Abstract
The socioeconomic implications of trypanosomosis in sub-Saharan Africa and the limitations of its current control regimes have stimulated research into alternative control methods. Considering the pro- and anti-inflammatory properties of transforming growth factor β1 (TGF-β1) and its potential to enhance immunity against protozoan parasites, we examined the effects of intraperitoneally delivered TGF-β1 in C57BL/6 mice infected with Trypanosoma congolense, the hemoprotozoan parasite causing nagana in cattle. A triple dose of 10 ng TGF-β1 significantly reduced the first parasitemic peak and delayed mortality of infected mice. Furthermore, exogenous TGF-β1 significantly decreased the development of trypanosome-induced anemia and splenomegaly. The apparent TGF-β1-induced antitrypanosome protection, occurring mainly during the early stage of infection, correlated with an enhanced parasite antigen-specific Th1 cell response characterized by a skewed type I cytokine response and a concomitant stronger antitrypanosome immunoglobulin G2a antibody response. Infected TGF-β1-pretreated mice exhibited a significant reduction in the trypanosome-induced hyperexpansion of B cells. Furthermore, evidence is provided herein that exogenous TGF-β1 activates macrophages that may contribute to parasite control. Collectively, these data indicate that exogenous TGF-β1 is immunostimulative, inducing partial protection against T. congolense infection, possibly through mechanisms involving innate immune responses.
African trypanosomosis is a tsetse-transmitted, chronic debilitating disease of humans and livestock in sub-Saharan Africa. It is caused by various species of extracellular hemoflagellate protozoan parasites, including Trypanosoma congolense, which causes nagana in cattle, and T. brucei species causing human sleeping sickness. Both human trypanosomosis and animal trypanosomosis have socioeconomic repercussions such that the disease remains a major obstacle to the overall development of the affected regions (13). The trypanosome's ability to constantly modify its variant surface glycoproteins has severely hampered the design of an effective antitrypanosome vaccine (28, 37). Moreover, current treatment and control regimes against trypanosomosis are costly and unsustainable (37). This has stimulated research into alternative control/preventive regimes in an attempt to serve the millions of people and livestock at risk of contracting trypanosomosis.
Several cytokines and their antagonists have potential in both human and veterinary medicine. Although the interferon (IFN) family has been used more extensively over the last 2 decades (5), there is a need to expand the study of the protective potential that other groups of cytokines may provide where the IFNs have been less effective. Furthermore, the mechanisms of action of cytokine therapy remain unclear.
Transforming growth factor β1 (TGF-β1), a pleiotropic cytokine having both stimulatory and suppressive effects on the immune response (26), could be one such candidate. Produced by a wide range of cells, TGF-β1 has both pro- and anti-inflammatory properties, depending on its environment and concentration (1, 42). Important proinflammatory properties of TGF-β1 include its ability to recruit monocytes, T cells, and neutrophils to the site of inflammation early in infection (43). TGF-β1 therapy has also been shown to exert enhancing systemic effects on interleukin-12 (IL-12) production and NK cell activities (1, 11). TGF-β1 may thus enhance innate/acquired immunity against hemoprotozoan parasites such as trypanosomes. Moreover, TGF-β1 could be critical in maintaining the balance between the control and clearance of organisms on the one hand and the prevention of immune-mediated pathology on the other (6, 26). The present study is thus aimed at evaluating the protective ability of exogenous TGF-β1 against murine African trypanosomosis.
MATERIALS AND METHODS
Animals and parasites.
Two-month-old female C57BL/6 mice (Nihon CLEA Inc., Tokyo, Japan) were inoculated intraperitoneally (i.p.) with the IL-1180 strain of T. congolense, previously obtained from the International Livestock Research Institute (Nairobi, Kenya) and maintained in our institute. All animal experiments were conducted in accordance with the standards relating to the Care and Management of Experimental Animals of Obihiro University of Agriculture and Veterinary Medicine (Hokkaido, Japan).
Experimental design.
For each experiment, 20 C57BL/6 mice were divided into two equal groups. One group was treated i.p. with 200 μl of different concentrations of recombinant human TGF-β1 (Sigma, Saint Louis, MS) dissolved in phosphate-buffered saline (PBS), initially as a single dose on day −5 postinfection (p.i.) and in subsequent studies as a triple dose on days −5, −1, and +3 p.i. The bioactivity of TGF-β1 was confirmed by a growth inhibition assay of Mv.1.Lu mink lung epithelial cells as previously described (27). Control mice were treated with 200 μl PBS.
On day 0 p.i., each mouse was inoculated i.p. with 2,000 T. congolense parasites. Parasitemia, morbidity development in mice, and survival rates were monitored in the initial series of experiments. These included one single-dose study followed by two parallel studies of single and triple doses. Weight gain, packed cell volume (PCV), and other parameters (see below) were also monitored in five independent triple-dose experiments. At various times p.i., immune parameters were quantified in splenocytes (SPC), sera, or peritoneal exudate cells (PEC) of three TGF-β1-pretreated mice and three control animals. Additionally, the effect of triple-dose TGF-β1 was examined in three independent experiments following subcutaneous inoculation of parasites into mice.
For each parameter, the pooled results of all similar experiments performed were expressed as the mean responses of infected mice (±standard errors [SE]) compared to the same parameters assessed for noninfected mice. Statistical analysis was performed by two-tailed Student's t test to validate the data. P values of <0.05 were considered statistically significant.
Preparation of soluble parasite antigens for enzyme-linked immunosorbent assay (ELISA).
At peak parasitemia, whole blood was collected from mice by cardiac puncture and the parasites were purified using DE52 anion-exchange column chromatography (Whatman, Brantford, United Kingdom) as previously described (14). The purified parasites were washed three times with PBS and disrupted by four cycles of freeze-thawing to obtain total parasite lysates (TPL) after sonication and centrifugation.
Serum collection and cell preparation.
At different times following infection, blood samples collected by heart puncture were centrifuged (10,000 × g at 4°C for 10 min), and serum samples were stored at −80°C until use. SPC and PEC single-cell suspensions were prepared as previously described (2).
Quantification of cytokine mRNA.
Total RNA was extracted from TRIzol (Invitrogen, Carlsbad, CA)-homogenized plastic-adherent or unfractioned PEC or SPC from untreated, PBS-pretreated, or TGF-β1-pretreated uninfected mice or from infected TGF-β1-pretreated or control mice according to the manufacturer's suggested protocol and kept at −80°C until use. Specific primer pairs for mouse IFN-γ, tumor necrosis factor alpha (TNF-α), inducible nitric oxide synthase (iNOS), IL-2, IL-4, IL-10, IL-12p40, TGF-β1, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) for reverse transcriptase PCR (RT-PCR) analysis of mRNA expression were purchased from R&D Systems (Abingdon, United Kingdom). RT-PCR was performed using a one-step RT-PCR kit (TaKaRa Biomedicals, Shiga, Japan) according to the manufacturer's instructions, employing a 35-cycle program of denaturation at 94°C for 45 s, annealing at 55°C for 45 s, and extension at 72°C for 45 s. The PCR products were harvested and resolved on a 1.5% agarose gel containing ethidium bromide and visualized by UV light.
Cell cultures for soluble cytokine quantification.
At different times following infection, 2 × 106 SPC were cultured with or without 50 μg/ml TPL in 1 ml (24-well plates) of RPMI 1640 medium (Sigma) supplemented with 10% fetal bovine serum and 100 U penicillin-100 μg/ml streptomycin. Cultures were incubated at 37°C in a humidified atmosphere containing 5% CO2 and culture supernatants collected after 24 to 96 h and frozen at −20°C until analysis.
Measurement of soluble cytokines.
Cytokines were quantified in sera or cell culture supernatants by specific ELISA kits from Endogen (Rockford, IL) for IFN-γ or from R&D Systems (Minneapolis, MN) for IL-10 and TGF-β1 following the manufacturers' protocols. The TGF-β1 ELISA kit measures both bioactive and total/latent peptides.
NK cell cytotoxic activity.
NK cell cytotoxic activity was determined by a nonradioactive method as previously described (22), using nonadherent SPC from untreated uninfected mice and from infected TGF-β1-pretreated and control mice as effector NK cell sources and YAC-1 target cells at 40:1, 20:1, and 10:1 effector/target (E:T) ratios. At every E:T ratio, triplicate wells of effector and target cells and triplicate wells of the effector cells alone were established in parallel. Eight replicates of the target cells alone, as well as four wells of media alone, were established for every experiment to determine the background. A total of 10 μl alamarBlue (Biosource International, Camarillo, CA) was added to each well, after which plates were incubated for 24 h at 37°C in a humidified atmosphere containing 5% CO2. The optical density (OD) of the alamarBlue-supplemented cell culture medium was read at 570-nm and 600-nm wavelengths. The percent specific lysis was calculated as follows: 100 × [AA of targets alone − (AA of mix − AA of effectors alone)] × (AA of targets alone)−1, where AA represents the mean of absolute OD for triplicate wells minus the average OD of the media alone.
Quantification of antibody isotypes.
ELISA plates were coated with T. congolense IL-1180 TPL or with bovine serum albumin (BSA) (10 μg/ml, 4°C, overnight). Plates were washed (0.05% Tween 20 in PBS) and blocked with 3% skim milk in PBS (1 h, 37°C). Following a 1-h incubation (37°C) of plates with serum samples diluted 1/100 in the blocking buffer and subsequent washing, biotin-conjugated rat anti-mouse isotype-specific antibodies (BD PharMingen, Japan) were added (1 h, 37°C). After further washings, plates were incubated (30 min, 37°C) with streptavidin-horseradish peroxidase conjugate (BD PharMingen) and washed and the assay was developed by adding 2,2′-azinobis(2-ethylbenzthiazolinesulfonic acid (ABTS; Sigma). For each sample, the OD (at 490 nm) determined on BSA-coated plates was subtracted from the OD value obtained on TPL-coated plates. Preliminary experiments showed that serum samples diluted 1/100 had an OD in the linear zone of a serial dilution curve.
Flow cytometric analysis.
Nonadherent SPC were stained directly after isolation using the following primary monoclonal antibodies: phycoerythrin-conjugated anti-CD4 (clone GK1.5, immunoglobulin G2b [IgG2b]) (PharMingen), phycoerythrin-conjugated anti-NK1.1 (clone PK136, IgG2a) (ImmunoTech, France), fluorescein isothiocyanate (FITC)-conjugated anti-CD8 (clone KT15, IgG2a), FITC-conjugated anti-CD25 (clone 7D4, IgM), and FITC-conjugated anti-CD19 (clone 6D5, IgG2a) (Chemicon International, Germany). After incubation, cells were washed, analyzed, and fluorescence quantified using a Coulter EPICS-XL flow cytometer (Beckman Coulter Inc., Fullerton, CA).
In vitro antitrypanosome assays.
To test whether TGF-β1 had any direct effects against parasites, about 105/ml DE52-purified bloodstream forms of trypanosomes, obtained from infected mice around peak parasitemia, were cocultured in twofold serially diluted TGF-β1 (500 ng/ml to 31.25 ng/ml) in Eagle's minimum essential medium (EMEM; Sigma) containing 2 mg/ml BSA in 25-cm2 culture flasks (Nunc A/S, Roskilde, Denmark). As a positive control, a twofold serial dilution (10% to 0.625%) of normal human serum (NHS) was used (25), while EMEM-BSA alone was used as a negative control. Cultures were incubated at 33°C for 2 h in a humidified atmosphere containing 5% CO2 and viable parasites counted by microscopy.
RESULTS
TGF-β1 therapy reduces parasitemia and increases the survival rate of T. congolense-infected mice.
In a preliminary experiment, we treated C57BL/6 mice i.p. with a single dose of 5 ng to 100 ng TGF-β1, challenged them with T. congolense 1 to 7 days posttreatment, and monitored the development of parasitemia. The antitrypanosome effect of TGF-β1 treatment was more apparent when animals were challenged 5 days following treatment. In the range of 5 ng to 20 ng, TGF-β1 therapy significantly reduced the first parasitemic peak (P < 0.05) compared to controls (Fig. 1A). In contrast, 100 ng TGF-β1 showed no effect. Further experiments showed that a 10-ng triple dose of TGF-β1 (days −5, −1, and +3 p.i.) produced maximal response and was used in the rest of the experiments. As shown in Fig. 1B, this greatly reduced the first parasitemic peak compared to controls (P < 0.001), whereas the difference in subsequent peaks was not significant. Figure 1C further shows that compared to controls, TGF-β1-pretreated mice had delayed mortality (P < 0.05) while about 33% of control mice failed to resolve the first parasitemic peak and died. The results were similar when parasites were inoculated subcutaneously (data not shown), suggesting that exogenous TGF-β1 induces systemic beneficial effects.
FIG. 1.
Effects of exogenous TGF-β1 on parasitemia, development of pathology, and survival rate of T. congolense-infected mice. Female C57BL/6 mice were pretreated i.p. with a single dose of 5 to 100 ng TGF-β1 on day −5 p.i. (A) or with triple doses of 10 ng TGF-β1 on days −5, −1, and +3 p.i. (B and C), while control animals received PBS. On day 0, mice were challenged with T. congolense via the i.p. route. (D) To test whether TGF-β1 had any direct effects against parasites, about 105/ml DE52-purified bloodstream forms of trypanosomes were cocultured in 500 ng/ml TGF-β1 or 10% NHS diluted in EMEM containing 2 mg/ml BSA. Levels of parasites in blood (A and B) or culture medium (D), the survival rate (C), and the development of anemia (E) and splenomegaly (F) were monitored at different times p.i. Statistical analysis was performed by two-tailed Student's t test, with P values of <0.05 considered statistically significant. Dashed horizontal lines represent average PCV levels and spleen weights in noninfected mice or viable parasite count in untreated medium. *, statistically significant difference compared to noninfected mice; #, statistically significant difference between infected TGF-β1- and PBS-pretreated mice; $, statistically significant difference compared to PBS cocultures.
The possible direct effects of TGF-β1 on the parasites were tested in an in vitro system. Figure 1D shows representative results for cocultures of parasites and 500 ng/ml TGF-β1. The viable parasite count in nontreated control cultures was about 93,000 ± 10,000/ml. In agreement with previous reports (44), the parasite count was significantly reduced in the presence of 10% NHS (P < 0.05). However, TGF-β1 did not affect the parasite count, suggesting that it has no direct effect on trypanosomes.
Exogenous TGF-β1 reduces pathology in T. congolense-infected mice.
We examined whether the protection of TGF-β1-pretreated mice had any ameliorating effect on trypanosome-induced pathology. The PCV of noninfected control mice was 55.0% ± 2.3%, while the spleen weight was 0.10 ± 0.01 g. As shown in Fig. 1E, infected control mice developed pronounced anemia during early-stage infection (week 2 p.i.) compared to TGF-β1-pretreated (P < 0.05) or noninfected (P < 0.01) mice. Infected control mice also developed significant splenomegaly compared to TGF-β1-pretreated or noninfected mice (P < 0.05) during early-stage infection (Fig. 1F). However, during late-stage disease (week 4 p.i.), both infected groups developed severe anemia (P < 0.01) and splenomegaly (P < 0.01), respectively, compared to noninfected mice.
TGF-β1 therapy correlates with a reduction in parasite-induced hyperexpansion of B cells.
Levels of specific lymphocyte populations in nonadherent SPC were analyzed on day 9 p.i. As shown in Table 1, compared to levels in SPC from untreated uninfected mice, absolute numbers of NK1.1+ cells remained unaltered while those of CD8+ cells tended to be lower in SPC from infected TGF-β1-pretreated and infected control mice. Interestingly, while a tendency towards a reduction in CD4+ cells was observed in SPC from infected control mice, that cell population remained at baseline levels in SPC from infected TGF-β1-pretreated mice (Table 1). Moreover, a profound increase in CD19+ cells was observed in SPC from infected control mice (P < 0.01) and to a lesser extent in SPC from infected TGF-β1-pretreated mice (P < 0.05) compared to preinfection levels. Similarly, SPC from both infected control and infected TGF-β1-pretreated mice had higher numbers of CD4+CD25+ cells than SPC from untreated uninfected mice (P < 0.05). However, it is noteworthy that compared to those in SPC from infected control mice, CD19+ (P < 0.01) and CD4+CD25+ (P < 0.05) cell populations in SPC from infected TGF-β1-pretreated mice were smaller.
TABLE 1.
Flow cytometric analysis of surface phenotypes of nonadherent SPC from uninfected and T. congolense-infected (peak parasitemia) mice pretreated or not treated with TGF-β1
| Surface molecule | Absolute no. of cells (106) ina:
|
||
|---|---|---|---|
| SPC-N | SPC-I | SPC-βI | |
| CD4+ | 19.6 ± 2.0 | 15.2 ± 1.8 | 18.7 ± 1.3 |
| CD8+ | 9.6 ± 1.0 | 6.4 ± 0.6 | 5.5 ± 1.2 |
| CD4+CD25+ | 0.9 ± 0.1 | 1.8 ± 0.1b | 1.4 ± 0.1c,d |
| CD19+ | 36.7 ± 2.4 | 89.6 ± 1.0e | 53.2 ± 1.6f,g |
| NK1.1+ | 3.8 ± 0.9 | 4.1 ± 0.2 | 3.9 ± 0.2 |
Pooled data from five independent experiments, expressed as means ± SE, are shown. SPC-N, SPC from untreated uninfected mice; SPC-I, SPC from infected control mice; SPC-βI, SPC from infected TGF-β1-pretreated mice.
Higher (P < 0.05) than that for SPC-N.
Higher (P < 0.05) than that for SPC-N.
Lower (P < 0.05) than that for SPC-I.
Higher (P < 0.01) than that for SPC-N.
Higher (P < 0.05) than that for SPC-N.
Lower (P < 0.01) than that for SPC-I.
TGF-β1 pretreatment enhances NK cell activity in noninfected mice but does not further modulate NK cell activity in T. congolense-infected mice.
Compared to NK cells from untreated or PBS-treated mice, NK cells from TGF-β1-treated noninfected animals tended to exhibit increased cytotoxic activity (Fig. 2A). Furthermore, as shown in Fig. 2B and C, infection progressively increased the NK cytotoxic activity to similar extents in both TGF-β1-pretreated and control animals compared to that in noninfected mice. Thus, although a tendency towards increased NK cytotoxic activity in both infected groups was observed during early-stage infection (Fig. 2B), that effect became more apparent and significant (P < 0.05) during late-stage disease (Fig. 2C).
FIG. 2.
Effects of exogenous TGF-β1 on NK cell activity in T. congolense-infected mice. NK cell cytotoxic activity was measured in TGF-β1-pretreated mice by using alamarBlue dye to assess cell viability during preinfection (A), early (week 2 p.i.) (B), and late (week 4 p.i.) (C) stages of infection. Pooled data from five independent experiments are expressed as the mean cytotoxicities of the nonadherent spleen cells from untreated (SPC-N), PBS-pretreated (SPC-PBS), and TGF-β1-pretreated (SPC-β) uninfected mice or from infected PBS-pretreated (SPC-I) or infected TGF-β1-pretreated (SPC-βI) mice against YAC-1 target cells at the indicated E:T ratios (±SE). Statistical analysis was performed by two-tailed Student's t test, with P values of <0.05 considered statistically significant. *, statistically significant difference compared to noninfected mice.
TGF-β1 pretreatment correlates with a type-I-skewed cytokine response in the spleen and peritoneal cavity of mice.
Since exogenous TGF-β1 was delivered i.p., we analyzed the cytokine mRNA expression in PEC following 10 ng TGF-β1 administration. TGF-β1 induced increased expression of iNOS, TNF-α, and IL-12p40 mRNA in adherent PEC from TGF-β1-pretreated uninfected mice compared to that in adherent PEC from PBS-pretreated or untreated uninfected mice (Fig. 3A). When unfractioned PEC were used, IFN-γ mRNA was also increased in PEC from TGF-β1-pretreated uninfected mice (data not shown).
FIG. 3.
Effects of exogenous TGF-β1 on cytokine levels in the spleen compartment and peritoneal cavity. The mRNA expression levels of GAPDH and type I (IFN-γ, TNF-α, and IL-12p40) and type II (IL-4, IL-10, and TGF-β1) cytokines were determined in adherent PEC (A) or SPC (B) from untreated (lane 1), PBS-pretreated (lane 2), or TGF-β1-pretreated (lane 3) noninfected mice or from noninfected (lane 1), infected PBS-pretreated (SPC-I) (lane 2), or infected TGF-β1-pretreated (SPC-βI) (lane 3) mice on day 9 p.i. (C) by RT-PCR. The product size of each gene is shown in parentheses. Secreted bioactive TGF-β1 (D), IL-10 (E), and IFN-γ (F) levels in culture supernatants of SPC or IFN-γ/IL-10 ratios (G), in the presence or absence of TPL, were quantified by ELISA. Statistical analysis was performed by two-tailed Student's t test, with P values of <0.05 considered statistically significant. Pooled data from five independent experiments are expressed as means ± SE. Dashed horizontal lines represent average cytokine levels in noninfected mice. #, statistically significant difference between infected TGF-β1- and PBS-pretreated mice.
The expression of cytokine mRNA was also examined in the spleen to verify possible systemic effects of exogenous TGF-β1. TGF-β1 treatment strongly up-regulated the expression mainly of IL-12p40 in adherent SPC from TGF-β1-pretreated uninfected mice (Fig. 3B). When unfractioned SPC were used, IL-2 mRNA was also increased in SPC from TGF-β1-pretreated uninfected mice (data not shown).
Cytokine mRNA expression levels in unfractioned SPC were also analyzed on day 9 p.i. Figure 3C shows that except for TGF-β1, there were hardly any detectable cytokine transcripts in SPC from untreated uninfected mice. SPC from both infected control and infected TGF-β1-pretreated mice had higher type I cytokine (IFN-γ and TNF-α) mRNA levels than SPC from untreated uninfected mice. Compared to SPC from TGF-β1-pretreated uninfected mice, those from infected control mice exhibited higher mRNA levels for both type I and type II (IL-4, IL-10, and TGF-β1) cytokines, while IL-10 and TGF-β1 transcripts in SPC from infected TGF-β1-pretreated mice remained roughly at baseline levels.
We also examined the profile of secreted cytokines in SPC cultures. SPC from untreated uninfected mice did not produce detectable levels of bioactive TGF-β1, IL-10, or IFN-γ spontaneously or after stimulation with TPL (Fig. 3D to G). However, while SPC from early-stage infected control mice produced moderate amounts of bioactive TGF-β1, SPC from infected TGF-β1-pretreated mice had no detectable levels. SPC from infected control mice tended to spontaneously produce larger amounts of both IL-10 and IFN-γ (Fig. 3E and F) during early-stage infection than SPC from infected TGF-β1-pretreated mice. Following stimulation with TPL, SPC from infected control mice further secreted higher IL-10 but lower IFN-γ levels than SPC from infected TGF-β1-pretreated mice (P < 0.05). As illustrated in Fig. 3G, a net type-I-skewed cytokine response, characterized by a higher IFN-γ/IL-10 ratio (P < 0.05), was observed in cultures of SPC from infected TGF-β1-pretreated mice during early-stage infection, whereas cultures of SPC from infected control mice exhibited a type II cytokine-inclined response (lower IFN-γ/IL-10 ratio). During late-stage infection, cytokine levels in both groups were comparable to baseline levels (not shown).
TGF-β1 pretreatment correlates with a net type-I-skewed response in sera of T. congolense-infected mice.
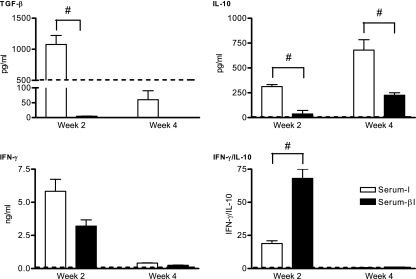
The amount of bioactive TGF-β1 in sera from noninfected mice (Serum-N) was 500.0 ± 90.3 pg/ml. Bioactive TGF-β1 levels in sera from infected controls (Serum-I) tended to be higher in early-stage infection and lower during late-stage infection than those in Serum-N (Fig. 4). During early-stage infection, Serum-I had higher levels of bioactive TGF-β1 than sera from TGF-β1-pretreated mice (Serum-βI) (P < 0.01).
FIG. 4.
Effects of exogenous TGF-β1 on serum cytokine responses in T. congolense-infected mice. At week 2 and week 4 p.i., TGF-β1, IL-10, and IFN-γ levels as well as IFN-γ/IL-10 ratios in Serum-N, Serum-I, and Serum-βI were quantified by ELISA. Statistical analysis was performed by two-tailed Student's t test, with P values of <0.05 considered statistically significant. Pooled data from five independent experiments are expressed as means ± SE. Dashed horizontal lines represent average cytokine levels in noninfected mice. #, statistically significant difference between infected TGF-β1- and PBS-pretreated mice.
Figure 4 further shows that Serum-I had higher levels of IL-10 than Serum-βI during early- and late-stage infections (P < 0.05). Moreover, during early-stage infection, Serum-I tended to have higher IFN-γ levels than Serum-βI, although both groups exhibited similar trace IFN-γ levels only during late-stage infection. Thus, as observed in the spleen, during early-stage infection, Serum-βI exhibited a net type I cytokine response.
Exogenous TGF-β1 induces increased IgG2a antibody titers during the early stage of T. congolense infection.
Serum levels of parasite-specific antibody isotypes were quantified at 2 weeks p.i. While control mice had higher IgM levels (P < 0.05), TGF-β1-pretreated mice mounted a stronger anti-TPL IgG2a antibody response (P < 0.05), whereas IgG1 levels remained lower in both groups (Fig. 5). Consequently, a higher IgG2a/IgG1 ratio was observed in Serum-βI (P < 0.05) than in Serum-I, further indicating a type-I-inclined response in the former and a type II response in the latter.
FIG. 5.
Effects of exogenous TGF-β1 on anti-T. congolense serum antibody production in infected mice. At week 2 p.i., anti-TPL-specific antibody isotypes (IgM, IgG1, and IgG2a) or IgG2a/IgG1 ratios were determined by ELISA, using Serum-N, Serum-I, and Serum-βI. Statistical analysis was performed by two-tailed Student's t test, with P values of <0.05 considered statistically significant. Pooled data from five independent experiments are expressed as means ± SE. Serum-N had no detectable antibody titers. #, statistically significant difference between infected TGF-β1- and PBS-pretreated mice.
DISCUSSION
We have demonstrated that exogenous TGF-β1 induces temporary protection against T. congolense infection in mice. Indeed, in agreement with previous reports that correlate the survival of T. congolense-infected mice with their ability to control the first parasitemic peak (24), treatment of mice with TGF-β1 retarded the rate of parasite proliferation and prolonged their survival. Exogenous TGF-β1 seems to induce beneficial systemic effects mainly during the early phase of T. congolense infection, as described for other infection models (26, 33). During that period, TGF-β1-pretreated mice effectively controlled the development of pathology. Moreover, these mice had a delayed onset of clinical trypanosomosis compared with controls (data not shown). Exogenous TGF-β1 has also been reported to induce similar effects against murine malaria (27). Lower TGF-β1 doses (5 ng) produced maximal benefits, higher doses (20 ng) were less effective, and in another study (38), very high doses (10 μg) exacerbated the infection. Similarly, in our model, 5 to 20 ng TGF-β1, which induced proinflammatory effects, conferred partial protection whereas higher doses (100 ng), possibly with anti-inflammatory effects, conferred no benefit. This highlights the pleiotropic nature of TGF-β1 and further shows that TGF-β1 operates effectively within a narrow range.
How might TGF-β1 induce protection against trypanosomosis? Considering that most of the beneficial effects induced by TGF-β1 were observed during early-stage infection and that TGF-β1 had no direct toxic effects on trypanosomes, it is conceivable that innate responses could be particularly important in this model. Indeed, the contribution of innate immune responses to resistance during African trypanosomosis was recently reported (7). Following infection with African trypanosomes, the induction of an innate immune response is thought to be mediated by interactions between parasite molecules and several Toll-like receptors that signal via the MyD88 pathway, leading to an early type 1 immune response and a resistance phenotype. In the present study, the induction of an innate immune response could be suppressed in infected control mice whereas pretreatment with TGF-β1 may augment it. Indeed, we observed that exogenous TGF-β1 led to increased expression of proinflammatory and related mediators (TNF-α, iNOS, IL-12p40, and IFN-γ) at the delivery site and in the spleen, indicating classical macrophage (MΦ) activation (19). Such MΦs could contribute to parasite elimination by phagocytosis (35) or through the secretion of trypanotoxic molecules, including TNF-α (16) and nitric oxide (41). Indeed, the induction of phagocytic cell chemotaxis and enhanced MΦ phagocytic activities early in infection by exogenous TGF-β1 has previously been documented (26, 43).
The induction of a parasite antigen-specific Th1 cell response in vitro and a net type I cytokine environment in vivo by TGF-β1 pretreatment was observed in early-stage-infected mice. This is in agreement with recent reports indicating that while TGF-β1 inhibits the development of IL-4-producing cells, it also enhances the development of IFN-γ-producing cells (8, 33). The resultant proinflammatory environment could contribute to parasite control (7, 17) and may explain why TGF-β1-pretreated mice exhibit lower parasitemia and reduced pathology. Indeed, type I cytokines, including IFN-γ, TNF-α, and IL-12, have been reported to contribute to resistance in murine T. congolense (23, 40) and other trypanosome infection models (10, 20, 29). In particular, type I cytokines and classically activated MΦs have been documented to be important in the control of the initial and most aggressive parasitemic waves (19).
Of particular interest was the level of TGF-β1 in pretreated and control mice. While partially protected TGF-β1-pretreated mice had no detectable bioactive TGF-β1 throughout the infection, susceptible control animals exhibited enhanced bioactive TGF-β1 production in vitro and in vivo during early-stage infection. Thus, trypanosomes might deliberately elicit increased production of bioactive TGF-β1 to induce immunosuppression (21, 26, 30, 34), possibly for their own survival.
Through signaling molecules such as IL-12, MΦs and/or dendritic cells may activate other cells, including NK cells and γδ T cells. The direct cytotoxic role of activated NK cells on African trypanosomes remains unclear (12). Nevertheless, activated NK cells may be one of the early sources of IFN-γ which could further activate antitrypanosome activities of MΦs as in other infection models (15, 32, 36, 39). In almost all protozoan diseases where NK cells have been reported to play a protective role, they seem to exert their effect through the cytokine secretory pathway rather than their direct lytic activity (12). In this study, evidence is provided that (i) NK cytotoxic activity is enhanced and (ii) there is increased expression of IFN-γ following TGF-β1 therapy, prior to infection. Such activated NK cells may also secrete cytokines (12). We cannot exclude the possibility that these cells contribute to the relative resistance induced by TGF-β1 pretreatment, at least at the very beginning of the infection. However, shortly after the infection is established, the antitrypanosome role of NK cells no longer seems significant.
Adaptive responses do not appear to be very effective in this model since most of the beneficial effects in TGF-β1 pretreated mice are no longer apparent following the clearance of the first parasitemic wave. This study, however, further supports the contribution of humoral responses against trypanosomosis (4, 18, 31, 40). The profound increase in CD19+ B cells in infected control mice herein is in agreement with previous reports and is thought to be a result of polyclonal B-cell activation by a trypanosome mitogen-like molecule analogous to endotoxin (9). Such polyclonal B cells produce mainly polyspecific IgM antibodies, which are associated with trypanosusceptibility (3, 9, 40). In agreement with this, control mice had higher IgM antibody titers while TGF-β1-pretreated mice, with reduced B-cell hyperexpansion, exhibited higher trypanosome-specific IgG2a antibody titers. Increased levels of IgG2a in those mice, paralleled by a concomitant reduction in IgG1 titers, fit with their elevated IFN-γ levels and a net type I cytokine environment (31). Of note, IgG2a has been closely linked with resistance in T. congolense-infected mice (40).
Although lower levels of TGF-β1 are associated with beneficial inflammatory responses against some protozoan parasites, the production of TGF-β1 above physiological levels has been reported to reduce resistance to leishmaniasis, toxoplasmosis, and Chagas' disease (38). Increased levels of circulating TGF-β1 are also associated with hepatic cirrhosis, autoimmune diseases, systemic lupus erythematosis, human immunodeficiency virus type 1 infection, arthritis, and tumorigenesis (42, 43). Moreover, caution should be exercised in the design of therapeutic trials of TGF-β1 since prolonged treatment may lead to liver fibrosis and glomerulosclerosis (33).
In conclusion, this study supports the idea that TGF-β1 is pleiotropic, inducing temporary protection against murine T. congolense infection at lower doses while relatively higher doses do not confer any beneficial effects. Exogenous TGF-β1 may exert this effect mainly through innate mechanisms, possibly involving MΦs and NK cells.
Acknowledgments
B.N. is supported by a research grant fellowship from the Japanese Society for the Promotion of Science (JSPS) for young scientists. This study was also supported by (i) a grant-in-aid for scientific research to N.I. and C.S. from the JSPS, (ii) a grant-in-aid for the 21st Century COE program from the JSPS, and (iii) the Program for Promotion of Basic Research Activities for Innovative Biosciences (PROBRAIN), Japan, to N.I.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 29 January 2007.
REFERENCES
- 1.Bellone, G., M. Aste-Amezaga, G. Trinchieri, and U. Rodeck. 1995. Regulation of NK cell functions by TGF-β1. J. Immunol. 155:1066-1073. [PubMed] [Google Scholar]
- 2.Beschin, A., L. Brys, S. Magez, M. Radwanska, and P. De Baetselier. 1998. Trypanosoma brucei infection elicits nitric oxide-dependent and nitric oxide-independent suppressive mechanisms. J. Leukoc. Biol. 63:429-439. [DOI] [PubMed] [Google Scholar]
- 3.Buza, J., and J. Naessens. 1999. Trypanosome non-specific IgM antibodies detected in serum of Trypanosoma congolense-infected cattle are polyreactive. Vet. Immunol. Immunopathol. 69:1-9. [DOI] [PubMed] [Google Scholar]
- 4.Campbell, G. H., K. M. Esser, and F. L. Weinbaum. 1977. Trypanosoma rhodesiense infection in B-cell-deficient mice. Infect. Immun. 18:434-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cummins, J. M., G. S. Krakowka, and C. G. Thompson. 2005. Systemic effects of interferons after oral administration in animals and humans. Am. J. Vet. Res. 66:164-176. [DOI] [PubMed] [Google Scholar]
- 6.de Kossodo, S., and G. E. Grau. 1993. Profiles of cytokine production in relation with susceptibility to cerebral malaria. J. Immunol. 151:4811-4820. [PubMed] [Google Scholar]
- 7.Drennan, M. B., B. Stijlemans, J. Van den Abbeele, V. J. Quesniaux, M. Barkhuizen, F. Brombacher, P. De Baetselier, B. Ryffel, and S. Magez. 2005. The induction of a type 1 immune response following a Trypanosoma brucei infection is MyD88 dependent. J. Immunol. 175:2501-2509. [DOI] [PubMed] [Google Scholar]
- 8.Gorelik, L., P. E. Fields, and R. A. Flavell. 2000. TGF-β inhibits Th type 2 development through inhibition of GATA-3 expression. J. Immunol. 165:4773-4777. [DOI] [PubMed] [Google Scholar]
- 9.Greenwood, B. M. 1974. Possible role of a B-cell mitogen in hypergammaglobulinaemia in malaria and trypanosomiasis. Lancet i:435-436. [DOI] [PubMed] [Google Scholar]
- 10.Hertz, C. J., H. Filutowicz, and J. M. Mansfield. 1998. Resistance to the African trypanosomes is IFN-γ dependent. J. Immunol. 161:6775-6783. [PubMed] [Google Scholar]
- 11.Ishizaka, S., M. Kimoto, S. Kanda, and S. Saito. 1998. Augmentation of natural killer cell activity in mice by oral administration of transforming growth factor-β. Immunology 95:460-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korbel, D. S., O. C. Finney, and E. M. Riley. 2004. Natural killer cells and innate immunity to protozoan pathogens. Int. J. Parasitol. 34:1517-1528. [DOI] [PubMed] [Google Scholar]
- 13.Kuzoe, F. A. 1993. Current situation of African trypanosomiasis. Acta Trop. 54:153-162. [DOI] [PubMed] [Google Scholar]
- 14.Lanham, S. M., and D. G. Godfrey. 1970. Isolation of salivarian trypanosomes from man and other mammals with DEAE-cellulose. Exp. Parasitol. 28:521-534. [DOI] [PubMed] [Google Scholar]
- 15.Laurenti, M. D., M. Gidlund, D. M. Ura, I. L. Sinhorini, C. E. Corbett, and H. Goto. 1999. The role of NK cells in the early period of infection in murine cutaneous leishmaniasis. Braz. J. Med. Biol. Res. 32:323-325. [DOI] [PubMed] [Google Scholar]
- 16.Magez, S., M. Radwanska, A. Beschin, K. Sekikawa, and P. De Baetselier. 1999. Tumor necrosis factor alpha is a key mediator in the regulation of experimental Trypanosoma brucei infections. Infect. Immun. 67:3128-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magez, S., M. Radwanska, M. Drennan, L. Fick, T. N. Baral, F. Brombacher, and P. De Baetselier. 2006. Interferon-gamma and nitric oxide in collaboration with antibodies are key protective host immune factors during trypanosome Tc13 infections. J. Infect. Dis. 193:1575-1583. [DOI] [PubMed] [Google Scholar]
- 18.Morrison, W. I., S. J. Black, J. Paris, C. A. Hinson, and P. W. Wells. 1982. Protective immunity and specificity of antibody responses elicited in cattle by irradiated Trypanosoma brucei. Parasite Immunol. 4:395-407. [DOI] [PubMed] [Google Scholar]
- 19.Namangala, B., P. De Baetselier, L. Brys, W. W. Noël, and A. Beschin. 2001. Alternative versus classical macrophage activation during experimental African trypanosomosis. J. Leukoc. Biol. 69:387-396. [PubMed] [Google Scholar]
- 20.Namangala, B., P. De Baetselier, W. Noël, L. Brys, S. Magez, and A. Beschin. 2001. Relative contribution of IL-10 and IFN-γ towards resistance to African trypanosomosis. J. Infect. Dis. 183:1794-1800. [DOI] [PubMed] [Google Scholar]
- 21.Namangala, B., P. De Baetselier, B. Stijlemans, W. Noël, E. Pays, M. Carrington, and A. Beschin. 2000. Attenuation of Trypanosoma brucei is associated with reduced immunosuppression and concomitant production of TH2 lymphokines. J. Infect. Dis. 181:1110-1120. [DOI] [PubMed] [Google Scholar]
- 22.Nociari, M. M., A. Shavlev, P. Benias, and C. Russo. 1998. A novel one-step, highly sensitive fluorometric assay to evaluate cell-mediated cytotoxicity. J. Immunol. Methods 213:157-167. [DOI] [PubMed] [Google Scholar]
- 23.Noël, W., G. Hassanzadeh, G. Raes, B. Namangala, I. Daems, L. Brys, F. Brombacher, P. De Baetselier, and A. Beschin. 2002. Infection stage-dependent modulation of macrophage activation in Trypanosoma congolense-resistant and -susceptible mice. Infect. Immun. 70:6180-6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogunremi, O., and H. Tabel. 1995. Genetics of resistance to Trypanosoma congolense in inbred mice: efficiency of apparent clearance of parasites correlates with long-term survival. J. Parasitol. 81:876-881. [PubMed] [Google Scholar]
- 25.Oli, M. W., L. F. Cotlin, A. M. Shiflett, and S. L. Hajduk. 2006. Serum resistance-associated protein blocks lysosomal targeting of trypanosome lytic factor in Trypanosoma brucei. Eukaryot. Cell 5:132-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Omer, F. M., J. A. L. Kurtzals, and E. M. Riley. 2000. Maintaining the immunological balance in parasitic infections: a role for TGF-β? Parasitol. Today 16:18-23. [DOI] [PubMed] [Google Scholar]
- 27.Omer, F. M., and E. M. Riley. 1998. TGF-β production is inversely correlated with severity of murine malaria infection. J. Exp. Med. 188:39-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pays, E., L. Vanhamme, and M. Berberof. 1994. Genetic control for expression of surface antigens in African trypanosomes. Annu. Rev. Microbiol. 48:25-52. [DOI] [PubMed] [Google Scholar]
- 29.Schleifer, K. W., H. Filutowicz, L. R. Schopf, and J. M. Mansfield. 1993. Characterization of T helper responses to the trypanosome variant surface glycoprotein. J. Immunol. 150:2910-2919. [PubMed] [Google Scholar]
- 30.Schleifer, K. W., and J. M. Mansfield. 1993. Suppressor macrophages in African trypanosomiasis inhibit T cell proliferative responses by nitric oxide and prostaglandins. J. Immunol. 151:5492-5503. [PubMed] [Google Scholar]
- 31.Schopf, L., H. Filutowicz, X.-J. Bi, and J. M. Mansfield. 1998. Interleukin-4-dependent immunoglobulin G1 isotype switch in the presence of a polarized antigen-specific Th1-cell response to the trypanosome variant surface glycoprotein. Infect. Immun. 66:451-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sher, A., C. Collazzo, C. Scanga, D. Jankovic, G. Yap, and J. Aliberti. 2003. Induction and regulation of IL-12-dependent host resistance to Toxoplasma gondii. Immunol. Res. 27:521-528. [DOI] [PubMed] [Google Scholar]
- 33.Smeltz, R. B., J. Chen, and E. M. Shevach. 2005. TGF-β1 enhances the interferon-γ-dependent, interleukin-12-independent pathway of helper 1 cell differentiation. Immunology 114:484-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sternberg, J., and F. McGuigan. 1992. Nitric oxide mediates suppression of T cell responses in murine Trypanosoma brucei infection. Eur. J. Immunol. 22:2741-2744. [DOI] [PubMed] [Google Scholar]
- 35.Stevens, D. R., and J. E. Moulton. 1978. Ultrastructural and immunological aspects of the phagocytosis of Trypanosoma brucei by mouse peritoneal macrophages. Infect. Immun. 19:972-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stevenson, M. M., Z. Su, H. Sam, and K. Mohan. 2001. Modulation of host responses to blood-stage malaria by interleukin-12: from therapy to adjuvant activity. Microbes Infect. 3:49-59. [DOI] [PubMed] [Google Scholar]
- 37.Taylor, K. A. 1998. Immune responses of cattle to African trypanosomes: protective or pathogenic? Int. J. Parasitol. 28:219-240. [DOI] [PubMed] [Google Scholar]
- 38.Tsutsui, N., and T. Kamiyama. 1999. Transforming growth factor β-induced failure of resistance to infection with blood-stage Plasmodium chabaudi in mice. Infect. Immun. 67:2306-2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Une, C., J. Anderson, and A. Orn. 2003. Role of IFN-alpha/beta and IL-12 in activation of natural killer cells and IFN-γ production during experimental infection with Trypanosoma cruzi. Clin. Exp. Immunol. 34:195-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uzonna, J. E., R. S. Kaushiki, J. R. Gordon, and H. Tabel. 1999. Cytokines and antibody responses during Trypanosoma congolense infections in two inbred mouse strains that differ in resistance. Parasite Immunol. 21:57-71. [DOI] [PubMed] [Google Scholar]
- 41.Vincendeau, P., S. Daulouede, B. Veyrt, M. L. Darde, B. Bouteille, and J. L. Lemesre. 1992. Nitric oxide-mediated cytostatic activity on Trypanosoma brucei gambiense and T. brucei brucei. Exp. Parasitol. 75:353-360. [DOI] [PubMed] [Google Scholar]
- 42.Wahl, S. M. 1994. Transforming growth factor-beta: the good, the bad and the ugly. J. Exp. Med. 180:1587-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wahl, S. M., D. A. Hunt, L. M. Wakefield, N. McCartney-Francis, L. M. Wahl, A. B. Roberts, and M. B. Sporn. 1987. TGF-β induces monocyte chemotaxis and growth factor production. Proc. Natl. Acad. Sci. USA 84:5788-5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xong, H. V., P. De Baetselier, and S. Magez. 2002. Selective pressure can influence the resistance of Trypanosome congolense to normal human serum. Exp. Parasitol. 102:61-65. [DOI] [PubMed] [Google Scholar]