Abstract
Template melting is an essential step in the initiation of DNA replication, but the mechanism of template melting is unknown for any replicon. Here we demonstrate that melting of the bovine papillomavirus type 1 ori is a sequence-dependent process which relies on specific recognition of TA base pairs in the minor groove by the E1 initiator. We show that correct template melting is a prerequisite for the formation of a stable double hexamer with helicase activity and that ori mutants that fail to melt correctly are defective for ori unwinding and DNA replication in vivo. Our results also indicate that melting of the DNA is achieved by destabilization of the double helix along its length through multiple interactions with E1, each of which is responsible for melting of a few base pairs, resulting in the extensive melting that is required for initiation of DNA replication.
Template melting is an essential step in the initiation of replication of double-stranded DNA. In spite of its fundamental importance for DNA replication, the melting process is almost entirely uncharacterized. For Escherichia coli, the initiator DnaA has long been known to melt OriC, as can be detected by the use of single-stranded DNA (ssDNA)-specific nucleases (6, 32). The mechanism by which the DNA is melted is unknown, but it is established that melting is dependent on nucleotide binding by DnaA (for a review, see reference 16). For eukaryotes, although the origin recognition complex has been identified as the factor that marks the replicator (2), an activity that can melt the DNA in preparation for replication has still not been identified. Viral initiator proteins such as the simian virus 40 (SV40) large T antigen (T-Ag) and the papillomavirus E1 protein have long been known to melt their respective origins of DNA replication, as detected by oxidation with KMnO4 (4, 5, 14, 22, 24, 25). However, little information exists about which forms of these proteins execute melting, which parts of the polypeptide are responsible for the melting activity, and whether this process is DNA sequence dependent (5, 27).
The E1 proteins from papillomaviruses are ∼70-kDa polypeptides which, in addition to DNA melting activity, have DNA helicase activity (19, 20, 30, 33, 35, 37, 38, 40) and also bind DNA. DNA binding by E1 is the result of two different DNA binding activities. Site-specific DNA binding is provided by the E1 DNA binding domain (DBD), which recognizes and binds to two pairs of E1 binding sites (E1 BS) in the origin of replication (7-9, 11, 15, 17, 31, 36). The E1 helicase domain binds DNA with low sequence specificity, and this activity is required for the ability of the E1 helicase domain to contact the DNA sequences flanking the E1 BS, including a region that has been termed the A/T-rich region (34).
Recent advances in the study of E1 and T-Ag have opened up the melting process for more detailed study. Structural studies of E1 and T-Ag have provided important information about the domain structure, how these proteins oligomerize, and how they bind and hydrolyze nucleotides (1, 10, 13, 18). Biochemical analysis has demonstrated that one particular form of E1, a double trimer (DT), generates permanganate reactivity in the ori in the presence of ADP, indicating that the DT provides template melting activity (28). The DT is a required precursor for formation of the double hexameric (DH) helicase, and we have therefore suggested that template melting is required for the assembly of a DH helicase that engages the DNA (28). In addition, a structural element in E1, a β-hairpin (specifically a highly conserved His at the tip of the β-hairpin), has also been implicated in template melting, possibly by a direct interaction between the β-hairpin histidine and the ori DNA (28).
Here we have dissected E1-dependent template melting to identify its components and to define the role that is played by the template. We demonstrate that template melting is sequence dependent and that E1 relies on a series of TA base pairs on the flank of the E1 BS for melting to occur. These TA base pairs are recognized in the minor groove. Mutations of the template that affect melting simultaneously affect DH formation, unwinding, and DNA replication, demonstrating that correct melting is a prerequisite for formation of a functional DH. Our results also indicate that melting of the DNA is achieved by destabilization of the double helix along its length through multiple interactions with E1, each of which is responsible for melting of a few base pairs.
MATERIALS AND METHODS
Permanganate reactivity assays.
Permanganate reactivity assays were performed essentially as described previously (5, 25). Binding reaction mixtures (20 mM HEPES [pH 7.9], 100 mM NaCl, 0.1% NP-40, 5% glycerol, 5 mM dithiothreitol [DTT], 5 mM MgCl2, and 5 mM ATP) containing ∼10 fmol of end-labeled probe were assembled and incubated with E1 at room temperature. After 30 min, KMnO4 was added to a final concentration of 6 mM, and reactions were incubated for a further 2 min. Modification was terminated by adding β-mercaptoethanol to 80 mM, sodium dodecyl sulfate to 0.3%, and EDTA to 10 mM. Reaction products were then digested with proteinase K (20 μg/ml) for 60 min at 37°C, and the DNA was recovered by phenol-chloroform extraction and ethanol precipitation. Cleavage at modified bases was achieved with piperidine (30 min at 90°C).
EMSA.
Acrylamide gels (4% [39:1 acrylamide:bis]) containing 0.5× Tris-borate-EDTA were used for all electrophoretic mobility shift assays (EMSAs). E1 was added to the probe (5,000 cpm) in a solution containing 20 mM HEPES, pH 7.5, 100 mM NaCl, 0.7 mg/ml bovine serum albumin, 0.1% NP-40, 5% glycerol, 5 mM DTT, 5 mM MgCl2, and 2 mM ATP or ADP. After incubation at room temperature for 60 min, the samples were loaded and run for 2 h at 9 V/cm. The ability to generate discrete complexes, especially the DT and DH, was critically dependent on the quality of the acrylamide, a freshly made ammonium persulfate solution, allowing overnight polymerization of the gels, and a precise prerunning time (9 V/cm for 4 h).
Combined EMSA and unwinding assays.
Unwinding assays were performed by incubating 2 fmol of probe with E1 under EMSA conditions (28). The 10-μl reaction system contained 20 mM HEPES (pH 7.9), 75 mM NaCl, 0.1% NP-40, 5% glycerol, 5 mM DTT, 5 mM MgCl2, 5 mM ATP, and 10 ng/μl E. coli ssDNA binding protein (SSB), and the reaction mixes were incubated for 30 min at 32°C. Prior to gel loading, the concentration of NaCl was increased to 500 mM, which disrupts most E1-DNA complexes but does not affect ssDNA-SSB complexes. The ssDNA was detected as an SSB-ssDNA complex under these EMSA conditions.
In vivo DNA replication assays.
In vivo DNA replication assays were performed as described previously (39). Briefly, expression vectors for the E1 and E2 proteins, i.e., pCGE1 (2 μg) and pCGE2 (0.5 μg), were cotransfected together with 0.5 μg of the respective ori plasmids into CHO cells, using electroporation. The mutant ori plasmids were all constructed in the context of the plasmid 11/12/X (26), which contains 110 bp of the bovine papillomavirus (BPV)ori sequence cloned into the polylinker of pUC 19. Two and 3 days after transfection, low-molecular-weight DNA was harvested using alkaline lysis, digested with DpnI, linearized with HindIII, and analyzed by Southern blotting. Quantitation of the mitochondrial DNA in the samples was used to ascertain equal loading.
Probes.
All probes used for permanganate reactivity assays and for EMSAs were generated by PCR amplification, using one primer end labeled with [γ-32P]ATP and T4 polynucleotide kinase. Our standard probe for permanganate reactivity assays and EMSAs contains 84 bp of the BPV ori sequence centered on the E1 BS. In one experiment (see Fig. 4), we used a shorter probe (56 bp) lacking 28 bp from the right end of the 84-bp probe. The purpose of this shorter probe was to reduce the length of the oligonucleotides required to insert inosine substitutions in the bottom strand of the probe from 84 bp to 56 bp. In another experiment (see Fig. 2A), a longer probe, which includes pUC 19 polylinker sequences at both ends, was used to resolve the melting of the T14, AT8, and TA8 probes. GA ladders generated by treatment of the probes according to the Maxam and Gilbert sequencing protocol (21) were used as markers.
FIG. 4.
(A) Comparison of permanganate reactivities of the A16 and T16/17 probes. Permanganate reactivity assays were performed on the A16 and T16/17 templates in parallel. E1 was incubated with the probe in the absence of nucleotide (lanes 1 and 8), in the presence of ADP (lanes 2 to 4 and 9 to 11) or in the presence of ATP (lanes 5 to 7 and 12 to 14). In the absence of nucleotide, 800 fmol of E1 was used, and in the presence of ADP or ATP, 200, 400, and 800 fmol of E1 was used. (B) Summary of the permanganate reactivities generated by E1 on the A16 and T16/17 templates in the presence of ADP and ATP. For comparison, the permanganate reactivity on the wt template (from Fig. 1) is shown. (C) The template T6(A16) was generated by insertion of six T residues into the A16 context at positions 12 to 17. Permanganate reactivity assays were performed in the presence of ATP on the bottom-strand labeled probe in the absence (lane 1) or presence (lane 2) of 800 fmol E1. (D) Permanganate reactivity of the T16/17 template. Permanganate reactivity assays were performed using the top-strand labeled T16/17 template. E1 (400 and 800 fmol, respectively) was incubated with the probe in the presence of ADP (lanes 2 and 3) or ATP (lanes 4 and 5) and treated with permanganate as described in the legend to Fig. 1. For lane 1, no E1 was added. Below the gel is a summary of the observed permanganate reactivity. (E) Schematic representation of distal and proximal T-dependent melting showing that TA bp (bold) direct melting 5 or 6 bp away from the inserted TA bp.
FIG. 2.
Permanganate reactivities of ori probes with altered sequences. (A) Permanganate reactivity assays in the presence of ATP were performed with the top and bottom strands of probes AT8 (lanes 1, 2, 9, and 10) and TA8 (lanes 3, 4, 7, and 8) and the bottom strand of T14 (lanes 5 and 6). The probes were incubated in the absence of E1 (lanes 1, 3, 5, 7, and 9) or in the presence of 800 fmol of E1 (lanes 2, 4, 6, 8, and 10) in the presence of ATP and treated with permanganate as described in the legend to Fig. 1. (B) Permanganate reactivity assays in the presence of ATP were performed with the top (lanes 1 to 8) and bottom (lanes 9 to 12) strands of the A16 (lanes 1 to 4, 11, and 12) and wt (lanes 5 to 10) templates. For lanes 2 to 4 and 6 to 8, 200, 400, and 800 fmol of E1 was used, respectively. For lanes 10 and 12, 800 fmol of E1 was used. (C) E1 complex formation on the wt and A16 probes. EMSA was performed on the wt (lanes 1 to 9) and A16 (lanes 10 to 18) probes. Three quantities of E1 (30, 60, and 120 fmol) were used in the presence of ADP (lanes 2 to 5 and 11 to 14) or ATP (lanes 6 to 9 and 15 to 18). For lanes 1 and 10, no E1 was added.
RESULTS
We recently demonstrated that the assembly of a DH helicase is a process that depends on formation of an E1 DT, which melts the ori and serves as a precursor for the DH. The DH helicase, in turn, is the helicase that unwinds the ori. The DT forms in the presence of ADP, while formation of the DH requires ATP hydrolysis (28). To relate template melting to these complexes, we performed permanganate reactivity assays on the top and bottom strands of the ori in the absence of nucleotide or in the presence of ADP or ATP (Fig. 1). The melting pattern on the bottom strand in the absence of nucleotide showed very slight differences compared to melting in the presence of ADP or ATP (Fig. 1A, lanes 1 to 8). However, on the top strand we observed some clear differences. A T6 stretch in the A/T-rich region which did not melt at all in the absence of nucleotide showed modest reactivity in the presence of ADP (lanes 11 to 13) and significantly greater reactivity in the presence of ATP (lanes 14 to 16). In the presence of ATP, we could also observe a low level of permanganate reactivity in the E1 BS (lanes 15 and 16). These results indicated that although the intensity of permanganate reactivity varied, the melting pattern of the wild-type (wt) ori was not greatly altered under conditions where DT or DH were assembled on the template.
FIG. 1.
(A) Permanganate reactivity of the wt ori probe in the absence of nucleotide or in the presence of ADP or ATP. Permanganate reactivity assays were performed by incubating the bottom-strand (lanes 1 to 8) and top-strand (lanes 9 to 16) labeled probes with E1 in the absence of nucleotide (lanes 2 and 10) or in the presence of ADP (lanes 3 to 5 and 11 to 13) or ATP (lanes 6 to 9 and 14 to 16). In the absence of nucleotide, 800 fmol of E1 was used, and in the presence of nucleotide 200, 400, and 800 fmol of E1 was used. After 30 min at room temperature, the samples were treated with 6 mM KMnO4 for 2 min. For lanes 1 and 9, the top- and bottom-strand probes were treated with KMnO4 in the absence of E1. (B) Summary of the permanganate reactivity of the wt ori probe. Black bars indicate the positions and relative levels of permanganate reactivity.
Template melting is sequence dependent.
A caveat in the above conclusion is that T is the only permanganate-reactive nucleotide and melting can only be detected in positions containing a TA or AT bp. Differences in melting between the DT and the DH may therefore be obscured. To generate more information about ori melting, we replaced the wt sequence with stretches of T residues to generate permanganate-reactive substrates (Fig. 2A). We focused on the left half of the ori (the A/T-rich region), replacing the top-strand sequence with alternating AT bp (AT8) (Fig. 2A, lanes 1, 2, 9, and 10), TA bp (TA8; lanes 3, 4, 7, and 8), and T residues (T14; lanes 5 and 6), and performed permanganate reactivity assays in the presence of ATP. The result using the T14 template clearly demonstrated that all bp between positions 3 and 18 were melted (Fig. 2A, lanes 5 and 6). Similarly, the AT8 (lanes 1, 2, 9, and 10) and TA8 (lanes 3, 4, 7, and 8) templates showed extensive melting, as summarized in the lower panel. These results are consistent with the melting observed on the wt template and indicate that extensive melting (∼15 bp) occurs on the wt probe.
Interestingly, the probe with homopolymeric T residues on the bottom strand (A16) gave rise to a very different pattern (Fig. 2B). We performed a side-by-side comparison of the melting patterns of the A16 template and the wt ori. We observed no melting with the A16 template on the top strand, as expected, since the A residues are not reactive with permanganate (lanes 2 to 4). Surprisingly, we also observed very little permanganate reactivity on the bottom strand, although this strand contains many T residues that could react with permanganate (lanes 11 and 12). Melting was observed only at positions 3 to 7 in the left half of the ori (Fig. 2B, lower panel). This result demonstrates that T residues on the top strand (or A residues on the bottom strand) are specifically required to generate the wt melting pattern.
What first occurred to us was that the defect in melting was caused by a defect in E1 binding. We therefore tested the probes by EMSA (Fig. 2C). As described previously, in the presence of ADP, E1 forms a DT on the wt probe (lanes 2 to 5). In the presence of ATP, an additional, larger complex is formed, corresponding to a DH (lanes 6 to 9). The E1 complex formed on the A16 probe in the presence of ADP was virtually identical to that formed on the wt probe (Fig. 2C, compare lanes 2 to 5 and 11 to 14). In the presence of ATP, however, we observed a less intense band at the position of the DH and higher levels of intermediate complexes on the A16 probe (compare lanes 6 to 9 and 15 to 18). This demonstrates that while the A16 probe formed the DT at the same level as the wt probe, the A16 probe showed a modest defect in DH formation.
Melting depends on TA base pairs flanking the E1 BS.
To determine how the TA bp affected melting, we inserted pairs of T residues at different positions in the A16 stretch on the top strand, generating the templates T10/11, T12/13, T14/15, T16/17, and T20/21 (Fig. 3C). We tested these templates for melting in the absence and presence of ATP (Fig. 3A). In the absence of ATP, these insertions had small effects on permanganate reactivity (compare lane 1 to lanes 3, 5, 7, 9, and 11), mostly generating permanganate reactivity adjacent to the inserted T residues, demonstrating that even in the absence of nucleotide some permanganate reactivity can be observed. In the presence of ATP, however, we observed systematic, qualitative effects of the T insertions on melting. The insertion of pairs of T residues resulted in altered permanganate reactivity, and the changes that we observed were related to the position of the T insertion such that melting in each case was observed 5 or 6 bp to the right of the inserted T residues, towards the E1 BS. Because some of these changes overlapped with the melting at positions 3 to 7 observed with the A16 template, we scanned each lane from the gel and aligned the scans (Fig. 3B). Here the changes in melting due to the inserted T pairs can be observed clearly. Insertion of T's at positions 16 and 17 resulted in the appearance of a new peak at positions 10 and 11 (Fig. 3A, lanes 9 and 10, and B), insertion of T's at positions 14 and 15 resulted in the appearance of a new peak at positions 7 and 8 (Fig. 3A, lanes 7 and 8, and B), etc.
FIG. 3.
Permanganate reactivities of probes with TT insertions. (A) The A16 ori probe was modified by insertion of pairs of T's at different positions in the A16 sequence, as shown in panel C. These probes were tested for permanganate reactivity in the presence of 800 fmol of E1 as described in the legend to Fig. 1. For lanes 1, 3, 5, 7, 9, and 11, no ATP was added. For lanes 2, 4, 6, 8, 10, and 12, 5 mM ATP was added. (B) The bracketed part of the gel in panel A was scanned, and the scans for lanes 2, 4, 6, 8, 10, and 12 were aligned. (C) Summary of the permanganate reactivities generated with the TT-substituted A16 probes. The positions of the substitutions are boxed and shaded. Black bars indicate the positions and levels of permanganate reactivity observed on the A16 probe. The gray bars correspond to the pattern obtained after subtraction of the A16 pattern from each lane. (D) Bottom-strand labeled probes for the A16 template and the TT insertions T12/13, T14/15, T16/17, and T20/21 in the A16 context were tested for permanganate reactivity in the absence (lanes 1, 3, 5, 7, and 9) or presence (lanes 2, 4, 6, 8, and 10) of 800 fmol of E1 in the presence of ADP. (E) Summary of the permanganate reactivities generated by E1 on TT-substituted templates in the presence of ADP. For comparison, the permanganate reactivity on the wt template (from Fig. 1) is also shown.
To resolve these overlapping peaks, we subtracted the pattern obtained with the A16 template (black bars) from the other patterns and plotted the resulting permanganate reactivities as gray bars (Fig. 3C). Clearly, with the exception of the T20/21 template, the insertion of T residues had similar effects on the melting pattern. In each case, melting of 3 or 4 bp was observed 5 or 6 bp away from the inserted residues, towards the E1 BS. This phenomenon was apparent only when the T residues were inserted in a 9-bp window between positions 9 and 17, and insertion of T residues had no apparent effect outside this region, as indicated by the lack of effect of insertions at T4/5, T6/7, and T8/9 (data not shown). Similarly, there were no effects of T insertions at T18/19 (data not shown) or T20/21 (Fig. 3A, compare lanes 2 and 12). We also tested some of the TT insertion templates for melting in the presence of ADP (Fig. 3D and E). In the presence of ADP, we did not observe the same phenomenon of shifting reactivity relative to the inserted T residues, indicating that this phenomenon is dependent on ATP hydrolysis. This notion was supported by the effects of the nonhydrolyzable ATP analog ATP-γ-S, which failed to support the T-induced melting and essentially behaved like ADP in these assays (data not shown). From previous experiments, we know that ATP-γ-S is bound and hydrolyzed extremely slowly by E1 (28).
To extend these observations, we performed a side-by-side comparison of the A16 and T16/17 templates in the absence of nucleotide, in the presence of ADP, and in the presence of ATP (Fig. 4A and B). In the absence of nucleotide, the two templates showed identical patterns, with low-level reactivity at positions 11 and 12 (Fig. 4A, lanes 1 and 8). In the presence of ADP at three concentrations of E1, the melting patterns for the A16 and T16/17 templates were still identical (lanes 2 to 4 and 9 to 11, respectively), corresponding to permanganate reactivity at positions 9 to 14. In the presence of ATP, the patterns for both templates changed dramatically (compare lanes 5 to 7 and 12 to 14). We now observed melting at positions 3 to 7 for both templates, as well as permanganate reactivity between positions 9 and 14 for the T16/17 template. We also observed robust permanganate reactivity in the sequences corresponding to the E1 BS (lanes 6, 7, 13, and 14). In summary, these results demonstrate that although TA bp play no apparent role in the ADP-dependent melting associated with the DT, they are essential for generating the melting pattern observed in the presence of ATP.
The wt template contains six TA bp within the 9-bp window where insertion of TA bp has an effect on permanganate reactivity. Collectively, the six TA bp could account for the extensive melting observed with the wt and T14 templates, since these TA bp are melted and also direct melting 5 or 6 bp away. To determine whether the six TA bp had the expected cumulative effect in the A16 context, we generated the template T6 (A16), which contains the six TA bp in the context of the A16 template (Fig. 4C). As predicted, when we performed permanganate reactivity assays on this template in the presence of ATP, we observed strong melting over all positions between positions 3 and 11, demonstrating that the inserted T6 stretch induces melting in a cumulative manner in the sequences between the point where the T6 is inserted and the E1 BS. To determine whether the inserted pairs of TA bp on the top strand were also melted, we performed permanganate reactivity assays on the top strand of the T16/17 template (Fig. 4D). As expected from the results with the wt template, the two inserted T residues reacted with permanganate. In summary, these results demonstrate that the six TA bp are largely responsible for the generation of the melting of positions 3 to 18 observed on the wt and T14 templates (Fig. 4E). We have termed these components of melting distal and proximal T-dependent melting.
TA bp are recognized in the minor groove.
To determine which aspect of the TA bp was important for melting, we generated various substitutions at positions 16 and 17 in the A16 template and used the resultant mutants for permanganate reactivity assays (Fig. 5). As observed previously, TA bp inserted at positions 16 and 17 clearly induced melting at positions 9 to 11 (lanes 3 and 4), in addition to the melting between positions 3 and 7 observed with the A16 template (lanes 1 and 2). GC and CG bp clearly did not direct melting at positions 9 to 11 (lanes 5 to 8), while UA bp functioned as well as TA bp (lanes 9 and 10). This demonstrates that the methyl group present in the major groove in the TA bp is not important for the generation of additional permanganate reactivity and indicates that an interaction might take place in the minor groove. To determine whether this was the case, we generated CI substitutions at both the 14-15 and the 16-17 positions. A CI bp is identical to a TA bp in the minor groove and identical to a CG bp in the major groove (Fig. 5B). As shown in Fig. 5A, lanes 11 to 14, CI substitutions at positions 14-15 or 16-17 behaved similarly to the corresponding TA bp, demonstrating that the dependence on TA bp for melting reflects minor groove recognition.
FIG. 5.
Melting requires minor groove recognition of TA bp. Permanganate reactivity assays were performed on E1 templates with substitutions at the 14-15 and 16-17 positions, as indicated in the figure. For lanes 1, 3, 5, 7, 9, 11, and 13, no E1 was added. For lanes 2, 4, 6, 8, 10, 12, and 14, 800 fmol of E1 was added in the presence of ATP. The bracket indicates the position of T-induced melting. (B) Schematic representation of the minor and major grooves of AT, IC, and GC bp.
DNA replication in vivo is dependent on the six TA base pairs.
One of the advantages of using a viral replication system is the ability to test ori mutants in vivo in transient DNA replication assays. We generated two sets of ori mutants and tested them for DNA replication in vivo (Fig. 6B). In one set, we changed the T6 stretch in the context of the wt ori and replaced either all six T's with A (A6) or the first two T's (A2T4) or first 4 T's (A4T2) with A, leaving the rest of the wt ori sequence intact. In the second set, we inserted T's into the A16 template. We inserted T's at positions 16 and 17 (T16/17) or positions 12 to 17 (T6). These correspond to the first two T's and all six T's, respectively, in the wt T6 stretch. Because we had observed effects of T insertion at positions 10 and 11 (Fig. 2), we also generated the template T9, which inserts nine TA bp between positions 9 and 17. We then transfected these ori plasmids together with expression vectors for E1 and E2 and measured DNA replication 2 and 3 days after transfection (Fig. 6A).
FIG. 6.
In vivo DNA replication and unwinding are dependent on six TA bp. (A) Mutant ori's, as shown in panel B, were generated and tested for transient DNA replication in vivo by cotransfection of the respective mutant ori plasmids with expression vectors for E1 and E2. Two and three days after transfection, low-molecular-weight DNAs were prepared, and replicated DpnI-resistant plasmid DNA was detected by Southern blotting. (B) Sequences of the two sets of ori mutants that were tested in panel A. The top set is based on the wt ori sequence, and the bottom set is based on the A16 ori sequence. The substitutions compared to the parent template are indicated in shaded boxes. The level of in vivo DNA replication, relative to that with the wt ori, is indicated for each mutant.
The A6 (Fig. 6A, lanes 3 and 4), A2T4 (lanes 11 and 12), and A4T2 (lanes 13 and 14) mutants all had 5 to 10% of the wt activity, demonstrating the importance of an intact T6 sequence in the context of the wt ori (Fig. 6A and B). In the context of the A16 mutant, which has a severe defect in DNA replication (lanes 17 and 18), the replacement of the AT bp with TA bp between positions 11 and 17 (T6), which corresponds to the position of the T stretch in the wt ori, restored significant levels of activity (∼40% of the wt activity) (lanes 5 and 6). Replacement of the first nine AT bp with TA bp (T9) restored replication to 70% of the wt activity (lanes 9 and 10). T16/17, corresponding to the insertion of T's at positions 16 and 17, did not restore activity to the ori in a detectable way (lanes 7 and 8). These results demonstrate that the six T residues that are present in the wt ori are essential for ori function and that the rest of the ori sequence in this region is of minor importance for replication. These results also indicate that the T6 stretch functions as a unit and that restoration of only parts of this unit does not result in partial activity.
The DH formed on the A16 template has reduced stability.
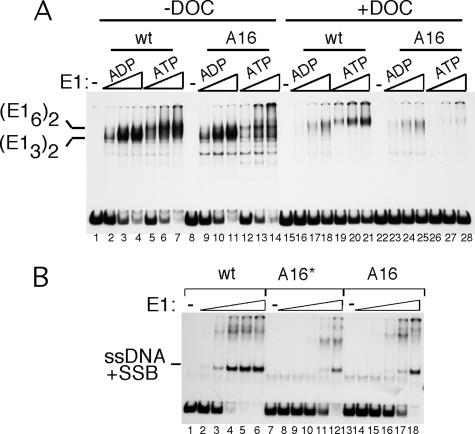
The results described above demonstrate that the T6 stretch serves an essential function in DNA replication and that the failure to melt the ori DNA correctly likely results in the severe defect in DNA replication. As shown in Fig. 2, the A16 template showed no defects in DT formation, and melting in the presence of ADP also appeared normal. This was an expected result, since the six TA bp have no detectable effect on either DT formation or melting in the presence of ADP. However, the A16 template did show defects in DH formation (Fig. 2C). To determine whether the complexes formed on the A16 template differed in stability from the complexes formed on the wt probe, we performed an EMSA in which we challenged the complexes formed on the wt and A16 probes with detergent. In the absence of detergent, DT formation was similar on the two probes (Fig. 7A, compare lanes 2 to 4 and 9 to 11), while the DH complex formed poorly on the A16 template compared to the wt probe (Fig. 7A, compare lanes 5 to 7 and 12 to 14). In a parallel set of reactions, we added low levels of detergent (0.2% sodium deoxycholate) after the binding reaction but before loading of the sample into the gel. Under these conditions, the DT on both the wt and A16 probes were largely disrupted, indicating that the complexes on the wt and A16 templates have similar low stabilities (Fig. 7A, compare lanes 2 to 4 to lanes 16 to 18 and lanes 9 to 11 to lanes 23 to 25). Interestingly, the DH complex formed on the wt template remained largely intact (compare lanes 5 to 7 and 19 to 21), while the DH complex formed on the A16 template was largely disrupted (compare lanes 12 to 14 and 26 to 28). This demonstrates that the DH complex formed on the A16 template has reduced stability, likely caused by the melting defect.
FIG. 7.
The DH formed on the A16 probe is unstable. EMSA was performed using the wt (lanes 1 to 9) or A16 (lanes 10 to 18) 84-bp ori probe. Three quantities of E1 (30, 60, and 120 fmol) were used in the presence of ADP (lanes 2 to 4, 9 to 11, 16 to 18, and 23 to 25) or ATP (lanes 5 to 7, 12 to 14, 19 to 21, and 26 to 28). For the left half of the figure, lanes 1 to 14, the samples were loaded directly into the gel. For the right half of the figure, lanes 15 to 28, 0.2% deoxycholate was added to the samples prior to their being loaded into the gel. (B) ori fragment unwinding assays. ori fragment unwinding assays were performed by incubating the wt, A16*, and A16 probes with E. coli SSB in the absence of E1 (lanes 1, 7, and 13) or in the presence of 30, 60, 120, 240, or 480 fmol of E1 (lanes 2 to 6, 8 to 12, and 14 to 18). Unwinding (ssDNA) was detected as a specific ssDNA-SSB complex by EMSA.
Template unwinding is dependent on the six TA bp.
To determine the direct consequences of the melting defect on the mutants lacking the TA bp stretch (such as A16), we performed unwinding assays with the wt and A16 probes (Fig. 7B). ori fragment unwinding is dependent on both the melting and the helicase activities of E1 to generate free single-stranded DNA from a completely double-stranded ori template. The single-stranded DNA can be detected by EMSA in the presence of E. coli SSB. We compared unwinding of the wt ori, A16, and A16*, which in addition to the A16 changes has mutations that disable the E1 BS. On the wt template, we could observe the appearance of the ssDNA-SSB complex at an approximately fourfold lower concentration of E1 than the level of E1 required to unwind the A16 ori (compare lanes 2 to 6 and lanes 14 to 18). The A16* probe, which lacks E1 binding sites, behaved similarly to the A16 probe (lanes 8 to 12), indicating that at these high concentrations of E1, some unwinding can occur without ori specificity. This demonstrates that the melting defect of the A16 template results in a corresponding defect in unwinding.
DISCUSSION
We initially set out to characterize melting of the ori by replacing the natural ori sequences with sequences with which we could detect permanganate reactivity. We focused on one-half of the ori to simplify the analysis. The results from this analysis demonstrate that ∼18 bp adjacent to the E1 BS are melted in the presence of ATP. As discussed below, the fact that we can distinguish several different components of melting indicates that several different mechanisms are required in combination for complete melting and that melting likely corresponds to a process rather than a single event. This idea is supported by the fact that the permanganate reactivity, which is first detected upon formation of the E1 DT, changes concurrently with the conversion of the DT to a DH. As discussed below, melting is a prerequisite for DH formation, supporting the idea that the substrate for DH formation is a protein-DNA complex in which the DNA is melted. The ori is not symmetrical, and the six TA bp that are present in the left half of the ori are not present in the correct position in the other half. Clearly, the sequences in the two halves of the ori are not melted in the same way. Indeed, it is surprising how different the melting patterns are for the two halves of the ori. Most likely, the events that we have determined are essential for initiation of DNA replication on the left flank of the E1 BS need to occur in only one-half of the ori for function.
Comparison to earlier studies.
Pioneering work on template melting using permanganate reactivity assays was carried out with the SV40 system more than 15 years ago (4, 5). As expected, there are similarities between the permanganate reactivities induced by E1 and T-Ag, although precise comparisons are hampered by the limited sequence similarity of the two ori's. In both cases, permanganate reactivity is observed at similar distances flanking the BS for the respective initiator, and a TA-rich region with the same polarity as that in the BPV ori is also present in the SV40 ori, at roughly the same position. Furthermore, based on limited mutational analysis, a similar dependence on the TA bp both for permanganate reactivity and for DNA replication was observed (3). There are, however, also significant differences. One conclusion of the early SV40 studies was that untwisting of the DNA, rather than melting, caused the permanganate reactivity in the TA-rich region (5). For the BPV ori, both the clear sequence dependence and the fixed relationship between the distal and proximal T-dependent melting make such a mechanism for the generation of permanganate reactivity unlikely. Nevertheless, the clear similarities indicate that template melting by E1 and T-Ag likely occurs in a similar manner.
Template melting is a process with multiple components.
Through mutational analysis of the ori, we found that certain sequences are incompatible with melting and that E1 shows distinctive sequence dependence for template melting. Within a 9-bp window between positions 9 and 17, multiple TA bp are required for in vivo DNA replication (Fig. 6). In this same window, multiple TA bp are required to generate the wt melting pattern. However, all of the base pairs within this window need not be TA bp, as indicated by the TA8 and AT8 templates, both of which have substantial activity in permanganate reactivity assays (Fig. 2) and for DNA replication (data not shown).
We used the E1-dependent permanganate reactivity assay with different mutant templates in the presence of both ADP and ATP to distinguish multiple components of melting. The first permanganate reactivity is generated upon binding of E1 as a DT in the presence of ADP. This reactivity, although it is superficially similar to that observed in the presence of ATP, is clearly quite different, since melting in the presence of ADP is not affected by the presence of TA bp (Fig. 3 and 4). In the presence of ATP, we observed four additional discrete components of melting (Fig. 8A). The six TA bp are melted (distal T-dependent melting), and in addition, they direct melting half a helical turn away from the six TA bp (proximal T-dependent melting). Both of these melting types appear only in the presence of ATP and are therefore likely dependent on ATP hydrolysis. In addition, by using the A16 probe, we observed two additional ATP-dependent components. These were proximal T-independent melting and melting of the E1 BS (Fig. 8A).
FIG. 8.
Models for template melting. (A) Four different components of ATP-dependent template melting. Distal and proximal T-dependent melting accounts for the majority of permanganate reactivity. In addition, permanganate reactivity of the E1 BS and of proximal T-independent melting can be observed under specific conditions (see Fig. 4A). (B) Six TA bp in the wt ori direct melting. Within a 9-bp window, the presence of TA bp results in melting of the TA bp (distal T-dependent melting). The TA bp within this window also induce melting half a helical turn away (proximal T-dependent melting). (C) Model for how multiple E1 DNA interactions generate large-scale melting. The E1 molecules in the DT bind in a helical arrangement, wrapping around the DNA duplex. Each E1 molecule contacts DNA at two positions, separated by a one-half turn of the helix. These contacts correspond to distal and proximal T-dependent melting. Together, these six contacts account for the ∼15 to 18 bp of permanganate reactivity observed in the left half of the ori. For clarity, only two of the three staggered E1 molecules are shown.
Our experiments demonstrate that two of these components (the distal and proximal T-dependent melting) are coupled. In contrast, other components of the melting process are clearly independent of each other. Both E1 BS melting and proximal T-independent melting, which are prominent on the A16 template, are executed, although T-dependent melting does not happen on this template. It seems likely that these types of ori distortion are generated in different ways. Our recent results demonstrate that proximal T-independent melting and E1 BS melting are likely due to template untwisting (S. Schuck and A. Stenlund, unpublished data).
The ATP-dependent melting of the E1 BS is interesting since this is the first demonstration that in the process of DH formation, the binding sites for the initiator are melted. The fact that the A16 template shows prominent E1 BS melting although this template is defective for DH formation indicates that E1 BS melting precedes DH formation and likely is required for DH formation (Fig. 4A). Although we have little specific information about how E1 BS melting comes about, we recently identified a mutant on the surface of the E1 DBD, K269A, which is competent to form the DT but fails to form the DH and, consequently, is defective for ori unwinding (29). The effect of this mutation demonstrates that melting of the ori and DH formation require specific contributions from the E1 DBD other than the initial binding of E1 to the ori.
Correct template melting is required for DH formation.
The melting defect of the A16 template causes severe defects in DH formation, unwinding, and DNA replication in vivo, providing good evidence that the wt melting pattern is required for these subsequent processes. This is consistent with our previous proposal that template melting is a prerequisite for formation of the active DH helicase (28). Because the DT forms on the A16 template without obvious defects (Fig. 2C) and generates essentially the same melting pattern as the wt template, the defect in complex formation appears to be confined to formation of the DH (Fig. 4B). The requirement for template melting indicates that formation of the DH helicase, similar to a single E1 hexamer, depends on ssDNA for its formation (12, 30). Recent structural data demonstrate that the hexamer of the E1 helicase domain encircles ssDNA, and this is likely also the case for the DH (10).
The requirement for multiple TA bp is related to binding of multiple E1 molecules.
Proximal and distal T-dependent melting, which in combination accounts for most of the melting that we observe on the wt template, is particularly interesting. Distal T-dependent melting is sequence and groove specific, demonstrating that it represents a direct interaction between E1 and the TA stretch. The observations that multiple TA bp are required within a particular window and that these function as a unit in the in vivo replication experiments indicate that the TA stretch is the target for multiple interactions with E1. We previously proposed that the E1 molecules in the DT are positioned in a helical arrangement which encircles the double-stranded DNA (28). In such a helical structure, which is based on the positions of the E1 BS, each molecule would be shifted 3 bp relative to its neighbor. This arrangement would provide an explanation for the requirement for the long TA stretch, since interaction of the same element in three E1 molecules that are staggered 3 bp relative to each other would require a 6- to 9-bp TA stretch, depending on the flexibility of the interaction. Together, these data fit very well with the idea that the three E1 molecules in one-half of the DT wrap around the DNA helix and interact with the stretch of six TA bp present in the wt ori (Fig. 8B and C).
How does the β-hairpin function in melting?
A prime candidate for the interaction with the TA bp is a β-hairpin structure, which is highly conserved in E1 and other papovavirus initiator proteins (1, 18). Our previous studies have demonstrated that H507 at the tip of this β-hairpin is required for DT formation and for template melting (28). We have suggested that this side chain interacts with the DNA to generate the initial opening of the double-stranded DNA that is observed in the presence of ADP. The same interaction is likely responsible for melting in the presence of ATP.
How melting is generated half a helical turn away from the TA bp (proximal T-dependent melting) is less clear. The fact that this melting occurs at a fixed distance from the inserted TA bp indicates that it is caused by a second interaction between E1 and DNA, since this would explain how the fixed distance is maintained (Fig. 7C). Such a secondary interaction is likely to reside in the linker region between the helicase domain and the oligomerization domain, based on the distance between the points of melting (∼20 Å). Together, these data suggest a very interesting model for how E1 may be able to melt relatively large regions of DNA by using simple tools. One consequence of the dual interactions of each E1 molecule with DNA is that each interaction need only be responsible for melting of a few bp to account for melting of ∼18 bp, since three E1 molecules and a total of six interactions are involved. This would allow the use of a fairly simple mechanism, e.g., some form of base flipping (23), to generate the ∼18 bp of melting that we observe in the left flank of the wt template.
Acknowledgments
This work was supported by NIH grant CA 13106 to A.S.
We thank K. Fien for critically reading the manuscript.
Footnotes
Published ahead of print on 3 January 2007.
REFERENCES
- 1.Abbate, E. A., J. M. Berger, and M. R. Botchan. 2004. The X-ray structure of the papillomavirus helicase in complex with its molecular matchmaker E2. Genes Dev. 18:1981-1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell, S. P., and B. Stillman. 1992. ATP-dependent recognition of eukaryotic origins of DNA replication by a multiprotein complex. Nature 357:128-134. [DOI] [PubMed] [Google Scholar]
- 3.Borowiec, J. A. 1992. Inhibition of structural changes in the simian virus 40 core origin of replication by mutation of essential origin sequences. J. Virol. 66:5248-5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borowiec, J. A., F. B. Dean, and J. Hurwitz. 1991. Differential induction of structural changes in the simian virus 40 origin of replication by T antigen. J. Virol. 65:1228-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borowiec, J. A., and J. Hurwitz. 1988. Localized melting and structural changes in the SV40 origin of replication induced by T-antigen. EMBO J. 7:3149-3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bramhill, D., and A. Kornberg. 1988. Duplex opening by dnaA protein at novel sequences in initiation of replication at the origin of the E. coli chromosome. Cell 52:743-755. [DOI] [PubMed] [Google Scholar]
- 7.Chen, G., and A. Stenlund. 2002. Sequential and ordered assembly of E1 initiator complexes on the papillomavirus origin of DNA replication generates progressive structural changes related to melting. Mol. Cell. Biol. 22:7712-7720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, G., and A. Stenlund. 1998. Characterization of the DNA-binding domain of the bovine papillomavirus replication initiator E1. J. Virol. 72:2567-2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, G., and A. Stenlund. 2001. The E1 initiator recognizes multiple overlapping sites in the papillomavirus origin of DNA replication. J. Virol. 75:292-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Enemark, E. J., and L. Joshua-Tor. 2006. Mechanism of DNA translocation in a replicative hexameric helicase. Nature 442:270-275. [DOI] [PubMed] [Google Scholar]
- 11.Enemark, E. J., A. Stenlund, and L. Joshua-Tor. 2002. Crystal structures of two intermediates in the assembly of the papillomavirus replication initiation complex. EMBO J. 21:1487-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fouts, E. T., X. Yu, E. H. Egelman, and M. R. Botchan. 1999. Biochemical and electron microscopic image analysis of the hexameric E1 helicase. J. Biol. Chem. 274:4447-4458. [DOI] [PubMed] [Google Scholar]
- 13.Gai, D., R. Zhao, D. Li, C. V. Finkielstein, and X. S. Chen. 2004. Mechanisms of conformational change for a replicative hexameric helicase of SV40 large tumor antigen. Cell 119:47-60. [DOI] [PubMed] [Google Scholar]
- 14.Gillette, T. G., M. Lusky, and J. A. Borowiec. 1994. Induction of structural changes in the bovine papillomavirus type 1 origin of replication by the viral E1 and E2 proteins. Proc. Natl. Acad. Sci. USA 91:8846-8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holt, S. E., G. Schuller, and V. G. Wilson. 1994. DNA binding specificity of the bovine papillomavirus E1 protein is determined by sequences contained within an 18-base-pair inverted repeat element at the origin of replication. J. Virol. 68:1094-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaguni, J. M. 2006. DnaA: controlling the initiation of bacterial DNA replication and more. Annu. Rev. Microbiol. 60:351-375. [DOI] [PubMed] [Google Scholar]
- 17.Leng, X., J. H. Ludes-Meyers, and V. G. Wilson. 1997. Isolation of an amino-terminal region of bovine papillomavirus type 1 E1 protein that retains origin binding and E2 interaction capacity. J. Virol. 71:848-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li, D., R. Zhao, W. Lilyestrom, D. Gai, R. Zhang, J. A. DeCaprio, E. Fanning, A. Jochimiak, G. Szakonyi, and X. S. Chen. 2003. Structure of the replicative helicase of the oncoprotein SV40 large tumour antigen. Nature 423:512-518. [DOI] [PubMed] [Google Scholar]
- 19.Lin, B. Y., A. M. Makhov, J. D. Griffith, T. R. Broker, and L. T. Chow. 2002. Chaperone proteins abrogate inhibition of the human papillomavirus (HPV) E1 replicative helicase by the HPV E2 protein. Mol. Cell. Biol. 22:6592-6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu, J. S., S. R. Kuo, T. R. Broker, and L. T. Chow. 1995. The functions of human papillomavirus type 11 E1, E2, and E2C proteins in cell-free DNA replication. J. Biol. Chem. 270:27283-27291. [DOI] [PubMed] [Google Scholar]
- 21.Maxam, A. M., and W. Gilbert. 1980. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 65:499-560. [DOI] [PubMed] [Google Scholar]
- 22.Parsons, R., M. E. Anderson, and P. Tegtmeyer. 1990. Three domains in the simian virus 40 core origin orchestrate the binding, melting, and DNA helicase activities of T antigen. J. Virol. 64:509-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts, R. J., and X. Cheng. 1998. Base flipping. Annu. Rev. Biochem. 67:181-198. [DOI] [PubMed] [Google Scholar]
- 24.Sanders, C. M., and A. Stenlund. 2001. Mechanism and requirements for bovine papillomavirus, type 1, E1 initiator complex assembly promoted by the E2 transcription factor bound to distal sites. J. Biol. Chem. 276:23689-23699. [DOI] [PubMed] [Google Scholar]
- 25.Sanders, C. M., and A. Stenlund. 1998. Recruitment and loading of the E1 initiator protein: an ATP-dependent process catalysed by a transcription factor. EMBO J. 17:7044-7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanders, C. M., and A. Stenlund. 2000. Transcription factor-dependent loading of the E1 initiator reveals modular assembly of the papillomavirus origin melting complex. J. Biol. Chem. 275:3522-3534. [DOI] [PubMed] [Google Scholar]
- 27.Scheffner, M., R. Wessel, and H. Stahl. 1989. Sequence independent duplex DNA opening reaction catalysed by SV40 large tumor antigen. Nucleic Acids Res. 17:93-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schuck, S., and A. Stenlund. 2005. Assembly of a double hexameric helicase. Mol. Cell 20:377-389. [DOI] [PubMed] [Google Scholar]
- 29.Schuck, S., and A. Stenlund. 2006. Surface mutagenesis of the bovine papillomavirus E1 DNA binding domain reveals residues required for multiple functions related to DNA replication. J. Virol. 80:7491-7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sedman, J., and A. Stenlund. 1998. The papillomavirus E1 protein forms a DNA-dependent hexameric complex with ATPase and DNA helicase activities. J. Virol. 72:6893-6897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sedman, T., J. Sedman, and A. Stenlund. 1997. Binding of the E1 and E2 proteins to the origin of replication of bovine papillomavirus. J. Virol. 71:2887-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sekimizu, K., D. Bramhill, and A. Kornberg. 1987. ATP activates dnaA protein in initiating replication of plasmids bearing the origin of the E. coli chromosome. Cell 50:259-265. [DOI] [PubMed] [Google Scholar]
- 33.Seo, Y. S., F. Muller, M. Lusky, and J. Hurwitz. 1993. Bovine papilloma virus (BPV)-encoded E1 protein contains multiple activities required for BPV DNA replication. Proc. Natl. Acad. Sci. USA 90:702-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stenlund, A. 2003. E1 initiator DNA binding specificity is unmasked by selective inhibition of non-specific DNA binding. EMBO J. 22:954-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun, S., L. Thorner, M. Lentz, P. MacPherson, and M. Botchan. 1990. Identification of a 68-kilodalton nuclear ATP-binding phosphoprotein encoded by bovine papillomavirus type 1. J. Virol. 64:5093-5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Titolo, S., K. Brault, J. Majewski, P. W. White, and J. Archambault. 2003. Characterization of the minimal DNA binding domain of the human papillomavirus E1 helicase: fluorescence anisotropy studies and characterization of a dimerization-defective mutant protein. J. Virol. 77:5178-5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Titolo, S., A. Pelletier, A. M. Pulichino, K. Brault, E. Wardrop, P. W. White, M. G. Cordingley, and J. Archambault. 2000. Identification of domains of the human papillomavirus type 11 E1 helicase involved in oligomerization and binding to the viral origin. J. Virol. 74:7349-7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Titolo, S., A. Pelletier, F. Sauve, K. Brault, E. Wardrop, P. W. White, A. Amin, M. G. Cordingley, and J. Archambault. 1999. Role of the ATP-binding domain of the human papillomavirus type 11 E1 helicase in E2-dependent binding to the origin. J. Virol. 73:5282-5293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ustav, M., and A. Stenlund. 1991. Transient replication of BPV-1 requires two viral polypeptides encoded by the E1 and E2 open reading frames. EMBO J. 10:449-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang, L., I. Mohr, E. Fouts, D. A. Lim, M. Nohaile, and M. Botchan. 1993. The E1 protein of bovine papilloma virus 1 is an ATP-dependent DNA helicase. Proc. Natl. Acad. Sci. USA 90:5086-5090. [DOI] [PMC free article] [PubMed] [Google Scholar]