Abstract
Enhanced survival of both individual cells and whole organisms following a heat stress is termed thermotolerance. In organisms, the maintenance of tissue function rather than the survival of individual cells ultimately determines outcome following thermal challenge. We used MDCK kidney epithelial cells to compare alterations in chaperone activity (as a measure of cellular tolerance) and epithelial barrier function (as a measure of physiological tolerance) after thermal challenge. Quercetin, an inhibitor of heat shock factor–dependent transcriptional activity, both potentiated the effects of heat on naive monolayers and blocked conditioning of monolayers following moderate heat shock, suggesting a central role of heat shock protein (HSP) family members in the maintenance of epithelial integrity. We used MDCK cells that constitutively overexpressed HSP70 to demonstrate 2 functionally distinct components of the response of monolayers to thermal stress. The maintenance of epithelial barrier function during exposure to elevated temperatures is regulated by a complex network of processes that involve the actions of HSP70 but that are independent of alterations in chaperone activity as reflected by changes in the thermal inactivation/refolding of luciferase. In contrast, the restoration of barrier function following a heat stress is directly modulated by HSP70 in a manner that can be fully accounted for by changes in chaperone activity. This study demonstrates an important, albeit complex, protective role for heat shock proteins in the modulation of MDCK epithelial barrier function following a thermal stress.
INTRODUCTION
Cells respond to stress by synthesizing the highly conserved family of protein chaperones and foldases termed heat shock proteins (HSPs) (Lindquist and Craig 1988; DeMaio 1999). The expression of HSPs in cells following a mild “conditioning” stress is associated with an enhanced ability of cells to survive a subsequent exposure to a potentially lethal stress (Beere and Green 2001; Garrido et al 2001). This increase in cell survival, generally termed tolerance, is independent of the exact nature of the conditioning or type of stress. For example, an initial exposure to moderate heat stress sufficient to increase HSP expression causes cells to become tolerant to any number of more severe second insults (Musch et al 1996, 1999; Chu et al 1997). The fact that HSP synthesis is triggered by alterations in global protein translation has led to the hypothesis that the HSP-mediated chaperone activity is responsible, at least in part, for enhanced cellular survival (Laszlo 1988; Craig et al 1994; Zeng et al 2004). Although the relationship between HSP expression and individual cell survival is well established, much less is understood about the role of HSPs in maintaining intercellular functions during challenges sufficient to disrupt cell-to-cell interactions without causing heat-induced cell death. The ability of cells to maintain critical cell-to-cell interactions following stress is an important determinant of overall tissue function, which is, in turn, required for the survival of the whole animal (Moseley 1994; Bouchama and Knochel 2002). Deficits in the maintenance of epithelial barrier integrity are the hallmarks of diseases as varied as sepsis, inflammatory bowel disease, heat stroke, and asthma (Moseley 1997, 1998).
In previous work using MDCK and Caco-2 epithelial monolayers, we have demonstrated that a nonlethal heat challenge causes a rapid but reversible alteration in epithelial barrier integrity (Moseley et al 1994; Dokladny et al 2006). Interestingly, these alterations in heat-induced epithelial barrier function were attenuated in epithelial monolayers that had been preconditioned by mild heat exposure to express increasing levels of HSPs (Moseley et al 1994). Thus, cellular HSP expression appeared to be a potential regulator of heat-induced modulations in epithelial permeability (ie, physiological thermotolerance).
We have now extended our previous studies to provide further insight into the role of HSPs, particularly HSP70, in the protection against heat-induced alterations in epithelial barrier function. The ability of in vitro monolayers to restore epithelial barrier function following severe heat stress (heat shock) is observed in either heat-conditioned monolayers or in cells that overexpress HSP70, and correlates with an enhancement in the refolding of heat-denatured proteins. In contrast, the loss of epithelial barrier function observed immediately after a severe insult is much less dependent on the actions of HSP70 alone and is independent of the level of chaperone activity.
Taken as a whole, our results indicate a more complex role for HSPs in both preserving the important cell-to-cell interactions that maintain the epithelial barrier integrity as well as in the restoration of epithelial barrier integrity following severe heat-induced epithelial damage. Our results expand the concept of thermotolerance from that of cellular survival to preservation of cell-to-cell interaction and epithelial function.
MATERIALS AND METHODS
Cell culture
MDCK cells (CCL-34), obtained from the American Type Culture Collection (Manassas, VA, USA), were maintained in Dulbecco modified Eagle medium supplemented with 2 mM glutamine, 100 U/mL penicillin, 100 μg/ mL streptomycin, 1 mM sodium pyruvate, and 10% fetal calf serum in a humidified atmosphere containing 5% CO2. Dulbecco modified Eagle medium, glutamine, penicillin, streptomycin, and sodium pyruvate were purchased from GIBCO-BRL (Grand Island, NY, USA). Stock cultures were maintained in 100-mm dishes from Corning (Corning, NY, USA) and were subcultured every 3–4 days. Cells to be used in transepithelial resistance experiments were seeded on 12-mm culture plate insert (0.45-μm pores) Millicell-HA (Millipore, Billerica, MA, USA) and allowed to grow until a confluent monolayer was formed. Cultures for Western blots analysis or protein refolding assays were maintained in 60-mm tissue culture plates from Corning.
Stable transfectants
A construct containing the coding region for human HSP70 (accession no. NM_005345) was obtained from Dr. Anne Knowlton (University of California, Davis, CA, USA). The HSP70 insert was amplified with thermal cycler (Applied Biosystems, Foster City, CA, USA) and recloned into a pRC/CMV backbone. Parent MDCK cells were transfected with either a control pRC/CMV plasmid lacking an exogenous insert, or with pRC/CMV-HSP70, and incubated in medium containing 75 μg/mL G418 (Life Technologies, Grand Island, NY, USA). Individual colonies were isolated by limiting dilution and screened for expression of HSP70 by Western blots analysis. A clone transfected with pRC/CMV that expressed control levels of HSP70 protein (MDCK_pRC.1), as well as a clone transfected with pRC/CMV-HSP70 that expressed elevated levels of HSP70 protein (MDCK_p70.2) were used in subsequent experiments.
Induction of thermal stress
Cells grown on 60-mm plates were placed in a bath in a manner that the dishes rested directly on water that was heated to the appropriate temperature. Air containing 5% CO2 was circulated through the sealed bath for the duration of the stress.
Determination of transepithelial resistance
An epithelial voltohmeter (World Precision Instruments, Sarasota, FL, USA) was used to determine transepithelial resistance as a marker of tight junction integrity in confluent monolayers grown on permeable filters as previously described (Ma TY et al 2000; Dokladny et al 2006). Inserts without cells served as blanks. Transepithelial resistance (× cm2) was calculated as follows: (Rsample − Rblank) × 0.6 cm2.
Western blots analysis
To study the effect of heat shock protein inhibitor quercetin (100 μM) on the heat stress–induced increase in heat shock protein expression, MDCK cells were exposed to 42°C in the presence or absence of quercetin. At the end of the experimental period, MDCK monolayers were immediately rinsed with ice-cold phosphate-buffered saline (PBS), and cells were lysed with lysis buffer containing 0.1% Nonidet P-40 as previously described (Roigas et al 2004). Cell lysates were placed in microfuge tubes and centrifuged to yield a clear lysate. Supernatant was collected and was followed by protein measurement using Bio-Rad Protein Assay kit (Bio-Rad Laboratories, Hercules, CA, USA). Aliquots containing 15 μg of total protein were mixed with Laemmli gel loading buffer and boiled for 7 minutes, after which proteins were separated on 8% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel. Proteins from the gel were transferred to the membrane (Trans-Blot Transfer Medium, Nitrocellulose Membrane; Bio-Rad Laboratories) overnight. The membranes were blocked for 2 hours in a blocking solution of TBS (10 mM Tris, 150 mM NaCl, pH 8.0) containing 5% dry milk (Bio-Rad Laboratories) and Tween 20 (Bio-Rad Laboratories). The membrane was probed with appropriate primary antibodies in blocking solution. Anti-HSP antibodies were purchased from StressGen Biotechnologies (Victoria, British Columbia, Canada). Following several rinses with TBS-Tween buffer, the membranes were incubated in appropriate secondary antibodies in blocking solution. Horseradish peroxidase (HRP)–conjugated secondary antibodies were purchased from Zymed Laboratories (San Francisco, CA, USA). After being washed with TBS-Tween buffer, membranes were developed using the Santa Cruz Western Blotting Luminol Reagents (Santa Cruz Biotechnology, Santa Cruz, CA, USA) on the Kodak BioMax MS film (Fisher Scientific, Pittsburgh, PA, USA).
Luciferase refolding assay
Cells were transfected with pGL3 (Clontech Laboratories, Mountain View, CA, USA) and incubated for 48 hours to allow for optimal luciferase expression. Cells were either maintained at 37°C (control) or exposed to 42°C for 2 hours (heat conditioning). Following a further 24-hour incubation at 37°C, cells were exposed to 45°C for 45 minutes in the presence of protein synthesis inhibitor cycloheximide (10 μg/mL) (Sigma, St Louis, MO, USA). Luciferase activity was determined either immediately after heating or following a 4-hour recovery period at 37°C. At the end of the experimental period, MDCK cells were washed twice with 1 mL of ice-cold PBS. Cells were lysed with 1× passive lysis buffer (500 μL) and incubated at room temperature for 15 minutes, scraped, and placed in microfuge tubes. Cell lysates were centrifuged (13 000 rpm for 20 seconds) to yield a clear lysate. Twenty microliters of the supernatant were used for each assay. Luciferase activity was performed using the luciferase assay system (Promega, Madison, WI, USA). Luciferase values were determined by Lumat LB 9501 (Berthold, Wildbad, Germany).
Statistical analysis
All data are expressed as the mean ± SE. The comparisons between groups were conducted using an analysis of variance. If any interaction terms were observed to be statistically significant and there were more than 2 comparison groups, subtesting was performed using t-tests for unpaired data. All reported significance represents 2-tailed P value. A value of P < 0.05 was used to indicate statistical significance.
RESULTS
The effect of heat preconditioning on the recovery of epithelial barrier function in heat shock–exposed MDCK monolayers
We have previously shown that a preconditioning heat stress resulting in HSP70 expression attenuates the increase in MDCK epithelial permeability observed after a more severe heat shock (Moseley et al 1994). In all of the experiments reported here, we use the term heat shock to refer to an exposure to 45°C for 45 minutes, whereas preconditioning refers to an exposure to 42°C for 2 hours. Figure 1 extends our previous findings by demonstrating that inhibition of HSP accumulation by quercetin (100 μM), a blocker of heat shock factor 1 (HSF1)-dependent HSP synthesis (Hosokawa et al 1992; Nagai et al 1995), abrogates the protective effects afforded by the preconditioning stimulus. In Figure 1A, transepithelial resistance was measured in MDCK monolayers immediately following an exposure to 45°C for 45 minutes (ie, after a heat shock as defined above). The short pulse of severe heat caused a dramatic drop in transepithelial resistance in all examined groups. The conditioned cells (42°C for 2 hours) demonstrated a more rapid recovery than monolayers of nonconditioned cells (P < 0.001 at the 4-hour time point and P < 0.05 at the 8-hour time point). The presence of quercetin both potentiated the effects of an acute heat stress and completely abolished the ability of a conditioning heat to provide protection to the monolayers.
Fig 1.
The effect of HSP inhibitor quercetin on the recovery of epithelial barrier function in heat shock–exposed MDCK monolayers. (A) Filter-grown MDCK cells were heated to 45°C for 45 minutes either with no preconditioning (37°C/45°C) or 24 hours after a preconditioning exposure to 42°C for 2 hours (42°C/45°C) in the absence or in the presence of quercetin (100 μM) (Q). Transepithelial resistance was measured sequentially over the 24-hour experimental period after the exposure to 45°C. Each symbol represents the mean ± SE of sets of 10 filters. ***P < 0.001 and *P < 0.05 represent a significant difference between 42°C/45°C vs Q/42 °C/45°C; ###P < 0.001 and ##P < 0.01 represent a significant difference between 37°C/45°C vs Q/37°C/45°C. (B) MDCK cells were maintained at 37°C or were heated to 42°C for 2 hours in the presence of the solvent control (DMSO/42°C) or quercetin (100 μM) (Q/42°C). After a further 24-hour incubation at 37°C, HSP70, HSP90, and HSC70 protein expressions were determined by Western blot analysis as described in Materials and Methods. The numbers below each lane of the HSP autoradiogram represent relative densitometry determined using Adobe Photoshop 7.0 software
The effects of quercetin on heat-induced expression of HSPs in MDCK cells are shown in Figure 1B. Control cells expressed very low levels of HSP70, whereas both HSP90 and heat shock cognate protein 70 (HSC70) were readily detectable. Twenty-four hours after heat conditioning (42°C for 2 hours), there were marked increases in HSP70 protein expression in the MDCK cells. Both HSP90 and HSC70 protein expression remained unaffected by the heat conditioning. Addition of quercetin (100 μM) during the heat conditioning prevented the increase in HSP70 expression. The HSF-1 inhibitor also caused a slight decrease in HSC70 and HSP90 expression.
These data indicate that heat conditioning (42°C for 2 hours) resulting in HSP70 up-regulation enhances the recovery of the epithelial barrier function in MDCK cells subjected to severe heat shock (45°C). The protective effect of the conditioning is removed when MDCK cells undergo conditioning in the presence of heat shock protein inhibitor quercetin. We take these results to suggest, although not prove, that an HSF-1-dependent increase in HSP expression underlies at least some aspects of physiological thermotolerance. The next series of experiments were designed to directly test this hypothesis.
The recovery of epithelial barrier function in heat shock–exposed MDCK monolayers that constitutively overexpress HSP70
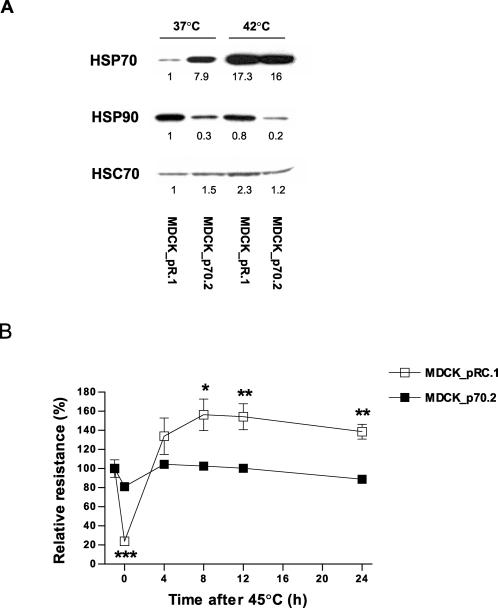
The above results suggest a correlation between HSP expression (and particularly HSP70 expression) and the ability of the MDCK monolayer to reestablish control levels of transepithelial resistance following heat-induced damage. To determine whether HSP70 expression itself could confer some of the protective effects in the maintenance of MDCK monolayer barrier function caused by heat conditioning and blocked by quercetin, we established a stable clone of MDCK cells that constitutively expressed elevated levels of HSP70. A stable clone (MDCK_p70.2) expressing high levels of HSP70 and a matching pRC/CMV-transfected control clone (MDCK_pRC.1) were chosen for further study. As shown in Figure 2A, MDCK_pRC.1 cells, which were transfected with empty pRC/CMV, expressed levels of HSP70 similar to untransfected parent MDCK cells. In contrast, MDCK_p70.2 cells expressed markedly elevated levels of HSP70 protein. There were no differences in the expression of HSC70 between the parent MDCK cells and the transfected clones, although the expression of HSP90 was somewhat decreased in MDCK_p70.2 cells.
Fig 2.
The recovery of epithelial barrier function in heat shock-exposed MDCK monolayers that constitutively overexpress HSP70. (A) HSP70, HSP90, and HSC70 protein expressions were determined in either parent untransfected MDCK cells or in MDCK_pRC.1 that contain the empty vector, or MDCK_p70.2 cells that were transfected with a vector that directs the expression of human HSP70. The numbers below each lane of the HSP autoradiogram represent relative densitometry determined using Adobe Photoshop 7.0 software. In the experiment shown in (B), transfected MDCK cells grown on permeable filters were exposed to heat shock (45°C for 45 minutes). The cells were then returned to control temperature (37°C) and transepithelial resistance was measured sequentially over the 24-hour experimental period. Data represent means ± SE (n = 4). ***P < 0.001 vs MDCK_pRC.1
The effects of HSP70 overexpression on the modulation of MDCK epithelial barrier function after heat shock exposure are shown in Figure 2B. Exposure of MDCK_pRC.1 monolayers to 45°C for 45 minutes resulted in a marked decrease in transepithelial resistance that was similar to that seen in parent MDCK cells (Fig 1A). Although the exogenous expression of HSP70 did not alter the drop in resistance measured immediately after heat shock, the recovery of epithelial barrier function during the subsequent recovery period was significantly enhanced in MDCK_p70.2 clones compared to MDCK_pRC.1 cells (P < 0.001). These data demonstrate that the overexpression of HSP70 markedly enhanced the epithelial barrier recovery following heat shock with a pattern of recovery similar to that seen in heat-conditioned monolayers. These findings indicate that, although the elevations in HSP70 expression do not have a direct protective effect during heat shock, they enhance the recovery of barrier function during the subsequent incubation at 37°C.
Heat preconditioning modulates the recovery of epithelial barrier function in heat shock–exposed MDCK monolayers that constitutively overexpress HSP70
In order to determine whether HSP70 overexpression could account for all of the protective effects conferred to epithelial monolayers by heat conditioning, we examined the effect of the heat conditioning on epithelial barrier function in stably transfected MDCK cells. Figure 3A shows the effect of heat conditioning on HSP expression in the clones. Control MDCK_pRC.1 cells express very low levels of HSP70 under control conditions. Heat preconditioning (42°C for 2 hours) caused a marked induction of HSP70 expression in MDCK_pRC.1 cells measured 24 hours after the stress. Under basal conditions, expression of HSP70 was significantly elevated in MDCK_p70.2 cells, as described above (Fig 2A), and heat preconditioning of MDCK_p70.2 monolayers resulted in a further increase in HSP70 protein levels. However, the expression levels of HSP70 24 hours following heat preconditioning was similar in the 2 clones, despite the fact that the MDCK_p70.2 had higher levels of HSP70 at basal state. HSC70 protein was expressed at equivalent levels in the control and MDCK_p70.2 cells, and was not induced by heat conditioning. As described above, MDCK_pRC.1 cells expressed higher levels of HSP90 compared to MDCK_p70.2 cells. Heat conditioning did not affect levels of expression of HSP90.
Fig 3.
The effect of heat preconditioning on changes in HSP expression and the recovery of epithelial barrier function in heat shock– exposed MDCK monolayers that constitutively overexpress HSP70. (A) One set of MDCK_pRC.1 or MDCK_p70.2 cells was maintained continuously at 37°C. The other set of cells was exposed to 42°C for 2 hours followed by 24-hour incubation at 37°C. Cellular levels of HSP70, HSP90, and HSC70 protein expressions were determined by Western blot analysis as described in Materials and Methods. The numbers below each lane of the HSP autoradiogram represent relative densitometry determined using Adobe Photoshop 7.0 software. (B) Filter-grown MDCK_pRC.1 or MDCK_p70.2 cells were exposed to a preconditioning heat stress (42°C for 2 hours) followed by incubation at 37°C. Twenty-four hours later, MDCK monolayers were exposed to 45°C for 45 minutes. The cells were then returned to control temperature (37°C) and transepithelial resistance was measured sequentially over the 24-hour experimental period. Data represent means ± SE (n = 4). *P < 0.05; **P < 0.01; ***P < 0.001 vs MDCK_p70.2
Figure 3B shows the effect of heat preconditioning on the heat shock–induced decrease in epithelial barrier function in MDCK_pRC.1 and MDCK_p70.2 cells. Heat preconditioning (42°C for 2 hours) of MDCK_pRC.1 cells resulted in the protection against the heat shock (45°C for 45 minutes)–induced decrease in transepithelial resistance similar to that seen for the parent MDCK monolayers (Fig 1A). Interestingly, heat conditioning the MDCK_p70.2 monolayers (which constitutively overexpress HSP70 and which demonstrate a heat-resistant phenotype) resulted in an even greater resistance to the barrier dysfunction induced by an acute heat shock, with only a minimal decrease (∼20%) in transepithelial resistance measured immediately after the severe stress.
Correlation between chaperone activity and HSP70 expression in MDCK_pRC.1 and MDCK_p70.2 monolayers following heat shock exposure
Given the demonstrated role of HSP70 overexpression as well as heat conditioning in attenuating the loss of epithelial barrier integrity caused by heat stress, we next examined the potential relationship between the impact of HSP70 on epithelial barrier function and protein-folding activity by measuring the denaturation/renaturation of luciferase.
Cultures of either MDCK_pRC.1 or MDCK_p70.2 cells were transfected with pGL3 basic vector encoding the luciferase gene. After a 48-hour incubation to allow for optimal luciferase expression, cells were subjected to a heat shock (45°C for 45 minutes). Levels of luciferase activity were measured immediately after the heat stress or after a 4-hour recovery at the control temperature (37°C) (Fig 4). Although luciferase activity was not significantly higher in MDCK_p70.2 monolayers than in MDCK_pRC.1 monolayers following exposure to the severe heat stress, there was more rapid recovery of luciferase activity in the MDCK_p70.2 cells than in MDCK_pRC.1 cells during a 4-hour recovery at 37°C (P < 0.001) (Fig 4A). As with epithelial barrier function, heat conditioning (42°C for 2 hours) resulted in a significantly greater preservation of luciferase activity in both MDCK_pRC.1 and MDCK_p70.2 cells at 4-hour time point following heat exposure (Fig 4B). Interestingly, the unconditioned MDCK_p70.2 showed more rapid recovery of luciferase activity than the heat-preconditioned MDCK_pRC.1 cells (P < 0.001), and monolayers composed of heat-conditioned MDCK_p70.2 cells were virtually impervious to the barrier-disrupting effects of the heat shock. Moreover, in MDCK_p70.2, heat preconditioning did further preserve luciferase stability over the unconditioned MDCK_p70.2 cells. These data indicate that the presence of elevated levels of HSP70 alone can promote the refolding of denatured proteins.
Fig 4.
Protection of luciferase activity from thermal degradation in transfected MDCK cells. Either MDCK_pRC.1 or MDCK_p70.2 cells were transfected with the luciferase expression vector pGL3. After 48-hour incubation period, cells were either maintained at 37°C (A) or preconditioned to 42°C for 2 hours (B). Following a further 24-hour incubation at 37°C, cells were exposed to 45°C for 45 minutes in the presence of protein synthesis inhibitor cycloheximide (10 μg/ mL). Luciferase activity was determined either immediately after the heat shock or following a 4-hour recovery period at 37°C. Data represent means ± SE (n = 4). ***P < 0.001 vs MDCK_pRC.1
DISCUSSION
The present studies expand on our previous work investigating the role of heat stress on epithelial barrier function. We have shown that HSP70 expression itself is sufficient to protect against the heat shock–induced disruption of MDCK epithelial barrier and confer improved epithelial barrier recovery following heat shock. Our results also indicate that the improved recovery of the MDCK epithelial barrier integrity mediated by HSP70 expression correlates with the improved protein-folding function in MDCK cells overexpressing HSP70. Moreover, we show that the mechanisms mediating the protection of MDCK epithelial barrier integrity during acute heat shock–induced damage is much more complex, and although involving the action of HSP70, is not directly related to chaperone activity.
Although heat stress sufficient to cause irreversible cell damage resulting in cell death also causes irreversible epithelial barrier disruption, little is understood about whether heat challenges can cause significant but reversible epithelial barrier changes. This is an important problem, because epithelial barrier integrity plays a key role in heat-induced illness. Early studies in a primate model demonstrated that prophylactic gut sterilization attenuated the effects of heat stress, allowing the animals to survive an otherwise lethal thermal insult (Gathiram et al 1998). These data support a crucial role for endotoxin leak across the intestinal epithelial barrier as an important determinant of the pathophysiology of heat stroke. In the intact organism, lethal core temperature elevations are associated with severe gut epithelial damage, epithelial cell sloughing and lysis, and concomitant leakage of large macromolecules such as fluorescein isothiocyanate– dextran and endotoxins across the intestinal epithelial barrier (Moseley and Gisolfi 1993). These effects have also been demonstrated in ex vivo studies using everted gut pouches (Lambert et al 2002). We have also recently shown, using in vitro intestinal epithelial model system consisting of Caco-2 intestinal epithelial monolayers grown on permeable filters, that moderate heat stress (37– 41°C) causes a reversible increase in tight junction permeability (Dokladny et al 2006). Similarly, we have shown that modest temperature elevation (38.5°C) causes an increase in MDCK epithelial monolayers (Moseley et al 1994). These changes in epithelial resistance occurred in the absence of measurable cell death and were completely reversible within 12 hours.
We have previously shown that exposure of rats to moderate elevations in temperatures (heat preconditioning) for a duration sufficient to induce a heat shock protein response affords protection against a subsequent exposure to an otherwise lethal temperature or challenge to a lethal dose of lipopolysaccharide (Ryan et al 1992; Hodchkiss et al 1993). Other studies of myocardial ischemia using both hyperthermic stress and HSP gene overexpression have demonstrated improvements in myocardial function following ischemia (Lau et al 1997; Jayakumar et al 2001). Taken as a whole, these whole-animal studies parallel cell survival studies by demonstrating that induction of HSP expression by heat preconditioning or HSP gene transfection also confers a survival advantage to the whole animal, as in individual cells. These prior investigations used a single remote endpoint to demonstrate the protective effects of heat conditioning and/or HSPs. In contrast, the present study assesses the role of heat conditioning in combination with HSP70 expression on the recovery from heat shock on both epithelial barrier function and protein folding.
The present studies provide a critical link between these 2 sets of data. We have for the first time demonstrated a direct role for HSP70 in the maintenance of epithelial barrier function in reconstituted MDCK epithelial monolayers. This protection was independent of cell survival, because all MDCK monolayers completely recovered barrier function by 12 hours regardless of the experimental treatment conditions used in this study. Moreover, we have previously demonstrated that the short duration of thermal stress used to alter MDCK epithelial permeability did not induce cell cytotoxicity (Moseley et al 1994). Placing the present studies in context with our prior works using cell culture and whole-organism systems, we previously showed that HSP expression was important for both individual cell survival and survival of an intact organism (Moseley et al 1994; Dokladny et al 2006). We now show that HSPs play a key protective role in the maintenance of epithelial barrier function during heat shock. In terms of the importance to the intact organism, maintenance of cell-to-cell function such as the maintenance of epithelial barrier function is likely to be far more important in determining the survival outcome of organism than is the regulation of individual cell death. Physiologically relevant stresses such as hyperthermia and endotoxemia have important effects on organ function. The death of the organism in heat shock or endotoxemia is not due to massive cell death, but secondary to cell-to-cell dysfunction such as the epithelial barrier defect seen in the gut endothelium or lungs in patients suffering heat stroke or during sepsis.
Acknowledgments
This work was supported by National Institutes of Health Grants AR40771 and HL61389 (P.L.M.) and DK64156 (T.Y.M.), as well as a Veterans Affairs Merit Review grant (T.Y.M.). Core support was provided by ES-012072.
REFERENCES
- Beere HM, Green DR. Stress management: heat shock protein and the regulation of cell death. Trends Cell Biol. 2001;11:6–10. doi: 10.1016/s0962-8924(00)01874-2.0962-8924(2001)011[0006:SMHSPA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bouchama A, Knochel JP. Heat stroke. N Engl J Med. 2002;346:1978–1988. doi: 10.1056/NEJMra011089.0028-4793(2002)346[1978:HS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Chu EK, Ribeiro SP, Slutsky AS. Heat stress increases survival rates in lipopolysaccharide-stimulated rats. Crit Care Med. 1997;25:1727–1732. doi: 10.1097/00003246-199710000-00025.0090-3493(1997)025[1727:HSISRI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Craig EA, Weissman JS, Horwich AL. Heat shock proteins and molecular chaperones: mediators of protein conformation and turnover in the cell. Cell. 1994;78:365–372. doi: 10.1016/0092-8674(94)90416-2.0092-8674(1994)078[0365:HSPAMC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- DeMaio A. Heat shock proteins: facts, thoughts and dreams. Shock. 1999;11:1–12. doi: 10.1097/00024382-199901000-00001.1073-2322(1999)011[0001:HSPFTA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Dokladny K, Moseley PL, Ma TY. Physiologically relevant increase in temperature causes an increase in intestinal epithelial tight junction permeability. Am J Physiol. 2006;290:G204–G212. doi: 10.1152/ajpgi.00401.2005.0002-9513(2006)290[G204:PRIITC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Garrido C, Gurbovani S, Ravagman L, Kroemer G. Heat shock proteins: endogenous modulators of apoptotic cell death. Biochem Biophys Res Commun. 2001;286:433–442. doi: 10.1006/bbrc.2001.5427.0006-291X(2001)286[0433:HSPEMO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Gathiram P, Wells MT, Brock-Utne JG, Gaffin SL. Prophylactic corticoid steroid increases survival in experimental heat stroke in primates. Aviat Space Environ Med. 1998;59:352–355.0095-6562(1998)059[0352:PCSISI]2.0.CO;2 [PubMed] [Google Scholar]
- Gathiram P, Wells MT, Brock-Utne JG, Wessels BC, Gaffin SL. Oral administered nonabsorbable antibiotics prevent endotoxemia in primates following intestinal ischemia. J Surg Res. 1998;45:187–193. doi: 10.1016/0022-4804(88)90064-9.0022-4804(1998)045[0187:OANAPE]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hodchkiss R, Nunnally I, Lindquist S, Taulien J, Perdrizet G, Karl I. Hyperthermia protects mice against the lethal effects of endotoxin. Am J Physiol. 1993;265:R1447–R1457. doi: 10.1152/ajpregu.1993.265.6.R1447.0002-9513(1993)265[R1447:HPMATL]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hosokawa N, Hirayoshi K, Kudo H, Takechi H, Aoike A, Nagata K. Inhibition of the activation of heat shock factor in vivo and in vitro by flavonoids. Mol Cell Biol. 1992;12:3490–3498. doi: 10.1128/mcb.12.8.3490.0270-7306(1992)012[3490:IOTAOH]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakumar J, Suzuki K, and Sammut IA. et al. 2001 Heat shock protein 70 gene transfection protects mitochondrial and ventricular function against ischemia-reperfusion injury. Circulation. 104:I303–I307. [DOI] [PubMed] [Google Scholar]
- Lambert GP, Gisolfi CV, Berg DJ, Moseley PL, Oberley LW, Kregel KC. Hyperthermia-induced intestinal permeability and the role of oxidative and nitrosative stress. J Appl Physiol. 2002;92:1750–1761. doi: 10.1152/japplphysiol.00787.2001.8750-7587(2002)092[1750:HIPATR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Laszlo A. The relationship of heat-shock proteins, thermotolerance, and protein synthesis. Exp Cell Res. 1988;178:401–414. doi: 10.1016/0014-4827(88)90409-0.0014-4827(1988)178[0401:TROHPT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lau S, Patnaik N, Sayen MR, Mestril R. Simultaneous expression of two stress proteins in rat cardiomyocytes and myogenic cells confers protection against ischemic-induced injury. Circulation. 1997;96:2287–2294. doi: 10.1161/01.cir.96.7.2287.0009-7322(1997)096[2287:SEOTSP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat shock proteins. Ann Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215.0066-4197(1988)022[0631:THSP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ma TY, Hoa NT, Tran DD, Bui V, Pedram A, Mills S, Merryfield M. Cytochalasin B modulation of Caco-2 tight junction barrier: role of myosin light chain kinase. Am J Physiol. 2000;279:G875–G885. doi: 10.1152/ajpgi.2000.279.5.G875.0002-9513(2000)279[G875:CBMOCT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Moseley PL. Mechanisms of heat adaptation: thermotolerance and acclimatization. J Lab Clin Med. 1994;123:48–52.0022-2143(1994)123[0048:MOHATA]2.0.CO;2 [PubMed] [Google Scholar]
- Moseley PL. Heat shock proteins and heat adaptation of the whole organism. J Appl Physiol. 1997;83:1413–1417. doi: 10.1152/jappl.1997.83.5.1413.8750-7587(1997)083[1413:HSPAHA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Moseley PL. Heat shock proteins and the inflammatory response. Ann NY Acad Sci. 1998;856:206–213. doi: 10.1111/j.1749-6632.1998.tb08327.x.0077-8923(1998)856[0206:HSPATI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Moseley PL, Gapen C, Wallen ES, Walter ME, Peterson MW. Thermal stress induces epithelial permeability. Am J Physiol. 1994;267:C425–C434. doi: 10.1152/ajpcell.1994.267.2.C425.0002-9513(1994)267[C425:TSIEP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Moseley PL, Gisolfi CV. New frontiers in thermoregulation. Sports Med. 1993;16:163–167. doi: 10.2165/00007256-199316030-00001.0112-1642(1993)016[0163:NFIT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Musch MW, Ciancio MJ, Sarge K, Chang EB. Induction of heat shock protein 70 protects intestinal epithelial IEC-18 cells from oxidant and thermal injury. Am J Physiol. 1996;270:C429–C436. doi: 10.1152/ajpcell.1996.270.2.C429.0002-9513(1996)270[C429:IOHSPP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Musch MW, Sugi K, Straus D, Chang EB. Heat shock protein 72 protects against oxidant-induced injury of barrier function of human epithelial Caco2/bbe cells. Gastroenterology. 1999;117:115–122. doi: 10.1016/s0016-5085(99)70557-3.0016-5085(1999)117[0115:HSPPAO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Nagai N, Nakai A, Nagata K. Quercetin suppresses heat shock response by down regulation of HSF1. Biochem Biophys Res Commun. 1995;208:1099–1105. doi: 10.1006/bbrc.1995.1447.0006-291X(1995)208[1099:QSHSRB]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Roigas J, Jensen CA, Wallen ES, Loening SA, Wharton W, Moseley PL. Repression of thermotolerance in Dunning R3327 prostate carcinoma cells by 2-deoxy-glucose. Int J Hyperthermia. 2004;20:557–566. doi: 10.1080/02656730310001625229.0265-6736(2004)020[0557:ROTIDR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ryan AJ, Flanagan SW, Moseley PL, Gisolfi CV. Acute heat stress protects rats against endotoxin shock. J Appl Physiol. 1992;73:1517–1522. doi: 10.1152/jappl.1992.73.4.1517.8750-7587(1992)073[1517:AHSPRA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Zeng XC, Bhasin S, and Wu X. et al. 2004 Hsp70 dynamics in vivo: effect of heat shock and protein aggregation. J Cell Sci. 117:4991–5000. [DOI] [PubMed] [Google Scholar]