Abstract
Maternal treatment with the synthetic glucocorticoid, dexamethasone has been reported to result in a nephron deficit and development of hypertension in the offspring of rats. However, it is not known whether elevated maternal corticosterone (CORT), the natural glucocorticoid, has similar effects on blood pressure and nephron endowment. The present study investigated the effects of CORT (0.8 mg kg−1 day−1) administration on embryonic day 14 (E14) and E15 of pregnancy on: (1) nephron number at postnatal day 30 (PN30); (2) blood pressure at PN120; and (3) receptors of the renal renin–angiotensin system (RRAS) (AT1Ra, AT1Rb and AT2Ra) during both embryonic (E16, E20) and adolescent (PN30) life. Plasma CORT concentrations were approximately doubled 30 min after injection. Unbiased stereological analysis revealed that maternal CORT treatment resulted in a nephron deficit of 21 and 19% in male and female offspring, respectively. Mean arterial pressures were significantly elevated in offspring of both sexes from the CORT group. Real-time PCR revealed that CORT treatment increased expression of AT1Ra and AT2R at E16, and at PN30. Expression of AT1Rb was downregulated in embryonic life but upregulated at PN30. We believe that these results are the first to demonstrate that maternal CORT treatment results in a nephron deficit and development of hypertension in the rat offspring. Changes in the RRAS may be contributing to these phenotypes. Critically, this study suggests that increased but physiological levels of the natural glucocorticoid can programme similar changes to those seen with pharmacological doses of the synthetic glucocorticoid. This may have important implications for women experiencing significant stress during pregnancy.
Exposure of the fetus to a suboptimal intrauterine environment has been implicated in development of adult onset diseases, such as hypertension, a phenomenon commonly known as ‘developmental programming’ (Barker & Osmond, 1986). The aetiology of this hypertension is as yet uncertain. However, it is becoming evident that exposure of the fetus to elevated maternal glucocorticoids for the entire duration, or at ‘critical windows’ of pregnancy, is likely to play a role. Maternal administration of the synthetic glucocorticoid dexamethasone, for just 2 days, results in hypertensive offspring in sheep (Dodic et al. 2002a) and rat (Ortiz et al. 2001, 2003) models. Interestingly, it has been demonstrated that maternal dexamethasone treatment results in adult hypertension and a reduced glomerular (nephron) number in the offspring when administered on embryonic day 15/16 (E15/16) or E17/18, but not when administered earlier (E11/12, E13/14) or later (E19/20) in pregnancy (Ortiz et al. 2001, 2003). Similarly, dexamethasone treatment in sheep only results in reduced nephron number and hypertension in the offspring when administered early in gestation (Dodic et al. 1998; Wintour et al. 2003) These observations highlight the existence of ‘critical windows’ during fetal development at which times the kidney may be most vulnerable.
Most studies of glucocorticoid programming have been undertaken using the synthetic glucocorticoids dexamethasone and betamethasone, which readily cross the placental barrier (Seckl, 1997). This is because natural glucocorticoids are at least, in part, inactivated in the placenta by the enzyme 11β-hydroxysteroid dehydrogenase type 2, thereby protecting the fetus from elevated maternal glucocorticoid concentrations (Seckl, 1997). However, the physiological relevance of these models is limited as the synthetic steroids are rarely administered in the ‘critical window’ of early pregnancy. The only studies to directly examine the effects of the natural glucocorticoid have been performed in sheep, where cortisol administration at 26–28 days, at concentrations sufficient to elevate plasma cortisol concentrations to high but physiological levels, has been reported to result in offspring that are born with a nephron deficit (Moritz et al. 2004) and develop hypertension in adult life (Dodic et al. 2002b).
Further highlighting the need for examination of the impact of natural glucocorticoids in programming hypertension are our recent findings in sheep that have demonstrated that the hypertension in dexamethasone-treated females is cardiac-output mediated (Dodic et al. 1999), whilst in the cortisol-treated males it is mediated by increases in total peripheral resistance (Wintour et al. 2005). There are also significant differences in renal and brain gene expression changes between the dexamethasone- and the cortisol-treated fetus (Moritz et al. 2002b; Dodic et al. 2002b). This suggests that the synthetic and natural glucocorticoids may have differential ‘programming’ effects, highlighting the importance of investigating the effect of the natural glucocorticoid.
As yet, no study has been undertaken to examine the direct effect of elevated concentrations of the natural glucocorticoid corticosterone (CORT) on blood pressure and nephron endowment in the rat. In the present study, the first aim was to determine blood pressure and nephron number in male and female offspring of rat dams exposed to high but physiological concentrations of CORT for 2 days. CORT was administered on E14/15, a similar age to which dexamethasone (Ortiz et al. 2001, 2003) had a maximal effect on these parameters. Maternal glucocorticoid exposure in sheep (Moritz et al. 2002a) and low protein exposure in the rat (Woods et al. 2001, 2004; McMullen & Langley-Evans, 2005) have been shown to induce marked changes in the renal renin–angiotensin system (RRAS) in the fetus and the offspring, suggesting that this system is of importance in many species and models of fetal programming. Thus, in the current study, we also examined the impact of CORT treatment on the RRAS of the embryo and adolescent offspring.
Methods
Animals
All experiments were approved by a Monash University Animal Ethics Committee in accordance with the guidelines of the National Health and Medical Research Council of Australia. Time-mated Sprague-Dawley rats were injected intraperitoneally twice daily on E14 and E15 of pregnancy with either saline (SAL; 0.2 ml, n= 10) or an equivalent volume of CORT (0.8 mg kg−1 day−1, n= 10; Sigma-Aldrich).
CORT assay
Blood collection
In a separate cohort of animals, time-mated Sprague-Dawley rats were injected intraperitoneally with either SAL (n= 4) or CORT (0.8 mg kg−1 min−1, n= 4) on E14/E15. Blood was collected via tail vein bleeds in chilled tubes 30 min after the first injection for determination of plasma CORT concentrations.
CORT assay
Plasma CORT concentrations were determined by radioimmunoassay using a commercially available [125I] kit (MP Biomedicals, NY, USA). The detection limit of the kit was 25 ng ml−1. The interassay coefficient of variation was 9.7%.
Tissue collection
Kidneys were collected at E16, E20, postnatal day 30 (PN30) and PN120. At E16 and E20, three pregnant animals from each treatment group (SAL and CORT) were culled with an overdose of sodium pentobarbitone (Nembutal Sodium; Abbott Australia). The abdomen was opened and the uterus was located. Embryos were removed, cleaned and weighed prior to decapitation. Kidneys were then removed, weighed, and frozen for later extraction of RNA. At E16, kidneys from two or three embryos were pooled, whilst kidneys from individual animals were collected at E20.
The remaining four animals per treatment group were allowed to litter down normally and offspring were weaned at PN22. At PN30, two offspring of each sex per litter were culled, as described above, for stereological studies. The remaining offspring from these litters were used for measurement of blood pressure at PN120.
Stereology
Kidney sampling
The excised left kidney from PN30 offspring was weighed, decapsulated, and fixed in 10% buffered formalin for determination of kidney volume, total nephron number and mean glomerular and mean renal corpuscle volumes.
Estimating kidney volume
Total kidney volume (Vkid) was estimated using the Cavalieri Principle (Gundersen et al. 1988), as previously described (Bertram et al. 1992; Cullen-McEwen et al. 2003). Total kidney volume was determined using the following equation:
where 2 is the inverse of the slice sampling fraction, 10 is the reciprocal of the section sampling fraction, T is section thickness, a(p) is the area associated with each grid point, and Ps is the total number of points landing on kidney tissue.
Determination of glomerular number and size
Total glomerular (and thus nephron) number was estimated by the physical disector/fractionator principle as previously described. (Bertram et al. 1992) Briefly, paired (10th and 11th) sections were placed on two Olympus BX50F4 light microscopes modified with projection arms and the fields were projected onto an orthogonal grid (2 cm × 2 cm).
The total number of glomeruli (Nglom,kid)was calculated using:
where 10 is the reciprocal of the section sampling fraction, Ps is the total number of grid points hitting kidney sections, Pf is the number of grid points overlying complete sections only, 1/(2fa) is the fraction of the total section area used to count glomeruli, and Q− is the actual number of glomeruli counted.
Estimating glomerular and renal corpuscle volumes
Stereological grid points overlying glomerular tufts (Pglom)and renal corpuscles (Pcorp) were also counted in order to estimate mean glomerular (Vglom) and renal corpuscle (Vcorp) volumes, as well as total volumes of glomeruli (Vglom,tot) and renal corpuscles (Vcorp,tot) per kidney. The following formulae were used:
Mean arterial pressure and heart rate
At PN120, conscious mean arterial pressure (MAP) and heart rate (HR) of CORT- and SAL-treated animals were measured from the caudal artery. The catheters of the animals were connected to a pressure transducer, and the Universal Acquisition Program (TracerDAQ; Measurement Computing, MA, USA) was used to obtain blood pressure and HR measurements. Recordings were made in the conscious, unrestrained animal as previously described (Bergstrom et al. 1998; Stevenson et al. 2000; Kett et al. 2004). At completion of all recordings, animals were humanely killed, and kidneys were excised and weighed.
Evaluation of systemic RAS
Systemic RAS activity was evaluated from plasma renin activity (PRA). Briefly, at the end of arterial pressure measurements, 2 ml of blood was drawn from the caudal artery, and collected in chilled tubes containing a cocktail of inhibitors. PRA was determined by an enzymatic technique adapted for small samples as previously described (Nussberger et al. 1985).
Renal histology
Kidneys of PN30 and PN120 male and female offspring from both experimental groups were fixed in 10% buffered-formalin, embedded in paraffin, and sectioned at 5 μm. Renal pathology was investigated by an expert renal pathologist (J.D.) who was blinded to the treatment groups.
Gene expression studies
Total RNA was extracted using RNeasy extraction kits (Qiagen). A 1 μg of RNA was then reverse transcribed into cDNA, as previously described (Dodic et al. 2002a). Gene expression for the AT1Ra, AT1Rb and AT2R receptors were determined using an ABI-PRISM 7700 real-time machine, as previously described (Dodic et al. 2002a). A comparative cycle of threshold fluorescence (CT) method was used with 18S as an internal control. The AT1Ra and AT1Rb receptor primer/probe sets were multiplexed with 18S. AT2R was run in a separate tube to 18S after optimization experiments revealed the CT was altered if run in a multiplex reaction.
Calculations of relative gene expression
The CT value for 18S was subtracted from the CT value for the gene of interest to give a ΔCT for each sample. The ΔCT of the calibrator (in this case the mean ΔCT of the saline group) was then subtracted from each sample to give a ΔΔCT value. This was then inserted into  to give a final relative expression relative to the calibrator. For data at E16 and E20, the mean ΔCT of the saline group was subtracted from each sample to obtain the ΔΔCT value. For data at PN30, the mean ΔCT of either SAL males or SAL females was used to obtain ΔΔCT value to determine relative gene expression within either sex.
to give a final relative expression relative to the calibrator. For data at E16 and E20, the mean ΔCT of the saline group was subtracted from each sample to obtain the ΔΔCT value. For data at PN30, the mean ΔCT of either SAL males or SAL females was used to obtain ΔΔCT value to determine relative gene expression within either sex.
Sex-specific relative expression of angiotensin II receptors
In order to determine whether angiotensin II receptor gene expression was affected by gender, a separate run of SAL males versus SAL females was performed, and gene expression was determined as described above, using male SAL as the calibrators.
Western blot analysis
The abundance of AT1Ra, AT1Rb and AT2R were determined with slight modifications of the Western blot analysis previously described by Graciano et al. (2004). (Western blots were only performed at PN30 due to the unavailability of kidney tissue at the other ages studied.) Briefly, 0.1 g tissue was homogenized for total protein isolation. The 2-D Quant kit protocol (Amersham Pharmacia Biotech) was used to standardize all protein concentrations for a 20 μl reaction. A 20 μg quantity of protein from each sample was loaded on a 12% SDS-PAGE gel and then electrophoretically transferred to a nitrocellulose membrane. The membrane was incubated in primary antibody overnight, following which the membranes were washed and incubated in secondary antibody for 1 h. Detection was performed by using the Enhance Chemiluminescence Western Blotting Kit (ECL system; Amersham Pharmacia Biotech) and quantified using the Gene Map Software. Rabbit anti-AT1R (1:1000) and the goat anti-AT2R (1:500) antibodies (Santa Cruz Biotechnology) were used as primary antibodies. Goat anti-rabbit IgG (Pierce Biotechnology) and protein-G–horseradish-peroxidase conjugate (Bio-Rad Laboratories) were used as secondary antibodies for anti-AT1R and anti-AT2R, respectively.
Statistical analyses
Values are means ±s.e.m. except where otherwise indicated. A two-way ANOVA was performed to analyse the effects of treatment and sex on stereological and physiological data. A post hoc analysis using the all pairwise multiple comparison Tukey test was used to determine the differences within treatment and sex. Gene expression data were analysed using unpaired t tests, where significance was accepted at P < 0.05.
Results
Maternal plasma CORT concentration
Maternal plasma CORT concentration was significantly elevated following CORT administration on both E14 and E15 (Ptreatment < 0.001, Fig. 1). Although maternal plasma CORT concentrations were higher on E15 compared with E14 in both SAL- and CORT-administered groups (Page < 0.001, Fig. 1) the degree of elevation was similar in both treatment groups (Ptreatment × age= 0.2, Fig. 1).
Figure 1. Effect of exogenous corticosterone (CORT) administration on maternal plasma CORT concentration at embryonic day 14 (E14) and E15.
P values reported here are from post hoc analysis using multiple comparison Tukey’s Test. ***P < 0.001, for treatment within age; ###P < 0.001, for age within saline (SAL) and for age within CORT. SAL, open bars, n= 4; CORT, hatched bars, n= 4.
Body and kidney weights
Body and left kidney weights for all different ages studied are presented in Table 1 (embryo body weights only, at E16 and E20). There was no difference in weight between the embryos or offspring from SAL- and CORT-infused animals at any age. Litter sizes were similar in both groups (SAL = 12 ± 1 pups, CORT = 11 ± 3 pups, pooled data from all ages).
Table 1.
Effect of prenatal CORT treatment at E14/15 on weight parameters of E16 and E20 embryos, and PN30 and PN120 male and female offspring
| E16 | E20 | PN30 | PN120 | |||
|---|---|---|---|---|---|---|
| Pooled | Pooled | Male | Female | Male | Female | |
| n (SAL/CORT) | 30/33 | 34/27 | 11/6 | 9/6 | 9/6 | 6/7 |
| Body weight (g) | ||||||
| SAL | 0.46 ± 0.01 | 3.60 ± 0.09 | 81 ± 7 | 82 ± 3 | 449 ± 13 | 289 ± 11 |
| CORT | 0.47 ± 0.01 | 3.40 ± 0.07 | 81 ± 9 | 73 ± 6 | 483 ± 5 | 288 ± 5 |
| Left kidney weight (g) | ||||||
| SAL | — | — | 0.45 ± 0.01 | 0.43 ± 0.01 | 1.81 ± 0.07 | 1.35 ± 0.07 |
| CORT | — | — | 0.43 ± 0.11 | 0.39 ± 0.02 | 1.35 ± 0.07 | 1.29 ± 0.07 |
| Kidney/body weight (mg g−1) | ||||||
| SAL | — | — | 5.61 ± 0.21 | 5.30 ± 0.11 | 4.03 ± 0.20 | 3.69 ± 0.24 |
| CORT | — | — | 5.26 ± 0.10 | 5.21 ± 0.09 | 4.69 ± 0.22 | 4.45 ± 0.28 |
CORT, corticosterone; SAL, saline; E, embryonic day; PN, postnatal day.
Kidney measurements
Total kidney volume
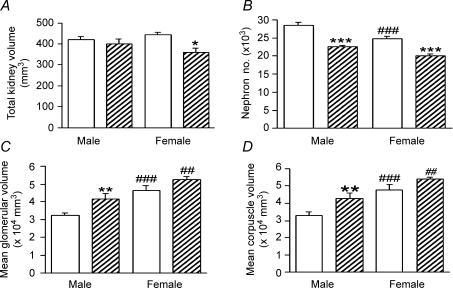
Two-way ANOVA revealed that CORT treatment decreased kidney volume (Ptreatment= 0.02); however, post hoc analysis revealed that this difference in treatment was only present in the female offspring (Ptreatment within females < 0.05, Fig. 2A).
Figure 2.
Effect of prenatal CORT exposure at E14/15 on total kidney volume, nephron number, mean glomerular volume, and mean corpuscle volume, of postnatal day 30 (PN30) male and female offspring Effect of CORT exposure total kidney volume (A), nephron number (B), mean glomerular volume (C) and mean corpuscle volume (D). P values reported here are from a post hoc multiple comparison Tukey test. ***P < 0.001, **P < 0.01, for treatment within sex; ###P < 0.001, for sex within SAL; ##P < 0.01, for sex within CORT. SAL (open bars), n= 5 males, n= 5 females; CORT (hatched bars), n= 5 males, n= 5 females.
Nephron number
Two-way ANOVA showed that total nephron number was significantly lower in the CORT group of both sexes (Ptreatment < 0.001). In addition, nephron number was lower in females than males in both SAL and CORT groups (Psex < 0.001, Psex within SAL < 0.001; Psex within CORT < 0.001, Fig. 2B). However, the effect of CORT treatment on nephron number was similar in both sexes (21% decrease in males, 19% decrease in females; Psex × treatment= 0.3).
Glomerular and renal corpuscle volumes
CORT treatment increased mean glomerular volumes and mean corpuscle volumes (Ptreatment= 0.006 for both) compared with SAL, and two-way ANOVA also showed that both parameters were increased in females compared to males (Psex < 0.001). However, the effect of treatment was not dependent on the sex of the offspring (Psex × treatment= 0.3). Post hoc analysis revealed that although females had higher mean glomerular volumes (Fig. 2C) and mean corpuscle volumes (Fig. 2D; Psex within SAL < 0.001; Psex within CORT < 0.001 for both) than males, CORT treatment increased these parameters in male offspring only (Ptreatment within male < 0.05).
Total renal glomerular and total renal corpuscle volumes were not different between treatment groups or between the sexes: total renal glomerular (mm3) (males: SAL, 9.3 ± 0.5; CORT, 9.3 ± 0.6; females: SAL, 10.5 ± 0.9; CORT, 10.8 ± 0.7); Total renal corpuscle volume (mm3) (males: SAL, 9.5 ± 0.6; CORT, 9.6 ± 0.6; females: SAL, 11.5 ± 0.7; CORT, 11.9 ± 0.8).
Blood pressure and heart rate at PN120
At PN120, MAP was significantly higher in both male and female offspring (Ptreatment < 0.001, Fig. 3A) of the CORT (male: 122 ± 1 mmHg; female: 122 ± 1 mmHg) than the SAL group (male: 114 ± 2 mmHg; female: 112 ± 1 mmHg). The effect of CORT treatment on MAP was similar in both sexes (Psex= 0.9, Psex × treatment= 0.8). There was no difference in heart rates between the treatment groups or sexes (Fig. 3B).
Figure 3.
Effect of prenatal CORT exposure at E14/15 on mean arterial pressure and heart rate in male and female offspring at PN120 A, mean arterial pressure; B, heart rate. ***P < 0.001, for treatment within male or female. SAL (open bars), n= 9 males, n= 6 females; CORT (hatched bars), n= 4 males; n= 6 females.
Plasma renin activity
No difference in PRA was observed between treatment groups or sexes. Male: SAL, 2.63 ± 0.45 ng Ang I ml−1 h−1; CORT, 2.54 ± 0.47 ng Ang I ml−1 h−1. Female: SAL, 2.57 ± 0.86 ng Ang I ml−1 h−1; CORT, 2.69 ± 0.28 ng Ang I ml−1 h−1.
Renal histology
No evidence of renal pathology was observed in the histological sections from any of the experimental groups. Glomeruli showed no signs of hypercellularity, sclerosis or enlargement. No evidence of renal interstitial fibrosis or cellular infiltration was observed. All renal blood vessels were normal (Fig. 4).
Figure 4.
Photomicrographs of representative kidney sections (5 μm paraffin sections) of PN120 offspring from the SAL- and CORT-treated groups A and B, SAL-treated group; C and D, CORT-treated group. A and C, low-power view (scale bar, 320 μm); B and D, high-power view (scale bar, 130 μm) of sections from PN120 SAL and CORT male offspring, respectively. No evidence of renal pathology was present in either treatment group.
Relative gene expression
AT1Ra, AT1Rb and AT2R expression during embryonic development
AT1Ra mRNA and AT2R mRNA expression in the CORT group was significantly elevated at E16, while AT1Rb mRNA was significantly lower (P < 0.05 in each case, Fig. 5A). The mRNA expression of AT1Rb remained downregulated at E20 (P < 0.05, Fig. 5B), while expression of AT1Ra and AT2R was similar in the SAL and CORT groups at E20 (Fig. 5B).
Figure 5.
Effect of maternal CORT treatment on relative expression of AT1Ra mRNA, AT1Rb mRNA, AT2R mRNA at E16, E20 and PN30, in males and females A, E16; B, E20; C, PN30 males; D, PN30 females. *P < 0.05; ***P < 0.001; SAL (open bars): E16, n= 5; E20, n= 8; PN30 male, n= 6; PN30 female, n= 6; CORT (hatched bars): E16, n= 7; E20, n= 7; PN30 male, n= 6; PN30 female, n= 6. All receptor expression is relative to a calibrator (see text).
AT1Ra, AT1Rb and AT2R expression at PN30
Expression of AT1Ra mRNA, AT1Rb mRNA and AT2R mRNA was significantly upregulated in PN30 male offspring following prenatal CORT exposure at E14/15 (P < 0.05 for AT1Ra, AT2R; P < 0.001 for AT1Rb; Fig. 5C). In PN30 female offspring, CORT treatment significantly upregulated the expression of AT1Rb mRNA (P < 0.05, Fig. 5D) and AT2R mRNA (P < 0.0001, Fig. 5D), but had no effect on expression of AT1Ra mRNA (Fig. 5D).
Sex-specific relative expression of AT1Ra, AT1Rb and AT2R mRNA at PN30
Relative expression of AT1Ra mRNA and AT1Rb mRNA was significantly higher in female than male offspring (Fig 6; P < 0.05, AT1Ra; P < 0.001, AT1Rb). No difference in expression of AT2R mRNA was observed between the sexes (Fig. 6).
Figure 6.
Relative expression of AT1Ra mRNA, AT1Rb mRNA, AT2R mRNA in SAL male versus SAL female offspring kidneys at PN30 ***P < 0.001; **P < 0.01; SAL: PN30 male, n= 6; PN30 female, n= 6. Males, open bars; females, filled bars.
AT receptor protein density at PN30
AT1 receptor protein abundance tended to be higher in male offspring from the CORT group (P= 0.065, Fig. 7A), although this difference was not statistically significant. AT1 and AT2 receptor protein abundance was significantly higher in female CORT offspring than in SAL offspring (P < 0.001 and P < 0.001, respectively, Fig. 7B).
Figure 7.
Effect of maternal CORT treatment on AT1R and AT2R protein density in male and female offspring kidneys, and representative Western blot for AT1R in males and AT2R in females at PN30 A, male offspring; B, female offspring; C, AT1R; D, AT2R; ***P < 0.001. SAL (open bars): male, n= 6; female, n= 6; CORT (hatched bars): male, n= 6; female, n= 5.
Discussion
We believe that this is the first report to demonstrate that a short prenatal exposure to elevated maternal CORT at an early period of metanephric development in the rat results in reduced nephron endowment and elevated arterial pressure in male and female offspring. Previous studies (Lindsay et al. 1996; Langley-Evans, 1997) using carbenoxolone to inhibit 11β-hydroxysteroid dehydrogenase-2, thus increasing fetal exposure to maternal CORT, have also reported elevated arterial pressure in the offspring. However these studies have administered carbenoxolone for either the entire pregnancy or longer durations of pregnancy. No study to reported to date has examined the effects of direct administration of CORT for a short duration on fetal/postnatal outcomes. Our findings in the presence of the natural glucocorticoid are very exciting, as the fetus is presumed to be protected, at least in part, from elevated maternal glucocorticoids by placental 11β-hydroxysteroid dehydrogenase-2 which inactivates the active corticosterone to the inert 11-dehydrocorticosterone (Seckl, 1997; Michael et al. 2003). However, even under ‘normal’ physiological conditions, approximately 20% of circulating maternal glucocorticoids cross the placental barrier (Michael et al. 2003). Our data show an increase in maternal plasma CORT concentration of approximately 150 ng ml−1 following CORT administration on E14 and E15. These elevated levels are similar to those reported by Giordano and colleagues (Giordano et al. 1996) following restraint stress. This suggests administration of CORT in this study resulted in elevated plasma CORT levels similar to those that would be seen in physiological stress. It is of interest that plasma levels in both the SAL and CORT groups were significantly higher on the second day of sampling. The reasons for this are unknown, but they may reflect increased release of endogenous corticosterone in response to the anticipated stress of a repeated tail bleed. Regardless of the higher levels, the increase in plasma CORT seen 30 min post injection in the CORT treatment group was similar on both days.
The association between reduced nephron number and development of hypertension is well documented (Brenner et al. 1988; Keller et al. 2003). It is hypothesized that a decrease in the number of nephrons results in a reduced filtration surface area, which in turn increases glomerular and systemic blood pressure, possibly through limiting sodium excretion and causing volume expansion. This increase in systemic pressure further impairs glomerular filtration and results in glomerular injury, and these parameters most often appear to be influenced by gender (Ortiz et al. 2001, 2003; Dodic et al. 2002a,b; Wintour et al. 2003; Moritz et al. 2004). Previous studies with maternal dexamethasone exposure at E15/16 resulted in a 30% reduction in nephron number in both male and female offspring; however, the degree of hypertension in the male offspring was higher than that of female offspring (Ortiz et al. 2001). In our study, the degree of nephron deficit (21% in males, 19% in females) or elevation in blood pressure is similar in both sexes. Of interest, glomerular size was affected by gender, as the male offspring from the CORT group exhibited signs of glomerular hypertrophy. Unlike in males, no significant compensatory glomerular hypertrophy was observed within the treatments in females, but, as discussed later, females from both the SAL and CORT groups had larger glomeruli than males. A decrease in nephron number often results in compensatory hypertrophy of the remnant glomeruli and would eventually cause glomerular hypertension and renal pathology (Brenner et al. 1988). In the present study, we observed no evidence of renal pathology in either male or female offspring at PN30 or PN120. Previously, Ortiz et al. (2003) reported glomerulosclerosis in 6- to 9-month-old male offspring following prenatal exposure to maternal dexamethasone. It is possible that a decrease in glomerular number and increase in glomerular size at PN30 in the presence of hypertension at PN120 may result in renal pathology in the offspring of this study at a later age.
Apart from a nephron deficit, low birth weight is often used as a predictor of future cardiovascular disease (Barker et al. 1993; Barker, 1999, 2002, 2003). Similar to findings in rats and sheep (Dodic et al. 1998; Ortiz et al. 2001), the present study reports no effect of maternal CORT treatment on body weight at any stage of development (E16, E20) or after birth (PN30, PN120) albeit reporting hypertension in the offspring. These results suggest that birth weight is a fairly crude measure and should not be used as a sole predictor of nephron number or risk of future disease.
The mechanisms resulting in the nephron deficit observed in models of developmental programming are not clearly understood. Studies by Moritz et al. (2002a) and Woods et al. (2001) have suggested that suppression of RRAS during nephrogenesis, may contribute towards the nephron deficit. In contrast, the present study demonstrates a nephron deficit whilst reporting an upregulation in expression of AT1Ra and AT2R at E16. However, this is likely to be a direct effect of maternal CORT exposure. The expression of AT1Rb is decreased, and this decrease in expression persists at E20, which is the active period of nephrogenesis in rats (Merlet-Benichou et al. 1994). Therefore, if suppression of RRAS contributes to nephron deficit, then it appears that in the present model, the AT1Rb may be mediating these effects. This is interesting as most renal effects of angiotensin II (Ang II) are reported to be mediated by AT1Ra rather than the AT1Rb subtype. However, studies on mouse knockouts have revealed that both receptors may be equally important for normal kidney development, but they also suggest redundancy of the receptor subtypes (Oliverio et al. 1998). Another possibility is that the upregulation of the AT2R may have had some growth restrictive effects, as it is documented to be an apoptotic mediator (Tufro-McReddie et al. 1995). In the present study upregulation in AT2R expression at E16 may have accelerated ongoing apoptosis of the differentiating mesenchyme, which is critical for nephron endowment. Exposure of the developing embryo to a maternal diet low in protein has also been reported to result in increased apoptosis of the mesenchyme (Welham et al. 2002), and recently an increase in expression of the proapoptotic gene Bax in the low protein model has been demonstrated (Welham et al. 2005). The net growth rate of the kidney is very tightly regulated, with mitotic and apoptotic rates of an equal 3% at E14.5 (Coles et al. 1993). This suggests that even slight alterations in pro-growth factors (such as AT1R) or antigrowth factors (such as AT2R) (Tufro-McReddie et al. 1995) in the ‘critical period’ may be enough to create an imbalance in growth of the rapidly developing kidney. Overall, these alterations in AT1Rb and AT2R expression observed in the present study may have restricted/inhibited growth and proliferation during metanephrogenesis and potentially contributed to the nephron deficit observed.
The upregulation of AT1R and AT2R at the gene level observed in the PN30 offspring in this study are generally consistent with other models of developmental programming (Woods & Rasch, 1998; Moritz et al. 2002a; Vehaskari et al. 2004; McMullen & Langley-Evans, 2005). However, as observed in the sheep (Moritz et al. 2002b) and rat models (Vehaskari et al. 2004), there seems to be alterations in the post-transcriptional activity of the genes as observed in the lack of correspondence between the levels of mRNA and protein. The present study demonstrates that the normal expression of these receptors is dependent on gender as expression of AT1Ra and AT1Rb is almost fivefold and twofold higher, respectively, in kidneys of normal (SAL) females than males. Furthermore, following CORT exposure, AT1Ra, AT1Rb and AT2R are upregulated in males whilst only AT1Rb and AT2R are increased in females. The full significance of these observations is unclear, but it suggests that there may be a sex-specific relationship between glomerular size and AT1Ra expression. As noted earlier, control female offspring have larger glomeruli than control male offspring. Furthermore, following prenatal exposure to CORT, the expression of AT1Ra is upregulated in male offspring at PN30 who exhibit an increase in glomerular size, whilst female offspring in whom AT1Ra expression is unaltered show insignificant changes in glomerular dimensions. It has to be noted that the location of the increased expression of AT1Ra was not determined in the current study. However, based on findings in sheep, it is thought to be glomerular (Moritz et al. 2002b). Antagonism of AT1R has been shown to reduce glomerular volume (Kett et al. 1996), suggesting that normal glomerular growth may be mediated by AT1R, but the receptor subtype was not stated in that study. Therefore, if the increased AT1Ra expression in this study is in glomeruli it may have led to the glomerular hypertrophy observed in males. We cannot discount that AT1Ra expression may be increased in the tubules, and this may have impaired Na+ excretion and resulted in the increased blood pressure in the offspring. Further studies are required to investigate this.
Conclusion
Maternal administration of the natural glucocorticoid CORT results in a reduced nephron number and hypertension in the offspring. Importantly, these outcomes are not influenced by birth weight. This strengthens the evidence suggesting that suboptimal maternal conditions may specifically impair organ development without affecting fetal growth. These findings may have major implications for normal birth weight babies born to women who experience stress during this early stage of pregnancy. The timing of CORT treatment for the stage of kidney development in this study is synonymous to that used in the sheep (Dodic et al. 2002b; Moritz et al. 2004) model and extrapolates to about the sixth week of pregnancy in humans (Vize et al. 1997; Moritz & Wintour, 1999). Finally, the altered expression of receptors of RRAS during development may have contributed to the nephron deficit, and the alterations at PN30 may have contributed to the hypertension seen in these animals. It must be recognized that kidney development is regulated by a number of genes, growth and transcription factors, not just components of RRAS. Therefore, the exact mechanism/s through which fetal exposure to maternal glucocorticoid result in a nephron deficit or programme for hypertension remains to be clearly defined. With the wide range of molecular approaches available in the rat, the next challenge in this field will be to identify these exact mechanisms.
Acknowledgments
This research was funded by a Monash University Small Grant. The authors thank Debbie Arena for help with real-time PCR and Jan Loose for help with the corticosterone assay. KM is supported by an NH&MRC RD Wright Fellowship.
References
- Barker D. Fetal origins of cardiovascular disease. Ann Med. 1999;31:3–6. doi: 10.1080/07853890.1999.11904392. [DOI] [PubMed] [Google Scholar]
- Barker D. Fetal programming of coronary heart disease. Trends Endocrinol Metab. 2002;13:364–368. doi: 10.1016/s1043-2760(02)00689-6. [DOI] [PubMed] [Google Scholar]
- Barker D. The developmental origins of adult disease. Eur J Epidemiol. 2003;18:733–736. doi: 10.1023/a:1025388901248. [DOI] [PubMed] [Google Scholar]
- Barker D, Gluckman P, Godfrey K, Harding J, Owens J, Robinson J. Fetal undernutrition and cardiovascular disease in adult life. Lancet. 1993;341:938–941. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- Barker D, Osmond C. Infant mortality, childhood undernutrition, and ischemic heart disease in England and Wales. Lancet. 1986;I:1077–1081. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- Bergstrom G, Johansson I, Stevenson KM, Kett MM, Anderson WP. Perindopril treatment affects both preglomerular renal vascular lumen dimensions and in vivo responsiveness to vasoconstrictors in spontaneously hypertensive rats. Hypertension. 1998;31:1007–1013. doi: 10.1161/01.hyp.31.4.1007. [DOI] [PubMed] [Google Scholar]
- Bertram J, Soosaipillai M, Ricardo S, Ryan G. Total numbers of glomeruli and individual glomerular cell types in the normal rat kidney. Cell Tissue Res. 1992;270:37–45. doi: 10.1007/BF00381877. [DOI] [PubMed] [Google Scholar]
- Brenner B, Garcia D, Anderson S. Glomeruli and blood pressure. Less one, more the other? Am J Hypertension. 1988;1:335–347. doi: 10.1093/ajh/1.4.335. [DOI] [PubMed] [Google Scholar]
- Coles H, Burne J, Raff M. Large scale normal cell death in the developing rat kidney and its reduction by epidermal growth factor. Development. 1993;118:777–784. doi: 10.1242/dev.118.3.777. [DOI] [PubMed] [Google Scholar]
- Cullen-McEwen LA, Kett MM, Dowling J, Anderson WP, Bertram JF. Nephron number, renal function, and arterial pressure in aged GDNF heterozygous mice. Hypertension. 2003;41:335–340. doi: 10.1161/01.hyp.0000050961.70182.56. [DOI] [PubMed] [Google Scholar]
- Dodic M, Abouantoun T, O’Connor A, Wintour E, Moritz K. Programming effects of short prenatal exposure to dexamethasone in sheep. Hypertension. 2002 a;40:729–734. doi: 10.1161/01.hyp.0000036455.62159.7e. [DOI] [PubMed] [Google Scholar]
- Dodic M, Hantzis V, Duncan J, Rees S, Koukoulas I, Johnson K, Wintour E, Moritz K. Programming effects of short prenatal exposure to cortisol. FASEB J. 2002 b;16:1017–1026. doi: 10.1096/fj.01-1045com. [DOI] [PubMed] [Google Scholar]
- Dodic M, May C, Wintour E, Coghlan J. An early prenatal exposure to excess glucocorticoid leads to hypertensive offspring in sheep. Clin Sci (Lond) 1998;94:149–155. doi: 10.1042/cs0940149. [DOI] [PubMed] [Google Scholar]
- Dodic M, Peers A, Coghlan J, May C, Lumbers E, Yu Z, Wintour E. Altered cardiovascular haemodynamics and baroreceptor-heart rate reflex in adult sheep after prenatal exposure to dexamethasone. Clin Sci (Lond) 1999;97:103–109. [PubMed] [Google Scholar]
- Giordano M, Vermeulen M, Trevani A, Dran G, Andonegui G, Geffner J. Nitric oxide synthase inhibitors enhance plasma levels of corticosterone and ACTH. Acta Physiol Scand. 1996;157:259–264. doi: 10.1046/j.1365-201X.1996.482222000.x. [DOI] [PubMed] [Google Scholar]
- Graciano M, Cavaglieri R, Delle H, Dominguez W, Casarini D, Malheiros D, Noronha I. Intra renin–angiotensin system is upregulated in experimental model of progressive renal disease induced by chronic inhibition of nitric oxide synthesis. J Am Soc Nephrol. 2004;15:1805–1815. doi: 10.1097/01.asn.0000131528.00773.a9. [DOI] [PubMed] [Google Scholar]
- Gundersen H, Bendtsen T, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard J, Pakkenberg B, Sorensen F, Vesterby A. Some new, simple and efficient stereological methods and their use in pathological and diagnosis. APMIS. 1988;96:379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- Keller G, Zimmer G, Mall G, Ritz E, Amann K. Nephron number in patients with primary hypertension. N Engl J Med. 2003;348:101–108. doi: 10.1056/NEJMoa020549. [DOI] [PubMed] [Google Scholar]
- Kett M, Alcorn D, Bertram J, Anderson W. Glomerular dimensions in spontaneously hypertensive rats: effects of AT1 antagonism. J Hypertension. 1996;14:107–113. [PubMed] [Google Scholar]
- Kett MM, Denton KM, Boesen EI, Anderson WP. Effects of early carvedilol treatment and withdrawal on the development of hypertension and renal vascular narrowing. Am J Hypertension. 2004;17:161. doi: 10.1016/j.amjhyper.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC. Maternal carbenoxolone treatment lowers birthweight and induces hypertension in the offspring of rats fed a protein-replete diet. Clin Sci. 1997;93:423–429. doi: 10.1042/cs0930423. [DOI] [PubMed] [Google Scholar]
- Lindsay R, Lindsay R, Edwards C, Seckl J. Inhibition of 11-beta-hydroxysteroid dehydrogenase in pregnant rats and the programming of blood pressure in the offspring. Hypertension. 1996;6:1200–1204. doi: 10.1161/01.hyp.27.6.1200. [DOI] [PubMed] [Google Scholar]
- McMullen S, Langley-Evans SC. Maternal low-protein diet in rat pregnancy programs blood pressure through sex-specific mechanisms. Am J Physiol Regul Integr Comp Physiol. 2005;288:R85–R90. doi: 10.1152/ajpregu.00435.2004. [DOI] [PubMed] [Google Scholar]
- Merlet-Benichou C, Gilbert T, Muffat-Joly M. Intrauterine growth retardation leads to a permanent nephron deficit in the rat. Pediatr Nephrol. 1994;8:175–180. doi: 10.1007/BF00865473. [DOI] [PubMed] [Google Scholar]
- Michael AE, Thurston LM, Rae MT. Glucocorticoid metabolism and reproduction: a tale of two enzymes. Reproduction. 2003;126:425–441. doi: 10.1530/rep.0.1260425. [DOI] [PubMed] [Google Scholar]
- Moritz K, Jefferies A, Douglas-Denton R, Wintour E, Dodic M. Reduced nephron number in the late gestation fetus after early maternal glucocorticoid treatment. Ninth International Workshop on Developmental Nephrology; September, 2004; Australia. Barossa Valley; [Google Scholar]
- Moritz K, Johnson K, Douglas-Denton R, Wintour E, Dodic M. Maternal glucocorticoid treatment programs alterations in the rennin–angiotensin system of the ovine fetal kidney. Endocrinology. 2002 a;143:4455–4463. doi: 10.1210/en.2002-220534. [DOI] [PubMed] [Google Scholar]
- Moritz KM, Johnson K, Douglas-Denton R, Wintour EM, Dodic M. Maternal glucocorticoid treatment programs alterations in the rennin–angiotensin system of the ovine fetal kidney. Endocrinology. 2002 b;143:4455–4463. doi: 10.1210/en.2002-220534. [DOI] [PubMed] [Google Scholar]
- Moritz K, Wintour E. Functional development of the meso-metanephros. Pediatr Nephrol. 1999;13:171–178. doi: 10.1007/s004670050587. [DOI] [PubMed] [Google Scholar]
- Nussberger J, Brunner D, BW, Brunner H. True versus immunoreactive angiotensin II in human plasma. Hypertension. 1985;7(suppl. 1):I1–I7. doi: 10.1161/01.hyp.7.3_pt_2.i1. [DOI] [PubMed] [Google Scholar]
- Oliverio M, Hyung-Suk K, Ito M, Thu L, Audoly L, Best C, Hiller S, Kluckman K, Maeda N, Smithies O, Coffman T. Reduced growth, abnormal kidney structure, and type 2 (AT2) angiotensin receptor-mediated blood pressure regulation in mice lacking both AT1A and AT1B receptors for angiotensin II. Proc Natl Acad Sci U S A. 1998;95:15496–15501. doi: 10.1073/pnas.95.26.15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz L, Quan A, Weinberg A, Baum M. Effect of prenatal dexamethasone on rat renal development. Kidney Int. 2001;59:1663–1669. doi: 10.1046/j.1523-1755.2001.0590051663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz L, Quan A, Zarzar F, Weinberg A, Baum M. Prenatal dexamethasone programs hypertension and renal injury in the rat. Hypertension. 2003;41:328–334. doi: 10.1161/01.hyp.0000049763.51269.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seckl JR. Glucocorticoids, feto-placental 11[beta]-hydroxysteroid dehydrogenase type 2, and the early life origins of adult disease. Steroids. 1997;62:89. doi: 10.1016/s0039-128x(96)00165-1. [DOI] [PubMed] [Google Scholar]
- Stevenson KM, Edgley AJ, Bergstrom G, Worthy K, Kett MM, Anderson WP. Angiotensin II infused intrarenally causes preglomerular vascular changes and hypertension. Hypertension. 2000;36:839–844. doi: 10.1161/01.hyp.36.5.839. [DOI] [PubMed] [Google Scholar]
- Tufro-McReddie A, Romano LM, Harris JM, Ferder L, Gomez RA. Angiotensin II regulates nephrogenesis and renal vascular development. Am J Physiol Renal Physiol. 1995;269:F110–F115. doi: 10.1152/ajprenal.1995.269.1.F110. [DOI] [PubMed] [Google Scholar]
- Vehaskari VM, Stewart T, Lafont D, Soyez C, Seth D, Manning J. Kidney angiotensin and angiotensin receptor expression in prenatally programmed hypertension. Am J Physiol Renal Physiol. 2004;287:F262–F267. doi: 10.1152/ajprenal.00055.2004. [DOI] [PubMed] [Google Scholar]
- Vize P, Seufert D, Carroll T, Wallingford J. Model systems for the study of kidney develpment: analysis of organ induction and patterning. Dev Biol. 1997;188:189–204. doi: 10.1006/dbio.1997.8629. [DOI] [PubMed] [Google Scholar]
- Welham SJM, Riley PR, Wade A, Hubank M, Woolf AS. Maternal diet programs embryonic kidney gene expression. Physiol Genomics. 2005;22:48–56. doi: 10.1152/physiolgenomics.00167.2004. [DOI] [PubMed] [Google Scholar]
- Welham S, Wade A, Woolf A. Protein restriction in pregnancy is associated with increased apoptosis of mesenchymal cells at the start of rat metanephrogenesis. Kidney Int. 2002;61:1231–1242. doi: 10.1046/j.1523-1755.2002.00264.x. [DOI] [PubMed] [Google Scholar]
- Wintour E, Dodic M, Matteo RD, McAlinden A, Jefferies A, Bartal D, Moritz K. Glucocorticoids and programming: evidence for different effects depending on the steroid used. Pediatr Res. 2005;58:1078. [Google Scholar]
- Wintour E, Moritz K, Johnson K, Ricardo S, Samuel C, Dodic M. Reduced nephron number in adult sheep, hypertensive as a result of prenatal glucocorticoid treatment. J Physiol. 2003;549:929–935. doi: 10.1113/jphysiol.2003.042408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods LL, Ingelfinger JR, Nyengaard JR, Rasch R. Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adult hypertension in rats. Pediatr Res. 2001;49:460–467. doi: 10.1203/00006450-200104000-00005. [DOI] [PubMed] [Google Scholar]
- Woods LL, Rasch R. Perinatal ANG II programs adult blood pressure, glomerular number, and renal function in rats. Am J Physiol Regul Integr Comp Physiol. 1998;275:R1593–R1599. doi: 10.1152/ajpregu.1998.275.5.R1593. [DOI] [PubMed] [Google Scholar]
- Woods LL, Weeks DA, Rasch R. Programming of adult blood pressure by maternal protein restriction: role of nephrogenesis. Kidney Int. 2004;65:1339–1348. doi: 10.1111/j.1523-1755.2004.00511.x. [DOI] [PubMed] [Google Scholar]