Abstract
Kawasaki disease is an acute febrile, systemic vasculitic syndrome of an unknown etiology that primarily occurs in children younger than five years of age. The principal presentations of Kawasaki disease include fever, bilateral nonexudative conjunctivitis, erythema of the lips and oral mucosa, changes in the extremities, rash, and cervical lymphadenopathy. Coronary artery aneurysms or ectasia develops in 15% to 25% of untreated children with the disease, which may later lead to myocardial infarction, sudden death, or ischemic heart disease. Treatment with intravenous gamma globulin (IVIG) is effective, but the mode of action is still unclear. The development of a diagnostic test, a more specific therapy, and ultimately the prevention of this potentially fatal illness in children are all dependent upon the continued advances in determining the etiopathogenesis of this fascinating disorder.
Keywords: Kawasaki disease
HISTORICAL BACKGROUND
Tomisaku Kawasaki saw his first case of an unusual illness in a four-year-old child with a rash and fever at the Red Cross Hospital in Tokyo, Japan in January of 1961. He published his first report on 50 similar Japanese patients in 1967.1
Initially, he believed that the clinical syndrome was a benign, self-limited process with no sequelae. Noboru Tanaka, a pathologist, discovered coronary artery thrombosis during an autopsy on a child who was previously diagnosed by Kawasaki as having the disorder. Takajiro Yamamoto, a pediatrician, noted that one of his patients with the clinical stigmata of the typical Kawasaki disease had a gallop rhythm associated with congestive heart failure. Yamamoto and colleagues2 published a report on 23 patients, of whom 11 (48%) had abnormalities detected by an electrocardiogram. These results persuaded Yamamoto that cardiac involvement was a common feature of this syndrome. The first Japanese nationwide survey was published in 1970 and documented ten autopsy cases of sudden cardiac death resulting from complications of coronary artery aneurysms after Kawasaki disease.3
It was not until 1974 that the first description of this disorder was published in the English language literature.4 The disease is now known to occur in both endemic and community-wide epidemic forms in children of all races in the Americas, Europe, and Asia. Although an infectious agent is suspected, the cause remains unknown. However, significant progress has been made towards understanding the natural history of the disease and in the development of therapeutic interventions that halt the immune-mediated destruction of the arterial wall.
DIAGNOSIS
In the absence of a specific diagnostic test or pathognomonic clinical feature, clinical criteria have been established to assist physicians in diagnosing Kawasaki disease. The syndrome is currently diagnosed by the use of a case definition that was initially created for epidemiological surveys (Table 1). In many cases, however, the clinical criteria for Kawasaki disease are not all present on any given day. The lack of a specific and sensitive diagnostic test remains a major obstacle to correctly identifying all patients with Kawasaki disease.
Table 1.
Diagnostic Criteria for Kawasaki Disease
Principal clinical findings
A remittent fever, often 40℃ or higher, is characteristic of the acute phase and usually heralds the onset of illness. The fever is unresponsive to antibiotics but partially responds to antipyretics. In untreated children, the febrile period lasts on average approximately ten days, but may range from 5 to 25 days.
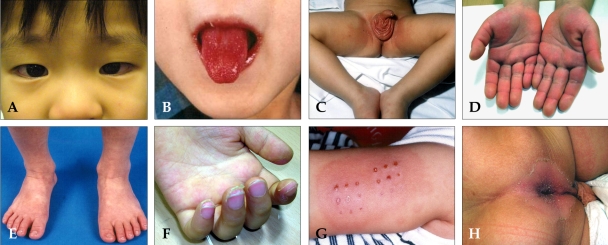
Bilateral conjunctival injection typically involves the bulbar conjunctivae, is not accompanied by suppuration (Fig. 1A), and it usually begins shortly after the onset of fever.
Fig. 1.
(A) Bilateral, non-exudative conjunctival injection with perilimbal sparing. (B) Strawberry tongue and bright red, swollen lips with vertical cracking and bleeding. (C) Erythematous rash involving perineum. (D) Erythema of the palms, which is often accompanied by painful, brawny edema of the dorsa of the hands. (E) Erythema of the soles, and swelling dorsa of the feet. (F) Desquamation of the fingers. (G) Erythema and induration at the site of a previous vaccination with Bacille Calmette-Gurin (BCG). (H) Perianal erythematous desquamation.
The most characteristic changes are the bright red, swollen lips with vertical cracking and bleeding (Fig. 1B). The mucosa of the oropharynx may be bright red, and the tongue may have a typical "strawberry" appearance.
The erythematous rash usually appears in the acute phase of the disease and lasts from one or two days to a week or more. The rash varies over time and is characteristically located on the trunk and may further spread to involve the face, extremities, and perineum (Fig. 1C). Scarlatiniform, macular, popular, multiforme, and purpuric lesions have all been described. The rash, however, is neither bullous nor vesicular.
Changes in the peripheral extremities include erythema of the palms and soles, which is often accompanied by painful, brawny edema of the dorsa of the hands (Fig. 1D) or feet (Fig. 1E) in the acute phase of the disease. Desquamation of the fingers and toes usually begins in the periungual region within two to three weeks after the onset of fever and may extend to include the palms and soles (Fig. 1F).
Cervical lymphadenopathy is the least common of the principal clinical features. It is usually unilateral and confined to the anterior cervical triangle, and its classic criteria include ≥ 1 lymph node that is > 1.5 cm in diameter. Imaging studies frequently demonstrate multiple enlarged nodes without suppuration.
Incomplete (atypical) Kawasaki disease
The concept of "incomplete" (atypical) Kawasaki disease has emerged in recent years. Many experienced clinicians have encountered patients with an inflammatory disorder who did not meet the clinical case definition, but in whom an echocardiogram documented coronary-artery abnormalities, thus confirming the diagnosis of Kawasaki disease. Therefore it may be reasonable to consider Kawasaki disease as a systemic vasculitic syndrome having heterogeneous features rather than a single clinical entity. Incomplete (atypical) Kawasaki disease is more common in young infants than in older children, and makes the accurate diagnosis and timely treatment especially important in these young patients who have a substantial risk of developing coronary abnormalities.5 The conventional diagnostic criteria should be viewed as guidelines that are particularly useful in preventing overdiagnosis but may fail to recognize incomplete forms of the disease.
Cardiac findings
Cardiovascular manifestations can be prominent in the acute phase of Kawasaki disease and are the leading cause of long-term morbidity and mortality. During this phase, the pericardium, myocardium, endocardium, valves, and coronary arteries all may be involved. Cardiac auscultation of the infant or child with Kawasaki disease in the acute phase often reveals a hyperdynamic precordium, tachycardia, a gallop rhythm, and an innocent flow murmur in the setting of anemia, fever, and depressed myocardial contractility secondary to myocarditis. Children with significant mitral regurgitation may have a pansystolic regurgitation murmur that is typical of this condition. Occasionally, patients with Kawasaki disease and poor myocardial function may have low cardiac output syndrome or shock.
Noncardiac findings
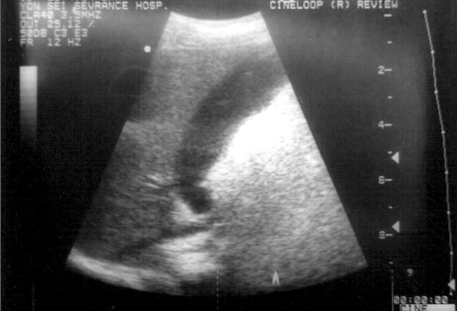
Multiple noncardiac clinical findings may be observed in patients with Kawasaki disease. Children with Kawasaki disease are often times more irritable than children with other febrile diseases. Arthritis or arthralgia can occur in the first week of the disease and are likely to involve multiple joints, especially the knees and ankles. Gastrointestinal complaints, including diarrhea, vomiting, and abdominal pain, occur in approximately one third of the patients. Hepatic enlargement and jaundice may occur. Acute acalculous distention of the gallbladder (hydrops) occurs during the first two weeks of illness and can be identified by an abdominal ultrasound (Fig. 2).6 Erythema and induration at the site of a previous vaccination with Bacille Calmette-Gurin (BCG) is common in patients, especially in those younger than a year old (Fig. 1G).7,8 These can help with the early diagnosis of Kawasaki disease along with perianal erythematous desquamation (Fig. 1H).
Fig. 2.
On the ninth day of Kawasaki disease, acute acalculous distention of the gallbladder (hydrops) was identified by an abdominal ultrasound in a one-year-old boy.
Laboratory findings
No specific diagnostic test for Kawasaki disease exists, but certain laboratory findings are characteristic. During the acute stage of Kawasaki disease, leukocytosis is typical with a predominance of immature and mature granulocytes, and peripheral lymphocytes are depleted by the upregulated apoptosis.9,10 Anemia may develop, usually with normal red blood cell indexes, particularly with a prolonged duration of active inflammation. Elevated erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and other acute phase reactants are almost universally present in the acute phase of the disease and may persist for four to six weeks. The platelet count is generally normal in the first week of the disease and rapidly increases within the next two weeks, sometimes exceeding 1,000,000/mm3. The antinuclear antibody and rheumatoid factor are not detectable.
Moderate elevations in serum transaminases occur in these patients. Hypoalbuminemia is common and is associated with more severe and more prolonged acute disease. Urinalysis reveals intermittent sterile pyuria in patients with Kawasaki disease, although suprapubic urine generally does not show pyuria, which suggests urethritis. Cerebrospinal fluid (CSF) is characterized by pleocytosis with a predominance of mononuclear cells, as well as normal glucose and protein levels.11 The synovial fluid has been described as inflammatory. Plasma lipids are markedly altered in acute Kawasaki disease, with depressed plasma cholesterol, high-density lipoprotein (HDL), and apolipoprotein A-I.12-14
EPIDEMIOLOGY
Kawasaki disease has been reported in all racial and ethnic groups, as well as across the entire pediatric age range, although in most cases 85% of the patients are younger than five years old. Patients younger than six months or older than eight years old are encountered infrequently but may be at risk of having coronary artery aneurysms.5,15-17 The frequency of the disease varies from one part of the world to another. Kawasaki disease is markedly more prevalent in Japan and in children of Japanese ancestry, with an annual incidence of 112 cases per 100,000 children younger than the age of five.18 The incidence rates have been steadily increasing for 11 years since 1987 and the rate had increased by over 1.5 times by 1998.18 In the United States, the incidence of Kawasaki disease has been best estimated by using hospital discharge data.19,20 An estimated 4,248 hospitalizations associated with Kawasaki disease occurred in the United States in 2000, with a median age of these patients being two years old.19 The race-specific incidence rate, derived from administrative data, indicate that Kawasaki disease is most common among Americans of Asian and Pacific Island descent, intermediate in non-Hispanic African American and Hispanics, and lowest in whites.19 Korea has the second highest incidence rate of Kawasaki disease with 86 cases per 100,000 children who are five years old or older.21
Rates of recurrence and familial occurrence of Kawasaki disease are best documented in the literature from Japan, and are reported to be 3% and 1%, respectively.22 Within one year after the onset of the first case in a family, the rate in a sibling is 2.1%, which is a relative ten-fold risk when compared to the unaffected Japanese population, in which 50% of the second cases develop within ten days of the first case.23 The risk of occurrence in twins is 13%.23,24 Higher rates of Kawasaki disease in the siblings of index cases and twins suggest a possible role for genetic predisposition that interacts with exposure to the etiologic agent or agents in the environment.23-25 The reported occurrence of Kawasaki disease in children of parents who themselves had the disease in childhood also supports the contribution of genetic factors.26-28
ETIOLOGY
The cause of Kawasaki disease remains unknown, although both clinical and epidemiological features strongly support an infectious cause. The peak incidence in the toddler age-group, with only rare cases in infants younger than three months of age and in adults, suggests a role for transplacental antibodies conferring protection and that the development of protective immunity is a result of asymptomatic infection in most individuals. Many of the clinical features are similar to those of other infectious diseases, such as adenovirus infection and scarlet fever. However, efforts to identify an infectious agent in Kawasaki disease with conventional bacterial and viral cultures, serological methods, as well as with animal inoculation, have failed to identify an infectious cause.
A controversial hypothesis has been proposed that Kawasaki disease is related to a bacterial superantigenic toxin because of the reported selective expansion of Vβ2 and Vβ8 T-cell receptor families.29-31 Recently, a multicenter, prospective study detected no difference in the rates of isolation of superantigen-producing bacteria between patients with the syndrome and febrile controls.32 Other investigators have found evidence of an oligoclonal antibody response and this suggests a conventional antigen.33-35
A genetic influence on disease susceptibility is suspected because Kawasaki disease is over-represented among Asian and Asian-American populations. There have been attempts to link susceptibility to Kawasaki disease or disease outcome to allelic variations.36-46 To date, only small cohorts of children have been analyzed in association studies in which the frequencies of the alleles of interest have been compared between Kawasaki disease and control individuals. The insufficient sample sizes of these studies preclude any general conclusions and additional studies are needed.
PATHOLOGY
Kawasaki disease causes severe vasculitis of all blood vessels but predominantly affects the medium-sized arteries, with predilection for the coronary arteries. The early stages of the formation and development of arteritis in Kawasaki disease have been well studied morphologically in relatively large muscular arteries.47 The media of affected vessels demonstrate edematous dissociation of the smooth muscle cells, which is most obvious outwardly. Endothelial cell swelling and subendothelial edema are seen, but the internal elastic lamina remains intact. An influx of neutrophils is found in the early stages (seven to nine days after onset), with a rapid transition to large mononuclear cells in concert with lymphocytes, predominantly CD8+ (cytotoxic) T cells.48 IgA-secreting plasma cells are also found in the vessel wall as the inflammation progresses.34 Destruction of the internal elastic lamina and eventually fibroblastic proliferation occur at this stage. Matrix metalloproteinases (MMPs) are prominent in the remodeling process. Active inflammation is replaced with scar formation over the course of several weeks to months by progressive fibrosis.49
IMMUNOPATHOGENESIS
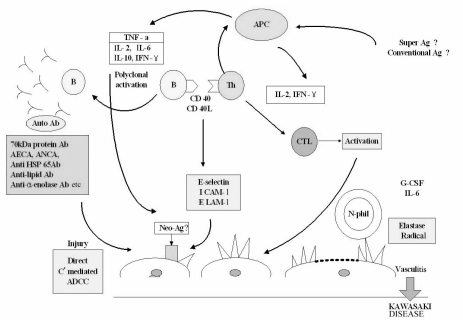
Striking immune perturbations occur in acute Kawasaki disease, including marked cytokine cascade stimulation and endothelial cell activation (Table 2). Concentrations of many proinflammatory cytokines and chemokines, including tumor necrosis factor α(TNFα), and interleukin (IL)s 1, 6, 8, 15, 17 and 18, are higher than normal during the acute phase of the disease.50-55 The key steps leading to coronary arteritis are still being clarified, but endothelial cell activation, CD68+ monocyte/macrophages, CD8+ T lymphocytes, and oligoclonal IgA plasma cells appear to be involved.34,48,56 The prominence of IgA plasma cells in the respiratory tract, which is similar to findings in fatal viral respiratory infections, suggests a respiratory portal of entry of an etiologic agent or agents.57 Enzymes, including MMPs, that are capable of damaging arterial wall integrity may be important in the development of aneurysmal dilatation.58 A role for MMPs in this process is suggested on the basis of high serum concentrations of these enzymes during acute Kawasaki disease and immunolocalisation of MMP9 and MMP2 in the arterial wall of the lesions.49,58 CD40 ligands (CD40L, CD154, and gp39), vascular endothelial growth factor (VEGF), monocyte chemotactic and activating factor (MCAF or MCP-1), and various interleukins also appear to play important roles in the vasculitic process.59-65 Increased expression of toll-like receptor 2 (TLR 2) suggests that the innate immune response may be associated with the pathogenesis of Kawasaki disease.66 Fig. 3 illustrates the proposed events in the evolution of vasculitis in Kawasaki disease.
Table 2.
Evidence of Immune System Activation in Kawasaki Disease
ANCA indicates anti-neutrophil cytoplasmic antibodies; CD40L, CD40 ligand; EBV, Epstein-Barr virus; HSP, heat shock protein; IL, interleukin; IL-2R, IL-2 receptor; INF, interferon; MCP, monocyte chemoattractant protein; MIF, macrophage migration inhibitory factor; MIP, macrophage inflammatory protein; RANTES, regulated upon activation normal T cell expressed and secreted; TGF, transforming growth factor; TLR2, toll-like receptor 2; TNF, tumor necrosis factor; TNF-R, tumor necrosis factor receptor; VEGF, vascular endothelial growth factor.
Fig. 3.
Schematic diagram of the pathogenesis of Kawasaki disease. ADCC indicates antibody-dependent cell-mediated cytotoxicity; AECA, anti-endothelial cell antibodies; ANCA, anti-neutrophil cytoplasmic antibodies; APC, antigen-presenting cell; CD40L, CD40 ligand; CTL, cytolytic T lymphocyte; ELAM-1, endothelial-leukocyte adhesion molecule-1; G-CSF, granulocyte colony stimulating factor; ICAM-1, intercellular adhesion molecule-1; IFN, interferon; IL, interleukin; HSP, heat shock protein; TNF, tumor necrosis factor.
TREATMENT
Treatment of Kawasaki disease in the acute phase is directed at reducing the inflammation in the coronary artery wall and preventing coronary thrombosis, whereas long-term therapy in individuals who develop coronary aneurysms is aimed at preventing myocardial ischemia or infarction.
Aspirin
Aspirin has been used to reduce inflammation and to inhibit platelet aggregation in children with Kawasaki disease, but it does not seem to decrease the number of patients who develop coronary abnormalities.67 Whether other non-steroidal anti-inflammatory agents might be less toxic and have a greater anti-inflammatory effect has not been studied. Currently, high doses of aspirin (80-100 mg/kg daily divided into four doses) are used during the acute inflammatory stage of the disease. Practices regarding the duration of high-dose aspirin administration vary across institutions, and many centers reduce the dose of aspirin to a single daily dose of 3-5 mg/kg after the child has been afebrile for 48 to 72 hours. This antiplatelet dose is continued until the patient shows no evidence of coronary changes within six to eight weeks after the onset of illness. For children who develop coronary abnormalities, aspirin may be continued indefinitely. Adverse effects of aspirin include gastrointestinal irritation and liver-function abnormalities. Children who take salicylates while they are experiencing an active infection with varicella or influenza are at risk for Reye syndrome and this syndrome has been reported in patients taking high-doses of aspirin for a prolonged period after Kawasaki disease. It is unclear whether the low-dose therapy used for the antiplatelet effect increases the risk of Reye syndrome. Children who are taking salicylates long-term should receive an annual influenza vaccine and their parents should be instructed to promptly contact their child's physician if the child develops symptoms of or is exposed to either influenza or varicella.
Intravenous immunoglobulin (IVIG)
The efficacy of IVIG administered in the acute phase of Kawasaki disease by reducing the prevalence of coronary artery abnormalities has been well established.67-72 The mechanism of action of IVIG remains unknown, although theories include cross-linking of the FcγII and FcγIII receptors on macrophages, induction of the immune inhibitory receptors, blocking of the interaction between endothelial cells and natural killer cells, augmenting the T-cell suppressor activity, suppression of antibody synthesis, neutralization of bacterial superantigens or other etiologic agents, and provision of the anti-idiotypic antibodies. In vitro findings suggest that IVIG blocks endothelial-cell proliferation and the synthesis of adhesion molecules, chemokines, and cytokines.73
A single dose of 2 g/kg IVIG infused over 10-12 h is the current standard of therapy. A variety of dose regimens have been used. Two meta-analyses have demonstrated a dose-response effect, with higher doses given in a single infusion having the greatest efficacy.67,71 Furthermore, peak adjusted serum IgG levels are lower among patients who subsequently develop coronary artery abnormalities and are inversely related to fever duration and laboratory indexes of acute inflammation.69,74 The association of lower peak IgG levels with worse outcomes lends further support to the concept of a relationship between serum IgG concentration and therapeutic effectiveness.
This therapy should be instituted within the first ten days of illness and when possible within seven days. Subsequent studies have suggested that the benefit of therapy is greatest when it is given early in the illness. An epidemiological survey of more than 5,000 patients in Japan who were treated with 2 g/kg IVIG showed that the patients treated within six days of illness had fewer cardiac complications one month after the onset of the syndrome than those treated later in the course of their illness.75 The benefits of starting treatment for Kawasaki disease prior to the fifth day of illness is controversial. Muta et. al.76 reported that starting treatment prior to the fourth day of the illness was no different than starting treatment between the fifth and ninth day of illness in regards to preventing cardiac sequelae. Any child with Kawasaki disease who has evidence of persisting inflammation, including fever or high concentrations of inflammatory markers with or without coronary artery abnormalities, should be treated even if the diagnosis is made after the tenth day of illness.77
Minor adverse reactions to IVIG vary with the specific product infused and may include fever, chills, and hypotension. Administration of live virus vaccines (measles, mumps, and rubella, or varicella) should be deferred for at least 11 months after the administration of IVIG due to the reduced immunogenicity of the vaccine related to the passive antibodies from the IVIG preparation.78
Glucocorticoids
The role of glucocorticoids in the treatment of Kawasaki disease remains controversial, although their use is the treatment of choice in other forms of vasculitis. Corticosteroids were used as the initial therapy for Kawasaki disease long before the first report of IVIG efficacy in 1984. An early study by Kato et al.79 suggested that steroids exert a detrimental effect when used as the initial therapy for Kawasaki disease. This study has been criticized because of the study design, the small number of patients in the prednisolone-treated group, and the high frequency of aneurysms, but it has profoundly influenced the treatment of this disease. In a retrospective review, Shinohara et. al.80 found that the treatment regimens that included prednisolone were associated with a significantly shorter duration of fever and a lower prevalence of coronary artery aneurysms. Most recently, a small randomized trial examined whether the addition of 30 mg/kg of intravenous methylprednisolone to conventional therapy with IVIG (2 g/kg) and aspirin improved outcomes.81 Patients who received steroids had a shorter duration of fever and shorter hospital stays, as well as a lower mean ESR and median CRP six weeks after the onset of illness. No differences between treatment groups in coronary outcomes were noted, with limited statistical power. Children, to whom corticosteroids and IVIG were administered, compared with those who received IVIG alone, had reduced levels of cytokines, including IL-2, IL-6, IL-8, and IL-10 within 24 hours of IVIG administration.82
Treatment of patients who failed to respond to initial therapy
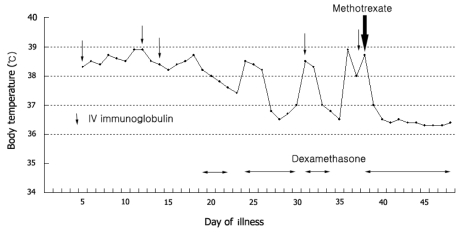
Ten to fifteen percent of the children diagnosed with Kawasaki disease who are treated with high-dose aspirin and 2 g/kg IVIG will have a persistent or recrudescent fever.83,84 Many studies have shown that children who do not become afebrile after the first dose of IVIG are at an increased risk of developing coronary artery aneurysms.85 Failure to respond is usually defined as a persistent or recrudescent fever 36 hours after the completion of the initial IVIG infusion. Most experts recommend retreatment with IVIG, at a dose of 2 g/kg. The putative dose-response effect of IVIG forms the theoretical basis for this approach. Corticosteroids also have been used to treat patients who have failed to respond to the initial therapy for Kawasaki disease,84,86 although their effects on coronary artery abnormalities are still uncertain. The most commonly used steroid regimen is intravenous pulse methylprednisolone, with 30 mg/kg for two to three hours, administered once daily for one to three days. Other treatments successfully used in children include a monoclonal antibody against TNF-α (infliximab), ulinastatin, plasmapheresis, and cytotoxic agents such as cyclophosphamide and cyclosporine A.87-90 Methotrexate (MTX), a dihydrofolate reductase inhibitor, has been used for its anti-inflammatory properties in several vasculitides, and was reported to be effective in patients who are refractory to IVIG.91,92 Low-dose oral MTX (10 mg/BSA, once weekly) resulted in a rapid defervescence without progression of coronary artery dilatation (Fig. 4). The mechanism for the anti-inflammatory action of MTX is still unclear, but some proposed mechanisms include the inhibition of the activity and secretion of proinflammatory cytokines such as IL-1 and IL-6.
Fig. 4.
Body temperature in a 7-year-old boy with refractory Kawasaki disease. After using low-dose oral methotrexate (10 mg/BSA, once weekly), body temperature has returned to normal rapidly.
Treatment of cardiovascular complications
The aims of therapy in patients who develop coronary artery aneurysms are to prevent thrombosis and the myointimal proliferation that leads to stenosis. Only anecdotal experience is available to determine the course of treatment for these children. Low-dose aspirin (3-5 mg/kg daily) has been the mainstay of therapy for children with small to medium aneurysms (< 8 mm). Use of other antiplatelet agents (e.g., clopidogrel and ticlopidine) alone or with aspirin may be beneficial for some patients.93 Randomised trials are needed to establish the appropriate role of low-molecular-weight heparins, monoclonal antibodies against the platelet IIB/IIIA receptor (abciximab), and warfarin in the long-term management of children with giant aneurysms (≥ 8 mm).94
PROGNOSIS
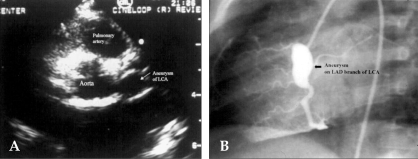
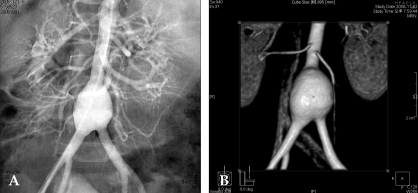
Coronary artery aneurysms occur as a sequela of the vasculitis in 20-25% of untreated children.95 Even when treated with high-dose IVIG regimens within the first ten days of illness, 5% of children with Kawasaki disease develop at the least transient coronary artery dilation and 1% develop giant aneurysms.67,71,96 Other cardiovascular complications of the syndrome include myocarditis, pericarditis with effusion, and valvulitis, which occurs in about 1% of the patients and most commonly involves the mitral valve.97 Echocardiography is a sensitive and reliable method to detect coronary artery aneurysms in the acute and subacute stages of the syndrome (Fig. 5A).98,99 Patients with no coronary artery aneurysms detected by echocardiography during the acute and subacute phases are clinically asymptomatic for at least ten years later.100 Coronary angiography offers a more detailed definition of coronary artery anatomy than the echocardiography, thus making it possible to detect coronary artery stenosis or thrombotic occlusion and to also determine the extent of the collateral artery formation in patients with Kawasaki disease (Fig. 5B). Aneurysms can occur in arteries outside the coronary system, most commonly the subclavian, brachial, axillary, iliac, or femoral vessels, and occasionally in the abdominal aorta and renal arteries (Fig. 6).97
Fig. 5.
(A) On the day 35 of Kawasaki disease, a 2D-echocardiogram demonstrates an aneurysm of the LCA in a six-year-old boy. (B) Coronary angiogram reveals diffuse dilatation in the left anterior descending (LAD) branch of the left coronary artery (LCA) in the same child.
Fig. 6.
Abdomial aortogram (A) and a 3D-image of the computed tomogram (B) showing the aneurysm of the distal abdominal aorta, just below the origin of inferior mesenteric artery (IMA) with an involving bifurcation.
Many studies have attempted to identify predictors of coronary artery aneurysms.101-106 Consistent risk factors across these studies include persistent fever after IVIG therapy, low hemoglobin concentrations, low albumin concentrations, high white-blood-cell count, high band count, high CRP concentrations, male sex, and age less than one year. Thus, laboratory evidence of increased inflammation combined with demographic features (male sex, age less than six months or greater than eight years) and incomplete response to IVIG therapy create a profile of a high-risk patient with Kawasaki disease.
Coronary artery lesions resulting from Kawasaki disease change dynamically with time. Angiographic resolution one to two years after the onset of the disease has been observed in 50% to 67% of vessels with coronary aneurysms.100,107 The likelihood that an aneurysm will resolve appears to be determined in large measure by the initial size of the aneurysm, in which the smaller aneurysms have a greater likelihood of regression.108,109 Other factors that are positively associated with the regression of aneurysms include being younger than a year old at the onset of Kawasaki disease, fusiform rather than saccular aneurysm morphology, and an aneurysm location in a distal coronary segment.107
About 20% of patients who develop coronary artery aneurysms during the acute disease will develop coronary artery stenosis100 and might subsequently need treatment for myocardial ischemia, including percutaneous transluminal angioplasty, rotational atherectomy, coronary artery stenting, bypass grafting, and even cardiac transplantation.100,110-113 Whereas aneurysm size tends to diminish with time, stenotic lesions that are secondary to marked myointimal proliferation are frequently progressive.100,114 The prevalence of stenosis continues to rise almost linearly over time.100,114 The highest rate of progression to stenosis occurs among patients with large aneurysms.114 The worst prognosis occurs in children with giant aneurysms.114-117 In these aneurysms, thrombosis is promoted by the combination of sluggish blood flow within the massively dilated vascular space and the frequent occurrence of stenotic lesions at the proximal or distal end of the aneurysms. Myocardial infarction caused by thrombotic occlusion in an aneurysmal, stenotic, or both aneurysmal and stenotic coronary artery is the principal cause of death from Kawasaki disease.118 The highest risk of myocardial infarction occurs in the first year after the onset of the disease, and the most fatal attacks are associated with an obstruction in either the left main coronary artery (LMCA) or both the right coronary artery (RCA) and the left anterior descending coronary artery (LAD).118 Serial stress tests and myocardial imaging are mandatory in the management of patients with Kawasaki disease and significant coronary artery disease in order to determine the need for a coronary angiography and for surgical or transcatheter intervention.
From a purely clinical perspective, children without known cardiac sequelae during the first month of Kawasaki disease appear to return to their previous (usually excellent) state of health, without signs or symptoms of cardiac impairment.100 However, identification of the appropriate follow-up of these children is difficult because no clear picture has yet emerged of the potential sequelae of the disease without coronary artery aneurysms. Meaningful knowledge about long-term myocardial function, late-onset valvar regurgitation, and coronary artery status in this population must await their careful surveillance in future decades.
References
- 1.Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children [in Japanese] Arerugi. 1967;16:178–222. [PubMed] [Google Scholar]
- 2.Yamamoto T, Oya T, Watanabe A, et al. Clinical features of Kawasaki disease [in Japanese] Shonika Rinsho (Jpn J Pediatr) 1968;21:291–297. [Google Scholar]
- 3.Kosaki F, Kawasaki T, Okawa S, et al. Clinicopathological conference on 10 fatal cases with acute febrile mucocutaneous lymph node syndrome [in Japanese] Shonika Rinsho (Jpn J Pediatr) 1971;24:2545–2559. [Google Scholar]
- 4.Kawasaki T, Kosaki F, Okawa S, Shigematsu I, Yanagawa H. A new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan. Pediatrics. 1974;54:271–276. [PubMed] [Google Scholar]
- 5.Burns JC, Wiggins JW, Jr, Toews WH, Newburger JW, Leung DY, Wilson H, et al. Clinical spectrum of Kawasaki disease in infants younger than 6 months of age. J Pediatr. 1986;109:759–763. doi: 10.1016/s0022-3476(86)80689-8. [DOI] [PubMed] [Google Scholar]
- 6.Suddleson EA, Reid B, Woolley MM, Takahashi M. Hydrops of the gallbladder associated with Kawasaki syndrome. J Pediatr Surg. 1987;22:956–959. doi: 10.1016/s0022-3468(87)80600-0. [DOI] [PubMed] [Google Scholar]
- 7.Roh TW, Park MC, Kim DS. Antibody response against 65kD heat shock protein in Kawasaki disease [in Korean] Pediatr Allergy Respir Dis. 1993;3:68–72. [Google Scholar]
- 8.Kim HY, Kim DS, Shin JS. NRAMP1 Gene Expression in Patients with Kawasaki Disease [in Korean] Pediatr Allergy Respir Dis. 2000;10:153–160. [Google Scholar]
- 9.Kim HS, Noh GW, Kim DS, Lee KY, Lee HS, Lee HK, et al. Decreased CD5+ B cells during the acute phase of Kawasaki disease. Yonsei Med J. 1996;37:52–58. doi: 10.3349/ymj.1996.37.1.52. [DOI] [PubMed] [Google Scholar]
- 10.Kim HY, Lee HG, Kim DS. Apoptosis of peripheral blood mononuclear cells in Kawasaki disease. J Rheumatol. 2000;27:801–806. [PubMed] [Google Scholar]
- 11.Dengler LD, Capparelli EV, Bastian JF, Bradley DJ, Glode MP, Santa S, et al. Cerebrospinal fluid profile in patients with acute Kawasaki disease. Pediatr Infect Dis J. 1998;17:478–481. doi: 10.1097/00006454-199806000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Okada T, Harada K, Okuni M. Serum HDL-cholesterol and lipoprotein fraction in Kawasaki disease (acute mucocutaneous lymph node syndrome) Jpn Circ J. 1982;46:1039–1044. doi: 10.1253/jcj.46.1039. [DOI] [PubMed] [Google Scholar]
- 13.Newburger JW, Burns JC, Beiser AS, Loscalzo J. Altered lipid profile after Kawasaki syndrome. Circulation. 1991;84:625–631. doi: 10.1161/01.cir.84.2.625. [DOI] [PubMed] [Google Scholar]
- 14.Cabana VG, Gidding SS, Getz GS, Chapman J, Shulman ST. Serum amyloid A and high density lipoprotein participate in the acute phase response of Kawasaki disease. Pediatr Res. 1997;42:651–655. doi: 10.1203/00006450-199711000-00017. [DOI] [PubMed] [Google Scholar]
- 15.Rosenfeld EA, Corydon KE, Shulman ST. Kawasaki disease in infants less than one year of age. J Pediatr. 1995;126:524–529. doi: 10.1016/s0022-3476(95)70344-6. [DOI] [PubMed] [Google Scholar]
- 16.Momenah T, Sanatani S, Potts J, Sandor GG, Human DG, Patterson MW. Kawasaki disease in the older child. Pediatrics. 1998;102:e7. doi: 10.1542/peds.102.1.e7. [DOI] [PubMed] [Google Scholar]
- 17.Stockheim JA, Innocentini N, Shulman ST. Kawasaki disease in older children and adolescents. J Pediatr. 2000;137:250–252. doi: 10.1067/mpd.2000.105150. [DOI] [PubMed] [Google Scholar]
- 18.Yanagawa H, Nakamura Y, Yashiro M, Oki I, Hirata S, Zhang T, et al. Incidence survey of Kawasaki disease in 1997 and 1998 in Japan. Pediatrics. 2001;107:E33. doi: 10.1542/peds.107.3.e33. [DOI] [PubMed] [Google Scholar]
- 19.Holman RC, Curns AT, Belay ED, Steiner CA, Schonberger LB. Kawasaki syndrome hospitalizations in the United States, 1997 and 2000. Pediatrics. 2003;112:495–501. doi: 10.1542/peds.112.3.495. [DOI] [PubMed] [Google Scholar]
- 20.Chang RK. The incidence of Kawasaki disease in the United States did not increase between 1988 and 1997. Pediatrics. 2003;111:1124–1125. doi: 10.1542/peds.111.5.1124. [DOI] [PubMed] [Google Scholar]
- 21.Park YW, Han JW, Park IS, Kim CH, Yun YS, Cha SH, et al. Epidemiologic picture of Kawasaki disease in Korea, 2000-2002. Pediatr Int. 2005;47:382–387. doi: 10.1111/j.1442-200x.2005.02079.x. [DOI] [PubMed] [Google Scholar]
- 22.Yanagawa H, Nakamura Y, Yashiro M, Ojima T, Tanihara S, Oki I, et al. Results of the nationwide epidemiologic survey of Kawasaki disease in 1995 and 1996 in Japan. Pediatrics. 1998;102:E65. doi: 10.1542/peds.102.6.e65. [DOI] [PubMed] [Google Scholar]
- 23.Fujita Y, Nakamura Y, Sakata K, Hara N, Kobayashi M, Nagai M, et al. Kawasaki disease in families. Pediatrics. 1989;84:666–669. [PubMed] [Google Scholar]
- 24.Harada F, Sada M, Kamiya T, Yanase Y, Kawasaki T, Sasazuki T. Genetic analysis of Kawasaki syndrome. Am J Hum Genet. 1986;39:537–539. [PMC free article] [PubMed] [Google Scholar]
- 25.Yanagawa H, Yashiro M, Nakamura Y, Kawasaki T, Kato H. Epidemiologic pictures of Kawasaki disease in Japan: from the nationwide incidence survey in 1991 and 1992. Pediatrics. 1995;95:475–479. [PubMed] [Google Scholar]
- 26.Kaneko K, Obinata K, Katsumata K, Tawa T, Hosaka A, Yamashiro Y. Kawasaki disease in a father and daughter. Acta Paediatr. 1999;88:791–792. doi: 10.1080/08035259950169152. [DOI] [PubMed] [Google Scholar]
- 27.Bruckheimer E, Bulbul Z, McCarthy P, Madri JA, Friedman AH, Hellenbrand WE. Images in cardiovascular medicine. Kawasaki disease: coronary aneurysms in mother and son. Circulation. 1998;97:410–411. doi: 10.1161/01.cir.97.4.410. [DOI] [PubMed] [Google Scholar]
- 28.Uehara R, Yashiro M, Nakamura Y, Yanagawa H. Kawasaki disease in parents and children. Acta Paediatr. 2003;92:694–697. doi: 10.1080/08035320310002768. [DOI] [PubMed] [Google Scholar]
- 29.Abe J, Kotzin BL, Jujo K, Melish ME, Glode MP, Kohsaka T, et al. Selective expansion of T cells expressing T-cell receptor variable regions V beta 2 and V beta 8 in Kawasaki disease. Proc Natl Acad Sci USA. 1992;89:4066–4070. doi: 10.1073/pnas.89.9.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim DS, Han BH, Lee SK, Lee HK, Chwae YJ, Lee KY. Evidence for selection of 11 amino acid CDR3 domains in V kappa III-derived immunoglobulin light chains in Kawasaki disease. Scand J Rheumatol. 1997;26:350–354. doi: 10.3109/03009749709065697. [DOI] [PubMed] [Google Scholar]
- 31.Leung DY, Giorno RC, Kazemi LV, Flynn PA, Busse JB. Evidence for superantigen involvement in cardiovascular injury due to Kawasaki syndrome. J Immunol. 1995;155:5018–5021. [PubMed] [Google Scholar]
- 32.Leung DY, Meissner HC, Shulman ST, Mason WH, Gerber MA, Glode MP, et al. Prevalence of super-antigen-secreting bacteria in patients with Kawasaki disease. J Pediatr. 2002;140:742–746. doi: 10.1067/mpd.2002.123664. [DOI] [PubMed] [Google Scholar]
- 33.Choi IH, Chwae YJ, Shim WS, Kim DS, Kwon DH, Kim JD, et al. Clonal expansion of CD8+ T cells in Kawasaki disease. J Immunol. 1997;159:481–486. [PubMed] [Google Scholar]
- 34.Rowley AH, Shulman ST, Spike BT, Mask CA, Baker SC. Oligoclonal IgA response in the vascular wall in acute Kawasaki disease. J Immunol. 2001;166:1334–1343. doi: 10.4049/jimmunol.166.2.1334. [DOI] [PubMed] [Google Scholar]
- 35.Lee JS, Park IH, Shin JS, Kim DS. Clonal Expansion of Peripheral B Cells in Kawasaki Disease [in Korean] Pediatr Allergy Respir Dis. 2002;12:299–314. [Google Scholar]
- 36.Shulman ST, Melish M, Inoue O, Kato H, Tomita S. Immunoglobulin allotypic markers in Kawasaki disease. J Pediatr. 1993;122:84–86. doi: 10.1016/s0022-3476(05)83493-6. [DOI] [PubMed] [Google Scholar]
- 37.Takeuchi K, Yamamoto K, Kataoka S, Kakihara T, Tanaka A, Sato S, et al. High incidence of angiotensin I converting enzyme genotype II in Kawasaki disease patients with coronary aneurysm. Eur J Pediatr. 1997;156:266–268. doi: 10.1007/s004310050597. [DOI] [PubMed] [Google Scholar]
- 38.Ahn SY, Jang GC, Shin JS, Shin KM, Kim DS. Tumor necrosis factor-alpha levels and promoter polymorphism in patients with Kawasaki disease in Korea. Yonsei Med J. 2003;44:1021–1026. doi: 10.3349/ymj.2003.44.6.1021. [DOI] [PubMed] [Google Scholar]
- 39.Huang Y, Lee YJ, Chen MR, Hsu CH, Lin SP, Sung TC, et al. Polymorphism of transmembrane region of MICA gene and Kawasaki disease. Exp Clin Immunogenet. 2000;17:130–137. doi: 10.1159/000019132. [DOI] [PubMed] [Google Scholar]
- 40.Tsukahara H, Hiraoka M, Saito M, Nishida K, Kobata R, Tsuchida S, et al. Methylenetetrahydrofolate reductase polymorphism in Kawasaki disease. Pediatr Int. 2000;42:236–240. doi: 10.1046/j.1442-200x.2000.01229.x. [DOI] [PubMed] [Google Scholar]
- 41.Jibiki T, Terai M, Shima M, Ogawa A, Hamada H, Kanazawa M, et al. Monocyte chemoattractant protein 1 gene regulatory region polymorphism and serum levels of monocyte chemoattractant protein 1 in Japanese patients with Kawasaki disease. Arthritis Rheum. 2001;44:2211–2212. doi: 10.1002/1529-0131(200109)44:9<2211::aid-art375>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 42.Sohn MH, Hur MW, Kim DS. Interleukin 6 gene promoter polymorphism is not associated with Kawasaki disease. Genes Immun. 2001;2:357–362. doi: 10.1038/sj.gene.6363785. [DOI] [PubMed] [Google Scholar]
- 43.Quasney MW, Bronstein DE, Cantor RM, Zhang Q, Stroupe C, Shike H, et al. Increased frequency of alleles associated with elevated tumor necrosis factor-alpha levels in children with Kawasaki disease. Pediatr Res. 2001;49:686–690. doi: 10.1203/00006450-200105000-00013. [DOI] [PubMed] [Google Scholar]
- 44.Ouchi K, Suzuki Y, Shirakawa T, Kishi F. Polymorphism of SLC11A1 (formerly NRAMP1) gene confers susceptibility to Kawasaki disease. J Infect Dis. 2003;187:326–329. doi: 10.1086/345878. [DOI] [PubMed] [Google Scholar]
- 45.Nishimura S, Zaitsu M, Hara M, Yokota G, Watanabe M, Ueda Y, et al. A polymorphism in the promoter of the CD14 gene (CD14/-159) is associated with the development of coronary artery lesions in patients with Kawasaki disease. J Pediatr. 2003;143:357–362. doi: 10.1067/S0022-3476(03)00330-5. [DOI] [PubMed] [Google Scholar]
- 46.Biezeveld MH, Kuipers IM, Geissler J, Lam J, Ottenkamp JJ, Hack CE, et al. Association of mannose-binding lectin genotype with cardiovascular abnormalities in Kawasaki disease. Lancet. 2003;361:1268–1270. doi: 10.1016/S0140-6736(03)12985-6. [DOI] [PubMed] [Google Scholar]
- 47.Naoe S, Takahashi K, Masuda H, Tanaka N. Kawasaki disease. With particular emphasis on arterial lesions. Acta Pathol Jpn. 1991;41:785–797. doi: 10.1111/j.1440-1827.1991.tb01620.x. [DOI] [PubMed] [Google Scholar]
- 48.Brown TJ, Crawford SE, Cornwall ML, Garcia F, Shulman ST, Rowley AH. CD8 T lymphocytes and macrophages infiltrate coronary artery aneurysms in acute Kawasaki disease. J Infect Dis. 2001;184:940–943. doi: 10.1086/323155. [DOI] [PubMed] [Google Scholar]
- 49.Gavin PJ, Crawford SE, Shulman ST, Garcia FL, Rowley AH. Systemic arterial expression of matrix metalloproteinases 2 and 9 in acute Kawasaki disease. Arterioscler Thromb Vasc Biol. 2003;23:576–581. doi: 10.1161/01.ATV.0000065385.47152.FD. [DOI] [PubMed] [Google Scholar]
- 50.Furukawa S, Matsubara T, Jujoh K, Yone K, Sugawara T, Sasai K, et al. Peripheral blood monocyte/macrophages and serum tumor necrosis factor in Kawasaki disease. Clin Immunol Immunopathol. 1988;48:247–251. doi: 10.1016/0090-1229(88)90088-8. [DOI] [PubMed] [Google Scholar]
- 51.Maury CP, Salo E, Pelkonen P. Circulating interleukin-1 beta in patients with Kawasaki disease. N Engl J Med. 1988;319:1670–1671. doi: 10.1056/NEJM198812223192515. [DOI] [PubMed] [Google Scholar]
- 52.Kim DS. Serum interleukin-6 in Kawasaki disease. Yonsei Med J. 1992;33:183–188. doi: 10.3349/ymj.1992.33.2.183. [DOI] [PubMed] [Google Scholar]
- 53.Jang GC, Kim HY, Ahn SY, Kim DS. Raised serum interleukin 15 levels in Kawasaki disease. Ann Rheum Dis. 2003;62:264–266. doi: 10.1136/ard.62.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sohn MH, Noh SY, Chang W, Shin KM, Kim DS. Circulating interleukin 17 is increased in the acute stage of Kawasaki disease. Scand J Rheumatol. 2003;32:364–366. doi: 10.1080/03009740410005034. [DOI] [PubMed] [Google Scholar]
- 55.Jang GC, Lee SY, Kim DS. Changes of Serum Interleukin-18 Levels in Kawasaki Disease [in Korean] Pediatr Allergy Respir Dis. 2001;11:130–137. [Google Scholar]
- 56.Kim DS, Lee KY. Serum soluble E-selectin levels in Kawasaki disease. Scand J Rheumatol. 1994;23:283–286. doi: 10.3109/03009749409103730. [DOI] [PubMed] [Google Scholar]
- 57.Rowley AH, Shulman ST, Mask CA, Finn LS, Terai M, Baker SC, et al. IgA plasma cell infiltration of proximal respiratory tract, pancreas, kidney, and coronary artery in acute Kawasaki disease. J Infect Dis. 2000;182:1183–1191. doi: 10.1086/315832. [DOI] [PubMed] [Google Scholar]
- 58.Takeshita S, Tokutomi T, Kawase H, Nakatani K, Tsujimoto H, Kawamura Y, et al. Elevated serum levels of matrix metalloproteinase-9 (MMP-9) in Kawasaki disease. Clin Exp Immunol. 2001;125:340–344. doi: 10.1046/j.1365-2249.2001.01608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang CL, Wu YT, Liu CA, Lin MW, Lee CJ, Huang LT, et al. Expression of CD40 ligand on CD4+ T-cells and platelets correlated to the coronary artery lesion and disease progress in Kawasaki disease. Pediatrics. 2003;111:E140–E147. doi: 10.1542/peds.111.2.e140. [DOI] [PubMed] [Google Scholar]
- 60.Maeno N, Takei S, Masuda K, Akaike H, Matsuo K, Kitajima I, et al. Increased serum levels of vascular endothelial growth factor in Kawasaki disease. Pediatr Res. 1998;44:596–599. doi: 10.1203/00006450-199810000-00021. [DOI] [PubMed] [Google Scholar]
- 61.Wong M, Silverman ED, Fish EN. Evidence for RANTES, monocyte chemotactic protein-1, and macrophage inflammatory protein-1 beta expression in Kawasaki disease. J Rheumatol. 1997;24:1179–1185. [PubMed] [Google Scholar]
- 62.Matsubara T, Furukawa S, Yabuta K. Serum levels of tumor necrosis factor, interleukin 2 receptor, and interferon-gamma in Kawasaki disease involved coronary-artery lesions. Clin Immunol Immunopathol. 1990;56:29–36. doi: 10.1016/0090-1229(90)90166-n. [DOI] [PubMed] [Google Scholar]
- 63.Lin CY, Lin CC, Hwang B, Chiang B. Serial changes of serum interleukin-6, interleukin-8, and tumor necrosis factor alpha among patients with Kawasaki disease. J Pediatr. 1992;121:924–926. doi: 10.1016/s0022-3476(05)80343-9. [DOI] [PubMed] [Google Scholar]
- 64.Kim DS, Lee HK, Noh GW, Lee SI, Lee KY. Increased serum interleukin-10 level in Kawasaki disease. Yonsei Med J. 1996;37:125–130. doi: 10.3349/ymj.1996.37.2.125. [DOI] [PubMed] [Google Scholar]
- 65.Kim KC, Kim DS. The soluble interleukin 2 receptor levels in Kawasaki disease [in Korean] J Korean Pediatr Soc. 1992;35:1657–1666. [Google Scholar]
- 66.Hwang DH, Han JW, Choi KM, Shin KM, Kim DS. Expression of Toll-like Receptor-2 on the Peripheral Blood Monocytes in Kawasaki Disease Patients [in Korean] J Korean Pediatr Soc. 2005;48:315–320. [Google Scholar]
- 67.Durongpisitkul K, Gururaj VJ, Park JM, Martin CF. The prevention of coronary artery aneurysm in Kawasaki disease: a meta-analysis on the efficacy of aspirin and immunoglobulin treatment. Pediatrics. 1995;96:1057–1061. [PubMed] [Google Scholar]
- 68.Furusho K, Kamiya T, Nakano H, Kiyosawa N, Shinomiya K, Hayashidera T, et al. High-dose intravenous gammaglobulin for Kawasaki disease. Lancet. 1984;2:1055–1058. doi: 10.1016/s0140-6736(84)91504-6. [DOI] [PubMed] [Google Scholar]
- 69.Newburger JW, Takahashi M, Burns JC, Beiser AS, Chung KJ, Duffy CE, et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. 1986;315:341–347. doi: 10.1056/NEJM198608073150601. [DOI] [PubMed] [Google Scholar]
- 70.Morikawa Y, Ohashi Y, Harada K, Asai T, Okawa S, Nagashima M, et al. A multicenter, randomized, controlled trial of intravenous gamma globulin therapy in children with acute Kawasaki disease. Acta Paediatr Jpn. 1994;36:347–354. doi: 10.1111/j.1442-200x.1994.tb03199.x. [DOI] [PubMed] [Google Scholar]
- 71.Terai M, Shulman ST. Prevalence of coronary artery abnormalities in Kawasaki disease is highly dependent on gamma globulin dose but independent of salicylate dose. J Pediatr. 1997;131:888–893. doi: 10.1016/s0022-3476(97)70038-6. [DOI] [PubMed] [Google Scholar]
- 72.Lee HK, Kim DS, Noh GW, Lee KY. Effects of intravenous immune globulin on the peripheral lymphocyte phenotypes in Kawasaki disease. Yonsei Med J. 1996;37:357–363. doi: 10.3349/ymj.1996.37.5.357. [DOI] [PubMed] [Google Scholar]
- 73.Xu C, Poirier B, Van Huyen JP, Lucchiari N, Michel O, Chevalier J, et al. Modulation of endothelial cell function by normal polyspecific human intravenous immunoglobulins: a possible mechanism of action in vascular diseases. Am J Pathol. 1998;153:1257–1266. doi: 10.1016/S0002-9440(10)65670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sawaji Y, Haneda N, Yamaguchi S, Kajino Y, Kishida K, Seto S, et al. Coronary risk factors in acute Kawasaki disease: correlation of serum immunoglobulin levels with coronary complications. Acta Paediatr Jpn. 1998;40:218–225. doi: 10.1111/j.1442-200x.1998.tb01915.x. [DOI] [PubMed] [Google Scholar]
- 75.Zhang T, Yanagawa H, Oki I, Nakamura Y. Factors relating to the cardiac sequelae of Kawasaki disease one month after initial onset. Acta Paediatr. 2002;91:517–520. doi: 10.1080/080352502753711623. [DOI] [PubMed] [Google Scholar]
- 76.Muta H, Ishii M, Egami K, Furui J, Sugahara Y, Akagi T, et al. Early intravenous gamma-globulin treatment for Kawasaki disease: the nationwide surveys in Japan. J Pediatr. 2004;144:496–499. doi: 10.1016/j.jpeds.2003.12.033. [DOI] [PubMed] [Google Scholar]
- 77.Marasini M, Pongiglione G, Gazzolo D, Campelli A, Ribaldone D, Caponnetto S. Late intravenous gamma globulin treatment in infants and children with Kawasaki disease and coronary artery abnormalities. Am J Cardiol. 1991;68:796–797. doi: 10.1016/0002-9149(91)90658-8. [DOI] [PubMed] [Google Scholar]
- 78.Pickering LK. Red Book. Chicago (IL): American Academy of Pediatrics; 2003. 2003 Report of the Committee on Infectious Diseases. [Google Scholar]
- 79.Kato H, Koike S, Yokoyama T. Kawasaki disease: effect of treatment on coronary artery involvement. Pediatrics. 1979;63:175–179. [PubMed] [Google Scholar]
- 80.Shinohara M, Sone K, Tomomasa T, Morikawa A. Corticosteroids in the treatment of the acute phase of Kawasaki disease. J Pediatr. 1999;135:465–469. doi: 10.1016/s0022-3476(99)70169-1. [DOI] [PubMed] [Google Scholar]
- 81.Sundel RP, Baker AL, Fulton DR, Newburger JW. Corticosteroids in the initial treatment of Kawasaki disease: report of a randomized trial. J Pediatr. 2003;142:611–616. doi: 10.1067/mpd.2003.191. [DOI] [PubMed] [Google Scholar]
- 82.Okada Y, Shinohara M, Kobayashi T, Inoue Y, Tomomasa T, Kobayashi T, et al. Effect of corticosteroids in addition to intravenous gamma globulin therapy on serum cytokine levels in the acute phase of Kawasaki disease in children. J Pediatr. 2003;143:363–367. doi: 10.1067/s0022-3476(03)00387-1. [DOI] [PubMed] [Google Scholar]
- 83.Burns JC, Capparelli EV, Brown JA, Newburger JW, Glode MP US/Canadian Kawasaki Syndrome Study Group. Intravenous gamma-globulin treatment and retreatment in Kawasaki disease. Pediatr Infect Dis J. 1998;17:1144–1148. doi: 10.1097/00006454-199812000-00009. [DOI] [PubMed] [Google Scholar]
- 84.Wallace CA, French JW, Kahn SJ, Sherry DD. Initial intravenous gammaglobulin treatment failure in Kawasaki disease. Pediatrics. 2000;105:E78. doi: 10.1542/peds.105.6.e78. [DOI] [PubMed] [Google Scholar]
- 85.Newburger JW. Kawasaki disease: who is at risk? J Pediatr. 2000;137:149–152. doi: 10.1067/mpd.2000.109025. [DOI] [PubMed] [Google Scholar]
- 86.Wright DA, Newburger JW, Baker A, Sundel RP. Treatment of immune globulin-resistant Kawasaki disease with pulsed doses of corticosteroids. J Pediatr. 1996;128:146–149. doi: 10.1016/s0022-3476(96)70447-x. [DOI] [PubMed] [Google Scholar]
- 87.Takagi N, Kihara M, Yamaguchi S, Tamura K, Yabana M, Tokita Y, et al. Plasma exchange in Kawasaki disease. Lancet. 1995;346:1307. doi: 10.1016/s0140-6736(95)91916-3. [DOI] [PubMed] [Google Scholar]
- 88.Zaitsu M, Hamasaki Y, Tashiro K, Matsuo M, Ichimaru T, Fujita I, et al. Ulinastatin, an elastase inhibitor, inhibits the increased mRNA expression of prostaglandin H2 synthase-type 2 in Kawasaki disease. J Infect Dis. 2000;181:1101–1109. doi: 10.1086/315332. [DOI] [PubMed] [Google Scholar]
- 89.Weiss JE, Eberhard BA, Chowdhury D, Gottlieb BS. Infliximab as a novel therapy for refractory Kawasaki disease. J Rheumatol. 2004;31:808–810. [PubMed] [Google Scholar]
- 90.Kuijpers TW, Biezeveld M, Achterhuis A, Kuipers I, Lam J, Hack CE, et al. Longstanding obliterative panarteritis in Kawasaki disease: lack of cyclosporin A effect. Pediatrics. 2003;112:986–992. doi: 10.1542/peds.112.4.986. [DOI] [PubMed] [Google Scholar]
- 91.Ahn SY, Kim DS. Treatment of intravenous immunoglobulin-resistant Kawasaki disease with methotrexate. Scand J Rheumatol. 2005;34:136–139. doi: 10.1080/03009740510026328. [DOI] [PubMed] [Google Scholar]
- 92.Lee MS, An SY, Jang GC, Kim DS. A case of intravenous immunoglobulin-resistant Kawasaki disease treated with methotrexate. Yonsei Med J. 2002;43:527–532. doi: 10.3349/ymj.2002.43.4.527. [DOI] [PubMed] [Google Scholar]
- 93.O'Brien M, Parness IA, Neufeld EJ, Baker AL, Sundel RP, Newburger JW. Ticlopidine plus aspirin for coronary thrombosis in Kawasaki disease. Pediatrics. 2000;105:E64. doi: 10.1542/peds.105.5.e64. [DOI] [PubMed] [Google Scholar]
- 94.Williams RV, Wilke VM, Tani LY, Minich LL. Does Abciximab enhance regression of coronary aneurysms resulting from Kawasaki disease? Pediatrics. 2002;109:E4. doi: 10.1542/peds.109.1.e4. [DOI] [PubMed] [Google Scholar]
- 95.Suzuki A, Kamiya T, Kuwahara N, Ono Y, Kohata T, Takahashi O, et al. Coronary arterial lesions of Kawasaki disease: cardiac catheterization findings of 1100 cases. Pediatr Cardiol. 1986;7:3–9. doi: 10.1007/BF02315475. [DOI] [PubMed] [Google Scholar]
- 96.Dajani AS, Taubert KA, Takahashi M, Bierman FZ, Freed MD, Ferrieri P, et al. Guidelines for long-term management of patients with Kawasaki disease. Report from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 1994;89:916–922. doi: 10.1161/01.cir.89.2.916. [DOI] [PubMed] [Google Scholar]
- 97.Akagi T, Kato H, Inoue O, Sato N, Imamura K. Valvular heart disease in Kawasaki syndrome: incidence and natural history. Am Heart J. 1990;120:366–372. doi: 10.1016/0002-8703(90)90081-8. [DOI] [PubMed] [Google Scholar]
- 98.Yoshikawa J, Yanagihara K, Owaki T, Kato H, Takagi Y, Okumachi F, et al. Cross-sectional echocardiographic diagnosis of coronary artery aneurysms in patients with the mucocutaneous lymph node syndrome. Circulation. 1979;59:133–139. doi: 10.1161/01.cir.59.1.133. [DOI] [PubMed] [Google Scholar]
- 99.Capannari TE, Daniels SR, Meyer RA, Schwartz DC, Kaplan S. Sensitivity, specificity and predictive value of two-dimensional echocardiography in detecting coronary artery aneurysms in patients with Kawasaki disease. J Am Coll Cardiol. 1986;7:355–360. doi: 10.1016/s0735-1097(86)80505-8. [DOI] [PubMed] [Google Scholar]
- 100.Kato H, Sugimura T, Akagi T, Sato N, Hashino K, Maeno Y, et al. Long-term consequences of Kawasaki disease. A 10- to 21-year follow-up study of 594 patients. Circulation. 1996;94:1379–1385. doi: 10.1161/01.cir.94.6.1379. [DOI] [PubMed] [Google Scholar]
- 101.Mori M, Imagawa T, Yasui K, Kanaya A, Yokota S. Predictors of coronary artery lesions after intravenous gamma-globulin treatment in Kawasaki disease. J Pediatr. 2000;137:177–180. doi: 10.1067/mpd.2000.107890. [DOI] [PubMed] [Google Scholar]
- 102.Asai T. Diagnosis and prognosis of coronary artery lesions in Kawasaki disease. Coronary angiography and the conditions for its application (a score chart) [Japanese] Nippon Rinsho. 1983;41:2080–2085. [PubMed] [Google Scholar]
- 103.Koren G, Lavi S, Rose V, Rowe R. Kawasaki disease: review of risk factors for coronary aneurysms. J Pediatr. 1986;108:388–392. doi: 10.1016/s0022-3476(86)80878-2. [DOI] [PubMed] [Google Scholar]
- 104.Harada K. Intravenous gamma-globulin treatment in Kawasaki disease. Acta Paediatr Jpn. 1991;33:805–810. doi: 10.1111/j.1442-200x.1991.tb02612.x. [DOI] [PubMed] [Google Scholar]
- 105.Beiser AS, Takahashi M, Baker AL, Sundel RP, Newburger JW US Multicenter Kawasaki Disease Study Group. A predictive instrument for coronary artery aneurysms in Kawasaki disease. Am J Cardiol. 1998;81:1116–1120. doi: 10.1016/s0002-9149(98)00116-7. [DOI] [PubMed] [Google Scholar]
- 106.Hwang DH, Sin KM, Choi KM, Choi JY, Sul JH, Kim DS. Statistical analysis of 1,000 cases of Kawasaki disease patients diagnosed at a single institute. Korean J Pediatr. 2005;48:416–424. [Google Scholar]
- 107.Takahashi M, Mason W, Lewis AB. Regression of coronary aneurysms in patients with Kawasaki syndrome. Circulation. 1987;75:387–394. doi: 10.1161/01.cir.75.2.387. [DOI] [PubMed] [Google Scholar]
- 108.Fujiwara T, Fujiwara H, Hamashima Y. Size of coronary aneurysm as a determinant factor of the prognosis in Kawasaki disease: clinicopathologic study of coronary aneurysms. Prog Clin Biol Res. 1987;250:519–520. [PubMed] [Google Scholar]
- 109.Nakano H, Ueda K, Saito A, Nojima K. Repeated quantitative angiograms in coronary arterial aneurysm in Kawasaki disease. Am J Cardiol. 1985;56:846–851. doi: 10.1016/0002-9149(85)90767-2. [DOI] [PubMed] [Google Scholar]
- 110.Ishii M, Ueno T, Akagi T, Baba K, Harada K, Hamaoka K, et al. Guidelines for catheter intervention in coronary artery lesion in Kawasaki disease. Pediatr Int. 2001;43:558–562. doi: 10.1046/j.1442-200x.2001.01464.x. [DOI] [PubMed] [Google Scholar]
- 111.Akagi T, Ogawa S, Ino T, Iwasa M, Echigo S, Kishida K, et al. Catheter interventional treatment in Kawasaki disease: a report from the Japanese Pediatric Interventional Cardiology Investigation group. J Pediatr. 2000;137:181–186. doi: 10.1067/mpd.2000.107164. [DOI] [PubMed] [Google Scholar]
- 112.Kitamura S. The role of coronary bypass operation on children with Kawasaki disease. Coron Artery Dis. 2002;13:437–447. doi: 10.1097/00019501-200212000-00009. [DOI] [PubMed] [Google Scholar]
- 113.Checchia PA, Pahl E, Shaddy RE, Shulman ST. Cardiac transplantation for Kawasaki disease. Pediatrics. 1997;100:695–699. doi: 10.1542/peds.100.4.695. [DOI] [PubMed] [Google Scholar]
- 114.Kamiya T, Suzuki A, Ono Y. Angiographic follow-up study of coronary artery lesion in the cases with a history of Kawasaki disease-with a focus on the follow-up more than ten years after the onset of the disease. In: Kato H, editor. Kawasaki Disease; Proceedings of the 5th International Kawasaki Disease Symposium; May 22-25, 1995; Fukuoka, Japan. New York (NY): Elsevier Science; 1995. pp. 569–573. [Google Scholar]
- 115.Fujiwara T, Fujiwara H, Hamashima Y. Frequency and size of coronary arterial aneurysm at necropsy in Kawasaki disease. Am J Cardiol. 1987;59:808–811. doi: 10.1016/0002-9149(87)91096-4. [DOI] [PubMed] [Google Scholar]
- 116.Nakano H, Saito A, Ueda K, Nojima K. Clinical characteristics of myocardial infarction following Kawasaki disease: report of 11 cases. J Pediatr. 1986;108:198–203. doi: 10.1016/s0022-3476(86)80982-9. [DOI] [PubMed] [Google Scholar]
- 117.Tatara K, Kusakawa S. Long-term prognosis of giant coronary aneurysm in Kawasaki disease: an angiographic study. J Pediatr. 1987;111:705–710. doi: 10.1016/s0022-3476(87)80246-9. [DOI] [PubMed] [Google Scholar]
- 118.Kato H, Ichinose E, Kawasaki T. Myocardial infarction in Kawasaki disease: clinical analyses in 195 cases. J Pediatr. 1986;108:923–927. doi: 10.1016/s0022-3476(86)80928-3. [DOI] [PubMed] [Google Scholar]
- 119.Furukawa S, Matsubara T, Umezawa Y, Okumura K, Yabuta K. Serum levels of p60 soluble tumor necrosis factor receptor during acute Kawasaki disease. J Pediatr. 1994;124:721–725. doi: 10.1016/s0022-3476(05)81361-7. [DOI] [PubMed] [Google Scholar]
- 120.Matsubara T, Umezawa Y, Tsuru S, Motohashi T, Yabuta K, Furukawa S. Decrease in the concentrations of transforming growth factor-beta 1 in the sera of patients with Kawasaki disease. Scand J Rheumatol. 1997;26:314–317. doi: 10.3109/03009749709105322. [DOI] [PubMed] [Google Scholar]
- 121.Lee TJ, Chun JK, Yeon SI, Shin JS, Kim DS. Increased serum levels of macrophage migration inhibitory factor in patients with Kawasaki disease. Scand J Rheumatol. 2006 doi: 10.1080/03009740701218790. In press. [DOI] [PubMed] [Google Scholar]
- 122.Leung DY, Collins T, Lapierre LA, Geha RS, Pober JS. Immunoglobulin M antibodies present in the acute phase of Kawasaki syndrome lyse cultured vascular endothelial cells stimulated by gamma interferon. J Clin Invest. 1986;77:1428–1435. doi: 10.1172/JCI112454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mason WH, Jordan SC, Sakai R, Takahashi M, Bernstein B. Circulating immune complexes in Kawasaki syndrome. Pediatr Infect Dis. 1985;4:48–51. doi: 10.1097/00006454-198501000-00012. [DOI] [PubMed] [Google Scholar]
- 124.Culora GA, Moore IE. Kawasaki disease, Epstein-Barr virus and coronary artery aneurysms. J Clin Pathol. 1997;50:161–163. doi: 10.1136/jcp.50.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Burns JC, Glode MP, Clarke SH, Wiggins J, Jr, Hathaway WE. Coagulopathy and platelet activation in Kawasaki syndrome: identification of patients at high risk for development of coronary artery aneurysms. J Pediatr. 1984;105:206–211. doi: 10.1016/s0022-3476(84)80114-6. [DOI] [PubMed] [Google Scholar]
- 126.Nash MC, Shah V, Reader JA, Dillon MJ. Anti-neutrophil cytoplasmic antibodies and anti-endothelial cell antibodies are not increased in Kawasaki disease. Br J Rheumatol. 1995;34:882–887. doi: 10.1093/rheumatology/34.9.882. [DOI] [PubMed] [Google Scholar]