Abstract
In a phenomenon referred to as “adaptive mutation,” a population of bacterial cells with a mutation in the lac operon (lac−) accumulates Lac+ revertants during prolonged exposure to selective growth conditions (lactose). Evidence was provided that selective conditions do not increase the mutation rate but instead favor the growth of rare cells with a duplication of the leaky lac allele. A further increase in copy number (amplification) improves growth and increases the likelihood of a sequence change by adding more mutational targets to the clone (cells and lac copies per cell). These duplications and amplifications are described here. Before selection, cells with large (134-kb) lac duplications and long junction sequences (>1 kb) were common (0.2%). The same large repeats were found after selection in cells with a low-copy-number lac amplification. Surprisingly, smaller repeats (average, 34 kb) were found in high-copy-number amplifications. The small-repeat duplications form when deletions modify a preexisting large-repeat duplication. The shorter repeat size allowed higher lac amplification and better growth on lactose. Thus, selection favors a succession of gene-amplification types that make sequence changes more probable by adding targets. These findings are relevant to genetic adaptation in any biological systems in which fitness can be increased by adding gene copies (e.g., cancer and bacterial drug resistance).
Keywords: gene duplication, genetic adaptation, natural selection, genome instability, mutation under selection
A genetic system devised by Cairns and Foster (1) has been used to investigate the effect of selection on the rate of appearance of adaptive mutations. The system uses an Escherichia coli strain with a leaky lac +1 frameshift mutation on the conjugative plasmid F'lac128 (2). On minimal lactose medium, ≈100 Lac+ revertant colonies accumulate linearly over 6 days above a lawn of 108 nongrowing cells. During nonselective growth, the lac mutation reverts at a rate of 10−8 per cell per division. Under selection, 100 colonies appear from 108 plated cells over 6 days. This behavior suggested that selective stress might activate a mechanism to increase the general mutation rate (3–5). However, modeling indicated that such a mechanism would reduce long-term fitness (6, 7).
The selective gene amplification model explains the effect of selection without stress-induced mutagenesis (8–10). Rare cells with a duplication of the leaky lac allele initiate slow-growing clones on selective medium. Within these clones, a succession of cells arises with progressive increases in lac copy number and consequently higher growth rates. The probability of a frameshift mutation to lac+ within one of these clones increases with the number of mutational targets (lac copies). The appearance of a lac+ allele relaxes selection on the mutant copies, which are then lost by unequal recombination (segregation), allowing a haploid lac+ cell to overgrow the clone. Aspects of this model are investigated here.
The generally accepted view of gene duplication and amplification is diagrammed in Fig. 1A. A duplication forms when two separated sequence elements (boxes) in different copies of a replicating chromosome recombine to leave a recombinant version of the sequence element at the junction between two copies of the intervening region. The sequences adjoining this junction element reveal the size of the repeated unit, and the sequence of the junction element itself provides evidence about how the duplication formed. In the Cairns–Foster system, these structures arise on the F'128 plasmid, which carries the lac region (Fig. 1B). The IS3 elements (A, B, and C) are the only large (>1 kb) sequence elements whose recombination can form a lac duplication. Shorter elements (35 bp) are the repetitive extragenic palindromic (REP) sequences (11), each of which is an imperfect 35-bp palindrome. Approximately 40 REP elements are found near lac in seven major (12 total) clusters of head-to-head REP dyads (12). Exchanges between REP elements can cause duplications and deletions (12–16).
Fig. 1.
Structures of amplification intermediates and the F′ plasmid. (A) Formation of duplications and amplifications by sister-strand exchanges. Boxes indicate sequence elements that recombine to form a duplication. Exchanges between the extensive repeated segments can lead to further increases and decreases in copy number. (B) Plasmid F'128 contains F plasmid sequence (dark portion of circle) with an IS3-flanked 134-kb fragment of the E. coli chromosome including lac, dinB, and REP elements (12). Each REP element (triangle) belongs to one of the three classes indicated (16).
The selective amplification model predicts that each revertant colony should contain some stable lac+ revertant cells and some progenitor cells with a lac amplification, which causes an unstable Lac+ phenotype. The predicted mixture was observed (9), but the model does not explain the two distinct classes of mixed revertant colonies (9, 17–19). In one colony type (stable-rich), >97% of cells are stable lac+ with a single revertant allele and only a few cells (<3%) have a lac amplification. The other type (unstable-rich) contains predominantly (>97%) unstable Lac+ cells and only a few stable Lac+ cells.
To better understand selective gene amplification and the two colony types, duplication junctions found before selection were compared with those found in unstable Lac+ cells of the two revertant colony types. Unexpectedly, the amplifications found in colonies rich in unstable Lac+ cells were very different from those found before selection or within stable-rich revertant colonies. In the unstable-rich colonies, amplifications had more copies of a shorter repeated unit and a very short (3- to 12-bp) junction element (defined in Fig. 1A). These short-repeat amplifications are called short junction (SJ) types to distinguish them from the common unselected duplications and large-repeat amplifications with more extensive junction sequences. The different junction sequences suggest distinct mechanisms of formation.
Because SJ junctions were common only after selection, one might imagine they are induced by stress (18, 19). On the contrary, the evidence presented here explains SJ duplications and the two observed revertant colony types without need for stress-induced events. The model is outlined below and diagrammed in Fig. 2, which will be discussed later.
Fig. 2.
Pathways of selected change during growth under selection. At the top above the horizontal line, are cell types with various structures of the lac region (black dots) and their frequencies before selection. Before selection, duplications (dup) arise between IS3 elements (boxes), and deletions (del) convert them to SJ duplications. Numbers at top indicate how many cells of each type are plated on selective medium (lactose). Events below the horizontal line occur under selection. Genotypes farther below the line allow better growth under selection. Copy number is indicated by the “n” after the diagrammed repeat. The final colonies are clonal mixtures of the stable lac+ revertant cells and its progenitor amplification cell; dotted lines indicate the minority type.
Most of the cells (108) plated on selective medium are haploid lac− cells, but a few (105) have a large-repeat lac duplication, and even fewer (102) have short-repeat SJ duplication (top of Fig. 2). Under selection, this mixture of Lac− cells gives rise to ≈100 Lac+ colonies, which arise by three different pathways.
The cells (105) with a large-repeat duplication initiate clones that grow poorly under selection, due to the instability of their large repeat and the fitness cost of amplifying it (downward arrow in Fig. 2). These 105 clones grow to 100–1,000 cells, each with ≈10 lac copies, but do not develop into visible colonies unless one of the following two events occurs.
(i) If any lac allele within a clone reverts to lac+, then stable Lac+ cells overgrow the clone and form a stable-rich visible colony in which unstable (progenitor) cells are rare (leftward slanted arrows in Fig. 2).
(ii) If any cell within a clone acquires a deletion that converts a long-repeat duplication into a short-repeat SJ type (as described below), then higher amplification is permitted, and a subclone of unstable Lac+ cells predominates (rightward slanted path of heavy arrows in Fig. 2). Revertants with sequence changes (lac+) arise late (dotted arrow) in these subclones and the colonies are therefore rich in unstable Lac+ cells.
Rare cells (102) in the unselected population with a short-repeat SJ type lac duplication can initiate an unstable rich clone immediately after plating (downward curved arrow in Fig. 2).
This model proposes that natural selection favors the growth of a succession of amplifications that focus progressively on the growth-limiting region and thereby stimulates formation of adaptive sequence changes without requiring a stress-induced increase in rate of mutation or amplification.
Experiments reported were done with Salmonella enterica, but the results apply equally well to the Escherichia coli version of the system. In both systems, selection enhances revertant yield only if (i) lac is on a conjugative F′ plasmid, (ii) the plasmid transfer system is operative, and (iii) the strain is recombination (RecA) proficient. The two colony types described here have been reported previously in both E. coli (19) and S. enterica (9, 20). Amplifications of the SJ type have been described in E. coli (21). In S. enterica, the two colony types show a less extreme ratio of stable to unstable Lac+ cell types, making it easier to identify the minority cell type. The reasons for this difference in behavior are described in supporting information, which is published on the PNAS web site.
Results
The Frequency and Structure of lac Duplications in an Unselected Culture.
Duplications were identified in the lac+ strain (TT24992), which is isogenic with the S. enterica reversion tester strain (TT18302). Duplication frequency was determined by a transduction cross (Materials and Methods) for cells grown in rich medium. Between 1,000 and 3,000 clones were screened from each of 20 independent cultures. Duplications of the lac region were present at a frequency of 18.0 × 10−4 (±1.4 × 10−4). Thus, when 108 cells are plated on selective medium, ≈105 (0.2%) are expected to carry a preexisting lac duplication.
Duplication junctions were identified and sequenced (Material and Methods). Most duplications had a copy of IS3 at the junction (20 of 24). Of these, 19 formed by an exchange between the identical IS3A and IS3C elements (repeat size of 134 kb); one arose between IS3B and IS3C, which differ by seven base substitutions. The remaining four duplications formed by exchanges between different pairs of REP sequences, which provide perfect repeats of 10- to 35-bp sequences; the duplicated regions ranged in size from 18 to 49 kb (Fig. 3, at the top).
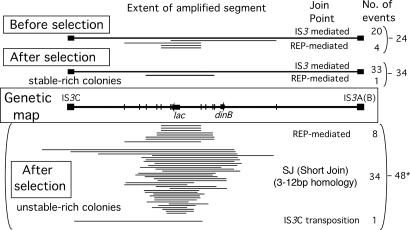
Fig. 3.
Duplications found before and after selection. The central box represents the bacterial chromosome segment on F'128; vertical bars indicate the position of REP element clusters. The top section depicts the repeat found in 24 duplications found in cells grown in rich medium. The second section presents the repeated segments of 34 amplifications found in the rare (<3%) unstable Lac+ cells from stable-rich Lac+ revertants. The bottom section shows the amplified segments of selected revertant colonies with mostly (>97%) unstable Lac+ cells. Junction sequences are described in supporting information. ∗, The total of 48 amplifications includes five inversion duplications, which are not diagrammed.
Amplifications in Stable-Rich Revertants Resemble Unselected Duplications.
Revertant colonies rich in stable Lac+ cells include rare cells (<3%) with an unstable Lac+ phenotype. Such amplifications were stabilized from 38 independent revertants. Junctions were successfully characterized from 34 of the amplifications (Materials and Methods) and resembled those found in unselected cells (Fig. 3, at the top).
To identify cells with these amplifications, revertant colonies were suspended, diluted, and plated on nutrient broth plates containing X-Gal, where stable Lac+ cells formed solid blue colonies, and unstable Lac+ cells formed blue-white sectored colonies due to loss of amplification. The sectored colonies (whose amplifications were later stabilized and sequenced) showed a very pale blue color compared with those from unstable-rich colonies (described below). This suggested a lower level of LacZ activity, indicating that the large IS3-flanked lac duplication (and the smaller REP duplications) found in these cells amplify poorly under selection and allow only slow growth on lactose. Previously published evidence shows that these faint blue-sectored clones had 10–15 lac copies, whereas the dark-blue sectored clones found in unstable-rich colonies (below) had 50–100 lac copies (8).
Amplifications in Unstable-Rich Colonies Have Higher Copy Number, Smaller Repeats, and a Short Junction (SJ) Sequence.
Of 30 previously described amplifications, 29 were shown to have a 50- to 100-fold copies of the lac sequence (8); one had a low-copy amplification with an IS3AC junction (like those described above). Twenty-seven additional independent amplifications were identified from unstable-rich revertant colonies, giving a total of 56. From these, 48 junctions were sequenced. The majority (34 of 48) were of a type not seen before selection or within stable-rich colonies. These 34 amplifications had a smaller repeat size and a short junction sequence (SJ), suggesting a distinct mode of formation. Amplified regions are diagrammed in Fig. 3 (below the map).
The 48 Amplifications Fell into Several Classes.
The predominant (SJ) type (34 of 48, or 71%) had an average repeat of 34 kb (85% were <40 kb), smaller than the IS3 duplications (134 kb) that predominated in the absence of selection and in stable-rich revertant colonies. They had very short junction sequences (3–12 bp; most are 7–8 bp), each of which was unique, suggesting there are many ways to form these structures.
Eight of the 48 amplifications had a REP element at their junctions, two of which were identical to the junction of a REP duplication identified before selection.
One amplification had an IS3 element at the junction, but a repeat size of only 60 kb; it is presumed to have formed by replicative transposition of the IS3C element from one sister chromosome to a site on the opposite side of lac on the other sister, producing the exchange event that forms a duplication in Fig. 1A.
Five amplifications originated from inversion duplications in which duplicated copies of a region (in the same orientation) flank a central inverse-order copy of a portion of the duplicated region. These inversion junctions are difficult to detect by the PCR methods used here, which are efficient at detecting simple duplication junctions. The eight amplifications (of 56) that could not be characterized are likely to be the inversion-duplication type, which will be described elsewhere (E. Kugelberg, unpublished work).
Multistep Formation of SJ Duplications: A Model.
It is difficult to imagine how SJ duplications (with 3- to 12-bp junction elements) could arise by exchanges between chromosomes (as diagrammed in Fig. 1A). We suggest that these short sequences did not contribute to duplication formation, but reflect deletions that remodeled a preexisting larger duplication. Deletions are known to form between short sequence repeats, one hybrid copy of which remains at the site of the deletion (22). We propose that the SJ sequence of an SJ duplication reflects a deletion that removed the junction of the original duplication. The model for formation of SJ duplications (Fig. 4) is outlined here and will be tested below.
Fig. 4.
Formation of an SJ duplication by a deletion. Boxes are large sequences (e.g., IS3) able to support recombination events that form the initial duplication. The black dots between b and c indicate the lac operon. The short arrows designate a repeated sequence (3–12 bp) that can mediate deletion formation.
A large duplication forms between long sequence repeats (as in Fig. 1A). These include the large IS3 type, which predominates before selection. Such duplications amplify poorly under selection because of their instability and fitness cost.
An SJ duplication forms when a deletion removes the junction sequence of the original larger, duplication. This shortens the repeated unit and replaces its junction with the short sequence that mediated deletion formation (Fig. 4). Such a deletion can form between any two members of a larger amplified array.
Time of Appearance of Stable- and Unstable-Rich Reverant Colonies.
The model being tested here predicts that two sorts of events can occur in any one of the many clones growing with a large-repeat amplification. A lac+ reversion event (frameshift mutation) generates a functional lac+ allele and initiates a subclone that can rapidly form a visible (stable-rich) colony. Alternatively, a deletion event forms an SJ duplication, which initiates a subclone whose growth rate increases slowly as more lac copies accumulate. In the clones with an SJ amplification, revertant alleles will appear only when sufficient lac copies have accumulated within the colony to allow the rare base change (10−8 per cell per division). These visible colonies will be rich in unstable Lac+ cells and are expected to appear later than colonies rich in stable revertant cells.
As predicted (Fig. 5), the stable-rich colonies appear first, and the accumulation of unstable-rich colonies is delayed. In many experiments, the number of unstable-rich clones surpasses the number of stable-rich colonies at late times. Results similar to these were first shown for the E. coli version of this system (18, 19).
Fig. 5.
Accumulation of stable- and unstable-rich Lac+ revertant colonies. The Salmonella version of the Cairns strain (TT18302) was grown and plated as described in Materials and Methods.
No Stress-Induced Event Is Required to Explain Formation of SJ Duplications Under Selection.
Because SJ duplications were initially seen only under selection, the possibility exists that their formation is induced by stress, as suggested previously (18, 19). To explore this, we estimated the rate at which SJ duplications form under nonselective and selective conditions.
During nonselective growth of cells with a large IS3 duplication, deletions forming an SJ duplication are expected to be frequent, because each end point can lie anywhere in a large region (≈50 kb). The measured frequency of IS3 duplications (0.2%) is close to the steady-state value achieved by balanced formation and segregation of duplications (A.B.R., unpublished results). Known unselected deletion rates are ≈10−7 per cell per division. Using this rate, the observed frequency of IS3 duplications, and the segregation rate of SJ duplications (measured below), the estimated frequency of SJ duplications is expected to be 10−6 in an unselected culture. That is, plated cells include ≈100 SJ duplications or ≈0.1% of total (105) plated lac duplications. This estimate is supported below.
During growth under selection, the likelihood of a deletion generating an SJ duplication increases as the parent IS3 duplication amplifies. This increase is described by the equation PSJ = N(N−1)/2, where PSJ is the factor by which deletion likelihood increases, and N is the copy number of the IS3 amplification. A cell with two copies has only one way to make an SJ duplication, delete the single junction. A cell with three copies has three ways, between copies 1 and 2, 2 and 3, or 1 and 3. A 10-copy array has 45 ways of forming a particular SJ duplication. Thus, in a clone of 100–1,000 cells, each with 10 copies of the IS3 duplication (as estimated), the probability of an SJ duplication increases 4,500- to 45,000-fold over that of the single plated parental duplication cell.
No stress-induced increase in deletion rate is required to explain formation of SJ duplications under selection. If 105 plated cells have a likelihood of deletion formation increased 4,500- to 45,000-fold by growth and amplification, the deletion rate of 10−7 expected for nonselective conditions is sufficient to explain formation of the observed (≈100) unstable rich colonies.
Evidence That SJ Duplications Can Arise Without Selective Stress.
No SJ types were observed among 24 duplications from unselected cultures (above), opening the possibility that stress is required to induce their formation, as suggested by others (18, 19). The estimates above, however, predicted that unselected SJ duplications would be only 0.1% of all duplications, a frequency too low to detect by the previous methods. Given the observation that each of >80 independent SJ junctions had a distinct SJ junction sequence, strong evidence for unselected formation of SJ duplications would be provided by finding multiple revertants from a single pregrowth culture with a single SJ junction, evidence that identical sibs were present in an unselected culture.
To test this possibility, multiple nonindependent Lac+ revertant colonies were selected (day 5) from each of two independent cultures of the wild-type tester strain (TT18302). Seven revertant duplication junctions were characterized from each culture. In one culture, two SJ duplications had the same junction sequence, and three other duplications had a single different sequence. One of the others was IS3-mediated, and the last was an SJ with a different sequence. The second culture was noninformative; four amplifications were IS3-mediated, one was REP-mediated, and the other two had distinct SJ junctions. The identical nonindependent SJ junctions found in revertants from the first culture could not be detected by PCR in that culture, consistent with their rarity. Identical nonindependent SJ junctions were also observed in E. coli (21).
An added support for formation of SJ duplication without selection is the finding (in a different experiment) of two SJ types among ≈1,000 duplications from an unselected culture (A.B.R., unpublished results). Although this is based on very small numbers, it fits with the calculation that ≈0.1% of unselected lac duplications would be of the SJ type.
Several Junction Types Can Be Found in a Single Lac+ Revertant Clone.
When an SJ duplication arises (by deletion) within a clone growing slowly with a low-copy IS3 amplification, the shorter SJ duplication type will amplify more highly and grow faster. As a consequence, the SJ amplification cells should predominate in the population, but some progenitor cells with the original IS3 junction should remain within the colony.
This expectation was supported by five revertant colonies found to contain two types of unstable Lac+ cells. When single cells from these colonies were distributed on nutrient broth plates with X-Gal, a few (5%) of the unstable Lac+ cells formed light-blue sectored colonies and were shown by PCR analysis to have amplifications with an IS3 junction. The more common unstable Lac+ cells (95%) in the same colonies formed a dark-blue sectored colony. Their amplifications had a shorter repeat; three had an SJ junction, and two were REP-mediated. The finding of both low- and high-copy amplification types within the same clones supports the idea that short-repeat duplications form within clones with a larger IS3 amplification and that the large repeat size of IS3 duplications limits selective amplification and growth.
Smaller Amplifications Are Less Prone to Loss by Segregation.
The difficulty of amplifying larger duplications may reflect in part their expected higher rate of loss by segregation. To test the effect of repeat size on segregational loss, three lac duplications were constructed by linear transformation, as described (8). The stability of these duplications was estimated by following the loss of a CmR element present at the duplication junction during nonselective growth.
After 10 generations in rich medium, the median frequency of CmS segregant cells was 25% for the large (134-kb) duplication (TT25133), compared with 7% and 3% for the 50- and 15-kb duplications (TT25150 and TT25149). Thus the segregant frequency was roughly proportional to repeat size. This is expected, because segregation occurs by an exchange between repeats, and its frequency should reflect the size of the repeat. Deleterious effects of sequences included in larger duplications may also contribute to these frequency differences.
Lack of IS3 Reduces the Yield of Lac+ Revertants Under Selection.
If IS3 amplifications are intermediates in formation both stable-rich revertants (lac+ revertants) and unstable-rich revertants (SJ duplications), then removal of IS3 repeats from the tester strain should reduce the revertant yield. The magnitude of the effect should reflect the fraction of revertants that depend on IS3 for their formation. This was tested by using a derivative of the tester strain (TT25215), whose IS3A and C elements were replaced by drug resistance cassettes (Kan and Rif).
Lack of the IS3 elements reduced the frequency of unselected duplications ≈5-fold (from 0.2% to 0.044%). Between 25,000 and 30,000 clones were screened for each of three cultures; the duplication frequency was 4.4 × 10−4 (±1 × 10−6). This 5-fold reduction was expected, because 80% of duplications in the normal strain had IS3 elements at the amplification junction. Revertant yield was reduced 2- to 3-fold, implying that about half of the revertants arising in the Cairns experiment depend on events that require the IS3 repeats (data in supporting information).
Seventeen unselected duplications from the strain lacking IS3 (TT25215) included 16 between REP sequences and one inversion duplication. The abundance of REP junctions is consistent with the fact that in the strain with IS3, 80% of the duplications had IS3 junctions, and the rest were of the REP type (top of Fig. 3).
From unstable-rich revertant colonies (derived from the strain lacking IS3), 17 amplifications were analyzed, and 10 junctions were successfully sequenced. Two had a REP element, three were of the SJ type, and four formed by transposition of IS5 or Tn1000 (γδ). One amplification had an inversion-duplication repeat, and the seven uncharacterized amplifications are likely to be of this type. The reduced frequency of SJ junctions and the corresponding increase in rare types (transpositions and inversion duplications) confirmed the important contribution of IS3 to SJ duplication formation. The finding of three SJ junctions among the 17 amplifications demonstrates that some SJ duplications form without prior IS3 duplication. We suggest these arose by deletions occurring in a large REP duplication or in a plasmid dimer.
Discussion
These experiments support the model described in the Introduction. The model explains why common unselected large-repeat duplications are inefficient at producing revertant clones under selection, and why the observed revertant colonies fall into two classes. Key points in the model are summarized below and are diagrammed at the top of Fig. 2.
Guided Tour of the Model.
Among the 108 cells plated on selective conditions, 105 have a large-repeat lac duplication that allows limited amplification and growth on lactose. These many clones contribute modestly to growth of the lawn (see supporting information) but seldom form a visible colony. In Fig. 2, this growth is indicated by the vertical arrow downward from the 105 cells with an unselected large-repeat duplication. Taken together, these clones provide a reservoir of growing cells, within which two sorts of rare events occur and allow formation of ≈100 visible colonies under selection. These events are described below.
A lac+ reversion event initiates a subclone (leftward heavy slanted arrow, Fig. 2) that can rapidly dominate the population of the small slow-growing parental clone and form a visible colony made up predominantly of stable lac+ cells. The rare reversion event does not require enhanced mutagenesis, because it can occur in any of the large number of tiny clones. If each of 105 clones has ≈100–1,000 cells with ≈10 lac copies, then multiple reversion events (to lac+) can occur at the basal unselected rate of 10−8 per copy per replication.
A deletion can shorten the repeat size and produce an SJ duplication (rightward slanted arrow, Fig. 2). The resulting cell increases its growth rate by further increases in lac copy number (slowly building to 100 copies). Although the initial cell grows faster than the parent with a large-repeat amplification, its growth rate approaches that of a lac+ revertant only as lac copy number approaches 100. Therefore, these colonies appear later than stable-rich colonies (see Fig. 5). Reversion events (to lac+) arise in these developing clones only when the total number of lac alleles (cells times lac copies/cell) approaches 108, because the reversion rate of the lac allele is 10−8 per copy per replication. Thus the reversion event occurs in a large clone of unstable lac+ cells. Stable lac+ cells cannot dominate this large population and the resulting colony is rich in unstable Lac+ cells. Colonies that reach substantial size without realizing a reversion event may show no stable lac+ cells.
The SJ duplications that form before selection can efficiently generate an unstable-rich revertant colony under selection (curved downward arrow, Fig. 2). The formation rate of SJ types is the same before and during selection; selection only provides more lac regions in which the SJ can arise. The behavior of the system does not require a stress-induced increase in the formation rate of either revertant alleles (lac+) or the deletions that form SJ duplications. Increases in general mutation rate that have been reported are due to occasional coamplification of dinB with lac, as described previously (8) and in supporting information.
Broader Implications.
These results show how multiple-selection-driven pathways of gene amplification can help cells escape growth limitation without increasing their mutation rate. Natural cell populations, whether free-living or within a multicellular organism, have growth limited by ambient conditions. We infer that selected gene amplification is a universal population-level response that uses frequent reversible increases in gene dosage to improve fitness and thereby to increase the probability of an adaptive sequence alteration. The biological importance of this mechanism is becoming more apparent (23–30).
Materials and Methods
Strains, Media, and Chemicals.
Strains are from S. enterica (Serovar Typhimurium, strain LT2; see supporting information). The tester strain (TT18302) has a chromosomal leu deletion and a proB::Tn10 insertion; it carries an F'128 plasmid with a mutant lac allele that includes a deletion fusing the lacI and lacZ genes (Ω), a mutation improving the lacI promoter (IQ), and a +1 frameshift mutation (lacI33) within the hybrid lacI-lacZ gene (1). Scavenger cells were wild-type S. enterica, LT2. Media and chemicals are described in supporting information.
Constructed Mutations.
The IS3A and IS3C elements were replaced by the KanR and RifR genes by linear transformation (8). Three similarly constructed lac duplications had lac in the middle of the repeat and a CmR determinant at the junction between copies.
The lac Reversion Assay.
Cells grown in no-citrate E salts (NCE) glycerol (0.2%) leucine were washed and plated (2 × 108 cells) on NCE lactose (0.2%) containing X-Gal and leucine. Scavenger cells (109) were added to consume carbon sources other than lactose present in the agar or released by the tester cells. During incubation (37°C), blue revertant colonies were scored every 24 h.
Identifying Unselected Duplications by Transduction.
The donor strain (TT17724) carried a lacZ::Tn10 insertion on F′128 (31). One recipient (TT24992) was a lac+ derivative of the standard Cairns strain (TT18302); the other (TT25460) was an isogenic strain lacking both IS3A and IS3C. Transductants (TcR) were selected on nutrient broth (NB)/tetracycline/X-Gal medium. A haploid recipient cell yields a white Lac− colony. A recipient cell with a lac+ duplication inherits the donor lac::Tn10 element in one lac+ copy and forms an unstable sectored blue colony. The duplication frequency was the fraction of TcR transductant colonies with sectors.
Isolation of Cells with a Selected lac Amplification.
Independent revertant colonies (day 5) were suspended, diluted, and plated on NB X-Gal medium. After 3 days, unstable Lac+ cells formed blue colonies with white (Lac−) sectors, which carry a tandem array of lac copies (19, 20). The plasmid was transferred from these unstable Lac+ cells to a recA recipient strain (DA7700) to stabilize the amplified array.
Determination of Junction Sequences.
Seventy-seven primers were designed with 3′ ends directed away from lacZ and spaced 2–3 kb apart across region including IS3B, lac, and a 30-kb region beyond IS3C (12). Four pools contained primers that directed replication clockwise, and three pools directed counterclockwise synthesis. Each counterclockwise pool was used in combination with each clockwise pool. A PCR fragment is seen when normally divergent replication tracks converge across a duplication junction. The responsible primer pair was identified, and its product was sequenced.
Supplementary Material
Acknowledgments
For helpful suggestions and stimulating objections, we thank colleagues Neil Hunter, Wei Liao, Semarhy Quiñones Soto, Fritz Roth, Emiko Sano, and Cristina Tun-Garrido. We thank Phil Hastings for sharing unpublished results. This work was supported in part by a fellowship from the Wenner–Gren Foundation (to E. Kugelberg), by a grant from the Swedish Research Council (to D.I.A.), and by National Institutes of Health Grant GM27068 (to J.R.R.).
Abbreviations
- REP
repetitive extragenic palindromic
- SJ
short junction.
Footnotes
The authors declare no conflict of interest.
References
- 1.Cairns J, Foster PL. Genetics. 1991;128:695–701. doi: 10.1093/genetics/128.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calos MP, Miller JH. J Mol Biol. 1981;153:39–64. doi: 10.1016/0022-2836(81)90525-8. [DOI] [PubMed] [Google Scholar]
- 3.Torkelson J, Harris RS, Lombardo M-J, Nagendran J, Thulin C, Rosenberg SM. EMBO J. 1997;16:3303–3311. doi: 10.1093/emboj/16.11.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ponder RG, Fonville NC, Rosenberg SM. Mol Cell. 2005;19:791–804. doi: 10.1016/j.molcel.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 5.Foster PL. Mutat Res. 2005;569:3–11. doi: 10.1016/j.mrfmmm.2004.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roth JR, Kofoid E, Roth FP, Berg OG, Seger J, Andersson DI. Genetics. 2003;163:1483–1496. doi: 10.1093/genetics/163.4.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roth JR, Kugelberg E, Reams AB, Kofoid E, Andersson DI. Annu Rev Microbiol. 2006;60:477–501. doi: 10.1146/annurev.micro.60.080805.142045. [DOI] [PubMed] [Google Scholar]
- 8.Slechta ES, L.Bunny K, Kugelberg E, Kofoid E, Andersson DI, Roth JR. Proc Natl Acad Sci USA. 2003;100:12847–12852. doi: 10.1073/pnas.1735464100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hendrickson H, Slechta ES, Bergthorsson U, Andersson DI, Roth JR. Proc Natl Acad Sci USA. 2002;99:2164–2169. doi: 10.1073/pnas.032680899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pettersson ME, Andersson DI, Roth JR, Berg OG. Genetics. 2005;169:1105–1115. doi: 10.1534/genetics.104.030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilson E, Clement JM, Brutlag D, Hofnung M. EMBO J. 1984;3:1417–1421. doi: 10.1002/j.1460-2075.1984.tb01986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kofoid E, Bergthorsson U, Slechta ES, Roth JR. J Bacteriol. 2003;185:660–663. doi: 10.1128/JB.185.2.660-663.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson RP, Roth JR. J Mol Biol. 1978;119:147–166. doi: 10.1016/0022-2836(78)90274-7. [DOI] [PubMed] [Google Scholar]
- 14.Shyamala V, Schneider E, Ames GFL. EMBO J. 1990;9:939–946. doi: 10.1002/j.1460-2075.1990.tb08192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Ende A, Hopman CT, Dankert J. Infect Immun. 1999;67:2928–2934. doi: 10.1128/iai.67.6.2928-2934.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bachellier S, Saurin W, Perrin D, Hofnung M, Gilson E. Mol Microbiol. 1994;12:61–70. doi: 10.1111/j.1365-2958.1994.tb00995.x. [DOI] [PubMed] [Google Scholar]
- 17.Slechta ES, Liu J, Andersson DI, Roth JR. Genetics. 2002;161:945–956. doi: 10.1093/genetics/161.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hastings PJ, Slack A, Petrosino JF, Rosenberg SM. PLoS Biol. 2004;2:e399. doi: 10.1371/journal.pbio.0020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hastings PJ, Bull HJ, Klump JR, Rosenberg SM. Cell. 2000;103:723–731. doi: 10.1016/s0092-8674(00)00176-8. [DOI] [PubMed] [Google Scholar]
- 20.Andersson DI, Slechta ES, Roth JR. Science. 1998;282:1133–1135. doi: 10.1126/science.282.5391.1133. [DOI] [PubMed] [Google Scholar]
- 21.Slack A, Thornton PC, Magner DB, Rosenberg SM, Hastings PJ. PLoS Genet. 2006;2:e48. doi: 10.1371/journal.pgen.0020048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albertini AM, Hofer M, Calos MP, Miller JH. Cell. 1982;29:319–328. doi: 10.1016/0092-8674(82)90148-9. [DOI] [PubMed] [Google Scholar]
- 23.Albertson DG. Trends Genet. 2006;22:447–455. doi: 10.1016/j.tig.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 24.Sebat J, Lakshmi B, Troge J, Alexander J, Young J, Lundin P, Maner S, Massa H, Walker M, Chi M, et al. Science. 2004;305:525–528. doi: 10.1126/science.1098918. [DOI] [PubMed] [Google Scholar]
- 25.Feuk L, Carson AR, Scherer SW. Nat Rev Genet. 2006;7:85–97. doi: 10.1038/nrg1767. [DOI] [PubMed] [Google Scholar]
- 26.Locke DP, Sharp AJ, McCarroll SA, McGrath SD, Newman TL, Cheng Z, Schwartz S, Albertson DG, Pinkel D, Altshuler DM, et al. Am J Hum Genet. 2006;79:275–290. doi: 10.1086/505653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raymond M, Berticat C, Weill M, Pasteur N, Chevillon C. Genetica. 2001;112-113:287–296. [PubMed] [Google Scholar]
- 28.Romero D, Palacios R. Annu Rev Genet. 1997;31:91–111. doi: 10.1146/annurev.genet.31.1.91. [DOI] [PubMed] [Google Scholar]
- 29.Price RN, Uhlemann AC, Brockman A, McGready R, Ashley E, Phaipun L, Patel R, Laing K, Looareesuwan S, White NJ, et al. Lancet. 2004;364:438–447. doi: 10.1016/S0140-6736(04)16767-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nilsson AI, Zorzet A, Kanth A, Dahlstrom S, Berg OG, Andersson DI. Proc Natl Acad Sci USA. 2006;103:6976–6981. doi: 10.1073/pnas.0602171103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson P, Roth J. Proc Natl Acad Sci USA. 1981;78:3113–3117. doi: 10.1073/pnas.78.5.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.