Abstract
Background
Most new tuberculosis vaccines will be administered as a booster to subjects primed with bacille Calmette-Guérin (BCG) during childhood.
Methods
We investigated in vivo and in vitro immune responses to mycobacteria in human immunodeficiency virus (HIV)–positive subjects in Tanzania primed with BCG during childhood and entering a tuberculosis booster vaccine trial. Tests included intradermal skin testing for Mycobacterium tuberculosis purified protein derivative (PPD) and Mycobacterium avium sensitin (MAS); lymphocyte proliferation assays and interferon (IFN)–γ levels after stimulation with Mycobacterium vaccae sonicate (MVS), M. tuberculosis early secreted antigen (ESAT)–6, M. tuberculosis antigen 85 (Ag85), or M. tuberculosis whole-cell lysate (WCL); and determination of serum antibody to lipoarabinomannin (LAM).
Results
A total of 888 subjects with CD4 cell counts ≥200 cells/mm3 were enrolled. PPD and MAS test results were positive in 34% and 30% of the subjects, respectively. Proliferative responses were detected as follows: MVS, 6%; Ag85, 24%; ESAT-6, 21%; and WCL, 59%. IFN-γ responses were 2%, 6%, 12%, and 38%, respectively. LAM antibody was detected in 28% of the subjects. Subjects were more likely to have detectable proliferative and IFN-γ responses if they had positive PPD test results or CD4 cell counts ≥500 cells/mm3. Overall, 94% of the subjects had evidence of primed mycobacterial immune responses.
Conclusion
Of HIV-positive BCG-immunized adults with CD4 cell counts ≥200 cells/mm3 in Tanzania, 94% are primed for booster mycobacterial immunization.
Evolving data suggest that the optimal immunological approach to an improved immunization strategy against tuberculosis is a prime-boost strategy: administration of a priming antigen followed by a heterologous boosting antigen [1–5]. Because most countries in which tuberculosis is endemic administer bacille Calmette-Guérin (BCG) at birth, many candidate tuberculosis vaccines will first be tested as booster vaccines in subjects who were primed with BCG at birth [6]. Persons with HIV infection are at the highest risk of morbidity and mortality from tuberculosis and represent a priority for the development of improved tuberculosis vaccine strategies [7]. Baseline cellular and humoral immune responses to mycobacteria after BCG vaccination have been characterized in HIV-negative individuals [8, 9] but not in a large cohort of HIV-infected adults with a history of childhood BCG immunization, to evaluate their suitability for boosting. Here, we present data on skin test results and in vivo responses to mycobacterial antigens among HIV-infected subjects entering a phase 3 tuberculosis booster vaccine trial in Tanzania (the DARDAR Study).
METHODS
Study subjects and protocol
The DARDAR Study is a phase 3 trial of a prime-boost vaccine strategy for the prevention of HIV-associated tuberculosis (the prime is BCG during childhood; the boost is multiple-dose whole-cell inactivated mycobacterial vaccine) that is being conducted in Dar es Salaam, Tanzania [10]. Ambulatory HIV-infected subjects ≥18 years old are referred from voluntary HIV counseling and testing centers in Dar es Salaam or by other study subjects. All subjects are screened via a standardized interview (which includes history of treatment for active tuberculosis), physical examination, measurement of CD4 cell count, and single-view chest radiography. Eligible subjects who provide informed consent are enrolled if they have 2 positive ELISA antibody test results for HIV, a CD4 cell count ≥200 cells/mm3, a BCG scar, and no evidence of active tuberculosis. Subjects with CD4 cell counts ≥200 cells/mm3 who are potentially eligible for the study are further evaluated via 3 expectorated sputum samples collected for acid-fast bacilli smear and culture and a single mycobacterial blood culture. All subjects undergo baseline phlebotomy for assessment of mycobacterial immune responses and then have 2 intradermal skin tests performed: 0.1-mL tuberculin (RT-23; State Serum Institute, Denmark) and 0.1-mL Mycobacterium avium sensitin (MAS; State Serum Institute). Skin tests are read as millimeters of induration at 48–72 h, and reactions ≥5 mm are considered to be positive. The human-experimentation guidelines of the US Department of Health and Human Services, as well as those of the Research Ethics Committee of the Muhumbili University College of Health Sciences, were followed in the conduct of this research. This study is registered through the US National Institutes of Health (NCT00052195).
Laboratory methods
Serum samples were tested by 2 different HIV ELISA methods. The Vironostika HIV Uni-Form II Ag/Ab test (bioMérieux) was used first, with reactive samples being retested by the Vironostika HIV Uni-Form II Plus O test (bioMérieux). Only samples that were reactive in both tests were accepted as being positive. Blood was collected in EDTA tubes for enumeration of CD4 cells by use of a FACSCount machine (Becton Dickinson) after staining with monoclonal antibodies [11].
Lymphocyte proliferation assays (LPAs) were done on freshly isolated peripheral-blood mononuclear cells by a standard [3H]-thymidine incorporation method, with a 5-day stimulation with either medium alone, 2 mg/mL M. vaccae sonicate (MVS), 1 mg/mL M. tuberculosis antigen 85 (Ag85), 2 mg/mL M. tuberculosis early secreted antigen (ESAT)–6, or 1 mg/mL M. tuberculosis whole-cell lysate (WCL). Results were expressed as a proliferation index (PI; counts per minute for the antigen-stimulated cells divided by that for the unstimulated cells) and a PI ≥3 was considered to be positive. LPAs were considered to be valid if the positive control (2.5 µg/mL phytohemagglutinin) or one of the experimental antigens had a PI ≥3, there was no spillover between wells, and the counts per minute for the unstimulated cells was <5000.
Interferon (IFN)–γ levels in cell culture supernatants after a 5-day stimulation with the same antigens used in the LPAs were determined by ELISA. Levels of antibody to M. tuberculosis lipoarabinomannin (LAM) were measured by standard ELISA. Serum from an M. tuberculosis–experienced individual, aliquotted into small samples and diluted 1:10 in 5% goat serum in PBS, was used as a positive control, and the color reaction in each assay was allowed to develop until the OD of the positive control reached 2.0. A pool of serum samples from tuberculosis-naive individuals was used as a negative control. Positive results for the IFN-γ supernatant and LAM antibody ELISAs were defined as ≥3 SDs above the mean.
Statistical analysis
Standard descriptive statistical methods were used to characterize baseline immunological responses to mycobacterial antigens. Comparisons between groups were done using 2-sided t tests; P < .05 was considered to be statistically significant. Data analysis was undertaken on Stata software (version 8; StataCorp).
RESULTS
Characteristics of the study subjects are shown in table 1. All subjects were HIV positive, had BCG scars, and had CD4 cell counts ≥200 cells/mm3. Of the 888 subjects enrolled in the study to date, baseline LPA results were available for 549, IFN-γ results were available for 742, and paired results were available for 454. LAM results were available for all 888 subjects. Prior treatment for tuberculosis was reported by 9% of the subjects. Skin test results are summarized in table 2. Approximately one-third of the subjects had positive PPD skin test results, with equivalent rates in patients with CD4 cell counts <500 and ≥500 cells/mm3.
Table 1.
Characteristics of the study subjects.
| Characteristic | Value |
|---|---|
| Age, median, years | 33 |
| Female | 668 (75) |
| BCG scar | 888 (100) |
| CD4 cell count | |
| ≥200 cells/mm3 | 888 (100) |
| ≥500 cells/mm3 | 320 (36) |
| Median, cells/mm3 | 421 |
| HIV load, median, log10 copies/mL | 4.09 |
| Prior tuberculosis | 78 (9) |
NOTE. Data are no. (%) of subjects, unless otherwise indicated. BCG, bacille Calmette-Guérin.
Table 2.
Detectable in vivo immune responses to mycobacterial antigens, by CD4 cell count.
| CD4 cell count | ||||
|---|---|---|---|---|
| Skin test | 200–499 cells/mm3 | ≥500 cells/mm3 | P | All |
| PPD ≥5 mm | 176/555 (32) | 113/306 (37) | .121 | 289/861 (34) |
| MAS ≥5 mm | 115/416 (28) | 75/212 (35) | .046 | 190/628 (30) |
NOTE. Data are proportion (%) of subjects, unless otherwise indicated. MAS, Mycobacterium avium sensitin; PPD, Mycobacterium tuberculosis purified protein derivative.
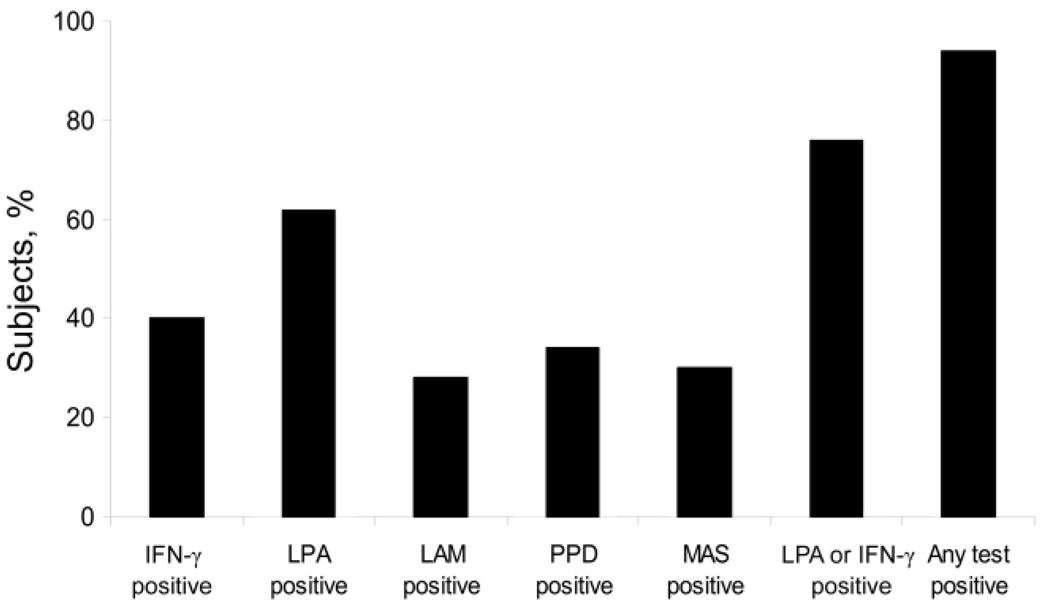
Figure 1 shows the percentage of subjects who had detectable immune responses to mycobacterial antigens on in vivo testing. Fully 94% of the subjects had detectable mycobacterialimmune responses by any assay and 76% had detectable immune responses by LPA or IFN-γ ELISA, whereas only 34% had a positive PPD skin test result. Table 3 provides data on in vivo immune responses to mycobacterial antigens. Responses were highest to WCL, with recognition by 58% of the subjects by LPA and by 38% of the subjects by IFN-γ ELISA. Responses to the tuberculosis-specific antigen ESAT-6 were lower, at 21% by LPA and 12% by IFN-γ ELISA. Differences in response rates by CD4 cell count were more pronounced for the IFN-γ ELISA, by which responses were seen more frequently in the subjects with CD4 cell counts ≥500 cells/mm3. However, LPA responses to ESAT-6 were more pronounced in the lower CD4 cell count stratum. Levels of antibody to the nonspecific mycobacterial antigen LAM were similar in subjects with CD4 cell counts <500 and ≥500 cells/mm3. Table 4 shows that 94% of all HIV-infected subjects responded to either an in vivo or in vitro assay of mycobacterial immune responses. Table 5 shows that detectable cellular, but not humoral, immune responses to Ag85, ESAT-6, and WCL were more frequent in the subjects with a positive PPD skin test result than in those with a negative result. Proliferative responses to MVS were slightly more frequent in the subjects with a positive PPD result, whereas the frequency of IFN-γ responses was not different between the 2 groups.
Figure 1.
Percentage of subjects with detectable immune responses to mycobacterial antigens by in vivo and in vitro tests. IFN, interferon; LAM, antibody to Mycobacterium tuberculosis lipoarabinomannin; LPA, lymphocyte proliferation assay; MAS, Mycobacterium avium sensitin; PPD, M. tuberculosis purified protein derivative.
Table 3.
Detectable in vitro immune responses to mycobacterial antigens, by CD4 cell count.
| CD4 cell count | ||||
|---|---|---|---|---|
| Assay, antigen | 200–499 cells/mm3 | ≥500 cells/mm3 | P | All |
| Positive LPA index | ||||
| MVS | 11/242 (5) | 9/115 (8) | .241 | 20/357 (6) |
| Ag85 | 62/242 (26) | 23/115 (20) | .721 | 85/357 (24) |
| ESAT-6 | 59/236 (25) | 16/115 (14) | .007 | 75/351 (21) |
| WCL | 143/242 (59) | 66/115 (57) | .784 | 209/357 (59) |
| Positive IFN-γ response | ||||
| MVS | 2/448 (0.4) | 11/294 (4) | .001 | 13/742 (2) |
| Ag85 | 15/448 (3) | 30/294 (10) | <.001 | 56/742 (6) |
| ESAT-6 | 37/430 (9) | 41/290 (14) | .019 | 84/720 (12) |
| WCL | 139/448 (31) | 143/294 (49) | <.001 | 282/742 (38) |
| Positive response, LAM | 158/566 (28) | 94/324 (29) | .727 | 252/890 (28) |
NOTE. Data are proportion (%) of subjects, unless otherwise indicated. Ag85, Mycobacterium tuberculosis antigen 85; ESAT, M. tuberculosis early secreted antigen; IFN, interferon; LAM, M. tuberculosis lipoarabinomannin; LPA, lymphocyte proliferation assay; MVS, Mycobacterium vaccae sonicate (vaccine antigen); WCL, M. tuberculosis whole-cell lysate.
Table 4.
Combined in vivo and in vitro immune responses to mycobacterial antigens, by CD4 cell count.
| CD4 cell count | No. (%) positivea | Pb |
|---|---|---|
| 200–499 cells/mm3 | 420/443 (95) | .167 |
| ≥500 cells/mm3 | 263/285 (92) | |
| All | 683/728 (94) |
Positive if any mycobacterial response detected (by skin test, lymphocyte proliferation assay, interferon-γ ELISA, or lipoarabinomannin antibody ELISA).
For the comparison between the subjects with CD4 cell counts 200–499 cells/mm3 and those with counts ≥500 cells/mm3.
Table 5.
Detectable in vitro immune responses to mycobacterial antigens, by Mycobacterium tuberculosis purified protein derivative (PPD) skin test result.
| PPD | ||||
|---|---|---|---|---|
| Assay, antigen | 0–4 mm | ≥5 mm | P | All |
| Positive LPA index | ||||
| MVS | 6/154 (4) | 5/88 (6) | .040 | 20/357 (6) |
| Ag85 | 10/154 (6) | 52/88 (59) | <.001 | 85/357 (24) |
| ESAT-6 | 20/150 (13) | 39/86 (45) | <.001 | 75/351 (21) |
| WCL | 63/154 (41) | 80/88 (91) | <.001 | 209/357 (59) |
| Positive IFN-γ response | ||||
| MVS | 8/467 (2) | 5/249 (2) | .789 | 13/742 (2) |
| Ag85 | 10/467 (2) | 32/249 (13) | <.001 | 55/742 (6) |
| ESAT-6 | 17/452 (4) | 58/244 (24) | <.001 | 78/720 (11) |
| WCL | 96/467 (21) | 177/249 (71) | <.001 | 282/742 (38) |
| Positive response, LAM | 252/572 (27) | 82/287 (29) | .729 | 252/890 (28) |
NOTE. Data are proportion (%) of subjects, unless otherwise indicated. P values are for any PPD ≥5 mm compared with PPD <5 mm. Ag85, Mycobacterium tuberculosis antigen 85; ESAT, M. tuberculosis early secreted antigen; IFN, interferon; LAM, M. tuberculosis lipoarabinomannin; LPA, lymphocyte proliferation assay; MVS, Mycobacterium vaccae sonicate (vaccine antigen); WCL, M. tuberculosis whole-cell lysate.
The 45 subjects without detectable immune responses to mycobacteria by skin test, LPA, IFN-γ ELISA, or LAM antibody ELISA had similar ages (median, 31 vs. 33 years; P = .088) and CD4 cell counts (median, 495 vs. 485 cells/mm3;P = .792)as those with detectable immune responses. The subjects without detectable immune responses to mycobacteria were more likely to be female than were those with detectable antimycobacterial immune responses (89% vs. 74%; P = .016).
DISCUSSION
We have shown that the vast majority of HIV-infected adults in Tanzania who have scar evidence of childhood BCG immunization have detectable immune responses to mycobacterial antigens during adulthood. This suggests that most HIV-infected adults in Tanzania are primed for boosting with a new tuberculosis vaccine.
We used 4 different methods to detect mycobacterialimmune responses and assayed reactions to both specific and nonspecific mycobacterial antigens. In vivo intradermal skin testing with tuberculin, a nonspecific preparation of proteins derived from M. tuberculosis, detected delayed-type hypersensitivity (DTH) reactions in only 34% of subjects. Notably, the proportion of subjects with positive results for tuberculin skin testing was not different by CD4 cell count strata (<500 and ≥500 cells/mm3).
In vitro testing detected a higher proportion of mycobacterial immune responses than did in vivo testing. The most widely recognized antigen was WCL. As with tuberculin, it is likely that reactions to this reagent may have been due to latent tuberculosis, prior infection with nontuberculous mycobacteria, immunization with BCG [12], or some combination thereof. Proliferative responses to the M. tuberculosis antigen ESAT-6 were present in 35% of the subjects with proliferative responses to WCL, whereas 26% of the subjects with IFN-γ responses to WCL also had detectable responses to ESAT-6. These data reflect the minimum proportion of subjects whose mycobacterial immune responses are the result of prior infection with or disease due to M. tuberculosis [13].
Although IFN-γ responses were more common in the subjects with CD4 cell counts ≥500 cells/mm3, LPA responses to ESAT-6 were notably higher in the lower CD4 cell count stratum. This is an unexpected result, because proliferative responses to alloantigens typically wane during the progression of HIV infection [14]. We postulate that lower CD4 cell counts may correlate with a longer time at risk for HIV-related recrudescent tuberculosis and a consequent increase in tuberculosis-specific T cell proliferation, and we will continue to assess this pattern as enrollment continues.
The results of IFN-γ ELISAs and LPAs did not correlate perfectly. One major reason for this observation is the fact that the >3 SD threshold used to consider an IFN-γ result to be positive in a population highly exposed to mycobacteria is more stringent than the PI used in the LPAs. It is likely that a greater proportion of IFN-γ responses would have been considered positive if the cutoff used was based instead on the mean response in an unexposed population. Beyond this, the discordant results from IFN-γ and LPA testing may in part reflect the complex immune response to mycobacteria, which may not always involve coordinated and simultaneous lymphoproliferative and cytokine responses. Last, some part of this discordance is likely attributable to HIV infection, which clearly alters immune responses to mycobacteria, as supported by the higher frequency of IFN-γ responses in the higher CD4 cell count stratum.
Our findings have several implications for the interpretation of previous BCG studies and for the design and interpretation of newer studies of prime-boost tuberculosis immunization strategies. Most trials of BCG in mycobacteria-naive infants and newborns have shown efficacy rates of 75%–80% [15]. Importantly, BCG trials in older children and adults were designed to exclude subjects with preexisting mycobacterial immune responses but relied on in vivo DTH skin testing to detect such immune responses. However, we have shown that, in an HIV-infected population, DTH skin testing is much less sensitive than combined contemporary in vivo or ex vivo assays of immune response to mycobacteria. These data raise the possibility that prior studies of BCG efficacy in HIV-uninfected subjects designated a significant fraction of subjects as being mycobacteria naive when more-contemporary assays would instead have demonstrated detectable mycobacterial immunity. This possibility, which warrants additional investigation, could result in an artifactual diminution of the apparent efficacy of BCG in these populations, because BCG vaccination may not have been undertaken in an entirely mycobacteria-naive population.
Studies in HIV-negative subjects who received BCG during childhood have shown demonstrable in vivo responses to mycobacterial antigens during adulthood [8, 9]. In vitro cytokine secretion to tuberculin PPD has also been reported in as many as 49% of HIV-positive adults with scars from childhood BCG vaccination [16]. The present study is the first to examine both in vivo and in vitro responses to multiple mycobacterial antigens in HIV-positive adults who received BCG during childhood and is the first to show that such responses are detectable in almost all subjects. Even when our analysis is restricted to LPA and IFN-γ responses, which reflect critical cellular immunity to mycobacteria, the present data demonstrate that the great majority of HIV-infected adults in Tanzania have primed mycobacterial immunity.
Because tuberculosis-specific cellular immune responses are impaired in BCG-vaccinated HIV-infected adults [17], higher rates of prior mycobacterial immune responses would be likely in adults without HIV infection from the same area. The 6% of subjects without detectable responses to mycobacterial antigens by any assay are demographically similar to the subjects with immune responses to mycobacteria. These 6% may represent a mix of subjects with HLA types who did not optimally present the antigens used in this study, subjects with HIV-induced functional impairment of immune responses, and adults who remain truly mycobacteria naive. More-sensitive assays (such as the enzyme-linked immunospot assay) and the use of additional antigens (e.g., culture filtrate protein 10) may have shown a greater prevalence of detectable mycobacteria-specific immune responses in subjects with exposure to mycobacteria.
We demonstrated a low baseline frequency of responses to MVS. This shows that, although HIV-infected subjects in Tanzania have a likelihood of prior exposure to mycobacterial antigens, preexisting immune responses to M. vaccae are uncommon. This means that detection of vaccine-induced responses to M. vaccae will be possible even in a mycobacteria-experienced population.
In summary, HIV-infected adults in Tanzania who had received childhood BCG vaccination were highly likely to demonstrate detectable immune responses to mycobacteria and, thus, are effectively primed for booster vaccination with MVS.
Acknowledgments
Financial support: Department of AIDS, National Institutes of Health (grant AI 45407); Fogarty International Center (grant D43-TW006807).
Footnotes
Potential conflicts of interest: none reported.
References
- 1.McConkey SJ, Reece WH, Moorthy VS, et al. Enhanced T-cell immunogenicity of plasmid DNA vaccines boosted by recombinant modified vaccinia virus Ankara in humans. Nat Med. 2003;9:729–735. doi: 10.1038/nm881. [DOI] [PubMed] [Google Scholar]
- 2.Kaufmann SH. Recent findings in immunology give tuberculosis vaccines a new boost. Trends Immunol. 2005;26:660–667. doi: 10.1016/j.it.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 3.McShane H, Hill A. Prime-boost immunisation strategies for tuberculosis. Microbes Infect. 2005;7:962–967. doi: 10.1016/j.micinf.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Goonetilleke NP, McShane H, Hannan CM, Anderson RJ, Brookes RH, Hill AV. Enhanced immunogenicity and protective efficacy against Mycobacterium tuberculosis of bacille Calmette-Guerin vaccine using mucosal administration and boosting with a recombinant modified vaccinia virus Ankara. J Immunol. 2003;171:1602–1609. doi: 10.4049/jimmunol.171.3.1602. [DOI] [PubMed] [Google Scholar]
- 5.Tanghe A, D’Souza S, Rosseels V, et al. Improved immunogenicity and protective efficacy of a tuberculosis DNA vaccine encoding Ag85 by protein boosting. Infect Immun. 2001;69:3041–3047. doi: 10.1128/IAI.69.5.3041-3047.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McShane H, Pathan AA, Sander CR, et al. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat Med. 2004;10:1240–1244. doi: 10.1038/nm1128. [DOI] [PubMed] [Google Scholar]
- 7.Dye C. Global epidemiology of tuberculosis. Lancet. 2006;367:938–940. doi: 10.1016/S0140-6736(06)68384-0. [DOI] [PubMed] [Google Scholar]
- 8.Ravn P, Boesen H, Pedersen BK, Andersen P. Human T cell responses induced by vaccination with Mycobacterium bovis bacillus Calmette-Guerin. J Immunol. 1997;158:1949–1955. [PubMed] [Google Scholar]
- 9.Black GF, Weir RE, Floyd S, et al. BCG-induced increase in interferon-gamma response to mycobacterial antigens and efficacy of BCG vaccination in Malawi and the UK: two randomised controlled studies. Lancet. 2002;359:1393–1401. doi: 10.1016/S0140-6736(02)08353-8. [DOI] [PubMed] [Google Scholar]
- 10.Vuola JM, Ristola MA, Cole B, et al. Immunogenicity of an inactivated mycobacterial vaccine for the prevention of HIV-associated tuberculosis: a randomized, controlled trial. AIDS. 2003;17:2351–2355. doi: 10.1097/00002030-200311070-00010. [DOI] [PubMed] [Google Scholar]
- 11.Landay A, Ohlsson-Wilhelm B, Giorgi JV. Application of flow cytometry to the study of HIV infection. AIDS. 1990;4:479–497. doi: 10.1097/00002030-199006000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Kemp EB, Belshe RB, Hoft DF. Immune responses stimulated by percutaneous and intradermal bacille Calmette-Guerin. J Infect Dis. 1996;174:113–119. doi: 10.1093/infdis/174.1.113. [DOI] [PubMed] [Google Scholar]
- 13.Johnson PD, Stuart RL, Grayson ML, et al. Tuberculin-purified protein derivative-, MPT-64-, and ESAT-6-stimulated gamma interferon responses in medical students before and after Mycobacterium bovis BCG vaccination and in patients with tuberculosis. Clin Diagn Lab Immunol. 1999;6:934–937. doi: 10.1128/cdli.6.6.934-937.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyaard L, Otto SA, Hooibrink B, Miedema F. Quantitative analysis of CD4+ T cell function in the course of human immunodeficiency virus infection: gradual decline of both naive and memory alloreactive T cells. J Clin Invest. 1994;94:1947–1952. doi: 10.1172/JCI117545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Reyn CF, Vuola JM. New vaccines for the prevention of tuberculosis. Clin Infect Dis. 2002;35:465–474. doi: 10.1086/341901. [DOI] [PubMed] [Google Scholar]
- 16.Elliott AM, Hodsdon WS, Kyosiimire J, et al. Cytokine responses and progression to active tuberculosis in HIV-1-infected Ugandans: a prospective study. Trans R Soc Trop Med Hyg. 2004;98:660–670. doi: 10.1016/j.trstmh.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Sutherland R, Yang H, Scriba TJ, et al. Impaired IFN-gamma-secreting capacity in mycobacterial antigen-specific CD4 T cells during chronic HIV-1 infection despite long-term HAART. AIDS. 2006;20:821–829. doi: 10.1097/01.aids.0000218545.31716.a4. [DOI] [PubMed] [Google Scholar]