Abstract
Obliterative bronchiolitis is a devastating illness that limits the long-term success of lung transplantation. Its high prevalence and overall poor response to current therapeutic measures demands further research to elucidate pathogenic mechanisms. Toward this goal, there is a role for animal models to study the mechanisms of obliterative bronchiolitis, such as the murine heterotopic tracheal allograft model. This review compares the tracheal allograft model to human obliterative bronchiolitis pathology and highlights the important mechanisms of airway rejection described using this model. Although certain limitations exist, the pursuit of proof-of-concept studies in this model, as well as other animal models, can provide the basis for genetic and cellular translational human studies directed toward post-transplant obliterative bronchiolitis pathogenesis. To meet these challenges, we call for the establishment of a National Institutes of Health–supported Lung Transplant Network to better orchestrate translational research efforts in obliterative bronchiolitis pathogenesis and treatment, and to advance the field of lung transplantation.
Keywords: allospecific T cells, lung allograft rejection, obliterative airway disease, obliterative bronchiolitis, tracheal transplant
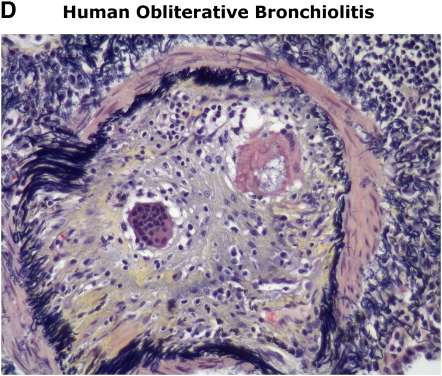
Lung transplantation is a life-saving procedure for select individuals with end-stage pulmonary disorders. However, its long-term success is most limited by obliterative bronchiolitis (OB), the manifestation of chronic lung allograft rejection. Despite standard three-drug calcineurin inhibitor–based immunosuppression regimens, OB occurs in up to 70% of patients by 5 yr post-transplant, and largely accounts for a 5-yr survival rate of only 45% after lung transplantation (1–3). Although OB is histologically characterized by connective tissue obliteration of the airway lumen, a tissue diagnosis of OB is not consistently available in the majority of patients. Therefore, the term “bronchiolitis obliterans syndrome” (BOS) is used to describe the clinical finding of progressive airflow obstruction, a hallmark of chronic lung allograft rejection, in the absence of infection or acute cellular rejection (4). Transplant centers worldwide use the BOS classification system most recently updated by a consensus panel of experts in 2002 and summarized in Table 1 (5). Unfortunately, the pathogenesis of OB/BOS remains poorly understood, with augmentation of conventional immunosuppressive therapies rarely effective in reversing disease progression. Although clinical studies have increased our understanding of important risk factors associated with OB/BOS development, further research is necessary to elucidate the underlying pathogenic mechanisms. To this end, the use of relevant animal models, in conjunction with basic translational studies in lung transplant recipients, is critical toward achieving this goal.
TABLE 1.
BRONCHIOLITIS OBLITERANS SYNDROME 2002 CLASSIFICATION SYSTEM
| BOS Stage | Pulmonary Function |
|---|---|
| 0 | FEV1 > 90% of baseline and |
| FEF25–75 > 75% of baseline | |
| 0p | FEV1 81–90% of baseline and/or |
| FEF25–75 ⩽ 75% of baseline | |
| 1 | FEV1 66–80% of baseline |
| 2 | FEV1 51–65% of baseline |
| 3 | FEV1 ⩽ 50% of baseline |
Definition of abbreviation: BOS = bronchiolitis obliterans syndrome.
PATHOGENESIS OF HUMAN OB
Although the underlying mechanisms leading to OB after lung transplantation remain incompletely understood, the majority of evidence suggests progressive alloimmune-mediated injury resulting from an ongoing adaptive immune response to the lung allograft. In support of this concept, multiple studies indicate that acute cellular rejection is a major risk factor for the development of OB/BOS (6–8). Recently, several studies have demonstrated that the detection of donor histocompatibility-linked antigen-specific (donor HLA-specific) antibody responses are associated with, and precede, clinical OB/BOS (9, 10). Together, these data support a central role for adaptive immune mechanisms in the pathogenesis of OB. Indeed, a recent gene expression profile study performed on bronchoalveolar lavage (BAL) cells from lung transplant recipients showed that a number of up-regulated candidate genes were associated with T-cell responses. Specifically, major components of the T-cell receptor (TCR)/CD3 complex, TCRα, TCRβ, and CD3ε; the costimulatory molecules CD28 and CTLA4; the cytolytic effector molecules granzyme A and perforin; the chemokine receptors CCR7 and CXCR3; and the T-cell effector molecules IFN-γ and lymphotoxin-β were all up-regulated on gene expression profiling of BAL-derived cells during episodes of acute cellular rejection compared with samples without rejection (11). These data, combined with the high prevalence of OB/BOS, may suggest that current immunosuppression strategies are frequently inadequate in successfully limiting the alloimmune response in lung transplant recipients. It should be noted that the standard calcineurin inhibitor–based regimens, primarily targeting interleukin (IL)-2 production, may not affect other important T-cell growth factors such as IL-7, IL-12, and IL-15, which may potentially drive the expansion and function of allospecific T cells, resulting in acute and chronic rejection (12, 13).
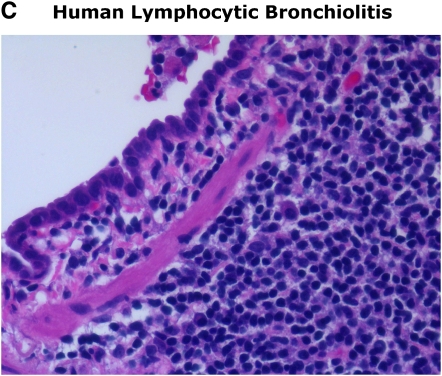
In addition to acute rejection, other probable risk factors include lymphocytic bronchiolitis in the absence of cytomegalovirus (CMV) infection or other infection (5). Several studies have implicated lymphocytic bronchiolitis as a risk factor and pathologic lesion indicative of ongoing alloimmune airway injury that may precede the development of OB (7, 8, 14, 15). Indeed, the similar progression of periairway mononuclear inflammation with epithelial cell injury followed by fibroproliferative obliteration observed in animal tracheal transplant models strongly supports this concept of a linear progression from lymphocytic bronchiolitis to OB. The role of active CMV infection in OB/BOS is controversial, although the majority of studies suggest it is a probable risk factor (5). Several lines of evidence support this, including early studies in lung transplant recipients before current antiviral prophylactic regimens and the progressive allograft decline observed in individuals with recurrent, poorly controlled active CMV infection and/or those infected with ganciclovir-resistant virus. In addition, high-risk lung transplant recipients mismatched for CMV serologic status (donor positive/recipient negative; D+R−) have an increased risk for 1-yr mortality according to recent data from the International Society of Heart and Lung Transplant registry (3). It is possible that some of the differences among studies examining whether CMV infection or donor/recipient CMV serologic mismatching is a risk factor for BOS may be due to heterogeneity in CMV-specific immunity, particularly among high-risk individuals as recent data suggest (16). Thus, active CMV infection may be important as a cofactor in OB/BOS development in individuals unable to adequately control virus due to an inability to develop and/or maintain protective antiviral immunity. It should also be pointed out that CMV may potentiate acute cellular rejection, although the mechanisms remain unclear (17, 18). Therefore, further studies are needed, including the development of animal models, to explore the potential role of CMV infection in the pathogenesis of OB/BOS.
In addition to the alloimmune factors cited above as playing a role in the development of OB, nonalloimmune factors, including infection (bacterial, viral, and fungal), ischemia–reperfusion injury, as well as gastroesophogeal reflux disease (GERD), have been implicated as etiologic factors in OB/BOS resulting in progressive allograft dysfunction (4, 18, 19). The role of the innate immune system and the operative mechanisms involved in airway allograft injury are major areas of ongoing investigation. Several studies have shown an association with the presence of BAL neutrophilia and BOS, as well as the neutrophil chemoattractant IL-8 (CXCL8) (20–22). However, a major question remains as to whether airway neutrophilia may be important in the pathogenesis of progressive allograft dysfunction, or whether these cells may be a biological surrogate for injured airways with impaired mucociliary clearance due to alloimmune-mediated impairment of airway epithelial cells. The clinical observation that patients with BOS often demonstrate radiographic evidence of bronchiectasis, together with frequent colonization with Pseudomonas aeruginosa and Aspergillus fumigatus, supports the injured airway hypothesis. This may also account for the observation that a subset of patients with allograft dysfunction improve with maintenance macrolide therapy, similar to other disease processes in which bronchiectasis is a prominent feature (23–25). In addition to infectious factors, other injurious graft insults, such as ischemia–reperfusion injury and GERD, are believed to contribute to the initiation and/or progression of allograft dysfunction though the mechanisms that remain largely undefined. One hypothesis is that these insults, as well as infection, may serve as “danger signals” to the immune system, which may result in immune-mediated graft injury (26). Indeed, emerging evidence suggests that activated T cells may play an important, and perhaps underappreciated, role in ischemia–reperfusion injury, in addition to innate immune cells such as neutrophils and monocytes/macrophages (27, 28). Moreover, it should be mentioned that certain infectious insults, particularly viral infections, might contribute to allograft dysfunction via other mechanisms, such as heterologous immunity, in which alloreactive T-cell responses develop in the setting of acute viral infection (29). Together, these emerging concepts suggest complex and dynamic interactions between the innate immune system and the adaptive immune system in the pathogenesis of OB/BOS, leading to the progression of allograft dysfunction. Thus, previous categorizations of alloimmune factors versus nonalloimmune factors may represent an artificial distinction in our understanding of OB/BOS pathogenesis.
Alternatively, it is conceivable that various insults to the lung allograft may enhance the alloimmune response by immune activation, resulting in the progression of allograft dysfunction by yet undefined mechanisms. In this regard, genetic factors of the host may also play an important role in the quality and magnitude of the immune response to these various injurious stimuli, as gene polymorphisms in Toll-like receptor 4 (TLR4), tumor necrosis factor (TNF)-α, IFN-γ, IL-10, IL-6, and transforming growth factor-β have been implicated in the susceptibility to BOS development (30–32). Together, differences in both the number and severity of injurious stimuli sustained by the lung allograft and factors regulating the host immune response may account for the marked heterogeneity of BOS progression observed among lung allograft recipients, although significantly more work needs to be done in this area.
MURINE HETEROTOPIC TRACHEAL TRANSPLANT MODEL
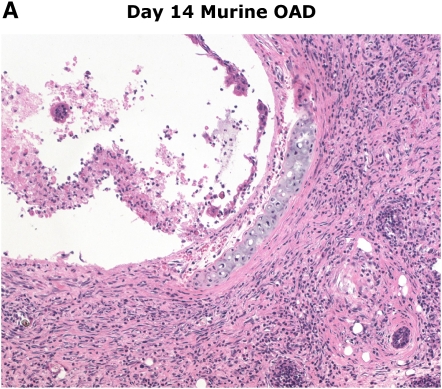
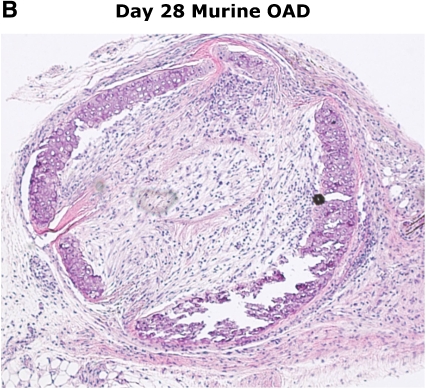
The murine heterotopic tracheal transplant model (HTT) was first described as a highly reproducible animal model for the study of OB by Hertz and colleagues in 1993 (33). These initial studies demonstrated that transplantation of a trachea from a genetically discordant donor mouse into a subcutaneous pouch in the upper back of a recipient mouse resulted in rejection of the tracheal airway that bore similar histologic features to those seen in human OB. Airway rejection in the HTT model, often referred to as obliterative airway disease (OAD), is preceded by substantial periairway/subepithelial mononuclear cell infiltration similar to lymphocytic bronchiolitis that peaks between Days 10 and 14, followed by lumen obliteration comparable to human OB by Days 21 to 28, as illustrated in Figure 1. In contrast to the rejection pathology observed in tracheal airway allografts, genetically identical airway isografts have normal-appearing tracheal histology at Day 28, with evidence of neovascularization.
Figure 1.
Similarities in early/late murine obliterative airway disease (OAD) pathology compared with human lymphocytic bronchiolitis and obliterative bronchiolitis. Images of human pathology courtesy of Dr. Rubin Tuder.
PATHOGENESIS OF OAD
Inflammatory Cells in OAD
Early studies in the HTT model demonstrated a critical role for the adaptive cellular immune response in the development of OAD, with T cells and macrophages infiltrating rejecting airway allografts (34, 35). Moreover, mice with a genetic T-cell deficiency, such as severe combined immunodeficient (SCID) mice or recombinase activating gene 1-deficient (RAG−/−) do not develop OAD (36). Because these mice have natural killer (NK) cells, macrophages, and dendritic cells, these data, together with NK cell depletion studies, provide strong evidence that these cell types are not sufficient alone to induce OAD pathology, and that T cells are required. In addition, Belperio and colleagues have recently shown that neutrophil depletion had no effect on the development of murine OAD (37). With regard to the adaptive immune system, B cells may only play a secondary role in murine OAD pathogenesis, based on the observation that immunoglobulin-deficient mice (Ig knockout [KO]) and B-cell–deficient mice readily reject airway allografts (38, 39). However, adaptive humoral immunity, as discussed above, may play an important role in human OB, as well as a role for the innate complement system in promoting acute vascular injury in the lung allograft, as demonstrated in the rat orthotopic transplant model (40). Regarding T cells in murine OAD pathogenesis, the relative contributions of CD4+ and CD8+ T cells appear to be complex, with CD8+ T cells outnumbering CD4+ T cells by an approximately 2.5:1 ratio in major histocompatibility (MHC)–mismatched airway transplants (BALB/c into C57BL/6). In previous studies, CD4+ T-cell–deficient mice (CD4-KO) have been shown to exhibit delayed airway rejection, whereas CD8+ T-cell–deficient mice (CD8-KO) rejected grafts at the same pace as wild-type animals (38, 41). However, one should exercise caution in extrapolating these results to the physiologic contributions of these T-cell subsets in wild-type allograft recipients and OAD pathogenesis, as compensatory mechanisms have been well described in animals with genetically targeted T-cell subset deficiencies (42). Furthermore, studies of in vivo depletion of either CD4+ T cells and/or CD8+ T cells using specific antibodies have shown this resulted in attenuated OAD pathology, suggesting that both of these T-cell subsets play an important role in pathogenesis (38, 43). Finally, it should be noted that the degree of genetic mismatch might also influence the predominant T-cell subset in OAD pathology. Although CD8+ T cells are the predominant cell type in complete MHC-mismatched HTT studies (43), CD4+ T cells appear to play a major role in HTT models based on a single antigen mismatch that is more comparable with a minor histocompatibility antigen (44, 45). Thus, experimental differences within the HTT model should be considered when assessing the relative contribution of CD4+ and CD8+ T cells in OAD.
Cytokines and Chemokines in OAD
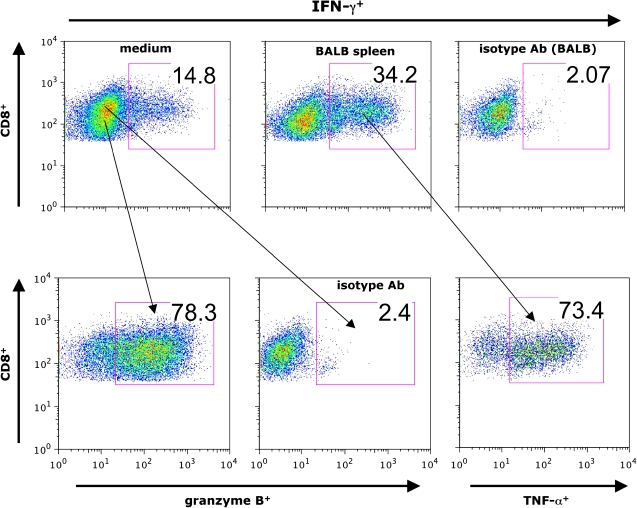
Inflammatory cytokines play a major role in the alloimmune response that leads to airway rejection. The T-cell growth factor IL-2, targeted by calcineurin inhibitors, appears to be important in OAD pathogenesis as evidenced by the fact that cyclosporine treatment attenuates fibroproliferative obliteration in the HTT model, although significant graft injury still occurs (46). In addition, in vivo antibody-mediated blockade of the inflammatory cytokine TNF-α or the TNF receptor 1 (TNFR1) have been reported to attenuate OAD pathology (47). The role of IFN-γ, the prototypical effector cytokine of a T-helper type 1 (Th1) response and produced largely by CD4+ and CD8+ T cells, as well as NK cells, remains controversial in allograft rejection (48). However, two recent studies show that a substantial percentage of airway allograft–infiltrating CD8+ T cells are constitutively IFN-γ positive (41), with West and colleagues demonstrating that pluripotent allospecific CD8+ T cells produce effector cytokine on in vitro restimulation with alloantigen, in addition to coexpressing other effector molecules such as TNF-α and granzyme B, as shown in Figure 2 (43). Moreover, evidence for systemic allosensitization in the HTT model is present because these pluripotent allospecific CD8+ effector T cells are also detectable in the draining lymph nodes, spleen, and the lung of allograft, but not isograft recipients (43). Thus, although the role of IFN-γ remains incompletely understood in the process of allograft rejection, it is a specific surrogate marker of allospecific T-cell responses during murine OAD. Moreover, given its broad effects on immune activation including the up-regulation of MHC expression, activation of macrophages to produce inflammatory cytokines including TNF-α, the induction of costimulatory molecules on antigen presenting cells (APCs), and the induction of multiple chemokines, it is unlikely that IFN-γ does not play an important role in airway rejection.
Figure 2.
Pluripotent allospecific CD8+ effector T cells contribute to murine OAD. On Day 14 post-transplant, tracheal allograft mononuclear cells from CD45.1+B6 recipients were isolated and cocultured with/without donor (BALB/c, CD45.2+) splenocytes for 6 h followed by determination of IFN-γ, tumor necrosis factor (TNF)-α, and granzyme B expression using intracellular cytokine staining (anticytokine or isotype antibody staining) and flow cytometric analysis gating on CD45.1+CD8+ T cells.
Chemokines are important regulators of alloimmune cellular responses that contribute to allograft rejection. In the HTT model, targeted disruption of specific chemokine/chemokine receptors alters the quality and magnitude of the allogeneic response. For example, CCR2−/− airway allograft recipients deficient for the receptor that binds the CC chemokine monocyte chemoattractant protein 1 (MCP-1 or CCL2) have been demonstrated to attenuate OAD, with reduced macrophage influx into tracheal allografts, although no differences were seen in lymphocyte infiltration of the grafts (49). In another study, the glutamine-leucine-arginine (ELR)–negative CXC chemokines, which include monokines induced by IFN-γ (MIG/CXCL9), IFN-inducible protein 10 (IP-10/CXCL10), and IFN-inducible T-cell α chemoattractant (ITAC/CXCL11), all of which are ligands that bind through the receptor CXCR3, were found to play an important role in OAD pathogenesis (50). These studies demonstrated that neutralizing antibody (Ab) experiments targeting CXCR3, MIG, or IP-10 resulted in attenuation of OAD, significantly reducing both lymphocyte and macrophage infiltration of airway grafts. In addition, these investigators reported significantly elevated levels of MIG, IP-10, and ITAC in the BAL fluid from lung transplant recipients with acute or chronic rejection, compared with healthy transplant controls. In contrast, the CXCR2/CXCR2 ligands belong to the ELR+ group of CXC chemokines known for their potent neutrophil chemoattraction as well as the promotion of angiogenesis. Most recently, the CXCR2/CXCR2 ligand subfamily, which includes CXCL3, CXCL7, and CXCL8 (IL-8), which all bind the endothelial cell receptor CXCR2, were shown to play a distinct role in vascular remodeling during the fibroproliferative phase of murine OAD (37). Finally, the CC chemokine RANTES (Regulated upon Activation, Normal T-cell Expressed and Secreted) or CCL5, which binds the receptors CCR1 and CCR5, has been shown to have an important role in mononuclear cell recruitment during acute lung allograft rejection in the rat orthotopic lung transplant model (51, 52). Taken together, these studies indicate that different groups of chemokines play critical roles in both mononuclear cell recruitment and vascular remodeling during airway rejection in the HTT model and other transplant models, including human studies. However, as with cytokines, potential functional redundancy among chemokines may present a significant challenge in the development of therapeutic targets to limit their effects on the alloimmune response after lung transplantation.
Costimulation Blockade in Airway Transplant
Optimal T-cell activation requires secondary costimulatory signals (e.g., B7 family of ligands) often provided by APCs in addition to engagement of the TCR. Studies examining costimulation in the HTT model have provided mixed results, with CTLA4Ig treatment, which blocks B7 (APC) interactions with CD28 (T cell), resulting in attenuation of OAD (both in mouse and rat) (53, 54); in another study, however, CD28−/− mice were shown to develop OAD by 5 wk post-transplant (39). In contrast, the CD40 ligand (CD40L, CD154)/CD40 costimulation pathway appears critical for murine OAD pathogenesis, because CD154−/− airway allograft recipients are OAD resistant (39). In addition, two recent studies using anti-CD154 Ab treatment alone or in combination with donor spleen cell transfusion significantly blocked OAD development (55, 56). However, the mechanisms leading to resistance to OAD in the absence of CD154/CD40 costimulation remain undefined, although recent studies in a skin transplant model suggest that CD4+CD25+ regulatory T cells may play an important role (57).
Limitations of the Murine HTT Model
Despite a high level of reproducibility in the HTT model, its notable similarities to human OB pathology, and its utility for controlled, mechanistic studies of airway rejection, there are several acknowledged limitations to the model. First, and perhaps most important, the tracheal allograft is not a functional airway. This limitation makes the study of various injurious stimuli, discussed above as playing potential roles in human BOS, problematic to address in the mouse model. Experimental efforts to transplant a functional tracheal allograft have been reported; however, the technical challenges of advanced microsurgery limit the widespread use of these approaches (58). To this end, it should be pointed out that no successful orthotopic lung transplant in the mouse has been reported thus far in the literature, although this approach has been successful in the rat and in miniature swine (59). A second limitation to the HTT model often cited is that the transplanted airway is not primarily vascularized, limiting its translatability to human lung transplant. In particular, it has been argued, early inciting events that potentially contribute to allograft dysfunction in the human, such as ischemia–reperfusion injury, are therefore not accounted for in the murine model. However, it should be noted that murine tracheal grafts develop blood supply from neovascularization that develops in the early post-transplant time period. In fact, in vivo administration of an anti-CXCR2 antibody starting at the time of transplant blocks early angiogenesis and appears to enhance tracheal graft injury (J. Belperio and R. Strieter, personal communication). Moreover, airway epithelial cell sloughing has been observed at early post-transplant time points, with subsequent regeneration in allograft as well as isograft tracheal airways. Taken together, these observations suggest a form of ischemia–reperfusion injury appears to occur in the HTT model, despite the absence of a primary vascular anastomosis. Another criticism of the HTT model is that human OB is a pathologic process predominantly involving the small peripheral airways and not the large conducting airways. Although this is certainly accurate, it should be pointed out that a subset of lung transplant recipients have large airway stenosis and/or obliteration, which is often recurrent and progressive, requiring techniques such as airway stenting, dilatation, and other interventions. Nevertheless, the pathologic progression from mononuclear cell infiltration of the airway subepithelial layer to airway epithelial injury and destruction, followed by fibroproliferative obliteration of the airway lumen, suggests that the similarities between murine OAD and human OB outweigh the differences at the single airway level, regardless of whether it is a small peripheral airway in the human or a tracheal allograft in the mouse. Thus, although limitations in the murine HTT experimental model certainly exist, similarities to human OB pathology make it very useful to study immune mechanisms of airway rejection. In fact, one could argue that the absence of confounding variables, such as infection or GERD, enhances its utility to study alloimmune mechanisms in a more controlled manner.
The Lung as a Reservoir Site for Allospecific T Cells
In the past several years, an increasing interest and recognition of the presence of effector memory T cells in nonlymphoid tissues, such as the lung, liver, kidney, and other sites has developed. Two major studies demonstrated the distribution and preferential localization of effector memory CD4+ and CD8+ T cells to nonlymphoid sites, with high frequencies of antigen-specific T cells detectable in the lung parenchyma and airways in these models (60, 61). A subsequent study showed functional differences between antigen-specific CD4+ T cells in the lung and airways compared with the mediastinal lymph node, with only cells in the lymphoid compartment demonstrating the capacity to proliferate in vivo (62). However, in other experiments, ex vivo isolation of antigen-specific lung/airway T cells followed by antigen restimulation in vitro indicate these cells are capable of cell division and expansion (62, 63). These apparently conflicting findings suggest that more studies are needed in different systems, including the human, to elucidate the functional capacities of effector memory T-cell populations in nonlymphoid sites such as the lung. Recently, Moyron-Quiroz and colleagues demonstrated the capacity for viral-specific T cells to develop in the lungs of mice devoid of secondary lymphoid tissues and Peyer's patches at sites of inducible bronchus-associated lymphoid tissue, suggesting that inducible bronchus-associated lymphoid tissue may function as inducible secondary lymphoid tissue for respiratory immune responses (64). The requirements for development, expansion, and homing to nonlymphoid sites such as the lung are highly relevant to solid organ transplantation, because these issues may significantly impact our understanding of acute and chronic allograft rejection. Recently, we described the trafficking of allospecific CD8+ effector memory T cells to the lung during airway rejection in the HTT model (43). Surprisingly, the frequency of allospecific CD8+ T cells was found to be significantly higher (10-fold) in the lung at the peak of the cellular response compared with the draining lymph nodes of animals rejecting tracheal allografts. This suggests that the lung may be an important reservoir for allospecific effector memory T cells, because trafficking of these cells occurred independent of the tissue type heterotopically transplanted (e.g., liver tissue in our studies). Recently, BLT1, the receptor for leukotriene B4, has been demonstrated to play a role in the trafficking of pathogenic effector T cells to the lung and in rejecting tracheal allografts (65). Future studies examining the factors that regulate the differentiation and trafficking of allospecific T cells to the lung are important to understand the regulation of T-cell responses involved in the pathogenesis of OB. Other animal models may also be useful in addressing questions regarding the immunobiology of allospecific cells homing to nonlymphoid tissue, namely the murine model of graft-versus-host disease (66–68). Studies in these models, in which genetically discordant cell populations (e.g., bone marrow with/without T cells, splenocytes) are adoptively transferred, suggest not only commonalities with the pathologic findings associated with acute and chronic rejection after lung transplant but also a critical role for allospecific effector T cells in mediating lung disease. The relevance of these models is supported by the well-established clinical observation that OB can be a major complication of graft-versus-host disease after hematopoietic cell transplantation (69). Therefore, the use of additional murine transplant models may provide further insights into the regulation of allospecific T cells that target lung/airway tissue.
FUTURE DIRECTIONS
The high prevalence of OB/BOS resulting in allograft dysfunction remains the major limitation to the long-term success of lung transplantation. Although significant advances have been made in identifying important factors that play a role in this process, the complex mechanisms underlying the pathogenesis of OB/BOS remain incompletely understood. To that end, the establishment of a multiinstitutional lung transplant network initiated and supported by the National Institutes of Health would represent a major advance toward the goal of improving research in the pathogenesis and treatment of OB/BOS and allograft dysfunction (70). Such a network could orchestrate multicenter clinical investigation, coordinate tissue-banking initiatives designed to facilitate genomic research, and establish core research facilities (e.g., flow cytometric analysis, gene expression profiling sites) to support multicenter translational studies in lung transplant recipients. In addition, the network could promote and support reagent development for animal models such as the HTT model, and perhaps expand into developing larger animal studies, such as in nonhuman primates at centers with expertise. These animal models could provide proof-of-concept studies that could be then explored through the network in translational human studies. Despite advances in recent years, it is becoming increasingly clear that a unified effort of these proportions is necessary to face the major clinical challenges of OB and progressive allograft dysfunction to advance the field of lung transplantation.
Acknowledgments
The author thanks Dr. Rubin Tuder for the illustrative human pathology presented in this article.
Supported by NIH grant HL68682 (J.F.M.).
Conflict of Interest Statement: J.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Trulock EP. Lung transplantation. Am J Respir Crit Care Med 1997;155:789–818. [DOI] [PubMed] [Google Scholar]
- 2.Arcasoy SM, Kotloff RM. Lung transplantation. N Engl J Med 1999;340:1081–1091. [DOI] [PubMed] [Google Scholar]
- 3.Trulock EP, Edwards LB, Taylor DO, Boucek MM, Keck BM, Hertz MI. The Registry of the International Society for Heart and Lung Transplantation: twenty-first official adult heart transplant report—2004. J Heart Lung Transplant 2004;23:804–815. [DOI] [PubMed] [Google Scholar]
- 4.Estenne M, Hertz MI. Bronchiolitis obliterans after human lung transplantation. Am J Respir Crit Care Med 2002;166:440–444. [DOI] [PubMed] [Google Scholar]
- 5.Estenne M, Maurer JR, Boehler A, Egan JJ, Frost A, Hertz M, Mallory GB, Snell GI, Yousem S. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant 2002;21:297–310. [DOI] [PubMed] [Google Scholar]
- 6.Keller CA, Cagle PT, Brown RW, Noon G, Frost AE. Bronchiolitis obliterans in recipients of single, double, and heart-lung transplantation. Chest 1995;107:973–980. [DOI] [PubMed] [Google Scholar]
- 7.Girgis RE, Tu I, Berry GJ, Reichenspurner H, Valentine VG, Conte JV, Ting A, Johnstone I, Miller J, Robbins RC, et al. Risk factors for the development of obliterative bronchiolitis after lung transplantation. J Heart Lung Transplant 1996;15:1200–1208. [PubMed] [Google Scholar]
- 8.Husain AN, Siddiqui MT, Holmes EW, Chandrasekhar AJ, McCabe M, Radvany R, Garrity ER. Analysis of risk factors for the development of bronchiolitis obliterans syndrome. Am J Respir Crit Care Med 1999;159:829–833. [DOI] [PubMed] [Google Scholar]
- 9.Jaramillo A, Smith MA, Phelan D, Sundaresan S, Trulock EP, Lynch JP, Cooper JD, Patterson GA, Mohanakumar T. Development of ELISA-detected anti-HLA antibodies precedes the development of bronchiolitis obliterans syndrome and correlates with progressive decline in pulmonary function after lung transplantation. Transplantation 1999;67:1155–1161. [DOI] [PubMed] [Google Scholar]
- 10.Palmer SM, Davis RD, Hadjiliadis D, Hertz MI, Howell DN, Ward FE, Savik K, Reinsmoen NL. Development of an antibody specific to major histocompatibility antigens detectable by flow cytometry after lung transplant is associated with bronchiolitis obliterans syndrome. Transplantation 2002;74:799–804. [DOI] [PubMed] [Google Scholar]
- 11.Gimino VJ, Lande JD, Berryman TR, King RA, Hertz MI. Gene expression profiling of bronchoalveolar lavage cells in acute lung rejection. Am J Respir Crit Care Med 2003;168:1237–1242. [DOI] [PubMed] [Google Scholar]
- 12.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol 2003;4:1191–1198. [DOI] [PubMed] [Google Scholar]
- 13.Judge AD, Zhang X, Fujii H, Surh CD, Sprent J. Interleukin 15 controls both proliferation and survival of a subset of memory-phenotype CD8(+) T cells. J Exp Med 2002;196:935–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reichenspurner H, Girgis RE, Robbins RC, Yun KL, Nitschke M, Berry GJ, Morris RE, Theodore J, Reitz BA. Stanford experience with obliterative bronchiolitis after lung and heart-lung transplantation. Ann Thorac Surg 1996;62:1467ndash;1472. [discussion, 1472–1473.] [DOI] [PubMed] [Google Scholar]
- 15.Ross DJ, Marchevsky A, Kramer M, Kass RM. Refractoriness of airflow obstruction associated with isolated lymphocytic bronchiolitis/bronchitis in pulmonary allografts. J Heart Lung Transplant 1997;16:832–838. [PubMed] [Google Scholar]
- 16.Shlobin OA, West EE, Lechtzin N, Miller SM, Borja M, Orens JB, Dropulic LK, McDyer JF. Persistent cytomegalovirus-specific memory responses in the lung allograft and blood following primary infection in lung transplant recipients. J Immunol 2006;176:2625–2634. [DOI] [PubMed] [Google Scholar]
- 17.Tolkoff-Rubin NE, Fishman JA, Rubin RH. The bidirectional relationship between cytomegalovirus and allograft injury. Transplant Proc 2001;33:1773–1775. [DOI] [PubMed] [Google Scholar]
- 18.Palmer SM, Miralles AP, Howell DN, Brazer SR, Tapson VF, Davis RD. Gastroesophageal reflux as a reversible cause of allograft dysfunction after lung transplantation. Chest 2000;118:1214–1217. [DOI] [PubMed] [Google Scholar]
- 19.Garantziotis S, Howell DN, McAdams HP, Davis RD, Henshaw NG, Palmer SM. Influenza pneumonia in lung transplant recipients: clinical features and association with bronchiolitis obliterans syndrome. Chest 2001;119:1277–1280. [DOI] [PubMed] [Google Scholar]
- 20.Martinez JA, Paradis IL, Dauber JH, Grgurich W, Richards T, Yousem SA, Ohori P, Williams P, Iacono AT, Nunley DR, et al. Spirometry values in stable lung transplant recipients. Am J Respir Crit Care Med 1997;155:285–290. [DOI] [PubMed] [Google Scholar]
- 21.DiGiovine B, Lynch JP III, Martinez FJ, Flint A, Whyte RI, Iannettoni MD, Arenberg DA, Burdick MD, Glass MC, Wilke CA, et al. Bronchoalveolar lavage neutrophilia is associated with obliterative bronchiolitis after lung transplantation: role of IL-8. J Immunol 1996;157:4194–4202. [PubMed] [Google Scholar]
- 22.Riise GC, Andersson BA, Kjellstrom C, Martensson G, Nilsson FN, Ryd W, Schersten H. Persistent high BAL fluid granulocyte activation marker levels as early indicators of bronchiolitis obliterans after lung transplant. Eur Respir J 1999;14:1123–1130. [DOI] [PubMed] [Google Scholar]
- 23.Gerhardt SG, McDyer JF, Girgis RE, Conte JV, Yang SC, Orens JB. Maintenance azithromycin therapy for bronchiolitis obliterans syndrome: results of a pilot study. Am J Respir Crit Care Med 2003;168: 121–125. [DOI] [PubMed] [Google Scholar]
- 24.Yates B, Murphy DM, Forrest IA, Ward C, Rutherford RM, Fisher AJ, Lordan JL, Dark JH, Corris PA. Azithromycin reverses airflow obstruction in established bronchiolitis obliterans syndrome. Am J Respir Crit Care Med 2005;172:772–775. [DOI] [PubMed] [Google Scholar]
- 25.Jaffe A, Francis J, Rosenthal M, Bush A. Long-term azithromycin may improve lung function in children with cystic fibrosis. Lancet 1998;351:420. [DOI] [PubMed] [Google Scholar]
- 26.Matzinger P. The danger model: a renewed sense of self. Science 2002;296:301–305. [DOI] [PubMed] [Google Scholar]
- 27.de Perrot M, Young K, Imai Y, Liu M, Waddell TK, Fischer S, Zhang L, Keshavjee S. Recipient T cells mediate reperfusion injury after lung transplantation in the rat. J Immunol 2003;171:4995–5002. [DOI] [PubMed] [Google Scholar]
- 28.Burne-Taney MJ, Yokota N, Rabb H. Persistent renal and extrarenal immune changes after severe ischemic injury. Kidney Int 2005;67:1002–1009. [DOI] [PubMed] [Google Scholar]
- 29.Adams AB, Williams MA, Jones TR, Shirasugi N, Durham MM, Kaech SM, Wherry EJ, Onami T, Lanier JG, Kokko KE, et al. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest 2003;111:1887–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palmer SM, Burch LH, Trindade AJ, Davis RD, Herczyk WF, Reinsmoen NL, Schwartz DA. Innate immunity influences long-term outcomes after human lung transplant. Am J Respir Crit Care Med 2005;171:780–785. [DOI] [PubMed] [Google Scholar]
- 31.Hutchinson IV, Pravica V, Perrey C, Sinnott P. Cytokine gene polymorphisms and relevance to forms of rejection. Transplant Proc 1999;31: 734–736. [DOI] [PubMed] [Google Scholar]
- 32.Lu KC, Jaramillo A, Lecha RL, Schuessler RB, Aloush A, Trulock EP, Mendeloff EN, Huddleston CB, Alexander Patterson G, Mohanakumar T. Interleukin-6 and interferon-gamma gene polymorphisms in the development of bronchiolitis obliterans syndrome after lung transplantation. Transplantation 2002;74:1297–1302. [DOI] [PubMed] [Google Scholar]
- 33.Hertz MI, Jessurun J, King MB, Savik SK, Murray JJ. Reproduction of the obliterative bronchiolitis lesion after heterotopic transplantation of mouse airways. Am J Pathol 1993;142:1945–1951. [PMC free article] [PubMed] [Google Scholar]
- 34.Kelly KE, Hertz MI, Mueller DL. T-cell and major histocompatibility complex requirements for obliterative airway disease in heterotopically transplanted murine tracheas. Transplantation 1998;66:764–771. [DOI] [PubMed] [Google Scholar]
- 35.Neuringer IP, Walsh SP, Mannon RB, Gabriel S, Aris RM. Enhanced T cell cytokine gene expression in mouse airway obliterative bronchiolitis. Transplantation 2000;69:399–405. [DOI] [PubMed] [Google Scholar]
- 36.Neuringer IP, Mannon RB, Coffman TM, Parsons M, Burns K, Yankaskas JR, Aris RM. Immune cells in a mouse airway model of obliterative bronchiolitis. Am J Respir Cell Mol Biol 1998;19:379–386. [DOI] [PubMed] [Google Scholar]
- 37.Belperio JA, Keane MP, Burdick MD, Gomperts B, Xue YY, Hong K, Mestas J, Ardehali A, Mehrad B, Saggar R, et al. Role of CXCR2/CXCR2 ligands in vascular remodeling during bronchiolitis obliterans syndrome. J Clin Invest 2005;115:1150–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Higuchi T, Jaramillo A, Kaleem Z, Patterson GA, Mohanakumar T. Different kinetics of obliterative airway disease development in heterotopic murine tracheal allografts induced by CD4+ and CD8+ T cells. Transplantation 2002;74:646–651. [DOI] [PubMed] [Google Scholar]
- 39.Rumbley CA, Silver SJ, Phillips SM. Dependence of murine obstructive airway disease on CD40 ligand. Transplantation 2001;72:1616–1625. [DOI] [PubMed] [Google Scholar]
- 40.Nakashima S, Qian Z, Rahimi S, Wasowska BA, Baldwin WM III. Membrane attack complex contributes to destruction of vascular integrity in acute lung allograft rejection. J Immunol 2002;169:4620–4627. [DOI] [PubMed] [Google Scholar]
- 41.Richards DM, Dalheimer SL, Hertz MI, Mueller DL. Trachea allograft class I molecules directly activate and retain CD8+ T cells that cause obliterative airways disease. J Immunol 2003;171:6919–6928. [DOI] [PubMed] [Google Scholar]
- 42.Doherty PC, Allan W, Eichelberger M, Carding SR. Roles of alpha beta and gamma delta T cell subsets in viral immunity. Annu Rev Immunol 1992;10:123–151. [DOI] [PubMed] [Google Scholar]
- 43.West EE, Lavoie TL, Orens JB, Chen ES, Ye SQ, Finkelman FD, Garcia JG, McDyer JF. Pluripotent allospecific CD8+ effector T cells traffic to lung in murine obliterative airway disease. Am J Respir Cell Mol Biol 2006;34:108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith MA, Jaramillo A, SivaSai KS, Naziruddin B, Kaleem Z, Patterson GA, Mohanakumar T. Indirect recognition and antibody production against a single mismatched HLA-A2-transgenic molecule precede the development of obliterative airway disease in murine heterotopic tracheal allografts. Transplantation 2002;73(2):186–193. [DOI] [PubMed] [Google Scholar]
- 45.Richards DM, Dalheimer SL, Ehst BD, Vanasek TL, Jenkins MK, Hertz MI, Mueller DL. Indirect minor histocompatibility antigen presentation by allograft recipient cells in the draining lymph node leads to the activation and clonal expansion of CD4+ T cells that cause obliterative airways disease. J Immunol 2004;172:3469–3479. [DOI] [PubMed] [Google Scholar]
- 46.King MB, Jessurun J, Savik SK, Murray JJ, Hertz MI. Cyclosporine reduces development of obliterative bronchiolitis in a murine heterotopic airway model. Transplantation 1997;63:528–532. [DOI] [PubMed] [Google Scholar]
- 47.Smith CR, Jaramillo A, Lu KC, Higuchi T, Kaleem Z, Mohanakumar T. Prevention of obliterative airway disease in HLA-A2-transgenic tracheal allografts by neutralization of tumor necrosis factor. Transplantation 2001;72:1512–1518. [DOI] [PubMed] [Google Scholar]
- 48.Fairchild RL. The yin and yang of IFN-gamma in allograft rejection. Am J Transplant 2003;3:913–914. [DOI] [PubMed] [Google Scholar]
- 49.Belperio JA, Keane MP, Burdick MD, Lynch JP III, Xue YY, Berlin A, Ross DJ, Kunkel SL, Charo IF, Strieter RM. Critical role for the chemokine MCP-1/CCR2 in the pathogenesis of bronchiolitis obliterans syndrome. J Clin Invest 2001;108:547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Belperio JA, Keane MP, Burdick MD, Lynch JP III, Xue YY, Li K, Ross DJ, Strieter RM. Critical role for CXCR3 chemokine biology in the pathogenesis of bronchiolitis obliterans syndrome. J Immunol 2002;169:1037–1049. [DOI] [PubMed] [Google Scholar]
- 51.Belperio JA, Burdick MD, Keane MP, Xue YY, Lynch JP III, Daugherty BL, Kunkel SL, Strieter RM. The role of the CC chemokine, RANTES, in acute lung allograft rejection. J Immunol 2000;165:461–472. [DOI] [PubMed] [Google Scholar]
- 52.Sekine Y, Yasufuku K, Heidler KM, Cummings OW, Van Rooijen N, Fujisawa T, Brown J, Wilkes DS. Monocyte chemoattractant protein-1 and RANTES are chemotactic for graft infiltrating lymphocytes during acute lung allograft rejection. Am J Respir Cell Mol Biol 2000;23:719–726. [DOI] [PubMed] [Google Scholar]
- 53.Tikkanen JM, Lemstrom KB, Koskinen PK. Blockade of CD28/B7-2 costimulation inhibits experimental obliterative bronchiolitis in rat tracheal allografts: suppression of helper T cell type 1-dominated immune response. Am J Respir Crit Care Med 2002;165:724–729. [DOI] [PubMed] [Google Scholar]
- 54.Yamada A, Konishi K, Cruz GL, Takehara M, Morikawa M, Nakagawa I, Murakami M, Abe T, Todo S, Uede T. Blocking the CD28-B7 T-cell costimulatory pathway abrogates the development of obliterative bronchiolitis in a murine heterotopic airway model. Transplantation 2000;69:743–749. [DOI] [PubMed] [Google Scholar]
- 55.Fernandez FG, McKane B, Marshbank S, Patterson GA, Mohanakumar T. Inhibition of obliterative airway disease development following heterotopic murine tracheal transplantation by costimulatory molecule blockade using anti-CD40 ligand alone or in combination with donor bone marrow. J Heart Lung Transplant 2005;24:S232–S238. [DOI] [PubMed] [Google Scholar]
- 56.Chalermskulrat W, McKinnon KP, Brickey WJ, Neuringer IP, Park RC, Sterka DG, Long BR, McNeillie P, Noelle RJ, Ting JP, et al. Combined donor specific transfusion and anti-CD154 therapy achieves airway allograft tolerance. Thorax 2006;61:61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Quezada SA, Bennett K, Blazar BR, Rudensky AY, Sakaguchi S, Noelle RJ. Analysis of the underlying cellular mechanisms of anti-CD154-induced graft tolerance: the interplay of clonal anergy and immune regulation. J Immunol 2005;175:771–779. [DOI] [PubMed] [Google Scholar]
- 58.Minamoto K, Pinsky DJ. Recipient iNOS but not eNOS deficiency reduces luminal narrowing in tracheal allografts. J Exp Med 2002;196:1321–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Allan JS, Wain JC, Schwarze ML, Houser SL, Benjamin LC, Madsen JC, Sachs DH. Modeling chronic lung allograft rejection in miniature swine. Transplantation 2002;73:447–453. [DOI] [PubMed] [Google Scholar]
- 60.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science 2001;291:2413–2417. [DOI] [PubMed] [Google Scholar]
- 61.Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature 2001;410:101–105. [DOI] [PubMed] [Google Scholar]
- 62.Harris NL, Watt V, Ronchese F, Le Gros G. Differential T cell function and fate in lymph node and nonlymphoid tissues. J Exp Med 2002;195:317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hogan RJ, Usherwood EJ, Zhong W, Roberts AA, Dutton RW, Harmsen AG, Woodland DL. Activated antigen-specific CD8+ T cells persist in the lungs following recovery from respiratory virus infections. J Immunol 2001;166:1813–1822. [DOI] [PubMed] [Google Scholar]
- 64.Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, Hartson L, Sprague F, Goodrich S, Woodland DL, Lund FE, Randall TD. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med 2004;10:927–934. [DOI] [PubMed] [Google Scholar]
- 65.Medoff BD, Seung E, Wain JC, Means TK, Campanella GS, Islam SA, Thomas SY, Ginns LC, Grabie N, Lichtman AH, et al. BLT1-mediated T cell trafficking is critical for rejection and obliterative bronchiolitis after lung transplantation. J Exp Med 2005;202:97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cooke KR, Krenger W, Hill G, Martin TR, Kobzik L, Brewer J, Simmons R, Crawford JM, van den Brink MR, Ferrara JL. Host reactive donor T cells are associated with lung injury after experimental allogeneic bone marrow transplantation. Blood 1998;92:2571–2580. [PubMed] [Google Scholar]
- 67.Niculescu F, Niculescu T, Nguyen P, Puliaev R, Papadimitriou JC, Gaspari A, Rus H, Via CS. Both apoptosis and complement membrane attack complex deposition are major features of murine acute graft-vs.-host disease. Exp Mol Pathol 2005;79:136–145. [DOI] [PubMed] [Google Scholar]
- 68.Wysocki CA, Burkett SB, Panoskaltsis-Mortari A, Kirby SL, Luster AD, McKinnon K, Blazar BR, Serody JS. Differential roles for CCR5 expression on donor T cells during graft-versus-host disease based on pretransplant conditioning. J Immunol 2004;173:845–854. [DOI] [PubMed] [Google Scholar]
- 69.Dudek AZ, Mahaseth H, DeFor TE, Weisdorf DJ. Bronchiolitis obliterans in chronic graft-versus-host disease: analysis of risk factors and treatment outcomes. Biol Blood Marrow Transplant 2003;9:657–666. [DOI] [PubMed] [Google Scholar]
- 70.Wilkes DS, Egan TM, Reynolds HY. Lung transplantation: opportunities for research and clinical advancement. Am J Respir Crit Care Med 2005;172:944–955. [DOI] [PMC free article] [PubMed] [Google Scholar]