Abstract
Cholesterol is an important constituent of cellular membranes. It has been suggested that cholesterol segregates into sterol-rich and -poor domains in the plasma membrane, although clear evidence for this is lacking. By fluorescence imaging of the natural sterol dehydroergosterol (DHE), the lateral sterol distribution has been visualized in living cells. The spatial labeling pattern of DHE coincided with surface structures such as ruffles, microvilli, and filopodia with correlation lengths in the range of 0.8–2.5 μm. DHE staining of branched tubules and of nanotubes connecting two cells was detected. Dynamics of DHE in folded and plane membrane regions was comparable as determined by fluorescence recovery after photobleaching. DHE colocalized with fluid membrane-preferring phospholipids in surface structures and at sites of cell attachment as well as in the cleavage furrow of dividing cells, but it was not particularly enriched in those regions. Fluorescent sterol showed homogeneous staining in membrane blebs induced by F-actin disruption. Cross-linking the ganglioside GM1—a putative raft marker—did not affect the cell surface distribution of DHE. The results suggest that spatial heterogeneities of plasma membrane staining of DHE resolvable by light microscopy reflect the cell surface topography but not phase-separated sterol domains in the bilayer plane.
INTRODUCTION
Cholesterol is the most abundant single lipid species in the plasma membrane of living cells. Here, cholesterol functions by increasing the permeability barrier of the phospholipid bilayer but also by mediating signal transduction and vesicle fission from the surface during endocytosis (Maxfield and Wüstner, 2002). It has been proposed that the many functions of cholesterol in cellular membranes relate to the ability of this sterol to form segregated lateral domains with specific properties in the bilayer plane (Maxfield, 2002). Elucidating the organization of cholesterol in the plasma membrane under physiological conditions is of crucial importance for determining mechanisms underlying trafficking of sterols in cells. It is well established for model membranes that cholesterol together with phospho- or sphingolipids bearing saturated fatty acyl chains forms a so-called liquid ordered (lo) phase (Ipsen et al., 1987; Vist and Davis, 1990; Ahmed et al., 1997). Ternary mixtures of lipid species, including cholesterol organized in giant unilamellar vesicles (GUVs), are commonly used to visualize sterol-induced phase separation (Korlach et al., 1999). A long-standing question is whether observations on those model membrane systems apply also to cellular membranes. Under pathological circumstances such as in cholesterol-loaded aortic smooth muscle cells, cholesterol microcrystallites paralleled by a bilayer thickening have been found by x-ray spectroscopy (Tulenko et al., 1998). However, it is unclear whether cholesterol can form lateral clusters in the plane of the plasma membrane under physiological conditions, as stated in connection with the “raft hypothesis” (Simons and Ikonen, 1997; Maxfield and Wüstner, 2002; Zuckermann et al., 2004; Zhang et al., 2005). Highly curved membrane regions such as tubules play an important role during intracellular membrane fission as well as at the cell surface during plasma membrane ruffling (Hirschberg et al., 1998; Sheetz, 2001). Both processes have been shown to require cholesterol (Grimmer et al., 2000, 2002). It remains to be established whether cholesterol partitions into those tubular structures as it is debated in models of biomembrane function (Corbeil et al., 2001; Huttner and Zimmerberg, 2001). To address these important questions requires a method to directly visualize the distribution of sterols in cellular membranes of living cells. Dehydroergosterol (DHE), a natural sterol from yeast, has very similar physicochemical properties to cholesterol in model and cellular membranes (Smutzer et al., 1986). DHE is a close analogue of cholesterol and has often been used to analyze lateral sterol organization in model membranes by using fluorescence spectroscopy (Yeagle et al., 1982; Chong and Thompson, 1986; Liu et al., 1997; Loura and Prieto, 1997). Microscopic visualization in living cells became recently possible by adapting a conventional epifluorescence microscope for UV imaging (Mukherjee et al., 1998). Imaging of DHE in living cells is limited by the fact that its emission maximum is between 370 and 400 nm in lipid membranes (Smutzer et al., 1986; Mukherjee et al., 1998). Using multiphoton fluorescence microscopy, Schroeder and colleagues have analyzed the distribution of DHE in the plasma membrane of fibroblasts (Zhang et al., 2005). Elaborated image analysis tools were required to extract plasma membrane from intracellular fluorescence of cells labeled to steady state with DHE. Using thresholding and intensity measurements along plasma membrane segments, Zhang et al. (2005) found evidence for clusters of DHE in the membrane with typical lengths of 565 nm. A problem with this approach is the low signal obtainable by multiphoton imaging of DHE. This makes interpretation of the findings very difficult.
Using wide field fluorescence microscopy and three-dimensional (3D) iterative deconvolution in concert with bleaching correction, the lateral distribution of DHE was determined in the plasma membrane of living polarized and nonpolarized cells. Highly improved spatial resolution of DHE in the cell membrane was obtained. Statistical image analysis was used to quantify distribution of DHE in the plasma membrane. Fluorescent phospholipid probes were selected based on their predicted propensities to partition into lo- or liquid-disordered (ld) phases in model membranes, and their plasma membrane distribution was compared with that of DHE. Fluorescence recovery after photobleaching (FRAP), bleach rate imaging, and time-integrated photobleaching were used to characterize the lateral distribution of DHE at the cell surface. To look at lipid distributions in the plasma membrane in the absence of cytoskeleton support, plasma membrane blebs were generated by actin disruption. In membrane blebs, lateral constraints on protein and lipid diffusion are released and only forces acting between membrane molecules should determine the lateral lipid distribution (Tank et al., 1982). These conditions are well suited to determine whether sterol phase segregates in cellular membranes and to assess the relevance of model membrane studies for analysis of biomembrane architecture. Finally, macroscopic domain formation was triggered by cross-linking gangliosides with cholera toxin in the plasma membrane. In colabeling studies, it was determined whether DHE redistributes with patched gangliosides in putative lipid rafts.
MATERIALS AND METHODS
Reagents
NBD-labeled phosphatidylcholine (PC) 1-palmitoyl-2-[6-[(7-nitro-2–1,3-benzooxadiazol-4-y) amino]caproyl]-sn-glycero-3-phosphatidylcholine (C6-NBD-PC), red fluorescent PC 2-(4,4-difluoro-5-(4-phenyl-1,3-butadienyl)-4-bora-3a, 4a-diaza-s-indacene-3-pentanoyl)-1-hexa-decanoyl-sn-glycero-3-phosphocholine (β-BODIPY-PC), 1,1′-didodecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate [DiIC12(3)], 1,1′-dihexadecyl-3,3,3′,3′tetramethylindocarbocyanine perchlorate [DiIC16(3)] Alexa488-labeled cholera toxin subunit B (Alexa488-CTxB), and the Vybrant kit for detecting membrane domains were purchased from Invitrogen (Carlsbad, CA). Fetal calf serum (FCS) and DMEM were from Invitrogen (Paisley, Scotland). All other chemicals were from Sigma-Aldrich (St. Louis, MO). Buffer medium contained 150 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 5 mM glucose, and 20 mM HEPES, pH 7.4, as described previously (Wüstner et al., 2002).
Cell Culture
HepG2 cells and J774 cells were grown in DMEM with 4.5 g/l glucose, supplemented with 10% heat-inactivated FCS and antibiotics. HepG2 cells were routinely passaged in plastic tissue culture dishes. J774 cells were passaged in 25-cm2 bacterial plastic culture dishes as described previously (Wüstner et al., 2005). For experiments, cells were plated onto glass coverslips coated with poly-d-lysine (Salzmann and Maxfield, 1989). HepG2 cells were used after reaching the highest degree of polarization as described previously (Wüstner et al., 2001). TRVb-1 is a modified Chinese hamster ovary (CHO) cell line that lacks endogenous transferrin (Tf) receptor and expresses the human Tf receptor (McGraw et al., 1987). TRVb-1 cells were grown in bicarbonate buffered Ham's F-12 medium supplemented with 5% FCS and antibiotics. Bovine mammary gland epithelial (BMGE) cells were grown in DMEM, supplemented with 20% heat-inactivated FCS, antibiotics, and l-glutamine as well as prolactin, insulin, and hydrocortisone (1 μg/ml each) as described previously (Schmid et al., 1983).
Labeling of Cells with DHE and Fluorescent Phospholipid Analogues
A stock solution of DHE (5 mM) was made in ethanol and stored under argon. For labeling cells with DHE, the analogue was loaded on methyl-β-cyclodextrin, giving a DHE/methyl-β-cyclodextrin (DHE/MCD) solution as described previously (Hao et al., 2002). HepG2 or TRVb-1 cells were routinely labeled with DHE/MCD for 1 min and J774 cells for 5 min at 37°C. Cells were washed and imaged on a wide field microscope as described below. DiI lipid- and β-BODIPY-PC–labeling solutions were prepared as described previously by binding analogues to fatty acid-free bovine serum albumin (BSA) (Mukherjee et al., 1999; Wüstner et al., 2001). HepG2 cells were labeled with either β-BODIPY-PC, DiIC12, or DiIC16 for 1 min at 37°C, washed, and imaged as described below. For detection of plasma membrane colocalization, HepG2 cells were labeled with DHE/MCD for 1 min at 37°C, washed, labeled with β-BODIPY-PC, washed, and imaged as described below.
Colabeling of Cells with DHE, DiIC12, and Cholera Toxin Subunit B
BMGE cells were labeled with DHE/MCD for 1 min at 37°C, washed, and chilled with ice-cold buffer solution, and then they were placed on ice and incubated for 25 min with 50 μg/ml Alexa488-CTxB. Cells were washed twice with cold (2°C) and once with warmed buffer and imaged on a wide field microscope. Alternatively, cells were after cold wash incubated with 10 μg/ml anti-CTxB antibody solution following the protocol of the Vybrant raft kit from Invitrogen. In some experiments, cells were—after labeling with DHE/MCD—incubated with DiIC12 for 1 min at 37°C, washed, and labeled with Alexa488-CTxB as described above.
Disruption of the Actin Cytoskeleton and Labeling of Plasma Membrane Blebs
HepG2 cells were incubated for 20 min at 37°C with 20 μM cytochalasin D to disrupt F-actin. Cells were washed and labeled either with C6-NBD-PC or double-labeled with DHE and β-BODIPY-PC as described above. During all subsequent incubations cytochalasin D was present. For staining of actin cytochalasin D-treated and nontreated cells were fixed with 3.3% paraformaldehyde (PFA) for 30 min, permeabilized in blocking buffer (1% Triton X-100, 1% BSA [wt/vol] in buffer medium), and incubated with Alexa488-phalloidin for 30 min (diluted 1:200 from a stock solution in blocking buffer) at room temperature. Cells were rinsed five times and observed by fluorescence microscopy.
Imaging of DHE in Crystals and Films
A drop containing 100 μl of DHE/MCD solution was added to a microscope dish, and the solvent was evaporated at room temperature in the dark and overnight. Under those conditions, DHE precipitates from the supersaturated solution and forms brightly fluorescent crystals, whereas the aqueous solvent evaporates. To generate a DHE film, 100 μl of the DHE stock solution in ethanol was added to a microscope slide, and the solvent was evaporated under a flow of nitrogen or argon. Crystals or films of DHE were imaged as described below.
Fluorescence Microscopy and Image Analysis
Wide field fluorescence microscopy and digital image acquisition were carried out using a Leica DMIRB microscope with a 63×, 1.4 numerical aperture oil immersion objective (Leica Lasertechnik, Wetzlar, Germany) equipped with a Princeton Scientific Instruments (Monmouth Junction, NJ) cooled charge-coupled device (CCD) camera driven by Image-1/MetaMorph Imaging System software (Molecular Devices, Sunnyvale, CA). Alternatively, a Leica DMIRBE microscope containing the same optical components equipped with a Hamamatsu Orca BT512 4-stage peltier and water-cooled (−80°C) CCD camera (Hamamatsu Photonics, Hamamatsu City, Japan) and a Lambda SC smartshutter (Sutter Instrument, Novato, CA), driven by Image-Pro Plus and ScopePro (Media Cybernetics, Silver Spring, MD) was used. DiIC12, DiIC16, and β-BODIPY-PC were imaged using a standard rhodamine filter set (535-nm [50-nm bandpass] excitation filter, 565-nm longpass dichromatic filter, and 610-nm [75-nm] bandpass emission filter). Detection of the emission of DHE was made possible by using a camera with back-thinned CCD chip (either Princeton Instruments Frame Transfer Pentamax with a 512 × 512 EEV chip or Hamamatsu Orca B 512 with similar characteristics). To optimize UV throughput in the excitation path, a lamp housing from Leica Lasertechnik with collector lenses having high transmittance in the UV was used. The light source was a mercury lamp (100 W) with a power density of irradiation of 170.000 cd/cm2. DHE was imaged using a specially designed filter cube obtained from Chroma Technology (Brattleboro, VT) with 335-nm (20-nm bandpass) excitation filter, 365-nm longpass dichromatic filter, and 405-nm (40-nm bandpass) emission filter as described previously (Wüstner et al., 2002). All other components of the microscope were adapted for UV imaging as described previously (Wüstner et al., 2002). Image analysis was carried out using the software packages Image-1/MetaMorph Imaging System (Molecular Devices), Image-Pro Plus (Media Cybernetics), or NIH Image in form of Scion Image (Scion, Frederick, MD) or ImageJ (developed at the National Institutes of Health and available at http://rsb.info.nih.gov/ij). For presentation purposes and contrast adjustment Adobe Photoshop (Adobe Systems, Mountain View, CA) was used. Differential interference contrast (DIC) imaging was performed on a wide field microscope as described above by using DIC optics. Time-lapse imaging of unlabeled cells or of DHE-labeled HepG2 cells was performed by image acquisition every 1 min at a temperature-controlled microscope stage of a wide field microscope maintained at 36 ± 1°C. Time-lapse sequences of DHE-labeled cells were corrected for bleaching as described previously (Wüstner, 2005a). Three-dimensional visualization of image stacks was performed using the ImageJ plugin SurfacePlot3D developed by Dr. Bartel (Technische Fachhochschule, Berlin, Germany).
Colocalization of DHE with Fluorescent Lipids and Proteins in the Plasma Membrane
This method is equal to that used previously to determine intracellular transport of DHE (Wüstner et al., 2002). Briefly, cells double labeled with DHE and β-BODIPY-PC were imaged by acquiring z-stacks 0.5 μm apart starting ∼2.5 μm above the central focal position. The axial chromatic shift between the DHE and β-BODIPY-PC channel was determined as described by measuring fluorescence beads (Wüstner et al., 2002). Lateral chromatic shift was corrected using the TurboReg plugin of ImageJ software written by Dr. Thevenaz (Swiss Federal Institute of Technology, Lausanne, Switzerland). Hereby, a rigid body transformation was applied to spatially register corresponding planes of DHE and β-BODIPY-PC (Thevenaz et al., 1998). After bleaching correction and deconvolution (nearest neighbor or iterative 3D) of the DHE stack, corresponding individual spatially aligned planes were compared and color coded for presentation. Alternatively, the sum projection of three individual corresponding planes was used. The same approach was applied to cells were the cytoskeleton was disrupted. For image deconvolution, a maximum likelihood restoration method using the Huygens software was applied (Scientific Volume Imaging, Hilversum, The Netherlands). This approach reduces apparent noise in the image by formulating a log-likelihood function that measures the likelihood of sampling the noisy data that were actually collected (Holmes et al., 1995; Verveer et al., 1999). Three-dimensional surface reconstructions were generated for the DHE channel or for areas of colocalization between DHE and β-BODIPY-PC by using Huygens software (Scientific Volume Imaging). Colocalization of DHE with β-BODIPY-PC was determined from wide field images of double-labeled cells by using Image-Pro Plus (Media Cybernetics). Pearson's correlation coefficient, rp, is given by the following equation:
 |
where Ri and Gi is the fluorescence intensity in red and green per pixel i, respectively. The average intensity in red and green in an image is given by RA and GA, respectively (Brown et al., 2000). Colocalization of DHE with DiIC12 or Alexa488-CTxB was determined in the same manner.
Ratio Imaging and Principal Component Analysis (PCA) of DHE and β-BODIPY-PC
Corresponding single spatially aligned planes of DHE and β-BODIPY-PC were background corrected as described previously (Ghosh et al., 1994; Mukherjee et al., 1999). The ratio image of DHE and β-BODIPY-PC was calculated using the public domain software ImageJ (NIH Image, http://rsb.info.nih.gov/ij). The ratio image allows for detection of spatially differing labeling intensity of two probes in the plasma membrane (Zamir et al., 1999). It was normalized and saved as 8-bit image for presentation or used in further calculations (see Results and above). Note that no deconvolution step was applied before ratio calculations. To assess the statistical differences in spatial labeling patterns of DHE and β-BODIPY-PC, a PCA was performed using the ImageJ plugin PCA written by Dr. Abrámoff (University of Iowa Hospitals and Clinics, Iowa City, IA). The principle of PCA is to consider every pixel of spatially aligned images as vector; to calculate the mean, variance, and covariance between vector components; and to perform a variable transformation that results in a set of orthogonal eigenvectors maximizing the data variation along the eigenvectors of the covariance matrix. Let x⃗ and m⃗x be column vectors representing actual and mean intensity values for one common pixel in the DHE and β-BODIPY-PC image, respectively. The covariance matrix of the vector population is defined as follows:
Using the so-called Hotelling transformation (Gonzalez and Woods, 2002):
whose mean vector m⃗y is zero, it follows that the covariance matrix of the transformed vector y⃗ is as follows:
The rows of the matrix A are orthonormal vectors (eigenvectors) named a⃗, each sequentially maximizing the percentage of variation by using the method of Lagrange multipliers under the orthogonality condition a⃗T · a⃗=1:
Here, λ corresponds to the eigenvalues being the main diagonal elements of the diagonal matrix Cy, which are determined by solving the eigenvalue problem:
where I is the unity matrix. The output of the PCA aligns the original data along the directions of greatest variance given by the eigenvectors (principal components) a⃗ of the covariance matrix Cx associated with the largest eigenvalues λ. As a result one obtains the first principal component as the direction within the original data has the largest variability, and the second component being perpendicular to the first component pointing toward the next large data variability (Gonzalez and Woods, 2002; Rowe and Hoffmann, 2006). The number of components is equal to the number of original variables, i.e., two when comparing images of DHE and β-BODIPY-PC.
Determination of Plasma Membrane Staining Patterns
After background subtraction a line scan along the plasma membrane outlining the perimeter of the cell labeled either with DHE, β-BODIPY-PC, DiIC12, DiIC16, or with Alexa488-CTxB was generated using Image-Pro Plus (Media Cybernetics). Fluorescence intensity along this line was measured, and results were exported to an Excel spread sheet (Microsoft, Redmond, WA) or saved as an ASCII file and later imported in SigmaPlot 9.0 (SPSS, Chicago, IL). To smooth data, a low pass filter or a running median implemented in SigmaPlot was occasionally applied. To determine whether the observed staining pattern of DHE could be caused by image noise, synthetic cell images were generated using ImageJ software. A cell outline was drawn using the free hand selection tool, and an pixel intensity value of 80 in an 8-bit image was added to this region. The “plasma membrane” image was generated by applying a variance filter of 7-pixel radius. To account for image noise either Gaussian or Poisson noise was added to the images. To this end, the RandomJ plugin to ImageJ written by Dr. Meijering (Swiss Federal Institute of Technology) was used. Next, the plasma membrane was manually outlined using ImagePro Plus (Media Cybernetics), and intensity was measured along this line as described above for real data. To compare staining patterns of DHE and β-BODIPY-PC in the plasma membrane of living HepG2 cells, the line scan was measured for the sum projection of three corresponding planes of double-labeled cells. Correlation coefficients between DHE and β-BODIPY-PC or DHE and Alexa488-CTxB scan data were calculated using a bivariate analysis routine implemented in SigmaPlot 9.0 (SPSS). Autocorrelation images were generated using the fast Fourier transform as implemented in Scion Image macros (Scion). Autocorrelation curves were measured from two-dimensional (2D) autocorrelation determined for selected regions (32 × 32 pixels) of double stained plasma membrane, exported as ASCII file and plotted using SigmaPlot 9.0 (SPSS). Note that no deconvolution was applied for all quantitative image measurements.
Bleach Rate Imaging and Time-integrated Photobleaching of DHE
Two images were acquired with 2-s integration time without interval for HepG2 cells labeled with DHE. Under those conditions some bleaching of DHE fluorescence in the plasma membrane can be found (Wüstner, 2005a). For selected regions of the plasma membrane, the ratio image (Ibleach) for the first (I1) and second image (I2) was calculated, which is related to the bleach rate according to (Brakenhoff et al., 1994):
Here, Ibleach is the bleach rate image, E is the time- and space invariant illumination intensity, α is the bleach rate constant, td is a delay time, and I∞ is the intensity in the last image of a bleach experiment. Because this intensity is almost zero for DHE-labeled HepG2 cells after repeated acquisition, the approximation in Eq. 7 is valid (Wüstner, 2005a). Brackets indicate pixel coordinates x, y for individual image planes. From Eq. 7, it becomes clear that in the bleach rate image only the pixel-dependent bleach rate is a contrast parameter. The method allows therefore to detect local differences in bleach rates of a fluorophore in a living cell (Brakenhoff et al., 1994).
Fluorescence measurements from single acquisitions may lead to erroneous estimates of total fluorescence due to the rapid photobleaching and/or self-quenching properties of DHE inserted into bilayers (Loura and Prieto, 1997; Wüstner, 2005a). Time-integrated fluorescence is independent of the quantum efficiency of the fluorophore, and its measurement allows for accurate and sensitive quantitation of total fluorescence (Mayor et al., 1993, and references therein). Total cell-associated fluorescence of DHE was determined by time-integrated photobleaching, where images of cells pulse-labeled with DHE/MCD were sequentially acquired until no fluorescence of DHE was left in the cells (Mayor et al., 1993; Wüstner, 2005a). A sum projection of such a bleach stack gives the total fluorescence of DHE in a cell. Importantly, the spatial labeling pattern of DHE in the plasma membrane did not differ between the sum projected image and a single acquisition image of the same field of cells (see Supplemental Material).
Image Segmentation by Active Contour Modeling
Snakes or active contour models are very suitable to segment irregular-shaped plasma membrane images of living cells. In this approach, a curve (snake) evolves from an initial position toward the boundary of an object (DHE-labeled cell) in such a way as to minimize some energy functional (Jacob et al., 2004). An ImageJ free-software Java plugin written by Dr. Bretschneider (Max-Planck-Institute of Biochemistry, Martinsried, Germany) was used (Dormann et al., 2002). Beside image energy minimization this plugin allows one to quantify fluorescence intensity along the defined boundary and to measure cell shape by defining second order geometric moments for calculation of a Legendre-Ellipse providing information about cell elongation and dispersion (for details, see Dormann et al., 2002; Gonzalez and Woods, 2002). The algorithm is therefore suitable to correlate plasma membrane intensity of DHE with membrane curvature and cell shape. These parameters were measured and analyzed by creating polar plots of curvature and intensity using SigmaPlot 9.0 (SPSS). To this end, node number i = 1, 100 was translated in degrees (0–360), whereas the polar plot radius represents either measured DHE intensity in the plasma membrane or membrane curvature. The algorithm was extensively tested using synthetic image sets which were generated as described above (see Supplemental Material).
Fluorescence Recovery after Photobleaching
HepG2 cells double labeled with DHE/MCD and β-BODIPY-PC were placed on a temperature-controlled stage of a wide field microscope maintained at 35 ± 1°C. Based on the red channel image of β-BODIPY-PC, the focus was set to the cell–substrate attachment region while taking the focal shift (chromatic aberration) of the DHE channel into account. FRAP experiments were performed as described previously with membrane folds visible in the red channel and labeled by β-BODIPY-PC defined as region of interest (ROI) (Wüstner et al., 2002). Intensity of DHE measured in the ROI was divided by total cell-associated DHE fluorescence, normalized, and plotted as described previously (Wüstner et al., 2005).
RESULTS
Cell Surface Dynamics and DHE Fluorescence in the Plasma Membrane of Polarized Cells
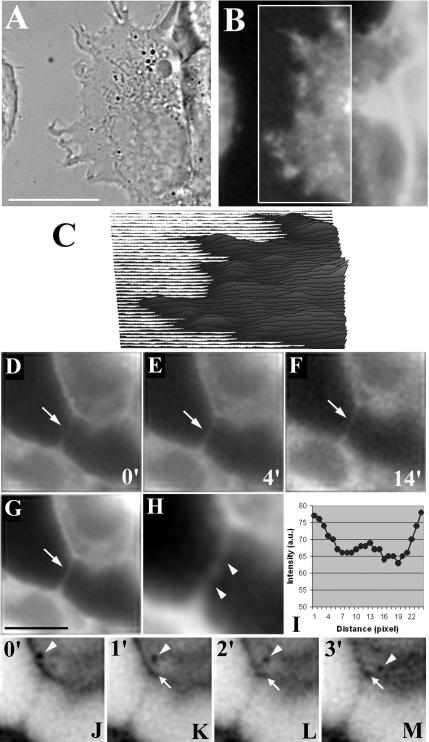
Important characteristics of the polarized hepatocyte phenotype are the ability of cell attachment to the substratum accompanied by dynamical cell shape changes and formation of apical bile canaliculi (BC) (Sormunen et al., 1993). These BC resemble the thin channels (canaliculi biliferi) of the liver into which the bile fluid is secreted. HepG2 cells are able to change the diameter of the BC in cell culture as shown in Figure 1, A–E, by time-lapse DIC microscopy: over a time course of 90 min, an “open” BC was found to close accompanied by large dynamics in cell protrusions at the basolateral cell surface (see Supplemental Video sequence). BC contraction is a very slow process as indicated by the apparent half-time of BC closing of t1/2 ∼40 min (Figure 1E). Because this process is so slow, it might have been missed in previous investigations. Fluorescence imaging of DHE combined with high-resolution DIC microscopy can be used to visualize the surface topology of DHE-containing specimen. Using three-dimensional imaging on a wide field microscope, fluorescence of DHE could be detected in filopodia at the basolateral membrane of HepG2 cells (Figure 1F). Filopodia were found to protrude and retract during the time course of the experiment (Figure 1, A–D, and Supplemental Material). Protrusions containing DHE were also found at the apical canalicular membrane of polarized HepG2 cells. From z-stacks of DHE-labeled HepG2 cell couplets postprocessed by 3D maximum likelihood deconvolution, microvilli protruding into the BC lumen can be identified (Figure 1G). Using the same technique, it can be shown that BC with low diameter probably caused by BC contraction (see above) have a highly folded canalicular membrane labeled by DHE (Figure 1, H–K). For such BC with small diameter, it is impossible to resolve differences in DHE staining intensity from the raw image data giving an apparent filled BC lumen (Wüstner et al., 2002, 2005a). By 3D maximum likelihood deconvolution, however, it is possible to show that the canalicular membrane of those small BC is folded, revealing a small open BC lumen. This is supported by 3D reconstructions of the large-diameter (Figure 1G) and small-diameter BC (Figure 1, L and M, compare with H–K). The results indicate that also in the canalicular membrane, the fluorescence intensity of DHE is uneven, resembling a highly folded apical cell surface. The observed varying diameter of DHE-labeled BC with often an apparently filled BC lumen can be explained by dynamic contraction of BC, resulting in strong folding of the apical membrane (Wüstner et al., 2002; Wüstner, 2005a).
Figure 1.
Surface dynamics and DHE staining pattern in polarized HepG2 cells. (A–D) DIC time-lapse series of a polarized HepG2 cell couplet showing slow closing of the central BC (big arrow) as well as periodic formation and retraction of filopodia at the basolateral cell surface (small arrows). E, quantification of BC closing from DIC images gives an approximative half-time of t1/2 ∼40 min. (F–K) Cells were labeled for 1 min with DHE/MCD, washed, and imaged. Images were acquired in a stack along the optical axis, corrected for bleaching, and 3D deconvolved. F, sum projection of three central planes of the HepG2 couplet shown in A–D reveals DHE staining of filopodia extending from the basolateral cell surface. (G–K) Representative planes of a z-stack of DHE-labeled polarized HepG2 cells. (G) Open BC with large diameter shows microvilli protruding into the BC lumen. (H–K) Single planes of BC with very small diameter acquired along the z-axis being 0.5 μm apart starting from the largest diameter in H. (L and M) 3D reconstruction of the large-diameter (L) and the small-diameter BC (M) calculated from the whole z-stacks as described in Materials and Methods, reveals that both BC have a lumen with reduced DHE intensity compared with the brightly stained canalicular membrane. Bar, 20 μm (A–F) or 5 μm (G–K).
To provide further insight into DHE-labeling patterns of the cell surface, images of DHE-labeled HepG2 cells were compared with the corresponding DIC image (Figure 2). The focus was set to the upper part of the cell to determine the surface topography. Clearly, fluorescence intensity of DHE is very heterogeneous and matches protrusions of the plasma membrane as identified in the DIC image. A 3D intensity plot of DHE created for the most left part of the cell (outlined box) shows the characteristics of a ruffled cell surface as often observed using surface scanning techniques (Figure 2C) (Edidin, 2003). Time-lapse analysis in combination with bleaching correction allows characteristics of DHE-labeled cell protrusions to be revealed: in Figure 2, D–M, representative images of a time-lapse sequence are presented that show a DHE-stained membrane tube connecting two HepG2 cells in a culture dish. This tubule emanates from one cell to the other and thereby bridges a space of ∼10 μm. The time-lapse sequence shows that this tube is stable for more than 10 min. Moreover, the fluorescence distribution of DHE along the tubule is heterogeneous as supported by the line scan (Figure 2I). During the time course of the experiment, a single vesicle moves in the peripheral region of the upper cell toward the tubule connecting the cells (Figure 2, J–M). Nanotubes connecting mammalian cells have been described previously in PC12 cells, J774 macrophages and B cells (Rustom et al., 2004; Önfelt and Davis, 2004; Önfelt et al., 2004). It has been demonstrated that these structures mediate one-directional exchange of material, including membrane lipids, proteins, and even vesicles between connected cells (Rustom et al., 2004). The results shown in Figure 2, D–M, are the first demonstration of nanotubes between hepatic cells and the first direct visualization of sterol in those structures.
Figure 2.
DHE staining of the plasma membrane reflects cell surface topography. HepG2 cells were pulse labeled with DHE/MCD for 1 min at 37°C, washed, and imaged. A 2D deconvolution algorithm implemented in the Huygens software (Scientific Volume Imaging) was applied to improve spatial resolution. DHE-labeled filopodia and microridges at the basolateral membrane (B), which match exactly structures in the corresponding DIC image (A). (C) Surface plot of the intensity distribution of DHE from the outlined region of the image in B. (D–M) Cells were pulse-labeled with DHE/MCD, washed, and immediately placed on a nitrogen-floated microscope stage of a wide field microscope maintained at 35 ± 1°C. Images were acquired every minute for a total time of 14 min. Images were postprocessed by applying a low pass filter implemented in Scion Image (Scion). D–G, a tubule connecting two HepG2 cells is shown that is labeled with DHE (arrows) and stable for at least 14 min (compare D with F). (G) Average image obtained from the first four acquisitions plus low pass filter to improve signal-to-noise ratio. (H) Zoomed version of G. Along the tubule, the DHE-labeling pattern is heterogeneous (arrowheads in H). This can be also inferred from the line scan along the central region of the tubule shown in H (I). (J–M) Representative inverted images of the time-lapse sequence show that a vesicle (arrowheads) moves from the cytoplasm toward the tubule in the upper cell being connected to the lower cell by the nanotube. Vesicles were also found at the site of nanotube–cell attachment in the upper cell (small arrows). Bar, 5 μm.
The distribution of DHE was also investigated in nonpolarized TRVb-1 cells by using wide field microscopy and image-processing techniques. TRVb-1 cells are flat and therefore very suitable for imaging of DHE. As shown in Figure 3, A–C, TRVb-1 cells growth partially on top of each other: overlapping plasma membrane staining was observed for DHE, where the signal in overlapping regions is exactly twice as large as in single flat cells (Figure 3C). Moreover, DHE is found in long tubules emanating from the TRVb-1 cell surface. Here, DHE matches exactly tubular structures visible in the corresponding DIC image (Figure 3, D–I). It is important to emphasize that heterogeneous DHE staining along a membrane tubule resembles the varying diameter of the tubule along its length (compare Figure 3, H and I). Membrane tubules emanating from the cell surface form a branched network visible in the DHE and DIC channel, respectively (Figure 3, F and G). Together, the results demonstrate that DHE stains intensely membrane tubules and nanotubes connecting cells in culture. The measured fluorescence of DHE along the tubes correlates with the tubule diameter visualized in the corresponding DIC image.
Figure 3.
Visualization of DHE in the plasma membrane of TRVb1 cells. Cells were pulse-labeled with DHE/MCD for 1 min at 37°C, washed, and imaged. (A–C) CHO cells grow occasionally in an overlapping mode. In overlapping regions, DHE fluorescence (B) is exactly twice as high as in other flat plasma membrane areas as inferred from a line scan (C) of the intensity in contact regions (line in B). (A) Bright field image corresponding to B. (D–I) DHE is found in membrane tubules emanating from the cell surface. The variation in tubule diameter visible in the DIC image (D and H) is exactly matched by DHE fluorescence in the tubule (E and I). This can be clearly seen in the zoomed region (H and I) outlined by the box 2 in D and E. Emanating membrane tubules are often branched as visible in the DIC (F) and DHE channel (G) of the zoomed box 1 of the field shown in D and E. Bar, 10 μm.
Heterogeneous Plasma Membrane Staining of DHE Measured along the Cell Perimeter
Heterogeneous membrane staining by DHE measured along the cell perimeter has been reported previously (Zhang et al., 2005). From weak signal intensities in multiphoton microscopy of fibroblasts labeled to steady state with DHE, Zhang et al. (2005) concluded that regions of slightly higher DHE intensity along the plasma membrane perimeter resemble sterol enriched lo-like domains. To analyze the spatial distribution of DHE in the cell bilayer requires selective and high plasma membrane staining. This was achieved here by pulse-labeling cells with DHE/MCD, which avoids artifacts potentially introduced by sophisticated image segmentation procedures (Zhang et al., 2005). A line scan along the membrane of a DHE-labeled TRVb1 cell indicates strong fluctuations in staining intensity (Figure 4, A–D). The same cell shows bright staining of DHE in surface protrusions such as filopodia visible in the corresponding bright field image. High-resolution fluorescence wide field images of TRVb-1 cells pulse labeled with DHE also reveals DHE staining in small membrane infolds (Figure 4F, arrowheads) as well as punctate structures probable being endocytic vesicles containing DHE (Figure 4F, arrows). Comparison with the corresponding DIC image shows that the heterogeneous staining of DHE resembles the surface topology of TRVb-1 cells (compare Figure 4E). The results indicate that the signal fluctuation observed by measuring DHE intensity along the cell perimeter is caused by the rough surface topology of the cells. This conclusion based on high resolution images of DHE specifically labeling the plasma membrane is in contrast to previous interpretations of low steady-state multiphoton signals of DHE in fibroblasts claiming visualization of lateral sterol domains (Zhang et al., 2005). It has to be ruled out that the fluctuations are caused by noise inherent to the imaging system (e.g., photon shot noise of the camera). Synthetic cell images were generated as described in Materials and Methods. Noise was added either based on a Gaussian or on a Poisson noise model (Figure 4, I and J). Signal intensity was measured along the plasma membrane outline of these synthetic images, plotted as function of pixel position, and compared with line scans of fluorescence images of DHE-labeled cells (Figure 4, G–L). For comparison, intensity of DHE along the cell perimeter was measured in DHE labeled HepG2 cells not touching neighboring cells (Figure 4, G, H and K). Although the fluctuations due to noise can be very large, they are regular along the outline (Figure 4L). In contrast, the measured staining intensity of DHE along the plasma membrane of living cells gives a clustered distribution (Figure 4K). This is even more obvious after applying a running median filter, which smoothes the data: DHE intensity along the line is still heterogeneous, whereas fluctuations caused by noise are removed after smoothing (black line in Figure 4, K and L). To further sustain that the detected heterogeneous DHE intensity is real fluctuating signal, the analysis was compared for cells with one image acquisition and cells were fluorescence of DHE was bleached by 20 acquisitions. When cellular fluorescence of DHE is bleached, the patched fluorescence pattern in the plasma membrane can no longer be detected, whereas the noise level is comparable to the first image acquisition (Figure 4M). From these results, it can be concluded that the patched signal intensity in the plasma membrane is caused by differences in fluorescence intensity of DHE. Fluctuations in detected intensity due to noise in the imaging system can be ruled out as cause for the patched distribution of DHE along the cell perimeter. Parallel staining of DHE in surface protrusions strongly suggests that signal fluctuations are caused by optical path lengths differences due to a folded cell surface topology.
Figure 4.
Quantification of heterogeneous plasma membrane staining of DHE. (A–F) TRVb1 cells were pulse labeled with DHE/MCD for 1 min at 37°C, washed, and imaged. The surface topology of TRVb1 cells is characterized by a network of branching filopodia as well as many small infolds clearly visible in the transmitted light (A and E) and DHE channel (B, C, and F). Note that in A–C the focus was set to the largest cell diameter close to the glass surface of the microscope dish to visualize the cell perimeter as well as the branched filopodia. Images of DHE were inverted (B, C, and F) for better visualization. A line scan (red in C) along the plasma membrane perimeter measures fluorescence intensity of DHE determined from the noninverted image (D). The inverted image of a DHE-labeled cell revealed fluorescence spots on the base of membrane infolds (arrowheads in F) as well as in small cytoplasmic vesicles (arrows in F). (G and H) HepG2 cells were pulse-labeled with DHE/MCD for 1 min at 37°C, washed, and imaged. (I and J) Synthetic images of a cell were generated as described in Materials and Methods. Using the variance filter with a 7-pixel filter width a synthetic membrane image was generated and either Gaussian (I) or Poisson noise (J) was added. (K and L) line intensities along the synthetic plasma membrane + Poisson noise (J) were measured (L) and compared with intensities from DHE-labeled HepG2 cell (K). A running median filter was applied to smooth data (gray lines, raw data; black lines, filtered data). (M) HepG2 cells labeled with DHE as described were repeatedly illuminated, and line scans were measured along the plasma membrane after 1 (red line), 7 (blue line), or 15 acquisitions (cyan line). See text for further explanations. Bar, 5 μm.
Intensity Fluctuations of DHE in the Plasma Membrane Correlate over Micrometers
Three-dimensional imaging of DHE in the plasma membrane of living cells was next combined with line measurements along the cell perimeter. J774 macrophages were pulse labeled with DHE, and z-stacks were acquired as described in Materials and Methods (Figure 5). Line scans measured from images taken with 0.5-μm step size reveal strong intensity fluctuations of DHE, which correlate between individual image planes (Figure 5, B–D). It can be concluded that heterogeneous staining along the cell perimeter is not restricted to fibroblast-like cells and hepatocytes but occurs in other cell types such as macrophages as well. An intensity plot of line scans along the first two planes (labeled 0 and 1 in Figure 5A) confirms that line intensities of DHE at the plasma membrane are highly correlated (Figure 5E). Moreover, the correlation sustains over several planes being acquired 0.5 μm apart. This was measured by determining correlation coefficients for intensities of individual planes. From Figure 5F, it can be inferred that the correlation length of the first component of the correlation curve is ∼0.8 μm. For larger distances between planes acquired along the optical (z-) axis, correlations in intensity fluctuations decline but are stable at about R = 0.4, even for a distance of 2.5 μm (equivalent to 5 planes). To determine whether heterogeneous intensity of DHE in the membrane is related to changes in overall cell shape, the cell border was tracked using an active contour model (see Materials and Methods; Figure 5G). From a x,z-scan of the acquired stack, it is clear that the DHE-labeled cell flattens considerably from top to bottom (Figure 5H). This flattening is accurately described by the snake algorithm measuring the cell contour along the optical axis (Figure 5I and Supplemental Material). Cell flattening is not symmetric but occurs mainly in the vertical direction when looking from top on the cell (Figure 5, A and H, and Supplemental Material). The observed cell shape change along the optical axis is accompanied by varying plasma membrane fluorescence intensity of DHE as determined in parallel for individual points (nodes) along the contour (see polar plot in Figure 5, J and K). Importantly, there is no correlation between measured fluorescence intensity of DHE in the plasma membrane and overall cell curvature. Note that the snake algorithm allows one to measure curvature in the range of several micrometers but not curvature caused by small-scale cell protrusions (see Supplemental Material). The results demonstrate that intensity fluctuations of DHE along the plasma membrane perimeter correlate over 0.8–2 μm, but they do not coincide with overall cell shape changes occurring on a 10- to 15-μm scale.
Figure 5.
Heterogeneous staining of DHE correlates locally but does not depend on large-scale membrane curvature. J774 cells were labeled for 5 min with DHE/MCD, washed, and imaged in z-stacking modus with planes being 0.5 μm apart. (A) individual planes of a J774 cell shown from the top. (B–D) Line scans along the perimeter of the cell shown in A for planes 0 and 1 (B), planes 2 and 3 (C), and planes 4 and 5 (D); the black and gray lines correspond to the first and second plane in B–D, respectively. (E) Correlation plot for the line scan data shown in B. Dots, data; straight line, linear fit. (F) Correlation coefficients calculated with a bivariate analysis between successive planes starting from plane 0 (black curve) or plane 2 (gray curve). (G–K) Parallel analysis of cell shape and DHE membrane intensity by using an active contour (snake) model. (G) Plane 3 of A with outlines snake contour (dotted line). (H) vertical slices of DHE from a 3D stack along the indicated directions. (I) Cell contour measured by the snake algorithm. (J) Curvature polar plot. (K) Intensity polar plot, where the angle (in degrees) indicates the node number for the snake, and the radius is measured curvature (J) or intensity (K), respectively. Lines indicate the different image planes (see A): plane 0 (black line), plane 3 (light gray line), and plane 5 (dark gray line).
DHE Colocalizes with a Fluid Phase Phospholipid Marker at Cell Attachment Sites
Previous studies have shown that cell attachment to a substratum is accompanied by pattern formation at cell–subtratum contact sites (Weikl and Lipowsky, 2004). These adhesion patterns evolve in time and require specific molecular recognition events between membrane receptors and the extracellular matrix (Dubin-Thaler et al., 2004). Measurements of adhesion energy of HepG2 cells on various substrates combined with confocal interference microscopy revealed a mean half-time of cell spreading of ∼10 min and an uneven membrane attachment pattern to the matrix (Yin et al., 2003). HepG2 cells cultured for 3 d on a poly-lysine–coated glass surface were double labeled with DHE and the red fluorescent fluid-phase preferring PC analogue β-BODIPY-PC (Wüstner et al., 2001, 2002). Under those conditions, selective plasma membrane staining without labeling of intracellular organelles can be achieved (Wüstner et al., 2002, 2005). Cells were washed, and z-stacks were acquired in the red (β-BODIPY-PC) and UV (DHE) channel. Maximum likelihood deconvolution was performed on image stacks of DHE, and colocalization of both membrane probes at the lower cell surface being attached to the substratum was analyzed (Figure 6, A–H). From three adjacent planes being 0.5 μm apart, it can be seen that fluorescent PC as well as DHE have a heterogeneous fluorescence pattern at cell attachment sites. Surface rendering of deconvolved 3D stacks reveal that fluorescence of DHE is high at cell borders as well as in the center of an almost circular cell (Figure 6, D and E). The pattern of DHE staining at the center of the plasma membrane resembles very closely cell attachment patterns visualized by other techniques (Yin et al., 2003; Dubin-Thaler et al., 2004; Weikl and Lipowsky, 2004). DHE was found to colocalize with β-BODIPY-PC in large patches at the site of attachment to the substratum (Figure 6, F–H). Moreover, DHE as well as β-BODIPY-PC show increased fluorescence at sites of cell–cell attachment (Figure 6, I–K). From a 3D reconstruction of a z-stack of DHE image planes colocalizing with β-BODIPY-PC, it becomes obvious that this local fluorescence increase of both probes is due to folding of the plasma membrane at cell–cell attachment sites (Figure 6, L and M). In summary, this experiment demonstrates that DHE fluorescence reveals characteristics of adhesion patterns of HepG2 cells to the substratum and to neighboring cells as visualized in parallel by β-BODIPY-PC fluorescence.
Figure 6.
DHE colocalizes with BODIPY-PC in attachment sites to the substratum. HepG2 cells were pulse labeled with DHE/MCD for 1 min at 37°C, washed, labeled with β-BODIPY-PC for 1 min at 37°C, washed, and imaged on a wide field microscope in z-stacking modus. (A–C) DHE image stack was 3D deconvolved using Huygens software (Scientific Volume Imaging). Using the surface renderer and isocolocalization modus implemented in Huygens, a 3D surface reconstruction was calculated for DHE colocalizing with β-BODIPY-PC in regions of cell–substrate attachment (D–E) or of two contacting cells (L and M). (F–H) DHE colocalizes with β-BODIPY-PC in the membrane approaching the microscope slide in small patches (arrows). (I–K) Areas of cell–cell contact show increased fluorescence intensity of DHE and β-BODIPY-PC (arrow). From the zoomed 3D surface reconstruction, it becomes obvious that this resembles a thickened membrane area, where the cells are in contact (M). (A, F, and I) DHE. (B, G, and J) β-BODIPY-PC. (C, H, and K) Color overlay with DHE (green) and β-BODIPY-PC (red) showing colocalizing regions in yellow/orange. Bar, 15 μm.
Bleaching Microscopy Reveals Fluorescence Properties and Mobility of DHE in Cell Surface Folds
In theory, it would be possible that spatially heterogeneous plasma membrane staining of DHE is caused by different fluorophore environments. For example, DHE could locally self-quench generating an inhomogeneous staining pattern. Alternatively, certain membrane regions could contain proteins that absorb DHE fluorescence, like sterol-binding proteins having a high tryptophan content (fluorescence quenching by resonance energy transfer) (Benson et al., 1985; de Almeida et al., 2004). It is also in principal possible that during image acquisition, DHE gets differently bleached due to spatial heterogeneous distribution of reactive oxygen in lipid environments (Benson et al., 1985; Wüstner, 2005a). To rule out these possibilities, a pixel-wise bleaching analysis of DHE in the plasma membrane was performed. It is well known that all of the above-described processes would interfere with fluorophore bleaching generated by repeated or sustained illumination at the excitation wavelength (Benson et al., 1985). Thus, in spatially different fluorophore environments, one would expect spatially differing bleaching rates of DHE in the plasma membrane. Bleach rate imaging is a convenient method to detect spatial heterogeneous bleaching rates of a fluorophore (Brakenhoff et al., 1994). The ratio image of two sequentially acquired images of the same field is proportional to the bleach rate (see Materials and Methods, Eq. 7; Brakenhoff et al., 1994). As shown in Figure 7, A–F, DHE in surface ruffles bleaches with the same rate as DHE surrounding those structures. This can be inferred from the absence of any image contrast in the ratio image (Figure 7F) (Brakenhoff et al., 1994). It can be concluded that heterogeneous plasma membrane staining of DHE is not caused by different fluorophore environments putatively altering the photophysical properties of membrane-inserted DHE. To determine the mobility of DHE in surface protrusions, a FRAP experiment was performed (Figure 7, G–L). Fluorescence of DHE was selectively bleached in the basolateral membrane of a HepG2 cell at sites of cell–substrate attachment, and recovery of DHE intensity in those regions was measured (Figure 7, G–K, arrows, and L) (see Materials and Methods). Fluorescence of DHE recovered rapidly in membrane folds given a half-time of t1/2 ∼1.4 min. Similar results were found for β-BODIPY-PC (data not shown). The same fluorescence recovery half-time was measured previously for plane membrane regions of the basolateral HepG2 cell membrane (Wüstner et al., 2002). The data show that DHE has the same dynamic properties in plane and folded plasma membrane regions. Together, these results argue strongly against lateral clustering of sterol in domains with reduced mobility in certain plasma membrane regions.
Figure 7.
Bleach rate imaging and fluorescence recovery after photobleaching of DHE in membrane folds. (A–F) Bleach rate imaging of DHE: HepG2 cells were pulse labeled with DHE/MCD for 1 min at 37°C, washed, and repeatedly imaged on a widefield microscope using 2-s acquisition time. (A) DIC image corresponding to the DHE image (B) of the first acquisition shows a surface protrusion highly labeled by DHE (outlined box). Zoomed version of the region outlined in A and B is shown in C and D, respectively. (E) Same region for the second image acqusition. (F) Ratio image of the first and second acquired image. The ratio image has only the bleaching rate constant as contrast parameter. The absence of any contrast shows that DHE bleaching in the membrane fold is indistinguishable from the rest of the plasma membrane. (G–L) FRAP: HepG2 cells were double-labeled with DHE/MCD and β-BODIPY-PC for 1 min at 37°C, washed, and placed on a temperature-controlled microscope stage maintained at 35 ± 1°C. The focus was set to the interface between cell and substrate (microscope slide). DHE was selectively bleached in regions of increased intensity likely representing folded membrane regions as judged based on the similar fluorescence pattern of β-BODIPY-PC in this area (data not shown, but see outlined circle and Materials and Methods). The field aperture was opened and images were acquired at the indicated time points. Fluorescence recovery was measured in the outlined region (circle in G and see arrows) and plotted as function of time after normalization to total cell-associated DHE fluorescence (L). Bar, 10 μm.
DHE Is Not Enriched in Surface Folds Compared with Fluid-preferring Phospholipids
The results presented above indicate that DHE is not particularly enriched in microvilli, filopodia, or other surface structures. However, previous work showed accumulation of sterol–filipin complexes in filopodia and at cell attachment sites (Robinson and Karnovsky, 1980). We found in independent experiments that DHE does not self-quench in membranes and that it forms highly fluorescent DHE crystals and films (see Supplemental Material). Absence of DHE self-quenching in cellular membranes allows one to accurately measure relative sterol accumulation in certain membrane regions such surface ruffles by using quantitative fluorescence imaging of DHE in living cells. Fluorescence ratio imaging is a suitable technique to quantify differences in spatial distribution of two fluorescence probes (Zamir et al., 1999; Dunn and Maxfield, 2003). It was used here to quantify the distribution of DHE in plane membrane regions versus surface folds. HepG2 cells were double labeled with DHE and β-BODIPY-PC, imaged, and the ratio image of DHE and β-BODIPY-PC was calculated after background correction. Assuming that heterogeneous staining of β-BODIPY-PC is completely caused by differences in optical path lengths created by the rough surface of the cell, the staining pattern of β-BODIPY-PC is a measure for the surface topology, including microvilli and membrane protrusions. This is supported by quantitative fluorescence anisotropy microscopy (Benninger et al., 2005). The very low contrast in the calculated ratio image demonstrates that the overall spatial labeling pattern of DHE and β-BODIPY-PC coincides at cell–substrate attachment sites (Figure 8, A–C). A line scan measured along surface speckles of double-labeled cells reveals that the staining pattern but also the absolute intensity of DHE is very similar to that of β-BODIPY-PC in surface folds (Figure 8, E–H). Thus, the fluorescence ratio of DHE and β-BODIPY-PC is almost constant (spatially invariant) over the plasma membrane and does not depend on the cell surface topology. This is also reflected by a colocalization coefficient of rp > 0.95 in selected cell surface regions. Note, that intensities of DHE and β-BODIPY-PC were corrected for different photobleaching kinetics during image acquisition as described in Materials and Methods. Spatial variance in pixel intensities was compared between DHE and β-BODIPY-PC by PCA (Figure 8D). This method allows one to quantify differences between images by a coordinate transformation according to maximal pixel variance between aligned multi-color images (see Materials and Methods) (Gonzalez and Woods, 2002). The first principal component (PC1) looks almost identical to the DHE and β-BODIPY-PC image, respectively, giving an eigenvector l1 close to 1 (l1 = 0.996; eigenvector being identical 1 would be found for identical images). Small differences between DHE and β-BODIPY-PC staining were found at intensity maxima of membrane folds (see component PC1) and close to brightly labeled surface patches (indicated by PC2, arrowheads). Together, the results demonstrate that DHE is not specifically enriched in bended or folded membrane regions compared with plane plasma membrane areas. Fluorescent sterol shows almost identical staining patterns like the fluid lipid phase marker β-BODIPY-PC. This is supported by a one-dimensional (line scan) and correlation analysis and was also found for DiI lipid probes having different acyl chain lengths (see Supplemental Material). These DiI probes have opposite partition preference in fluid and gel phases in model membranes (see Supplemental Material), but they showed the same intensity fluctuations as DHE in the plasma membrane of living cells (Spink et al., 1990).
Figure 8.
DHE is not enriched in surface protrusions compared with BODIPY-PC. HepG2 cells were double labeled with DHE/MCD and β-BODIPY-PC, washed, and imaged in z-stacking modus on a wide field microscope. Corresponding spatially registered planes were background corrected, and the ratio of the DHE image (B) and the β-BODIPY-PC image (A) was calculated. This ratio image (C) was normalized and printed as 8-bit file. (D) PCA of the images in A and B provides two principal components named PC1 and PC2. Pixel intensities in PCA images range from −0.035 to +0.075 (see scale bar) for an 8-bit image format (0–255 possible pixel values), indicating that the difference between DHE and β-BODIPY-PC membrane staining patterns is very low. Calculated eigenvalues l1 = 0.996 and l2 = 0.004 support that there is almost now data scattering (see Materials and Methods for further details on calculations). (E–H) Line intensities (F) measured for a plasma membrane region double labeled with DHE (green in E) and β-BODIPY-PC (red in E, colcoalizing regions are yellow to orange). The intensity ratio measured along the line shown in E according to the intensity profiles in F is almost constant and close to 1 (G). A correlation plot of the line intensity profiles reveals high correlation of fluorescence of DHE and β-BODIPY-PC (R = 0.86) (H, dots, pixel intensities; straight line, linear regression). Bar, 5 μm.
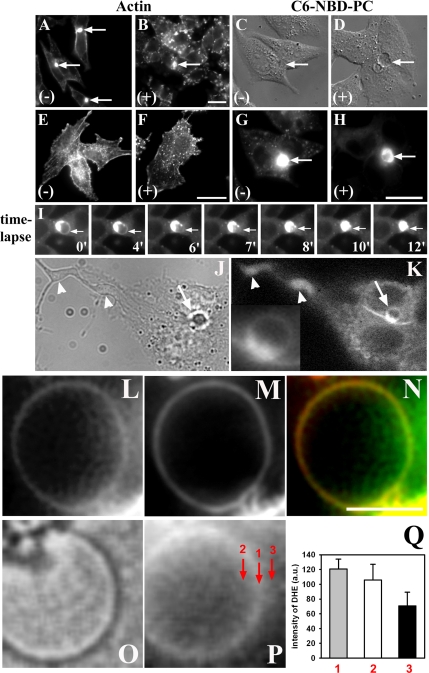
DHE Colocalizes with β-BODIPY-PC in Plasma Membrane Blebs after Actin Disruption
The ruffled surface topography of mammalian cells depends on the actin cytoskeleton being closely attached to the inner site of the plasma membrane. Dynamics of the cell surface is mediated by changes in the underlying actin network not only driving membrane protrusion and retraction but also membrane trafficking events such as endocytosis (Sheetz, 2001). In theory, it is possible that membrane attachment to the cytoskeleton masks phase separation into sterol-poor and -enriched regions in the plasma membrane. To directly test this hypothesis, HepG2 cells were treated with cytochalasin D, which disrupts F-actin and causes plasma membrane blebbing. Cellular F-actin was visualized by staining fixed cells with rhodamine-phalloidin after incubation in the presence or absence of cytochalasin D (Figure 9, A, B, E, and F). Whereas in control cells actin staining could be found along the lateral and basal plasma membrane, in the BC and in stress fibers (Figure 9, A and E), these structures were disrupted in cells treated with cytochalasin D (Figure 9, B and F). Both plasma membrane domains started to bleb in response to prolonged cytochalasin D treatment. This is shown for the BC stained with the fluorescent PC analog C6-NBD-PC (Figure 9, D and H). Cytochalasin D-induced blebbing is a reversible process in both plasma membrane domains as visualized by time-lapse microscopy: In cells treated with cytochalasin D, a blebbed canalicular membrane “self-healed” in a time course of 12 min as indicated by absorption of the blebbed membrane area into the spherical BC (Figure 9I, arrow). Similar results were found for the basolateral membrane of polarized HepG2 cells (data not shown). Partial reassembly of F-actin might be responsible for the self-healing process (Cunningham, 1995; Sheetz, 2001). DHE shows homogeneous staining along apical membrane blebs (Figure 9K, inset). Membrane tubules containing DHE were found more often in HepG2 cells after actin disruption (Figure 9, J and K, arrowheads). When cells were double-labeled with β-BODIPY-PC and DHE, both lipids colocalized along the blebbed plasma membrane (Figure 9, L–N). Importantly, no phase segregation between the fluorescent sterol- and ld-preferring β-BODIPY-PC was found. Parallel imaging of membrane blebs in brightfield and DHE fluorescence modus reveals homogeneous staining of DHE along the bleb perimeter (Figure 9, O–Q). Moreover, intensity fluctuations measured from a line scan along the bleb membrane in the DHE image are comparable (or even lower) than those caused from noise in the image background. This can be inferred from the SD of mean intensity measured along the bleb perimeter (gray bar) compared with a scan at the inner site (white bar) or outside of the membrane bleb (black bar) in Figure 9Q. Although the mean intensity is largest along the bleb membrane, signal SD is not higher than in the background region, indicating that observed intensity fluctuations in the image are caused by the low signal-to-noise ratio. These results rule out that cytoskeleton attachment of the plasma membrane mask phase segregation of sterol and argue strongly against the existence of optically resolvable sterol-enriched domains in the plasma membrane of living cells.
Figure 9.
Homogeneous staining of membrane blebs by DHE and β-BODIPY-PC. (A, B, E, and F) HepG2 cells incubated in buffer medium ([−], A and E) or in medium containing 20 μM cytochalasin D for 20 min at 37°C ([+], B and F) were fixed with 3.3% PFA for 30 min, permeabilized, and incubated with Alexa488-phalloidin for 30 min at room temperature. Actin disruption removed actin staining associated with the basolateral membrane, whereas actin staining around the BC (arrows) was maintained. Actin stress fibers associated with the membrane close to the coverslip as found in control cells ([−], E) were disturbed after cytochalasin D treatment ([+], F). (C, D, G, and H) Cells were preincubated in the presence ([+], D and H) or absence ([−], C and G) of cytochalasin D, washed, and labeled with C6-NBD-PC for 1 min at 37°C. These cells were washed and chased for 30 min at 37°C in the presence or absence of the drug. BC (arrows) was brightly labeled in control and drug-treated cells. Compared with control cells (C and G), the canalicular membrane looked blebbed in cytochalasin D-treated cells as inferred from the fluorescence image of C6-NBD-PC (H) and the corresponding DIC image (D). (I) Cells treated with cytochalasin D and labeled with C6-NBD-PC as described above were washed and imaged at 37°C on a temperature-controlled nitrogen floated microscope stage maintained at 35 ± 1°C of a wide field microscope. Images were acquired every 1 min after washout of the drug, allowing for observation of resealing of a blebbed BC (arrow) in a time course of 12 min. (J and K) Cells treated with cytochalasin D were labeled for 1 min at 37°C with DHE, washed, and imaged. A blebbing BC stained with DHE (arrow) was found. (L–O) Cytochalasin D-treated cells were double labeled with DHE and β-BODIPY-PC, washed, and imaged in z-stacking modus. Corresponding planes showing a membrane bleb were combined. DHE (L) colocalizes with β-BODIPY-PC (M) along the perimeter of the bleb. (N) Color image with DHE (green) and β-BODIPY-PC (red). (O and P) Bright field (O) and corresponding DHE image (Q) of a membrane bleb. A line scan was measured either exactly along the membrane bleb shown in P (line scan 1), shifted to the inner site of the bleb (line scan 2) or shifted parallel to the outside of the bleb measuring essential background intensity (line scan 3). (Q) Mean intensity ± SD of the line scans: 1, gray bar; 2, white bar; and 3, black bar. Bar, 10 μm (A–H) and 5 μm (L–N).
Cross-Linking of Ganglioside GM1 Does Not Affect the Membrane Distribution of DHE
The results presented above do not rule out that sterol forms submicrometer clusters, nor that it becomes preferentially enriched in very small domains (diameter ≪ 200 nm) together with certain proteins as stated in context with the raft hypothesis. Recent data, however, indicate that one can visualize putative membrane rafts by cross-linking molecules thought to be raft constituents. Moreover, it has been shown that cross-linking the ganglioside GM1 with cholera toxin triggers lipid demixing in model membranes: The fluid-preferring probe DiIC12 showing homogenous staining in GUVs becomes confined to circular domains excluding ganglioside after cross-linking GM1 in the GUV membrane (Hammond et al., 2005). It could be shown that this process resembles a liquid-ordered liquid-disordered phase transition in those model membranes with fluorescent Alexa488-CTxB in the ordered and DiIC12 in the disordered phase (Hammond et al., 2005). Partition of Alexa488-CTxB–patched GM1 into lo domains was later confirmed by atomic force microscopy (Shaw et al., 2006). Next, BMGE epithelial cells were used to visualize the plasma membrane distribution of DHE in the presence of Alexa488-CTxB (Figure 10). These cells form many surface protrusions and are therefore ideally suited to compare membrane staining of a particular probe with surface topology (Schmid et al., 1983). DHE and Alexa488-CTxB showed very homogeneous membrane staining at the cell perimeter, in lamellopodia, and in long, thin tubules emanating from the cell surface of BMGE cells (Figure 10, A–D). Similarly, when cells were triple labeled with DHE, Alexa488-CTxB, and DiIC12, all three probes colocalized in the plasma membrane with no indication for patching other than changes in surface topology revealed from the corresponding DIC images (Figure 10, E–L). Thus, in contrast to observations in model membranes, cholera toxin binding to GM1 does not trigger optically resolvable phase separation of sterol, DiIC12, or the toxin itself in the plasma membrane of living cells.
Figure 10.
DHE colocalizes with cholera toxin in surface protrusions and the cleavage furrow of dividing cells. (A–L) BMGE cells were labeled with DHE/MCD for 1 min at 37°C, washed, and chilled with ice-cold buffer solution, placed on ice, and incubated for 25 min with 50 μg/ml Alexa488-CTxB. Cells were washed twice with cold and once with warmed buffer and imaged on a wide field microscope. DHE (B) and Alexa488-CTxB (C) show equal membrane staining in lamellopodia-like structures (A–D, arrowhead) and in long, thin tubules emanating from the cell surface (A–D, arrows). Both probes colocalize also in smooth surface areas in the plasma membrane (A′–D′, inset). (D) Color overlay with DHE in green and Alexa488-CTxB in red. (E–S) BMGE cells were triple labeled with DHE (F, J, and O), Alexa488-CTxB (G, K, and N), and DiIC12 (H, L, and P). All three probes show homogeneous staining of the plasma membrane, including lamellopodia (E–H) and long branched tubules (I–L). DHE, Alexa488-CTxB, and DiIC12 show slight enrichment in the cleavage furrow of dividing BMGE cells (M–P). A line scan of color merged images of DHE and Alexa488-CTxB (R) or Alexa488-CTxB and DiIC12 (S) shows that DHE is less enriched in the furrow compared with the other two probes (Q). Colocalization is yellow to orange. (A, E, I, and M) DIC image of the respective fields. Bar, 10 μm, except 5 μm (M–P).
Based on experiments with the sterol binding probe filipin it has been proposed that cholesterol and Alexa488-CTxB but not fluid preferring DiI lipid become enriched in the cleavage furrow of dividing sea urchin eggs (Ng et al., 2005). In dividing BMGE cells, DHE, Alexa488-CTxB, and DiIC12 become slightly enriched in the cleavage furrow (Figure 10, M–S). However, there was no indication of preferred enrichment of sterol or the ganglioside over DiC12. Moreover, DiIC12 showed the highest relative enrichment, whereas DHE showed the lowest enrichment, as inferred from a line scan along the furrow (Figure 10, Q–S). It can be concluded that sterol or ganglioside enrichment in the cleavage furrow of dividing mammalian cells reflects an increase in total membrane area in this region but not formation of a specific domain in the plane of the bilayer.
The raft hypothesis states that in the unperturbed plasma membrane cholesterol-enriched microdomains are very small. These steady-state rafts should be between 20 and 70 nm in diameter, far below the resolution limit of the light microscope (approx. 250 nm under best conditions) (Pralle et al., 2000; Sharma et al., 2004). Coalescence of those small domains to large optically resolvable rafts can be triggered by cross-linking of raft components, for example, by binding of antibodies against CTxB on the cell surface. By this method, it has been shown that certain membrane proteins having a raft preference by biochemical criteria (detergent extraction) but not other proteins copatch with GM1 in the plasma membrane (Harder et al., 1998). To determine whether local sterol enrichment in the plasma membrane can be triggered by patching raft components, BMGE cells labeled with DHE and Alexa488-CTxB were incubated with anti-CTxB antibody (Figure 11). There was a profound patching of Alexa488-CTxB induced by antibody cross-linking (Figure 11, C, K, and T). Those patches did not resemble plasma membrane folds as inferred from the corresponding DIC images (Figure 11, A, I, and R). Importantly, DHE did not copatch with Alexa488-CTxB, i.e., the sterol showed homogeneous membrane staining and did not become particularly enriched in Alexa488-CTxB patches (Figure 11, B, J, and S). DHE showed homogeneous staining in nanotubes between two cells having a constant diameter (Figure 11, E and F, see DIC image). In contrast, Alexa488-CTxB showed clustered staining along the nanotube (Figure 11, E–H, arrows). In membrane blebs lacking cytoskeleton support, Alexa488-CTxB but not DHE clustered in certain regions along the bleb outline (Figure 11, N–Q). Fluorescence intensities of DHE and Alexa488-CTxB measured along the cell perimeter of the cell in Figure 11, I–L, showed only low correlation as determined by a bivariate analysis (R = 0.45). This was similarly found for many other cells giving correlation coefficients R = 0.41–0.5. In contrast, those for DHE and Alexa488-CTxB without antibody cross-linking where as high as between DHE and β-BODIPY-PC or DiIC12 (i.e., R >0.85; see above). Surface protrusions being homogeneously stained with DHE often completely lacked cross-linked Alexa488-CTxB (Figure 11, R–U). Together, the results demonstrate that fluorescent sterol does not become enriched in coalesced membrane rafts created by cross-linking of Alexa488-CTxB. These results were confirmed in other cell types (data not shown).
Figure 11.
Patching of cholera toxin by antibody cross-linking does not affect DHE membrane distribution. (A–L) BMGE cells were labeled with DHE/MCD for 1 min at 37°C, washed, and chilled with ice-cold buffer solution, placed on ice, and incubated for 25 min with 50 μg/ml Alexa488-CTxB. Cells were washed twice with cold buffer and incubated with anti-CTxB antibody for 10 min on ice. Cells were washed and imaged as described in text. Although Alexa488-CTxB occurs in patches after antibody cross-linking (C, G, K, P, and T), DHE shows homogeneous membrane staining (B, F, J, O, and S) as found in control cells (compare Figure 10). Plasma membrane labeling of both probes in lamellopodia (A–D), nanotubes connecting two cells (E–H), along the cell perimeter (I–L), and in membrane blebs (N–Q) is shown. Alexa488-CTxB occurs in large optically resolvable patches (arrows). (M) Line scan along the perimeter of the cell shown in I–L for DHE (see J, black line) and Alexa488-CTxB (see K, red line). Inset shows a correlation plot with linear regression of the line intensity profile revealing low correlation between DHE and Alexa488-CTxB fluorescence (R = 0.45). (R–U) Surface ruffles visible in the DIC image (R, arrowheads) are strongly labeled by DHE (S) but lack any patched Alexa488-CTxB (T). Corresponding DIC images (A, E, I, N, and R) reveal the cell surface shape, whereas color merged images (D, H, L, Q, and U) show DHE in green and Alexa488-CTxB in red, respectively. Bar, 10 μm, except 5 μm (N–Q).
DISCUSSION
Cholesterol is a crucial molecule in cellular membranes. Models on the molecular architecture of biomembranes reserve a unique place for cholesterol. This is because the lateral and transverse organization of this sterol molecule in bilayers plays a fundamental role for understanding the structure–function relationship of biological membranes. It is currently highly debated among membrane biophysicists and cell biologists whether cholesterol phase segregates laterally in the plane of the plasma membrane and whether cholesterol partitions into strongly curved membrane regions. DHE is a versatile tool to analyze sterol distribution in the plasma membrane of living cells by fluorescence microscopy. Using this approach in concert with advanced image processing and analysis, the results provide strong evidence that topological structuring of the cell surface but not lateral clusters in the bilayer plane cause an uneven labeling of fluorescent sterol in the plasma membrane of living cells. This conclusion is therefore somewhat different from that obtained by Schroeder and colleagues, who imaged DHE-labeled cells by multiphoton microscopy (Zhang et al., 2005). This study lacks a rigorous colocalization analysis to exclude the possibility of “apparent DHE enrichment” by membrane folding. Using the sterol-binding agent filipin, cell–substratum contact sites have been visualized previously after cell fixation (Robinson and Karnovsky, 1980; Bridgman and Nakajima, 1983). The authors argued that there is a local sterol enrichment at sites of cell attachment. However, fixation and filipin staining are harsh treatments for cells, and the labeling pattern is not a quantitative measure of sterol distribution (Maxfield and Wüstner, 2002). Only observation of sterol in living cells by using a close cholesterol analogue can provide reliable results. In the present study, ld-phase preferring PC analogue β-BODIPY-PC was used to show the distribution of DHE in plane and folded plasma membrane regions, including cell–cell and cell–substrate attachment sites. The absence of particular sterol enrichment and the same lateral diffusion dynamics of DHE in surface protrusions and plane membrane areas rule out optically resolvable sterol domains in the plasma membrane under physiological conditions. Similarly, induction of macroscopic membrane domains by cross-linking plasma membrane GM1 does not cause sterol copatching (Figures 10 and 11). These results rule out optically resolvable sterol domains in the plasma membrane of living cells. Instead, strong membrane folding results in higher optical path length and thereby higher heterogeneous membrane staining of DHE. The absence of local enrichment of DHE in response to raft protein patching by antibody cross-linking of GM1 argues strongly against coalescence of preexistent submicroscopic sterol domains, as stated by the raft hypothesis (Kusumi et al., 2004). Further evidence against sterol domains in the bilayer plane comes from the absence of DHE enrichment compared with the fluid phase lipid marker DiIC12 in the cleavage furrow of dividing cells (Figure 10, M–S). A slight enrichment of all three markers, i.e., DHE, DiIC12, and Alexa488-CTxB could be detected in this region. However, there was no preference of DHE or the raft marker Alexa488-CTxB compared with DiC12, indicating a general increase in membrane area in the cleavage furrow. The results presented here are in accordance with those of Koppel et al., (1982), ruling out selective accumulation of fluorescent CTxB in dividing J774 cells (Koppel et al., 1982). They seem to be in contradiction to observations made in sea urchin eggs by using filipin, DiI lipid, and Alexa488-CTxB (Ng et al., 2005). Further studies are required to reveal differences in membrane dynamics during cleavage furrow formation in mammalian culture cells and dividing embryos.
Apparent local enrichment of signaling proteins and lipid probes due to topological structuring of the cell surface has been reported previously: heterogeneous labeling pattern of DiI lipids in the plasma membrane could be assigned to surface folding-like microvilli at the apical membrane of polarized epithelial cells and at sites of cell–substrate attachment in macrophages, respectively (Sund et al., 1999; Colarusso and Spring, 2002). By confocal microscopy of adherent cells, it was found that green fluorescent protein (GFP)-tagged pleckstrin homology (PH) protein (GFP-PH) perfectly colocalizes with DiI lipid probes in membrane patches of medial and basal sections of neuroblastoma cells (van Rheenen and Jalink, 2002). These authors demonstrated with 15-nm resolution that observed membrane patches resemble surface folds and thereby higher local lipid content in the plasma membrane (van Rheenen and Jalink, 2002). Another study demonstrated that GFP-PH colocalizes with ld-preferring β-BODIPY-PC in lateral patches and ruffles, being several micrometers in size at the cell surface of adipocytes. Those patches were zones of increased membrane traffic as shown by endocytosis of transferrin (Huang et al., 2004). Moreover, atomic force microscopy and transmission electron microscopy have shown that the surface of mammalian cells is densely occupied by microvilli-like structures with diameters between 0.2 and 1.5 μm (Braet et al., 1998). Using the lipid phase state-sensitive probe Laurdan, evidence has been presented that filopodia and cell–cell contact areas are in a lo-like phase being enriched in cholesterol (Gaus et al., 2003). This is in contradiction to the results presented here showing not only the absence of sterol enrichment in these plasma membrane regions but also a relative depletion of antibody cross-linked Alexa488-CTxB in filopodia (Figure 11, R–U). One has to be aware that Laurdan-generalized polarization reflects water permeability into the bilayer and thereby measures, indirectly, membrane condensation (Bagatolli and Gratton, 1999). The physical parameters controlling Laurdan fluorescence can be well controlled in model but not in cellular membranes. Moreover, it was recently shown that Laurdan can sense also other factors such as membrane tension, which is controlled by cytoskeleton–membrane adhesion in cells (Sheetz, 2001; Zhang et al., 2006).
Owing to the much higher signal of DHE in wide field compared with two-photon fluorescence microscopy, DHE could be detected in long, thin tubules emanating from the cell surface of varying cell types (Figures 2 and 3 and 10 and 11). Along tubules, DHE shows an uneven distribution (Figures 2, 3, and 10). It is demonstrated that this staining pattern is caused by varying diameter of the tubules, ruling out curvature-induced phase separation as cause for uneven DHE staining along plasma membrane tubes (Roux et al., 2005). The latter mechanism has been shown to operate in model membranes having fluorescent phospholipid probes with different partition behavior (Roux et al., 2005). Interestingly, the raft marker Alexa488-CTxB clustered along nanotubes, but only after antibody cross-linking (see Figure 11). Despite the absence of relative enrichment, sterol might have important functions in cell surface protrusions. Moreover, cholesterol is strongly required for membrane ruffling and pinocytosis in mammalian cells (Grimmer et al., 2002). Biogenesis of cell protrusions and membrane ruffles was found to depend either directly on cholesterol or on proteins that bind cholesterol, such as prominin (Corbeil et al., 2001; Grimmer et al., 2002). Thus, cholesterol might be fundamental for generation and maintenance of cell protrusions via stabilization of curved membranes and by site-directed sterol-dependent membrane traffic. Moreover, it was found that cholesterol loading of macrophages induces actin-dependent surface protrusions. Based on the presented results, it is suggested that sterol plays a similar important role in exchange of material between cells via nanotube connections (Önfelt and Davis, 2004; Önfelt et al., 2004).
Here, it is shown that DHE does not phase segregate from the fluid (ld)-preferring phospholipid β-BODIPY-PC in plasma membrane blebs generated by actin disruption by using cytochalasin D (Figure 9). The homogeneous staining of DHE and β-BODIPY-PC along the membrane bleb rules out that sterol molecules segregate and form separate domains in biological membranes. Patching of Alexa488-CTxB in the plasma membrane, including membrane blebs, does not induce a copatching of DHE. According to the raft hypothesis, lipids in the patched region should be in a lo-like state (Harder et al., 1998). Here, it is clearly demonstrated that sterol does not become particularly enriched in these patched regions (Figure 11). It is possible that equivalents to the lo and ld phase exist in cellular membranes; however, the results shown here indicate that cholesterol would be equally distributed between these phases. Moreover, recent studies in model membranes indicate that cholesterol is only slightly enriched in the lo compared with the ld phase (Veatch and Keller, 2002; Oradd et al., 2005). Moreover, cholesterol seems to be able to exchange rapidly between different lipid phases in model membranes (Oradd et al., 2005).
Cholesterol in cell surface protrusions might have an important function in sterol exchange between cells and lipoproteins. For example, the initial interaction of atherogenic lipoproteins with macrophages in the intima of vessel walls occurs in deep surface-connected compartments formed by the macrophage cell surface (Myers et al., 1993; Kruth et al., 1995; Buton et al., 1999). Similarly, sterol efflux from fibroblasts and macrophages to apolipoproteins occurs preferentially from cell surface protrusions (Lin and Oram, 2000). The high-density lipoprotein (HDL) receptor scavenger receptor BI (SR-BI), being important for plasma-to-bile transport of cholesterol, clusters in microvilli channels at the cell surface of mammalian cells (Reaven et al., 2001; Peng et al., 2004). Moreover, it was shown that expression of SR-BI in insect cells mediates formation of such cell protrusions (Reaven et al., 2001). The observations presented here indicate that sterol in hepatocyte surface protrusions could play an important role in selective uptake of sterol from HDL mediated by SR-BI at the basolateral membrane domain (Wüstner et al., 2004, 2005b). Further studies will be directed toward analysis of SR-BI cell surface distribution and dynamics in polarized hepatic cells.
Supplementary Material
ACKNOWLEDGMENTS
I am grateful to Dr. Frederick R. Maxfield (Weill Medical College of Cornell University, New York, NY) who gave me the opportunity to use fluorescence microscopy instrumentation, reagents, and cell culture facilities for some of the experiments described in this article (supported by National Institutes of Health Grant DK-27083). Jesper S. Hansen is acknowledged for providing BMGE cells and for assistance in use. I acknowledge funding by grants of the Danish Heart Association Hjerteforeningen, the Diabetes Foundation Diabetesforeningen, as well as the Danish Research Agency Forskningsstyrelsen, Forskningsrådet for Natur og Univers.
Abbreviations used:
- Alexa488-CTxB
Alexa488-labeled cholera toxin subunit B
- BC
biliary canaliculus
- β-BODIPY-PC
2-(4,4-difluoro-5-(4-phenyl-1,3-butadienyl)-4-bora-3a,4a-diaza-s-indacene-3-pentanoyl)-1-hexa-decanoyl-sn-glycero-3-phosphocholine
- BSA
bovine serum albumin
- CE
cholesteryl ester
- CHO
Chinese hamster ovary
- CTxB
cholera toxin subunit B
- C6-NBD-PC
1-palmitoyl-2-[6-[(7-nitro-2–1,3-benzooxadiazol- 4-y) amino]caproyl]-sn-glycero-3-phosphatidylcholine
- DHE
dehydroergosterol
- DHE/MCD
dehydroergosterol/methyl-β-cyclodextrin complex
- DiIC12
1,1′-didodecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate
- DiIC16
1,1′-dihexadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate
- GFP-PH
green fluorescent protein-tagged pleckstrin homology protein
- PC
phosphatidylcholine
- PCA
principal component analysis
- PFA
paraformaldehyde
- ROI
region of interest
- SR-BI
scavenger receptor BI
- Tf
transferrin.
Footnotes

 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-05-0445) on October 25, 2006.
REFERENCES
- Ahmed S. N., Brown D. A., London E. On the origin of sphingolipid/cholesterol-rich detergent-insoluble cell membranes: physiological concentrations of cholesterol and sphingolipid induce formation of a detergent-insoluble, liquid-ordered lipid phase in model membranes. Biochemistry. 1997;36:10944–10953. doi: 10.1021/bi971167g. [DOI] [PubMed] [Google Scholar]
- Bagatolli L. A., Gratton E. Two-photon fluorescence microscopy observation of shape changes at the phase transition in phospholipid giant unilamellar vesicles. Biophys. J. 1999;77:2090–2101. doi: 10.1016/S0006-3495(99)77050-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benninger R. K., Önfelt B., Neil M. A., Davis D. M., French P. M. Fluorescence imaging of two-photon linear dichroism: cholesterol depletion disrupts molecular orientation in cell membranes. Biophys. J. 2005;88:609–622. doi: 10.1529/biophysj.104.050096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson D. M., Bryan J., Plant A. L., Gotto A.M.J., Smith L. C. Digital imaging fluorescence microscopy: spatial heterogeneity of photobleaching rate constants in individual cells. J. Cell Biol. 1985;100:1309–1323. doi: 10.1083/jcb.100.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braet F., Seynaeve C., De Zanger R., Wisse E. Imaging surface and submembranous structures with the atomic force microscope: a study on living cancer cells, fibroblasts and macrophages. J. Microsc. 1998;190:328–338. doi: 10.1046/j.1365-2818.1998.00333.x. [DOI] [PubMed] [Google Scholar]
- Brakenhoff G. J., Visscher K., Gijsbers E. J. Fluorescence bleach rate imaging. J. Microsc. 1994;175:154–161. [Google Scholar]
- Bridgman P. C., Nakajima Y. Distribution of filipin-sterol complexes on cultured muscle cells: cell-substratum contact areas associated with acetylcholine clusters. J. Cell Biol. 1983;96:363–372. doi: 10.1083/jcb.96.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. S., Wang E., Aroeti B., Chapin S. J., Mostov K. E., Dunn K. W. Definition of distinct compartments in polarized Madin-Darby canine kidney (MDCK) cells for membrane-volume sorting, polarized sorting and apical recycling. Traffic. 2000;1:124–140. doi: 10.1034/j.1600-0854.2000.010205.x. [DOI] [PubMed] [Google Scholar]
- Buton X., Mamdouh Z., Ghosh R. N., Du H., Kuriakose G., Beatini N., Grabowski G. A., Maxfield F. R., Tabas I. Unique cellular events occurring during the initial interaction of macrophages with matrix-retained or methylated aggregated low density lipoprotein (LDL). Prolonged cell-surface contact during which ldl-cholesteryl ester hydrolysis exceeds ldl protein degradation. J. Biol. Chem. 1999;274:32112–32121. doi: 10.1074/jbc.274.45.32112. [DOI] [PubMed] [Google Scholar]
- Chong P. L., Thompson T. E. Depolarization of dehydroergosterol in phospholipid bilayers. Biochim. Biophys. Acta. 1986;863:53–62. doi: 10.1016/0005-2736(86)90386-x. [DOI] [PubMed] [Google Scholar]
- Colarusso P., Spring K. R. Reticulated lipid probe fluorescence reveals MDCK cell apical membrane topography. Biophys. J. 2002;82:752–761. doi: 10.1016/S0006-3495(02)75437-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbeil D., Roper K., Fargeas C. A., Joester A., Huttner W. B. Prominin: a story of cholesterol, plasma membrane protrusions and human pathology. Traffic. 2001;2:82–91. doi: 10.1034/j.1600-0854.2001.020202.x. [DOI] [PubMed] [Google Scholar]
- Cunningham C. C. Actin polymerization and intracellular solvent flow in cell surface blebbing. J. Cell Biol. 1995;129:1589–1599. doi: 10.1083/jcb.129.6.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida R. F., Loura L. M., Prieto M., Watts A., Fedorov A., Barrantes F. J. Cholesterol modulates the organization of the gammaM4 transmembrane domain of the muscle nicotinic acetylcholine receptor. Biophys. J. 2004;86:2261–2272. doi: 10.1016/S0006-3495(04)74284-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormann D., Libotte T., Weijer C. J., Bretschneider T. Simultaneous quantification of cell motility and protein-membrane-association using active contours. Cell Motil. Cytoskeleton. 2002;52:221–230. doi: 10.1002/cm.10048. [DOI] [PubMed] [Google Scholar]
- Dubin-Thaler B. J., Giannone G., Döbereiner H. G., Sheetz M. P. Nanometer analysis of cell spreading on matrix-coated surfaces reveals two distinct cell states and STEPs. Biophys. J. 2004;86:1794–1806. doi: 10.1016/S0006-3495(04)74246-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn K. W., Maxfield F. R. Ratio imaging instrumentation. Methods Cell Biol. 2003;72:389–413. doi: 10.1016/s0091-679x(03)72019-6. [DOI] [PubMed] [Google Scholar]
- Edidin M. Lipids on the frontier: a century of cell-membrane bilayers. Nat. Cell Biol. 2003;4:414–418. doi: 10.1038/nrm1102. [DOI] [PubMed] [Google Scholar]
- Gaus K., Gratton E., Kable E. P., Jones A. S., Gelissen I., Kritharides L., Jessup W. Visualizing lipid structure and raft domains in living cells with two-photon microscopy. Proc. Natl. Acad. Sci. USA. 2003;100:15554–15559. doi: 10.1073/pnas.2534386100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh R. N., Gelman D. L., Maxfield F. R. Quantification of low density lipoprotein and transferrin endocytic sorting in HEp2 cells using confocal microscopy. J. Cell Sci. 1994;107:2177–2189. doi: 10.1242/jcs.107.8.2177. [DOI] [PubMed] [Google Scholar]
- Gonzalez R. C., Woods R. E. Upper Saddle River, NJ: Prentice Hall; 2002. Digital Image Processing, Chapter 11; pp. 675–683. [Google Scholar]
- Grimmer S., Iversen T. G., van Deurs B., Sandvig K. Endosome to Golgi transport of ricin is regulated by cholesterol. Mol. Biol. Cell. 2000;11:4205–4216. doi: 10.1091/mbc.11.12.4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimmer S., van Deurs B., Sandvig K. Membrane ruffling and macropinocytosis in A431 cells require cholesterol. J. Cell Sci. 2002;115:2953–2962. doi: 10.1242/jcs.115.14.2953. [DOI] [PubMed] [Google Scholar]
- Hammond A. T., Heberle F. A., Baumgart T., Holowka D., Baird B., Feigenson G. W. Crosslinking a lipid raft component triggers liquid ordered-liquid disordered phase separation in model plasma membranes. Proc. Natl. Acad. Sci. USA. 2005;102:6320–6325. doi: 10.1073/pnas.0405654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao M., Lin S. X., Karylowski O. J., Wüstner D., McGraw T. E., Maxfield F. R. Vesicular and non-vesicular sterol transport in living cells. The endocytic recycling compartment is a major sterol storage organelle. J. Biol. Chem. 2002;277:609–617. doi: 10.1074/jbc.M108861200. [DOI] [PubMed] [Google Scholar]
- Harder T., Scheiffele P., Verkade P., Simons K. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J. Cell Biol. 1998;141:929–942. doi: 10.1083/jcb.141.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg K., Miller C. M., Ellenberg J., Presley J. F., Siggia E. D., Phair R. D., Lippincott-Schwartz J. Kinetic analysis of secretory protein traffic and characterization of Golgi to plasma membrane transport intermediates in living cells. J. Cell Biol. 1998;143:1485–1503. doi: 10.1083/jcb.143.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes T. J., Bhattacharyya S., Cooper J. A., Hanzel D., Krishnamurthi V., Lin W.-C., Roysam B., Szarowski D. H., Turner J. N. Handbook of Biological Confocal Microscopy. New York: Plenum Press; 1995. Light microscopic images reconstructed by maximum likelihood deconvolution; pp. 389–402. [Google Scholar]
- Huang S., Lifshitz L., Patki-Kamath V., Tuft R., Fogarty K., Czech M. P. Phosphatidylinositol-4,5-bisphosphate-rich plasma membrane patches organize active zones of endocytosis and ruffling in cultured adipocytes. Mol. Cell. Biol. 2004;24:9102–9123. doi: 10.1128/MCB.24.20.9102-9123.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner W. B., Zimmerberg J. Implications of lipid microdomains for membrane curvature, budding and fission. Curr. Opin. Cell Biol. 2001;13:478–484. doi: 10.1016/s0955-0674(00)00239-8. [DOI] [PubMed] [Google Scholar]
- Ipsen J. H., Karlstrom G., Mouritsen O. G., Wennerstrom H., Zuckermann M. J. Phase equilibria in the phosphatidylcholine-cholesterol system. Biochim. Biophys. Acta. 1987;905:162–172. doi: 10.1016/0005-2736(87)90020-4. [DOI] [PubMed] [Google Scholar]
- Jacob M., Blu T., Unser M. Efficient energies and algorithms for parametric snakes. IEEE Trans. Image Process. 2004;13:1231–1244. doi: 10.1109/tip.2004.832919. [DOI] [PubMed] [Google Scholar]
- Koppel D. E., Oliver J. M., Berlin R. D. Surface functions during mitosis. III. Quantitative analysis of ligand-receptor movement into the cleavage furrow: diffusion vs. flow. J. Cell Biol. 1982;93:950–960. doi: 10.1083/jcb.93.3.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korlach J., Schwille P., Webb W. W., Feigenson G. W. Characterization of lipid bilayer phases by confocal microscopy and fluorescence correlation spectroscopy. Proc. Natl. Acad. Sci. USA. 1999;96:8461–8466. doi: 10.1073/pnas.96.15.8461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruth H. S., Skarlatos S. I., Lilly K., Chang J., Ifrim I. Sequestration of acetylated LDL and cholesterol crystals by human monocyte-derived macrophages. J. Cell Biol. 1995;129:133–145. doi: 10.1083/jcb.129.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumi A., Koyama-Honda I., Suzuki K. Molecular dynamics and interactions for creation of stimulation-induced stabilized rafts from small unstable steady-state rafts. Traffic. 2004;5:213–230. doi: 10.1111/j.1600-0854.2004.0178.x. [DOI] [PubMed] [Google Scholar]
- Lin G., Oram J. F. Apolipoprotein binding to protruding membrane domains during removal of excess cellular cholesterol. Atherosclerosis. 2000;149:359–370. doi: 10.1016/s0021-9150(99)00503-1. [DOI] [PubMed] [Google Scholar]
- Liu F., Sugar I. P., Chong P. L. Cholesterol and ergosterol superlattices in three-component liquid crystalline lipid bilayers as revealed by dehydroergosterol fluorescence. Biophys. J. 1997;72:2243–2254. doi: 10.1016/S0006-3495(97)78868-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loura L. M., Prieto M. Dehydroergosterol structural organization in aqueous medium and in a model system of membranes. Biophys. J. 1997;72:2226–2236. doi: 10.1016/S0006-3495(97)78866-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxfield F. R. Plasma membrane microdomains. Curr. Opin. Cell Biol. 2002;14:483–487. doi: 10.1016/s0955-0674(02)00351-4. [DOI] [PubMed] [Google Scholar]
- Maxfield F. R., Wüstner D. Intracellular cholesterol transport. J. Clin. Investig. 2002;110:891–898. doi: 10.1172/JCI16500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor S., Presley J. F., Maxfield F. R. Sorting of membrane components from endosomes and subsequent recycling to the cell surface occurs by a bulk flow process. J. Cell Biol. 1993;121:1257–1269. doi: 10.1083/jcb.121.6.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw T. E., Greenfield L., Maxfield F. R. Functional expression of the human transferrin receptor cDNA in Chinese hamster ovary cells deficient in endogenous transferrin receptor. J. Cell Biol. 1987;105:207–214. doi: 10.1083/jcb.105.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S., Soe T. T., Maxfield F. R. Endocytic sorting of lipid analogues differing solely in the chemistry of their hydrophobic tails. J. Cell Biol. 1999;144:1271–1284. doi: 10.1083/jcb.144.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S., Zha X., Tabas I., Maxfield F. R. Cholesterol distribution in living cells: fluorescence imaging using dehydroergosterol as a fluorescent cholesterol analog. Biophys. J. 1998;75:1915–1925. doi: 10.1016/S0006-3495(98)77632-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J. N., Tabas I., Jones N. L., Maxfield F. R. beta-Very low density lipoprotein is sequestered in surface-connected tubules in mouse peritoneal macrophages. J. Cell Biol. 1993;123:1389–1402. doi: 10.1083/jcb.123.6.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng M. M., Chang F., Burgess D. R. Movement of membrane domains and requirement of membrane signaling molecules for cytokinesis. Dev. Cell. 2005;9:781–790. doi: 10.1016/j.devcel.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Oradd G., Westerman P. W., Lindblom G. Lateral diffusion coefficients of separate lipid species in a ternary raft-forming bilayer: a Pfg-NMR multinuclear study. Biophys. J. 2005;89:315–320. doi: 10.1529/biophysj.105.061762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Önfelt B., Davis D. M. Can membrane nanotubes facilitate communication between immune cells? Biochem. Soc. Trans. 2004;32:676–678. doi: 10.1042/BST0320676. [DOI] [PubMed] [Google Scholar]
- Önfelt B., Nedvetzki S., Yanagi K., Davis D. M. Cutting edge: Membrane nanotubes connect immune cells. J. Immunol. 2004;173:1511–1513. doi: 10.4049/jimmunol.173.3.1511. [DOI] [PubMed] [Google Scholar]
- Peng Y., Akmentin W., Connelly M. A., Lund-Katz S., Phillips M. C., Williams D. L. Scavenger receptor BI (SR-BI) clustered on microvillar extensions suggests that this plasma membrane domain is a way station for cholesterol trafficking between cells and high-density lipoprotein. Mol. Biol. Cell. 2004;15:384–396. doi: 10.1091/mbc.E03-06-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pralle A., Keller P., Florin E. L., Simons K., Horber J. K. Sphingolipid-cholesterol rafts diffuse as small entities in the plasma membrane of mammalian cells. J. Cell Biol. 2000;148:997–1008. doi: 10.1083/jcb.148.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaven E., Leers-Sucheta S., Nomoto A., Azhar S. Expression of scavenger receptor class B type 1 (SR-BI) promotes microvillar channel formation and selective cholesteryl ester transport in a heterologous reconstituted system. Proc. Natl. Acad. Sci. USA. 2001;98:1613–1618. doi: 10.1073/pnas.98.4.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. M., Karnovsky M. J. Specializations in filopodial membranes at points of attachment to the substrate. J. Cell Biol. 1980;87:562–568. doi: 10.1083/jcb.87.3.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux A., Cuvelier D., Nassoy P., Prost J., Bassereau P., Goud B. Role of curvature and phase transition in lipid sorting and fission of membrane tubules. EMBO J. 2005;24:1537–1545. doi: 10.1038/sj.emboj.7600631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe D., Hoffmann R. G. Multivariate statistical analysis in fMRI. IEEE Eng. Med. Biol. 2006;25:60–64. doi: 10.1109/memb.2006.1607670. [DOI] [PubMed] [Google Scholar]
- Rustom A., Saffrich R., Markovic I., Walther P., Gerdes H.-H. Nanotubular highways for intercellular organelle transport. Science. 2004;303:1007–1010. doi: 10.1126/science.1093133. [DOI] [PubMed] [Google Scholar]
- Salzmann N. H., Maxfield F. R. Fusion accessibility of endocytic compartments along the recycling and lysosomal endocytic pathways in intact cells. J. Cell Biol. 1989;109:2097–2104. doi: 10.1083/jcb.109.5.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid E., Schiller D. L., Grund C., Stadler J., Franke W. W. Tissue type-specific expression of intermediate filament proteins in a cultured epithelial cell line from bovine mammary gland. J. Cell Biol. 1983;96:37–50. doi: 10.1083/jcb.96.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P., Varma R., Sarasij R. C., Ira, Gousset K., Krishnamoorthy G., Rao M., Mayor S. Nanoscale organization of multiple GPI-anchored proteins in living cell membranes. Cell. 2004;116:577–589. doi: 10.1016/s0092-8674(04)00167-9. [DOI] [PubMed] [Google Scholar]
- Shaw J. E., Epand R. F., Epand R. M., Li Z., Bittman R., Yip C. M. Correlated fluorescence-atomic force microscopy of membrane domains: structure of fluorescence probes determines lipid localization. Biophys. J. 2006;90:2170–2178. doi: 10.1529/biophysj.105.073510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz M. P. Cell control by membrane cytoskeleton adhesion. Nat. Rev. Mol. Cell Biol. 2001;2:392–396. doi: 10.1038/35073095. [DOI] [PubMed] [Google Scholar]
- Simons K., Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Smutzer G., Crawford B. F., Yeagle P. L. Physical properties of the fluorescent sterol probe dehydroergosterol. Biochim. Biophys. Acta. 1986;862:361–371. doi: 10.1016/0005-2736(86)90239-7. [DOI] [PubMed] [Google Scholar]
- Sormunen R., Eskelinen S., Lehto V. P. Bile canaliculus formation in cultured HEPG2 cells. Lab. Investig. 1993;68:652–662. [PubMed] [Google Scholar]
- Spink C. H., Yeager M. D., Feigenson G. W. Partitioning behavior of indocarbocyanine probes between coexisting gel and fluid phases in model membranes. Biochim. Biophys. Acta. 1990;1023:25–33. doi: 10.1016/0005-2736(90)90005-9. [DOI] [PubMed] [Google Scholar]
- Sund S. E., Swanson J. A., Axelrod D. Cell membrane orientation visualized by polarized total internal reflection fluorescence. Biophys. J. 1999;77:2266–2283. doi: 10.1016/S0006-3495(99)77066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tank D. W., Wu E.-S., Webb W. W. Enhanced molecular diffusibility in muscle membrane blebs: release of lateral constraints. J. Cell Biol. 1982;92:207–212. doi: 10.1083/jcb.92.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevenaz P., Ruttimann U. E., Unser E. A pyramid approach to subpixel registration based on intensity. IEEE Trans. Image Process. 1998;7:27–41. doi: 10.1109/83.650848. [DOI] [PubMed] [Google Scholar]
- Tulenko T. N., Chen M., Mason P. E., Mason R. P. Physical effects of cholesterol on arterial smooth muscle membranes: evidence of immiscible cholesterol domains and alterations in bilayer width during atherogenesis. J. Lipid Res. 1998;39:947–956. [PubMed] [Google Scholar]
- van Rheenen J., Jalink K. Agonist-induced PIP(2) hydrolysis inhibits cortical actin dynamics: regulation at a global but not at a micrometer scale. Mol. Biol. Cell. 2002;13:3257–3267. doi: 10.1091/mbc.E02-04-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veatch S. L., Keller S. L. Organization in lipid membranes containing cholesterol. Phys. Rev. Lett. 2002;89:268101. doi: 10.1103/PhysRevLett.89.268101. [DOI] [PubMed] [Google Scholar]
- Verveer P. J., Gemkow M. J., Jovin T. M. A comparison of image restoration approaches applied to three-dimensional confocal and wide-field fluorescence microscopy. J. Microsc. 1999;193:50–61. doi: 10.1046/j.1365-2818.1999.00421.x. [DOI] [PubMed] [Google Scholar]
- Vist M. R., Davis J. H. Phase equilibria of cholesterol/ dipalmitoylphosphatidylcholine mixtures: 2H nuclear magnetic resonance and differential scanning calorimetry. Biochemistry. 1990;29:451–464. doi: 10.1021/bi00454a021. [DOI] [PubMed] [Google Scholar]
- Weikl T. R., Lipowsky R. Pattern formation during T-cell adhesion. Biophys. J. 2004;87:3665–3678. doi: 10.1529/biophysj.104.045609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wüstner D. Improved visualization and quantitative analysis of fluorescent membrane sterol in polarized hepatic cells. J. Microsc. 2005a;220:47–64. doi: 10.1111/j.1365-2818.2005.01516.x. [DOI] [PubMed] [Google Scholar]
- Wüstner D. Mathematical analysis of hepatic high density lipoprotein transport based on quantitative imaging data. J. Biol. Chem. 2005b;280:6766–6779. doi: 10.1074/jbc.M413238200. [DOI] [PubMed] [Google Scholar]
- Wüstner D., Herrmann A., Hao M., Maxfield F. R. Rapid nonvesicular transport of sterol between the plasma membrane domains of polarized hepatic cells. J. Biol. Chem. 2002;277:30325–30336. doi: 10.1074/jbc.M202626200. [DOI] [PubMed] [Google Scholar]
- Wüstner D., Mondal M., Huang A., Maxfield F. R. Different transport routes for high density lipoprotein and its associated free sterol in polarized hepatic cells. J. Lipid Res. 2004;45:427–437. doi: 10.1194/jlr.M300440-JLR200. [DOI] [PubMed] [Google Scholar]
- Wüstner D., Mondal M., Tabas I., Maxfield F. R. Direct observation of rapid internalization and intracellular transport of sterol by macrophage foam cells. Traffic. 2005;6:396–412. doi: 10.1111/j.1600-0854.2005.00285.x. [DOI] [PubMed] [Google Scholar]
- Wüstner D., Mukherjee S., Maxfield F. R., Müller P., Hermann A. Vesicular and nonvesicular transport of phosphatidylcholine in polarized HepG2 cells. Traffic. 2001;2:277–296. doi: 10.1034/j.1600-0854.2001.9o135.x. [DOI] [PubMed] [Google Scholar]
- Yeagle P. L., Bensen J., Boni L., Hui S. W. Molecular packing of cholesterol in phospholipid vesicles as probed by dehydroergosterol. Biochim. Biophys. Acta. 1982;692:139–146. [Google Scholar]
- Yin C., Liao K., Mao H. Q., Leong K. W., Zhuo R. X., Chan V. Adhesion contact dynamics of HepG2 cells on galactose-immobilized substrates. Biomaterials. 2003;24:837–850. doi: 10.1016/s0142-9612(02)00416-7. [DOI] [PubMed] [Google Scholar]
- Zamir E., Katz B. Z., Aota S., Yamada K. M., Geiger B., Kam Z. Molecular diversity of cell-matrix adhesions. J. Cell Sci. 1999;112:1655–1669. doi: 10.1242/jcs.112.11.1655. [DOI] [PubMed] [Google Scholar]
- Zhang W., McIntosh A. L., Xu H., Wu D., Gruninger T., Atshaves B., Liu J. C., Schroeder F. Structural analysis of sterol distributions in the plasma membrane of living cells. Biochemistry. 2005;44:2864–2884. doi: 10.1021/bi048172m. [DOI] [PubMed] [Google Scholar]
- Zhang Y. L., Frangos J. A., Chachisvillis M. Laurdan fluorescence senses mechanical strain in the lipid bilayer membrane. Biochem. Biophys. Res. Commun. 2006;347:838–841. doi: 10.1016/j.bbrc.2006.06.152. [DOI] [PubMed] [Google Scholar]
- Zuckermann M. J., Ipsen J. H., Miao L., Mouritsen O. G., Nielsen M., Polson J., Thewalt J., Vattulainen I., Zhu H. Modeling lipid-sterol bilayers: applications to structural evolution, lateral diffusion, and rafts. Methods Enzymol. 2004;383:198–229. doi: 10.1016/S0076-6879(04)83009-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.