Abstract
To determine how norepinephrine affects the basic physiological properties of catecholaminergic neurons, brain slices containing the Substantia Nigra Pars Compacta and Locus Coeruleus were studied with cell-attached and whole-cell recordings in control and dopamine β-hydroxylase knockout (Dbh −/−) mice that lack norepinephrine. In the cell-attached configuration, the spontaneous firing rate and pattern of Locus Coeruleus neurons recorded from Dbh −/− mice was the same as the firing rate and pattern recorded from heterozygous littermates (Dbh +/−). During whole-cell recordings, synaptic stimulation produced an α-2 receptor-mediated outward current in the Locus Coeruleus of control mice that was absent in Dbh −/− mice. Normal α-2 mediated outward currents were restored in Dbh −/− slices after pre-incubation with norepinephrine. Locus Coeruleus neurons also displayed similar changes in holding current in response to bath application of norepinephrine, UK 14304, and methionine-enkephalin. Dopamine neurons recorded in the Substantia Nigra Pars Compacta similarly showed no differences between slices harvested from Dbh −/− and control mice. These results indicate that endogenous norepinephrine is not necessary for the expression of catecholaminergic neuron firing properties or responses to direct agonists, but is necessary for auto-inhibition mediated by indirect α-2 receptor stimulation.
Keywords: dopamine β-hydroxylase knockout, locus coeruleus, substantia nigra pars compacta, cocaine
The activity of norepinephrine (NE) neurons in the locus coeruleus (LC) has an important role in selective attention, general arousal, and stress reactions upon challenging environmental situations (Foote et al., 1983; Levine et al., 1990; Berridge and Waterhouse, 2003; Aston-Jones and Cohen, 2005), and NE depletion may underlie some affective disorders and disease states (Ressler and Nemeroff, 1999). In addition, the LC projects directly to, and modulates the activity of, midbrain dopamine (DA) neurons (Swanson and Hartman, 1975; Jones and Moore, 1977; Grenhoff et al., 1993; Grenhoff and Svensson, 1993; Liprando et al., 2004), which are critical for reward and motor related behaviors (Girault and Greengard, 2004; Montague et al., 2004). In an intact animal, NE neurons recorded in vitro fire spontaneously in a single-spike pattern at a rate of up to 5 spikes per second (Williams et al., 1984). However, it is not known whether NE depletion affects the basic physiological properties of NE or DA neurons.
Dopamine β-hydroxylase knockout (Dbh −/−) mice completely lack NE and have many brain-mediated behavioral abnormalities, including impaired maternal and social behavior, defects in memory retrieval, increased seizure susceptibility, dysregulation of DA signaling, and hypersensitivity to psychostimulants (Thomas et al., 1995; Thomas and Palmiter, 1997; Thomas et al., 1998; Szot et al., 1999; Weinshenker and Szot, 2002; Murchison et al., 2004; Marino et al., 2005; Schank et al., 2005). NE neurons are intact and make proper connections in Dbh −/− mice, and receptor levels and co-transmitter expression are remarkably normal (Weinshenker et al., 2002b; Jin et al., 2004; Sanders et al., in press; D. Weinshenker unpublished observations). Many Dbh −/− phenotypes are reversible by acute pharamcological restoration of NE, indicating that the phenotypes are specifically due to NE deficiency (Thomas and Palmiter., 1997b; Thomas et al., 1998; Szot et al., 1999; Weinshenker et al., 2000). However, it is unclear whether NE replacement truly restores the NE system to a “wild-type” state. In addition, other Dbh −/− phenotypes cannot be pharmacologically rescued (Thomas and Palmiter, 1997b; Weinshenker et al., 2002a), provoking the question of whether the chronic absence of NE affects the firing properties of LC neurons and contributes to these deficits. Here we assessed the properties of NE neurons from Dbh −/− mice to gain insights into how this neurotransmitter influences LC activity. We also examined the firing properties of DA neurons to determine whether the dysregulation of DA release in Dbh −/− mice is an inherent abnormality in DA neurons.
Experimental Procedures
Animals
Mice were used in accordance with guidelines for animal care and use established by the National Institutes of Health, and the Animal Care Committees at the University of Texas at San Antonio, Emory University, and Oregon Health and Science University. Dbh −/− mice were bred as described and maintained on a mixed C57BL6/J and 129SvEv genetic background (Thomas et al., 1998). Dbh +/− mice have normal brain NE content and are behaviorally indistinguishable from wild-type mice, and were used as controls (Thomas & Palmiter, 1997, 1998; Szot et al., 1999; Marino et al., 2005). Because complete NE deficiency is lethal embryonically in mice (Thomas et al., 1995), NE was restored to knockout embryos by supplementing the drinking water of pregnant dams with adrenergic agonists from E 9.5 to E 14.5, and L-3,4-dihydroxyphenylserine (DOPS) from E 14.5 until birth (Thomas et al., 1995; Thomas et al., 1998). DOPS can be converted to NE by the enzyme aromatic acid decarboxylase, thus bypassing the requirement for DBH. NE is not required for postnatal survival, so the mice used in the study lacked NE since birth. Dbh −/− and littermate Dbh +/− mice used for recordings in vitro were 6-12 weeks old.
Slice Recordings
Horizontal brain slices (200–300 μm) were prepared from 26 mice (11 Dbh −/− mice and 15 Dbh +/− mice) as described previously (Torrecilla et al., 2002). The experimenter was blind to the genotype of the animal until after the experiments were completed. Horizontal slices were placed in a chamber (0.5 ml) superfused with physiological saline (35°C) at a rate of 1.5 ml/min. The solution was equilibrated with 95% O25% CO2 (pH 7.4) and contained 126 mM NaCl, 2.5 mM KCl, 1.2 mM MgCl2, 2.4 mM CaCl2, 1.4 mM NaH2PO4, 25 mM NaHCO3, and 11 mM Dglucose. The internal solution used for NE neuron whole-cell recordings contained 115 mM K-methyl sulfate, 20 mM KCl, 1 mM MgCl2, 10 mM Hepes, 0.1 mM EGTA, 2 mM ATP, 0.3 mM GTP, and 10 mM creatine phosphate. DA neuron recordings contained the same internal solution except 10 mM BAPTA was used instead of 0.1 mM EGTA. Patch recordings were made by using an Axopatch 1D amplifier (Axon Instruments, Foster City, CA). Midbrain DA neurons were identified by their electrical properties, which included slow spontaneous activity and a hyperpolarization-induced inward current (H-current Johnson & North, 1992; Mercuri et al., 1995; Neuhoff et al., 2002). NE neurons were identified visually within the locus coeruleus and fired action potentials at a rate up to 5 Hz (Williams et al., 1984; Alvarez-Maubecin et al., 2000).
Evoked Responses
Iontophoretic pipettes (20 –50 MΩ) were filled with NE or DA (0.5 M, pH 7.5) and placed within 10 μm of the soma or proximal dendrite. Iontophoretic pulses (50 nA, 50 ms; −1 nA backing current) were applied once per minute. Synaptic currents were evoked with bipolar tungsten stimulating electrodes with a tip separation of 300–600 μm and placed rostral to the recording site (within 1 mm). A train of 5–10 stimuli (400 μsec at 0.3– 0.5 mA) was delivered at 66 Hz once every 60 sec. Evoked responses were measured as the peak amplitude and width of the response at 50% of amplitude, relative to baseline holding current directly before stimulation. Picrotoxin (100 μM) and strychnine (1 μM) were used to block γ-aminobutyric acid type A (GABAA) and glycine receptors, respectively. The α-2-adrenergic receptor inhibitory postsynaptic potential was isolated by using 2,3-dihydroxy-6-nitro-7-sulfamoylbenzo[f]-quinoxaline (NBQX) (5 μM), MK-801 (50 μM), and CGP 56999a (100 nM) to block α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), N-methyl-D-aspartate (NMDA), and γ-amino-butyric acid type B (GABAB) receptors, respectively.
Drugs
Drugs were applied to the slice by superfusion, except when norepinephrine or dopamine was iontophoresed (see above). Adenosine trisphosphate, baclofen, dopamine-HCl, guanosine trisphosphate, norepinephrine, methionine-enkephalin, picrotoxin, yohimbine, and strychnine were from Sigma. S(−)-eticlopride and MK-801 were from Research Biochemicals (Natick, MA). NBQX, (S)- methyl-4-carboxyphenylglycine (MCPG), and (S)-3,5-dihydroxyphenylglycine were from Tocris Cookson (St. Louis). CGP 56999a was a gift from Novartis Pharmaceuticals (Basel). Cocaine-HCl was from the National Institute on Drug Abuse.
Data Analysis
Values are given as means ± SEM. For all experiments, P < 0.05 was considered as a significant difference. The firing pattern coefficient of variation, defined as the standard deviation divided by the mean firing rate, was also measured. For in vitro recordings, the change produced by a drug was calculated as the mean holding evoked current amplitude 30 s after equilibrium had been reached relative to the holding current before drug superfusion. Unpaired comparisons between two groups were made with a Mann–Whitney U test, whereas paired comparisons were made by using a Wilcoxon signed-rank test. Concentration-response curves were compared with a two way repeated-measures ANOVA. Maximum D2 receptor-mediated currents and IH were analyzed with unpaired Student's t-tests.
Results
Basic electrophysiological properties of LC neurons
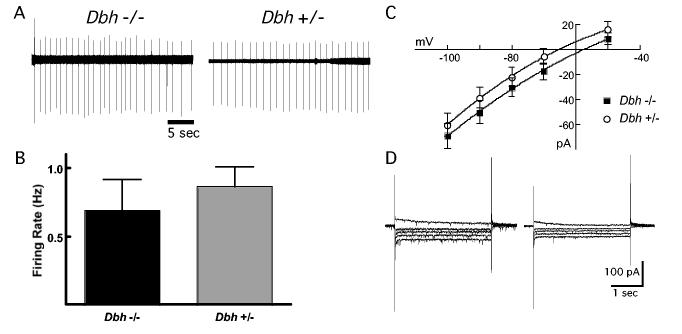
NE neurons in the LC fire spontaneously in vitro in a single-spike pattern. A typical firing pattern of a LC neuron recorded in the cell-attached mode for both a Dbh −/− and control mouse is shown in Fig. 1A. The average firing rates from Dbh −/− and control mice were the same (Fig. 1B; Dbh −/−: 0.8 ± 0.2 spikes per s, n = 15; Dbh +/−: 0.9 ± 0.1 spikes per s, n = 17; p = 0.10). There was also no difference in firing pattern between Dbh −/− and control mice. The coefficient of variation in control mice and Dbh −/− was 49.0 ± 12.2% and 47.6 ± 18.8%, respectively (n = 12, 9; p = 0.95). NE neurons also display a characteristically linear current response to hyperpolarizing voltage commands (10 mV steps −50 to −80 mV) (Williams et al., 1984). The currents were measured and compared between Dbh −/− and control mice, and no difference in amplitude could be detected (Fig. 1C, D; n = 24; p = 0.62). Thus, the postnatal formation and maintenance of these basic electrophysiological properties of LC neurons are similar in Dbh −/− and control mice.
Figure 1.
The firing pattern and membrane conductances in vitro are the same between Dbh −/− and control (Dbh +/−) mice. (A) Cell-attached voltage recording of a noradrenergic neuron firing rate and pattern in a slice containing the locus coeruleus from a Dbh −/− mouse (left) and a control mouse (right). (B) No differences were observed between Dbh −/− (n=16) and control mice (n=18). (C) The current-voltage relationships of Dbh −/− (n=21) and control (n=24) mice are indistinguishable. (D) Currents evoked from noradrenergic neurons in response to voltage steps from −50 to −90 mV (holding current = −60 mV) in a Dbh −/− mouse (left) and a control mouse (right).
α-2 receptor mediated currents in LC neurons and cocaine. Iontophoretic application of NE on the recorded LC neuron induced an α-2 mediated outward current in control mice (36.3 ± 7.9 pA, n = 4). Synaptic stimulation of the slice in the presence of antagonists for GABAergic and glutamatergic inputs also induced an average outward current of 28.4 ± 3.0 pA (n = 12). The effects of cocaine on the iontophoretically- and synaptically-induced currents were also measured. By interacting with biogenic amine transporters, like the NE transporter, cocaine blocks neurotransmitter reuptake, resulting in increased extracellular concentrations of neurotransmitter (Amara and Sonders, 1998). Cocaine-induced increase of NE concentration in the midbrain is known to inhibit the activity of LC neurons through activation of α-2 autoreceptors (Pitts and Marwah, 1987). When cocaine was perfused in the slice, the peak amplitude of the outward currents induced by electrical stimulation (control mice only) was increased to 137 ± 9% of baseline levels (n = 7; p < 0.05). The peak amplitude of the outward currents induced by iontophoretic stimulation (both control and Dbh −/− mice) were increased by cocaine to 127 ± 6% and 125 ± 8% of baseline levels (n = 10, 5; p < 0.05 for each case) in knockout and control mice, respectively (Fig. 2A). Both outward currents, induced by electrical and iontophoretic stimulation, were abolished by bath application of the specific α-2 receptor antagonist, yohimbine (n = 7; p < 0.05).
Figure 2.
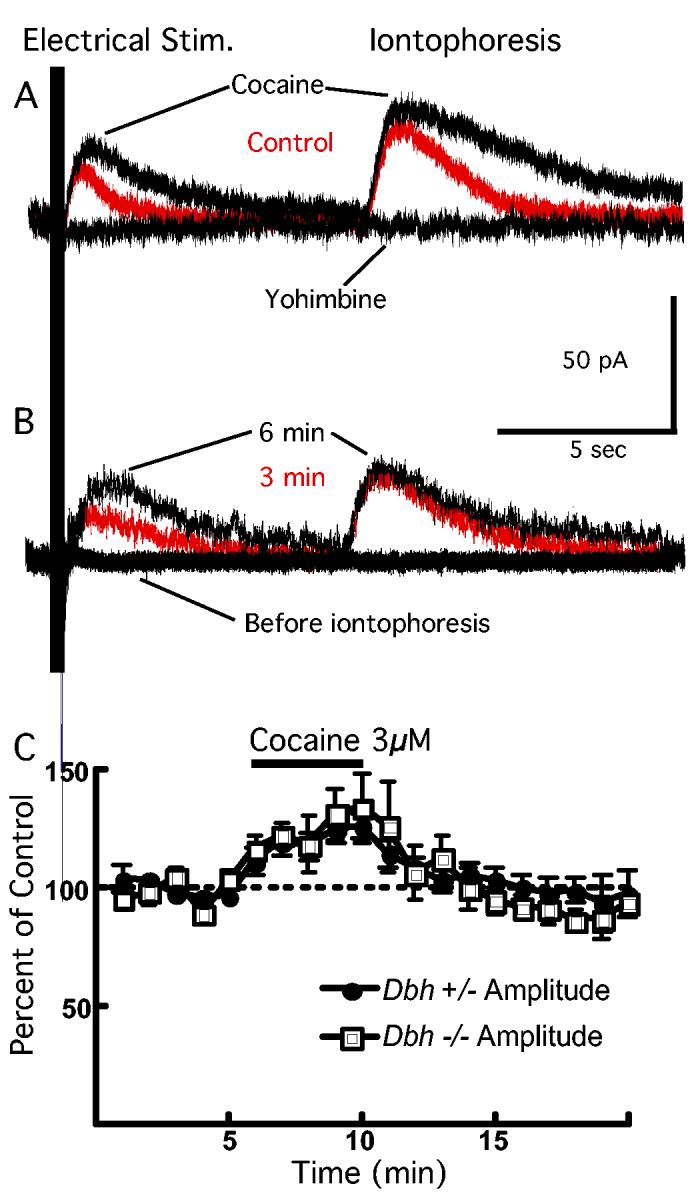
Response of LC neurons to cocaine is the same between Dbh −/− and control mice. (A) Synaptic stimulation of noradrenergic receptors (Electrical Stim.) and iontophoretic application of NE (Iontophoresis) during a voltage clamp recording of an LC neuron obtained from a Dbh +/− mouse. Bath application of cocaine (3μM) induces an increase in the peak amplitude and duration of the outward current (“cocaine” black trace). The outward current is blocked by bath application of the α-2 antagonist, yohimbine (10μM; “yohimbine” black trace). (B) Before iontophoretic application of NE, electrical stimulation of the slice results in no response of the recorded neuron obtained from a Dbh −/− mouse. However, after the third transient application of NE iontophoresis (50 ms application, once per minute), electrical stimulation of the slice produces a synaptically-induced outward current (“3 min” red trace). After the sixth iontophoretic application of NE, the outward current induced by electrical stimulation has reached its maximum amplitude (“6 min” black trace). (C) Summarized data showing that cocaine induces the same increase in peak amplitude of the outward current induced by synaptic stimulation in Dbh −/− and control mice.
Iontophoretic application of NE on LC neurons from Dbh −/− mouse slices also induced an outward current that was similar in amplitude (38.0 ± 5.3 pA, n = 4; p = 0.54) to the outward currents measured from control mouse recordings indicating that LC neurons in the Dbh −/− are capable of detecting NE postsynaptically. However, in contrast to control mice, synaptic stimulation of the slice from Dbh −/− mice produced no measurable current. After bath application of NE (30μM) to the slice, synaptic stimulation produced an outward current comparable to the current induced in control mice (38.0 ± 6.3 pA, n = 7; p = 0.45). Indeed, accumulation of NE in the neurons within the slice after a few minutes of iontophoretic NE application onto the recorded neuron was sufficient to enable the neurons in the LC to release NE and produce an outward current during synaptic stimulation. During the first minute of electrical stimulation, no outward current was detectable whereas a robust outward current was induced with direct iontophoretic application of NE onto the cell. However, after a few minutes, electrical stimulation began to induce an outward current, presumably due to the accumulation of the NE in the recorded and other neurons in the slice within the region where NE was being iontophoresed (Fig. 2B). Thus, although the Dbh −/− mice lacked NE, the reuptake and vesicular release mechanisms remained intact and functional.
Addition of cocaine after NE restoration also induced an increase in the peak amplitude of the outward current induced by synaptic stimulation to 134 ± 14% of the amplitude before cocaine application in slices from Dbh −/− mice (n = 8; p < 0.05). Similarly, cocaine caused an increase in the peak amplitude of the outward current induced by NE iontophoresis (126 ± 12% of baseline, n = 4; p < 0.05). The increase in outward current induced by exposure to cocaine was the same in both Dbh −/− and control mice (Fig. 2C).
Sensitivity of LC neurons to G protein coupled receptor activation
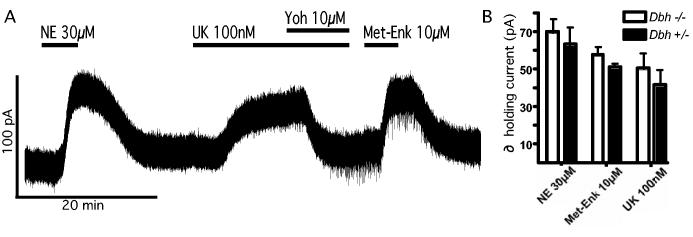
NE α-2 and μ-opioid receptor stimulation increase a G protein coupled, inward rectifier potassium (GIRK) conductance to induce an outward current in LC neurons (e.g. Osborne et al., 2002; e.g. Dang and Williams, 2005). The sensitivity of LC neurons to α-adrenergic receptor activation was compared in Dbh −/− and control mice. The outward current induced by bath perfusion of NE (30μM) was identical in Dbh −/− (70.0 ± 7.1 pA, n = 12) and control mice (63.2 ± 9.5 pA, n = 10; Fig. 3A). Similarly, the outward current induced by the α-2 receptor agonist, UK 14304 (100 nM), was identical in Dbh −/− (50.6 ± 8.2 pA, n = 5) and control mice (41.9 ± 7.8 pA, n = 5; Fig. 3), indicating that the sensitivity of LC neurons to α-2 receptor activation is unaffected by loss of NE tone. The effect of UK 14304 was reversed with the α-2-adrenergic receptor antagonist yohimbine (10 μM). The sensitivity of LC neurons to α-adrenergic receptor activation by dopamine was also compared in Dbh −/− and control mice. Dopamine evoked modest current amplitudes in LC neurons even at high concentrations (100 μM) and no differences were observed between the two genotypes (control = 21.3 ± 3.8 pA, n = 5; Dbh −/− = 22.9 ± 3.1 pA, n = 5; p = 0.33; not shown).
Figure 3.
Response of LC neurons to α-2-adrenerigc and μ-opioid receptor stimulation is the same between Dbh −/− and control mice. (A) Current recording from a Dbh −/−mouse. Bath application of norepinephrine (NE, 30μM) induces an outward current that washes out. The α-2-adrenergic agonist UK 14304 (UK, 100 nM) induces an outward current that is blocked by the α-2-adrenergic antagonist, yohimbine (Yoh, 10μM). The μ-opioid agonist, methionine-enkepahlin (Met-Enk, 10μM), also induced an outward current (single trace). (B) Summarized data. No difference was observed in the outward currents induced by bath application of any agent tested.
The LC is also often used as a native system to study the signaling of μ-opioid receptors (e.g. Dang and Williams, 2005). The sensitivity to opioid receptor stimulation was also tested in this study. The μ-opioid receptor agonist, methionine-enkephalin (10 μM), caused an outward current in LC neurons from both Dbh −/− (57.7±4.1 pA, n = 4) and control mice (51.2±1.8 pA, n = 6). There were no differences observed between Dbh −/− and control mice (p = 0.88). Dbh −/− mice show no measurable differences in μ-opioid receptor sensitivity from control animals. Therefore, the depletion of NE in Dbh −/−mice did not affect the GIRK conductance per se in LC neurons.
Sensitivity of SNC neurons to DA
Because basal striatal DA release is compromised in Dbh −/− mice (Schank et al., 2005), the sensitivity of DA neurons in the SNC to DA was also examined. A maximal response to DA was measured in DA neurons by iontophoresing DA onto the recorded neuron for 5 seconds. The maximum outward current evoked was 278 ± 39 pA (n = 5) in control mice, and 345 ± 47 pA (n = 7; p = 0.33) in Dbh −/− mice. Bath application of DA at different concentrations (3 – 100 μM) was measured as a percentage of the maximal (iontophoretic) outward current, and compared between Dbh −/− and control mice. No difference in DA concentration-response was observed between Dbh −/− and control mice (Fig. 4; p = 0.88 for main effect of genotype, p = 0.89 for genotype-concentration interaction, n = 4-7).
Figure 4.
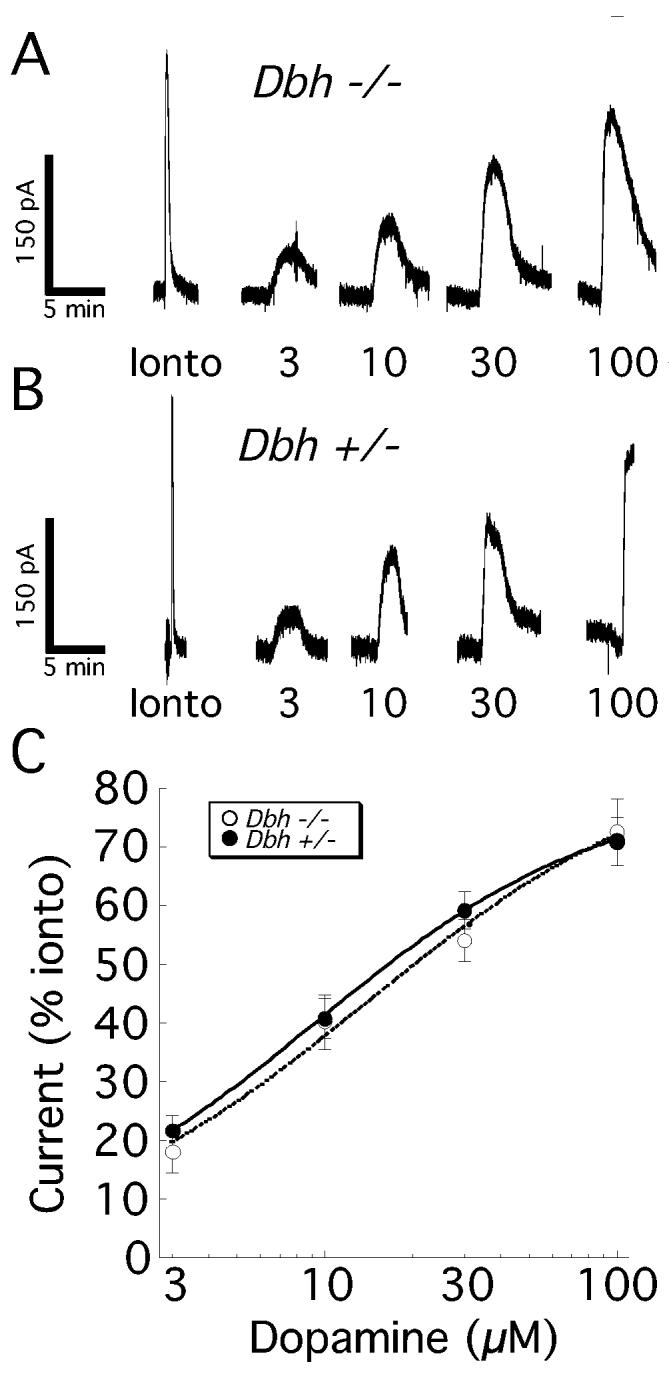
D2 dopamine receptor sensitivity on DA neurons is unchanged in Dbh −/− mice. The DA sensitivity was assessed by measuring D2 autoreceptor-mediated currents from DA neurons of the substantia nigra pars compacta (SNC). The maximal DA response was determined with a 5 second iontophoretic application (+190 nA) of 1M DA (Ionto). Subsequently, neurons from Dbh −/− mice (A) and control mice (B) exhibited similar sensitivity to bath perfusion of DA (3-100 μM). Analysis of the summary data (C) indicated that there was no significant main effect of genotype or genotype-concentration interaction.
The basic electrophysiological properties of DA neurons possibly affected by NE depletion in Dbh −/− mice were also measured. The current measured in response to a −50 mV step was 392 ± 133 pA (n=6) in control mice and 367±43 pA (n=10) in Dbh −/−mice (not shown; p = 0.83). Thus, similar to studies in which other catecholamines were made deficient in genetically altered mice (Paladini et al., 2003), the basic in vitro electrophysiogical measurements were the same in Dbh −/− and control mice.
Discussion
We used Dbh −/− mice to evaluate the contribution of endogenous NE to the measured basic electrophysiological properties of catecholaminergic neurons. An important attribute of Dbh −/− mice is that although brain NE is undetectable, LC neurons are intact, and their ability to make NE can be controlled by the addition of DOPS, which can be converted to NE by the enzyme aromatic acid decarboxylase, thus bypassing the requirement for DBH (Thomas et al., 1995; Thomas et al., 1998). Development of LC neurons occurs both pre- and post-natally. In rodents, LC neurons begin to differentiate and express noradrenergic markers, such as tyrosine hydroxylase and α-2 receptors, between E10-E13 (Lauder and Bloom, 1974; Dreyfus et al., 1983; Winzer-Serhan et al., 1997), and synaptogenesis commences in late gestation (Lauder and Bloom, 1975). Some aspects of the noradrenergic system undergo further changes between birth and adulthood, such as expression of the NE transporter and tyrosine hydroxylase abundance and distribution (Zyzek et al., 1990; Bezin et al., 1997; Le Saux et al., 2002; Sanders et al., 2005). Importantly for our study, while LC expression of α-2 mRNA remains fairly constant throughout gestation and postnatal development (Winzer-Serhan et al., 1997), the functional sensitivity of these receptors (α-2 mediated autoinhibition of LC neurons) decreases between birth and postnatal day 34 (Kimura and Nakamura, 1987). Furthermore, the firing patterns of LC neurons in vivo also change during this time period (Nakamura et al., 1987). Because NE is required for embryonic development, we pharmacologically rescue Dbh −/− mice during gestation, but they lack NE starting at birth. Our results presented here indicate that these postnatal changes can still occur in the absence of NE.
Administration of DOPS partially restores brain NE levels and reverses most of the known Dbh −/− phenotypes (Thomas and Palmiter, 1997, 1998; Szot et al., 1999; Weinshenker et al., 2000; Murchison et al., 2004). We have previously shown that NE transporter expression is normal in Dbh −/− mice (Weinshenker et al., 2002), and our current study indicates that normal LC neuron firing and α-2 autoreceptor function are also preserved. There were two main findings: (1) the basic electrophysiological characteristics studied in this report of DA and NE neurons do not depend on NE tone, and (2) NE is required for the endogenous α-2 receptor mediated autoinhibition of LC neurons. The firing rate of brainstem NE neurons recorded in vitro from Dbh −/− mice was indistinguishable from control mice indicating that NE tone does not significantly influence the firing rate of LC cells. Additionally, the lack of difference in firing rate between control and Dbh −/− mice indicates that α-2 autoreceptors are not sufficiently activated in LC neurons of control mice to cause any decrease in firing rate. Further, the activity of μ-opioid receptors was unaffected in Dbh −/− mice as demonstrated by the similarity in sensitivity to methionine-enkephalin. Thus, LC neurons do not depend on NE tone to maintain these intrinsic properties. The results provided here support the notion that the altered phenotypes of Dbh −/− mice are due primarily to the lack of NE per se and not to an alteration in the basic physiology of individual neurons in the LC. Exogenous application of NE or an α-2 agonist induced outward currents of equal magnitude from Dbh −/− and control mice that were blocked by an α-2 receptor antagonist, indicating that the number and sensitivity of α-2 autoreceptors was not affected by complete NE deficiency.
Synaptic stimulation was used to verify the NE-depleted state of LC neurons in Dbh −/− mice. Slices from Dbh −/− mice failed to elicit an outward current with synaptic stimulation, indicating that NE signaling through α-2 autoreceptors is required for this property of LC neurons. Importantly, bath application of NE restored the ability of Dbh −/−LC neurons to mount an α-2-mediated response, indicating that the neurons were able to transport, store, and release NE. DA can activate α-2 receptors in vitro (Zheng et al., 1999; Zhang et al., 2004), and has been proposed to be an endogenous α-2 receptor ligand in vivo (Zhang et al., 2004). Because Dbh converts DA to NE, Dbh −/− mice produce DA instead of NE in noradrenergic cells (Thomas et al., 1998). If endogenously released DA could activate α-2 receptors, we would have expected the α-2-mediated synaptic inhibition of LC neurons to remain intact in slices from Dbh −/− mice. However, we found that all endogenous α-2 autoreceptor activation was abolished in the absence of NE even though DA was present, suggesting that the levels of DA released may not be sufficient to activate α-2 autoreceptors in the LC in vivo. One caveat to this interpretation is that we do not have direct evidence that LC neurons from Dbh −/− mice release DA upon stimulation, although it is highly likely (Weinshenker et al., 2002), and we do know that DA is released from Dbh −/− adrenal chromaffin cells (D. Weinshenker and E. Pothos, unpublished observations). Additionally, bath application of dopamine at high concentrations only modestly induces an outward current in both control and Dbh −/−mice, and this effect may be mediated by D2-like receptors (Yokoyama et al., 1994; Suzuki et al., 1998).
The LC and other NE nuclei (A1/A2 NE cell region) project directly to, and modulate the activity of, midbrain DA neurons (Swanson and Hartman, 1975; Jones and Moore, 1977; Grenhoff et al., 1993; Grenhoff and Svensson, 1993; Liprando et al., 2004), and both basal and amphetamine-induced striatal DA release is compromised in Dbh −/− mice (Schank et al., 2005). Therefore, the sensitivity of DA neurons to exogenous application of DA was also tested. Similar to NE neurons, no differences were discernible in pharmacology or electrophysiology of DA neurons in the brain slice preparation between Dbh −/− and control mice. The severe behavioral abnormalities associated with impaired DA release in Dbh −/− mice may reflect both the absence of tonic NE signaling and phasic increases in DA release associated with bursting activity in vivo (Schank et al., 2005). These abnormalities may arise from differences in afferent input that do not irreversibly affect the intrinsic physiological properties of catecholaminergic neurons.
NE deficiency is associated with a number of neurological and neuropsychiatric diseases, including depression (Ressler and Nemeroff, 1999), Parkinson's disease (Soldani and Fornai, 1999), and Alzheimer's disease (Mann and Yates, 1986). In addition, serum DBH activity is a highly variable trait across individuals, and this variability has a strong genetic component. Individuals homozygous for the low activity allele of the major putative functional Dbh polymorphism (approximately 4% of the population) have less than 10% of “normal” DBH activity, and may have deficient noradrenergic function (Cubells and Zabetian, 2004). This report is, to our knowledge, among the first physiological assessments of neuronal function following chronic genetic absence of the primary neurotransmitter. Because appropriate intrinsic LC firing patterns and agonist-induced stimulation of α-2 receptors are preserved in Dbh −/− mice, pharmacological NE replacement could potentially restore not only NE levels, but also normal LC function. Indeed, LC neurons synthesize and release a host of other neuromodulators, including neuropeptide Y (Xu et al., 1998), galanin (Xu et al., 1998), enkephalin (Morita et al., 1990), cocaine- and amphetamine-regulated transcript (Koylu et al., 1999), and brain-derived neurotrophic factor (Castren et al., 1995). Our present results allow us to make predictions about the release of LC “co-transmitters” under conditions of chronic NE deficiency. We hypothesize that under basal conditions, co-transmitters would be released normally, but under high-frequency stimulation, we would expect an increase in release due to attenuated α-2 autoreceptor stimulation. Pharmacological NE replacement could potentially restore normal co-transmitter release in these individuals by reinstating α-2 mediated negative feedback.
Acknowledgements
We thank Dr. J.T. Williams for helpful comments on work and manuscript and C. Liles for mouse breeding and genotyping. We thank Sumitomo Pharmaceuticals (Osaka, Japan) for their generous donation of DOPS. This work was supported by National Institutes of Health Grants – National Institute on Drug Abuse DA4523, DA016262 and DA16467
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- DA
Dopamine
- Dbh
Dopamine β-hydroxylase
- DOPS
L-3,4-dihydroxyphenylserine
- GABAA
γ-aminobutyric acid type A
- GABAB
γ-amino-butyric acid type B
- GIRK
G protein coupled inward rectifier potassium channel
- LC
locus coeruleus
- MCPG
(S)- methyl-4-carboxyphenylglycine
- NBQX
2,3-dihydroxy-6-nitro-7-sulfamoylbenzo[f]-quinoxaline
- NMDA
N-methyl-D-aspartate
- NE
norepinephrine
- SNC
substantia nigra pars compacta
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Alvarez-Maubecin V, Garcia-Hernandez F, Williams JT, Van Bockstaele EJ. Functional coupling between neurons and glia. J Neurosci. 2000;20:4091–4098. doi: 10.1523/JNEUROSCI.20-11-04091.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amara SG, Sonders MS. Neurotransmitter transporters as molecular targets for addictive drugs. Drug Alcohol Depend. 1998;51:87–96. doi: 10.1016/s0376-8716(98)00068-4. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. Adaptive gain and the role of the locus coeruleusnorepinephrine system in optimal performance. J Comp Neurol. 2005;493:99–110. doi: 10.1002/cne.20723. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Bezin L, Diaz JJ, Marcel D, Le Cavorsin M, Madjar JJ, Pujol JF, Weissmann D. Controlled targeting of tyrosine hydroxylase protein toward processes of locus coeruleus neurons during postnatal development. Brain Res Mol Brain Res. 1997;50:23–32. doi: 10.1016/s0169-328x(97)00138-1. [DOI] [PubMed] [Google Scholar]
- Castren E, Thoenen H, Lindholm D. Brain-derived neurotrophic factor messenger RNA is expressed in the septum, hypothalamus and in adrenergic brain stem nuclei of adult rat brain and is increased by osmotic stimulation in the paraventricular nucleus. Neuroscience. 1995;64:71–80. doi: 10.1016/0306-4522(94)00386-j. [DOI] [PubMed] [Google Scholar]
- Cubells JF, Zabetian CP. Human genetics of plasma dopamine beta-hydroxylase activity: applications to research in psychiatry and neurology. Psychopharmacology (Berl) 2004;174:463–476. doi: 10.1007/s00213-004-1840-8. [DOI] [PubMed] [Google Scholar]
- Dang VC, Williams JT. Morphine-Induced mu-opioid receptor desensitization. Mol Pharmacol. 2005;68:1127–1132. doi: 10.1124/mol.105.013185. [DOI] [PubMed] [Google Scholar]
- Dreyfus CF, Markey KA, Goldstein M, Black IB. Development of catecholaminergic phenotypic characters in the mouse locus coeruleus in vivo and in culture. Dev Biol. 1983;97:48–58. doi: 10.1016/0012-1606(83)90062-3. [DOI] [PubMed] [Google Scholar]
- Foote SL, Bloom FE, Aston-Jones G. Nucleus locus ceruleus: new evidence of anatomical and physiological specificity. Physiol Rev. 1983;63:844–914. doi: 10.1152/physrev.1983.63.3.844. [DOI] [PubMed] [Google Scholar]
- Girault JA, Greengard P. The neurobiology of dopamine signaling. Arch Neurol. 2004;61:641–644. doi: 10.1001/archneur.61.5.641. [DOI] [PubMed] [Google Scholar]
- Grenhoff J, Nisell M, Ferre S, Aston-Jones G, Svensson TH. Noradrenergic modulation of midbrain dopamine cell firing elicited by stimulation of the locus coeruleus in the rat. J Neural Transm Gen Sect. 1993;93:11–25. doi: 10.1007/BF01244934. [DOI] [PubMed] [Google Scholar]
- Grenhoff J, Svensson TH. Prazosin modulates the firing pattern of dopamine neurons in rat ventral tegmental area. Eur J Pharmacol. 1993;233:79–84. doi: 10.1016/0014-2999(93)90351-h. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA. Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J Physiol. 1992;450:455–468. doi: 10.1113/jphysiol.1992.sp019136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BE, Moore RY. Ascending projections of the locus coeruleus in the rat. I. Autoradiographic study. Brain Res. 1977;127:25–53. [PubMed] [Google Scholar]
- Kimura F, Nakamura S. Postnatal development of alpha-adrenoceptor-mediated autoinhibition in the locus coeruleus. Brain Res. 1987;432:21–26. doi: 10.1016/0165-3806(87)90004-6. [DOI] [PubMed] [Google Scholar]
- Koylu EO, Smith Y, Couceyro PR, Kuhar MJ. CART peptides colocalize with tyrosine hydroxylase neurons in rat locus coeruleus. Synapse. 1999;31:309–311. doi: 10.1002/(SICI)1098-2396(19990315)31:4<309::AID-SYN10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Lauder JM, Bloom FE. Ontogeny of monoamine neurons in the locus coeruleus, Raphe nuclei and substantia nigra of the rat. I. Cell differentiation. J Comp Neurol. 1974;155:469–481. doi: 10.1002/cne.901550407. [DOI] [PubMed] [Google Scholar]
- Lauder JM, Bloom FE. Ontogeny of monoamine neurons in the locus coeruleus, raphe nuclei and substantia nigra of the rat. II. Synaptogenesis. J Comp Neurol. 1975;163:251–264. doi: 10.1002/cne.901630302. [DOI] [PubMed] [Google Scholar]
- Le Saux F, Besson MJ, Maurin Y. Abnormal postnatal ontogeny of the locus coeruleus in the epileptic mutant mouse quaking. Brain Res Dev Brain Res. 2002;136:197–205. doi: 10.1016/s0165-3806(02)00386-3. [DOI] [PubMed] [Google Scholar]
- Levine ES, Litto WJ, Jacobs BL. Activity of cat locus coeruleus noradrenergic neurons during the defense reaction. Brain Res. 1990;531:189–195. doi: 10.1016/0006-8993(90)90773-5. [DOI] [PubMed] [Google Scholar]
- Liprando LA, Miner LH, Blakely RD, Lewis DA, Sesack SR. Ultrastructural interactions between terminals expressing the norepinephrine transporter and dopamine neurons in the rat and monkey ventral tegmental area. Synapse. 2004;52:233–244. doi: 10.1002/syn.20023. [DOI] [PubMed] [Google Scholar]
- Mann D,M,, Yates PO. Neurotransmitter deficits in Alzheimer's disease and in other dementing disorders. Hum Neurobiol. 1986;5:147–158. [PubMed] [Google Scholar]
- Marino MD, Bourdelat-Parks BN, Cameron Liles L, Weinshenker D. Genetic reduction of noradrenergic function alters social memory and reduces aggression in mice. Behav Brain Res. 2005;161:197–203. doi: 10.1016/j.bbr.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Mercuri NB, Bonci A, Calabresi P, Stefani A, Bernardi G. Properties of the hyperpolarization-activated cation current Ih in rat midbrain dopaminergic neurons. Eur J Neurosci. 1995;7:462–469. doi: 10.1111/j.1460-9568.1995.tb00342.x. [DOI] [PubMed] [Google Scholar]
- Montague PR, Hyman SE, Cohen JD. Computational roles for dopamine in behavioural control. Nature. 2004;431:760–767. doi: 10.1038/nature03015. [DOI] [PubMed] [Google Scholar]
- Morita Y, Zhang JH, Hironaka T, Tateno E, Noguchi K, Sato M, Kiyama H, Tohyama M. Postnatal development of preproenkephalin mRNA containing neurons in the rat lower brainstem. J Comp Neurol. 1990;292:193–213. doi: 10.1002/cne.902920204. [DOI] [PubMed] [Google Scholar]
- Murchison CF, Zhang XY, Zhang WP, Ouyang M, Lee A, Thomas SA. A distinct role for norepinephrine in memory retrieval. Cell. 2004;117:131–143. doi: 10.1016/s0092-8674(04)00259-4. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Kimura F, Sakaguchi T. Postnatal development of electrical activity in the locus ceruleus. J Neurophysiol. 1987;58:510–524. doi: 10.1152/jn.1987.58.3.510. [DOI] [PubMed] [Google Scholar]
- Neuhoff H, Neu A, Liss B, Roeper J. I(h) channels contribute to the different functional properties of identified dopaminergic subpopulations in the midbrain. J Neurosci. 2002;22:1290–1302. doi: 10.1523/JNEUROSCI.22-04-01290.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne PB, Vidovic M, Chieng B, Hill CE, Christie MJ. Expression of mRNA and functional alpha(1)-adrenoceptors that suppress the GIRK conductance in adult rat locus coeruleus neurons. Br J Pharmacol. 2002;135:226–232. doi: 10.1038/sj.bjp.0704453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paladini CA, Robinson S, Morikawa H, Williams JT, Palmiter RD. Dopamine controls the firing pattern of dopamine neurons via a network feedback mechanism. Proc Natl Acad Sci U S A. 2003;100:2866–2871. doi: 10.1073/pnas.0138018100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts DK, Marwah J. Electrophysiological actions of cocaine on noradrenergic neurons in rat locus ceruleus. J Pharmacol Exp Ther. 1987;240:345–351. [PubMed] [Google Scholar]
- Ressler KJ, Nemeroff CB. Role of norepinephrine in the pathophysiology and treatment of mood disorders. Biol Psychiatry. 1999;46:1219–1233. doi: 10.1016/s0006-3223(99)00127-4. [DOI] [PubMed] [Google Scholar]
- Sanders JD, Happe HK, Bylund DB, Murrin LC. Development of the norepinephrine transporter in the rat CNS. Neuroscience. 2005;130:107–117. doi: 10.1016/j.neuroscience.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Schank JR, Liles LC, Weinshenker D. Reduced anticonvulsant efficacy of valproic acid in dopamine beta-hydroxylase knockout mice. Epilepsy Res. 2005;65:23–31. doi: 10.1016/j.eplepsyres.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Soldani P, Fornai F. The functional anatomy of noradrenergic neurons in Parkinson's disease. Funct Neurol. 1999;14:97–109. [PubMed] [Google Scholar]
- Swanson LW, Hartman BK. The central adrenergic system. An immunofluorescence study of the location of cell bodies and their efferent connections in the rat utilizing dopamine-beta-hydroxylase as a marker. J Comp Neurol. 1975;163:467–505. doi: 10.1002/cne.901630406. [DOI] [PubMed] [Google Scholar]
- Szot P, Weinshenker D, White SS, Robbins CA, Rust NC, Schwartzkroin PA, Palmiter RD. Norepinephrine-deficient mice have increased susceptibility to seizure-inducing stimuli. J Neurosci. 1999;19:10985–10992. doi: 10.1523/JNEUROSCI.19-24-10985.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Hurd YL, Sokoloff P, Schwartz JC, Sedvall G. D3 dopamine receptor mRNA is widely expressed in the human brain. Brain Res. 1998;779:58–74. doi: 10.1016/s0006-8993(97)01078-0. [DOI] [PubMed] [Google Scholar]
- Thomas SA, Marck BT, Palmiter RD, Matsumoto AM. Restoration of norepinephrine and reversal of phenotypes in mice lacking dopamine beta-hydroxylase. J Neurochem. 1998;70:2468–2476. doi: 10.1046/j.1471-4159.1998.70062468.x. [DOI] [PubMed] [Google Scholar]
- Thomas SA, Matsumoto AM, Palmiter RD. Noradrenaline is essential for mouse fetal development. Nature. 1995;374:643–646. doi: 10.1038/374643a0. [DOI] [PubMed] [Google Scholar]
- Thomas SA, Palmiter RD. Disruption of the dopamine beta-hydroxylase gene in mice suggests roles for norepinephrine in motor function, learning, and memory. Behav Neurosci. 1997;111:579–589. doi: 10.1037//0735-7044.111.3.579. [DOI] [PubMed] [Google Scholar]
- Thomas SA, Palmiter RD. Impaired maternal behavior in mice lacking norepinephrine and epinephrine. Cell. 1997;91:583–592. doi: 10.1016/s0092-8674(00)80446-8. [DOI] [PubMed] [Google Scholar]
- Thomas SA, Palmiter RD. Examining adrenergic roles in development, physiology, and behavior through targeted disruption of the mouse dopamine beta-hydroxylase gene. Adv Pharmacol. 1998;42:57–60. doi: 10.1016/s1054-3589(08)60695-x. [DOI] [PubMed] [Google Scholar]
- Torrecilla M, Marker CL, Cintora SC, Stoffel M, Williams JT, Wickman K. G-protein-gated potassium channels containing Kir3.2 and Kir3.3 subunits mediate the acute inhibitory effects of opioids on locus ceruleus neurons. J Neurosci. 2002;22:4328–4334. doi: 10.1523/JNEUROSCI.22-11-04328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshenker D, Miller NS, Blizinsky K, Laughlin ML, Palmiter RD. Mice with chronic norepinephrine deficiency resemble amphetamine-sensitized animals. Proc Natl Acad Sci U S A. 2002;99:13873–13877. doi: 10.1073/pnas.212519999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshenker D, Rust NC, Miller NS, Palmiter RD. Ethanol-associated behaviors of mice lacking norepinephrine. J Neurosci. 2000;20:3157–3164. doi: 10.1523/JNEUROSCI.20-09-03157.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshenker D, Szot P. The role of catecholamines in seizure susceptibility: new results using genetically engineered mice. Pharmacol Ther. 2002;94:213–233. doi: 10.1016/s0163-7258(02)00218-8. [DOI] [PubMed] [Google Scholar]
- Weinshenker D, White SS, Javors MA, Palmiter RD, Szot P. Regulation of norepinephrine transporter abundance by catecholamines and desipramine in vivo. Brain Res. 2002;946:239–246. doi: 10.1016/s0006-8993(02)02889-5. [DOI] [PubMed] [Google Scholar]
- Williams JT, North RA, Shefner SA, Nishi S, Egan TM. Membrane properties of rat locus coeruleus neurones. Neuroscience. 1984;13:137–156. doi: 10.1016/0306-4522(84)90265-3. [DOI] [PubMed] [Google Scholar]
- Xu ZQ, Shi TJ, Hokfelt T. Galanin/GMAP- and NPY-like immunoreactivities in locus coeruleus and noradrenergic nerve terminals in the hippocampal formation and cortex with notes on the galanin-R1 and -R2 receptors. J Comp Neurol. 1998;392:227–251. doi: 10.1002/(sici)1096-9861(19980309)392:2<227::aid-cne6>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Yokoyama C, Okamura H, Nakajima T, Taguchi J, Ibata Y. Autoradiographic distribution of [3H]YM-09151-2, a high-affinity and selective antagonist ligand for the dopamine D2 receptor group, in the rat brain and spinal cord. J Comp Neurol. 1994;344:121–136. doi: 10.1002/cne.903440109. [DOI] [PubMed] [Google Scholar]
- Zhang W, Xie Y, Gu J, Ai S, Wang J, Yamamoto K, Jin L. Liquid chromatography with amperometric detection at a nano crystalline Ce-doped lead dioxide film modified electrode for determination of (R)-Salsolinol, (R)-N-methylsalsolinol and monoamine neurotransmitters in Parkinsonian patients' cerebrospinal fluid. Analyst. 2004;129:229–234. doi: 10.1039/b314277a. [DOI] [PubMed] [Google Scholar]
- Zheng P, Zhang XX, Bunney BS, Shi WX. Opposite modulation of cortical N-methyl-D-aspartate receptor-mediated responses by low and high concentrations of dopamine. Neuroscience. 1999;91:527–535. doi: 10.1016/s0306-4522(98)00604-6. [DOI] [PubMed] [Google Scholar]
- Zyzek E, Richard F, Bouilloux JP, Pujol JF. Ontogeny of tyrosine hydroxylase concentration in locus coeruleus of newborn rats: long-term effects of RU24722. J Neurochem. 1990;55:849–853. doi: 10.1111/j.1471-4159.1990.tb04569.x. [DOI] [PubMed] [Google Scholar]