Abstract
The pharmacokinetic behavior of ibafloxacin was studied after intravenous administration of a single dose of 15 mg/kg to 6 healthy lactating goats. Plasma concentrations of ibafloxacin were determined by high-performance liquid chromatography with fluorescence detection. The data for concentration versus time could best be described by a 2-compartment model. The mean plasma ibafloxacin clearance (and standard error) was 1.05 (0.10) L/h·kg. The mean steady-state volume of distribution was 1.65 (0.42) L/kg. The mean elimination half-life was 3.76 (0.30) h. Ibafloxacin penetration from the blood to the milk was poor. The ratio between the areas under the concentration–time curve of milk and plasma was 0.20 (0.01), indicating scant penetration of ibafloxacin into the milk.
Résumé
Les propriétés pharmacocinétiques de l’ibafloxacine ont été étudiées après administration intraveineuse d’une dose unique de 15 mg/kg à six chèvres en santé en lactation. Les concentrations plasmatiques d’ibafloxacine ont été mesurées par chromatographie en phase liquide à haute performance avec détection par fluorescence. Les données de la concentration en fonction du temps peuvent être le mieux décrites par un modèle à 2 compartiments. La clairance plasmatique moyenne d’ibafloxacine (et l’erreur type) était 1,05 (0,10) L/h·kg. Le volume moyen de distribution à l’équilibre dynamique était 1,65 (0,42) L/kg. La demi-vie moyenne d’élimination était 3,76 (0,30) h. La pénétration d’ibafloxacine du sang vers le lait était faible. Le ratio entre les surfaces sous la courbe de la relation concentration–temps du sang et du plasma était 0,20 (0,01), indiquant une pénétration peu marquée de l’ibafloxacine dans le lait.
(Traduit par Docteur Serge Messier)
Ibafloxacin is a new fluoroquinolone that was developed exclusively for veterinary use. It has the pharmacodynamic properties expected of a fluoroquinolone; that is, bactericidal activity and broad-spectrum antibacterial effects (1).
Veterinary medicine requires studies of the value of individual fluoroquinolones for particular purposes in individual species, including the establishment of optimal dosage and the microorganisms against which the drug is effective, as this group of drugs is used for the treatment of multidrug-resistant infection in humans (2). Thus, these agents must not be used as first-line drugs. Articles about milk kinetics and depletion of fluoroquinolones in lactating animals are scant (3–5), yet the pharmacokinetics of antibiotics may change in lactating animals (6–8), and this must be considered when extrapolating dosing regimens from data for nonlactating animals. The aim of this study was to determine the pharmacokinetic behavior of ibafloxacin after intravenous (IV) administration of a single dose to lactating goats.
Six Murciano–Granadina female goats 52 to 58 kg in body weight (BW) and 3 to 4 y of age were used. The study was approved by the Bioethics Committee of the University of Murcia, Murcia, Spain. A 5% aqueous solution of ibafloxacin (Intervet, Salamanca, Spain) was prepared from the pure substance and sterilized in our laboratory. The solution was administered by the IV route as a single dose of 15 mg/kg BW. Blood samples (4 mL) were collected from the contralateral jugular vein into heparinized tubes at 0, 5, 10, 15, 30, and 45 min and at 1, 1.5, 2, 4, 6, 8, 10, 12, 24, 32, 48, and 72 h after drug administration. The samples were centrifuged at 1500 × g for 15 min, and then the plasma was removed and stored at −45°C until assayed. Milk samples were collected before and at 1, 2, 4, 6, 12, 24, 32, 48, and 72 h after complete evacuation of the udder at each collection period.
The concentration of ibafloxacin was measured in plasma and milk by a modified high-performance liquid chromatography (HPLC) method for moxifloxacin that was previously described (9). After the addition of 10 μL of the internal standard (1-pyrenebutanoic acid, 4-sulfo-2,3,5,6-tetrafluorophenol ester, and sodium salt, 1 mg/mL) to 200 μL of plasma or milk, 200 μL of acetonitrile was added. Proteins were precipitated by means of shaking in an ultrasonic bath followed by centrifugation for 10 min at 1600 × g. The supernatant was diluted 4-fold with 0.067 M disodium hydrogen phosphate buffer, pH 7.5, and injected directly into the HPLC/fluorescence apparatus (LC-10ASVP pump, RF-10AXL fluorescence detector, and SIL-10ADVP autoinjector; Shimadzu, Kyoto, Japan). The HPLC separation was performed with the use of a reverse-phase Discovery C18 column (150 × 4.6 mm, particle diameter 5 μm; Supelco, Bellefonte, Pennsylvania, USA). The mobile phase was composed of acetonitrile (40%) and tetrabutylammonium hydrogen sulfate solution, 5 g/L (60%), pH 3.5; an isocratic method with a flow rate of 1.0 mL/min was used. Fluorescence detection was performed at an excitation wavelength of 330 nm and an emission wavelength of 368 nm.
Quality controls were prepared from a pool of blank goat plasma and milk spiked with 8 concentrations (10 to 2000 μg/L) of ibafloxacin. The plasma and milk aliquots were stored at −45°C until assayed and then extracted as described for the test samples. After injection of 25 μL of each control into the chromatographic system, standard curves were obtained by unweighted linear regression of the ibafloxacin peak areas versus known concentrations. Each point was established from an average of 5 determinations. The correlation coefficient (r) was greater than 0.97% for the calibration curves. The percentage recovery of ibafloxacin was determined by comparing the peak areas of plasma and milk blank samples spiked with different amounts of drug and treated as any samples with the peak areas of the same standards prepared in phosphate buffer. Each point was established from an average of 5 determinations. The mean percentage recovery (and standard error) from plasma was 93.23 (3.84) and from milk 88.24 (5.66). The assay precision (relative standard deviation [RSD]) was assessed by expressing the standard deviation of repeated measurements as a percentage of the mean value. Intraday precision was estimated from 6 replicates of 3 plasma or milk standard samples used for the calibration curves (RSD < 4.8%). Interday precision was estimated from the analysis of plasma or milk standard samples on 3 separate days (RSD < 5%). The limit of quantification was 10 μg/L for plasma and milk.
The data for plasma and milk concentrations versus time were fitted with the WinNonlin computer program (version 5.0; Pharsight Corporation, Cary, North Carolina, USA). Pharmacokinetic parameters for plasma were obtained from the individual fitted equations. A noncompartmental analysis was used to determine the area under the concentration–time curve (AUC) and the area under the first moment curve (AUMC), with use of the linear trapezoidal rule and extrapolation to infinity. Mean residence time was calculated as MRT = AUMC/AUC. Systemic clearance was calculated as Cl = dose/ AUC. The drug concentration in milk at each sampling time and the volume of milk at each time were used for calculating the cumulative amounts of ibafloxacin eliminated in milk, the percentage of the dose recovered in the milk (recovery), and the elimination half-life. The AUCmilk was calculated with use of the linear trapezoidal rule and extrapolation to infinity. Penetration of ibafloxacin from blood into the milk was expressed as AUCmilk/AUCplasma. The statistical software SPSS for Windows (version 11.0; Chicago, Illinois, USA) was used to calculate the mean, standard error, standard deviation, and geometric mean (for half-life).
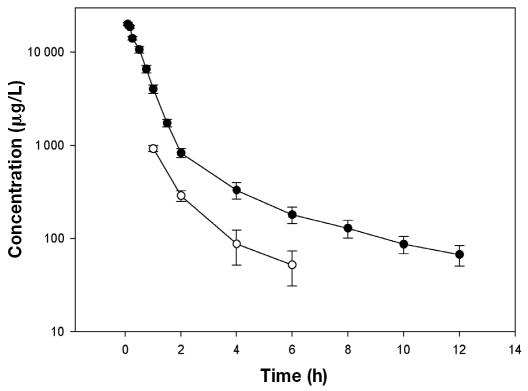
The mean concentrations (and standard errors) of ibafloxacin in plasma and milk after IV administration are plotted in Figure 1, and the mean values of the pharmacokinetic parameters are shown in Table I. Measurable concentrations of ibafloxacin were detected until 12 h in plasma and 6 h in milk. The data for ibafloxacin concentrations versus time after IV administration were best fitted to a 2-compartment open model.
Figure 1.
Semilogarithmic plot of the mean concentrations (and standard errors) of ibafloxacin in plasma (—•—) and milk (—○—) of 6 healthy lactating goats after a single intravenous dose of 15 mg/kg body weight.
Table I.
Mean values of pharmacokinetic parameters for ibafloxacin in 6 healthy lactating goats after intravenous administration of a single dose of 15 mg/kg body weight
| Parameter | Unit | Mean (and standard error) |
|---|---|---|
| In plasma | ||
| Initial distribution phase (λ1) | h−1 | 2.00 (0.09) |
| Elimination phase (λz) | h−1 | 0.19 (0.02) |
| Initial-distribution half-life (t1/2λ1)a | h | 0.35 |
| Elimination half-life (t1/2λz)b | h | 3.76 |
| Vz | L/kg | 5.86 (0.85) |
| Vss | L/kg | 1.65 (0.42) |
| AUC | mg·h/L | 15.19 (0.88) |
| AUMC | mg·h2/L | 22.83 (3.48) |
| MRT | h | 1.50 (0.22) |
| Cl | L/h·kg | 1.05 (0.10) |
| In milk | ||
| Recovery | % | 0.36 (0.003) |
| t1/2λzb | h | 2.37 |
| AUCmilk/AUCplasma | — | 0.20 (0.01) |
Vz — apparent volume of distribution, calculated by the area method; Vss — apparent volume of distribution at steady state; AUC — area under the plasma concentration–time curve from zero to infinity; AUMC — area under the moment curve; MRT — mean residence time; Cl — total body clearance of the drug from the plasma.
Disposition half-life associated with the initial slope (λl) of a semilogarithmic concentration–time curve.
Elimination half-life associated with the terminal slope (λz) of a semilogarithmic concentration–time curve.
It is widely reported that fluoroquinolone pharmacokinetics are different in lactating animals compared with nonlactating animals of the same species (6–8). Therefore, since pharmacokinetic studies of fluoroquinolones in lactating goats are scant, and ibafloxacin has not been studied in this species, it is important to compare the results of our experiment mainly with those from studies of other fluoroquinolones in lactating goats.
In our study, after IV injection ibafloxacin showed very rapid initial distribution, with a mean half-life of 0.35 h, followed by slower elimination, with a mean half-life of 3.76 h. The elimination half-life was shorter than has been reported after IV administration of danofloxacin (4.67 h) (10) and marbofloxacin (7.18 h) (11) to nonlactating goats and longer than has been reported after IV administration of enrofloxacin (2.39 h) (4) and pefloxacin (1.6 h) (5) to lactating goats. The pharmacokinetic behavior of ibafloxacin after IV administration has been studied only in dogs (12), the reported elimination half-life being 5.19 h, longer than that for goats in our study. The elimination half-life of ibafloxacin after oral administration has been reported to be 3.83 h in dogs (12) and 3.00 h in cats (13).
In goats, fluorquinolones are widely distributed (10). The high volume of distribution (mean 5.86 [standard error 0.85] L/kg) calculated by the area method in our study suggests good tissue penetration. The volume was greater than calculated for danofloxacin in nonlactating goats (3.80 L/kg) (10) and less than that calculated for pefloxacin in lactating goats (8.3 L/kg) (5), although differences in dosage regimen must be taken into account.
The total body clearance of ibafloxacin from the plasma of the goats in our study was high (mean 1.05 [standard error 0.10] L/h·kg), greater than that reported for ibafloxacin in dogs (0.52 L/h·kg) (12) and for moxifloxacin in goats (0.43 L/h·kg) (3). The high total body clearance explains the short persistence of the drug after IV administration (mean MRT 1.50 [standard error 0.22] h).
Effective systemic treatment of mastitis requires that drugs penetrate extensively from the blood into the milk. In contrast to other fluoroquinolones studied in goats, ibafloxacin penetrated poorly from the blood into the milk after IV administration, as shown by the AUCmilk/AUCplasma ratio, and it was detectable in milk for only 6 h. Ibafloxacin persisted for less time in milk than in plasma (t1/2 milk/t1/2 plasma < 1), in disagreement with several reports from studies of other fluoroquinolones in lactating animals (3,4,14).
For a concentration-dependent drug such as ibafloxacin, clinical response usually correlates with AUC/minimum inhibitory concentration (MIC) and Cmax (peak plasma concentration after extravascular routes)/MIC. A high Cmax/MIC ratio has also been associated with a lower incidence of resistance development (15). Up to now, MIC data for ibafloxacin against caprine bacterial isolates have not been reported. However, the previously reported pharmacokinetic and pharmacodynamic ratios (15) should be taken with caution. Clinical doses of fluoroquinolones used in veterinary medicine often result in AUC/MIC values that are less than ideal, and clinical cures have been reported (16), especially in immunocompetent animals.
In conclusion, on the basis of the results we have obtained, ibafloxacin shows favorable plasma pharmacokinetic behavior and limited milk penetration in lactating goats. Further studies are needed to establish its clinical effectiveness.
Acknowledgment
Thanks are due to Intervet for supplying the ibafloxacin as pure substance.
References
- 1.Coulet M, van Borssum Waalkes M, Cox P, Lohuis J. In vitro and in vivo pharmacodynamic properties of the fluoroquinolone ibafloxacin. J Vet Pharmacol Ther. 2002;25:401–411. doi: 10.1046/j.1365-2885.2002.00438.x. [DOI] [PubMed] [Google Scholar]
- 2.Walker RD. Fluoroquinolones. In: Prescott JF, Baggot JD, Walker RD, eds. Antimicrobial Therapy in Veterinary Medicine. Ames, Iowa: Iowa State University Press, 2000:315–338.
- 3.Fernández-Varón E, Villamayor L, Escudero E, Espuny A, Cárceles CM. Pharmacokinetics and milk penetration of moxifloxacin after intravenous and subcutaneous administration to lactating goats. Vet J. 2006;172:302–307. doi: 10.1016/j.tvjl.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 4.Abo El-Sooud K. Influence of albendazole on the disposition kinetics and milk antimicrobial equivalent activity of enrofloxacin in lactating goats. Pharmacol Res. 2003;48:389–395. doi: 10.1016/s1043-6618(03)00179-8. [DOI] [PubMed] [Google Scholar]
- 5.Abd El-Aty AM, Goudah A. Some pharmacokinetic parameters of pefloxacin in lactating goats. Vet Res Commun. 2002;26:553–561. doi: 10.1023/a:1020243615928. [DOI] [PubMed] [Google Scholar]
- 6.Oukessou M, Benlamlih S, Toutain PL. Benzylpenicillin kinetics in the ewe: influence of pregnancy and lactation. Res Vet Sci. 1990;49:190–193. [PubMed] [Google Scholar]
- 7.Petraca K, Riond JL, Graser T, Wanner M. Pharmacokinetics of the gyrase inhibitor marbofloxacin: influence of pregnancy and lactation in sows. Zentralbl Veterinarmed A. 1993;40:70–79. doi: 10.1111/j.1439-0442.1993.tb00602.x. [DOI] [PubMed] [Google Scholar]
- 8.Soback S, Gips M, Bialer M, Bor A. Effect of lactation on single dose pharmacokinetics of norfloxacin in ewes. Antimicrob Agents Chemother. 1994;38:2336–2339. doi: 10.1128/aac.38.10.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siefert HM, Kohlsdorfer C, Steinke W, Witt A. Pharmacokinetics of the 8-methoxyquinolone, moxifloxacin: tissue distribution in male rats. J Antimicrob Chemother. 1999;43(Suppl B):61–67. doi: 10.1093/jac/43.suppl_2.61. [DOI] [PubMed] [Google Scholar]
- 10.Aliabadi FS, Lees P. Pharmacokinetics and pharmacodynamics of danofloxacin in serum and tissue fluids of goats after intravenous and intramuscular administration. Am J Vet Res. 2001;62:1979–1989. doi: 10.2460/ajvr.2001.62.1979. [DOI] [PubMed] [Google Scholar]
- 11.Waxman S, Rodríguez C, González F, De Vicente ML, San Andrés MI, San Andrés MD. Pharmacokinetic behaviour of marbofloxacin after intravenous and intramuscular administrations in adult goats. J Vet Pharmacol Ther. 2001;24:375–378. doi: 10.1046/j.1365-2885.2001.00357.x. [DOI] [PubMed] [Google Scholar]
- 12.Coulet M, van Borssum Waalkes M, Leeuwenkamp OR, Cox P, Lohuis J. Pharmacokinetics of ibafloxacin after intravenous and oral administration to healthy beagle dogs. J Vet Pharmacol Ther. 2002;25:89–97. doi: 10.1046/j.1365-2885.2002.00361.x. [DOI] [PubMed] [Google Scholar]
- 13.Coulet M, Morello C, Cox P, Lohuis J. Pharmacokinetics of ibafloxacin in healthy cats. J Vet Pharmacol Ther. 2005;28:37–44. doi: 10.1111/j.1365-2885.2004.00623.x. [DOI] [PubMed] [Google Scholar]
- 14.Shem-Tov M, Ziv G, Glickman A, Saran A. Pharmacokinetics and penetration of marbofloxacin from blood into the milk of cows and ewes. Zentralbl Veterinarmed A. 1997;44:511–519. doi: 10.1111/j.1439-0442.1997.tb01137.x. [DOI] [PubMed] [Google Scholar]
- 15.Drusano GL, Johnson DE, Rosen M, Standiford H. Pharmacodynamics of a fluoroquinolone antimicrobial agent in a neutropenic rat model of Pseudomonas sepsis. Antimicrob Agents Chemother. 1993;37:483–490. doi: 10.1128/aac.37.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papich MG. Current concepts in antimicrobial therapy. In: Davenport DJ, Paradis MR, eds. Proceedings of the 18th Annual Veterinary Medical Forum of the American College of Veterinary Internal Medicine, Denver, Colorado, 2001:513–516.