Developments in anaesthetic and surgical techniques—that is, loco‐regional anaesthesia and minimally invasive surgery—have improved postoperative cardiac outcome considerably in recent years. For example, patients with a severely reduced left ventricular function used to be at increased risk, but because of the implementation of these new techniques they are now scheduled for surgery at relatively low risk. In other words, the improvement of perioperative care has altered the impact of established cardiac risk factors.
However, as more patients with cardiac co‐morbidity survive surgery, long‐term cardiac outcome has gained interest. Therefore, the focus of preoperative risk evaluation should also take into consideration the impact of cardiac co‐morbidity on long‐term survival. After all, patients should live long enough to enjoy the benefits of surgery.
It is estimated that the incidence of cardiac complications after non‐cardiac surgical procedures is between 0.5–1.0%.1,2 Annually around 100 million adults undergo some form of non‐cardiac surgery. Consequently, approximately 500 000 to 1 000 000 people will suffer from perioperative cardiac complications. Moreover, one out of every four of these patients will die. For the prevention of perioperative cardiac complications it remains of critical importance to identify those at increased risk and treat them accordingly, to improve both perioperative and long‐term survival.
This article gives an overview of the current status of preoperative cardiac screening. In a stepwise approach the use and prognostic value of clinical cardiac risk factors, laboratory measurements, non‐invasive and invasive coronary testing, and consequently medical and interventional strategies to alter cardiac risk will be discussed (fig 1).
Figure 1 Nine steps to optimal preoperative cardiac risk evaluation and modification.
STEP 1: IDENTIFICATION OF CLINICAL RISK FACTORS
The first, most simple and least costly step in preoperative cardiac risk stratification is the identification of clinical cardiac risk factors. In the last three decades much attention has focused on the identification of patients at risk by using simple clinical cardiac risk factors. This research has led to numerous cardiac risk indices for non‐cardiac surgical procedures. In 1977 Goldman et al proposed the first cardiac risk stratification model based on prospectively collected data.3 In this study of 1001 patients, nine independent predictors were found to be correlated with postoperative life‐threatening and fatal cardiac complications: preoperative third heart sound or jugular venous distension; myocardial infarction in the preceding six months; more than five premature ventricular contractions per minute documented at any time before operation; rhythm other than sinus rhythm or presence of premature atrial contractions on preoperative ECG; age over 70 years; intraperitoneal, intrathoracic or aortic operation; emergency operation; important valvular aortic stenosis; and poor general medical condition. The incidence of adverse cardiac events was 1% in the group at lowest risk (class I), and increased to 7%, 14%, and 78% in class II, III, and IV patients, respectively. However, it must be noted that only 18 patients were in the group at highest risk. As pointed out by Ridley, the Goldman index has a 96.8% negative predictive value, and thus is an excellent tool to rule out coronary artery disease (CAD).4 The value of the Goldman index for diagnosing patients with CAD on the other hand was less optimal—that is, a positive predictive value of 21.6%. In 1986 Detsky et al prospectively validated and modified the Goldman index and presented a simple normogram, introducing the pre‐test likelihood of perioperative cardiac events for cardiac risk stratification.5
The Detsky modified multifactorial risk index has been in use ever since and is considered to be a good and practical index. In 1999 Lee et al reviewed the performance of several clinical risk indices in patients who underwent elective non‐cardiac surgery.2 They found that the Goldman risk index and the Detsky modified cardiac risk index had a similar performance for predicting major cardiac complications. However, when the Goldman risk index was revised and validated, the predictive value of the risk index had substantially improved. In the validation cohort the receiver operating characteristic (ROC) area improved from 0.70 for the original Goldman index to 0.81 for the Revised Cardiac Risk Index by Lee et al. The Revised Cardiac Risk Index identified six predictors of major cardiac complications: high‐risk surgery, ischaemic heart disease, congestive heart failure, cerebrovascular disease, insulin dependent diabetes mellitus, and renal failure. Based on the presence of 0, 1, 2, or ⩾ 3 of these predictors, the rate of major cardiac complications was estimated to be 0.4%, 0.9%, 7%, and 11%, respectively. Interestingly the Lee index has better prognostic value than the Goldman and Detsky indices, though the number of cardiac risk factor variables in the Lee index is smaller. This might be explained by the improvement of perioperative care in the time between the development of the Goldman and Lee risk indices. Nowadays, the Lee index is considered the most relevant index for predicting perioperative cardiac risk in non‐cardiac surgery by many clinicians and researchers. However, the patients studied by Lee et al can hardly be considered an average non‐cardiac surgical population. Thoracic, vascular and orthopaedic patients were overrepresented in this study population.
Step 1: Clinical cardiac risk factors based on the Revised Cardiac Risk Index
High‐risk surgery
Ischaemic heart disease
Congestive heart failure
Cerebrovascular disease
Insulin dependent diabetes mellitus
Renal failure
STEP 2: TYPE OF SURGERY
After specifying patients' clinical cardiac risk factors it is important to consider the surgical procedure the patient is scheduled for. However, the clinical cardiac risk indices of Lee, Detsky and Goldman include only high‐risk surgery in their models as other types of surgery were not associated with adverse outcome. However, this simplification might not be sufficient to predict perioperative cardiac outcome accurately. Recently Boersma et al validated the Lee risk index in a large cohort (n = 108 593) of all types of non‐cardiac surgical procedures.1 When the Lee index was adapted and more detailed information on the surgical risk of the procedure was added, the predictive value improved substantially (C‐statistic improved from 0.63 to 0.85). In this model surgical procedures were classified as low‐risk, intermediate‐low, intermediate‐high, and high‐risk surgical procedures (see box).
Step 2: Surgical risk in elective non‐cardiac surgery
Low‐risk: breast, carotid, dental, endocrine, eye, gynaecology, reconstructive
Intermediate‐low risk: orthopaedic and urologic procedures
Intermediate‐high risk: abdominal, ENT (ear, nose and throat), neurologic, pulmonary, renal transplant, carotid
High risk: aortic, peripheral vascular
STEP 3: ELECTROCARDIOGRAM
Recently, Noordzij et al showed that the addition of a simple classification of preoperative ECG (that is, normal or abnormal) improved the predictive value of the combination of clinical cardiac risk factors and type of surgery.6 An ECG was considered abnormal in the case of atrial fibrillation, left or right bundle branch block, left ventricular hypertrophy, premature ventricular complexes, pacemaker rhythm, Q wave, or ST changes. This study was performed in a group of 23 036 patients undergoing non‐cardiac surgery. Though ECGs added extra information on perioperative cardiac risk, it was also shown that in absolute numbers, the increase in predictive value was small in patients undergoing low‐risk or intermediate‐risk procedures and a “routine preoperative ECG” in this population should be precluded.
Step 3: Electrocardiogram
Adding preoperative ECG improves the predictive value compared to clinical cardiac risk factors and type of surgery alone
In low‐risk patients and low‐risk procedures the value of preoperative ECGs seems limited
SUMMARISING STEPS 1 TO 3
With these simple risk predictors (that is, clinical risk factors; type of surgery; and ECG) it is possible to make an initial crude assessment of a patient's perioperative cardiac risk. This risk estimation can be used to identify those patients at increased risk who should undergo further cardiac testing. Recently Boersma et al proposed an interesting risk estimation based on the evaluation of 108 593 non‐cardiac surgical procedures.7 In this model, clinical risk factors, type of surgery and ECG are all included (fig 2).
Figure 2 Example of a risk model including clinical risk factors, type of surgery, and ECG to assess perioperative cardiac risk (derived from Boersma et al7). CVD, cardiovascular disease; PVC, premature ventricular contractions.
STEP 4: LABORATORY MEASUREMENTS
Apart from those measurements indicating clinical risk factors (for example, serum creatinine for renal failure, fasting glucose for diabetes mellitus, etc) currently no routine laboratory measurements are related to perioperative cardiac complications. However, two recent studies showed that increased plasma N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) was associated with adverse postoperative outcome.8,9 NT‐proBNP is increased in patients with left ventricular dilatation caused by fluid overload (that is, heart failure and renal dysfunction), pressure overload (that is, aortic valve stenosis) and myocardial ischaemia, which might explain the excellent relation with adverse postoperative outcome.10 Diabetes mellitus is known to be a strong predictor for perioperative events. Therefore fasting glucose values should be obtained from all patients. Recently it was shown that the level of preoperative glycosylated haemoglobin in diabetic patients is strongly related to perioperative cardiac outcome.11 In the same patient population it was also shown that in patients with high preoperative glycosylated haemoglobin it is more difficult to regulate glucose values in the perioperative period. This might partly explain the strong relation between preoperative glycosylated haemoglobin and outcome, since it is known from critically ill patients and patients with myocardial infarction that tight glucose control is of imminent importance.
In the Lee risk index renal insufficiency is taken into account. The serum creatinine cut‐off value Lee et al used is 2.0 mg/dl. However, it might be argued that patients with less pronounced renal insufficiency also do worse compared to patients with normal serum creatinine values. A continuous variable for creatinine would probably be better, though not very user‐friendly in every day practice. Recent studies have also shown that glomerular filtration rate might be a better predictor than serum creatinine since this takes into account the different creatinine concentrations between sexes.12
Step 4: Laboratory measurements
Serum creatinine and glucose are related to perioperative cardiac events
Glycosylated haemoglobin is a strong predictor for perioperative cardiac events, possibly related to the more difficult glucose regulation associated with high HbA1c
Glomerular filtration rate might be a stronger predictor than serum creatinine
High concentrations of plasma NT‐proBNP seem to be related to adverse cardiac events
STEP 5: NON‐INVASIVE RESTING CARDIAC IMAGING
If steps 1 to 4 indicate an increased cardiac risk or if there is evidence or suspicion of CAD at physical examination—for example, peripheral atherosclerotic disease, valve abnormalities or left ventricular dysfunction—further cardiac testing might be required. The most simple, inexpensive form of cardiac imaging is resting echocardiography, for the detection of impaired left ventricular function and valve stenosis and sclerosis. Impaired left ventricular function was long considered a strong predictor for adverse perioperative cardiac events. However, due to improved perioperative care it is no longer a strong predictor for short‐term outcome but remains a significant predictor for long‐term adverse cardiac events.
The presence of aortic stenosis is associated with a fivefold increased risk of perioperative cardiac events.13 Also, the severity of aortic stenosis is related to an increased risk of perioperative events. Considering this, it is important to detect the presence and significance of valve disease. Though physical examination is reliable in detecting abnormal heart sounds, the estimation of the severity of stenosis by physical examination alone is difficult and echocardiography is recommended in patients with abnormal heart sounds.
Step 5: Non‐invasive resting cardiac imaging
Resting echocardiography is useful in patients at increased risk for coronary artery disease (CAD)
Low costs, easily accessible
Presence and severity of valve stenosis is related to perioperative outcome
Left ventricular dysfunction is currently a strong predictor for long‐term outcome, not for perioperative outcome
STEP 6: NON‐INVASIVE STRESS CARDIAC IMAGING
According to the guidelines of the American College of Cardiology/American Heart Association,14 preoperative cardiac exercise or pharmacological stress testing is recommended for: patients with intermediate pre‐test probability of CAD; prognostic assessment of patients undergoing initial evaluation for suspected or proven CAD; evaluation of subjects with significant change in clinical status; demonstration of proof of myocardial ischaemia before coronary revascularisation; evaluation of adequacy of medical treatment; and prognostic assessment after an acute coronary syndrome. For stress testing, the evaluation of exercise capacity when subjective assessment is unreliable seems to be a valid reason as well. Patients with CAD or at risk for CAD can be frequently found in the group of patients with limited every day exercise—for example, patients with severe intermittent claudication. In these patients pharmacological stress echocardiography or nuclear imaging are elegant ways to exclude subclinical CAD. However, stress testing should not be performed in asymptomatic patients without evidence of CAD; patients with severe co‐morbidity likely to limit the life expectancy or candidacy for revascularisation; and patients with resting ECG abnormalities that preclude adequate assessment.
The sensitivity and specificity of available exercise and pharmacological stress tests (including exercise electrocardiography, radionuclide ventriculography, myocardial perfusion scintigraphy, and dobutamine stress echocardiography) were compared in several meta‐analyses. The meta‐analysis of Kertai et al showed a trend in favour of dobutamine stress echocardiography, though other tests had satisfying sensitivity and specificity as well (fig 3).15 An upcoming elegant new diagnostic tool is dobutamine stress magnetic resonance imaging, though no randomised trials or large series have reported the sensitivity and specificity of this test yet.
Figure 3 Sensitivity and specificity of different types of preoperative non‐invasive cardiac testing modalities (derived from Kertai et al15).
Step 6: Non‐invasive stress cardiac imaging
Intermediate pre‐test probability of CAD
Pharmacological or nuclear stress imaging in patients with limited exercise capacity
Meta‐analyses show no stress test modality to be superior over other stress tests
Dobutamine stress magnetic resonance imaging is an elegant upcoming method for stress testing
STEP 7: MEDICAL TREATMENT
β blockers
Although widely prescribed during non‐cardiac surgery, the evidence for perioperative β blocker use is mainly based on only two landmark studies and several observational studies. The first trial evaluated the effect of atenolol in high‐risk patients undergoing non‐cardiac surgery.16 In this study 200 patients with risk factors for, or with known, ischaemic heart disease were randomised to treatment with atenolol or placebo before surgery. Atenolol treatment was not associated with an improved in‐hospital outcome (cardiac death or myocardial infarction); however, continuous three‐lead Holter monitoring showed a 50% reduction of myocardial ischaemia in the atenolol treated group during the first 48 hours after surgery. The second trial showed in a selected high‐risk—that is, stress‐induced myocardial ischaemia during preoperative dobutamine echocardiography—population of 112 vascular surgery patients, a 10‐fold reduction in the incidence of perioperative cardiac death and myocardial infarction in patients receiving β blocker treatment compared to patients without β blocker therapy (3.4% v 34%).17
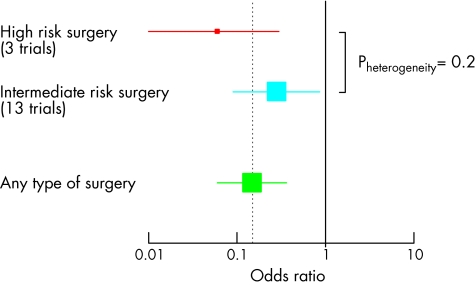
These promising results were confirmed by a meta‐analysis, of prospective randomised studies, evaluating the incidence of perioperative ischaemic episodes in 1077 patients in 15 studies (fig 4). β blocker treatment was associated with a 65% relative risk (RR) reduction in perioperative myocardial ischaemia and a 56% RR reduction in non‐fatal myocardial infarctions. Also, β blocker treatment was associated with a significant RR reduction of 67% in the composite end point of cardiac death and non‐fatal myocardial infarction. Though other meta‐analyses found a similar benefit of β blockers,18,19 a recent meta‐analysis by Devereaux et al reported no benefit of β blockers on perioperative outcome.20 However, in that meta‐analysis studies that had not yet undergone peer‐review were also included which might have seriously influenced the outcome of their meta‐analysis.
Figure 4 Meta‐analysis of 15 randomised β blocker trials. Odds ratio of β blocker treatment for cardiovascular outcome per type of non‐cardiac surgery. Derived from Schouten et al, Cor Artery Dis 2006;17:173–9.
The promising results were not supported by two recent trials evaluating the effect of β blockers in patients at intermediate cardiac risk. In the POBBLE trial, low‐risk patients (those with a history of ischaemic heart disease were excluded) scheduled for vascular surgery were randomised to receive a fixed dose of metoprolol (n = 55) or placebo (n = 48). No difference was observed in the incidence of perioperative cardiovascular events. The only difference was observed in the length of hospital stay, which was significantly shorter in those taking metoprolol, 10 versus 12 days. More recently, the DIPOM study, evaluating the cardioprotective effect of a fixed dose of metoprolol on the evening before major non‐cardiac surgery in 921 diabetics, showed no difference in 30‐day morbidity and mortality. However, this study was powered for a one year follow‐up period. Currently at least two large trials, POISE and DECREASE‐IV, are ongoing, evaluating the effect of β blockers in patients at intermediate risk for perioperative cardiac events. Though the results of these trials have to be awaited, in the mean time it seems to be safe and effective to prescribe β blockers in patients with a Lee index score of 2 or more, as Lindenauer found in a large cohort study of 663 635 patients.21
Statins
Several recent studies address the beneficial effect of statin use in patients undergoing non‐cardiac surgery (fig 5). In a case–control study involving 2816 patients who underwent major vascular surgery, statin use was associated with a significant fourfold reduction in all‐cause mortality compared to patients with no statin use.22 The first blinded, placebo‐controlled, randomised trial, in which the influence of statin use on perioperative cardiovascular complications was investigated, was reported by Durazzo et al.23 In their study, 100 patients were randomly assigned to treatment with either 20 mg atorvastatin or placebo. Patients received treatment for 45 days which was started at least two weeks before surgery. The outcome of this trial was the end point of cardiovascular events, defined as cardiac death, non‐fatal MI, stroke or unstable angina pectoris. Patients were followed up to six months after the surgical procedure. Of 100 patients, 90 (44 statin users and 46 non‐users) underwent elective vascular surgery. The six‐month incidence of cardiovascular events was reduced 3.1‐fold in statin users compared with non‐users. Finally, Lindenauer et al24 and O'Neil‐Callahan et al25 confirmed the beneficial effects of statins based on the results of their large‐scale retrospective studies. Lindenauer performed a retrospective cohort study based on the hospital discharge and pharmacy records of over 780 000 patients (70 159 statin users) in 329 hospitals throughout the United States. All patients underwent elective major surgical procedures and survived the first two postoperative days at least. After correction for numerous baseline differences, statin users had a 1.4‐fold reduced risk of in‐hospital mortality. Subsequently, Lindenauer concluded that perioperative statin use might result in a reduced risk of death after major surgical procedures. Though the studies published so far are in favour of perioperative statin treatment, this needs to be confirmed in large, adequately powered randomised trials, such as the recently started DECREASE‐IV trial.
Figure 5 Overview of odds ratios and 95% confidence intervals of currently known studies on the influence of statins on perioperative cardiac outcome in non‐cardiac surgical procedures.
Other medical treatments
A meta‐analysis by Nishina showed that use of clonidine, an α2 agonist, was associated with a reduction in the incidence of perioperative ischaemia.26 However, this study was underpowered (358 non‐cardiac surgical patients in two studies) and effects were only reported on ischaemia. In two more recent meta‐analyses, the beneficial effect of perioperative α2 agonist use was shown in the reduction of myocardial ischaemia and perioperative cardiovascular complications. But similarly to the study of Nishina, the results of these two meta‐analyses were mainly driven by the European Mivazerol Trial, the only large‐scale study available to date.27 The results of the European Mivazerol Trial showed no overall effect of mivazerol on the pre‐specified combined end point of cardiac death and myocardial infarction in the total study population of 2854 patients. Only a post‐hoc analysis revealed that, in 904 patients who underwent high‐risk major vascular surgery, mivazerol use was associated with a significantly lower incidence of cardiac death and myocardial infarction.
Nitrates are the most frequently used drugs in the treatment of myocardial ischaemia. However, studies on the prophylactic use of intravenous glyceryl trinitrate (GTN, nitroglycerine) failed to find any difference in the incidence of intraoperative and perioperative myocardial ischaemia in patients receiving GTN compared to placebo. A potential harmful effect might be a vagal withdrawal caused by peripheral vasodilatation and subsequent cardiac stimulation and induction of myocardial ischaemia in patients with CAD.
In the perioperative setting calcium channel blockers are effectively used in cardiac surgery, reducing myocardial ischaemia and arrhythmias. In a meta‐analysis Wijeysundera evaluated the use of calcium channel blockers in 11 studies involving 1007 patients, all undergoing non‐cardiac surgery.28 Calcium channel blockers significantly reduced perioperative myocardial ischaemia (RR 0.49, 95% confidence interval (CI) 0.30 to 0.80) and supraventricular arrhythmias (RR 0.52, 95% CI 0.37 to 0.72). However, mortality was not significantly reduced (RR 0.40, 95% CI 0.14 to 1.16).
Step 7: Medical treatment
Perioperative β blocker use is effective in high‐risk patients
Perioperative β blocker use in intermediate‐risk patients is still controversial
Statins seem to be associated with improved perioperative outcome
Results for other medical treatments are less conclusive for hard cardiac end points
Step 8: Preoperative coronary interventions
Preoperative coronary revascularisation in intermediate‐risk patients is not effective
Results for patients at very high risk are unknown
Cardiac event rate increases if non‐cardiac surgical procedure is within six weeks of percutaneous coronary intervention
STEP 8: PREOPERATIVE CORONARY INTERVENTIONS
Recent findings of the Coronary Artery Revascularization Prophylaxis Trial showed no survival benefit of preoperative coronary revascularisation in cardiac stable patients.29 Among 5859 patients scheduled for elective vascular operations a selection was made of patients considered at increased risk for cardiac events with evidence of severe coronary stenosis at coronary angiography. Anatomical criteria of exclusion included > 50% stenosis of the left main coronary artery, left ventricular ejection fraction < 20%, and severe stenosis of the aorta. The 510 patients selected were randomised to: optimal medical treatment (more than 80% were on β blocker therapy in both groups) with or without coronary revascularisation; either percutaneous coronary intervention (PCI) (59%, mean 18 days before surgery) or coronary artery bypass graft surgery (CABG) (41%, mean 54 days before surgery). No differences in mortality in the long‐term outcome (median follow up of 2.7 years) were found: 22% in the revascularisation group versus 23% in the non‐revascularisation group. Although the primary end point was late mortality, even the findings at 30 days did not show any difference in terms of mortality or postoperative myocardial infarction, nor did “prophylactic” revascularisation result in a reduction of the length of hospital stay.
Other, non‐randomised studies on preoperative coronary revascularisation are conflicting. The study by Eagle et al, based on the Coronary Artery Surgical Study (CASS) database, showed a significant benefit in patients with previous CABG. The same was found by the BARI investigators after both PCI and CABG. However, the time interval between coronary revascularisation and non‐cardiac surgery in these non‐randomised studies was relatively long. Two studies by Kaluza et al and Wilson et al showed that perioperative complications after PCI occur mainly when the patient undergoes non‐cardiac surgery within six weeks after PCI. Placement of coronary stents induces a denudation of the endothelial surface of the coronary artery, thereby greatly increasing the risk of thrombosis. This is further reinforced by the hypercoagulable state during surgery and the problematic use of antiplatelet treatment during surgery.
CONCLUSION
Clinical cardiac risk markers combined with ECG and the risk of the planned surgical procedure can effectively divide patients into a truly low‐risk, intermediate‐risk, and high‐risk population. Low‐risk patients probably can be operated on without any additional cardiac testing since these tests will not alter perioperative management. β blockers are recommended in patients with ischaemic heart disease and should be continued in patients on chronic β blocker treatment.
Intermediate‐risk patients are referred for cardiac testing to exclude extensive stress induced myocardial ischaemia, as β blockers provide insufficient myocardial protection in this case and preoperative coronary revascularisation should be considered, taking into account the coronary anatomy and the delay of the index surgical procedure due to revascularisation. Whether patients at intermediate risk without ischaemic heart disease should be treated with statins and/or β blockers is still the subject of debate. The currently ongoing POISE and DECREASE IV studies will probably provide us with the necessary evidence for this group of patients. Furthermore preoperative screening should not only focus on perioperative cardiac risk reduction but should also be considered as a unique opportunity to improve patients' long‐term cardiac outcome by proper medical treatment such as statins and β blockers.
Additional references appear on the Heart website—http://www.heartjnl.com/supplemental
Additional references appear on the Heart website—http://www.heartjnl.com/supplemental
Supplementary Material
Acknowledgements
Dr O Schouten is supported by unrestricted research grants from The Netherlands Organization for Health Research and Development (ZonMw), The Hague, The Netherlands, and the “Lijf en Leven” Foundation, Rotterdam, The Netherlands.
Footnotes
In compliance with EBAC/EACCME guidelines, all authors participating in Education in Heart have disclosed potential conflicts of interest that might cause a bias in the article
Additional references appear on the Heart website—http://www.heartjnl.com/supplemental
References
- 1.Boersma E, Kertai M D, Schouten O.et al Perioperative cardiovascular mortality in noncardiac surgery: validation of the Lee cardiac risk index. Am J Med 20051181134–1141.Validation and improvement by adding surgical risk and age of the Lee risk index in a cohort of 108 593 patients undergoing non‐cardiac surgery. [DOI] [PubMed] [Google Scholar]
- 2.Lee T H, Marcantonio E R, Mangione C M.et al Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 19991001043–1049.Most widely used preoperative index for the prediction of cardiac risk, based on six clinical risk factors. [DOI] [PubMed] [Google Scholar]
- 3.Goldman L, Caldera D L, Nussbaum S R.et al Multifactorial index of cardiac risk in noncardiac surgical procedures. N Engl J Med 1977297845–850. [DOI] [PubMed] [Google Scholar]
- 4.Ridley S. Cardiac scoring systems—what is their value? Anaesthesia 200358985–991. [DOI] [PubMed] [Google Scholar]
- 5.Detsky A S, Abrams H B, McLaughlin J R.et al Predicting cardiac complications in patients undergoing non‐cardiac surgery. J Gen Intern Med 19861211–219. [DOI] [PubMed] [Google Scholar]
- 6.Noordzij P G, Schreiner F, Galal W.et al Prognostic value of preoperative electrocardiography in patients undergoing non‐cardiac surgery. Circulation 2005112II833 [Google Scholar]
- 7.Boersma E, Kertai M D, Bax J J. A risk‐model to assess perioperative cardiovascular mortality, developed in 108,593 patients undergoing non‐cardiac surgery. Circulation 2003108652 [Google Scholar]
- 8.Yeh H M, Lau H P, Lin J M.et al Preoperative plasma N‐terminal pro‐brain natriuretic peptide as a marker of cardiac risk in patients undergoing elective non‐cardiac surgery. Br J Surg 2005921041–1045. [DOI] [PubMed] [Google Scholar]
- 9.Feringa H H, Bax J J, de Jonge R J.et al Plasma N‐terminal pro‐B‐type natriuretic peptide for preoperative cardiac risk stratification in patients scheduled for major non‐cardiac vascular surgery. Circulation 2005112II362 [Google Scholar]
- 10.Sabatine M S, Morrow D A, de Lemos J A.et al Acute changes in circulating natriuretic peptide levels in relation to myocardial ischemia. J Am Coll Cardiol 2004441988–1995. [DOI] [PubMed] [Google Scholar]
- 11.Schreiner F, Bax J J, Feringa H H.et al Poor glycaemic control in diabetic patients prior to major vascular surgery is associated with an increased mortality. Circulation 2005112II828 [Google Scholar]
- 12.Kertai M D, Boersma E, Bax J J.et al Comparison between serum creatinine and creatinine clearance for the prediction of postoperative mortality in patients undergoing major vascular surgery. Clin Nephrol 20035917–23. [DOI] [PubMed] [Google Scholar]
- 13.Kertai M D, Bountioukos M, Boersma E.et al Aortic stenosis: an underestimated risk factor for perioperative complications in patients undergoing noncardiac surgery. Am J Med 20041168–13.This article addresses the impact of the presence and severity of aortic valve abnormalities in patients undergoing non‐cardiac (vascular) surgery. [DOI] [PubMed] [Google Scholar]
- 14.Eagle K A, Berger P B, Calkins H.et al ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). Circulation 20021051257–1267.Most recent ACC/AHA guidelines for perioperative cardiovascular evaluation in non‐cardiac surgery. The ACC/AHA guidelines provide a framework for assessing cardiac risk in a variety of patients and surgical situations using a combination of evidence‐based and authority‐based approaches. The guidelines give an extensive overview of the literature. [PubMed] [Google Scholar]
- 15.Kertai M D, Boersma E, Bax J J.et al A meta‐analysis comparing the prognostic accuracy of six diagnostic tests for predicting perioperative cardiac risk in patients undergoing major vascular surgery. Heart 2003891327–1334.Meta‐analysis of the sensitivity and specificity of different types of preoperative non‐invasive cardiac tests for predicting adverse perioperative cardiac events. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mangano D T, Layug E L, Wallace A.et al Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter Study of Perioperative Ischemia Research Group. N Engl J Med 19963351713–1720.First large scale randomised study on the use of β blockers for the prevention of perioperative cardiac complications. [DOI] [PubMed] [Google Scholar]
- 17.Poldermans D, Boersma E, Bax J J.et al The effect of bisoprolol on perioperative mortality and myocardial infarction in high‐risk patients undergoing vascular surgery. Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. N Engl J Med 19993411789–1794.Randomised study in high‐risk patients undergoing non‐cardiac vascular surgery. This study shows an impressive improvement in cardiac outcome in high‐risk patients on β blockers compared to patients not on β blockers. [DOI] [PubMed] [Google Scholar]
- 18.Stevens R D, Burri H, Tramer M R. Pharmacologic myocardial protection in patients undergoing noncardiac surgery: a quantitative systematic review. Anesth Analg 200397623–633. [DOI] [PubMed] [Google Scholar]
- 19.Auerbach A D, Goldman L. Beta‐blockers and reduction of cardiac events in noncardiac surgery: scientific review. JAMA 20022871435–1444. [DOI] [PubMed] [Google Scholar]
- 20.Devereaux P J, Beattie W S, Choi P T.et al How strong is the evidence for the use of perioperative beta blockers in non‐cardiac surgery? Systematic review and meta‐analysis of randomised controlled trials. BMJ 2005331313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindenauer P K, Pekow P, Wang K.et al Perioperative beta‐blocker therapy and mortality after major noncardiac surgery. N Engl J Med 2005353349–361. [DOI] [PubMed] [Google Scholar]
- 22.Poldermans D, Bax J J, Kertai M D.et al Statins are associated with a reduced incidence of perioperative mortality in patients undergoing major noncardiac vascular surgery. Circulation 20031071848–1851.First study describing the beneficial effects perioperative statin use in patients undergoing non‐cardiac surgery. [DOI] [PubMed] [Google Scholar]
- 23.Durazzo A E, Machado F S, Ikeoka D T.et al Reduction in cardiovascular events after vascular surgery with atorvastatin: a randomized trial. J Vasc Surg 200439967–975.First randomised double‐blinded trial describing the beneficial effects perioperative statin use in patients undergoing non‐cardiac surgery. [DOI] [PubMed] [Google Scholar]
- 24.Lindenauer P K, Pekow P, Wang K.et al Lipid‐lowering therapy and in‐hospital mortality following major noncardiac surgery. JAMA 20042912092–2099. [DOI] [PubMed] [Google Scholar]
- 25.O'Neil‐Callahan K, Katsimaglis G, Tepper M R.et al Statins decrease perioperative cardiac complications in patients undergoing noncardiac vascular surgery: the Statins for Risk Reduction in Surgery (StaRRS) study. J Am Coll Cardiol 200545336–342. [DOI] [PubMed] [Google Scholar]
- 26.Nishina K, Mikawa K, Uesugi T.et al Efficacy of clonidine for prevention of perioperative myocardial ischemia: a critical appraisal and meta‐analysis of the literature. Anesthesiology 200296323–329. [DOI] [PubMed] [Google Scholar]
- 27.Oliver M F, Goldman L, Julian D G.et al Effect of mivazerol on perioperative cardiac complications during non‐cardiac surgery in patients with coronary heart disease: the European Mivazerol Trial (EMIT). Anesthesiology 199991951–961. [DOI] [PubMed] [Google Scholar]
- 28.Wijeysundera D N, Beattie W S. Calcium channel blockers for reducing cardiac morbidity after noncardiac surgery: a meta‐analysis. Anesth Analg 200397634–641. [DOI] [PubMed] [Google Scholar]
- 29.McFalls E O, Ward H B, Moritz T E.et al Coronary‐artery revascularization before elective major vascular surgery. N Engl J Med 20043512795–2804.Large randomised trial evaluating the effect of preoperative cardiac revascularisation on postoperative outcome. This study showed no benefit for patients who underwent preoperative cardiac revascularisation (PCI or CABG) compared to patients without preoperative cardiac revascularisation. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.