Abstract
Toxin-antitoxin loci belonging to the yefM-yoeB family are located in the chromosome or in some plasmids of several bacteria. We cloned the yefM-yoeB locus of Streptococcus pneumoniae, and these genes encode bona fide antitoxin (YefMSpn) and toxin (YoeBSpn) products. We showed that overproduction of YoeBSpn is toxic to Escherichia coli cells, leading to severe inhibition of cell growth and to a reduction in cell viability; this toxicity was more pronounced in an E. coli B strain than in two E. coli K-12 strains. The YoeBSpn-mediated toxicity could be reversed by the cognate antitoxin, YefMSpn, but not by overproduction of the E. coli YefM antitoxin. The pneumococcal proteins were purified and were shown to interact with each other both in vitro and in vivo. Far-UV circular dichroism analyses indicated that the pneumococcal antitoxin was partially, but not totally, unfolded and was different than its E. coli counterpart. Molecular modeling showed that the toxins belonging to the family were homologous, whereas the antitoxins appeared to be specifically designed for each bacterial locus; thus, the toxin-antitoxin interactions were adapted to the different bacterial environmental conditions. Both structural features, folding and the molecular modeled structure, could explain the lack of cross-complementation between the pneumococcal and E. coli antitoxins.
The gram-positive, spherical bacterium Streptococcus pneumoniae (pneumococcus) is the cause of many human diseases, such as pneumonia, bacterial blood poisoning (bacteremia), inflammation of the membranes surrounding the brain and spinal cord (meningitis), middle-ear infection (otitis media), osteomyelitis, septic arthritis, endocarditis, peritonitis, pericarditis, and sinusitis; pneumonia is the most severe disease (15, 28). Although the pneumococcus can normally be found in the noses and throats of healthy individuals, it can grow and cause infection when the immune system is weakened. The people who are most at risk of developing pneumococcal pneumonia have a weakened immune system. These people include the elderly, infants, cancer patients, AIDS patients, postoperative patients, alcoholics, and people with diabetes. The global rate of mortality is more than 1,000,000 people per year, and this figure represents about 15 to 20% of the people infected. Epidemiological studies of community-acquired pneumonia have shown that S. pneumoniae is still one of the most significant etiologic agents in all age groups in developing and industrialized countries (28). A recently published analysis estimated that 1.6 to 2.2 million children die from acute respiratory infection worldwide each year and that about 30% of the deaths are in Southeast Asia (52). Historically, the treatment for pneumococcal pneumonia has been penicillin. However, an increasing number of pneumococcal strains are partially or completely resistant not only to penicillin but also to macrolides, trimethoprim-sulfamethoxazole, and cephalosporins, making it increasingly difficult to treat this disease (3, 50). Vaccination is an available solution, but unfortunately, the people for whom vaccination is most recommended are the people who are least likely to respond favorably to it. Therefore, the overall effectiveness of a vaccine remains questionable, and the need for novel drugs is increasing (51).
Seeking new approaches and finding new targets for pneumococci comprise an important field. In this sense, chromosomally encoded proteic toxin-antitoxin (TA) systems can be considered suitable targets for antibiotics (17). Originally, these systems were discovered in bacterial plasmids, but they are also abundant in the chromosomes of bacteria and archaea (37). They consist of two proteins that form a harmless complex in which the unstable antitoxin neutralizes the toxicity of the cognate stable toxin. When cells encounter nutritional or environmental stresses, cellular proteases (Lon or Clp) activate the toxins by actively degrading their antitoxin counterparts (1, 10, 11). However, the toxin activity does not necessarily lead to cell death provided that within a certain window of time synthesis of the antitoxin is resumed (16, 36, 38). The chromosomal TA systems seem to operate by modulating the global level of translation, with the toxins functioning as specific endoribonucleases (12, 20) that cleave either free mRNA, like YoeB or MazF (26, 55), or mRNA associated with actively translating ribosomes, like RelE (39). Thus, the bacterial toxins are potentially interesting as new antibiotics (17, 20, 36). Ideally, an inhibitor that mimics the most relevant toxin residues and their interactions with the antitoxin, leading to a new complex (compound-antitoxin) that frees the toxin to cause bacterial damage, would be an excellent antimicrobial agent.
In the chromosome of S. pneumoniae R6 (24), at least three TA loci have been identified. Two of these loci, relBE1Spn and relBE2Spn, exhibit homology with the relBE genes of Escherichia coli (19), whereas the third locus, yefM-yoeBSpn, is homologous to the yefM-yoeB (also designated relBE3) genes of E. coli (5). A similar locus has been reported to be present in plasmid pRUM of Enterococcus faecium (22). DNA fragments containing the putative toxin genes relE1Spn and relE2Spn were cloned in an E. coli expression vector. Overproduction of both toxins showed that RelE2Spn was toxic to E. coli, whereas RelE1Spn was innocuous, indicating that relE2Spn is a toxin gene (36). The relB2Spn and relE2Spn genes were shown to be organized in a single operon. Overexpression of the relE2Spn toxin gene both in S. pneumoniae and in E. coli led to cell growth arrest and a concomitant reduction in the number of viable cells, which could be reversed by expression of the cognate antitoxin (36).
The yefM-yoeB TA locus was first identified on the basis of its similarity to the axe-txe TA system of plasmid pRUM (22). Later, the E. coli yefM-yoeB locus was analyzed, and the chromosomal proteins YefM and YoeB were characterized in detail. The YefM antitoxin of E. coli was shown to be unfolded in its native state (5), although the YoeB toxin was folded and formed a physical complex with the unfolded YefM antitoxin (6). YoeB exhibited endogenous endoribonuclease activity, and its interaction with YefM induced a conformational change in the toxin around the putative active site, leading to inhibition of the RNase activity of YoeB (26). Although the antitoxins associated more efficiently with their cognate toxins, effective cross-complementation between Axe and YoeB and, to lesser extent, between YefM and Txe has been shown to occur in vivo (22), demonstrating the broad specificity of the TA of the two bacterial species and suggesting that there is a common mechanism of toxin-antitoxin interaction.
In this paper, we describe cloning, characterization, and analysis of the yefM-yoeBSpn pneumococcal TA system. We analyzed the cross-complementation between the two YefM antitoxins from E. coli and S. pneumoniae with the corresponding heterologous YoeB toxins. Unlike the interaction of Axe and YefMEco, the YefMSpn antitoxin was able to interact only with its cognate toxin, indicating that there are some structural differences between the antitoxins which could hinder heterologous TA interactions. Far-UV circular dichroism (CD) and molecular modeling analyses indicated that even though the toxins of E. coli and S. pneumoniae exhibited a high level of similarity, the YefM antitoxin counterparts seemed to be structurally different, thus explaining the lack of cross-complementation. Therefore, similar TA systems harbored by different bacteria may have specifically evolved to respond to different environmental conditions, thereby optimizing the TA interactions in their hosts.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The E. coli strains used were TOP-10 [F− mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 recA1 deoR araD139 Δ(ara-leu)7697 galU galK rpsL(Strr) endA1 nupG] (Invitrogen), BL21 [F− ompT gal (dcm) (lon) hsdSB(rB mB−)] (an E. coli B strain) (47), BL21(DE3)(pLysS) (Novagen), MG1655 (wild-type E. coli) (53), and two isogenic derivatives of MG1655, SC36 (MG1655ΔyefM-yoeB) (7) (provided by K. Gerdes) and MG1655lon (a gift from L. van Melderen). For bacterial two-hybrid assays, E. coli XL-Blue MRF′ Km (Stratagene) was used as the host during construction of the recombinant bait and target vectors. The recombinant bait and target pairs were then cotransformed into competent cells of the Bacteriomatch II E. coli validation reporter (Stratagene), a derivative of E. coli XL1-Blue MRF′ [Δ(mcrA)183 Δ(mcrCB-hsdSMR-mrr)173 endA1 hisB supE44 thi-1 recA1 gyrA96 relA1 lac (F′ lacIq his-3 aadA Kanr)], which allowed detection of protein-protein interactions through transcriptional activation of the His reporter gene. All cultures were grown in TY medium (33), which was supplemented with 100 μg/ml ampicillin or with 50 μg/ml kanamycin. All cultures were grown at 37°C. The source of S. pneumoniae chromosomal DNA was strain R61, which was grown as reported previously (29) and which is a derivative of the R6 sequenced strain (24). The growth conditions and details concerning induction of synthesis of the pneumococcal toxin and its cognate antitoxin in E. coli have been described elsewhere (36); briefly, the procedures included measuring the turbidity of the cultures by monitoring the optical density at 600 nm (OD600) and determining the number of CFU by plating suitable dilutions of the cultures.
Plasmids used.
Plasmids used in this work were constructed as follows.
(i) pYBS2.
The yoeBSpn gene with its own Shine-Dalgarno sequence was amplified by PCR from chromosomal DNA (prepared from S. pneumoniae R61) using primers yoeBN2 (5′-CTGGAATTCCGCAGGTCCATGTGATTGAGGAGT-3′) and yoeBC2 (5′-CGCGGATCCGGTAGAGACTTGAGAAAAAGCCTA-3′). The resulting 338-bp PCR fragment was doubly digested with EcoRI and BamHI and ligated into plasmid pFUS2 (31) digested with EcoRI and BglII (compatible ends with BamHI sites). This construction placed the yoeBSpn gene under control of the arabinose-inducible PBAD promoter. Thus, when cells were grown in arabinose-containing medium, AraC was active and promoted transcription from the PBAD promoter, whereas when cells were grown in the presence of glucose, AraC was inactive and transcription from PBAD was switched off (54).
(ii) pYFS10.
A DNA fragment encompassing the yefMSpn gene with its putative Shine-Dalgarno sequence was amplified by PCR from pneumococcal chromosomal DNA using primers yefMN (5′-CGCGGATCCGCTTGTACAAGTTCCTGACAATTTC-3′) and yefMc (5′-CTGGAATTCCGTTTTGCCAGTAGCAATAATCTGC-3′). The 403-bp PCR fragment was digested with BamHI and EcoRI before ligation into the equivalent sites of plasmid pNM220, placing yefMSpn under control of the isopropyl-β-d-1-thiogalactopyranoside (IPTG)-inducible Plac promoter (21).
(iii) pFYBE.
The yoeBEco gene was amplified from E. coli genomic DNA by PCR using primers yoeBNE (5′-CTGGAATTCCTGAAATCAGGCAAAGGAACGGAAA-3′) and yoeBCE (5′-CGCGATCCGTATCAAAACTGACAATTCATT-3′). The resulting 350-bp PCR product was digested with EcoRI and BamHI before ligation into the EcoRI and BglII sites of pFUS2, as described above.
(iv) pNMYE.
The yefMEco gene was amplified from E. coli genomic DNA by PCR using primers yefMNE (5′-CGCGGATCCGTTAATTAACGCTCATCATTGAT-3′) and yefMCE (5′-CTGGAATTCCTCAGACCAGATTAGTTTCA-3′). The resulting 341-bp product was digested with EcoRI and BamHI before ligation into the equivalent sites of pNM220.
(v) pRES2.
A PCR DNA fragment obtained from the chromosome of S. pneumoniae containing the relE2Spn gene with its own Shine-Dalgarno sequence was generated using primers relE2N (5′-GCGAATTCGATGCATGATTTAGGCTTGAAG-3′) and relE2C (5′-CGGGATCCGAATGAAAATTTACTTGAAAAAAGT-3′). The 325-bp DNA fragment was digested with BamHI and EcoRI and ligated into pFUS2 digested with EcoRI and BglII.
(vi) pEMH10.
The yefMSpn gene from the S. pneumoniae chromosome was amplified by PCR using primers yefMNHis (5′-GCTCTAGAATGGTTATGGAAGCAGTCCTT-3′) and yefMc (5′-CTGGAATTCCGTTTTGCCAGTAGCAATAATCTGC-3′). The resulting 337-bp PCR fragment was doubly digested with XbaI and EcoRI and inserted into plasmid pET28a digested with NheI (compatible with XbaI) and EcoRI. This construction yielded a His-tagged YefMSpn protein.
(vii) pEMBH13.
A DNA fragment from the pneumococcal chromosome spanning both the yefMSpn and yoeBSpn genes was PCR amplified using primers yefMNHis (5′-GCTCTAGAATGGTTATGGAAGCAGTCCTT-3′) and yoeBCHis (5′-GAACTCGAGGTAATGATCTTTAAAGGACAAG-3′). The resulting 535-bp fragment was digested with XbaI and AvaI and ligated into plasmid pET24b digested with NheI and XhoI. This construction yielded His-tagged pneumococcal toxin-antitoxin proteins.
(viii) pBT-YefMSpn.
A DNA fragment containing the coding sequence of the yefMSpn gene was obtained by PCR using the chromosomal DNA of S. pneumoniae as the template and primers yefM1Spn-F (5′-GAATCCGGTGTATAATAGTGGAAAAGAGCTAAAAC-3′) and yefMSpn-R (5′-CTCGAGTCACTCCTCAATCACATGG-3′). The resulting 262-bp PCR fragment was digested with EcoRI and XhoI and ligated into the equivalent sites of pBT, generating pBT-YefMSpn, which expressed YefMSpn as a fusion protein with λcI at the N terminus.
(ix) pBT-YoeBSpn.
The yoeBSpn gene was amplified by PCR from chromosomal DNA of S. pneumoniae using primers yoeB1Spn-F (5′-GAATCCAATGCTACTCAAGTTTACAG-3′) and yoeBSpn-R (5′-CTCGAGTTAGTAATGATCTTTAAAGG-3′). The resulting 257-bp PCR fragment was digested with EcoRI and XhoI and ligated into similarly digested pBT, yielding pBT-YoeBSpn, which produced YoeBSpn as a fusion protein with λcI at the N erminus.
(x) pTRG-YefMSpn.
The yefMSpn gene from the S. pneumoniae chromosome was amplified by PCR using primers yefM2Spn-F (5′-GAATCCGGGTGTATAATAGTGGAAAAGAGC-3′) and yefMSpn-R (5′-CTCGAGTCACTCCTCAATCACATGG-3′). The resulting 263-bp fragment was digested with EcoRI and XhoI prior to ligation into EcoRI- and XhoI-digested pTRG to generate the pTRG-YefMSpn recombinant, which expressed YefMSpn as an N-terminal fusion protein with the RNA polymerase α subunit (RNAPα).
(xi) pTRG-YoeBSpn.
A DNA fragment encompassing the yoeBSpn gene was PCR amplified from DNA of the S. pneumoniae chromosome using primers yoeB2Spn-F (5′-GAATCCATATGCTACTCAAGTTTACAG-3′) and yoeBSpn-R (5′-CTCGAGTTAGTAATGATCTTTAAAGG-3′). The resulting 258-bp fragment was digested with EcoRI and XhoI and then ligated with similarly digested pTRG to obtain the pTRG-YoeBSpn recombinant, which expressed YoeBSpn as a fusion protein with RNAPα at its N terminus.
Purification of S. pneumoniae His-YefM antitoxin and the YefM-YoeB-His antitoxin-toxin pair.
The pEMH10 vector, containing the coding sequence of yefMSpn, and the pEMBH13 vector, containing the coding sequences of yefM-yoeBSpn, were transformed into E. coli BL21(DE3)(pLysS). Transformed bacteria were grown in 2YT broth at 37°C and 200 rpm to an OD600 of 0.4. Protein expression was induced by addition of 1 mM IPTG at 30°C. After 3 h, cells were harvested and resuspended in buffer A (300 mM NaCl, 20 mM NaH2PO4; pH 8.0) to which 1 ml protease inhibitor cocktail (Sigma) and 0.5 mM phenylmethylsulfonyl fluoride were added. Cells were disrupted by sonication (four 20-s pulses; 0°C; 1-min breaks between pulses). The insoluble material was removed by centrifugation at 4°C and 20,000 × g for 20 min, followed by filtration (pore size, 0.45 μm), and the supernatant was applied to an XK 16/20 fast protein liquid chromatography column (GE Healthcare) packed with Ni-CAM HC affinity resin (Sigma) and preequilibrated with buffer A. Following extensive washing, the bound proteins were eluted from the column in a single broad peak using buffer A containing 500 mM imidazole. Fractions containing the His-YefMSpn protein or the coeluted YoeBSpn-His and YefMSpn proteins were identified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analyses, combined, and dialyzed against phosphate-buffered saline (PBS) (pH 7.4) (10 mM Na2HPO4, 1.8 mM KH2PO4, 140 mM NaCl, 2.7 mM KCl). The identities of the His-YefMSpn protein and the (YefM-YoeB)Spn-His complex were verified by mass spectrometry analysis.
The fractions were then pooled, concentrated, and exchanged with buffer B (50 mM sodium phosphate [pH 7.2], 150 mM NaCl) using a spin column concentrator (Vivaspin). The identity of the expressed His-YefMSpn protein was verified by Western hybridization using anti-His antibodies. Protein concentrations were calculated using extinction coefficients at 280 nm of 1,197 M−1 cm−1 for a single tyrosine and 5,559 M−1 cm−1 for a single tryptophan under neutral conditions, as described by Mihalyi (35). The protein solution was then applied to a HiPrep 16/60 Sephacryl S-100 high-resolution size exclusion column (GE Healthcare) which had a molecular weight (Mr) separation range of 1 × 103 to 1 × 105 and which was preequilibrated with buffer B. Albumin (Mr, 67,000), ovalbumin (Mr, 43,000), chymotrypsinogen A (Mr, 25,000), and RNase A (Mr, 13,700) were used as molecular weight standards to plot a calibration curve for log Mr against mean Kav values, which were determined as follows: Kav = (Ve − V0)/(Vt − V0), where Ve is the elution volume for the target protein, V0 is the column void volume (i.e., elution of Blue Dextran 2000), and Vt is the total bed volume (120 ml for the HiPrep 16/60 Sephacryl S-100 column) (44). The target proteins were eluted from the column in a single peak using buffer B containing 150 mM NaCl, and 0.5-ml fractions were collected. The desired fractions were pooled and concentrated prior to SDS-PAGE analysis. The target protein was then stored at −20°C. For the E. coli YefM-YoeB-His complex, the size exclusion column used was a Superdex 75 10/300 column (GE Healthcare). The column was preequilibrated with PBS (pH 7.3), and the complex was eluted at a flow rate of 1 ml/min at the ambient temperature. Ovalbumin (Mr, 44,000), myoglobin (Mr, 17,000), and vitamin B12 (Mr, 1,350) were used as the molecular weight standards (Bio-Rad) to plot a calibration curve as described above. The column void volume was determined using Blue Dextran 2000, and the total bed volume was 24 ml. The elution spectrum was deconvoluted into Gaussian-shaped peaks, and peaks values were determined using the PeakFit analysis software (Seasolve).
Detection of protein-protein interactions using a bacterial two-hybrid system.
A BacterioMatch II two-hybrid system vector kit (Stratagene) was employed to investigate whether there were any interactions between the YefMSpn antitoxin and its cognate YoeBSpn toxin. This system uses a new HIS3-aadA reporter cassette, and detection of protein-protein interactions is based on transcriptional activation of the HIS3 reporter gene, which allows growth in the presence of 3-amino-1,2,4-triazole (3-AT), a competitive inhibitor of the His3 enzyme. The DNA fragments encoding the bait proteins (yefMSpn as well as yoeBSpn) were amplified and cloned in frame with the λcI repressor of the pBT bait vector via the EcoRI and XhoI restriction sites. The corresponding DNA fragments that encoded the target proteins (yoeBSpn and yefMSpn) were similarly cloned in frame with the RNAPα reading frame of the pTRG target vector via the EcoRI and XhoI sites. Each of the recombinant bait and target pairs (i.e., the pBT-yefMSpn-pTRG-yoeBSpn pair or the pBT-yoeBSpn-pTRG-yefMSpn pair) was cotransformed into the E. coli reporter strain provided by the supplier. A positive interaction between the λcI fusion protein produced by the recombinant pBT plasmid and the RNAPα fusion protein produced by the recombinant pTRG plasmid resulted in growth of the reporter strain cotransformants on the selective medium containing 5 mM 3-AT.
Circular dichroism.
CD spectra were obtained using an AVIV 202 spectropolarimeter equipped with a temperature-controlled sample holder and a 10-mm-path-length cuvette. The mean residual ellipticity ([θ]) was calculated as follows: [θ] = (100 × θ × m)/(c × L), where θ is the observed ellipticity, m is the mean residual weight, c is the concentration (in mg/ml), and L is the path length (in cm). All experiments were performed in PBS (pH 7.3) using a protein concentration of 2 μM. Prior to analysis, all protein samples were dialyzed against PBS (pH 7.3) at 4°C and centrifuged for 10 min at 12,000 × g to remove insoluble protein aggregates. The protein concentration used was approximately 2 μM. Evaluation of the secondary structure composition based on the far-UV CD spectra obtained was facilitated by use of the Selcon3, ContiLL, and CDsstr programs (25, 41, 45) included in the CDpro software package (46) and the K2d program (2) on the K2d server (http://www.embl-heidelberg.de/∼andrade/k2d/).
Model construction.
Three-dimensional models for the sequences of YefMSpn, YoeBSpn, Axe, and Txe were constructed using a knowledge-based protein modeling method based on the given pairwise sequence-template alignments for the sequences extracted from the multiple alignments of members of the YefM and YoeB protein families (5) and the 2.05-Å-resolution X-ray crystallographic structure of the E. coli YefM-YoeB heterotrimeric complex as the template (PDB code 2a6q) (26). The homology modeling program Nest (40) was used to build and refine by energy minimization the final structures of the models. Computations were carried out using an SGI Octane R10000 workstation. Calculations and surface mapping of the electrostatic potentials for all the structures, as well as the graphic display, were performed with the Swiss-PdbViewer v3.7 computer program (23). Potentials were computed using a Poisson-Boltzmann interaction, with the protein structure simulated at pH 7.0, with the default protonation state for all residues, and taking into account only charged residues (i.e., Arg, Lys, Glu, and Asp). For superposition of each model on the structure of the corresponding toxin and antitoxin templates (E. coli YoeB and YefM, respectively) in order to perform an accurate comparative analysis of the surface electrostatic potentials, we used a combinatorial extension algorithm for calculating each pairwise structure alignment (43).
RESULTS
Chromosomal location of the yefM-yoeBSpn operon.
Genome analyses showed that the two sequenced pneumococcal strains, TIGR4 and R6, harbor homologs of the E. coli yefM-yoeB and E. faecium plasmid pRUM-encoded axe-txe TA systems (5, 22, 37). By PCR amplification of chromosomal DNA of the R6 pneumococcal strain, we were able to clone the region spanning the yefM-yoeB locus in a pneumococcal plasmid vector and show that (i) the genes constitute an operon and (ii) the toxin was able to stop the growth of pneumococcal cells (unpublished results). The antitoxins exhibited more sequence divergence than the toxins; YefMEco exhibited 24% identity with YefMSpn and 27% identity with Axe. For the toxin interaction domains, the levels of similarity were higher, 67% for YefMSpn and YefMEco and 56% for Axe and YefMEco.
Overproduction of YoeBSpn inhibits cell growth and colony formation in different strains of E. coli.
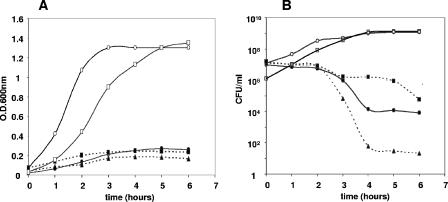
The yoeBSpn gene was amplified by PCR from the pneumococcal chromosome and cloned in a plasmid vector, pFUS2 (31), which placed the toxin gene under control of the arabinose-inducible PBAD promoter of the araBAD operon and the araC gene for its transcriptional activator. Plasmid pYBS2, harboring the pneumococcal toxin gene, was transferred into E. coli strain BL21, MG1655, or TOP-10, and transformants were selected in TY medium plates containing 0.4% glucose to minimize toxin expression. When the toxicity of YoeBSpn was tested, normal cell growth was observed when the E. coli cells were grown in glucose-containing medium (Fig. 1A) (note that for these conditions only the results for strain MG1655 are shown) or when antitoxin expression was induced by addition of IPTG in E. coli cells containing plasmid pYFS2 (yefMSpn). However, total arrest of cell growth and a great reduction in the number of viable cells were observed for the three E. coli strains (BL21, MG1655, and TOP-10) harboring pYBS2 when the cultures were shifted to arabinose-containing medium. The reduction in the number of CFU depended on the strain tested, but at the end of the 6-h exposure to YoeBSpn, the differences between the viability and the viability of the control cultures decreased about 8 and 4 to 5 logarithmic units for BL21 and the other two strains, respectively (Fig. 1B), demonstrating that YoeBSpn is a potent toxin against E. coli cells. Furthermore, when the pneumococcal yefM-yoeBSpn genes were cloned into a segregationally unstable mini-F replicon and the rate of plasmid loss was calculated, it was apparent that there was an increase in plasmid stability (not shown).
FIG. 1.
Effect of yoeBSpn expression in E. coli strains. (A) Cell growth arrest after induction of yoeBSpn expression in strains MG1655 (○ and •), TOP-10 (▪), and BL21 (▴) harboring pYBS2 (yoeBSpn). Cells were grown exponentially in medium containing 0.2% glucose (repression conditions) and kanamycin to an OD600 of 0.03 to 0.04. Cultures were divided in two. One half of each culture was grown in the presence of glucose (open symbols; for clarity, only the data for strain MG1655 are shown), and the other half was induced by addition of 0.2% arabinose (solid symbols). Growth was monitored by determining the OD600 of the cultures. (B) At the times indicated, the numbers of CFU in the cultures were determined by plating appropriate dilutions on TY agar supplemented with 0.4% glucose and kanamycin and incubating the preparations overnight at 37°C. Neither cell growth arrest nor a reduction in the number of CFU was observed for E. coli TOP-10 cultures containing the pYFS2 plasmid (YefMSpn) (□) after induction of antitoxin synthesis by addition of 2 mM IPTG. This culture was grown and plated as described above but using ampicillin instead of kanamycin for selection. All experiments were performed at least in duplicate.
Cytotoxic effect of YoeBSpn is alleviated only by its cognate antitoxin, YefMSpn.
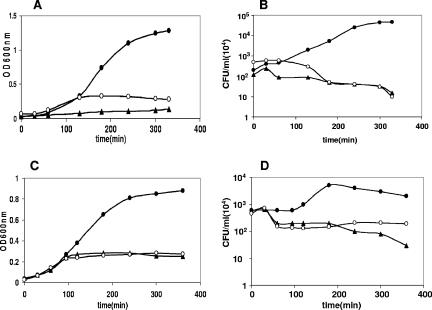
In addition to the chromosomal yefM-yoeB of E. coli (5-7), another yefM-yoeB analog, axe-txe, has been studied in some detail (22). Overproduction of the Txe toxin in E. coli severely impaired bacterial growth, but no reduction in the number of viable cells was observed. The toxic effect of the Txe toxin was alleviated by expression of the cognate antitoxin gene (axe) or was partially alleviated by expression of the heterologous E. coli yefM gene. Likewise, synthesis of the Axe antitoxin alleviated the toxic effect of YoeBEco, showing the capacity of the two TA systems to interact with each other in vivo (22). When a similar experiment was performed with the pneumococcal yefM-yoeB TA, it was apparent that overproduction of YoeBSpn or YoeBEco in E. coli cultures not only resulted in growth inhibition but also led to a reduction in the number of viable cells (Fig. 2). It is interesting that after 6 h of YoeBEco synthesis about 27% of the bacteria were still able to form colonies (Fig. 2D). This could have been due to neutralization of the toxin by the endogenous levels of the antitoxin encoded by the E. coli chromosomal copy of the yefM-yoeB locus, in agreement with previous results (7, 36). To test whether there was cross-complementation between yoeBSpn and yefMEco, E. coli TOP-10 cells were transformed with DNA of plasmids pYBS2 (yoeBSpn) and pYFS10 (yefMSpn) or with DNA of plasmids pYBS2 (yoeBSpn) and pNMYE (yefMEco). Whereas synthesis of the pneumococcal toxin was inducible by arabinose, the genes encoding the antitoxins (either the pneumococcal antitoxin or the E. coli antitoxin) were inducible by IPTG. E. coli cultures containing only pYBS2 (yoeBSpn) were used as toxicity controls. Coinduction of both the pneumococcal yefM and yoeB genes (by addition of arabinose and IPTG) resulted in a normal rate of cell growth (Fig. 2A), indicating that the toxic effect of YoeBSpn was neutralized by coexpression of the yefMSpn antitoxin gene. However, coinduction of yoeBSpn and the noncognate yefMEco antitoxin gene did not neutralize the YoeBSpn toxicity, and the reduction in the number of CFU was similar to that observed after arabinose addition in control cultures harboring only plasmid pYBS2 (yoeBSpn) (Fig. 2A and B). To determine whether the toxicity of E. coli YoeB could be counterbalanced by coexpression of the pneumococcal antitoxin, a similar approach was used with E. coli cells that harbored different pairs of plasmids, either pFYBE (yoeBEco) and pNMYE (yefMEco) or pFYBE (yoeBEco) and pYFS10 (yefMSpn). E. coli cells harboring only plasmid pFYBE (yoeBEco) were used as a toxicity control. The results (Fig. 2C and D) confirmed that as in the previous experiment, there was no interaction between the noncognate toxin and the antitoxin proteins.
FIG. 2.
Toxicity of YoeBSpn is neutralized only by combined expression of the cognate antitoxin in E. coli. (A and B) E. coli TOP-10 strains harboring pYBS2 (yoeBSpn) (○), pYBS2 (yoeBSpn), pYFS10 (yefMSpn) (•), or pYBS2 (yoeBSpn) and pNMYE (yefMEco) (▴) were grown exponentially in TY medium containing 0.2% glucose, kanamycin, and ampicillin to an OD600 of 0.03 to 0.04. Then 0.2% arabinose (toxin synthesis induction) and 1 mM IPTG (antitoxin synthesis induction) were added. (A) Absorbance at 600 nm was used to monitor the growth of the cultures. (B) At the times indicated, samples were diluted and plated on TY agar containing 0.4% glucose, kanamycin, and ampicillin and incubated overnight at 37°C. (C and D) E. coli TOP-10 strains harboring pFYBE (yoeBEco) (○), pFYBE (yoeBEco) and pNMYE (yefMEco) (•), or pFYBE (yoeBEco) and pYFS10 (yefMSpn) (▴) were grown and treated as described above. (C) Absorbance at 600 nm was used to monitor the growth of the cultures. (D) At the times indicated, samples were diluted and plated on TY agar containing 0.4% glucose, kanamycin, and ampicillin and incubated overnight at 37°C. All the experiments were performed at least in duplicate.
Recovery of cell viability after induction of YefMSpn antitoxin synthesis in cultures treated with the YoeBSpn toxin.
To determine whether the toxicity of the YoeBSpn pneumococcal toxin in E. coli could be eliminated by the cognate antitoxin YefMSpn, E. coli cells harboring plasmids pYBS2 (yoeBSpn) and pYFS10 (yefMSpn) were grown in the presence of 0.2% arabinose to induce toxin synthesis. Bacterial growth was measured by monitoring the optical densities of the cultures, and the number of CFU was determined by plating samples on glucose-containing medium (repressed conditions for the toxin) supplemented or not supplemented with IPTG (to induce antitoxin synthesis). The number of CFU was plotted as a function of the time of toxin induction, and the results indicated the fraction of surviving cells (Fig. 3). It was apparent that the number of viable cells decreased after induction of toxin expression, although the reduction was more dramatic when there was no induction of the antitoxin (plates without IPTG). After 6 h of toxin overproduction, plates with IPTG contained 3.5% of the original number of E. coli CFU, compared with the 0.3% survivors when the cells were grown in IPTG-free plates.
FIG. 3.
Recovery of cell viability due to antitoxin synthesis after overproduction of YoeBSpn. E. coli TOP-10 cells harboring plasmids pYBS2 (yoeBSpn) and pYFS10 (yefMSpn) were grown in TY medium without glucose containing kanamycin and ampicillin. When the culture reached an OD600 of 0.13, 0.2% arabinose was added. Absorbance data were obtained for 7 h after induction of toxin synthesis (inset). At different times, samples were plated on TY agar containing 0.4% glucose, kanamycin, and ampicillin supplemented (•) or not supplemented (○) with IPTG.
Effect of YoeBSpn overproduction in lon+ and lon E. coli strains.
Overproduction of the Lon protease in E. coli triggers bacterial growth inhibition mediated by YoeBEco (7). Furthermore, indirect results obtained with a strain in which lon was deleted suggested that this strain was less sensitive to YoeBEco overproduction, indicating that antitoxin degradation could be involved in Lon-dependent YoeBEco-mediated lethality (7). For pneumococcal YoeBSpn, overproduction in E. coli resulted in a reduction in bacterial growth, and this effect could not be alleviated by expression of the host yefMEco gene (Fig. 2). In addition, when E. coli strain SC36, which lacked the yefM-yoeB locus (7), was tested to determine the toxicity of pneumococcal YoeBSpn, we found no differences between this strain and strain MG1655, which is wild type for the yefM-yoeB locus (not shown). Both sets of results suggested that degradation of the YefMEco host antitoxin by the Lon protease was not necessary for YoeBSpn activity. To test directly whether the Lon protease has a role in YoeBSpn toxicity in E. coli, two isogenic strains that were defective and not defective for the lon gene (MG1655 and MG1655lon, respectively) were transformed with the yoeBSpn-containing plasmid pYBS2 (yoeBSpn). Cells were treated identically by growing them under toxin induction conditions (with arabinose), and the effect of pneumococcal toxin overproduction was determined by measuring cell growth and CFU counting. The results showed that synthesis of the pneumococcal toxins led to growth arrest in both strains (Fig. 4A), indicating that inhibition of growth by YoeBSpn does not seem to require lon expression. However, plotting the percentages of surviving cells of the lon+ and lon strains showed that there were differences between the Lon-proficient strain and the mutant. After 1 h of toxin synthesis, about 85% of the lon mutant bacteria were able to form colonies, but only 25% of the wild-type bacteria were able to form colonies (Fig. 4B). This result could be explained by a direct effect of the lon mutation, although at present we cannot rule out the possibilities that the two strains had different growth rates and that there were different levels of expression in the two strains. However, these possibilities seem unlikely since (i) the two strains had similar doubling times and at time zero the numbers of CFU were almost identical for the two strains (around 107 CFU/ml) and (ii) expression of the relE2Spn gene (encoding the pneumococcal toxin RelE2Spn), using the same expression vector, resulted in up to a 5% reduction in the number of viable cells of a lon strain after 1 h of toxin overproduction (36).
FIG. 4.
Differences in YoeBSpn and RelE2Spn toxicity in E. coli lon+ and lon strains. (A) Inhibition of cell growth by overproduction of the pneumococcal toxins YoeBSpn (○ and •) and RelE2Spn (▴ and ▵) in E. coli strain MG1655 (lon+) (solid symbols) or in the lon isogenic mutant MG1655lon (open symbols). E. coli MG1655 (• and ▴) or MG1655lon (○) was grown in TY medium supplemented with kanamycin and 0.2% glucose (▪) or arabinose (•, ○, ▴, and ▵). Absorbance data were obtained at different times after the addition of arabinose. (B) To determine the number of CFU, samples were removed at various times and spread on TY agar plates containing 0.4% glucose and kanamycin. The percentages of viable cells were calculated by comparison of the numbers of CFU in the induced cultures to the numbers of CFU in the noninduced cultures at time zero.
Purification of YefMSpn and of the (YefM-YoeB)Spn complex.
A DNA fragment spanning the pneumococcal region that included either the (yefM-yoeB)Spn genes or only the yefMSpn gene was cloned in two different pET vectors (see Materials and Methods). The pneumococcal genes were placed under control of promoter φ10 of phage T7 (47), so IPTG induction led to synthesis of a His-tagged toxin-antitoxin complex or only a His-tagged antitoxin. The YefMSpn antitoxin (9,953 Da) coprecipitated with the His-tagged YoeBSpn toxin (11,467 Da) and eluted in a single broad peak (Fig. 5A). The proteinaceous yield of the purified complex was relatively high, as a considerable amount of complex (>15 mg) could be obtained from 2 liters of a bacterial culture. Compared to the yield of the complex, the proteinaceous yield of the His-tagged YefMSpn antitoxin (12,457 Da) was lower (Fig. 5B). This was unexpected since the YefMEco antitoxin exhibited high solubility on the one hand and should not have provoked transcriptional or translational inhibition on the other hand, which may have led to such poor yields. However, we speculated that susceptibility of the antitoxin to proteases (due to partial unfolding [see below]) without an available cognate partner to stabilize it could have been the reason for the results observed.
FIG. 5.
Purification of (YefM-YoeB-His)Spn complex and His-YefMSpn antitoxin and indications of YefM-YoeBSpn interaction. (A and B) Chromatograms showing the elution pattern of proteins from the nickel affinity column. Note that although the yield of the YefMSpn protein (B) was poor, an increase in the absorbance was evident. However, no peak was observed, most likely because of the small amount of protein which was masked by the increase in the imidazole background absorbance. The results of SDS-PAGE analyses of eluted fractions are shown in the insets. RT, retention time; mAu, milli-absorbance units. (C) Elution profile of the YefM-YoeBSpn protein complex from a 16/60 Sephacryl S-100 HR gel filtration column, showing a void volume of 45.89. The 45.89-ml peak with a Kav value of 0.1351 corresponds to an Mr of approximately 49,500. (D) E. coli two-hybrid indicator strain (Stratagene) (derived from XL-1 Blue MRF′) harboring plasmids pBT-LGF2 and pTRG-Gal11P (positive control) (streak 1), pBT-yefMSpn and pTRG (streak 2), pBT and pTRG-yoeBSpn (streak 3), pBT and pTRG-Gal11P (streak 4), pBT-yefMSpn and pTRG-yoeBSpn (streak 5), or pBT-yoeBSpn and pTRG-yefMSpn (streak 6) was streaked on selective medium containing 3-AT and incubated overnight at 37°C. Cotransformants in streaks 2, 3, and 4 were used as negative controls.
Analysis of YefMSpn and YoeBSpn interactions.
Crude lysates of E. coli cells overproducing the pneumococcal toxin and antitoxin were loaded onto a nickel affinity column under native conditions. Two distinct protein bands were observed during an SDS-PAGE analysis following elution with 500 mM imidazole, and the positions of these bands corresponded to the expected sizes of YefMSpn and YoeB-HisSpn, indicating that under native conditions the two proteins copurified (Fig. 5A). This, in turn, suggests that the YefMSpn protein may have formed a tight complex with its toxin counterpart. This inference was validated when the purified proteins were subjected to size exclusion chromatography. A single peak at 49.5 kDa was detected, suggesting that YefMSpn and YoeBSpn generated a protein complex with an apparent stoichiometry of (YefMSpn)2(YoeBSpn)2 (Fig. 5C). This finding agrees with the 2:2 stoichiometry observed for the E. coli YefM-YoeB complex based on size exclusion chromatography of approximately 0.2 mg/ml of the YefM-YoeB-His complex, although 2:1 and 2:4 stoichiometries were also observed, but with fewer occurrences (I. Cherny and E. Gazit, unpublished). In the case of the Kis-Kid proteins, 1:2, 2:2, and 2:1 stoichiometries were also found, although the results depended on the relative protein concentrations (27). Subsequent Western hybridization using an anti-His6 antibody confirmed the identity of the protein.
To detect whether the two proteins interacted in vivo, a two-hybrid system was employed. To this end, the yefMSpn and yoeBSpn reading frames were cloned into both the pBT bait and pTRG target vectors for the two-hybrid assay as described in Materials and Methods. Positive protein-protein interactions, which were indicated by growth of the indicator strain harboring both the bait and target recombinants on the selection medium containing 5 mM 3-AT, were detected for both the positive control pBT-LGF2 and pTRG-Gal11P (Fig. 5D, streak 1) and for the pBT-yoeBSpn-pTRG-yefMSpn pair (Fig. 5D, streak 6). However, when the interaction pair was changed (i.e., pBT-yefMSpn and pTRG-yoeBSpn instead of pBT-yoeBSpn and pTRG-yefMSpn), there was sparser growth of the indicator strain harboring both plasmids on the 3-AT-containing selective medium (Fig. 5D, streak 5).
Far-UV CD analyses of YefMSpn and the (YefM-YoeB)Spn complex.
The CD spectra of His-YefMSpn at 4°C, 37°C, and 60°C included two negative bands at 217 nm and 208 nm (Fig. 6A). The first minimum corresponded to the presence of beta structures, whereas the second minimum, which was significantly lower, could be attributed to the presence of both α-helices and random coil conformations. Since the presence of α-helices contributed to the CD signals at both 208 nm and 222 nm (although it contributed more to the latter), the major negative band at 208 nm should be ascribed to the presence of considerable amounts of unstructured or flexible conformations. This is in agreement with the calculations for the secondary structure content using different deconvolution programs (Table 1), which estimated that 55 to 57% of the YefMSpn conformations were unordered at 4°C. Intriguingly, despite having a large disordered content, the YefMSpn antitoxin did not exhibit complete unfolding at elevated temperatures, even at 90°C (data not shown), like YefM or Phd homologues antitoxins exhibit (5, 18). The CD spectrum of the YefM-YoeB-HisSpn complex (Fig. 6B) at 4°C is compatible with an elevated occurrence of α-helices (47% α-helices, 10% β-strands, and 43% coils) (Table 1). With a temperature increase, part of the helical structures was lost in favor of β structures and, to some extent, in favor of an unordered conformation, as the CD minimum signals were weakened at both 221 and 208 nm and shifted to 218 and 207 nm.
FIG. 6.
Far-UV CD spectra of His-YefMSpn (A) and (YefM-YoeB-His)Spn complex (B) at 4°C (solid line), 37°C (dashed line), and 60°C (dotted line). (C) CD ellipticity of His-YefMSpn (▴) and (YefM-YoeB-His)Spn (○) was measured at 220 nm at various temperatures to estimate the thermal stability. The values were normalized between 0 and 1 and are expressed as fractional changes. (D) SDS-PAGE analysis of (YefM-YoeB-His)Spn sample that was examined by CD analysis before and after exposure to 85°C. Lane M, marker; lane 1, before treatment (sample at room temperature); lane 2, after treatment (soluble fraction after exposure to 85°C).
TABLE 1.
Secondary structure contents determined from CD spectra
| Protein(s) | Methoda | % in:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| α-Helices
|
β-Strands
|
Coilsb
|
|||||||||||
| 4°C | 37°C | 60°C | 65°C | 4°C | 37°C | 60°C | 65°C | 4°C | 37°C | 60°C | 65°C | ||
| YefM | K2d | 26 | 27 | 25 | 17 | 21 | 20 | 57 | 52 | 55 | |||
| Selcon3 | 26 | 24 | 19 | 19 | 20 | 23 | 55 | 56 | 58 | ||||
| ContinLL | 27 | 25 | 20 | 18 | 20 | 22 | 55 | 55 | 58 | ||||
| CDsstr | 25 | 23 | 17 | 19 | 19 | 24 | 56 | 58 | 59 | ||||
| Avg | 26 | 24.75 | 20.25 | 18.25 | 20 | 22.25 | 55.75 | 55.25 | 57.5 | ||||
| YefM-YoeB | K2d | 55 | 37 | 28 | 9 | 15 | 15 | 36 | 50 | 57 | |||
| Selcon3 | 50 | 32 | 30 | 8 | 13 | 14 | 42 | 55 | 56 | ||||
| ContinLL | 38 | 39 | 34 | 6 | 11 | 14 | 56 | 50 | 52 | ||||
| CDsstr | 45 | 42 | 38 | 17 | 15 | 24 | 38 | 43 | 38 | ||||
| Avg | 47 | 37.5 | 32.5 | 10 | 13.5 | 16.75 | 43 | 49.5 | 50.75 | ||||
The thermal stabilities of the antitoxin and the complex were estimated by monitoring the ellipticity at 220 nm (Fig. 6C) at various temperatures between 4 and 90°C. Both the antitoxin and the complex seemed to undergo unfolding when the temperature increased. However, two noteworthy differences were observed: (i) the unfolding rate of the antitoxin was higher than that of the complex (the difference in the melting temperatures was greater than 10°C, as estimated from the inflection points of the two melting curves), and (ii) following thermal melting the sample containing the complex became turbid, indicating that YoeB-HisSpn aggregation occurred during the melting. SDS-PAGE analysis of the soluble fraction which remained after heating (Fig. 6D) showed that the aggregation was predominantly related to the toxin, while the antitoxin remained soluble.
Molecular modeling of YoeB-YefM-like protein structures.
To shed some light on the structural basis of the interactions between members of the YefM and YoeB families of proteins, we constructed structural models for YefMSpn, YoeBSpn, Axe, and Txe (Fig. 7), based on the previously determined crystal structure of the E. coli YefM2-YoeB heterotrimeric complex (26). Because in this complex the C-terminal segment of the YefM monomer, which docks mainly with YoeB, folds into an ordered structure, we used this major monomer as the antitoxin template (YefM-ordered monomer). In spite of an intricate atomic interaction network supporting the E. coli YefM2-YoeB complex, recognition of the YoeBEco monomer is due to two sites of the YefMEco-ordered monomer; one site is composed of the H3 and H4 helices, and the other site is composed of an extended β-strand (26). For the toxin models, we used the YoeBEco monomer that belongs to the complex (YefM-bound YoeB). The levels of pairwise sequence identity for the alignments between the YoeB-like proteins (YoeBSpn and Txe) and the template (51% and 52%, respectively) made it possible to construct reliable homology models for the YoeBSpn and Txe toxins. On the other hand, the levels of pairwise sequence identity for the alignments between the YefM-like proteins (YefMSpn and Axe) and the template (24% and 27%, respectively) are in the so-called “twilight zone” for reliable homology modeling, although the levels of sequence similarity for the residues encompassing the two sites for recognition of YoeB by YefM are 67% and 56%, respectively. Additionally, the intrinsic qualities of the YefMSpn and Axe models were probed and confirmed by using several specific programs (WHAT_CHECK, PROCHECK, and Verify 3D). Thus, the aim of this modeling study was to perform a comparative analysis of some structural features of the interaction domains of the YefM-like and YoeB-like proteins.
FIG. 7.
Surface electrostatic potentials of the interaction domains of the antitoxins YefMEco (A), YefMSpn model (C), and Axe model (E) with their cognate YoeB toxins and surface electrostatic potentials of the toxins YoeBEco (B), YoeBSpn model (D), and Txe model (F). All the surfaces are oriented facing the interacting side and in equivalent positions (by superposition with the CE algorithm). Positive potentials are blue, and negative potentials are red.
With all the considerations described above, we focused on the interaction domain of YefM-YoeB, and we performed an analysis of the surface electrostatic potentials of the structural models. The positively charged hindrance defined by the YefMEco R72 residue in the neutral H4 helix was shown to be the main part of the electrostatic interaction with the negatively charged pocket on the interface of YoeBEco (26); the equivalent residue is K64 in the pneumococcal antitoxin. This positively charged moiety seems to be also exposed in the Axe model but not in the YefMSpn model (Fig. 7A, B, and C), and there are no other apparent differences between the surface electrostatic potentials of the antitoxins. The change in the distribution of charges in the residues that configure this region in the pneumococcal antitoxin could indicate that single amino acid substitutions may not necessarily lead to a total loss of antitoxin activity. The lack of this positively charged patch in the YefMSpn antitoxin could explain its failure to neutralize the heterologous YoeBEco toxic effect; in addition, it suggests that interaction of the pneumococcal TA could involve other residues. A detailed mutational analysis of this region should clarify this possibility. When the models of the toxins were examined, an analysis of the surface electrostatic potentials showed that there were no significant differences (Fig. 7D, E, and F). However, a quantitative analysis of the solvation energies of the surface areas calculated with the program GETAREA (http://www.scsb.utmb.edu/getarea/) proved that the YoeBSpn structural model has a larger exposed surface (7% larger polar area) than the other two toxins as a result of 9 surface atoms more than the YoeBEco-ordered monomer and 18 buried atoms less than the YoeBEco-ordered monomer. This structural feature of YoeBSpn increases the potential interaction surface and influences a wider range of protein interactions, leading to a likely explanation for the ineffectiveness of the YefMEco antitoxin for counteracting the toxic effect of the pneumococcal toxin. Definite insights into the structural interface of the pneumococcal YefM-YoeB complex, however, await X-ray crystallographic resolution data for this TA system.
DISCUSSION
The yefM-yoeBSpn locus has been identified as a new chromosomal TA system of S. pneumoniae. Overproduction of the pneumococcal YoeBSpn toxin in E. coli cells resulted in cytotoxic effects commonly linked to toxin activity. However, the size of the negative effect mediated by YoeBSpn depended on the strain used; whereas in the K-12 strains used, TOP-10 and MG1655, the reductions in the number of CFU were more than 104- to 105-fold, respectively, in the E. coli B strain (BL21) the reductions in the number of CFU were more than 108-fold (Fig. 1). This influence of the type of strain used on the YoeB toxicity could explain the previous inability to obtain transformants from plasmid DNA harboring the yoeBEco gene in an E. coli B strain (26). The differences in the reduction in the number of viable cells for the E. coli strains used could have been due to different levels of expression of yoeBSpn or to different sensitivities of the genetic backgrounds. The yefM-yoeBSpn cassette functions as an antitoxin-toxin module both in S. pneumoniae (unpublished results) and in E. coli (Fig. 1 to 4), and it seems likely that degradation of the pneumococcal antitoxin by a Lon-type protease could trigger the YoeBSpn toxic activity in S. pneumoniae. In the case of E. coli, it has been shown that Lon overproduction stimulates the toxic activity of YoeBEco and that a Δlon strain is less sensitive to YoeBEco overproduction than a lon+ strain is, indicating that the Lon protease could be responsible for antitoxin degradation and the subsequent activation of YoeBEco (7). Our results (Fig. 4) suggested that the activity of YoeBSpn did not require degradation of the E. coli antitoxin. We hypothesized that Lon could mediate the toxicity of YoeB-like proteins by antitoxin degradation and/or by an antitoxin degradation-independent mechanism.
Overproduction of YoeBSpn resulted in cessation of cell growth and a substantial decrease in the number of CFU, which then increased gradually as the time of toxin synthesis increased (Fig. 1 and 2). The reduction in cell viability was alleviated by antitoxin expression for almost 2 h, so that at this time the number of CFU was reduced only 60%. After 4 h of exposure to the toxin, neutralization by the antitoxin resulted in 4% viability, compared to the 0.4% viability in the absence of antitoxin synthesis (Fig. 3). The YoeBSpn toxin could inhibit protein synthesis by mRNA degradation like its E. coli homolog (7, 26). Overproduction of the pneumococcal toxin for extended periods of time could lead to very low levels of translation, thus favoring the senescence processes and finally leading to cell death. We believe that the function of the YoeBSpn toxin is to promote cell growth arrest under stress conditions; in a physiological situation, the amount of free toxin could be enough to reduce cell growth but permit a residual level of protein synthesis, so that the cells could be protected from irreversible damage and the metabolism could adapt to the unfavorable conditions.
The toxic effect of YoeBSpn could be neutralized by its cognate antitoxin, YefMSpn, but not by the E. coli counterpart (Fig. 2). This behavior is different from that of the YoeBEco toxin, whose activity was counteracted by both the cognate YefMEco antitoxin and antitoxin Axe encoded by the axe-txe locus of plasmid pRUM (22). The lack of cross-complementation suggested that there is not a favorable interaction between the two heterologous proteins, namely, YoeBSpn and YefMEco. Far-UV CD analysis of the YefMSpn protein and of the (YefM-YoeB)Spn complex indicated that unlike YefMEco, YefMSpn does not seem to be an unfolded protein in its native state, although this does not necessarily mean that is a well-structured protein. It may include exposed regions that are not available for proteases in its bound form (in a complex with the toxin). This hypothesis is supported both by its lower thermal stability (melting temperature, ∼45°C) compared to that of the complex (melting temperature, ∼70°C) and by its resistance to heat (at least 85°C) (Fig. 6D). The latter property also suggests that YefMSpn lacks a significant hydrophobic core, which in turn may be important to keep it proteolytically unstable. The difference between the YefM proteins was also detectable in the analysis of their amino acid sequences; alignment of the proteins revealed that their C-terminal portions (the region that binds the YoeB toxin) are different and cannot be aligned.
The fact that there is a considerable unstructured region in YefMEco may explain the structural difference between the two proteins and may also explain the lack of complementation between the toxins and the heterologous antitoxins, which was apparent from the molecular modeling of the proteins (Fig. 7). This in turn may indicate that the specific interactions of a toxin with its cognate antitoxin could be related to the mechanism of survival in the different niches that the bacteria colonize (e.g., the gut for E. coli and the nasopharynx for pneumococci). In the case of strain R6 of S. pneumoniae (24), two different functional TA loci, namely relBE2Spn and yefM-yoeBSpn, the residual nonfunctional relBE1Spn genes (36), and a putative homolog of omega-epsilon-zeta (13; J. C. Alonso, personal communication) are present in the chromosome. In the case of virulent strain TIGR4 (48), in addition to these three loci, there are two more putative TA loci (37), one locus homologous to P1 phage phd/doc (30) and the other homologous to Rts1 plasmid higBA (49). YoeB and RelE toxins have similar toxic activities, but they have different mRNA cleavage patterns and sets of features that could enable the two TA systems to play different roles. First, RelE cleaves at UAG, UAA, UGA, UCG, and CAG sequences with preference for the stop codon UAG (39), and a similar pattern has been observed for pneumococcal RelE2Spn (9); cleavage of mRNA by YoeBEco occurs predominantly at the 3′ end of adenine or guanine residues (26). Second, RelE-dependent cleavage occurs only in the ribosome (39), whereas YoeBEco has an intrinsic RNase activity and is able to cleave RNA in the absence of ribosomes (26). Third, structure-based comparisons (26) revealed that the residues involved in the RNase activity of YoeBEco (D46, H83, and Y84) are not conserved in RelE, with the exception of R65, suggesting that RelE could be an incomplete RNase which lacks the essential residues for catalytic activity. Finally, the signal that triggers mRNA cleavage could be different, since RelE activity in E. coli is induced by amino acid starvation (10), whereas the Lon protease triggers YoeBEco-dependent RNA cleavage (7). Thus, either TA systems could act through independent mechanisms or they could work together under stress conditions (i.e., amino acid starvation), as has recently been suggested for RelE and MazF (12). The combined action of the two toxins with RNase activity (namely, YoeBEco with free mRNA and RelE with mRNA bound to ribosomes) could lead to significant depletion of mRNA and an effective, but not total, block of translation, leaving low levels of antitoxin synthesis which could result in toxin-mediated arrest of cell growth but not in induction of a bacterial death response.
Even though TA loci appear to be nonessential, it is curious that in many bacterial species more than one TA locus is conserved in the chromosome and in plasmids. The role of the TA systems in the extrachromosomal elements is related to stable inheritance, or the TA systems may be triggered when a plasmid cannot replicate (4, 14). Furthermore, all E. coli TA loci can be deleted without causing significant growth differences in the wild-type strains (7, 8, 36, 38). However, antibiotics that inhibit transcription and/or translation in E. coli and induce mazEF-dependent cell death do not reduce cell viability in mazEF mutants (42). Mutations affecting both MazF and RelE activities in Streptococcus mutans resulted in cells whose acid tolerance and growth in glucose-based medium were affected (32). In the case of Neisseria gonorrhoeae workers have identified a mutant with a mutation in a putative TA system (designated FitAB for fast intracellular trafficking; function unknown) which grows normally extracellularly but has a higher rate of intracellular replication, and there is a concomitant increase in the rate at which this mutant traverses a monolayer of polarized epithelial cells (34).
Taking into account the complexity of the “biological niches” of S. pneumoniae and other pathogenic bacteria, it is very simplistic to attempt to ascertain the function of the TA system when cells are growing in a culture medium. Bacteria grow in communities with other living cells and surely compete with each other for the surrounding nutrients. In these bacterial environments the levels of nutrients are not as high as those used in laboratory conditions. For S. pneumoniae, the mucosal epithelium of the nasopharynx is the primary site of colonization, where it does not cause any symptoms of illness. Occasionally, bacteria can switch from colonization to infection in the lungs, blood, and other tissues, and many times they traverse the blood-brain barrier and infect the meninges. Consequently, S. pneumoniae has to adapt to different situations, and perhaps in these different situations the TA systems play an important role in survival when the bacteria are exposed to host defenses.
Acknowledgments
We thank K. Gerdes and L. van Melderen for providing bacterial strains and M. T. Alda for technical help.
This research was financed by grant BFU2004-00687/BMC to M.E. and by a SAGA/MUST/MZA-YCC/1 grant from the Malaysian Academy of Sciences to C.C.Y.
Footnotes
Published ahead of print on 27 October 2006.
Dedicated to Martine Thilly-Couturier, a retired pioneer in toxins-antitoxins and a continuous friend.
REFERENCES
- 1.Aizenman, E., H. Engelberg-Kulka, and G. Glaser. 1996. An Escherichia coli chromosomal “addiction module” regulated by guanosine 3′,5′-bispyrophosphate: a model for programmed bacterial cell death. Proc. Natl. Acad. Sci. USA 93:6059-6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrade, M. A., P. Chacón, J. J. Merolo, and F. Morán. 1993. Evaluation of secondary structure of proteins from UV circular dichroism spectra using an unsupervised learning neural network. Protein Eng. 6:443-454. [DOI] [PubMed] [Google Scholar]
- 3.Baquero, F. 1996. Epidemiology and management of penicillin-resistant pneumococci. Curr. Opin. Infect. Dis. 9:372-379. [Google Scholar]
- 4.Bravo, A., G. de Torrontegui, and R. Díaz. 1987. Identification of components of a new stability system of plasmid R1, Par D, that is close to the origin of replication of this plasmid. Mol. Gen. Genet. 210:101-110. [DOI] [PubMed] [Google Scholar]
- 5.Cherny, I., and E. Gazit. 2004. The YefM antitoxin defines a family of natively unfolded proteins: implications as a novel antibacterial target. J. Biol. Chem. 279:8252-8261. [DOI] [PubMed] [Google Scholar]
- 6.Cherny, I., L. Rockah, and E. Gazit. 2005. The YoeB toxin is a folded protein that forms a physical complex with the unfolded YefM antitoxin. J. Biol. Chem. 280:30063-30072. [DOI] [PubMed] [Google Scholar]
- 7.Christensen, S. K., G. Maenhauf-Michel, N. Mine, S. Gothesman, K. Gerdes, and L. Van Melderen. 2004. Overproduction of the Lon protease triggers inhibition of translation in Escherichia coli: involvement of the yefM-yoeB toxin-antitoxin system. Mol. Microbiol. 51:1705-1717. [DOI] [PubMed] [Google Scholar]
- 8.Christensen, S. K., and K. Gerdes. 2004. Delayed-relaxed response explained by hyperactivation of RelE. Mol. Microbiol. 53:587-597. [DOI] [PubMed] [Google Scholar]
- 9.Christensen, S. K., and K. Gerdes. 2003. RelE toxins from bacteria and Archaea cleave mRNAs on translating ribosomes, which are rescued by tmRNA. Mol. Microbiol. 48:1389-1400. [DOI] [PubMed] [Google Scholar]
- 10.Christensen, S. K., M. Mikkelsen, K. Pedersen, and K. Gerdes. 2001. RelE, a global inhibitor of translation, is activated during nutritional stress. Proc. Natl. Acad. Sci. USA 98:14328-14333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christensen, S. K., K. Pedersen, F. G. Hansen, and K. Gerdes. 2003. Toxin-antitoxin loci as stress-response-elements: ChpAK/MazF and ChpBK cleave translated RNAs and are counteracted by tmRNA. J. Mol. Biol. 332:809-819. [DOI] [PubMed] [Google Scholar]
- 12.Condon, C. 2006. Shutdown decay of mRNA. Mol. Microbiol. 61:573-583. [DOI] [PubMed] [Google Scholar]
- 13.de la Hoz, A. B., S. Ayora, I. Sitkiewicz, S. Fernandez, R. Pankiewicz, J. C. Alonso, and P. Ceglowski. 2000. Plasmid copy-number control and better-than-random segregation genes of pSM19035 share a common regulator. Proc. Natl. Acad. Sci. USA 97:728-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Díaz-Orejas, R., A. J. Muñoz-Gómez, and M. Lemonnier. 2003. The Kid-Kis toxin-antitoxin: a tale of selfishness, survival and death. ELSO Gaz. Rev. http://www.the-elso-gazette.org/magazines/issue17/reviews/reviews1.asp.
- 15.Di Guilmi, A. M., and A. Dessen. 2002. New approaches towards the identification of antibiotic and vaccine targets in Streptococcus pneumoniae. EMBO Rep. 3:728-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engelberg-Kulka, H., and G. Glaser. 1999. Addiction modules and programmed cell death and antideath in bacterial cultures. Annu. Rev. Microbiol. 53:43-70. [DOI] [PubMed] [Google Scholar]
- 17.Engelberg-Kulka, H., B. Sat, M. Reches, S. Amitai, and R. Hazan. 2004. Bacterial programmed cell death systems as targets for antibiotics. Trends Microbiol. 12:66-71. [DOI] [PubMed] [Google Scholar]
- 18.Gazit, E., and R. T. Sauer. 1999. Stability and DNA binding of the Phd protein of the phage P1 plasmid addiction system. J. Biol. Chem. 274:2652-2657. [DOI] [PubMed] [Google Scholar]
- 19.Gerdes, K., S. Ayora, I. Canosa, P. Ceglowski, R. Díaz-Orejas, T. Franch, A. P. Gultyaev, R. Bugge Jensen, I. Kobayashi, C. Macpherson, D. Summers, C. M. Thomas, and U. Zielenkiewicz. 2000. Bacterial plasmids and gene spread, p. 49-85. In C. M. Thomas (ed.), The horizontal gene pool. Harwood Academic Publishers, Amsterdam, The Netherlands.
- 20.Gerdes, K., K. S. Christensen, and A. Lobner-Olensen. 2005. Prokaryotic toxin-antitoxin stress response loci. Nat. Rev. Microbiol. 3:371-382. [DOI] [PubMed] [Google Scholar]
- 21.Gotfredsen, M., and K. Gerdes. 1998. The Escherichia coli relBE genes belong to a new toxin-antitoxin gene family. Mol. Microbiol. 29:1065-1076. [DOI] [PubMed] [Google Scholar]
- 22.Grady, R., and F. Hayes. 2003. Axe-Txe, a broad-spectrum proteic toxin-antitoxin system by a multidrug-resistant, clinical isolate of Enterococcus faecium. Mol. Microbiol. 47:1419-1432. [DOI] [PubMed] [Google Scholar]
- 23.Guex, N., and M. C. Peitsch. 1997. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18:2714-2723. [DOI] [PubMed] [Google Scholar]
- 24.Hoskins, J., W. E. J. Alborn, J. Arnold, L. C. Blaszczak, S. Burgett, B. S. DeHoff, S. T. Estrem, L. Fritz, D. J. Fu, W. Fuller, C. Geringer, R. Gilmour, J. S. Glass, H. Khoja, A. R. Kraft, R. E. Lagace, D. J. LeBlanc, L. N. Lee, E. J. Lefkowitz, J. Lu, P. Matsushima, S. M. McAhren, M. McHenney, K. McLeaster, C. W. Mundy, T. I. Nicas, F. H. Norris, M. O'Gara, R. B. Peery, G. T. Robertson, P. Rockey, P. M. Sun, M. E. Winkler, Y. Yang, M. Young-Bellido, G. Zhao, C. A. Zook, R. H. Baltz, S. R. Jaskunas, P. R. J. Rosteck, P. L. Skatrud, and J. I. Glass. 2001. Genome of the bacterium Streptococcus pneumoniae strain R6. J. Bacteriol. 183:5709-5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson, W. C. 1999. Analyzing protein circular dichroism spectra for accurate secondary structures. Proteins 35:307-312. [PubMed] [Google Scholar]
- 26.Kamada, K., and F. Hanaoka. 2005. Conformational change in the catalytic site of the ribonuclease YoeB toxin by YefM antitoxin. Mol. Cell 19:497-509. [DOI] [PubMed] [Google Scholar]
- 27.Kamphuis, M. B., M. C. Monti, R. H. H. van den Heuvel, S. Santos-Sierra, G. E. Folkers, M. Lemonnier, R. Díaz-Orejas, A. J. R. Heck, and R. Boelens. Interactions between the toxin Kid of the bacterial parD system and the antitoxins Kis and MazE. Proteins Struct. Funct. Bioinformatics, in press. [DOI] [PubMed]
- 28.Kaplan, S. L., and E. O. J. Mason. 1998. Management of infections due to antibiotic-resistant Streptococcus pneumoniae. Clin. Microbiol. Rev. 11:628-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lacks, S. A. 1968. Genetic regulation of maltosaccharide utilization in Pneumococcus. Genetics 60:685-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lehnherr, H., E. Maguin, S. Jafri, and M. B. Yarmolinsky. 1993. Plasmid addiction genes of bacteriophage P1: doc, which causes cell death on curing of prophage, and phd, which prevents host death when prophage is retained. J. Mol. Biol. 233:414-428. [DOI] [PubMed] [Google Scholar]
- 31.Lemonnier, M., G. Ziegelin, T. Reick, A. Muñoz-Gómez, R. Díaz-Orejas, and E. Lanka. 2003. P1 Ban protein is a hexameric DNA helicase that interacts with and substitutes for Escherichia coli DnaB. Nucleic Acids Res. 31:3918-3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lemos, J. A., T. A. Brown, Jr., J. Abranches, and R. A. Burne. 2005. Characteristics of Streptococcus mutans strains lacking the MazEF and RelBE toxin-antitoxin modules. FEMS Microbiol. Lett. 253:251-257. [DOI] [PubMed] [Google Scholar]
- 33.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 34.Mattison, K., J. S. Wilbur, M. So, and R. G. Brennan. 2006. Structure of FitAB from Neisseria gonorrhoeae bound to DNA reveals a tetramer of toxin-antitoxin heterodimers containing PIN domains and ribbon-helix-helix motifs. J. Biol. Chem. 281:37942-37951. [DOI] [PubMed] [Google Scholar]
- 35.Mihalyi, E. J. 1968. Numerical values of the absorbances of the aromatic amino acids in acid, neutral, and alkaline solutions. Chem. Eng. Data 13:179-182. [Google Scholar]
- 36.Nieto, C., T. Pellicer, D. Balsa, S. K. Christensen, K. Gerdes, and M. Espinosa. 2006. The chromosomal relBE2 toxin-antitoxin locus of Streptococcus pneumoniae: characterization and use of a bioluminescence resonance energy transfer assay to detect toxin-antitoxin interaction. Mol. Microbiol. 59:1280-1296. [DOI] [PubMed] [Google Scholar]
- 37.Pandey, D. P., and K. Gerdes. 2005. Toxin-antitoxin loci are highly abundant in free-living but lost from host-associated prokaryotes. Nucleic Acids Res. 33:966-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pedersen, K., K. S. Christensen, and K. Gerdes. 2002. Rapid induction and reversal of a bacteriostatic conditions by controlled expression of toxins and antitoxins. Mol. Microbiol. 45:501-510. [DOI] [PubMed] [Google Scholar]
- 39.Pedersen, K., A. V. Zavialov, M. Y. Pavlov, J. Elf, K. Gerdes, and M. Ehrenberg. 2003. The bacterial toxin RelE displays codon-specific cleavage of mRNAs in the ribosomal A site. Cell 112:131-140. [DOI] [PubMed] [Google Scholar]
- 40.Petrey, D., Z. Xiang, C. L. Tang, L. Xie, M. Gimpelev, T. Mitros, C. S. Soto, S. Goldsmith-Fischman, A. Kernytsky, A. Schlessinger, I. Y. Koh, E. Alexov, and B. Honig. 2003. Using multiple structure alignments, fast model building, and energetic analysis in fold recognition and homology modeling. Proteins 53(Suppl. 6):430-435. [DOI] [PubMed] [Google Scholar]
- 41.Provencher, S. W., and J. Glockner. 1981. Estimation of globular protein secondary structure from circular dichroism. Biochemistry 6:33-37. [DOI] [PubMed] [Google Scholar]
- 42.Sat, B., R. Hazan, T. Fisher, H. Khaner, G. Glaser, and H. Engelberg-Kulka. 2001. Programmed cell death in Escherichia coli: some antibiotics can trigger mazEF lethalithy. J. Bacteriol. 183:2041-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shindyalov, I. N., and P. E. Bourne. 1998. Protein structure alignment by incremental combinatorial extension (CE) of the optimal path. Protein Eng. 11:739-747. [DOI] [PubMed] [Google Scholar]
- 44.Siegel, L. M., and K. J. Monty. 1966. Determination of molecular weights and frictional ratios of proteins in impure systems by the use of gel filtration and density gradient centrifugation. Applications to crude preparations of sulfite and hydroxylamine reductases. Biochim. Biophys. Acta 112:346-362. [DOI] [PubMed] [Google Scholar]
- 45.Sreerama, N., S. Y. Venyaminov, and R. W. Woody. 1999. Estimation of the number of alpha-helical and beta-strand segments in proteins using circular dichroism spectroscopy. Protein Sci. 8:370-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sreerama, N., and R. W. Woody. 2000. Estimation of protein secondary structure from circular dichroism spectra: comparison of CONTIN, SELCON, and CDSSTR methods with an expanded reference set. Anal. Biochem. 15:252-260. [DOI] [PubMed] [Google Scholar]
- 47.Studier, F. W., A. H. Rosenberg, J. J. Dunn, and J. W. Dubendorff. 1990. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 185:60-89. [DOI] [PubMed] [Google Scholar]
- 48.Tettelin, H., K. E. Nelson, I. T. Paulsen, J. A. Eisen, T. D. Read, S. Peterson, J. Heidelberg, R. T. DeBoy, D. H. Haft, R. J. Dodson, A. S. Durkin, M. Gwinn, J. F. Kolonay, W. C. Nelson, J. D. Peterson, L. A. Umayam, O. White, S. L. Salzberg, M. R. Lewis, D. Radune, E. Holtzapple, H. Khouri, A. M. Wolf, T. R. Utterback, C. L. Hansen, L. A. McDonald, T. V. Feldblyum, S. Angiuoli, T. Dickinson, E. K. Hickey, I. E. Holt, B. J. Loftus, F. Yang, H. O. Smith, J. C. Venter, B. A. Dougherty, D. A. Morrison, S. K. Hollingshead, and C. M. Fraser. 2001. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293:498-506. [DOI] [PubMed] [Google Scholar]
- 49.Tian, Q.-B., M. Ohnishi, T. Murata, K. Nakayama, Y. Terawaki, and T. Hayashi. 2001. Specific protein-DNA and protein-protein interaction in the hig gene system, a plasmid-borne proteic killer gene system of plasmid Rts1. Plasmid 45:63-74. [DOI] [PubMed] [Google Scholar]
- 50.Walsh, F. M., and S. G. B. Amyes. 2004. Microbiology and drug resistance mechanisms of fully resistant pathogens. Curr. Opin. Microbiol. 7:439-444. [DOI] [PubMed] [Google Scholar]
- 51.Whitney, C. G. 2000. Vaccination against pneumococcal disease: current questions and future opportunities. BioMed. Central 1:7. [Google Scholar]
- 52.Williams, B. G., E. Gouws, C. Boschi-Pinto, J. Bryce, and C. Dye. 2002. Estimates of world-wide distribution of child deaths from acute respiratory infections. Lancet Infect. Dis. 2:25-32. [DOI] [PubMed] [Google Scholar]
- 53.Xiao, H., M. Kalman, K. Ikehara, S. Zemel, G. Glaser, and M. Cashel. 1991. Residual guanosine 3′,5′-bis-pyrophosphate synthetic activity of relA null mutants can be eliminated by spot null mutations. J. Biol. Chem. 266:5980-5990. [PubMed] [Google Scholar]
- 54.Zhang, X., T. Reeder, and R. Schleif. 1996. Transcription activation parameters at ara pBAD. J. Mol. Biol. 258:14-24. [DOI] [PubMed] [Google Scholar]
- 55.Zhang, Y., J. Zhang, K. P. Hoeflich, M. Ikura, and M. Inouye. 2003. MazF cleaves cellular mRNAs specifically at ACA to block protein synthesis in Escherichia coli. Mol. Cell 12:913-923. [DOI] [PubMed] [Google Scholar]