Abstract
Most patients with herpes simplex virus (HSV) central nervous system (CNS) infection have abnormal cerebrospinal fluid (CSF) indices. Therefore, we implemented screening criteria based on CSF values and host immune status to guide testing. All CSF samples submitted for HSV PCR analysis from January 1999 through December 2004 were included in the study. Specimens from patients with human immunodeficiency virus, a history of transplants, an age of <2 years, a CSF white blood cell count of >5 cells/mm3, or a protein level of >50 mg/dl were tested upon request. All other samples were rejected and frozen. To validate our screening criteria, rejected specimens were pooled and tested retrospectively. Electronic medical records were also reviewed. A total of 1,659 HSV PCR requests from 1,458 patients were screened. Of the 1,296 specimens (78.1%) accepted for testing, 1,213 were negative, 7 were positive for HSV type 1 (HSV-1), 26 were positive for HSV-2, and 50 had unavailable results. Sixteen requests were rejected because an alternative microbiologic diagnosis had been established. Of the 347 samples rejected based on criteria, 222 (64.0%) remained available for pooled testing. No HSV-1-positive samples were identified in the rejected specimens. Two rejected specimens tested positive for HSV-2 DNA, but both met acceptance criteria which had not been communicated to the laboratory. Few patients (7.8%) with rejected specimens were treated with acyclovir, which suggests a low clinical concern for HSV encephalitis. Acceptance criteria based on CSF parameters and host immune status saved time and cost and did not miss patients with HSV CNS infection. Communication between the clinician and the laboratory is imperative for a successful screening program.
Early recognition of herpes simplex virus (HSV) encephalitis is crucial because prompt initiation of antiviral therapy improves clinical outcomes (17, 18). The detection of HSV DNA directly in cerebrospinal fluid (CSF) by PCR has been well validated for the diagnosis of herpes central nervous system (CNS) infections, and given its high sensitivity and specificity, HSV PCR is now recognized as the reference standard (1, 4, 9, 12). HSV PCR has become one of the most commonly requested tests of CSF in our clinical microbiology laboratory, and there has been a costly trend toward overusing molecular diagnostics to evaluate patients with nonspecific complaints.
Most patients with CNS infections have an elevated CSF leukocyte count and/or protein level (5, 7, 16). Screening the CSF cell count and protein values prior to performing HSV PCR has been suggested as a way to save healthcare costs without reducing sensitivity (13, 16). In addition, laboratory acceptance criteria for HSV testing of CSF have been shown to increase the proportion of positive results (from 1.9% to 4.0% at one institution) (13). Based on these findings, we implemented criteria for HSV PCR testing that are founded on CSF profiles in addition to host characteristics but had not previously been validated with our protocol. In the present study, we retrospectively tested rejected CSF specimens for HSV DNA and reviewed available medical records to ascertain the clinical characteristics of patients with viral CNS infections as well as those of patients with rejected specimens.
(This study was presented in part at the 43rd Annual Meeting of the Infectious Diseases Society of America, San Francisco, CA, October 2005.)
MATERIALS AND METHODS
Acceptance criteria.
Every request for HSV PCR testing of CSF is reviewed by the Duke University Clinical Microbiology Laboratory as part of our standard operating procedure. Specimens are accepted for analysis if there is an elevated CSF white blood cell count (>5 cells/mm3) or abnormal protein level (>50 mg/dl) or if the sample was collected from an immunocompromised patient (human immunodeficiency virus [HIV]-positive patient or transplant recipient) or a child of <2 years of age. Through our electronic physician order entry system, providers are presented with these acceptance criteria and prompted to indicate a patient's immunocompromised status when applicable. A rejection notice is issued by the laboratory when a request does not meet the prespecified criteria, and the remaining CSF is then catalogued and frozen immediately at −80°C in the event that future testing is warranted.
Pooling strategy and HSV-1/2 PCR analysis.
During the study period, specimens for HSV PCR analysis submitted to our laboratory were sent to the Mayo Clinic Reference Laboratory for testing. We reviewed all HSV PCR requests between 1 January 1999 and 31 December 2004 and identified the corresponding frozen CSF samples. Rejected specimens with adequate volume were combined into pools and batch tested retrospectively for HSV type 1 and 2 (HSV-1/2) DNA in the Duke University Molecular Microbiology Laboratory, using the same primer-probe set as the Mayo Clinic Reference Laboratory. Pools consisted of 100 μl of CSF collected from 10 individual patients, and 200 μl from each pool was used for PCR analysis. Each pool was vortexed briefly to mix the specimen prior to sampling. Nucleic acid was then extracted from CSF using a High Pure viral nucleic acid kit (Roche Applied Science, Mannheim, Germany) per the manufacturer's instructions. HSV-1/2 primers and hybridization probes (Roche Molecular Systems, Branchburg, NJ) targeting a 215-bp fragment of the DNA polymerase gene (GenBank accession numbers M12356 and M16321) were used for amplification and detection on a LightCycler 1.5 instrument (Roche Diagnostics, Indianapolis, IN). If HSV DNA was identified in a CSF pool, then the individual samples comprising the pool were retested separately.
Medical record review.
Medical and laboratory records were reviewed for all patients with CSF submitted for HSV PCR testing during the study period. Demographic information and laboratory and microbiologic test results were recorded in an Access database (Microsoft Office 2003) for all study subjects. For patients with CSF samples not meeting acceptance criteria and for those with documented CNS infection, additional review was performed to assess the clinical presentation prompting lumbar puncture, antiviral therapy (if any), and CNS radiographic findings. The protocol was approved by the Duke Institutional Review Board.
Case definitions.
Abnormal CSF parameters were defined as a nucleated cell count of >5 cells/mm3 (13, 16) or a protein concentration of >50 mg/dl (Duke Laboratory reference value). Antiviral therapy was classified as at least one dose of intravenous acyclovir or oral valacyclovir given to treat suspected HSV CNS infection. A diagnosis of meningitis required signs of meningeal inflammation (i.e., headache, stiff neck, and/or photophobia) plus fever (≥38°C) or CSF pleocytosis (white blood cell count of ≥5 cells/mm3). Encephalitis was characterized by involvement of the brain parenchyma (i.e., altered or depressed mentation, behavioral disturbance, focal or diffuse neurologic findings, and/or seizures) plus fever (≥38°C) or CSF pleocytosis. Meningoencephalitis included a combination of the signs and symptoms of both meningitis and encephalitis.
Statistical analyses.
Proportions were compared with the chi-square test. Statistical significance was set at the 5% level. All P values were two-tailed. Statistical analyses were performed using Analyze-It software (Analyze-It Software Ltd., Leeds, England).
RESULTS
HSV PCR requests.
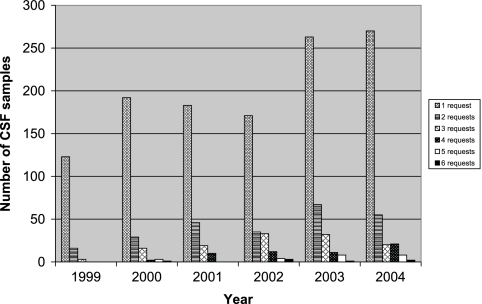
During the 6-year study period, a total of 1,659 CSF specimens from 1,458 individual patients were submitted to the clinical microbiology laboratory for HSV PCR testing. The number of HSV PCR requests increased over the course of the study, from 142 requests in 1999 to 376 requests in 2004. The number of CSF specimens with multiple molecular microbiologic tests ordered in addition to HSV testing also increased (13.3% [19/142] of samples in 1999 compared to 28.2% [106/376] of samples in 2004; P = 0.0007) (Fig. 1). Despite these trends, there was not a significant rise in the proportion of requests for immunocompromised patients (21.8% [31/142] of requests in 1999 compared to 24.4% [92/376] of requests in 2004; P = 0.6).
FIG. 1.
Yearly distribution of PCR requests for CSF.
The majority of HSV test requests, i.e., 78.1% (1,296/1,659 requests), met acceptance criteria. Most of the samples accepted for testing had abnormal CSF profiles (46.0% [596/1,296] had both an elevated white blood cell count and protein concentration, 6.9% [89/1,296] had pleocytosis only, and 32.6% [423/1,296] had proteinosis only). An additional 172 samples with normal CSF parameters were accepted based on host characteristics (36 from HIV-positive patients, 105 from transplant recipients, and 31 from pediatric patients). The remaining 16 tests were performed following discussion with the ordering clinician.
PCR results.
Of the 1,296 HSV PCR tests performed, 33 (2.5%) were positive for HSV DNA (7 for HSV-1 and 26 for HSV-2), 1,213 were negative for HSV DNA, and 50 results from the reference laboratory were not available in the electronic medical record. The CSF cell count and protein concentration were elevated in all samples collected from patients with HSV-1 infection, regardless of immune status (Table 1). All patients with HSV-1 (six adults and one neonate) met our case definition of encephalitis. Every HSV-1-positive adult had altered mental status and fever as well as temporal lobe abnormalities seen by magnetic resonance imaging. The neonate had disseminated HSV disease in association with CNS involvement.
TABLE 1.
Clinical characteristics of patients with HSV-1 encephalitis
| Patient | Age (yr) | Underlying condition(s)a | Cell count (cells/mm3) | Protein concnb (mg/dl) |
|---|---|---|---|---|
| 1 | 24 | HSCT | 80 | 193 |
| 2 | 28 wk | Premature infant | 11 | 158 |
| 3 | 28 | None | 67 | 130 |
| 4 | 67 | None | 1,680 | 456 |
| 5 | 42 | HIV positive | 22 | 269 |
| 6 | 84 | DM, PMR, on steroids | 104 | 77 |
| 7 | 67 | CNS B-cell lymphoma | 29 | 83 |
HSCT, hematopoietic stem cell transplant; none, no identifiable predisposing condition; DM, diabetes mellitus; PMR, polymyalgia rheumatica.
Reference range for CSF protein, 15 to 50 mg/dl.
Twenty-six specimens from 25 adults and 1 neonate had HSV-2 DNA detected in CSF. All had either an elevated CSF cell count (median, 333 cells/mm3; range, 0 to 1,353 cells/mm3) or an increased protein concentration (median, 110 mg/dl; range, 49 to 165 mg/dl), and almost all patients with HSV-2 infection (24/26) had both CSF abnormalities. All but one patient, an adult with AIDS, fever, and an elevated CSF protein concentration but no documented CNS signs or symptoms, had meningitis or meningoencephalitis.
Additional PCR tests in conjunction with HSV testing were requested for 27.5% (457/1,659) of the CSF specimens. Table 2 displays the diagnostic yield in relation to the number of tests performed per sample. Of the 1,213 samples with negative HSV PCR results, an alternative organism was identified by molecular techniques in 12.0% (55/457) of those with additional PCR tests performed. The most common pathogen identified after HSV was enterovirus (n = 21). All enterovirus RNA-positive specimens had either an elevated cell count (median, 131 cells/mm3; range, 0 to 2,160 cells/mm3) or elevated protein concentration (median, 161 mg/dl; range, 32 to 192 mg/dl) and were received during the summer or fall months (June thru November). The majority of samples with two PCR tests ordered for which a viral pathogen was identified were positive for either HSV or enterovirus (Table 2).
TABLE 2.
Diagnostic yield in relation to number of PCR tests requested
| No. of PCR tests requested | No. of specimensa | No. of PCR tests
|
% Positive | |
|---|---|---|---|---|
| Performed | Positive | |||
| 1 | 1,202 | 873 | 29 | 3.3 |
| 2 | 248 | 459 | 38 | 8.3 |
| 3 | 123 | 349 | 14b | 4.0 |
| 4 | 56 | 221 | 4 | 1.8 |
| 5 | 24 | 119 | 2 | 1.7 |
| 6 | 6 | 36 | 0 | 0 |
| Total | 1,659 | 2,057 | 87 | 4.2 |
All CSF specimens had an HSV PCR requested (n = 1,659), and 457 had an additional PCR requested.
Includes a patient with EBV and JC virus coinfection.
Epstein-Barr virus (EBV) (n = 12), human herpesvirus 6 (HHV-6) (n = 9), and varicella-zoster virus (VZV) (n = 9) were the next most common organisms detected in our cohort. All of the patients with EBV DNA detected in CSF were immunosuppressed (HIV-positive patient or transplant recipient). Similarly, all HHV-6 DNA-positive samples were collected from solid organ or stem cell transplant recipients. Specimens that contained VZV DNA typically had an elevated cell count (median, 9 cells/mm3; range, 1 to 42 cells/mm3) and/or protein concentration (median, 86 mg/dl; range, 45 to 172 mg/dl). One of the nine patients with VZV infection had normal CSF indices (transplant recipient), and three had documented CNS infection without a rash. There was only one instance in which multiple pathogens were detected simultaneously (JC virus and EBV in the CSF of an adult patient with AIDS).
Rejected specimens.
Over the 6-year period, 21.9% (363/1,659) of requests were rejected (347 specimens did not meet acceptance criteria, and 16 had an alternative microbiologic diagnosis made by the time the PCR order was reviewed). Of the 347 specimens from 344 patients that did not meet the criteria for HSV testing, 222 (64.0%) were frozen and had an adequate volume for pooled analysis. Two of 23 CSF pools tested positive for HSV-2 DNA. No HSV-1 was detected in any of the pools. When CSF samples from the individual patients in the positive pools were tested separately, two patients with HSV-2 infection were identified. In retrospect, both would have met the acceptance criteria for PCR testing. One had normal CSF parameters with advanced HIV disease, but this diagnosis was not communicated to the laboratory. The second patient had fever and a headache associated with a CSF lymphocytic pleocytosis and an elevated protein concentration. Upon review of the laboratory record, only an enterovirus PCR and viral culture had been performed.
Few patients, i.e., 7.8% (27/344), with rejected specimens were treated with acyclovir. Pharmacologic records were not available for 33 of the 344 patients with rejected specimens. Only 3.8% (13/344) of patients with rejected specimens had clinical signs or symptoms consistent with meningitis or encephalitis, and just 2 of the 13 were treated with acyclovir.
DISCUSSION
Molecular diagnostic testing for infectious diseases is particularly useful for organisms that are difficult or slow to grow in the laboratory. PCR testing of CSF has become the diagnostic method of choice for CNS viral infections because of the low yield from CSF cultures (15). When a causative agent is found, HSV and enterovirus are the most commonly identified viral pathogens (3, 6, 11), which is in agreement with our findings. Prompt diagnosis of herpes simplex virus encephalitis is especially important because treatment with intravenous acyclovir decreases morbidity and mortality (17). Furthermore, a rapid diagnosis of enteroviral meningitis can facilitate early discharge from the hospital and prevent unnecessary antibiotic treatment and further laboratory testing (10, 14). The majority of viral PCR tests performed on CSF, however, yield negative results, and the etiology of aseptic meningoencephalitis remains unknown >50% of the time (3, 8). At our institution as well as others (16), there has been a rise in PCR test ordering as part of the evaluation of suspected CNS infection, often without prioritization in order of diagnostic likelihood.
Based on the observation that most patients with CNS infection have abnormal CSF indices, and given a trend of increased molecular test utilization with variable returns, we implemented acceptance criteria for HSV PCR testing that are predicated on CSF profiles, patient age, and immune status. The inclusion of host immune status differentiates our analysis from previous studies and is particularly important because HSV encephalitis has been reported for immunocompromised patients with normal CSF parameters (2).
Each morning, a technologist and the clinical microbiology fellow review HSV PCR requests and document the patient's age, immune status (if known), and corresponding CSF cell count and protein level. This process routinely takes less than 10 min. Over a 6-year period, approximately one in four requests failed to meet our acceptance criteria. Retrospective analysis of rejected CSF specimens did not reveal any missed cases of HSV-1 infection. Two cases of HSV-2 infection were identified, but upon review, both patients actually met the acceptance criteria. Fewer than 10% of patients with rejected specimens were treated with acyclovir, and even fewer met standard case definitions for meningoencephalitis, which suggests a low clinical concern for HSV encephalitis in this subgroup of patients. Our HSV PCR acceptance criteria saved the laboratory approximately $40,900 in direct costs over the study period and reduced patient charges (data not shown). The time taken to review the PCR requests and to catalogue and freeze the remaining CSF samples was less than the cumulative technologist and instrument time required to process and test low-yield samples (data not shown). More importantly, patient care might have been improved by avoiding false-positive PCR results for patients with a low pretest probability of HSV CNS infection.
In most cases, when an HSV PCR is ordered in combination with additional PCRs with CSF, the HSV PCR should be performed first. This is in accordance with the conclusions of Tang et al. (16). Testing for enterovirus concurrently with HSV testing (when both are requested during the summer and fall months) may increase the diagnostic yield of PCR and has the potential to expedite appropriate care of patients with enteroviral CNS infections, since the clinical and CSF features of HSV-2 and enteroviral CNS infections often overlap substantially. Therefore, we do advocate simultaneous testing for these organisms during enterovirus season. Immunosuppressed patients are another subset where simultaneous PCR testing may also be warranted. The most common viruses detected in the CSF of HIV-positive and transplant patients for whom an HSV PCR was ordered were EBV and HHV-6, respectively. The clinical significance of these viruses was not always readily apparent, but this observation highlights a greater breadth of possible pathogens for this group. Lastly, in the appropriate clinical setting, VZV infection should also be considered (even in the absence of a rash) when the initial HSV PCR is negative.
Our findings also suggest that screening criteria incorporating CSF values and host factors could reasonably be extended beyond HSV PCR testing to other commonly requested tests for viral pathogens, including enterovirus, EBV, HHV-6, VZV, and cytomegalovirus. The majority of patients with confirmed viral CNS infection, regardless of immune status, had either an abnormal CSF cell count or abnormal protein level. There were a few patients with normal CSF values and a positive PCR test, but all were heavily immunosuppressed and had infections with viruses other than HSV or enterovirus.
There are several limitations related to our study design. First, not all of the rejected specimens were available for retrospective testing. It is unlikely, however, that this significantly biased the results, as there was no obvious pattern (e.g., clinical diagnosis or number of other microbiologic tests performed) distinguishing rejected specimens that were frozen from those that were not. We may also have missed low levels of HSV DNA in the CSF pools, but despite the 1:10 dilution factor, we were successful in detecting two HSV-2-positive samples, which suggests a reasonable level of analytic sensitivity. Finally, given the retrospective nature of the study, various clinical features, such as documentation of fever, neurologic examination, and antiviral treatment, were not available for all patients.
In conclusion, molecular diagnostic testing for viral nucleic acid in CSF should be used judiciously and based on the probability of particular pathogens in the given clinical context. Important factors, such as clinical presentation, CSF values, seasonality, and host immune status, must be assessed. Until affordable, sensitive, multiplex panels of probable CNS pathogens become widely available, laboratory acceptance criteria can be used to save time and cost and do not miss patients with HSV CNS infection.
Acknowledgments
This study was supported by an NIH T32 training grant (CA93245-03) and a K12 (RR17630-03) award (K.E.H.).
We give special thanks to Lauren Hicks, Chelsea Castellano, and Natalie Pitts for assistance with data entry and medical record review.
Footnotes
Published ahead of print on 3 January 2007.
REFERENCES
- 1.Aurelius, E., B. Johansson, B. Skoldenberg, A. Staland, and M. Forsgren. 1991. Rapid diagnosis of herpes simplex encephalitis by nested polymerase chain reaction assay of cerebrospinal fluid. Lancet 337:189-192. [DOI] [PubMed] [Google Scholar]
- 2.Fodor, P. A., M. J. Levin, A. Weinberg, E. Sandberg, J. Sylman, and K. L. Tyler. 1998. Atypical herpes simplex virus encephalitis diagnosed by PCR amplification of viral DNA from CSF. Neurology 51:554-559. [DOI] [PubMed] [Google Scholar]
- 3.Glaser, C. A., S. Gilliam, D. Schnurr, B. Forghani, S. Honarmand, N. Khetsuriani, M. Fischer, C. K. Cossen, and L. J. Anderson. 2003. In search of encephalitis etiologies: diagnostic challenges in the California Encephalitis Project, 1998-2000. Clin. Infect. Dis. 36:731-742. [DOI] [PubMed] [Google Scholar]
- 4.Guffond, T., A. Dewilde, P. E. Lobert, D. Caparros-Lefebvre, D. Hober, and P. Wattre. 1994. Significance and clinical relevance of the detection of herpes simplex virus DNA by the polymerase chain reaction in cerebrospinal fluid from patients with presumed encephalitis. Clin. Infect. Dis. 18:744-749. [DOI] [PubMed] [Google Scholar]
- 5.Hayward, R. A., M. F. Shapiro, and R. K. Oye. 1987. Laboratory testing on cerebrospinal fluid. A reappraisal. Lancet i:1-4. [DOI] [PubMed] [Google Scholar]
- 6.Huang, C., D. Morse, B. Slater, M. Anand, E. Tobin, P. Smith, M. Dupuis, R. Hull, R. Ferrera, B. Rosen, and L. Grady. 2004. Multiple-year experience in the diagnosis of viral central nervous system infections with a panel of polymerase chain reaction assays for detection of 11 viruses. Clin. Infect. Dis. 39:630-635. [DOI] [PubMed] [Google Scholar]
- 7.Jeffery, K. J., S. J. Read, T. E. Peto, R. T. Mayon-White, and C. R. Bangham. 1997. Diagnosis of viral infections of the central nervous system: clinical interpretation of PCR results. Lancet 349:313-317. [DOI] [PubMed] [Google Scholar]
- 8.Khetsuriani, N., R. C. Holman, and L. J. Anderson. 2002. Burden of encephalitis-associated hospitalizations in the United States, 1988-1997. Clin. Infect. Dis. 35:175-182. [DOI] [PubMed] [Google Scholar]
- 9.Lakeman, F. D., and R. J. Whitley. 1995. Diagnosis of herpes simplex encephalitis: application of polymerase chain reaction to cerebrospinal fluid from brain-biopsied patients and correlation with disease. J. Infect. Dis. 171:857-863. [DOI] [PubMed] [Google Scholar]
- 10.Ramers, C., G. Billman, M. Hartin, S. Ho, and M. H. Sawyer. 2000. Impact of a diagnostic cerebrospinal fluid enterovirus polymerase chain reaction test on patient management. JAMA 283:2680-2685. [DOI] [PubMed] [Google Scholar]
- 11.Read, S. J., K. J. Jeffery, and C. R. Bangham. 1997. Aseptic meningitis and encephalitis: the role of PCR in the diagnostic laboratory. J. Clin. Microbiol. 35:691-696. (Erratum, 35:1649.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rowley, A. H., R. J. Whitley, F. D. Lakeman, and S. M. Wolinsky. 1990. Rapid detection of herpes-simplex-virus DNA in cerebrospinal fluid of patients with herpes simplex encephalitis. Lancet 335:440-441. [DOI] [PubMed] [Google Scholar]
- 13.Simko, J. P., A. M. Caliendo, K. Hogle, and J. Versalovic. 2002. Differences in laboratory findings for cerebrospinal fluid specimens obtained from patients with meningitis or encephalitis due to herpes simplex virus (HSV) documented by detection of HSV DNA. Clin. Infect. Dis. 35:414-419. [DOI] [PubMed] [Google Scholar]
- 14.Stellrecht, K. A., I. Harding, A. M. Woron, M. L. Lepow, and R. A. Venezia. 2002. The impact of an enteroviral RT-PCR assay on the diagnosis of aseptic meningitis and patient management. J. Clin. Virol. 25(Suppl. 1):S19-S26. [DOI] [PubMed] [Google Scholar]
- 15.Storch, G. A. 2000. Diagnostic virology. Clin. Infect. Dis. 31:739-751. [DOI] [PubMed] [Google Scholar]
- 16.Tang, Y. W., J. R. Hibbs, K. R. Tau, Q. Qian, H. A. Skarhus, T. F. Smith, and D. H. Persing. 1999. Effective use of polymerase chain reaction for diagnosis of central nervous system infections. Clin. Infect. Dis. 29:803-806. [DOI] [PubMed] [Google Scholar]
- 17.Whitley, R. J., C. A. Alford, M. S. Hirsch, R. T. Schooley, J. P. Luby, F. Y. Aoki, D. Hanley, A. J. Nahmias, and S. J. Soong. 1986. Vidarabine versus acyclovir therapy in herpes simplex encephalitis. N. Engl. J. Med. 314:144-149. [DOI] [PubMed] [Google Scholar]
- 18.Whitley, R. J., S. J. Soong, R. Dolin, G. J. Galasso, L. T. Chien, C. A. Alford, Jr., et al. 1977. Adenine arabinoside therapy of biopsy-proved herpes simplex encephalitis: National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study. N. Engl. J. Med. 297:289-294. [DOI] [PubMed] [Google Scholar]