Abstract
Background
There are limited systematic data on the incidence, clinical characteristics and outcomes of congestive heart failure (CHF) in patients with hyperthyroidism. The aim of this study was to investigate the incidence, clinical characteristics and outcome of CHF as the initial presentation in patients with primary hyperthyroidism.
Methods
The prevalence, clinical characteristics and outcome of CHF was studied in 591 consecutive patients (mean (SD) age 45 (1) years, 140 men) who presented with primary hyperthyroidism.
Results
CHF was the presenting condition in 34 patients (5.8%) with hyperthyroidism. The presence of atrial fibrillation at presentation (OR 37.4, 95% CI 9.72 to 144.0, p<0.001) was an independent predictor for the occurrence of CHF. Of the 34 patients with CHF, 16 (47%) had systolic left ventricular dysfunction with left ventricular ejection fraction (LVEF)<50%. They were predominantly male (OR 26.6, 95% CI 2.6 to 272.5, p = 0.006) and had a lower serum thyroxine level (OR 0.93, 95% CI 0.87 to 0.99, p = 0.044) than patients with preserved left ventricular systolic function. In these patients, LVEF (55 (4)% vs 30 (2)%, p<0.001) and New York Heart Association functional class (1.2 (0.1) vs 2.5 (0.2), p<0.001) improved significantly 3 months after achieving euthyroid status. Systolic left ventricular dysfunction (mean (SD) LVEF 38 (4)%) persisted on long‐term follow‐up in five patients: no clinical parameter could be identified to predict the occurrence of this persistent cardiomyopathy (p>0.05).
Conclusion
CHF was the initial clinical presentation in approximately 6% of patients with hyperthyroidism, and half of them had left ventricular systolic dysfunction. Symptoms of CHF subsided and LVEF improved after treatment for hyperthyroidism. Nonetheless, one‐third of these patients developed persistent dilated cardiomyopathy.
Hyperthyroidism is a common metabolic disorder with prominent cardiovascular manifestations.1,2 It creates a hyperdynamic circulatory state because of a marked fall in peripheral vascular resistance and an increased total blood volume and heart rate.2,3,4,5 These cardiovascular changes can aggravate pre‐existing cardiac disease or directly lead to thyrotoxic heart disease.1,6 Although symptoms and signs of congestive heart failure (CHF) are common in patients with hyperthyroidism,2,7 dilated cardiomyopathy with impaired left ventricular systolic function is only rarely reported.8,9,10,11 Hyperthyroidism is a very rare (<1%) cause of dilated cardiomyopathy.12 Nonetheless, there has been no systematic study of the incidence, clinical characteristics and outcome of heart failure caused by hyperthyroidism. The purpose of this study was to determine the incidence, clinical characteristics and outcome of heart failure in a large cohort of patients with hyperthyroidism.
Methods
Study population
We studied 618 consecutive Chinese patients diagnosed with hyperthyroidism from January 2001 to December 2002 in the Cardiology Division, Department of Medicine and Metabolic and Endocrinology Division, Department of Medicine, Queen Mary Hospital, The University of Hong Kong, Hong Kong, China, the only large regional general hospital to accept emergency referrals from the western region of Hong Kong Island. Patients were referred directly or from hospital wards to our thyroid and general medical outpatient clinics for treatment of primary hyperthyroidism. Data were prospectively collected on their demographic characteristics, symptoms of heart failure and hyperthyroidism, ECG at presentation and clinical outcome during follow‐up. Patients were excluded from study if they had documented pre‐existing coronary artery disease, or a history of myocardial infarction, CHF and cerebrovascular and peripheral vascular disease (n = 8) or incomplete clinical data (n = 12). A further seven patients with previously diagnosed persistent atrial fibrillation in the presence of a normal thyroid function test, and who subsequently developed hyperthyroidism after treatment with amiodarone were also excluded from the analysis. A total of 591 patients were included in the final analysis.
Definitions
The diagnosis of primary hyperthyroidism was established in the presence of a serum‐free thyroxine (T4) level >23 pmol/l, and a concomitant suppressed thyroid‐stimulating hormone level <0.03 pmol/l. A diagnosis of Graves' disease was based on the clinical presentation of hyperthyroidism, usually in the presence of a diffuse goitre with or without thyroid eye signs and positive immunological markers. Presence of CHF diagnosis was retrieved from the medical records and validated with the use of modified Framingham criteria.13,14 Hypertension was defined as a systolic blood pressure of ⩾140 mm Hg, a diastolic blood pressure of ⩾90 mm Hg, or if the patient was prescribed drugs for hypertension. Smoking status was also recorded, with patients classified as non‐smoker or current smoker.
Transthoracic echocardiography
Two‐dimensional and M‐mode transthoracic echocardiographic examinations were performed in all subjects using the System V machine (GE Medical System, Waukesha, Wisconsin, USA) with a 3.5 MHz transducer, according to the recommendations of the American Society of Echocardiography.15 Left ventricular systolic dysfunction was defined as left ventricular ejection fraction (LVEF)⩽50%. All studies were performed and analysed by the same experienced operator who was unaware of the patients' clinical status.
Follow‐up
In patients with hyperthyroidism and CHF, initial transthoracic echocardiography was performed after stabilisation of heart failure symptoms and adequate ventricular rate control in those with atrial fibrillation. Clinical and ECG evaluation, laboratory measurements and transthoracic echocardiography were repeated 3 months after completion of treatment (antithyroid drugs or radioactive iodine‐131) for hyperthyroidism to achieve euthyroid status. In patients in whom ventricular systolic dysfunction persisted after achievement of euthyroid status, cardiac catheterisation or exercise thallium was performed to exclude concomitant coronary artery disease. Transthoracic echocardiography was also repeated at 12 months after establishment of a euthyroid state to detect delayed recovery of left ventricular systolic dysfunction.
Statistical analysis
Continuous variables are expressed as mean±1SEM. Statistical comparisons were performed using Student's t test or Fisher's exact test, as appropriate. A logistic regression model was applied to determine clinical predictors for the occurrence of CHF and left ventricular systolic dysfunction in patients with hyperthyroidism. Multivariate analyses were performed with an enter regression model, in which each variable with a p⩽0.1 (based on the univariate analysis) was entered into the model. Calculations were performed using SPSS software (V.10.0). p<0.05 was considered significant.
Results
Incidence of CHF
Table 1 presents the baseline demographic and clinical variables of 591 consecutive patients diagnosed with hyperthyroidism. Their mean age was 45 (1) years (range 18–97 years), and 140 patients (24%) were male. All patients were Chinese in origin. A total of 34 patients (5.8%) had symptoms of CHF at presentation. The Hong Kong West Island adult population remained stable at approximately 300 000 during the study period giving an annual incidence of CHF related to hyperthyroidism of 5.6 per 100 000 general population.
Table 1 Demographic characteristics of study population.
| All (n = 591) | Without heart failure (n = 557) | With heart failure (n = 34) | p Value | |
|---|---|---|---|---|
| Age, years | 45 (1) | 44 (1) | 66 (3) | <0.01 |
| Male | 140 (24) | 120 (22) | 20 (59) | <0.01 |
| Hypertension | 50 (9) | 39 (7) | 11 (32) | <0.01 |
| Diabetes mellitus | 32 (5) | 27 (5) | 5 (15) | 0.01 |
| Smoking history | 116 (20) | 103 (18) | 13 (34) | 0.01 |
| Free thyroxine, pmol/l | 52 (1) | 52 (1) | 53 (7) | 0.77 |
| Aetiology of hyperthyroidism | ||||
| Graves' diseases | 362 (61) | 346 (62) | 16 (47) | 0.10 |
| Toxic multinodular goitre | 226 (38) | 208 (37) | 18 (52) | |
| Others | 3 (1) | 3 (1) | 0 (0) | |
| Duration of symptoms of hyperthyroidism, months | 35 (14) | 32 (16) | 39 (12) | 0.53 |
| Atrial fibrillation at presentation | 82 (14) | 50 (9) | 32 (94) | <0.01 |
| Heart rate at presentation, bpm | 110 (5) | 100 (3) | 138 (3) | <0.01 |
Values are in n (%) or mean (SEM).
Clinical characteristics of CHF
Patients with CHF were older, most of them were male, had a higher prevalence of a history of hypertension and diabetes, and cigarette smoking, and had a higher incidence of atrial fibrillation and resting heart rate at presentation compared with patients without CHF (table 1; p<0.05). There were no significant differences in the duration of symptoms of hyperthyroidism, serum free T4 level or the incidence of Graves' disease (table 1; p>0.05). In multivariate analysis, only the presence of atrial fibrillation at presentation (OR 37.4, 95% CI 9.72 to 144.0, p<0.001) was an independent predictor for the occurrence of CHF in patients with hyperthyroidism.
In the 34 patients who presented with symptoms of CHF, an echocardiogram showed no evidence of marked valvular lesion, pericardial disease, or hypertrophic or infiltrative cardiomyopathy, and 16 (47%) had left ventricular systolic dysfunction with LVEF⩽50%. None had a history of alcohol or illicit drug misuse or exposure to cardiotoxic agents. Patients with left ventricular systolic dysfunction were significantly younger, with a higher prevalence of males, lower serum free T4 level and LVEF and a larger left ventricular end‐diastolic dimension compared with patients without left ventricular systolic dysfunction (table 2; p<0.05). There were no significant differences in the prevalence of hypertension, diabetes and smoking, duration of symptoms of hyperthyroidism, incidence of atrial fibrillation and resting heart rate at presentation or incidence of antithyroid antibodies (table 2; p>0.05). In multivariate analysis, male sex (OR 26.6, 95% CI 2.6 to 272.5, p = 0.006) and lower serum T4 level (OR 0.93, 95% CI 0.87 to 0.99, p = 0.044) were independent predictors of left ventricular systolic dysfunction with LVEF⩽50% in patients with hyperthyroidism.
Table 2 Demographic characteristics and clinical parameters of patients with heart failure, with or without left ventricular systolic dysfunction at presentation.
| LV systolic dysfunction | p Value | ||
|---|---|---|---|
| Absent (n = 18) | Present (n = 16) | ||
| Age, years | 72 (3) | 58 (4) | 0.03 |
| Male | 6 (33) | 14 (88) | <0.01 |
| Hypertension | 3 (17) | 8 (50) | 0.07 |
| Diabetes mellitus | 3 (17) | 2 (13) | 1.0 |
| Smoking history | 4 (22) | 9 (56) | 0.07 |
| Free thyroxine, pmol/l | 66 (12) | 39 (4) | 0.04 |
| Aetiology of hyperthyroidism | |||
| Graves' diseases | 11 (61) | 5 (31) | 0.10 |
| Toxic multinodular goitre | 7 (39) | 11 (69) | |
| Duration of symptoms of hyperthyroidism, days | 40 (25) | 38 (16) | 0.85 |
| Atrial fibrillation | 18 (100) | 14 (88) | 0.21 |
| Heart rate, bpm | 140 (5) | 137 (9) | 0.79 |
| LV end‐diastolic dimension, cm | 4.3 (0.1) | 5.3 (0.2) | <0.01 |
| LVEF | 62 (2) | 29 (2) | <0.01 |
LV, left ventricle; LVEF, left ventricular ejection fraction.
Values are in n (%) or mean (SEM).
Clinical outcome
In the 34 patients with CHF, all achieved euthyroid status with either radioactive iodine (n = 21) or carbimazole (n = 12) treatment, except one patient who died of refractory heart failure during the initial presentation.
In the 18 patients with LVEF ⩾50%, all had atrial fibrillation at presentation. The time to achieve satisfactory resting mean heart rate control (<90 bpm) was 3.5 (0.5) days. Symptoms of heart failure subsided after treatment with diuretics, β‐blocker and carbimazole at initial presentation. Spontaneous sinus conversion from atrial fibrillation was observed in 8 of 18 patients (44%) at a mean of 121 (46) days after treatment of hyperthyroidism. During a mean follow‐up of 42 (6) months, none reported any symptoms of CHF, including the 10 patients in whom atrial fibrillation persisted.
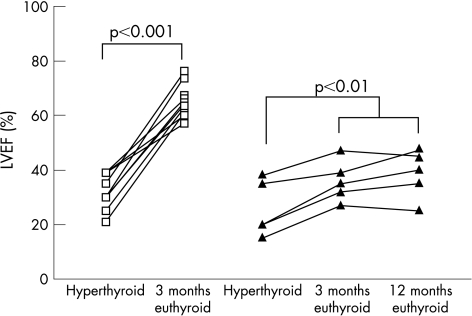
In the remaining 15 patients with LVEF⩽50%, 13 had atrial fibrillation at presentation. The time to achieve satisfactory mean resting heart rate control (<90 bpm) was 4.7 (0.8) days. Spontaneous sinus conversion from atrial fibrillation was observed in 6 of 13 patients (46%) at a mean of 328 (131) days after treatment of hyperthyroidism. Three months after achieving euthyroid status, LVEF (55 (4)% vs 30 (2)%, p<0.001) and New York Heart Association (NYHA) functional class (1.2(0.1) vs 2.5 (0.2), p<0.001) had significantly improved compared with initial presentation. In 10 patients (67%), LVEF had completely recovered (65 (2)% vs 32 (2)%, p<0.01, fig 1) and none of them reported any symptoms of CHF 3 months after achieving euthyroid status. In the remaining five patients (33%), left ventricular systolic dysfunction persisted with LVEF⩽50%, although LVEF (36 (4)% vs 26 (5)%, p<0.01, fig 1) and NYHA functional class (1.4 (1) vs 3.2 (1), p = 0.03) had significantly improved.
Figure 1 Serial changes in left ventricular ejection fraction (LVEF) in patients with (▴) or without (□) persistent left ventricular systolic dysfunction at presentation, 3 months and 12 months after achieving euthyroid status.
There were no significant differences (p>0.05) in the demographic features of patients with or without persistent left ventricular systolic dysfunction in terms of duration of symptoms of hyperthyroidism, incidence of atrial fibrillation and resting heart rate, serum free T4 level, incidence of Graves' disease, LVEF and left ventricular end‐diastolic dimension at presentation of hyperthyroidism and time to achieve satisfactory heart rate (table 3). In addition, among those patients with atrial fibrillation at presentation, there were no significant differences in the percentage of patients with spontaneous conversion to sinus rhythm (50% vs 44%, p = 1.0) and their time to conversion (265 (260) days vs 361 (67) days, p = 0.3) between patients with and without persistent left ventricular systolic dysfunction.
Table 3 Demographic characteristics and clinical parameters of patients with and without persistent left ventricular systolic dysfunction.
| Persistent LV systolic dysfunction | p Value | ||
|---|---|---|---|
| Absent (n = 10) | Present (n = 5) | ||
| Age, years | 63 (5) | 56 (8) | 0.43 |
| Male | 8 (80) | 5 (100) | 0.52 |
| Hypertension | 4 (40) | 80 (4) | 0.28 |
| Diabetes mellitus | 2 (20) | 0 (0) | 0.52 |
| Smoking history | 4 (40) | 3 (60) | 0.61 |
| Free thyroxine, pmol/l | 41 (5) | 38 (6) | 0.81 |
| Aetiology of hyperthyroidism | |||
| Graves' diseases | 4 (40) | 1 (20) | 0.60 |
| Toxic multinodular goitre | 6 (60) | 4 (80) | |
| Duration of symptoms of hyperthyroidism, days | 37 (12) | 40 (13) | 0.78 |
| Atrial fibrillation at presentation | 9 (90) | 4 (80) | 1.00 |
| Heart rate at presentation bpm | 139 (12) | 122 (3) | 0.21 |
| Time to achieve resting heart rate control (<90/min), days | 4.6 (0.6) | 4.8 (1.4) | 0.90 |
| LV end‐diastolic dimension at presentation, cm | 5.4 (0.3) | 5.8 (0.6) | 0.49 |
| LVEF at presentation | 32 (2) | 26 (5) | 0.16 |
| Time to achieve euthyroid status, days | 138 (53) | 113 (70) | 0.79 |
LV, left ventricle; LVEF, left ventricular ejection fraction.
Values are in n (%) or mean (SEM).
In five patients with persistent left ventricular systolic dysfunction after achieving a euthyroid state, a coronary angiogram was normal in four, and a thallium SPECT scan in the remaining patient showed no evidence of myocardial ischaemia. At 1‐year follow‐up, three patients were in sinus rhythm and two had persistent atrial fibrillation with satisfactory heart rate control (average heart rate <80 bpm at 24 h ECG recording). Despite optimal medical treatment for heart failure in these patients, including use of an ACE inhibitor (n = 5), digoxin (n = 4), frusemide (n = 4), β‐blocker (n = 3), spironolactone (n = 2) and angiotensin II antagonists (n = 2), there was no further improvement in LVEF at 1 year compared with those at 3 months after achieving a euthyroid state (36 (3)% vs 38 (4)%, p = 0.29, fig 1).
Discussion
Although an increased workload caused by the induction of a hyperdynamic circulation during hyperthyroidism can trigger the onset of heart failure in patients with pre‐existing heart disease, it may also directly induce CHF.2,7 Nevertheless, there are limited data on the incidence of CHF in patients with hyperthyroidism. This study showed that 6% of patients with hyperthyroidism developed CHF and the annual incidence is 5.6 per 100 000 general population. Consistent with previous studies,16 this study showed that elderly patients with pre‐existing hypertension or with risk factors for coronary artery disease, such as smoking and diabetes, were more susceptible to adverse haemodynamic changes related to hyperthyroidism and developed CHF. In addition, the occurrence of atrial fibrillation could worsen cardiac function, and was an independent predictor for the development of CHF.
In this study, about half of the patients who presented with CHF had normal LVEF. Their heart failure symptoms may have reflected a congestive circulation secondary to excess sodium and fluid retention related to hyperthyroidism or caused by diastolic heart failure triggered by sustained tachycardia and/or atrial fibrillation.2,16,17 These patients responded well to treatment with diuretics and rate control treatment with a β‐blocker.
More importantly, left ventricular systolic dysfunction was observed in the remaining patients with CHF at presentation. Compared with patients in whom left ventricular function was preserved, those with left ventricular systolic dysfunction showed a male predominance and had a significantly lower serum T4 level at presentation. Although the exact mechanism remains unclear, multiple factors are likely to contribute to the development of left ventricular systolic dysfunction in patients with hyperthyroidism. First, chronic persistent tachyarrhythmias caused by a combination of hyperthyroidism and atrial fibrillation can lead to CHF due to tachycardia‐induced cardiomyopathy. Despite this, we found no significant difference in the duration of symptoms of hyperthyroidism, incidence of atrial fibrillation and resting heart rate at presentation between patients with and without left ventricular systolic dysfunction. Furthermore, in those patients with atrial fibrillation, the time to achieve satisfactory heart rate control, percentage of patients with spontaneous conversion to sinus rhythm, and their time to conversion were similar between the two groups. In the five patients with persistent left ventricular systolic dysfunction, three were in sinus rhythm and two had persistent atrial fibrillation with satisfactory heart rate control at 1‐year follow‐up after establishment of a euthyroid state. Therefore, in approximately two‐thirds of patients with left ventricular dysfunction at presentation, sustained tachyarrhythmias and atrial fibrillation contributed to the development of left ventricular dysfunction which recovered after rate or rhythm control of atrial fibrillation. In the remaining one‐third, there was only partial recovery of left ventricular dysfunction despite successful rate or rhythm control of tachyarrhythmias and atrial fibrillation by drugs and treatment of hyperthyroidism. This suggests that other mechanisms may contribute to the development of left ventricular dysfunction in these patients. Second, the presence of concurrent autoimmune myocarditis related to Graves' disease has been suggested to be a contributing factor.18 Nonetheless, several histological studies in patients with dilated cardiomyopathy complicated hyperthyroidism showed no evidence of active myocarditis.19,20,21 In this study, there was no significant difference in the incidence of Graves' disease between patients with and without left ventricular systolic dysfunction. Finally, direct myocardial damage in hyperthyroidism has also been shown by radioactive22 or magnetic resonance23 cardiac imaging. Interestingly, a lower rather than a higher serum T4 level was associated with the development of left ventricular systolic dysfunction in this study. Although there was no significant difference in the duration of onset of symptoms of hyperthyroidism, patients with left ventricular systolic dysfunction may have a more prolonged duration of subclinical hyperthyroidism, possibly because of a lower serum T4 level as in elderly patients with multinodular goitre. We found that male patients were more likely to develop left ventricular systolic dysfunction with hyperthyroidism. In experimental studies, male animals were more susceptible to the cardiotoxic effects of overexpression of β‐adrenergic receptors than female animals, possibly because of upregulation of a hypertrophy‐related gene by androgens.24 This increased cardiac sensitivity to β‐adrenergic stimulation in males may account for their higher incidence of left ventricular systolic dysfunction related to hyperthyroidism.
The availability of effective treatment for primary hyperthyroidism has led to the widespread belief that it is a benign disorder and that all the associated adverse effects are reversible with no long‐term sequelae. Although prior case studies have shown that left ventricular function improves in the majority of patients after treatment of hyperthyroidism, persistent dilated cardiomyopathy was observed in a significant proportion of patients.8,9,10,11,20,21 This is the first systematic study to show that persistent and potentially fatal dilated cardiomyopathy developed in approximately 1% (6/519 patients, including one patient who died of refractory heart failure) of patients with primary hyperthyroidism. Up to one‐third of patients with left ventricular systolic dysfunction at presentation had persistent dilated cardiomyopathy during long‐term follow‐up. Long‐term follow‐up is thus vital to detect the persistence of cardiomyopathy in those patients with hyperthyroidism who have left ventricular systolic dysfunction at initial presentation.
Study limitation
In this study, echocardiography was performed only in patients with signs and symptoms of CHF. The incidence of subclinical left ventricular systolic dysfunction in those patients without CHF thus remains unknown.
Abbreviations
CHF - congestive heart failure
LVEF - left ventricular ejection fraction
NYHA - New York Heart Association
Footnotes
Competing interests: None.
References
- 1.Polikar R, Burger A G, Scherrer U.et al The thyroid and the heart. Circulation 1993871435–1441. [DOI] [PubMed] [Google Scholar]
- 2.Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med 2001344501–509. [DOI] [PubMed] [Google Scholar]
- 3.Klein I. Thyroid hormone and the cardiovascular system. Am J Med 199088631–637. [DOI] [PubMed] [Google Scholar]
- 4.Kahaly G J, Kampmann C, Mohr‐Kahaly S. Cardiovascular hemodynamics and exercise tolerance in thyroid disease. Thyroid 200212473–481. [DOI] [PubMed] [Google Scholar]
- 5.Feldman T, Borow K M, Sarne D H.et al Myocardial mechanics in hyperthyroidism: importance of left ventricular loading conditions, heart rate and contractile state. J Am Coll Cardiol 19867967–974. [DOI] [PubMed] [Google Scholar]
- 6.Forfar J C, Muir A L, Sawers S A.et al Abnormal left ventricular function in hyperthyroidism. Evidence for a possible reversible cardiomyopathy. N Engl J Med 19823071165–1170. [DOI] [PubMed] [Google Scholar]
- 7.Polikar R, Burger A G, Scherrer U.et al The thyroid and the heart. Circulation 1993871435–1441. [DOI] [PubMed] [Google Scholar]
- 8.Shirani J, Barron M M, Pierre‐Louis M L.et al Congestive heart failure, dilated cardiac ventricles, and sudden death in hyperthyroidism. Am J Cardiol 199372365–368. [DOI] [PubMed] [Google Scholar]
- 9.Umpierrez G E, Challapalli S, Patterson C. Congestive heart failure due to reversible cardiomyopathy in patients with hyperthyroidism. Am J Med Sci 199531099–102. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe E, Ohsawa H, Noike H.et al Dilated cardiomyopathy associated with hyperthyroidism. Intern Med 199534762–767. [DOI] [PubMed] [Google Scholar]
- 11.Boccalandro C, Boccalandro F, Orlander P.et al Severe reversible dilated cardiomyopathy and hyperthyroidism: case report and review of literature. Endocr Pract 20039140–146. [DOI] [PubMed] [Google Scholar]
- 12.Kasper E K, Agema W R, Hutchins G M.et al The causes of dilated cardiomyopathy: a clinicopathologic review of 673 consecutive patients. J Am Coll Cardiol 199423586–590. [DOI] [PubMed] [Google Scholar]
- 13.Senni M, Tribouilloy C M, Rodeheffer R J.et al Congestive heart failure in the community: a study of all incident cases in Olmsted County, Minnesota, in 1991. Circulation 1998982282–2289. [DOI] [PubMed] [Google Scholar]
- 14.Redfield M M, Jacobsen S J, Burnett J C., Jret al Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 2003289194–202. [DOI] [PubMed] [Google Scholar]
- 15.Schiller N B, Shah P M, Crawford M.et al Recommendations for quantitation of the left ventricle by two‐dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two‐Dimensional Echocardiograms. J Am Soc Echocardiogr 19892358–367. [DOI] [PubMed] [Google Scholar]
- 16.Klein I, Ojamaa K. Thyrotoxicosis and the heart. Endocrinol Metab Clin North Am 19982751–62. [DOI] [PubMed] [Google Scholar]
- 17.DeGroot W J, Leonard J J. Hyperthyroidism as a high cardiac output state. Am Heart J 197079265–275. [DOI] [PubMed] [Google Scholar]
- 18.Koshiyama H, Sellitti D F, Akamizu T.et al Cardiomyopathy associated with Graves' disease. Clin Endocrinol (Oxf) 199645111–116. [PubMed] [Google Scholar]
- 19.Sachs R N, Vignot M, Modigliani E.et al Absence of ultra‐structural histological lesions of the myocardium in cardiac insufficiency of hyperthyroidism. Ann Med Interne 1986137375–378. [PubMed] [Google Scholar]
- 20.Ebisawa K, Ikeda U, Murata M.et al Irreversible cardiomyopathy due to thyrotoxicosis. Cardiology 199484274–277. [DOI] [PubMed] [Google Scholar]
- 21.Fatourechi V, Edwards W D. Graves' disease and low‐output cardiac dysfunction: implications for autoimmune disease in endomyocardial biopsy tissue from eleven patients. Thyroid 200010601–605. [DOI] [PubMed] [Google Scholar]
- 22.Marti V, Ballester M, Rigla M.et al Myocardial damage does not occur in untreated hyperthyroidism unless associated with congestive heart failure. Am Heart J 19971341133–1137. [DOI] [PubMed] [Google Scholar]
- 23.Matsuoka H, Hamada M, Honda T.et al Precise assessment of myocardial damage associated with secondary cardiomyopathies by use of Gd‐DTPA‐enhanced magnetic resonance imaging. Angiology 199344945–950. [DOI] [PubMed] [Google Scholar]
- 24.Gao X M, Agrotis A, Autelitano D J.et al Sex hormones and cardiomyopathic phenotype induced by cardiac beta 2‐adrenergic receptor overexpression. Endocrinology 20031444097–4105. [DOI] [PubMed] [Google Scholar]