capillary HPLC (Agilent 1100; Hewlett-Packard) equipped with a Jupiter C4 microbore column (2 × 200 mm, 5-μm particle diameter, 100-Å pore size; Phenomenex). SPs (50 μg), dissolved in water, were injected, and proteins were eluted with a two-step linear gradient at a flow rate of 50 μL per min by mixing mobile phases A (0.1% trifluoroacetic acid in water) and B (0.065% trifluoroacetic acid in CH3CN). The gradient was as follows: 5% to 45% of B for 30 min, 45% of B for 5 min, and then 45% to 90% of B for 50 min. All the fractions were collected manually. The solvents were evaporated, and the pellets were dissolved in water.
Adhesion Assay
The in vitro assay was performed as described previously (Park et al., 2000) with several modifications. SPs (5 μg) and pectin (25 μg) were immobilized on nitrocellulose membrane by application of vacuum using a device modified from a dot-blot apparatus, and the membrane was air-dried. The dried nitrocellulose membranes were submerged in a petri dish containing 20 mL of germination medium (Jauh et al., 1997) with pollen tubes from nine anthers pregerminated in vitro for 2 h. Pollen tubes were evenly distributed over the membranes and incubated for 4 to 5 h in the dark. The membranes were washed in germination medium to remove nonadhered pollen tubes and stained with fixing solution (50% methanol, 10% acetic acid, and 0.1% Coomassie Brilliant Blue R-250). The pollen with tubes adhering to the membranes was counted under a dissecting microscope.
Western Blot
For western blots, the fractionated proteins were analyzed by SDS-PAGE (15%) and transferred onto a nitrocellulose membrane. The procedure for western blots was followed as described above for antibody purification. Secondary goat anti-rabbit IgG conjugated with alkaline phosphatase or horseradish peroxidase in TTBS (50 mm Tris-Cl, pH 7.5, 50 mm NaCl, 0.2% Tween 20) was used for immunodetection. After the blots were washed with TTBS, the immunoblot signals were detected using NBT/BCIP (Bio-Rad) or enhanced chemiluminescent (PerkinElmer Life Sciences).
ESI-MS and Nano LC-MS/MS Analysis
Molecular weight of intact proteins was measured by nano ESI ion source using a quadrupole time-of-flight Ultima-Global instrument (Micromass). For sequencing, fractions from HPLC were dissolved in 10 μL of 25 mm NH4HCO3. After reduction of Cys residues by 25 μL of 10 mm dithiothreitol and alkylation by 25 μL of 55 mm iodoacetamide, proteins were digested with trypsin (modified sequencing grade; Roche Molecular Biochemicals) at 37°C, overnight, and extracted with 10 μL of acetonitrile/0.1% formic acid in water (50/50) solution. Sequence analysis of peptides was done by nano ESI-MS/MS with a collision energy varying from 20 to 40 eV applied in the hexapole collision cell with argon (12 psi) as the collision gas. The quadrupole time-of-flight mass spectrometer was run at a capillary voltage of 1.0 kV and a cone voltage of 77 V. The source block temperature was 80°C. Approximately 4 μL of sample was loaded into the nanoelectrospray tip (Protana) for each ESI-MS or ESI-LC-MS/MS analysis.
SCA Labeling with Sulfo-SBED
SCA was first labeled with Sulfo-SBED using the procedure described by the supplier (Pierce). In brief, SCA (1 mg) was dissolved in 400 μL of PBS. Then, 15 mg of Sulfo-SBED was dissolved in 25 μL of dimethylformamide and 3 μL of Sulfo-SBED stock was added to this mixture and incubated in the dark at 4°C for 2 h. Following this procedure, LSCA was dialyzed and then tested for function in the pollen tube adhesion assay. It was then added to pollen tube cultures for localization studies.
Localization Studies Using LSCA and LUb in Pollen Tube Cultures
Lily pollen grains (three anthers) were added to 10 mL of lily pollen tube germination medium (Jauh et al., 1997), pregerminated, and treated with LSCA (100 μg/mL), Sulfo-SBED alone, N-terminal LUb (10 μg/mL; Boston Biochem), or LBSA (100 μg/mL). For localization, pollen tubes treated with LSCA and Sulfo-SBED alone for 2.5, 5, and 10 min were fixed in 2% (w/v) paraformaldehyde in 50 mm PIPES buffer (50 mm PIPES, 2 mm EGTA, 2 mm MgSO4·7H20, pH 6.8) with 10% Suc for overnight. The pollen tubes were examined under a confocal microscope (TCS SP2; Leica) at 488 nm excitation.
Preparation of Adhered Pollen Tube Sections and Immunolocalization
Cellophane sheets were used to prepare the adhesion assays because nitrocellulose dissolved in the ethanol used for dehydration. The in vitro assay was performed as described above. Adhered pollen tubes were fixed in 2% paraformaldehyde in 50 mm PIPES buffer with 10% Suc for overnight, then washed with PIPES buffer and PBS. Tissues were then dehydrated in a series of ethanol concentrations (30%, 50%, 70%, and 100%) for 1 h and infiltrated with LR White resin (London Resin) according to the manufacturer's instructions. Finally, tissues were embedded in 100% resin in gelatin capsules and cast in a 60°C oven. Sections are cut in 3 μm thickness and collected on poly-l-Lys-coated slides. The adhered pollen tube sections were incubated with purified polyclonal antibodies raised from HPLC-purified SCA (dilution 1/50). After three washes in PBS containing 0.1% Tween 20, adhered pollen tubes were incubated with streptavidin conjugated with FITC (dilution 1/200; Sigma) for 2 h at room temperature or overnight in 4°C and then washed three times with PBS containing 0.1% Tween 20. The pollen tubes were examined under a confocal microscope (TCS SP2; Leica) at 488 nm excitation.
Immunogold Localization
Pollinated styles of lily were prepared for localization of SCA and Ub. They were cut and fixed with 1.5% glutaraldehyde in 100 mm phosphate buffer (PBS; pH 7.0). Tissues were then dehydrated in a series of ethanol concentrations (30%, 50%, 70%, and 100%) and infiltrated with LR White resin (London Resin) for 4 d by gradually increasing its concentration (33%, 67%, and 100%). Finally, tissues were embedded in 100% resin in gelatin capsules under UV at −20°C. Sections were cut at 70 to 90 nm thickness and collected on nickel grids. Ultrathin sections were incubated with a blocking solution (1% BSA, 0.1% Tween 20 in PBS) for 1 h at room temperature and then treated with affinity-purified anti-SCA and anti-Ub at primary dilutions of 1/5 and 1/10, respectively. This was followed by incubation with 18-nm gold particle-conjugated rabbit IgG at a dilution of 1/40 in PBS supplemented with 0.1% BSA and 0.01% Tween 20 overnight at 4°C after three washes. Secondary antibodies alone were used as a negative control. Specimens are stained with 1% uranyl acetate and Reynold's lead citrate, and then rinsed with water. Sections were examined with a Philips Tecnai 12 TEM at 80 kV.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Dosage-dependent adhesion assay.
Supplemental Figure S2. Optical-sectioned confocal images.
Supplemental Figure S3. Colocalization of FITC-labeled SCA and biotin-labeled SCA.
Acknowledgments
We thank Dr. Zhenbiao Yang, Dr. Glenn Hicks, and Dr. Sunran Kim for their critical reading of the manuscript. We thank Kimberly Tan for general lab assistance.
This work was supported by the National Science Foundation (grant no. IBM0420445 to E.M.L.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Elizabeth M. Lord (lord@ucr.edu).
The online version of this article contains Web-only data.
References
- Bahaji A, Cornejo MJ, Ortiz-Zapater E, Contreras I, Aniento F (2001) Uptake of endocytic markers by rice cells: variations related to the growth phase. Eur J Cell Biol 80: 178–186 [DOI] [PubMed] [Google Scholar]
- Beers EP, Moreno TM, Callis J (1992) Subcellular localization of ubiquitin and ubiquitinated proteins in Arabidopsis thaliana. J Biochem (Tokyo) 267: 15432–15439 [PubMed] [Google Scholar]
- Blackbourn HD, Jackson AP (1996) Plant clathrin heavy chain: sequence analysis and restricted localisation in growing pollen tubes. J Cell Sci 109: 777–787 [DOI] [PubMed] [Google Scholar]
- Blein JP, Coutos-Thevenot P, Marion D, Ponchet M (2002) From elicitins to lipid-transfer proteins: a new insight in cell signalling involved in plant defence mechanisms. Trends Plant Sci 7: 293–296 [DOI] [PubMed] [Google Scholar]
- Buhot N, Douliez JP, Jacquemard A, Marion D, Tran V, Maume BF, Milat ML, Ponchet M, Mikes V, Kader JC, et al (2001) A lipid transfer protein binds to a receptor involved in the control of plant defence responses. FEBS Lett 509: 27–30 [DOI] [PubMed] [Google Scholar]
- Buhot N, Gomes E, Milat ML, Ponchet M, Marion D, Lequeu J, Delrot S, Coutos-Thevenot P, Blein JP (2004) Modulation of the biological activity of a tobacco LTP1 by lipid complexation. Mol Biol Cell 15: 5047–5052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daino H, Matsumura I, Takada K, Odajima J, Tanaka H, Ueda S, Shibayama H, Ikeda H, Hibi M, Machii T, et al (2000) Induction of apoptosis by extracellular ubiquitin in human hematopoietic cells: possible involvement of STAT3 degradation by the proteasome pathway in interleukin 6-dependent hematopoietic cells. Blood 95: 2577–2585 [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Estelle M (2005) The F-box protein TIR1 is an auxin receptor. Nature 435: 441–445 [DOI] [PubMed] [Google Scholar]
- Dong J, Kim ST, Lord EM (2005) Plantacyanin plays a role in reproduction in Arabidopsis. Plant Physiol 138: 778–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emans N, Zimmermann S, Fischer R (2002) Uptake of a fluorescent marker in plant cells is sensitive to brefeldin A and wortmannin. Plant Cell 14: 71–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagne JM, Smalle J, Gingerich DJ, Walker JM, Yoo SD, Yanagisawa S, Vierstra RD (2004) Arabidopsis EIN3-binding F-box 1 and 2 form ubiquitin-protein ligases that repress ethylene action and promote growth by directing EIN3 degradation. Proc Natl Acad Sci USA 101: 6803–6808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford ML, Robertson FC, Soares DC, Ingram GC (2005) Arabidopsis CRINKLY4 function, internalization, and turnover are dependent on the extracellular crinkly repeat domain. Plant Cell 17: 1154–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldraij A, Kondo K, Lee CB, Hancock CN, Sivaguru M, Vazquez-Santana S, Kim S, Phillips TE, Cruz-Garcia F, McClure B (2006) Compartmentalization of S-RNase and HT-B degradation in self-incompatible Nicotiana. Nature 439: 805–810 [DOI] [PubMed] [Google Scholar]
- Hepler PK, Vidali L, Cheung AY (2001) Polarized cell growth in higher plants. Annu Rev Cell Dev Biol 17: 159–187 [DOI] [PubMed] [Google Scholar]
- Hicke L (1999) Gettin′ down with ubiquitin: turning off cell-surface receptors, transporters and channels. Trends Cell Biol 9: 107–112 [DOI] [PubMed] [Google Scholar]
- Hicke L (2001) A new ticket for entry into budding vesicles-ubiquitin. Cell 106: 527–530 [DOI] [PubMed] [Google Scholar]
- Holstein SEH (2002) Clathrin and plant endocytosis. Traffic 3: 614–620 [DOI] [PubMed] [Google Scholar]
- Horak J (2003) The role of ubiquitin in down-regulation and intracellular sorting of membrane proteins: insights from yeast. Biochim Biophys Acta 1614: 139–155 [DOI] [PubMed] [Google Scholar]
- Horn MA, Heinstein PF, Low PS (1990) Biotin-mediated delivery of exogenous macromolecules into soybean cells. Plant Physiol 93: 1492–1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn MA, Heinstein PF, Low PS (1992) Characterization of parameters influencing receptor-mediated endocytosis in cultured soybean cells. Plant Physiol 98: 673–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huet JC, Salle-Tourne M, Pernollet JC (1994) Amino acid sequence and toxicity of the α elicitin secreted with ubiquitin by Phytophthora infestans. Mol Plant-Microbe Interact 7: 302–304 [DOI] [PubMed] [Google Scholar]
- Jauh GY, Eckard KJ, Nothnagel EA, Lord EM (1997) Adhesion of lily pollen tubes on an artificial matrix. Sex Plant Reprod 10: 178–180 [Google Scholar]
- Kader J-C (1997) Lipid transfer proteins: a puzzling family of plant proteins. Trends Plant Sci 2: 66–70 [Google Scholar]
- Kepinski S, Leyser O (2005) The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature 435: 446–451 [DOI] [PubMed] [Google Scholar]
- Kieffer AE, Goumon Y, Ruh O, Chasserot-Golaz S, Nullans G, Gasnier C, Aunis D, Metz-Boutigue MH (2003) The N- and C-terminal fragments of ubiquitin are important for the antimicrobial activities. FASEB J 17: 776–778 [DOI] [PubMed] [Google Scholar]
- Kim S, Mollet J-C, Dong J, Zhang K, Park S-Y, Lord EM (2003) Chemocyanin, a small, basic protein from the lily stigma, induces pollen tube chemotropism. Proc Natl Acad Sci USA 100: 16125–16130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutty BC, Pasupathy K, Mishra KP (2005) Effects of exogenous ubiquitin on cell division cycle mutants of Schizosaccharomyces pombe. FEMS Microbiol Lett 244: 187–191 [DOI] [PubMed] [Google Scholar]
- Lord EM (2000) Adhesion and cell movement during pollination: cherchez la femme. Trends Plant Sci 5: 368–373 [DOI] [PubMed] [Google Scholar]
- Lord EM (2001) Adhesion molecules in lily pollination. Sex Plant Reprod 14: 57–62 [Google Scholar]
- Lord EM, Russell SD (2002) Mechanisms of pollination and fertilization in plants. Annu Rev Cell Dev Biol 18: 81–105 [DOI] [PubMed] [Google Scholar]
- Lord EM, Walling LL, Jauh GY (1996) Cell adhesion in plants and its role in pollination. In M Smallwood, JP Knox, DJ Bowles, eds, Membranes: Specialized Functions in Plants. BIOS Scientific Publishers, Oxford, pp 21–37
- Luu DT, Xike Q, Morse D, Cappadocia M (2000) S-RNase uptake by compatible pollen tubes in gametophytic self-incompatibility. Nature 407: 649–651 [DOI] [PubMed] [Google Scholar]
- Majetschak M, Krehmeier U, Bardenheuer M, Denz C, Quintel M, Voggenreiter G, Obertacke U (2003) Extracellular ubiquitin inhibits the TNFα response to endotoxin in peripheral blood mononuclear cells and regulates endotoxin hyporesponsiveness in critical illness. Blood 101: 1882–1890 [DOI] [PubMed] [Google Scholar]
- Majetschak M, Ponelies N, Hirsch T (2006) Targeting the monocytic ubiquitin system with extracellular ubiquitin. Immunol Cell Biol 84: 59–65 [DOI] [PubMed] [Google Scholar]
- Mollet J, Park S, Nothnagel EA, Lord EM (2000) A lily stylar pectin is necessary for pollen tube adhesion to an in vitro stylar matrix. Plant Cell 12: 1737–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy AS, Bandyopadhyay A, Holstein SE, Peer WA (2005) Endocytotic cycling of PM proteins. Annu Rev Plant Biol 56: 221–251 [DOI] [PubMed] [Google Scholar]
- Muschietti J, Eyal Y, McCormick S (1998) Pollen tube localization implies a role in pollen-pistil interactions for tomato receptor-like protein kinases LePRK1 and LePRK2. Plant Cell 10: 319–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Driscoll D, Hann C, Read SM, Steer MW (1993) Endocytotic uptake of fluorescent dextrans by pollen tubes grown in vitro. Protoplasm 175: 126–130 [Google Scholar]
- Park S-Y, Jauh GY, Mollet J-C, Eckard KJ, Nothnagel EA, Walling LL, Lord EM (2000) A lipid transfer-like protein is necessary for lily pollen tube adhesion to an in vitro stylar matrix. Plant Cell 12: 151–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S-Y, Lord EM (2003) Expression studies of SCA in lily and confirmation of its role in pollen tube adhesion. Plant Mol Biol 51: 183–189 [DOI] [PubMed] [Google Scholar]
- Parton RM, Fischer-Parton S, Watahiki MK, Trewavas AJ (2001) Dynamics of the apical vesicle accumulation and the rate of growth are related in individual pollen tubes. J Cell Sci 114: 2685–2695 [DOI] [PubMed] [Google Scholar]
- Qiao H, Wang H, Zhao L, Zhou J, Huang J, Zhang Y, Xue Y (2004) The F-box protein AhSLF-S2 physically interacts with S-RNases that may be inhibited by the ubiquitin/26S proteasome pathway of protein degradation during compatible pollination in Antirrhinum. Plant Cell 16: 582–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran S, Kim S, Martin R, Lord EM, Ozkan CS (2005) Quantum dots as bio-labels for the localization of a small plant adhesion protein. Nanotechnology 16: 1–4 [Google Scholar]
- Robatzek S, Chinchilla D, Boller T (2006) Ligand-induced endocytosis of the pattern recognition receptor FLS2 in Arabidopsis. Genes Dev 20: 537–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron M, Avni A (2004) The receptor for the fungal elicitor ethylene-inducing xylanase is a member of a resistance-like gene family in tomato. Plant Cell 16: 1604–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russinova E, Borst JW, Kwaitaal M, Cano-Delgado A, Yin Y, Chory J, de Vries SC (2004) Heterodimerization and endocytosis of Arabidopsis brassinosteroid receptors BRI1 and AtSERK3 (BAK1). Plant Cell 16: 3216–3229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaj J, Baluska F, Voigt B, Schlicht M, Volkmann D, Menzel D (2004) Endocytosis, actin cytoskeleton, and signaling. Plant Physiol 135: 1150–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaj J, Read ND, Volkmann D, Menzel D, Baluska F (2005) The endocytic network in plants. Trends Cell Biol 15: 425–433 [DOI] [PubMed] [Google Scholar]
- Sanchez AM, Bosch M, Bots M, Nieuwland J, Feron R, Mariani C (2004) Pistil factors controlling pollination. Plant Cell (Suppl) 16: S98–S106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada H, Sakai N, Abe Y, Tanaka E, Takahashi Y, Fujino J, Kodama E, Takizawa S, Yokosawa H (2002) Extracellular ubiquitination and proteasome-mediated degradation of the ascidian sperm receptor. Proc Natl Acad Sci USA 99: 1223–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo HS, Watanabe E, Tokutomi S, Nagatani A, Chua NH (2004) Photoreceptor ubiquitination by COP1 E3 ligase desensitizes phytochrome A signaling. Genes Dev 18: 617–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah K, Russinova E, Gadella TW Jr, Willemse J, de Vries SC (2002) The Arabidopsis kinase-associated protein phosphatase controls internalization of the somatic embryogenesis receptor kinase 1. Genes Dev 16: 1707–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speranza A, Scoccianti V, Crinelli R, Calzoni GL, Magnani M (2001) Inhibition of proteasome activity strongly affects kiwifruit pollen germination. Involvement of the ubiquitin/proteasome pathway as a major regulator. Plant Physiol 126: 1150–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone SL, Anderson EM, Mullen RT, Goring DR (2003) ARC1 is an E3 ubiquitin ligase and promotes the ubiquitination of proteins during the rejection of self-incompatible Brassica pollen. Plant Cell 15: 885–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Dale GL (1987) Biotinylated erythrocytes: in vivo survival and in vitro recovery. Blood 70: 791–795 [PubMed] [Google Scholar]
- Swanson R, Edlund AF, Preuss D (2004) Species specificity in pollen-pistil interactions. Annu Rev Genet 38: 793–818 [DOI] [PubMed] [Google Scholar]
- Tang W, Ezcurra I, Muschietti J, McCormick S (2002) A cysteine-rich extracellular protein, LAT52, interacts with the extracellular domain of the pollen receptor kinase LePRK2. Plant Cell 14: 2277–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Kelley D, Ezcurra I, Cotter R, McCormick S (2004) LeSTIG1, an extracellular binding partner for the pollen receptor kinases LePRK1 and LePRK2, promotes pollen tube growth in vitro. Plant J 39: 343–353 [DOI] [PubMed] [Google Scholar]
- Ushijima K, Sassa H, Dandekar AM, Gradziel TM, Tao R, Hirano H (2003) Structural and transcriptional analysis of the self-incompatibility locus of almond: identification of a pollen-expressed F-box gene with haplotype-specific polymorphism. Plant Cell 15: 771–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie DX, Feys BF, James S, Nieto-Rostro M, Turner JG (1998) COI1: an Arabidopsis gene required for jasmonate-regulated defense and fertility. Science 280: 1091–1094 [DOI] [PubMed] [Google Scholar]
- Zeng LR, Qu S, Bordeos A, Yang C, Baraoidan M, Yan H, Xie Q, Nahm BH, Leung H, Wang GL (2004) Spotted leaf11, a negative regulator of plant cell death and defense, encodes a U-box/armadillo repeat protein endowed with E3 ubiquitin ligase activity. Plant Cell 16: 2795–2808 [DOI] [PMC free article] [PubMed] [Google Scholar]