Abstract
Cannabinoids exert complex actions on neurotransmitter systems involved in cognition, locomotion, appetite, but no information was available so far on the interactions between the endocannabinoid system and histaminergic neurons that command several, similar behavioural states and memory. In this study, we investigated the effect of cannabimimetic compounds on histamine release using the microdialysis technique in the brain of freely moving rats. We found that systemic administration of the cannabinoid receptors 1 (CB1-r) agonist arachidonyl-2′chloroethylamide/N-(2chloroethyl)-5Z,8Z,11Z,14Z-eicosatetraenamide (ACEA; 3 mg/kg) increased histamine release from the posterior hypothalamus, where the histaminergic tuberomamillary nuclei (TMN) are located. Local infusions of ACEA (150 nM) or R(+)-methanandamide (mAEA; 1μM), another CB1-r agonist, in the TMN augmented histamine release from the TMN, as well as from two histaminergic projection areas, the nucleus basalis magnocellularis and the dorsal striatum. When the endocannabinoid uptake inhibitor AM404 was infused into the TMN, however, increased histamine release was observed only in the TMN. The cannabinoid-induced effects on histamine release were blocked by co-administrations with the CB1-r antagonist AM251. Using double-immunofluorescence labelling and confocal laser-scanning microscopy, CB1-r immunostaining was found in the hypothalamus, but was not localized onto histaminergic cells. The modulatory effect of cannabimimetic compounds on histamine release apparently did not involve inhibition of γ-aminobutyric acid (GABA)ergic neurotransmission, which provides the main inhibitory input to the histaminergic neurons in the hypothalamus, as local infusions of ACEA did not modify GABA release from the TMN. These profound effects of cannabinoids on histaminergic neurotransmission may partially underlie some of the behavioural changes observed following exposure to cannabinoid-based drugs.
Keywords: GABA, hypothalamus, microdialysis, tuberomamillary nucleus
Abbreviations: Δ9-THC, Δ9-tetrahydrocannabinol; ACEA, arachidonyl-2′chloroethylamide/N-(2chloroethyl)-5Z,8Z,11Z,14Z-eicosatetraenamide; ACh, acetylcholine; BSA, bovine serum albumin; CB1-r, cannabinoid receptors 1; GABA, γ-aminobutyric acid; HDC, histidine decarboxylase; HPLC, high-performance liquid chromatography; mAEA, R(+)-methanandamide; NBM, nucleus basalis magnocellularis; NDS, normal donkey serum; OPA, o-phthalaldehyde; PB, phosphate buffer; TMN, tuberomamillary nuclei
Introduction
The endocannabinoid system plays an important neuromodulatory role in brain physiology, fine-tuning information flow in neuronal networks associated with cognitive processing, emotion, pain perception and appetite (Di Marzo & Matias, 2005). Endocannabinoids are generally made on demand, bind with high affinity to cannabinoid receptors 1 (CB1-r), and are rapidly eliminated by a carrier-mediated transport followed by intracellular enzymatic metabolism (Piomelli, 2003). For example, endocannabinoids release in the hypothalamus regulates appetitive behaviour (Kirkham et al., 2002), whereas in the basal ganglia it counteracts the stimulation of movement induced by dopamine agonists (Beltramo et al., 2000). Furthermore, recent studies indicated that endocannabinoids content rises in restricted brain areas engaged in the processing of emotional information (Marsicano et al., 2002). CB1-r mediate not only the physiological effects of endocannabinoids, but also the psychotropic effects of Δ9-tetrahydrocannabinol (Δ9-THC). Exogenous cannabinoids induce a broad array of behavioural responses that include catalepsy, reduced movement and hypothermia (Pertwee, 1997). Furthermore, behaviours ranging from relaxation and sedation to anxiety and panic attacks have been observed (Zuardi et al., 1982).
Histaminergic neurons are located in the hypothalamic tuberomamillary nuclei (TMN) and send projections to various brain regions, hence regulating the sleep–wake cycle, energy and endocrine metabolism, and learning (Haas & Panula, 2003). Histaminergic axonal arborizations in the hypothalamus are involved in the release of several hypothalamic hormones (Knigge et al., 1999), and food consumption is accompanied by increased histamine release in the hypothalamus (Itoh et al., 1991). Histamine can enhance cortical activity by stimulating the cholinergic neurons of the nucleus basalis magnocellularis (NBM; Dringenberg & Kuo, 2003), as activation of histamine H1 receptors in the NBM increases acetylcholine (ACh) release from the cortex (Cecchi et al., 2001). Histaminergic afferents also innervate the striatum, a brain region that contains a high density of H2 (Traiffort et al., 1992) and H3 (Pollard et al., 1993) receptors and is important for proper motor function and in mediating stimulus–response habit formation (Gerdeman et al., 2003). In the striatum, histamine induces hypokinetic effects that are accompanied by altered dopaminergic transmission (Chiavegatto et al., 1998).
We therefore explored the possibility that the cannabinoid system exerts some of its behavioural effects by modulating, or acting in concert with, the activity of histaminergic neurons. Using the microdialysis technique in freely moving rats, selective, directly acting cannabinoid CB1-r agonists or an indirectly acting inhibitor of endocannabinoid uptake were given locally into the TMN, and their effect on histamine release was monitored in the TMN itself, in the NBM and striatum. We show that cannabinoids, at the low concentrations used in this study, have an excitatory effect on the histaminergic system.
CB1-r activation modulates neuronal activity by hyperpolarizing the cell and/or inhibiting the release of neurotransmitters such as γ-aminobutyric acid (GABA). As histaminergic neurons receive prominent innervations from sleep active GABAergic neurons of the ventrolateral preoptic nucleus (Sherin et al., 1998), we also measured the effect of cannabinoids on GABA release from the hypothalamus. Finally, we used immunohistochemical techniques to establish whether there is a similar distribution of CB1-r and histaminergic fibres in the hypothalamus.
Materials and Methods
Male, 8–9-week-old Sprague–Dawley rats (250–280 g/wt, Harlan, Italy) were housed in groups of three in a temperature-controlled room (20–24 °C), on a 12 h light : dark cycle, and were allowed free access to food and water. All the experiments were done in strict compliance with the EEC recommendations for the care and use of laboratory animals (86/609/CEE), and were approved by the Animal Care Committee of the ‘Dipartimento di Farmacologia Preclinica e Clinica’ of the ‘Universitá di Firenze’.
Surgical procedures
Rats, anaesthetized with chloral hydrate (400 mg/kg i.p.) and positioned in a stereotaxic frame (Stellar, Stoelting, Wood Dale, IL, USA), were implanted with one or two guide cannulae (Metalant, Sweden) according to the following coordinates from bregma (Paxinos & Watson, 1998): TMN, AP = −4.3, L = −1.1, DV = −7.2; NBM, AP = −0.8; L = −2.8; DV = −6.5; dorsal striatum, AP = 0, L = −4, DV = −4. A surgical screw served as an anchor and the cannulae were fixed to the skull with acrylic dental cement.
In vivo microdialysis measurements of histamine
The microdialysis experiments were performed 24 h after surgery, during which rats, housed one per cage, recovered from surgery. The stylet was removed from the guide cannulae and the microdialysis probes (molecular weight cut-off, 6000 Da; Metalant) were inserted; the dialysing membrane protruded 2 mm from the tip of the cannula. Both probes were perfused with Ringer’s solution (in mM: NaCl, 147; CaCl2, 1.2; KCl, 4.0; pH 7.0) at a flow rate of 2 μL/min using a microperfusion pump (Carnegie Medicine, Sweden; Mod CMA/100). Histamine release stabilized 2 h after insertion of the microdialysis probes, and fractions were collected at 15-min intervals. Spontaneous release was defined as the average value of the four 15-min fractions collected during 1 h of perfusion with Ringer’s solution prior to drug treatment. All subsequent fractions were expressed as a percentage of this value. To prevent degradation of histamine, 1.5 μL of 5 mM HCl was added to each sample. The dialysates were kept at −80 °C until analysis. Drugs were supplied in 100% EtOH that was diluted 1 : 90 000 in the final solution. For arachidonyl-2′chloroethylamide/N-(2chloroethyl)-5Z,8Z,11Z,14Z-eicosatetraenamide (ACEA) i.p. administration, EtOH concentration was 60%. Control injections with saline contained 60% EtOH.
Histology
The placement of microdialysis membranes was verified post mortem. Rats were overdosed with chloral hydrate, the brains removed and stored in 10% formalin for 10 days. Forty-micrometre sections were then sliced on a cryostat, mounted on gelatin-coated slides and then stained with Cresyl violet for light microscopic observation. Data from rats in which the membranes were not correctly positioned were discarded. Typical probe placements are shown in Fig. 1.
Fig. 1.

Schematic diagram and photomicrographs showing the position of the microdialysis probes. Rats were implanted with one probe in the tuberomamillary nuclei (TMN) to deliver drugs locally and measure neurotransmitter release, and another probe in either the nucleus basalis magnocellularis (NBM) (A) or the dorsal striatum (B) to measure histamine release. A single probe was implanted in the TMN, when measuring γ-aminobutyric acid (GABA), or when drugs were administered i.p. (C). (D–F) Representative histological structures showing the actual site of probe placement.
Determination of histamine concentration by high-performance liquid chromatography (HPLC)-fluorimetry
Histamine contents in the dialysates were determined by HPLC-fluorimetry using a modified version of the protocol by Yamatodani et al. (1985). Briefly, the column (Hypersil ODS, 3 μm, 2.1 × 100 mm; Thermo Electron Corporation, Bellefonte, PA, USA) was eluted with 0.25 M potassium dihydrogen phosphate containing 5% octanesulphonic acid (Sigma) at a flow rate of 0.4 mL/min. The eluate from the column was mixed first with 0.1% o-phthalaldehyde (OPA) solution at a flow rate of 0.1 mL/min and then to a solution containing 4 M sodium hydroxide and 0.2 M boric acid (flow rate 0.137 mL/min) to adjust the reaction mixture to pH 12.5. The reaction took place at 45 °C. Then 17% orthophosphoric acid was added to the solution (flow rate 0.137 mL/min) to reach a final reaction mixture at pH 3. The fluorescent intensity was measured with a spectrofluorometer (Agilent series 1100, Waldbronn, Germany) at 450 nm with excitation at 360 nm.
Assay of GABA in the dialysate
GABA analysis was carried out by HPLC with fluorimetric detection after OPA derivatization as previously described (Bianchi et al., 1999). The OPA-derivatives were separated on a 5 μm reverse-phase Nucleosil C18 column (200 × 4.6 mm i.d., Machery-Nagel, Duren, Germany) at room temperature, using as mobile phase methanol and potassium acetate (0.1 M, pH adjusted to 5.52 with glacial acetic acid), eluting at 1 mL/min flow rate in a three-step linear gradient, from 25% to 90% methanol.
Immunohistochemistry
Young adult male Sprague–Dawley rats were deeply anaesthetized with chloral hydrate and perfused transcardially with 50 mL physiological saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer (PB), pH 7.4. Brains were then postfixed in 4% paraformaldehyde in PB for 2 h at 4 °C. Brains were cryoprotected in 30% sucrose in PB, and 40-μm-thick sections were cut on a cryostat microtome and collected in PB. All immunostaining procedures were performed on free-floating sections. For double-labelling of CB1-r and histaminergic cells, sections were preincubated with 5% normal donkey serum (NDS, Jackson Immunoresearch, West Grove, PA, USA), 2.5% bovine serum albumin (BSA, Sigma) and 0.5% Triton X-100 in PB for 1 h at room temperature. Sections were then incubated in a cocktail of goat anti-CB1-r antibodies (1 : 1000, directed against the C-terminal; Hájos et al., 2000) and rabbit antihistidine decarboxylase (HDC, 1 : 1000; Acris, Bad Nauheim, Germany) primary antibodies in PB containing 0.5% Triton X-100, 0.1% BSA and 1% NDS for 48 h at 4 °C. After thorough rinsing in PB, sections were incubated in Cy3-conjugated donkey anti-goat IgG (1 : 200; Jackson Immunoresearch) for 2 h at room temperature. After extensive rinses in PB, sections were incubated in Alexa Fluor 488-labelled, donkey anti-rabbit IgG (Molecular Probes, Eugene, OR, USA) diluted 1 : 200 in PB containing 2% BSA for 1 h at room temperature. Sections were then mounted on glass slides and coverslipped with 70% glycerol in PB. Preadsorption of the CB1-r antibodies with glutathione-S-transferase-conjugated fusion protein (1 μg/mL), or elimination of the primary antibodies resulted in no immunostaining. Observations were performed with a Bio-Rad MCR 1024 ES confocal laser-scanning microscope (Bio-Rad, Hercules, CA, USA) equipped with a Krypton/Argon laser source 15 mW for fluorescence measurements. Series of optical sections (512 × 512 pixels) were taken through the depth of the specimens with a thickness of 1 μm at the intervals of 0.8 μm by using a Nikon Planapo × 60 (× 40) 1.4 oil immersion objective. Twenty optical sections for each sample were examined and projected as a single composite image by superimposition. To avoid bleed-through, dual channel scanning of signals from Cy3 and Alexa Fluor 488 were recorded separately and saved in two different files.
Drugs
ACEA, R(+)-methanandamide (mAEA), AM251 and AM404 were purchased from Tocris Cookson (Avonmouth, UK). For systemic administrations, ACEA was injected i.p. diluted in physiological saline. Bicuculline methiodide was purchased from Sigma.
Statistics
All values are expressed as means ± SEM, and the number of rats used in each experiment is also indicated. The presence of significant treatment effects was first determined by a one-way ANOVA followed by Fisher’s PSLD test or with two-way ANOVA. For all statistical tests, P < 0.05 was considered significant. For clarity purposes we reported in figures and figure legends only the significant differences vs. the last sample before drug treatment. However, differences were significant vs. all baseline samples. Statistical analysis was performed using StatView (Abacus Concepts, Berkley, CA, USA).
Results
After 120 min of equilibration following the insertion of the dialysing membranes, histamine was released spontaneously at a stable rate of 0.19 ± 0.03 pmol/15 min from the TMN (n = 70), 0.25 ± 0.03 pmol/15 min from the NBM (n = 33), and 0.09 ± 0.04 pmol/15 min from the dorsal striatum (n = 7).
Systemic administration of a CB1 receptor agonist increased histamine release from the TMN
The effect of a single intraperitoneal injection of the selective and potent CB1-r agonist ACEA (Hillard et al., 1999) on histamine release from the TMN is shown in Fig. 2. ACEA (3 mg/kg, i.p.; n = 8) significantly increased histamine efflux from the TMN 60 min after the injection compared with vehicle treatment (n = 4), up to a peak value of 84 ± 30%. Histamine release slowly returned to basal levels over the next 60 min.
Fig. 2.
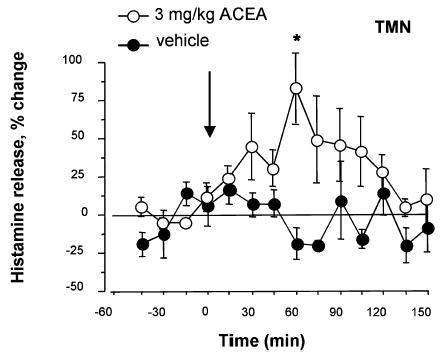
Time course of histamine release from the tuberomamillary nuclei (TMN) of freely moving rats after systemic administration of the CB1-r agonist, arachidonyl-2′chloroethylamide/N-(2chloroethyl)-5Z,8Z,11Z,14Z-eicosatetraenamide (ACEA). Histamine release was measured in fractions collected every 15 min. ACEA was administered i.p., whereas control animals received 1 mL/kg of vehicle containing 60% ethanol. Control values of spontaneous histamine release were calculated for each experiment by averaging the mean of four initially collected 15 min samples. Histamine release was expressed as a percentage of spontaneous release. The arrow indicates the time of i.p. injections. Represented are means ± SEM of eight–four experiments. *P < 0.05 vs. last sample before drug treatment (ANOVA and Fischer’s test).
Perfusion of CB1-r agonists into the TMN increased histamine release from the TMN and NBM
Using a double-probe microdialysis protocol, ACEA was infused locally in the TMN and histamine release was monitored from the TMN and NBM. As shown in Fig. 3A, 60 min perfusion with 150 nM ACEA induced a significant increase in histamine release from both the TMN and the NBM. In the TMN a significant increase of histamine release was present during the first 15-min fraction of ACEA administration and reached a maximum level of 122 ± 7% of spontaneous release (n = 5). Histamine release returned gradually to baseline values during ACEA wash out. Histamine release also increased in the NBM when ACEA was infused into the TMN and it reached a maximal level of approximately 400 ± 146% within the first 30 min of TMN perfusion. A similar, significant increase of histamine release from both the TMN and NBM was also observed following perfusion of the TMN for 60 min with 1 μM mAEA, a synthetic analogue of the endocannabinoid anandamide resistant to degradation (Abadji et al., 1994). In the TMN, the maximal effect was of similar magnitude and time course to that observed with ACEA (the maximal increase was 155 ± 78%; n = 4; Fig. 3B). In the NBM, histamine release increased significantly during the first 15-min fraction of TMN perfusion with 1 μM mAEA, and the maximal effect was 98 ± 34% of spontaneous, baseline release (n = 4). Histamine output tended to remain elevated for the duration of mAEA application to the TMN and then returned quickly to baseline values in both the TMN and NBM after mAEA perfusion ended.
Fig. 3.
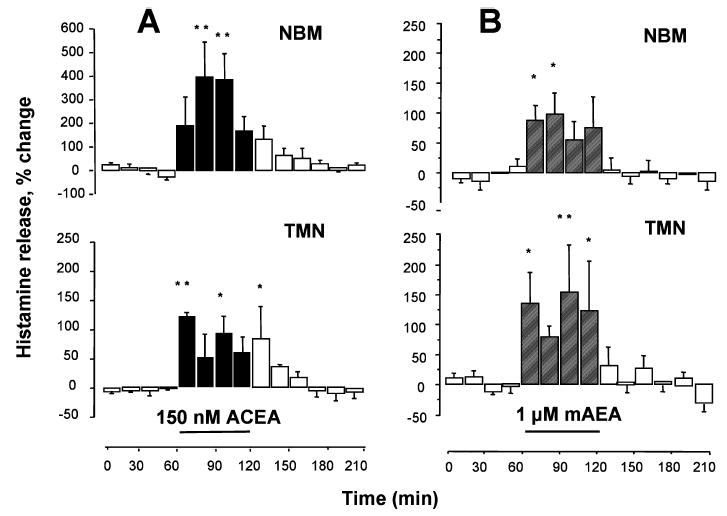
Influence of cannabinoid administration into the tuberomamillary nuclei (TMN) on histamine release from the TMN and nucleus basalis magnocellularis (NBM) of freely moving rats. Histamine was measured in 15-min fractions and expressed as a percentage of spontaneous release, calculated as described in Fig. 2. (A) Arachidonyl-2′chloroethylamide/N-(2chloroethyl)-5Z,8Z,11Z,14Z-eicosatetraenamide (ACEA) was infused into the TMN and histamine release was measured from the TMN (lower panel) and the NBM (upper panel). (B) R(+)-Methanandamide (mAEA) was infused in the TMN and histamine release was measured in the TMN (lower panel) and NBM (upper panel). Bars indicate the period of drug application. Shown are means ± SEM of four–five experiments. **P < 0.01; *P < 0.05 vs. last sample before drug treatment (ANOVA and Fisher’s test).
Perfusion of CB1-r agonists into the TMN increased histamine release from the dorsal striatum
Perfusion of the TMN for 60 min with either 150 nM ACEA (n = 3; Fig. 4A) or 1 μM mAEA (n = 4; Fig. 4B) increased histamine release in the TMN as well as the dorsal striatum. Histamine levels returned to basal values during wash out of the compounds. ACEA increased histamine release significantly, up to 91 ± 35% in the TMN and up to 173 ± 65% of basal levels in the striatum during the third, 15-min period of pharmacological stimulation. Similarly, 1 μM mAEA infused in the TMN augmented histamine release locally to a maximum of 88 ± 40% of basal values (Fig. 4B), whereas histamine release from the dorsal striatum was augmented up to a maximum of 145 ± 63%.
Fig. 4.

Effect of cannabinoid infusion into the tuberomamillary nuclei (TMN) on spontaneous histamine release from the TMN and dorsal striatum of freely moving rats. Histamine was measured in 15-min fractions and expressed as a percentage of spontaneous release, calculated as described in Fig. 2. (A) Arachidonyl-2′chloroethylamide/N-(2chloroethyl)-5Z,8Z,11Z,14Z-eicosatetraenamide (ACEA) was infused into the TMN and histamine release was measured from the TMN (lower panel) and the dorsal striatum (upper panel). (B) R(+)-Methanandamide (mAEA) was infused in the TMN and histamine release was measured in the TMN (lower panel) and dorsal striatum (upper panel). Bars indicate the period of drug application. Shown are means ± SEM of four–five experiments. **P < 0.01; *P < 0.05 vs. last sample before drug treatment (ANOVA and Fisher’s test).
Effect of TMN perfusion with AM404 or AM251 on histamine release
As endocannabinoid action is presumably terminated by cellular uptake via an endocannabinoid membrane transporter (Piomelli, 2003) and by amidohydrolysis (Deutsch & Chin, 1993; McKinney & Cravatt, 2005), we tested whether endocannabinoids reached a sufficient concentration to activate their receptors and modulate the activity of histaminergic cells by blocking their uptake and metabolism in the hypothalamus. Presumably, both mechanisms are blocked by AM404 (Jarrahian et al., 2000). When AM404 (100 μM) was added to the TMN-perfusing medium for 60 min, histamine release in the TMN increased by a maximum of 165 ± 58%, but was not significantly changed in the NBM (Fig. 5A; n = 5). In the TMN, a significant increase was achieved during the fourth, 15-min period of perfusion in the presence of AM404 and persisted for approximately 45 min during wash out, after which basal histamine levels were attained. Perfusion of the TMN for 60 min with the selective CB1-r antagonist AM251 at a concentration of either 200 nM (Fig. 5B; n = 5) or 10 μM (not shown; n = 3) did not change spontaneous histamine release significantly from the TMN (Fig. 5B, lower panel) and consequently from the NBM (Fig. 5B, upper panel).
Fig. 5.
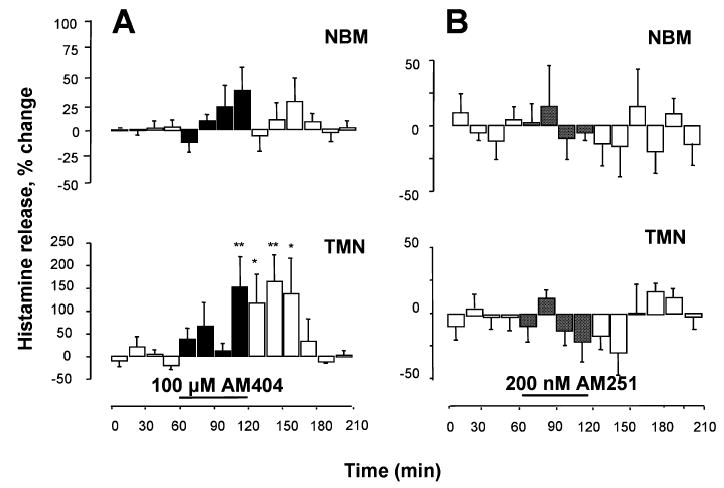
Endocannabinoids modulate histamine release in the tuberomamillary nuclei (TMN) of freely moving rats. Histamine was measured in 15-min fractions and expressed as a percentage of spontaneous release, calculated as described in Fig. 2. (A) AM404 was infused into the TMN and histamine release was measured from the TMN (lower panel) and nucleus basalis magnocellularis (NBM; upper panel). (B) AM251 was infused into the TMN and histamine release was measured from the TMN and (lower panel) and NBM (upper panel). Bars indicate the period of drug application. Shown are means ± SEM of five experiments (A and B). ** P < 0.01; *P < 0.05 vs. last sample before drug treatment (ANOVA and Fisher’s test).
Perfusion of TMN with the CB1-r antagonist blocked the effect of mAEA on histamine release
To confirm that the excitatory effects of cannabimimetic compounds were mediated through activation of CB1-r, the CB1-r antagonist AM251 was co-administered together with the CB1-r agonist mAEA in the TMN, and histamine release was measured both in the TMN and NBM. After collection of four, 15-min baseline samples, 200 nM AM251 was added to the perfusing medium for 15 min, and then it was administered in combination with the CB1-r agonist for an additional 60 min. Co-application of AM251 with mAEA in the TMN completely abolished the stimulatory effect of mAEA on histamine release, both in the TMN and NBM (Fig. 6A and B; n = 6).
Fig. 6.
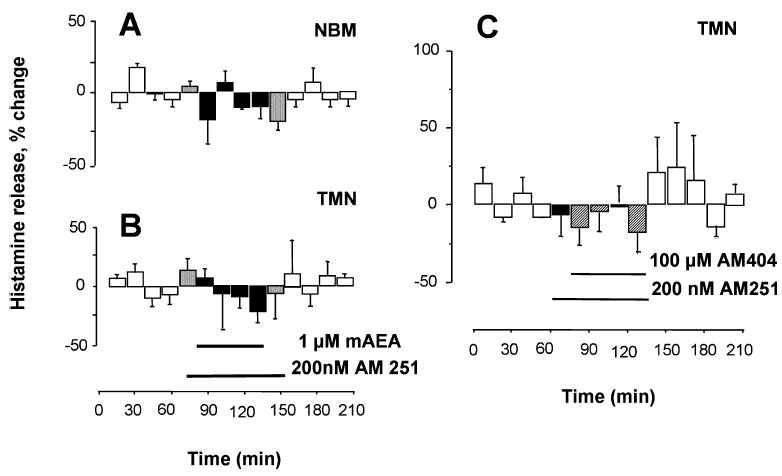
Cannabinoid-induced increase of histamine release involves activation of CB1-r in the tuberomamillary nuclei (TMN) of freely moving rats. Histamine was measured in 15-min fractions and expressed as a percentage of spontaneous release, calculated as described in Fig. 2. (A and B) The selective CB1-r antagonist AM251 was infused into the TMN 15 min before adding R(+)-methanandamide (mAEA) and maintained in the perfusing medium during administration of the agonist. (C) AM251 was infused in the TMN 15 min before adding AM404 and maintained during administration of the endocannabinoid uptake inhibitor. Bars indicate the period of drug application. Each point represents the means ± SEM of six (A and B) and three experiments (C).
Perfusion of TMN with the CB1-r antagonist blocked the effect of AM404 on histamine release
As shown in Fig. 6C, after collection of four 15-min baseline samples, 200 nM AM251 was added to the perfusing medium for 15 min, and then it was administered in combination with 100 μM AM404 for an additional 60 min. AM251 completely blocked the effect of AM404 on histamine release in the TMN (n = 3).
CB1-r immunostaining in the posterior hypothalamus
We examined if CB1-r are localized on histaminergic neurons in the hypothalamus by performing double immunofluorescence labelling of hypothalamic slices using a combination of anti-CB1-r antibodies and anti-HDC antibodies to identify histaminergic neurons. The distribution profile of HDC-immunoreactive neurons in the E2–E3 subdivisions of the TMN is shown in Fig. 7A and B. CB1-r immunostaining was sparse in this region, indicating the presence of very few CB1-r-expressing fibres. A higher density of CB1-r immunostaining was found in the E4–E5 subdivisions of the TMN (Fig. 7C and D), where CB1-r immunostaining apparently surrounds clusters of HDC-immunonegative cells. Analysis of optical scan volumes showed that CB1-r immunostaining did not co-localize to HDC positive cell bodies (Fig. 7, insets), nor dendrites (not shown). Despite the very low CB1-r immunostaining in the hypothalamus, CA3 hippocampal neurons were densely immunolabelled (Fig. 7E), as previously reported by Tsou et al. (1998). Analysis of optical scan volumes in this region as well failed to show co-localization of CB1-r and HDC immunostaining (not shown). The specificity of CB1-r immunostaining in the hypothalamus was further confirmed preabsorbing the anti-CB1-r antibodies with glutathione-S-transferase-conjugated fusion protein (Fig. 7F).
Fig. 7.

CB1 receptors are not detectable on TMN histaminergic neurons. (A and B) The photomicrographs show HDC-positive cells (green) in the E2–E3 subdivision of the TMN and the sparse immunoreactivity for CB1-r (red). (C and D) In the E4 subdivision, the density of immunoreactive fibres is higher than in E2–E3, although no co-localization of anti-HDC and anti-CB1-r immunoreactivity was observed. Note the denser CB1-r immunostaining surrounding what appear to be clusters of HDC-immunonegative cells (arrowhead). Insets show optical scan volumes of the histaminergic cell framed in (D). (E) Anti-CB1-r antibodies densely stained the neuropil in the CA3 region of the hippocampus. Pyramidal cells are immunonegative but surrounded by immunoreactive fibres. HDC positive projections are also visible. (F) The photomicrograph shows HDC positive cells in the TMN. Preadsorption of the anti-CB1-r antibodies with glutathione-S-transferase-conjugated fusion protein resulted in no anti-CB1-r immunostaining. PY, pyramidal cell layer; Scale bars: 120 μm (A and E); 20 μm (B and F); 30 μm (C and D).
Perfusion of the TMN with mAEA did not modify spontaneous release of GABA from the hypothalamus
We investigated whether the increased histamine release elicited by CB1-r agonists involved modulation of intrahypothalamic GABA release. GABA content measured in dialysates collected from the TMN perfused with 1 μM mAEA for 60 min did not significantly change (spontaneous release, 0.80 ± 0.14 pmol/15 min; n = 7; data not shown). As such, it is unlikely that the excitatory effect of CB1-r agonists on histaminergic neurons is mediated by the reduction of an inhibitory GABAergic tone. However, the activity of histaminergic cells is controlled by a tonic release of GABA, as perfusion of the TMN with the GABAA receptor antagonist bicuculline (10 μM) for 75 min significantly increased histamine spontaneous release from the TMN up to 91 ± 16% (Fig. 8A, lower panel) but, surprisingly, not from the NBM (Fig. 8A, upper panel; n = 4). Co-administration in the TMN of bicuculline and ACEA (150 nM) increased significantly histamine release from the NBM (196 ± 87.7% of baseline; Fig. 8B, upper panel), and induced an additional increase of TMN histamine release over that induced by 10 μM bicuculline alone (Fig. 8B, lower panel; n = 5). We compared the percentage of histamine release calculated by averaging histamine content in all samples collected during pharmacological stimulations, and found that ACEA increased histamine release from the TMN by 82 ± 14%, bicuculline by 65 ± 9% and both compounds together by 136 ± 21% (Fig. 8C), therefore bicuculline did not occlude the effect of ACEA on histamine release. The administration of bicuculline likely induces strong local depolarization, which, in turn, might induce release of endocannabinoids. We tested this hypothesis co-administering AM251, at the concentration (200 nM) that fully blocked the effect of mAEA, together with bicuculline (10 μM). After collection of four 15-min baseline samples, AM251 was added to the perfusing medium for 15 min, and then it was administered in combination with bicuculline (Fig. 8D). A significant increase (85 ± 17%) was achieved during the second, 15-min period of TMN perfusion with AM251 and bicuculline and returned to basal levels during wash out in the presence of AM251 (n = 6).
Fig. 8.
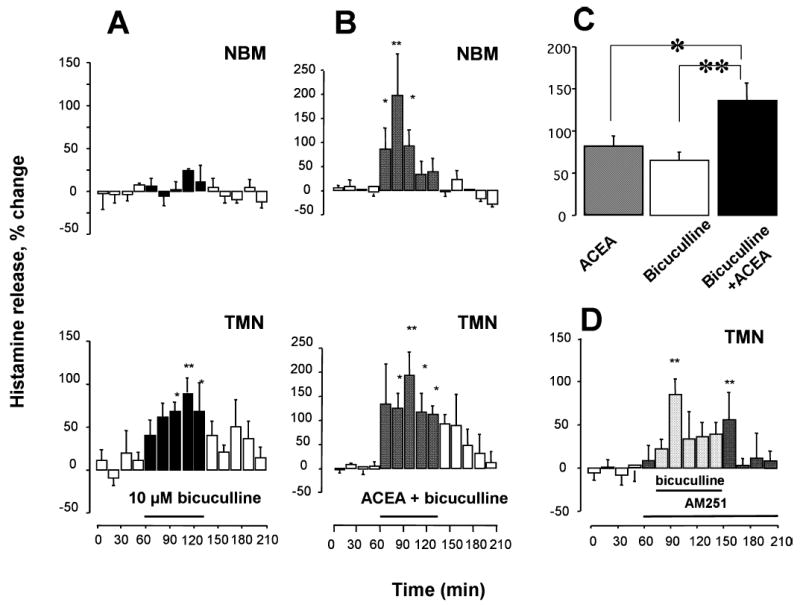
Comparison of the effects of bicuculline, and of bicuculline in the presence of selective CB1-r ligands. Bicuculline (A) or bicuculline plus arachidonyl-2′chloroethylamide/N-(2chloroethyl)-5Z,8Z,11Z,14Z-eicosatetraenamide (ACEA) (B) were infused in the tuberomamillary nuclei (TMN), and histamine release was measured in the TMN (lower panels) and nucleus basalis magnocellularis (NBM; upper panels). Histamine was measured in 15-min fractions and expressed as a percentage of spontaneous release, calculated as described in Fig. 2. Bars indicate the period of drug application. Each point represents the means ± SEM of six–seven experiments (ANOVA and Fisher’s test). (C) 15-min changes of histamine release in the presence of ACEA, bicuculline and ACEA + bicuculline. Percentage histamine release was calculated by averaging histamine content in the samples collected during pharmacological stimulations. **P < 0.01, *P < 0.05 (ANOVA and Fisher’s test). (D) AM251 (200 nM) was infused in the TMN 15 min before adding bicuculline (10 μM) and maintained in the perfusion medium throughout the experiment. Histamine was measured in 15-min fractions and expressed as a percentage of spontaneous release, calculated as described in Fig. 2. Bars indicate the period of drug application. Each point represents the means ± SEM of six experiments. **P < 0.01, *P < 0.05 (ANOVA and Fisher’s test).
Discussion
In summary, cannabinoids activate the histaminergic system in the rat brain in vivo. Acute i.p. administration of the CB1-r agonist ACEA augmented histamine release from the TMN, where histaminergic somata are located. This effect was mimicked when the selective CB1-r agonists ACEA or mAEA were infused locally into the TMN of freely moving rats via a microdialysis probe, indicating that modulation of histamine release is a local phenomenon mediated within the hypothalamus. Supposedly, histamine is released by short histaminergic projections within the posterior hypothalamus, as TMN neurons have extensive axonal arborizations within this brain region (Haas & Panula, 2003). TMN perfusion with CB1-r agonists increased histamine release not only from the TMN, but also from two histaminergic projection areas, the NBM and striatum. Modulation of histamine release in these regions presumably results from the activation of ascending histaminergic projections. Furthermore, we found that exogenous and endogenous cannabinoids differed significantly in the regulation of histaminergic cells activity: CB1-r agonists affected histamine release not only at the site of perfusion, the TMN, but also in the NBM and striatum, whereas the endocannabinoid membrane uptake blocker AM404 when administered in the hypothalamus increased histamine release only in the TMN.
Specificity of cannabinoid action
The CB-r ligands used in this study are selective for the CB1-r and the concentrations used in the microdialysis experiments were consistent with the range of concentrations considered selective for this receptor. In our study, cannabimimetic compounds were used at similar or lower concentrations than those effective to modulate synaptic activity in vitro (e.g. Melis et al., 2004; Marcaggi & Attwell, 2005). The CB1-r antagonist AM251 effectively blocked both mAEA- and AM404-induced increase of histamine release at a concentration (200 nM) well below the range that decreases glutamate release from striatal synaptosomes (Köfalvi et al., 2003), and that inhibits the depolarization of synaptoneurosomes induced by the sodium channel, site 2-specific neurotoxin veratridine (Liao et al., 2004). Furthermore, the observation that AM251 blocked AM404-induced increase of histamine release in the TMN argues against the possibility that AM404 effect may be explained by its affinity for the vanilloid VR1 receptor (De Petrocellis et al., 2000).
Exogenous vs. endogenous cannabinoid action
The current theory indicates that endocannabinoids are released via activity-dependent cleavage of membrane lipid precursors and are immediately released from the cells and metabolized (Di Marzo et al., 1994; Freund et al., 2003; Piomelli, 2003). Endocannabinoid release for a single neuron is a relatively rare occurrence, but release probability may be increased by the convergence of synchronous synaptic events (Varma et al., 2001; Kim et al., 2002). Indeed, intrahypothalamic administration of AM251 did not modify histamine release, suggesting that under resting conditions endocannabinoids tone is insufficient to modulate the activity of histaminergic neurons. Furthermore, the lack of an effect on histamine release at both AM251 concentrations tested (200 nM and 10 μM) suggests that CB1-r are not constitutively active. However, AM404 increased histamine release from the TMN, strongly suggesting that AM404 inhibited local endocannabinoid clearance and allowed sufficient accumulation to activate CB1-r. The increased endocannabinoid tone produced by AM404 augmented histamine release only in the TMN presumably by activating a more restricted, or different population of CB1-r than those activated by the administration of direct-acting CB1-r agonists. In this regard, it was recently shown that constitutive release of endocannabinoids by hypothalamic, proopiomelanocortin neurons inhibits a GABAergic tone, whereas exogenous CB1-r agonists inhibit glutamate release as well (Hentges et al., 2005). Alternatively, endocannabinoids may increase histamine release in a subpopulation of TMN neurons and, in turn, the released histamine may activate autoinhibitory H3 receptors on other parts of the TMN. Consistent with these observations, in vivo studies demonstrated that administrations of AM404 or URB597, an inhibitor of the metabolic pathway of the endocannabinoid anandamide, do not mimic the full spectrum of pharmacological responses produced by classical CB1-r agonists (Gaetani et al., 2003; Solinas et al., 2005). Therefore, understanding in what circumstances endocannabinoids are released and activate histaminergic cells warrants further investigations (see below).
CB1-r are not expressed on histaminergic neurons and do not attenuate GABA release in the hypothalamus
Extensive analysis of the histaminergic neuropil within the hypothalamus and of histaminergic projections to the hippocampus did not reveal co-expression of CB1-r protein and HDC in the same cells. Therefore, our data strongly suggest that CB1-r are not localized onto histaminergic neurons, which makes a direct excitatory effect of CB1-r activation on histamine release unlikely. CB1-r immunoreactivity in the hypothalamus was overall very low, as previously reported (Moldrich & Wenger, 2000), although CB1-r mRNA is expressed in hypothalamic neurons that release neuropeptides known to modulate food intake (Cota et al., 2003). This study did not examine if histaminergic neurons express CB1-r mRNA, therefore this possibility cannot be completely excluded. A possible explanation for the excitatory effects of CB1-r agonists on histamine release is therefore the blockade of an inhibitory action exerted on histaminergic neurons. Four lines of evidence strongly suggest that the cannabinoids modulate the activity of histaminergic neurons independently of GABAergic neurotransmission in the hypothalamus: (1) CB1-r agonists did not decrease GABA release in the TMN; (2) ACEA, but not bicuculline, administered in the TMN induced a significant increase of histamine release in the NBM suggesting different modes of action; (3) bicuculline did not occlude the increment in histamine release induced by ACEA in the TMN; (4) the CB1-r antagonist AM251 did not block the effect of bicuculline on histamine release from the TMN.
Direct inhibitory actions on TMN neurons have been found also for galanin and nociceptin (Eriksson et al., 2000). However, preliminary studies in our laboratory showed that intra-TMN administration of UFP-101, an antagonist of the nociceptin receptor ORL1, did not modify histamine release (unpublished observations), indicating that TMN neurons are not tonically inhibited by nociceptin. Therefore, it is unlikely that cannabinoids effect on histamine release depend on depression of nociceptin-mediated hyperpolarization of histaminergic neurons. The mechanism by which cannabinoids increase histamine release therefore remains to be elucidated.
Different histaminergic neuronal populations?
It is known that histamine release from the anterior hypothalamus varies during natural sleep–wakefulness cycles, being lowest during rapid eye movement sleep (Strecker et al., 2002). In our microdialysis experiments rats were quiescent or sleeping, and presumably the activity of histaminergic cells was low. Therefore, we assume that histaminergic modulation of signal processing in other brain regions, which depends on behavioural state (Weiler et al., 1998), did not vary considerably during microdialysis sample collection. Nevertheless, both ACEA and bicuculline significantly increased histamine release from the TMN, but had different effects on histamine release from the NBM. These observations indicate that excitation of histaminergic neurons might not necessarily produce a broad activation of all histaminergic projections, and suggests the existence of subpopulations of histaminergic cells that respond differently to pharmacological manipulations and/or project to different brain regions. Although anatomical tracing studies did not reveal any topographical organization of the histaminergic projections originating in the TMN (Köhler et al., 1985; Ericson et al., 1987), histamine-containing neurons were recently shown to be a functional heterogeneous population, based on differential activation by acute stress (Miklos & Kovacs, 2003), and on the expression of different γ-subunits that confer different sensitivity to exogenous GABA (Sergeeva et al., 2002). The observation that histaminergic neurons are not a homogenous neuronal population may have relevant consequences in the development of target-specific drugs that affect only a subset of histaminergic cells, and in reducing the occurrence of collateral or undesired effects.
Functional implications
Cannabinoids and brain histamine have received much attention recently, because of the prominent role that they play in regulating appetite. CB1-r antagonists such as rimonabant reduce food intake and body weight in animals and humans, and CB1-r agonists have been approved for the treatment of anorexia (Marx, 2006), whereas antagonists of the H3 receptor are being developed as anti-obesity (Malmlöf et al., 2005). For both the cannabinoid and histaminergic systems though, the mechanisms involved in regulating food intake are not clear. Hypothalamic levels of the endocannabinoid 2-arachydonoylethanolamide increase during fasting and are lowest during food consumption (Kirkham et al., 2002); on the other hand, histaminergic cells activity increases during food presentation to fasted rats (Meynard et al., 2005) and remains sustained during feeding (Itoh et al., 1991). However, nothing is known about the temporal and causal relationship between the histaminergic and cannabinoid systems in controlling appetitive behaviour, an issue that deserves further investigation.
Administration of CB1-r agonists in the TMN facilitates histamine release from the NBM, the major source of cholinergic innervation to the neocortex. This may explain why systemic, but not local, administration of CB1-r agonists increases ACh release from the neocortex (Verrico et al., 2003). Activation of cholinergic neurons innervating the cortex contributes to arousal mechanism; activation of histamine H1 receptors in the NBM increases cortical ACh release (Cecchi et al., 2001) and improves rat performance in the object recognition test (Orsetti et al., 2002; Malmberg-Aiello et al., 2003). It may seem counterintuitive that cannabinoids facilitate histamine release from the NBM, given that cannabinoids have deleterious effects on cognitive processes (Schneider & Koch, 2002). However, augmented histamine release is also an indicator of stress (Westerink et al., 2002), and it is conceivable that protracted occupancy of CB1-r, as produced following local or systemic administration of CB1-r agonists, disrupts the spatiotemporal specificity of histamine release in different brain regions, contributing to maladaptive behavioural responses.
Administration of CB1-r agonists in the TMN facilitates histamine release from the striatum as well. The striatum provides the anatomical substrate for the integration of movements (Brown, 1992), and takes part in learning and executing adequate behavioural responses to environmental stimuli (Hyman & Malenka, 2001). Systemic administration of CB1-r agonists reduces locomotion (McLaughlin et al., 2005), and histamine induces hypokinetic effects that are accompanied by altered dopaminergic transmission in the striatum (Chiavegatto et al., 1998). Therefore, the augmented histamine release in the dorsal striatum following cannabinoid administration in the TMN may contribute to direct actions of cannabinoids on striatal neurons (Sañudo-Peña et al., 1999; Huang et al., 2001; Köfalvi et al., 2005), worsening locomotor activity.
Further research is necessary to determine what role a hyperhistaminergic state may play in cannabinoid detrimental effects on cognitive and locomotor performance, and whether it contributes to drug-motivated habits that are crucial for the establishment of addiction.
Acknowledgments
This research was supported by Ministero dell’Universita’ e della Ricerca Scientifica e tecnologica-FIRB (RBAU017SWS), Fondazione Cassa di Risparmio di Firenze Funds and the NIH (DA11322 and DA00286).
References
- Abadji V, Lin S, Taha G, Griffin G, Stevenson L, Pertwee RG, Makriyannis A. (R)-methanandamide: a chiral novel anandamide possessing higher potency and metabolic stability. J Med Chem. 1994;37:1889–1893. doi: 10.1021/jm00038a020. [DOI] [PubMed] [Google Scholar]
- Beltramo M, de Fonseca FR, Navarro M, Calignano A, Gorriti MA, Grammatikopoulos G, Sadile AG, Giuffrida A, Piomelli D. Reversal of dopamine D(2) receptor responses by an anandamide transport inhibitor. J Neurosci. 2000;20:3401–3407. doi: 10.1523/JNEUROSCI.20-09-03401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi L, Della Corte L, Tipton KF. Simultaneous determination of basal and evoked output levels of aspartate, glutamate, taurine and 4-aminobutyric acid during microdialysis and from superfused brain slices. J Chromatogr B. 1999;723:47–59. doi: 10.1016/s0378-4347(98)00519-2. [DOI] [PubMed] [Google Scholar]
- Brown L. Somatotopic organization in the rat striatum: evidence for a combinatorial map. Proc Natl Acad Sci USA. 1992;89:7403–7407. doi: 10.1073/pnas.89.16.7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi M, Passani MB, Bacciottini L, Mannaioni PF, Blandina P. Cortical acetylcholine release elicited by stimulation of histamine H1 receptors in the nucleus basalis magnocellularis: a dual probe microdialysis study in the freely moving rat. Eur J Neurosci. 2001;13:68–78. [PubMed] [Google Scholar]
- Chiavegatto S, Nasello AG, Bernardi MM. Histamine and spontaneous motor activity: biphasic changes, receptors involved and participation of the striatal dopamine system. Life Sci. 1998;62:1875–1888. doi: 10.1016/s0024-3205(98)00154-4. [DOI] [PubMed] [Google Scholar]
- Cota D, Marsicano G, Tschöp M, Grübler Y, Flachskamm C, Schubert M, Auer D, Yassouridis A, Thöne-Reineke C, Ortmann S, Tomassoni F, Cervino C, Nisoli E, Linthorst ACE, Pasquali R, Lutz B, Stalla GK, Pagotto U. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest. 2003;112:423–431. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petrocellis L, Bisogno T, Davis JB, Pertwee RG, Di Marzo V. Overlap between the ligand recognition properties of the anandamide transporter and the VR1 vanilloid receptor: inhibitors of anandamide uptake with negligible capsaicin-like activity. FEBS Lett. 2000;483:52–56. doi: 10.1016/s0014-5793(00)02082-2. [DOI] [PubMed] [Google Scholar]
- Deutsch DG, Chin SA. Enzymatic synthesis and degradation of anandamide, a cannabinoid receptor agonist. Biochem Pharmacol. 1993;46:791–797. doi: 10.1016/0006-2952(93)90486-g. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, Piomelli D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Matias I. Endocannabinoid control of food intake and energy balance. Nat Neurosci. 2005;8:585–589. doi: 10.1038/nn1457. [DOI] [PubMed] [Google Scholar]
- Dringenberg H, Kuo M. Histaminergic facilitation of electrocorticographic activation: role of basal forebrain, thalamus, and neocortex. Eur J Neurosci. 2003;18:2285–2291. doi: 10.1046/j.1460-9568.2003.02975.x. [DOI] [PubMed] [Google Scholar]
- Ericson H, Watanabe T, Köhler C. Morphological analysis of the tuberomammillary nucleus of the rat brain: delineation of subgroups with antibody against 1-histidine decarboxylase as a marker. J Comp Neurol. 1987;263:1–24. doi: 10.1002/cne.902630102. [DOI] [PubMed] [Google Scholar]
- Eriksson KS, Stevens DR, Haas HL. Opposite modulation of histaminergic neurons by nociceptin and morphine. Neuropharmacology. 2000;39:2492–2498. doi: 10.1016/s0028-3908(00)00062-9. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signalling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Gaetani S, Cuomo V, Piomelli D. Anandamide hydrolysis: a new target for anti-anxiety drugs? Trends Mol Med. 2003;9:474–478. doi: 10.1016/j.molmed.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Gerdeman GL, Partridge JG, Lupica CR, Lovinger DM. It could be habit forming: drugs of abuse and striatal synaptic plasticity. Trends Neurosci. 2003;26:184–192. doi: 10.1016/S0166-2236(03)00065-1. [DOI] [PubMed] [Google Scholar]
- Haas H, Panula P. The role of histamine and the tuberomamillary nucleus in the nervous system. Nat Neurosci Rev. 2003;4:121–130. doi: 10.1038/nrn1034. [DOI] [PubMed] [Google Scholar]
- Hájos N, Katona I, Naiem SS, Mackie K, Ledent C, Mody I, Freund TF. Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. Eur J Neurosci. 2000;12:3239–3249. doi: 10.1046/j.1460-9568.2000.00217.x. [DOI] [PubMed] [Google Scholar]
- Hentges ST, Low MJ, Williams JT. Differential regulation of synaptic inputs by constitutively released endocannabinoids and exogenous cannabinoids. J Neurosci. 2005;25:9746–9751. doi: 10.1523/JNEUROSCI.2769-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard J, Manna S, Greenberg M, Dicamelli R, Ross R, Stevenson L, Murphy V, Pertwee R, Campbell W. Synthesis and characterization of potent and selective agonists of the neuronal cannabinoid receptor (CB1) J Pharmacol Exp Ther. 1999;289:1427–1433. [PubMed] [Google Scholar]
- Huang C-C, Lo S-W, Hsu K-S. Presynaptic mechanisms underlying cannabinoid inhibition of excitatory synaptic transmission in rat striatal neurons. J Physiol. 2001;532.3:731–748. doi: 10.1111/j.1469-7793.2001.0731e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Oishi R, Saeki K. Feeding-induced increase in the extracellular concentration of histamine in rat hypothalamus as measured by in vivo microdialysis. Neurosci Lett. 1991;125:235–237. doi: 10.1016/0304-3940(91)90037-t. [DOI] [PubMed] [Google Scholar]
- Jarrahian A, Manna S, Edgemond WS, Campbell WB, Hillard CJ. Structure–activity relationships among N-arachidonoylethanolamine (anandamide) head group analogues for the anandamide transporter. J Neurochem. 2000;74:2597–2606. doi: 10.1046/j.1471-4159.2000.0742597.x. [DOI] [PubMed] [Google Scholar]
- Kim J, Isokawa M, Ledent C, Alger B. Activation of muscarinic acetylcholine receptors enhances the release of endogenous cannabinoids in the hippocampus. J Neurosci. 2002;22:10182–10191. doi: 10.1523/JNEUROSCI.22-23-10182.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham TC, Williams CM, Fezza F, Di Marzo V. Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: stimulation of eating by 2-arachidonoyl glycerol. Br J Pharmacol. 2002;136:550–557. doi: 10.1038/sj.bjp.0704767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knigge U, Willems E, Kjaer A, Jorgensen H, Warberg J. Histaminergic and catecholaminergic interactions in the central regulation of vasopressin and oxytocin secretion. Endocrinology. 1999;140:3713–3719. doi: 10.1210/endo.140.8.6891. [DOI] [PubMed] [Google Scholar]
- Köfalvi A, Rodrigues RJ, Ledent C, Mackie K, Vizi ES, Cunha RA, Sperlágh B. Involvement of cannabinoid receptors in the regulation of neurotransmitter release in the rodent striatum: a combined immunochemical and pharmacological analysis. J Neurosci. 2005;25:2874–2884. doi: 10.1523/JNEUROSCI.4232-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köfalvi A, Vizi ES, Ledent C, Sperlágh B. Cannabinoids inhibit the release of [3H]glutamate from rodent hippocampal synaptosomes via a novel CB1 receptor-independent action. Eur J Neurosci. 2003;18:1973–1978. doi: 10.1046/j.1460-9568.2003.02897.x. [DOI] [PubMed] [Google Scholar]
- Köhler C, Swanson L, Haglund L, Wu JY. The cytoarchitecture, histochemistry and projections of the tuberomammillary nucleus in the rat. Neuroscience. 1985;16:85–110. doi: 10.1016/0306-4522(85)90049-1. [DOI] [PubMed] [Google Scholar]
- Liao C, Zheng J, David LS, Nicholson RA. Inhibition of voltage-sensitive sodium channels by the cannabinoid 1 receptor antagonist AM 251 in mammalian brain. Pharmacol Toxicol. 2004;94:73–78. doi: 10.1111/j.1742-7843.2004.pto940204.x. [DOI] [PubMed] [Google Scholar]
- Malmberg-Aiello P, Ipponi A, Blandina P, Bartolini L, Schunack W. Pro-cognitive effect of a selective H1 receptor agonist, 2-(3-trifluoromethylphenyl) histamine, in the rat object recognition test. Inflam Res. 2003;52:S33–S34. doi: 10.1007/s000110300042. [DOI] [PubMed] [Google Scholar]
- Malmlöf K, Zaragoza F, Golozoubova V, Refsgaard HH, Cremers T, Raun K, Wulff BS, Johansen PB, Westerink B, Rimvall K. Influence of a selective histamine H3 receptor antagonist on hypothalamic neural activity, food intake and body weight. Int J Obes. 2005;29:1402–1412. doi: 10.1038/sj.ijo.0803036. [DOI] [PubMed] [Google Scholar]
- Marcaggi P, Attwell D. Endocannabinoid signaling depends on the spatial pattern of synapse activation. Nat Neurosci. 2005;8:776–781. doi: 10.1038/nn1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad S, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Marx J. Drug development. Drugs inspired by a drug. Science. 2006;311:322–325. doi: 10.1126/science.311.5759.322. [DOI] [PubMed] [Google Scholar]
- McKinney MK, Cravatt BF. Structure and function of fatty acid amide hydrolase. Annu Rev Biochem. 2005;74:411–432. doi: 10.1146/annurev.biochem.74.082803.133450. [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Lu D, Winston KM, Thakur G, Swezey LA, Makriyannis A, Salamone JD. Behavioral effects of the novel cannabinoid full agonist AM 411. Pharmacol Biochem Behav. 2005;81:78–88. doi: 10.1016/j.pbb.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Melis M, Pistis M, Perra S, Muntoni A, Pillolla G, Gessa G. Endocannabinoids mediate presynaptic inhibition of glutamatergic transmission in rat ventral tegmental area dopamine neurons through activation of CB1 receptors. J Neurosci. 2004;24:53–62. doi: 10.1523/JNEUROSCI.4503-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meynard MM, Valdes JL, Recabarren M, Seron-Ferre M, Torrealba F. Specific activation of histaminergic neurons during daily feeding anticipatory behavior in rats. Behav Brain Res. 2005;158:311–319. doi: 10.1016/j.bbr.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Miklos I, Kovacs K. Functional heterogeneity of the responses of histaminergic neuron subpopulations to various stress challenges. Eur J Neurosci. 2003;18:3069–3079. doi: 10.1111/j.1460-9568.2003.03033.x. [DOI] [PubMed] [Google Scholar]
- Moldrich G, Wenger T. Localization of the CB1 cannabinoid receptor in the rat brain. An immunohistochemical study. Peptides. 2000;21:1735–1742. doi: 10.1016/s0196-9781(00)00324-7. [DOI] [PubMed] [Google Scholar]
- Orsetti M, Ferretti C, Gamalero R, Ghi P. Histamine H3-receptor blockade in the rat nucleus basalis magnocellularis improves place recognition memory. Psychopharmacology. 2002;159:133–137. doi: 10.1007/s002130100892. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1998. [Google Scholar]
- Pertwee RG. Pharmacology of cannabinoid CBI and CB2 receptors. Pharmacol Ther. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Pollard H, Moreau J, Arrang JM, Schwartz JC. A detailed autoradiographic mapping of histamine H3 receptors in rat brain areas. Neuroscience. 1993;52:169–189. doi: 10.1016/0306-4522(93)90191-h. [DOI] [PubMed] [Google Scholar]
- Sañudo-Peña MC, Tsou K, Walker JM. Motor actions of cannabinoids in the basal ganglia output nuclei. Life Sci. 1999;65:703–713. doi: 10.1016/s0024-3205(99)00293-3. [DOI] [PubMed] [Google Scholar]
- Schneider M, Koch M. The cannabinoid agonist WIN 55,212–2 reduces sensorimotor gating and recognition memory in rats. Behav Pharmacol. 2002;13:29–37. doi: 10.1097/00008877-200202000-00003. [DOI] [PubMed] [Google Scholar]
- Sergeeva OA, Eriksson KS, Sharonova IN, Vorobjev VS, Haas HL. GABA(A) receptor heterogeneity in histaminergic neurons. Eur J Neurosci. 2002;16:1472–1482. doi: 10.1046/j.1460-9568.2002.02221.x. [DOI] [PubMed] [Google Scholar]
- Sherin JE, Elmquist JK, Torrealba F, Saper CB. Innervation of tuberomammillary neurons by Gabaergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J Neurosci. 1998;18:4705–4721. doi: 10.1523/JNEUROSCI.18-12-04705.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Panlilio LV, Tanda G, Makriyannis A, Matthews SA, Goldberg SR. Cannabinoid agonists but not inhibitors of endogenous cannabinoid transport or metabolism enhance the reinforcing efficacy of heroin in rats. Neuropsychopharmacology. 2005;30:2046–2057. doi: 10.1038/sj.npp.1300754. [DOI] [PubMed] [Google Scholar]
- Strecker RE, Nalwalk J, Dauphin LJ, Thakkar MM, Chen Y, Ramesh V, Hough LB, McCarley RW. Extracellular histamine levels in the feline preoptic/anterior hypothalmic area during natural sleep-wakefulness and prolonged wakefulness: an in vivo microdialysis study. Neuroscience. 2002;113:663–670. doi: 10.1016/s0306-4522(02)00158-6. [DOI] [PubMed] [Google Scholar]
- Traiffort E, Pollard H, Moreau J, Ruat M, Schwartz JC, Martinez-Mir MI, Palacios JM. Pharmacological characterization and autoradiographic localization of histamine H2 receptors in human brain identified with [125I]iodoaminopotentidine. J Neurochem. 1992;59:290–299. doi: 10.1111/j.1471-4159.1992.tb08903.x. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sañudo-Peña MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Varma N, Carlson GC, Ledent C, Alger BE. Metabotropic glutamate receptors drive the endocannabinoid system in hippocampus. J Neurosci. 2001;21:RC188. doi: 10.1523/JNEUROSCI.21-24-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrico CD, Jentsch JD, Dazzi L, Roth RH. Systemic, but not local, administration of cannabinoid CB1 receptor agonists modulate prefrontal cortical acetylcholine efflux in the rat. Synapse. 2003;48:178–183. doi: 10.1002/syn.10202. [DOI] [PubMed] [Google Scholar]
- Weiler HT, Hasenohrl RU, van Landeghem AAL, van Landeghem M, Brankack J, Huston JP, Haas HL. Differential modulation of hippocampal signal transfer by tuberomammillary nucleus stimulation in freely moving rats dependent on behavioral state. Synapse. 1998;28:294–301. doi: 10.1002/(SICI)1098-2396(199804)28:4<294::AID-SYN5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Westerink BH, Cremers TI, Vries JBD, Liefers H, Tran N, Boer PD. Evidence for activation of histamine H3 autoreceptors during handling stress in the prefrontal cortex of the rat. Synapse. 2002;15:238–243. doi: 10.1002/syn.10043. [DOI] [PubMed] [Google Scholar]
- Yamatodani A, Fukuda H, Wada H, Iwaeda T, Watanabe T. High-performance liquid chromatographic determination of plasma and brain histamine without previous purification of biological samples: cation-exchange chromatography coupled with post-column derivatization fluorometry. J Chromatogr. 1985;344:115–123. doi: 10.1016/s0378-4347(00)82012-5. [DOI] [PubMed] [Google Scholar]
- Zuardi AW, Shirakawa I, Finkelfarb E, Karniol IG. Action of cannabidiol on the anxiety and other effects produced by delta 9-THC in normal subjects. Psychopharmacology. 1982;76:245–250. doi: 10.1007/BF00432554. [DOI] [PubMed] [Google Scholar]


