Abstract
Little is known about how the brain regulates context appropriate communication. European starlings (Sturnus vulgaris) are seasonally-breeding songbirds that produce song in various social contexts. During the breeding season, males that acquire nest sites sing high levels of sexually-motivated song in response to a female. Outside of this context, however, song rates are not affected by female presence. The medial preoptic nucleus (POM) is a region outside of the song control system that regulates male sexual behavior, and several studies in songbirds implicate the POM in sexually-motivated song. However, recent data suggest that the role of the POM might extend to song produced in other contexts as well. To examine this possibility, effects of bilateral electrolytic lesions of the POM on singing and other behaviors in adult male starlings within sexually relevant and non-sexual contexts in response to various social stimuli were examined. Lesions to the POM exclusively reduced song and nest box-directed behaviors within highly sexually relevant contexts. Unexpectedly, POM lesions increased song in a non-sexual context, suggesting an inhibitory role for the POM in this context. These data suggest that the POM interacts with the song control system so that song occurs in an appropriate social context in response to appropriate stimuli.
Keywords: birdsong, vocal communication, social context, sexual motivation, courtship
INTRODUCTION
The ability to respond to social stimuli with appropriate vocal communication is critical for the establishment and maintenance of social relationships in many species. The ability of songbirds to learn and produce complex vocalizations and the identification of specific social cues to which males reliably respond with high levels of song production have made them a valuable model system for the study of neural mechanisms regulating appropriate vocal communication (Catchpole & Slater, 1995; Slater, 2003). Although a growing body of literature exists on the functions, social catalysts, and neural regulation of the learning and production of song (e.g., Bottjer & Johnson, 1997; Brenowitz, Margoliash, & Nordeen, 1997; Margoliash, 1997), little is known about how the brain adjusts communication so that it occurs in an appropriate context in response to appropriate stimuli.
European starlings (Sturnus vulgaris) are seasonally-breeding songbirds that sing year-round. Circulating concentrations of the steroid hormone testosterone (T) are elevated in spring, during the breeding season (Riters, Eens, Pinxten, & Ball, 2002; Riters et al., 2000). During this time, male song can be highly sexually relevant, serving two principal functions: to attract mates and to repel competitors. Unpaired spring males that have acquired nest sites (or nest boxes) respond to females by singing at extremely high rates, primarily from a nest box, gathering nest material, and wing waving (reviewed in Eens, 1997). Females preferentially approach nest boxes broadcasting conspecific song compared to no song and are more likely to enter nest boxes broadcasting complex song compared to simple song (Mountjoy & Lemon, 1991). After pairing, males reduce song production, generally restricting periods of singing to immediately prior to copulation (Eens, Pinxten, & Verheyen, 1990; Eens, Pinxten, & Verheyen, 1993; Eens, Pinxten, & Verheyen, 1994) or to periods when the mate is absent (Cuthill & Hindmarsh, 1985). Additionally, males with nest boxes in spring have been found to respond to intruding males by flying to the nest site and singing, but not gathering nest material or wing waving (Eens et al., 1990; Eens et al., 1993). Fighting between two males in a nest hole may be accompanied by singing (Eens, 1997), and males tend to avoid nest boxes broadcasting complex male starling song (Mountjoy & Lemon, 1991). These data suggest that song in the breeding season, specifically produced by nest box owners from the nest box, is highly sexually relevant, serving mate attraction, courtship and nest box defense purposes.
In contrast, males without nest sites in spring have lower concentrations of circulating T than do nest site owners, and although they also sing at high levels, they do not respond to females with an increase in song (Riters et al., 2000). Although the functions of and motivations behind song in males without nest boxes in spring are not clear, the lack of effect of female presence on song rate suggests that song in this context is not sexually relevant or at least does not function in immediate mate attraction or nest box defense.
Outside of the breeding season, in fall, song is involved in functions other than immediate mate attraction or nest box defense. At this time, starlings feed and roost in large mixed-sex flocks (Feare, 1984). Fall males have lower T concentrations than spring males both with and without a nest box, do not acquire nest sites, and display little or no courtship activity or nest box defense behaviors (Eens, 1997; Heimovics & Riters, 2005; Riters et al., 2000). Males do not respond to females with an increase in song during this time, suggesting that fall song is also not immediately sexually relevant (Riters et al., 2000). Rather, song in this context is proposed to be involved in advertising social associations, promoting tolerance (Hausberger, Richardyris, Henry, Lepage, & Schmidt, 1995), and individual recognition (Eens, 1997; Feare, 1984).
Converging evidence from across vertebrate taxa implicates the medial preoptic nucleus in the regulation of sexually-motivated male behavior (e.g., Hull et al., 1999; Panzica, Viglietti-Panzica, & Balthazart, 1996). In songbirds, a growing body of data implicates the medial preoptic nucleus (POM) in sexually-motivated song (Heimovics & Riters, 2005; Riters & Ball, 1999; Riters et al., 2000; Riters, Teague, Schroeder, & Cummings, 2004). The POM in male European starlings is largest and has the most dense aromatase (an enzyme that converts T into estrogen) immunostaining during the breeding season. POM volume relates positively to song bout length (Riters et al., 2000), a feature of song used by females to select mates and by males to assess potential rivals (Gentner & Hulse, 2000; Mountjoy & Lemon, 1991). Furthermore, positive relationships between the number of songs produced and the number of cFOS (an immediate early gene that indicates neuronal activity) labeled cells in the POM are found within, but not outside the breeding season. Within the breeding season, the POM is largest in males that occupy nest boxes and sing in response to females, compared to males without nest boxes (Riters et al., 2000) and males with nest boxes have significantly more cFOS labeled cells in the POM than those without nest boxes, even when singing at comparable levels (Heimovics & Riters, 2005). In house sparrows, another songbird species, the number of ZENK (another immediate early gene) and cFOS-labeled cells in rostral POM relates positively to vocalizations broadcast from a nest box (presumed sexually-motivated song), but there is no correlation with vocalizations produced away from the nest box (Riters et al., 2004). In individually housed male starlings with nest boxes, lesions to the POM disrupted singing and the gathering of nest materials in response to a female conspecific in spring (Riters & Ball, 1999). Together, the majority of data support a role for the POM specifically in sexually relevant song. However, recently, methionine-enkephalin (mENK; an opioid peptide) fiber densities in the POM were found to relate positively to song outside, but not within, the breeding season (Riters et al., 2005), suggesting that the role of the POM may not be restricted to sexually relevant song.
Here we tested the hypothesis that the POM specifically regulates sexually relevant song. If true, lesions to the POM should reduce courtship behavior and song in a sexually relevant context (in nest box owners in spring), but have little or no effect on song in social contexts that are non-sexual (in males without nest boxes in spring, or in fall). We tested this hypothesis by comparing the behavioral effects of electrolytic and sham lesions of the POM in male starlings in social contexts that were sexually relevant and non-sexual, in response to the introduction of female and male conspecifics.
METHODS
Animals
One-hundred ten adult male and 55 adult female starlings (Sturnus vulgaris) were captured during late fall and early winter of 2002 and 2003 at a farm outside of Madison, Wisconsin. They were immediately housed indoors in stainless steel cages in single-sex groups of up to six on a light cycle reflective of the natural outside photoperiod until the beginning of the experiment.
Subject Males
“Subject males” refers to the 55 males assigned to the experimental and control groups (described below). Each subject male was observed both in spring and in fall. Initially, the photoperiod was adjusted to mimic the natural photoperiod until spring, when testing began. However, birds on natural photoperiods were found to enter a fall-like reproductive state prior to the end of spring observations. To prevent this in later groups, birds to be tested in spring were placed on a photoperiod of 11L:13D for at least 12 weeks, a photoperiod under which birds will enter and remain in the reproductive state characteristic of spring (Falk & Gwinner, 1988). For fall testing, males were housed on a photoperiod of 16L:8D for at least 8 weeks, a photoperiod that induces photorefractoriness, a condition characterized by regressed gonads and a lack of sexual behavior (Falk & Gwinner, 1988). Approximately half of the subject males were tested in spring first and fall second, and half were tested in fall first and spring second in order to counterbalance the effect of season-order and the time lapsed since the lesion surgery.
In spring, some males occupied nest boxes and thus assigned themselves to the “spring males with nest boxes” (Springbox) condition. In contrast, other males did not occupy nest boxes in spring, thus assigning themselves to the “spring males without nest boxes” (Springno box) condition,. All fall males were assigned to the Fall condition.
Flock Males
The stimuli that best elicit song in males differ for each condition (Springbox, Springno box, and Fall). In order to facilitate song behavior during behavioral testing, subject males were housed in the test aviaries with four “flock males”, a situation necessary to stimulate song in Fall males. Although not necessary to stimulate Spring male song, flock males were also included in spring observations to maintain social consistency. These flock males were captured and housed in an identical manner to the subject males.
Stimulus Birds
During behavioral observation, subject males were presented with the following social stimuli: 1) a female in Spring condition, 2) a female in Fall condition, and 3) a male in the same condition (Fall or Spring) as the subject male at the time of testing. Spring condition females and Fall condition females were housed on 11L:13D and 16L:8D, respectively, for at least six months.
Steroid Hormone Manipulations
All subject males were gonadectomized and subsequently implanted with T in spring so that POM lesion effects could be examined independent of any involvement of the POM in the regulation of T. Males received blank implants in fall as a control. The castration procedure involved anesthetizing the birds with isoflurane gas anesthesia, making a small incision just anterior to the last rib to expose the gonads, and removing the gonads using forceps. The incision was sutured and males were allowed to recover on a heating pad. All castrations were performed in fall. Once birds recovered from surgery, they were returned to their home cages.
Seventeen days prior to behavioral observations, subject males received two subcutaneous 14 mm silastic implants (1.47 mm i.d., 1.96 mm o.d.; Dow Corning) filled for 10 mm with either crystalline T (Sigma; in spring) or empty (in fall). Although T was not assayed in this study, this treatment in castrated male starlings results in testosterone concentrations within the physiological range of breeding males for at least 8 weeks (Duffy, Bentley, Drazen, & Ball, 2000).
The hormone implantation procedure was as follows: Each bird to be implanted was lightly anesthetized using isoflurane. An incision was made in the skin just posterior to the last rib and implants were placed under the skin. The skin was sutured and the birds were allowed to recover in a heated cage. For the birds' first season of observations, hormone (or blank capsule) implantation was done at the same time as the electrolytic lesion so that birds only had to be anesthetized once.
At the time of hormone (or blank capsule) implantation for subject males, stimulus Spring condition females also received subcutaneous estrogen (E2) implants in order to facilitate males' sexual interest and associated song production. Each Spring condition female received two 18 mm silastic implants (1.47 mm i.d., 1.96 mm o.d., Dow Corning) filled for 13 mm with 17-beta-estradiol (Sigma). The hormone implantation procedure was identical to that described for male T implants.
Lesion Surgery
Subject males were randomly assigned to the bilateral POM lesion group or the bilateral POM sham lesion group, with the goal of having at least ten birds in each of four groups (sham lesioned and tested spring first and fall second, POM-lesioned and tested spring first and fall second, sham lesioned and tested fall first and spring second, and POM-lesioned and tested fall first and spring second). We assigned more birds to be lesioned than to receive a sham operation because we expected that, due to natural variation in brain size among wild-caught starlings, some of the attempted POM lesions would miss one or both hemispheres of the POM. Coordinates for the POM in starlings were determined during test surgeries, as no stereotaxic atlas is available for the starling brain.
Subject males, anesthetized with isoflurane, were placed into a small animal stereotaxic apparatus (Kopf) fitted with a beak cone for use with gas anesthesia. The beak was angled at a 45° angle below the horizontal plane. Ear bars can fit snugly in two possible positions in the ear canals of starlings. The ear bars were placed in as anterior a position as possible during surgery (otherwise the coordinates map onto habenula, rather than POM). The skull was opened above the POM and an electrode, consisting of an epoxy-insulated stainless steel insect pin (Wards, size 00), was lowered to the desired coordinates. The coordinates for bilateral POM lesions were: A = −0.5 mm, L = ±0.15 mm, V = 7.0 mm from the zero coordinate. The anterior-posterior and ventral-dorsal zero coordinates were taken directly from the ear bar before each surgery. The lateral-medial zero coordinate was taken from the midline at the surface of the brain. For each lesion 0.25 mA of anodal direct current, produced by an S88 stimulator (Grass) and held constant by a constant current unit (Grass), were passed for 20 s through the exposed tip of the electrode. Pilot tests using higher currents or longer stimulation times resulted in high subject mortality. The lesion parameters used here were chosen based on pilot data indicating that they result in small POM lesions (see Results), but minimal animal death. For a sham procedure the electrode remained in the brain for 20 s without the passage of current. The electrode was rapidly removed after each lesion, the skull was closed with dental cement and the skin was sutured. Males were allowed to recover on a heating pad and, once recovered (typically within one hour), they were returned to their cages.
Behavioral Testing
Seventeen days following hormone (or blank capsule) implantation (and lesion surgery in the first season), subject males were introduced into one of eight outdoor behavioral observation aviaries (approximately 2.5 × 2 × 2 m). Each aviary contained three nest boxes approximately 1.5 m from ground level, branches, and food and water ad libitum. Each subject male was allowed to habituate to his surroundings for two days before four flock males were released into the aviary. The subject male was given two days alone in the aviary prior to the introduction of flock males in order to maximize the probability that he would secure a nest box. The five males were then allowed one week to habituate to the aviary and to each other. Although flock males were reused multiple times, special care was taken that flock males had not been previously exposed to one another either in test runs or in their home cages in order to prevent previously established dominance hierarchies from affecting the behavior of the subject male.
One week following the introduction of the flock males, subject males were behaviorally tested for three consecutive days, each day in response to a different stimulus (a Spring condition female, a Fall condition female, and a male; Table 1). The order of stimuli presented for each bird was randomized. Spring behavioral observations all occurred during the months March through July between the hours of 0800 and 1200, with three subject males observed daily. Fall behavioral observations began once the birds completed molt and all occurred during the months September through December (before the winter solstice) between the hours of 0900 and 1300, with three subject males observed daily. These hours were based on the observation that in spring, starlings sing significantly more between the hours of 0900 and 1200 than between 0600 and 0900 (Eens, 1997). The fall observation times were shifted by one hour to compensate for daylight savings time. The order of males observed was randomized.
Table 1.
POM-lesioned males and sham males were each observed in six different contexts, of which only two (Springbox males with a female or male stimulus) were sexually relevant. Stimulus condition was counterbalanced.
| Spring | Fall | Spring | ||
|---|---|---|---|---|
| Lesion Group 1 |
Observe with:
as Springbox males and Springno box males |
Observe with:
not own nest boxes |
||
| Lesion Group 2 |
Observe with:
not own nest boxes |
Observe with:
as Springbox males and Springno box males |
||
Each behavioral observation period proceeded as follows: The stimulus bird and a handful of fresh green nest material were introduced into each aviary prior to each behavioral observation and the subject male was given 15 min to habituate. Males were then observed from behind a blind for 45 min. The following behaviors exhibited by the subject male were recorded: number of times each male sang from a nest box (presumably sexually-motivated song), the number of times he sang away from a nest box (presumably less sexually-motivated song), number of wing waves, gathering of green nest materials, entering a nest box, pecking the stimulus bird (generally a light touch with the beak which may indicate either aggressive or reproductive behavior (Kessel, 1957)), and aggressive interactions (determined by the number of times the subject displaced or was displaced by another bird; pecking was not observed during displacements). General behaviors (eating, drinking, bathing and preening) were also recorded to determine whether effects of POM lesions are specific to song and courtship behavior and not to behavior in general. Hormone or blank capsules were removed at the conclusion of the first season of behavioral observations.
Tissue Collection and Lesion Reconstruction
Once the three days of testing in the second season were complete, subject males were rapidly decapitated and brains were removed and placed on crushed dry ice. Males were checked at this time to confirm the presence of hormone or blank implants and testicular remnants. Frozen brains were cut in coronal sections at 40 μm using a cryostat. Sections were collected beginning just prior to the lateral magnocellular nucleus of the anterior nidopallium (lMAN) and area × through the locus coeruleus (LoC). Every third section was collected, except for the region containing POM, in which every section was collected. Tissue was Nissl stained with Thionin for lesion reconstruction.
The percentage of lesion damage located within and outside POM for each bird was quantified from the Nissl-stained tissue using a Spot camera (Diagnostic Instruments) attached to a Nikon microscope and a personal computer. The volumes of POM, lesions inside POM and lesions outside POM were traced with a mouse using MetaView software (Universal Imaging Corp.). Lesions were identified by examining the area in and around POM for cell death that would indicate the presence of the electrolytic lesion in the tissue. Lesions were reconstructed by tracing the areas that showed evidence of cell death. Because lesions only destroyed a small portion of the POM in each lesioned animal, it was possible to reconstruct the boundaries of the POM as they would be in the absence of a lesion with relative precision. Volume estimates for POM and lesions were then made by multiplying the area for each section by 0.04 mm for the section thickness and summing these volumes for the length of the nucleus.
Pooling of the Data for Analysis
Twenty males received sham operations, 22 had successful bilateral lesions to the POM, 6 had unilateral lesions, 6 had bilateral lesions that missed the POM, and 1 bird received a lesion which could not be located. Only data from males with sham and successful bilateral lesions were considered here. Three males were excluded from the study due to wounds present during the observation periods (2 from the sham group and 1 from the POM-lesioned group). One more male from the sham group was removed from the study because the spring condition stimulus female used during his spring observation had lost her E2 implants prior to the observation. Therefore, the data analyzed here were from 17 sham males and 21 POM-lesioned males.
A few males had testicular remnants (n = 6 in the lesion group and n = 7 in the sham group). However the remnants were extremely tiny (less than 1 mm) and the behavior of males with remnants was not different from other males. Therefore these males were included in the analysis. There was also no difference in any behavioral measure related to whether the animals were studied spring first and fall second or fall first and spring second. For this reason, we felt justified in pooling the data from these groups. Although a single observer collected behavioral data in spring, multiple observers collected behavioral data in fall. Observers were randomized across lesion treatment and stimulus condition and practiced collecting data simultaneously until collected values were within 95% of each other. There was no significant effect of observer on any behavior measured in the complete fall data set (p≥0.075). We therefore pooled these groups as well. Finally, there were no significant differences in responses of males to spring condition females compared to fall condition females in either the Fall or Spring data sets. Due to the lack of differences in responses to the two female conditions, we only considered the responses of males to spring condition females and males for the remainder of the analysis.
Statistics
Data were analyzed using statistical software programs SAS (SAS Institute, 2001) and Statistica (StatSoft, 2001). Due to the high variability of the data and the large number of zeros in the count data, traditional parametric statistics were not appropriate. Likewise, traditional nonparametric statistical methods were not able to take full advantage of the balanced design. Therefore, negative binomial regressions were used to make comparisons of behavioral responses across lesion condition and stimulus bird used (female versus male). The negative binomial regression model is the standard parametric model to account for overdispersion. The data are overdispersed if the variance of the distribution is greater than the mean. Another advantage of the negative binomial regression is that it allows for repeated measures. We used the bird identity and season (where appropriate) as repeated measurements. Assumptions for the negative binomial regression model were met, as checked using Pearson Chi-square tests to compare the log likelihood values for the Poisson model versus the log likelihood values for the negative binomial model and by comparing the sample variances with the sample means.
A logistic regression model with logit as linking function and a binomial error structure was used to analyze the proportion of animals that showed each behavior measured. In cases for which the negative binomial regressions were significant, post hoc Mann-Whitney U tests were used to assess the effects of lesion condition on the response to female versus male stimuli. To assess the effect of lesion size on behavior, we used simple linear regressions. Values given are mean ± standard error of the mean (SEM).
Separate negative binomial regressions were run for males in a sexually relevant context (i.e., Spring condition males with nest boxes [Springbox], and males in a non-sexual context (males without nest boxes in spring [Springno box], and males in fall [Fall]). The rationale for this is that we observed male behavior in six different social contexts (Springbox, Springno box, and Fall, each with both a novel female stimulus and a novel male stimulus). Of these six social contexts, only one (Springbox with a female stimulus) provides a social context with potential for immediate sexual behavior, and one (Springbox with a male stimulus) provides a social context in which males are presumably competing for potential mates. A direct comparison across social contexts would dilute the effect of POM lesions in these two highly sexually relevant contexts with the copious data in the four contexts that have less or no sexual relevance. Therefore separate analyses for each of the three primary contexts (Springbox, Springno box, and Fall) were run to address each component of the hypothesis that POM lesions a) will reduce song in a sexually relevant context and b) will not reduce song in non-sexual contexts.
RESULTS
Location and size of lesions
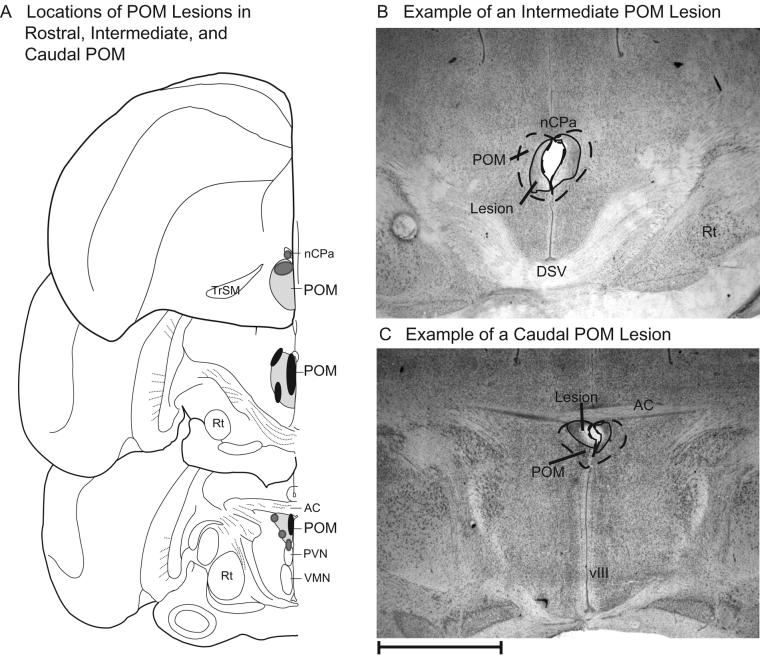
The lesion damage was small (POM damage ranging from 2% to 22% with a mean = 8.02 ± 1.24%, mean lesion volume = 150.85 ± 15.44 μ3), slightly asymmetrical, and the portion of POM damage varied slightly across males (Fig. 1). Of the 21 males in the bilaterally lesioned group, 2 had lesions that included rostral POM (POM located ventral to the tractus septomesencephalicus and dorsal to the supraoptic decussation) as well as intermediate POM, 8 had lesions confined to intermediate POM, 1 had a lesion confined to the caudal portion of POM (POM just ventral to anterior commissure), and 10 had lesions that included both intermediate and caudal portions of POM. Only the effects of intermediate lesions versus intermediate + caudal lesions could be statistically analyzed due to the small sample sizes of the other groups. There was no difference in the effects of intermediate POM lesions versus intermediate + caudal POM lesions in both the complete data set and the data for Springbox alone. All individuals with bilateral lesions to the POM were therefore pooled, regardless of the exact location of the lesion.
Figure 1. Location and size of lesion damage.
(A) Reconstruction of POM lesion damage, cumulative across all 21 POM lesioned subjects. The POM is highlighted with light shading. Dark gray ovals represent lesion damage located in one bird. Black ovals represent lesioned areas common to multiple males (see text for details). (B, C) Photomicrographs of approximate boundaries of POM and lesion damage for a rostral POM lesion and a caudal POM lesion in Nissl stained tissue. Abbreviations: POM, medial preoptic nucleus; TrSM, tractus septomesencephalicus; nCPa, nucleus commissurae pallii; DSV, ventral supraoptic decussation; Rt, nucleus rotundus; AC, anterior commissure; PVN, paraventricular nucleus; VMN, ventromedial nucleus of the hypothalamus; vIII, third ventricle. Scale bar at the lower left corner of (F) = 1 mm.
Males were only assigned to the Springbox condition if they were observed entering and exiting nest boxes during the spring observation periods. Otherwise they were assigned to the Springno box condition. Based on this definition, there were 10 Springbox males and 11 Springno box males in the POM-lesioned group, and 11 Springbox males and 6 Springno box males in the sham group. Although the ratio of males with nest boxes to males without nest boxes was lower in POM lesioned males compared to controls, this difference was not statistically significant. However, there were significant lesion condition × nest box status interactions in the number of males that sang from a nest box, and that pecked the stimulus bird (p=0.046 and p=0.020, respectively). There were also significant lesion condition × nest box status interactions in the overall counts of songs produced from a nest box, wing waves, and aggressive interactions (p=0.033, p=0.040 and p=0.043, respectively). These interaction terms confirmed what has been previously shown, that spring represents a different social context for males with nest boxes compared to those without, further justifying our decision to analyze Springbox, Springno box, and Fall males separately.
Song Production
Males With Nest Boxes in Spring (Springbox males)
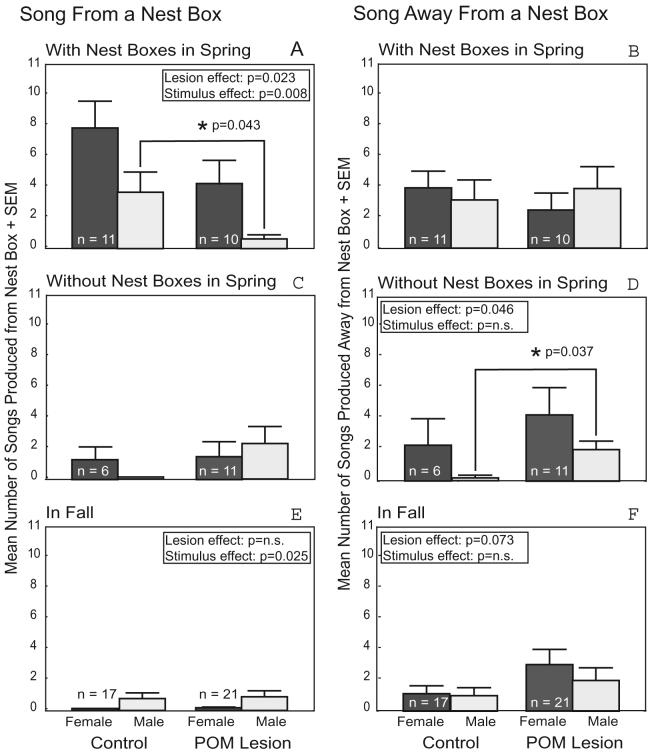
POM-lesioned Springbox males produced fewer songs from their nest boxes in response to a conspecific than controls (p=0.023) (Fig. 2A) and POM lesions significantly reduced the proportion of Springbox males observed to sing from their nest boxes in response to a conspecific (80% of control males, 50% of POM-lesioned males, p=0.040). Post hoc Mann-Whitney U tests revealed that POM-lesioned Springbox males reduced song frequency from nest boxes in response to males, but not females (U=28.5, p=0.043 and U=35.5, p=0.164, respectively; Fig. 2A). All Springbox males sang significantly more frequently from a nest box in the presence of a female stimulus compared to a male (p=0.008; Fig. 2A), and the proportion of Springbox males that sang from a nest box was higher in response to a female than a male (83% in response to a female, 48% in response to a male, p=0.022). POM lesions did not affect the frequency of songs produced away from a nest box by Springbox males (Fig. 2B), or the proportion of Springbox males producing song away from a nest box.
Figure 2. Graphs of Song Production.
Effect of POM lesions and stimulus sex on song produced from a nest box (presumably sexually relevant song) and on song produced away from a nest box in three different contexts: (A, B) among Springbox males, (C, D) among Springno box males, and (E, F) among Fall males. Dark bars indicate song produced in the presence of a female stimulus. Light bars indicate song produced in the presence of a male stimulus. N values reported in the dark bars also correspond to the associated light bar. Significant results of negative binomial tests are indicated. * indicates a significant post hoc effect.
Males Without Nest Boxes in Spring (Springno box males)
POM lesions did not affect the proportion of Springno box males to sing from nest boxes, or the overall frequency of songs produced from nest boxes by Springno box males (Fig. 2C). However, POM-lesioned Springno box males produced significantly more song away from a nest box compared to control Springno box males (p=0.046) (Fig. 2D) and a higher proportion POM-lesioned Springno box males tended to produce song away from a nest box than control Springno box males (p=0.074). Post hoc Mann-Whitney U tests revealed that this effect was in response to males, but not females (U=14.0, p=0.037 and U=29.0, p=0.671, respectively; Fig. 2D). Unlike Springbox males, no significant differences were found in the proportion of Springno box males to sing, or in the number of songs produced from or away from nest boxes in this group in response to a male versus a female conspecific (Fig. 2C,D).
Males in Fall
POM lesions did not affect either the proportion of Fall males to sing, or the frequency of songs produced from a nest box (Fig. 2E). However, similar to Springno box males, POM-lesioned Fall males tended to produce more song away from a nest box than Fall controls (p=0.073; Fig. 2F), and there was a trend for a higher proportion of POM-lesioned Fall males than control Fall males to produce song away from a nest box (p=0.089). Fall males produced more song from a nest box in the presence of a male stimulus compared to a female (p=0.025; Fig. 2E), but stimulus did not affect the frequency of song produced away from a nest box. Stimulus did not affect the proportion of Fall males to produce either song from nest boxes or song away from nest boxes.
Other Sexually-Motivated Behaviors
Males With Nest Boxes in Spring (Springbox males)
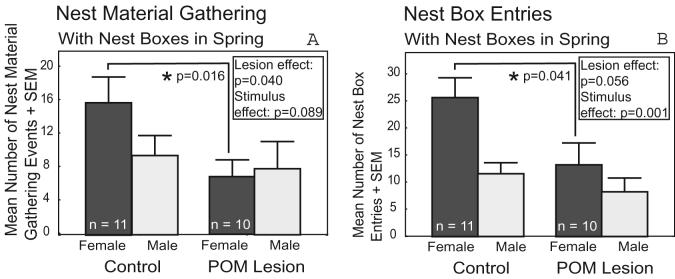
POM-lesioned Springbox males gathered significantly less nest material (control males mean=12.45±2.58, POM-lesioned males mean=7.2±2.38, p=0.040) and tended to enter nest boxes less frequently (p=0.056) compared to control Springbox males (Fig. 3). Post hoc Mann-Whitney U tests revealed that POM-lesioned Springbox males gathered less nest material (U=21.0, p=0.016) and entered nest boxes less (U=26.0, p=0.041) in response to females, but not males (Fig. 3). Furthermore, a higher proportion of Springbox males gathered nest material when exposed to a female compared to a male (100% in response to a female, 71% in response to a male, p=0.005). Wing waving frequency was not affected differentially by POM lesions with respect to the sex of the stimulus bird. The proportion of POM-lesioned Springbox males to peck the stimulus bird (a sign of sexual interest or nest box defense) was significantly higher than the proportion of control Springbox males to do the same (0% of control males, 25% of POM-lesioned males, p=0.008). Springbox males entered nest boxes and wing waved more frequently (p=0.001 and p=0.047, respectively; Fig. 3B), and tended to gather more nest material (p=0.089; Fig. 3A), in the presence of a female stimulus compared to a male.
Figure 3. Graphs of Nest Box-Directed Behavior.
Effect of POM lesions and stimulus sex on the number of times Springbox males (A) gathered nest material and (B) entered a nest box. Dark bars indicate behavioral response in the presence of a female stimulus. Light bars indicate behavioral response in the presence of a male stimulus. N values reported in the dark bars also correspond to the associated light bar. Significant results of negative binomial tests are indicated. * indicates a significant post hoc effect.
Males Without Nest Boxes in Spring (Springno box males)
A higher proportion of POM-lesioned than control Springno box males wing waved (14% of control males, 46% of POM-lesioned males, p=0.034), and POM-lesioned Springno box males tended to wing wave more frequently than control Springno box males (p=0.068). There were no other effects of lesion treatment. Female and male stimuli did not differentially affect behavioral responses in Springno box males.
Males in Fall
POM-lesioned Fall males tended to wing wave more than control Fall males (p=0.085). There were no other effects of lesion treatment. Female and male stimuli did not differentially affect behavior in this group.
Lesion and Stimulus Effects on Aggression
POM lesions did not affect aggression in any social context. Stimulus condition did not affect aggression in either Springbox males or Springno box males. However, Fall males showed aggression more frequently in the presence of a male stimulus than a female (female stimulus mean=7.45±0.7, male stimulus mean=10.45±0.97, p=0.011).
General Behaviors
Males With Nest Boxes in Spring (Springbox males)
POM lesions did not affect any general behaviors measured (bathing, preening, feeding and drinking) in Springbox males. Springbox males fed significantly more frequently when exposed to a male stimulus compared to a female stimulus (female stimulus mean=3.05±0.51, male stimulus mean=5.6±0.66, p=0.012). There was no effect of stimulus on bathing, preening, or drinking in Springbox males.
Males Without Nest Boxes in Spring (Springno box males)
POM lesions did not affect any general behaviors measured in Springno box males. Springno box males drank significantly more frequently (female stimulus mean=2.71±0.37, male stimulus mean=4.35±0.8, p=0.021) and tended to feed more often (p=0.087) when exposed to a male stimulus compared to a female stimulus. No other general behaviors were affected by stimulus.
Males in Fall
POM-lesioned Fall males preened less frequently than did sham-treated Fall males (control males mean=14.03.45±1.51, POM-lesioned males mean=10.21±1.3, p=0.047), although POM lesions did not affect any other general behavior measured in fall. Stimulus condition did not affect any general behaviors measured in fall.
Effect of Lesion Size
A student's t-test revealed no statistical difference in the volume of POM between lesioned birds sacrificed in spring compared to birds sacrificed in fall. Pooling these two groups, simple linear regression analysis showed a positive correlation of percent POM damage with the wing wave frequency in Fall males (r2=0.327, p=0.007). A similar trend, albeit non-significant, was observed for the frequency of aggressive encounters in Fall males (r2=0.177, p=0.058). The percent of POM damage did not correlate with any other behavior in Fall males, in Springbox males or in Springno box males.
DISCUSSION
The present study suggests that lesions to the POM differentially affect male song in sexually relevant and non-sexual social contexts. During the breeding season, nest box acquisition is a critical first step in mate attraction, and song from a nest box is considered more highly sexually-motivated than song from other locations. Our data show that POM lesions exclusively disrupted song in a sexually relevant context (i.e., song produced by Springbox males from nest boxes). This was expected given past data (Riters & Ball, 1999) and the established role of the POM in sexual motivation. Surprisingly however, unlike control males, POM lesioned males failed to display reductions in song within non-sexual contexts (in Springno box and Fall males). Rather, lesions appeared to stimulate song within these contexts. Thus, these data provide new insight into the role of the POM in vocal communication, suggesting that the POM is important for an individual to adjust vocal communication to match the social context. Furthermore, they are the first to suggest the intriguing possibility that the POM stimulates vocal communication in a sexually relevant context, but inhibits vocal communication outside of a sexual context.
The Role of the POM in Sexually Relevant Song
Singing, usually in close proximity to the nest box, occurs significantly more prior to pairing compared to after a female has been attracted (Eens et al., 1994) and males with nest boxes in spring respond to the introduction of females and intruding males by flying to the nest site and singing (Cuthill & Hindmarsh, 1985; Eens, 1997; Pinxten, de Ridder, & Eens, 2003; Riters et al., 2000), suggesting that song produced near a nest box is highly sexually-motivated. Spring box males sang significantly more song from a nest box (as opposed to away from a nest box) in response to female compared to male conspecifics, consistent with a primary role for song from a nest box in mate attraction (Eens, 1997).
As expected given the role of the POM in sexual motivation and past work implicating the POM in sexually-motivated song in songbirds (Heimovics & Riters, 2005; Riters & Ball, 1999; Riters et al., 2000; Riters et al., 2004), lesions to the POM significantly reduced song from a nest box in Springbox males. Unexpectedly however, this effect was only significant when the males were presented with a male stimulus and not with a female stimulus (although the pattern was similar with female stimuli). Song produced by nest box owners within a breeding context is highly sexually relevant, critical not only for mate attraction, but also for nest box defense (Eens, 1997; Eens et al., 1990; Eens et al., 1993; Mountjoy & Lemon, 1991). These data suggest a role for the POM in the regulation of song in the sexually relevant context of inter-male aggression during mate competition. Differences in song structure or the type of song produced by male starlings responding to male versus female conspecifics have not been examined, but the present data suggest that the way the brain regulates song in response to these stimuli might differ.
The finding that POM lesions reduced, but did not significantly disrupt, song in response to a female in Springbox males is somewhat in contrast to the study of Riters and Ball (1999). A major difference between these two studies is that males in the Spring conditions of the present study were implanted with T, whereas those of the past study were not. T treatment in the present study may thus have stimulated sexual motivation by acting in brain regions outside of the POM or within the remaining POM tissue of lesioned males. Furthermore, differences in social dynamics associated with differences in aspects of the experimental design might explain the different results between these two studies. In contrast to the present study in which males were required to compete with other males for nest boxes, males in the Riters and Ball study were singly housed with a single nest box and were never required to compete for nest box access.
Males of other avian species have been observed to adjust song in response to female behavior (e.g. Smith, King, & West, 2000). In starlings, females have the highest levels of circulating E2 concentrations when displaying the highest levels of sexual behavior (Dawson, 1983). Thus, it is somewhat surprising that males did not display differences in song behavior when presented with spring condition females (which were implanted with E2) compared to fall condition females (which were not implanted). This lack of difference may have been a consequence of the T implants in the Spring males causing indiscriminant sexual behavior. The possibility that males with natural T levels in Spring may respond differently to females in different reproductive states remains to be investigated.
The Role of the POM in Non-Sexual Song
Several studies in songbirds suggest a role for the POM specifically in sexually relevant song (Heimovics & Riters, 2005; Riters & Ball, 1999; Riters et al., 2000; Riters et al., 2004). However, the results of a study examining relationships between the density of enkephalin labeled fibers in the POM and song revealed tight positive relationships in fall, but not spring (Riters et al., 2005). The present results offer further support for a role of the POM in song produced outside of a sexual context. Specifically, POM-lesioned Springno box males sang at higher rates away from nest boxes than controls. Again, this difference was significant in the presence of a male stimulus, but not a female stimulus. Although not significant, Fall males showed this same trend, indicating that the POM normally inhibits song produced outside of a sexual context. These data are consistent with a role for the POM in integrating information so that males will display context-appropriate behaviors.
It is important to note that we do not suggest that the failure of POM lesions to influence song from nest boxes in Springno box (Fig. 2C) or Fall males (Fig. 2E) indicates a subtle difference in the effects of POM lesions on song from versus away from nest boxes. Very little song from nest boxes is observed in Springno box and Fall males, so the effects of lesions on this behavior are difficult to evaluate. If anything, the data from the Springno box males hint that lesions similarly affect song from and away from nest boxes. However, additional research is needed to address this issue.
Similar to song produced by Springno box males, male starling song in fall is thought to be involved in functions other than for immediate reproductive purposes (Eens, 1997). We found that Fall males increase the amount of song they produce from nest boxes in response to a male more than a female conspecific, suggesting that song broadcast from nest boxes may also serve functions outside the breeding season. Males rarely enter and exit or defend a nest box in fall. However, males use nest boxes for perching (in our aviaries they are often the highest perches located near a wall) and for shelter. Thus the increased song observed in response to males in the present study might relate to establishment or maintenance of prime perch position. Related to this possibility, dominant starlings tend to occupy the safest perches that are higher or in the center of flocks (Feare, Gill, Mckay, & Bishop, 1995).
Testosterone Effects on Song Rate?
Another factor that could influence male song rate in the present study is T. T levels in songbirds tend to be high in birds that sing at high rates (e.g., Ball, Riters, & Balthazart, 2002). In an attempt to investigate the effects of POM lesions on male song while controlling T effects, male starlings were castrated and administered T in spring, and blank implants in fall. This treatment should minimize differences in T concentrations across males; however it is possible that T from remnants or extra-gonadal sources of T (e.g., steroid hormone precursors from the adrenal glands, which can be converted into T (Soma & Wingfield, 2001) might result in non-uniform T concentrations in the males. Thus it is possible that POM lesions could produce variation in levels of singing indirectly by influencing concentrations of T. However, this appears to be unlikely given that the relationship between T and singing in starlings has not been found to be linear (Buchanan, Spencer, Goldsmith, & Catchpole, 2003; Duffy & Ball, 2002; Riters et al., 2000). Rather, the data suggest that song production increases once a threshold concentration of T is reached, a relationship consistent with T effects on other reproductive behaviors (e.g., Damassa, Smith, Tennet, & Davidson, 1977).
The Role of the POM in Aggression
The preoptic area has been identified as an important control center for aggressive as well as other sexual behaviors in multiple vertebrate species ( e.g., Hasen & Gammie, 2005; Schlinger & Callard, 1989; Wheeler & Crews, 1978). POM lesions reduced the number of Springbox males that pecked the stimulus bird (both female and male stimuli), but did not affect pecking behavior in Springno box males or Fall males. Pecking has been described in starlings in both agonistic encounters between males and in courtship, where females have been observed to peck a male prior to copulation (Kessel, 1957). The fact that POM lesions decreased pecking specifically in Springbox males is consistent with the idea that the POM may be involved in both intersexual and agonistic intrasexual interactions involved in reproduction. Furthermore, consistent with a role for the POM in aggression, when Springbox males were presented with a male stimulus, lesions to the POM significantly reduced song from a nest box, a behavior used for nest box defense (Eens, 1997; Eens et al., 1990; Eens et al., 1993; Mountjoy & Lemon, 1991).
The Role of the POM in Other Sexually-Motivated Behaviors
Only Springbox males gathered nest material and entered nest boxes more in response to a female than a male conspecific, confirming that only Springbox males are highly sexually-motivated. As in Riters and Ball (1999), POM lesions only reduced nest material gathering and nest box entering in Springbox males. A higher proportion of POM lesioned Springno box males wing waved when compared to controls. Wing waving is generally thought to serve as a visual signal in mate attraction (Eens et al., 1990; Eens et al., 1993; Eens et al., 1994; Feare, 1984). However, wing waving is also used in agonistic encounters between competing males in both spring and fall (Davis, 1959; Ellis, 1965; Feare, 1984). Davis (1959) suggested that wing waving in starlings is a long-distance signal of territory ownership. The increase in wing waving in non-sexual contexts among POM-lesioned males may indicate that these males are increasing a courtship behavior in an inappropriate context. Alternatively, POM-lesioned males may increase wing waving as an agonistic display. In either case, the data are consistent with a role for the POM in regulating appropriate behavioral displays within appropriate social contexts.
It is important to note that POM lesions only decreased sexually relevant behaviors and only in a sexually-relevant context. With the single exception of preening in Fall males, no control behaviors measured were affected by POM lesions. Wing waving and song produced away from a nest box were increased, not decreased, in POM-lesioned males outside of a sexual context. These results show that POM lesions do not uniformly depress all behaviors, but are selectively affecting behavioral responses depending on the social context of the animal.
Size and Specificity of the Lesions
POM lesions in this study were quite small, only damaging approximately 8% or 150 μm3 of the POM. Nonetheless, lesions damaging as little as 2% of the POM volume impaired sexual behaviors, indicating the critical importance of this structure in regulating sexual behavior in male starlings. We found no correlations of the size of the POM lesion with any behavior measured in spring, duplicating the finding of Riters and Ball (1999). However, the percent POM damage did positively correlate with the number of wing waves in Fall males, providing more evidence that the POM regulates this behavior specifically in a non-sexual context.
Evidence from various bird species suggests that the rostral and caudal portions of the POM differentially regulate appetitive and consummatory sexual behaviors, respectively (Balthazart, Absil, Gerard, Appeltants, & Ball, 1998; Riters & Ball, 1999; Riters et al., 2004). In starlings, Riters and Ball (1999) found that males with lesions confined to rostral POM never sang. However in the present study the two males with damage to rostral POM were not similarly impaired.
Conclusion
The data presented here contribute to a growing body of research highlighting the POM as a key area involved in the integration of sensory and hormonal information that regulates appropriate behavioral responses to environmental and conspecific social stimuli (Ball & Balthazart, 2004; Wood, 1998). The POM projects directly to the dorsal medial portion of nucleus intercollicularis (DM), a nucleus involved in song output, and is well-positioned to regulate song through multiple indirect connections with other song control nuclei (Riters & Alger, 2004). Through these pathways, the POM might provide contextual input to the song control system and this interaction could be what ensures that song occurs in an appropriate context and in response to appropriate stimuli.
Acknowledgements
The material presented in this paper is based upon work supported by NIMH grant (R01-MH 65645) to LVR and a NSF graduate research fellowship to SJA. We gratefully acknowledge Kate Skogen and Martin Lund for animal care, Nicole Hayes and Sydney Cummings for help with behavioral observations, Sara Dudgeon for help with tissue processing and Bill Feeny for assistance with illustrations.
References
- Ball GF, Balthazart J. Hormonal regulation of brain circuits mediating male sexual behavior in birds. Physiology & Behavior. 2004;83(2):329–46. doi: 10.1016/j.physbeh.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Ball GF, Riters LV, Balthazart J. Neuroendocrinology of song behavior and avian brain plasticity: multiple sites of action of sex steroid hormones. Front Neuroendocrinol. 2002;23(2):137–78. doi: 10.1006/frne.2002.0230. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Absil P, Gerard M, Appeltants D, Ball GF. Appetitive and consummatory male sexual behavior in Japanese quail are differentially regulated by subregions of the preoptic medial nucleus. The Journal of Neuroscience. 1998;18(16):6512–27. doi: 10.1523/JNEUROSCI.18-16-06512.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottjer SW, Johnson F. Circuits, hormones, and learning: vocal behavior in songbirds. Journal of Neurobiology. 1997;33(5):602–18. doi: 10.1002/(sici)1097-4695(19971105)33:5<602::aid-neu8>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Brenowitz EA, Margoliash D, Nordeen KW. An introduction to birdsong and the avian song system. Journal of Neurobiology. 1997;33(5):495–500. [PubMed] [Google Scholar]
- Buchanan KL, Spencer KA, Goldsmith AR, Catchpole CK. Song as an honest signal of past developmental stress in the European starling (Sturnus vulgaris) Proc Biol Sci. 2003;270(1520):1149–56. doi: 10.1098/rspb.2003.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catchpole C, Slater PJB. Birdsong : Biological Themes and Variations. Cambridge University Press; Cambridge, [England] ;: New York NY USA: 1995. [Google Scholar]
- Cuthill I, Hindmarsh A. Increase in starling song activity with removal of mate. Animal Behaviour. 1985;33(FEB):326–327. [Google Scholar]
- Damassa DA, Smith ER, Tennet B, Davidson JM. The relationship between circulating testosterone levels and male sexual behavior in rats. Hormones and Behavior. 1977;8:275–286. doi: 10.1016/0018-506x(77)90002-2. [DOI] [PubMed] [Google Scholar]
- Davis DE. Territorial rank in starlings. Animal Behaviour. 1959;7(34):214–221. [Google Scholar]
- Dawson A. Plasma gonadal steroid levels in wild starlings (Sturnus vulgaris) during the annual cycle and in relation to the stages of breeding. General and Comparative Endocrinology. 1983;49:286–294. doi: 10.1016/0016-6480(83)90146-6. [DOI] [PubMed] [Google Scholar]
- Duffy DL, Ball GF. Song predicts immunocompetence in male European starlings (Sturnus vulgaris) Proc R Soc Lond B Biol Sci. 2002;269(1493):847–52. doi: 10.1098/rspb.2002.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy DL, Bentley GE, Drazen DL, Ball GF. Effects of testosterone on cell-mediated and humoral immunity in non-breeding adult European starlings. Behavioral Ecology. 2000;11(6):654–662. [Google Scholar]
- Eens M. Understanding the complex song of the European starling: and integrated ethological approach. Advances in the Study of Behavior. 1997;26:255–435. [Google Scholar]
- Eens M, Pinxten R, Verheyen RF. On the function of singing and wing-waving in the European starling Sturnus vulgaris. Bird Study. 1990;37:48–52. [Google Scholar]
- Eens M, Pinxten R, Verheyen RF. Function of the song and song repertoire in the European starling (Sturnus vulgaris): an aviary experiment. Behaviour. 1993;125:51–66. [Google Scholar]
- Eens M, Pinxten R, Verheyen RF. Variation in singing activity during the breeding cycle of the European starling Sturnus vulgaris. Belgian Journal of Zoology. 1994;124(2):167–174. [Google Scholar]
- Ellis CR. Agonistic behavior in the male starling. Wilson Bulletin. 1965;78(2):208–224. [Google Scholar]
- Falk H, Gwinner E. Timing of photorefractoriness in the European starling -significance of photoperiod early and late in the reproductive cycle. Biology of Reproduction. 1988;39(5):1004–1008. doi: 10.1095/biolreprod39.5.1004. [DOI] [PubMed] [Google Scholar]
- Feare C. The Starling. Oxford University Press; Oxford [Oxfordshire] ; New York: 1984. [Google Scholar]
- Feare CJ, Gill EL, Mckay HV, Bishop JD. Is the distribution of starlings Sturnus vulgaris within roosts determined by competition? Ibis. 1995;137(3):379–382. [Google Scholar]
- Gentner TQ, Hulse SH. Female European starling preference and choice for variation in conspecific male song. Animal Behaviour. 2000;59:443–458. doi: 10.1006/anbe.1999.1313. [DOI] [PubMed] [Google Scholar]
- Hasen NS, Gammie SC. Differential fos activation in virgin and lactating mice in response to an intruder. Physiology and Behavior. 2005;84(5):681–95. doi: 10.1016/j.physbeh.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Hausberger M, Richardyris MA, Henry L, Lepage L, Schmidt I. Song sharing reflects the social organization in a captive group of European starlings (Sturnus vulgaris) Journal of Comparative Psychology. 1995;109(3):222–241. [Google Scholar]
- Heimovics SA, Riters LV. Immediate early gene activity in song control nuclei and brain areas regulating motivation relates positively to singing behavior during, but not outside of, a breeding context. Journal of Neurobiology. 2005;65(3):207–24. doi: 10.1002/neu.20181. [DOI] [PubMed] [Google Scholar]
- Hull EM, Lorrain DS, Du J, Matuszewich L, Lumley LA, Putnam SK, Moses J. Hormone-neurotransmitter interactions in the control of sexual behavior. Behavioral Brain Research. 1999;105(1):105–16. doi: 10.1016/s0166-4328(99)00086-8. [DOI] [PubMed] [Google Scholar]
- Kessel B. A study of the breeding biology of the European starling (Sturnus vulgaris L.) in North America. American Midland Naturalist. 1957;58(2):257–331. [Google Scholar]
- Margoliash D. Functional organization of forebrain pathways for song production and perception. Journal of Neurobiology. 1997;33(5):671–93. doi: 10.1002/(sici)1097-4695(19971105)33:5<671::aid-neu12>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Mountjoy DJ, Lemon RE. Song as an attractant for male and female European starlings, and the influence of song complexity on their rResponse. Behavioral Ecology and Sociobiology. 1991;28(2):97–100. [Google Scholar]
- Panzica GC, Viglietti-Panzica C, Balthazart J. The sexually dimorphic medial preoptic nucleus of quail: a key brain area mediating steroid action on male sexual behavior. Front Neuroendocrinol. 1996;17(1):51–125. doi: 10.1006/frne.1996.0002. [DOI] [PubMed] [Google Scholar]
- Pinxten R, de Ridder E, Eens M. Female presence affects male behavior and testosterone levels in the European starling (Sturnus vulgaris) Hormones and Behavior. 2003;44(2):103–109. doi: 10.1016/s0018-506x(03)00120-x. [DOI] [PubMed] [Google Scholar]
- Riters LV, Alger SJ. Neuroanatomical evidence for indirect connections between the medial preoptic nucleus and the song control system: possible neural substrates for sexually motivated song. Cell and Tissue Research. 2004;316(1):35–44. doi: 10.1007/s00441-003-0838-6. [DOI] [PubMed] [Google Scholar]
- Riters LV, Ball GF. Lesions to the medial preoptic area affect singing in the male European starling (Sturnus vulgaris) Hormones and Behavior. 1999;36(3):276–86. doi: 10.1006/hbeh.1999.1549. [DOI] [PubMed] [Google Scholar]
- Riters LV, Eens M, Pinxten R, Ball GF. Seasonal changes in the densities of alpha(2) noradrenergic receptors are inversely related to changes in testosterone and the volumes of song control nuclei in male European starlings. J Comp Neurol. 2002;444(1):63–74. doi: 10.1002/cne.10131. [DOI] [PubMed] [Google Scholar]
- Riters LV, Eens M, Pinxten R, Duffy DL, Balthazart J, Ball GF. Seasonal changes in courtship song and the medial preoptic area in male European starlings (Sturnus vulgaris) Hormones and Behavior. 2000;38(4):250–61. doi: 10.1006/hbeh.2000.1623. [DOI] [PubMed] [Google Scholar]
- Riters LV, Schroeder MB, Auger CJ, Eens M, Pinxten R, Ball GF. Evidence for opioid involvement in the regulation of song production in male European starlings (Sturnus vulgaris) Behavioral Neuroscience. 2005;119(1):245–55. doi: 10.1037/0735-7044.119.1.245. [DOI] [PubMed] [Google Scholar]
- Riters LV, Teague DP, Schroeder MB, Cummings SE. Vocal production in different social contexts relates to variation in immediate early gene immunoreactivity within and outside of the song control system. Behavioural Brain Research. 2004;155(2):307–18. doi: 10.1016/j.bbr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Schlinger BA, Callard GV. Aromatase activity in quail brain: correlation with aggressiveness. Endocrinology. 1989;124(1):437–43. doi: 10.1210/endo-124-1-437. [DOI] [PubMed] [Google Scholar]
- Slater PJB. Fifty years of bird song research: a case study in animal behaviour. Animal Behaviour. 2003;65:633–639. [Google Scholar]
- Smith VA, King AP, West MJ. A role of her own: female cowbirds, Molothrus ater, influence the development and outcome of song learning. Animal Behaviour. 2000;60:599–609. doi: 10.1006/anbe.2000.1531. [DOI] [PubMed] [Google Scholar]
- Soma KK, Wingfield JC. Dehydroepiandrosterone in songbird plasma: seasonal regulation and relationship to territorial aggression. Gen Comp Endocrinol. 2001;123(2):144–55. doi: 10.1006/gcen.2001.7657. [DOI] [PubMed] [Google Scholar]
- Wheeler JM, Crews D. The role of the anterior hypothalamus-preoptic area in the regulation of male reproductive behavior in the lizard, Anolis carolinensis: lesion studies. Hormones and Behavior. 1978;11(1):42–60. doi: 10.1016/0018-506x(78)90057-0. [DOI] [PubMed] [Google Scholar]
- Wood RI. Integration of chemosensory and hormonal input in the male Syrian hamster brain. Annals of the New York Academy of Sciences. 1998;855:362–72. doi: 10.1111/j.1749-6632.1998.tb10594.x. [DOI] [PubMed] [Google Scholar]