Abstract
In this work, three amino acids derived (L-leucinol, L-isoleucinol and L-valinol) sulfated chiral surfactants are synthesized and polymerized. These chiral sulfated surfactants are thoroughly characterized to determine critical micelle concentration, aggregation number, polarity, optical rotation and partial specific volume. For the first time the morphological behavior of polymeric sulfated surfactants is revealed using cryogenic high-resolution electron microscopy (cryo-HRSEM). The polysodium N-undecenoyl-L-leucine sulfate (poly-L-SUCLS) shows distinct tubular structure, while polysodium N-undecenoyl-L-valine sulfate (poly-L-SUCVS) also shows tubular morphology but without any distinct order of the tubes. On the other hand, polysodium N-undecenoyl-L-isoleucine sulfate (poly-L-SUCILS) displays random distribution of coiled/curved filaments with heavy association of tightly and loosely bound water. All three polymeric sulfated surfactants are compared for enantio-separation of broad range of structurally diverse racemic compounds at very acidic, neutral and basic pH conditions in micellar electrokinetic chromatography (MEKC). A small combinatorial library of 10 structurally related phenylethylamines (PEAs) is investigated for chiral separation under acidic and moderately acidic to neutral pH conditions using an experimental design. In contrast to neutral pH conditions, at acidic pH, significantly enhanced chiral resolution is obtained for class I and class II PEAs due to the compact structure of polymeric sulfated surfactants. It is observed that the presence of hydroxy group on the benzene ring of PEAs resulted in deterioration of enantioseparation. A sensitive MEKC-mass spectrometry (MS) method is developed for one of the PEA (e.g., (±)-pseudoephedrine) in human urine. Very low limit of detection (LOD) is obtained at pH 2.0 (LOD 325 ng/mL), which is ca 16 times better compared to pH 8.0 (LOD 5.2 µg/mL). Other broad range of chiral analytes (β-blockers, phenoxypropionic acid, benzoin derivatives, PTH-amino acids, and benzodiazepinones) studied also provided improved chiral separation at low pH compared to high pH conditions. Among the three polymeric sulfated surfactants, poly-L-SUCILS with two chiral centers on the polymer head group provided overall higher enantioresolution for the investigated acidic, basic and neutral compounds. This work clearly demonstrates for the first time the superiority of chiral separation and sensitive MS detection at low pH over conventional high pH chiral separation and detection employing anionic chiral polymeric surfactants in MEKC and MEKC-MS.
Over the last 20 years, the number of materials and products developed as pure enantiomers (eutomer) has continued to increase. With evidence of problems related to stereoselectivity in drug action, enantioselective analysis by separation are of particular importance for production, therapeutic monitoring or pharmacokinetic studies, and/or to validate the optical purity.1–2 Enantioseparation can be achieved in almost all separation techniques, including gas chromatography, liquid chromatography, thin-layer chromatography, supercritical fluid chromatography, counter current liquid chromatography, liquid-liquid extractions and electrodriven separation methods.3–5 Capillary electrophoresis (CE) is a family of a electrodriven separation technique which has many benefits for chiral analysis. Several advantages of CE includes its ability to analyze extremely small samples, less consumption of exotic chiral selector as well as possibility of highthroughput.6–10
Micellar electrokinetic chromatography (MEKC) is one of the CE mode, which is capable of separating both charged and uncharged molecules simultaneously.11 In MEKC, low molecular weight chiral surfactant has to be dissolved at or above its critical micelle concentration (CMC) for effective chiral separation.12–13 In many instances due to very high CMC, chiral surfactant is added in large amount. This results in significant joule heating and consequently poor resolution and baseline shift. In addition, it has been reported that coupling of MEKC with electrospray ionization mass spectrometry (ESI-MS) detection is very difficult. In particular, the accumulation of nonvolatile surfactants not only causes fouling of the ion source resulting in loss of sensitivity but also interfere with most solutes in the low molecular mass region.14–15 Two main techniques have been used for chiral analysis in CE-MS: (a) counter migration using charged cyclodextrins16–17 and vancomycin,18 (b) partial filling (PF) using derivitized cyclodextrins19–20 as well as crown ethers.21 Although both of the aforementioned techniques avoid the detrimental effects of chiral selector on the MS signal, the former is somewhat limited to the chiral analysis of charged compounds while the latter provides lower resolution and lower peak capacity compared to the standard MEKC-MS.22 These aforementioned disadvantages have prompted the combined use of counter-migration and PF which demonstrated higher CE resolution and higher MS sensitivity.23
In the past ten years, polymeric surfactants24–26 (aka. molecular micelle) has been widely employed as pseudostationary phases for chiral27–35 and achiral36–38 separations in MEKC. The polymeric surfactants are synthesized from surfactants having a polymerizable group on the hydrophobic tail end. Since polymerization is performed well above the CMC of the monomeric surfactant, the resulting structures are micelle like in many respects. However, once polymerized the monomers are held together by covalent bonds and act as molecular micelle at any concentration independent of CMC. Hence, polymeric surfactants exhibit essentially zero CMC and can be effective as pseudophase at any concentration independent of CMC. Furthermore, low surface activity and ability to solubilize hydrophobic compounds in the presence of a high content of organic solvents are some of the important advantages of polymeric surfactants over conventional micelles. Another aspect that makes polymeric surfactant preferential is their ability to provide stable electrospray, which has been more difficult to do with conventional micelles. Therefore, high molecular weight and low surface activity of polymeric surfactants aids in less suppression of analyte signal with essentially no background due to absence of surfactant monomers.39–41 Recently, our group conducted several studies with the chiral amino acid based polymeric surfactant which actually showed at least 5–14 fold better sensitivity in MEKC-MS compared to MEKC-UV method for chiral analysis.31, 40–41
The pH is considered one of the most important parameter for optimization of chiral resolution (Rs) in MEKC. This is because pH usually alters both the charge of the analyte and/or chiral selector (surfactant) possessing ionizable groups as well as influencing the magnitude of electroosmotic flow (EOF). Furthermore, it has been documented that low pH can be used to eliminate the interferences from matrix during MEKC analysis of the biological samples.42–43 The present state-of-the-art in developing chiral molecular micelles for MEKC-MS mostly involves the use of amino acid based surfactants with carboxylate head groups.26,44–45 However, the use of these surfactants is somewhat limited to basic pH range due to their poor water solubility in acidic pH range of 1.5–5.0. To date, there are no studies on polymeric chiral surfactant that can be utilized over a wide pH range in MEKC. In this work, we report the synthesis, characterization and chiral MEKC and MEKC-MS application of novel pH independent sulfated amino acid polymeric surfactants. Our data suggests that the use of sulfated polymeric surfactant not only enhances the solubility of these micelles in acidic media, but also significantly improves chiral separation at low pH conditions. We have compared three polymeric chiral surfactants such as, polysodium N-undecenoxycarbonyl-L-leucine sulfate (poly-L-SUCLS), polysodium N-undecenoxycarbonyl-L-isoleucine sulfate (poly-L-SUCILS) and polysodium N-undecenoxycarbonyl-L-valine sulfate (poly-L-SUCVS). These polymeric surfactants which are collectively referred to as polysodium N-undecenoxycarbonyl-L-amino acid sulfates (poly-L-SUCAAS) are utilized to achieve the optimal enantioselectivity of a small combinatorial library of several structurally similar basic, acidic and neutral chiral compounds.
The present study had four major goals. First, to synthesize chiral sulfated amino acid based surfactant and their polymers (Figure 1). Second, to characterize the synthesized monomeric and polymeric surfactants using a variety of techniques including most modern cryogenic high resolution scanning electron microscopy (cryo-HRSEM) to study the solution phase characteristic of these self-assembling molecules. Third, to achieve optimum enantioseparation of structurally similar phenylethylamines (PEAs) using experimental design strategy that utilizes the optimum MEKC-MS conditions for a sensitive assay of a nasal decongestant (pseudoephedrine) in human urine. The fourth and final goal was to achieve simultaneous enantioseparation of a broad range of racemic analytes by fine tuning MEKC parameters as well as to evaluate the role of chemical structure of both poly-L-SUCAAS and the structurally similar compounds on stereoselective recognition.
Figure 1.
Synthesis of the N-undecenoxy carbonyl-L-amino acid sulfated surfactants and their polymers.
EXPERIMENTAL SECTION
Standards and Chemicals
The analytes (±)-epinephrine, (±)-norepinephrine, (±)-isoproterenol, (±)-terbutaline, (±)-synephrine, (±)-octopamine, (±)-norphenylephrine, (±)-ephedrine, (±)-pseudoephedrine, (±)-norephedrine, (±)-atenolol, (±)-metoprolol, (±)-2-(2-chloro-phenoxy)-propionic acid [(±)-2-PPA], (±)-hydrobenzoin, (±)-benzoin, (±)-benzoin methylether, (±)-benzoin ethylether, (±)-phenylthiohydantoin-isoleucine {(±)-PTH-isoleucine}, (±)-PTH-tryptophan, (±)-PTH-tyrosine, (±)-lorazepam, (±)-temazepam and (±)-oxazepam were obtained as racemic mixture from Sigma Chemical Co (St. Louis, MO) or Aldrich (Milwaukee, WI). Dodecanophenone and chemicals used for the synthesis of surfactants, ω-undecylenyl alcohol, triphosgene, pyridine, dichloromethane, chlorosulfonic acid, L-leucinol, L-isoleucinol and L-valinol, were all obtained from Fluka (St. Louis, MO) or Aldrich (Milwaukee, WI) and were used as received.
Synthesis and Characterization of Monomeric and Polymeric Surfactants
The choloroformate of the undecenol was synthesized by reacting unsaturated alcohol with triphosgene in the presence of pyridine in dichloromethane (CH2Cl2).27 Next, chloroformate of the undecenol was added dropwise to an equimolar aqueous solution of chiral amino alcohol (L-leucinol, L-isoleucinol and L-valinol) and Na2CO3. After 2 hrs, the aqueous solution was extracted twice with CH2Cl2, the bottom layer of CH2Cl2 was collected, repeatedly washed with H2O, dried over Na2SO4 and concentrated in vacuo (yield, 91–95%). The chiral sulfated surfactants were synthesized by dropwise addition of chlorosulfonic acid over a period of 1 hr to the carbamate functionalized chiral amino alcohols in pyridine and CH2Cl2. The resulting mixture was diluted with water, copious amount of 6 M HCl (pH ~1) was added and extracted with CH2Cl2, the bottom aqueous layer of CH2Cl2 was collected, dried over Na2SO4 and concentrated in vacuo (yield, 60–65%). The resulting product was dissolved in equimolar aqueous solution of Na2CO3. This solution was then extracted with ethyl acetate, the bottom layer containing clear foamy surfactant solution was lyophilized (yield, 50–55%) on a Labconco 4.5L benchtop freeze dryer at −50 °C collector temperature and 0.05 mbar pressure. 1H-NMR spectra of L-SUCASS and poly-L-SUCASS were recorded on a Varian Unity+ 300 MHz spectrometer using D2O as the solvent. The surfactants were characterized by using electrospray ionization-mass spectrometry (ESI-MS), 1H-NMR and elemental analysis. The ESI-MS in negative scan mode of L-UCLS, L-UCILS and L-UCVS provided [M-H]+ peaks at 392.5 m/z, 392.5 m/z and 379.5 m/z respectively. Thus confirming the structure and identity of the synthesized surfactants.
The numerical values obtained from the NMR spectra are listed as follows: L-SUCLS, 1H-NMR δ (ppm): 0.806–0.814 (b, 6H), 1.177 (b, 14H), 1.501 (b, 2H), 1.881 (b, 3H), 3.365–3.428 (b, 1H), 3.7754–3.961 (b, 2H), 4.103–4.4.227 (b, 2H), 4.744–4.850 (m, 2H), 5.581–5.659 (m, 1H). L-SUCILS, 1H-NMR δ (ppm): 0.789–0.812 (b, 3H), 1.036 (b, 3H), 1.176 (b, 14H), 1.501 (b, 2H), 1.764–1.792 (m, 2H), 1.880 (b, 1H), 3.543 (b, 2H), 3.882–3.955 (m, 1H), 4.118 (b, 2H), 4.747–4.848 (m, 2H), 5.557–5.669 (m, 1H). L-SUCVS, 1H-NMR δ (ppm): 0.0784–0.848 (b, 6H), 1.166 (b, 12H), 1.486 (b, 2H), 1.755–1.786 (m, 2H), 1.874 (b, 1H), 3.505 (b, 2H), 3.931 (b, 1H), 4.089 (b, 2H), 4.779–4.845 (m, 2H), 5.612–5.636 (m, 1H).
The critical micelle concentration (CMC) was determined using a sigma 703 Digital Tensiometer (KVS Instruments USA, Monroe, Connecticut), by the Du NoÜy ring method at room temperature. Polymerization of the L-SUCLS, L-SUCLS and L-SUCVS were achieved by 60Co γ-irradiation (1.8 Mrad/h) of 100 mM aqueous solution of each surfactant for 30 hrs. The 1H-NMR indicated the disappearance of double bond protons signal in the region of 4.8–5.0 ppm and 5.7–5.9 ppm. Furthermore, all three polymers exhibited broadening of the signal, which is consistent with the classical spectrum. After irradiation, the polymeric surfactant solutions were dialyzed against triply deionized water using regenerated cellulose (RC) dialysis membrane (Spectrum Laboraties, Inc, Rancho Dominguez, CA, USA) with a 1000 Da molecular mass cutoff for 24 hrs. Finally, the dialyzed solutions were lyophilized to obtain the dried polymeric surfactants.
Further characterization, such as aggregation number and polarity of the L-SUCAAS and poly-L-SUCAAS were determined by using pyrene emission vibronic fine structure method.46–47 The partial specific volume (V¯) was determined as described in detail elsewhere.27,37 The optical rotation of monomeric and the polymeric surfactants was obtained by an AUTOPOL III automatic polarimeter (Rudolph Research Analytical, Flanders, New Jersey) by measuring the optical rotation at 589 nm of a 10 mg/mL solution of each in triply deionized water at 25 °C. Chromatographic parameters such as resolution (Rs), efficiency (N) and signal/noise (S/N) ratio were calculated using Chemstation software (V9.0). For MEKC-MS experiments, all chromatograms were smoothed utilizing the “Gaussian” option available in Agilent Chemstation software. The value of Gaussian width was set at 0.05 min before calculating the S/N ratio. Plackett-Burmann design was used to optimize the chiral resolution of phenylethylamines (PEAs). These experiments were performed in triplicate and the differences in retention times and peak width at half height were used to calculate efficiency and resolution between enantiomers by Chemstation software.
MEKC and MEKC-ESI-MS Instrumentation
All MEKC-UV and MEKC-MS experiments were performed on an Agilent CE system (Agilent Technologies, Waldbronn, Germany) which was interfaced to an Agilent 1100 series MSD quadrupole mass spectrometer (for ESI-MS detection) equipped with a CE-MS adaptor kit, sprayer kit, 0–30 kV high-voltage power supply, a diode array detector (for UV detection) and Chemstation software (V 9.0) for system control and data acquisition. Sheath liquid was delivered by an Agilent 1100 series HPLC pump equipped with a 1/100 split flow. The fused-silica capillary was obtained from Polymicro Technologies (Phoenix, AZ). The total length of the capillary used for MEKC-UV detection was 64.5 cm (56.0 cm from inlet to detector, 50 µm ID, 350 µm OD), prepared by burning about 3 mm polyimide to create a detection window. For MEKC-ESI-MS experiments, the total length of the capillary used was 70 cm.
Capillary Electrophoresis Procedures
The capillaries for all MEKC experiments were prepared by flushing with 1N aqueous NH3 for 1 hr at 50 °C followed by 30 min rinse with triply deionized water at a temperature desired for chiral separation, 2 min flush with buffer and finally 7 min with the running MEKC buffer containing surfactant. In addition, the capillary was flushed with 0.1 N aqueous NH3 and H2O for 3 min each and finally equilibrated with running buffer for 7 min in between the runs. All separations were performed at ± 20 kV and at 20 °C otherwise mentioned. All classes of analytes were evaluated for enantioseparation using a new capillary (cut to the same length from the same capillary bundle) and was preconditioned using the identical flushing procedure as mentioned above.
Preparation of MEKC Buffers, Analyte Solutions and Human Urine Sample
For separation of class I, II and III PEAs in acidic pH range (pH 2.0–3.0), the buffer was prepared by dissolving 25 mM triethylamine (TEA) in water and titrated with H3PO4 to the desired pH. The buffer for acidic to neutral pH range (pH 6.0–7.0) was prepared by dissolving either 15, 25 or 40 mM ammonium acetate in water and titrated with CH3COOH to the desired pH. For pseudoephedrine assay in human urine by MEKC-MS, 15 mM TEA and 15 mM NH4OAc were dissolve in water and HCOOH was used to obtain pH 2.0 buffer. For enantioseparation of β-blockers at pH 2.0, 25 mM NaH2PO4 and 25 mM CH3COONa were dissolved in water and pH was adjusted using H3PO4. For enantioseparation of β-blockers at basic pH 8.0, 25 mM NH4OAc and 25 mM TEA were dissolved in water and pH was adjusted using CH3COOH. For enantioseparation of 2-PPA, benzoin derivatives, PTH-amino acids and benzodiazepenes at acidic pH (2.0 or 3.0) and at basic pH (8.0), the same buffers were used as for enantioseparation of β-blockers (see above) at the respective acidic and basic pH. The desired pH value of all buffers was obtained before the addition of polymeric surfactants. All BGE solutions are finally filtered through a 0.45 µm Nalgene syringe filter (Rochester, NY).
The running MEKC buffer solution was prepared by addition of specific amount of surfactants to the BGE, followed by ultrasonication for about 25–30 minutes. The analytes were prepared in MeOH at various concentrations and diluted with water according to the separation conditions (exact dilutions and final analyte concentrations are mentioned in the figure caption). Blank human urine sample was collected from a healthy male subject and stored in a refrigerator at low temperature (4 °C). The analyte [(±) pseudoephedrine in 100% MeOH, 3 mg/mL] was diluted at levels of 0.00065, 0.00130, 0.00260, 0.00520, 0.0104, 0.0210, 0.0415, 0.0830, 0.1660 and 0.3330 mg/mL with freshly filtered (0.45 µm nylon syringe filter, Nalgene, Rochester, NY) human urine in all ten 10 mL volumetric flasks. To each flask, internal standard [(−)-phenylephrine in 100% MeOH, 3 mg/mL] was added at a constant concentration (0.1660 mg/mL). A portion from each flask was transferred into 300 µL sample vial and injected into the capillary by applying 15 mbar pressure for 2 sec.
Cryogenic-High-Resolution Scanning Electron Microscopy (Cryo-HRSEM) Sample Preparation and Imaging
Approximately 10 µL aliquots (5 mg/mL) of the polymeric sulfated surfactant (poly-L-SUCLS, poly-L-SUCILS and poly-L-SUCVS) solutions were loaded into flat-bottom-well gold planchets (Balzers BU 0120130T), plunge-frozen into liquid ethane and stored under liquid nitrogen. The frozen samples were transferred into a precooled (~ −170 °C) cryo-preparation stage (Gatan CT-3500) and were fractured with a prechilled blade and kept under liquid nitrogen. The shutters on the cryo-preparation stage were kept closed to avoid frost formation and stage was quickly transferred into a Denton DV-602 (Moorestown, NJ) chromium coater. Once the chromium coater was evacuated to 2 × 10−7 Torr, the stage shutters were opened and the stage temperature was ramped to −105 °C during the entire etching period and then finally the chamber was refilled to 5 × 10−3 Torr with argon gas.
With a series of experiments it was found that 6 min etch time at −105 °C was needed to remove sufficient amount of unbound water-ice, and 5 mg/mL surfactant concentration was needed to reveal any notable structural features. After etching, the temperature was returned to −170 °C and the frozen specimens were sputter-coated with 1–2 nm of chromium. The chamber of chromium coater was flushed with dry nitrogen gas (which allowed the specimen to return to atmospheric pressure) and cold stage was removed and quickly transferred to in-lens DS-130F Field Emission SEM. The temperature of the sample was increased from about −160 to −110 °C in order to allow any nanometer-size frost that may have condensed on the surface of the chromium film to sublime in the microscope prior to imaging. The specimens were imaged at 25 kV, digitally recorded in 30 s with a GW capture board at 17.4 Mbytes file size, and Adobe Photoshop 6.0 was used to adjust levels.48–49
RESULTS AND DISCUSSION
Physicochemical Properties of Surfactants
Table 1 represents the physicochemical properties of the enantiomerically pure synthetic sulfated amino acid surfactants L-SUCLS, L-SUCILS and L-SUCVS and their micelle polymers, poly-L-SUCLS, poly-L-SUCILS and poly-L-SUCVS. Comparing the physicochemical properties of monomeric and polymeric surfactants, it can be noticed that aggregation number (A) is always lower, while polarity, optical rotation and V¯ is always higher for polymeric surfactants compared to the corresponding monomers.
Table 1.
Physicochemical properties of the monomers and polymers of sodium N-undecenoxycarbonyl-L-amino acid sulfates (L-SUCAAS).
| Characteristic of the Monomeric surfactants | L-SUCLS | L-SUCILS | L-SUCVS |
|---|---|---|---|
| Critical micelle concentration (CMC) a)[mM] | 4.15 ± (0.07)* | 3.95 ± (0.36)* | 5.23 ± (0.04)* |
| Aggregation numberb) | 71 ± (1)* | 66 ± (1)* | 74 ± (1)* |
| Polarity (I1/I3) ratio c) | 1.0246 ± (0.0004)* | 1.0844 ± (0.0014)* | 1.0413 ± (0.0002)* |
| Optical rotationd) | −19.35 ± (0.07)* | −14.10 ± (0.14)* | −16.20 ± (0.14)* |
| Partial specific volumee) | 0.5590 ± (0.0006)* | 0.5134 ± (0.0009)* | 0.5426 ± (0.0018)* |
| Characteristic of the polymeric surfactants | poly-L-SUCLS | poly-L-SUCILS | poly-L-SUCVS |
| Aggregation numberb) | 32 ± (1)* | 42 ± (1)* | 36 ± (1)* |
| Polarity (I1/I3) ratioc) | 1.0630 ± (0.0008)* | 1.105 ± (0.007)* | 1.076 ± (0.003)* |
| Optical rotationd) | −22.65 ± (0.07)* | −18.10 ± (0.14)* | −19.80 ± (0.14)* |
| Partial specific volumee) | 0.8095 ± (0.0004)* | 0.7994 ± (0.0011)* | 0.7905 ± (0.0004)* |
Critical micelle concentration is determined by the surface tension measurements.
Aggregation number is determined by the florescence quenching experiment using pyrene as a probe and cetyl pyridinium chloride as a quencher.
Polarities of the surfactants are determined using ratio of the fluorescence intensity (I1/I3) of pyrene.
Optical rotation of 1%(w/v) of monomer and micelle polymers were determined in triply deionized water; were obtained at 589nm [sodium D line].
Partial specific volumes were determined by the density measurements at different surfactant concentrations.
Standard deviations are given in parentheses.
The cryo-HRSEM was used to investigate the morphology of polymeric surfactants. Cryo-etch HRSEM has two key advantages over atomic force microscopy (AFM) and transmission electron microscopy (TEM). First, the cryo-HRSEM does not require tedious and time-consuming sample preparation and image generated is free of surface artifacts usually noted during AFM imaging. Second, it has been observed that during imaging the AFM probe sometimes destroys the fine features of the sample being imaged.50 Due to this reason samples are treated with chemical fixing agents to stabilize the structure during AFM imaging. In our case, the polymeric surfactant samples are not chemically fixed and are fully hydrated. Hence, cryo-HRSEM mimic the actual behavior of surfactant in aqueous solution.51–52 The etched surface of the fractured drop of the poly-L-SUCLS revealed tubular or rod-like structures when cryo-etch for 6 min under low temperature-HRSEM (Figure 2A). Nanorods having a distinct order appeared to have 80–100 nm widths, which is dependent on the amount of loosely bound water around them. Furthermore, the tubular structure revealed by cryo-HRSEM is reminiscence of the fact that surfactants at concentration significantly higher than critical micelle concentration (CMC) quickly form rod like structures and spherical micelles only exist in dilute solutions.53–54 In contrast to the morphological behavior of poly-L-SUCLS, poly-L-SUCILS displayed random distribution of coiled/curved filaments with heavy association of tightly and loosely bound water (Figure 2B). Similar to poly-L-SUCLS, the poly-L-SUCVS (Figure 2C) also shows tubular morphology, but without any distinct order of the tubes having 120–180 nm widths which depends on the amount of loosely bound water around them.
Figure 2.
Intermediate magnification cryo-etch-HRSEM of (A) poly-L-SUCLS imaged at 10000 x, scale bar = 1.00 µm, (B) poly-L-SUCILS imaged at 20000, scale bar = 500 nm and (C) poly-L-SUCVS imaged at 15000 x, scale bar = 667 nm. For poly-L-SUCLS, poly-LSUCILS and poly-L-SUCVS, images were taken at 5 mg/mL, 6-min etch-time and −115 °C. Blue asterisk (*) represents the remnant patches of nonsublimed ice; red color and green color arrows point the loosely and tightly bound water around the nanorods, respectively.
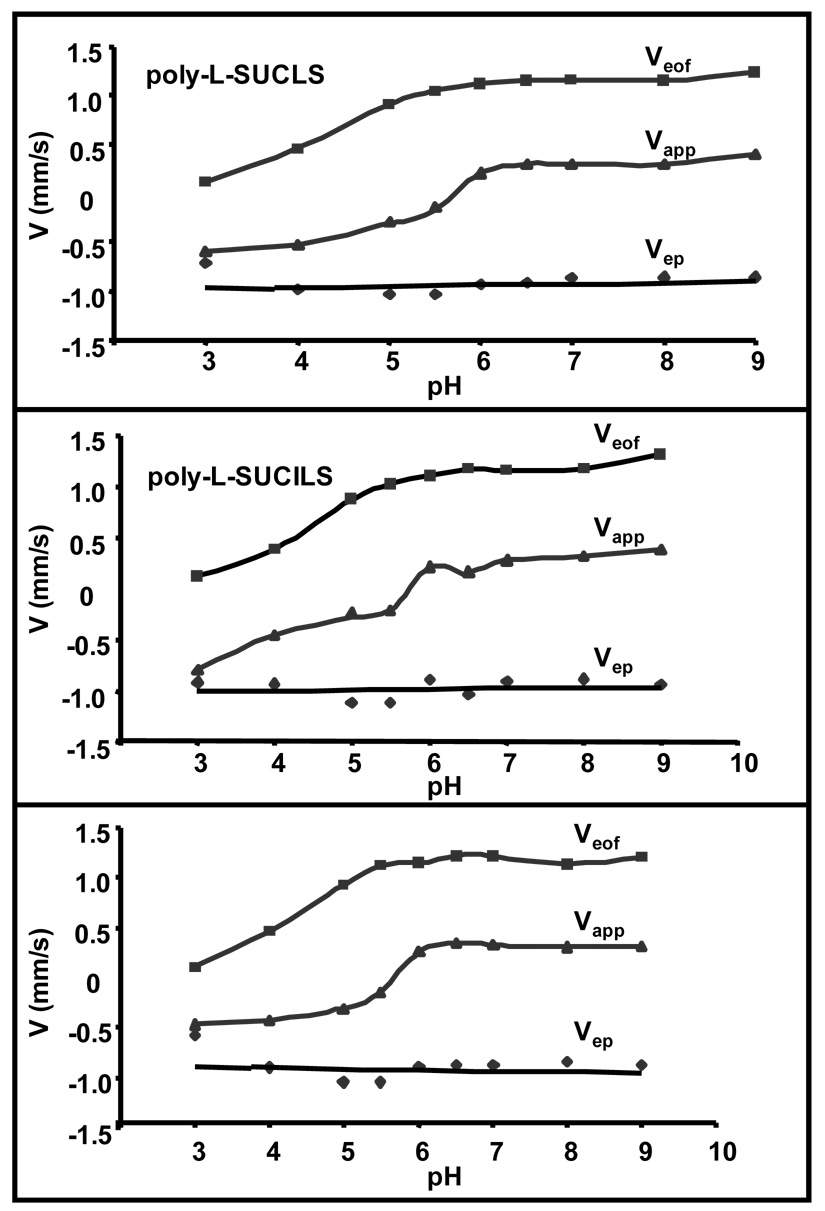
Figure 3 shows the dependence of electroosmotic velocity Veof (methanol, mms−1), micelle migration velocity Vapp (dodecanophenone, mms−1) and effective micelle electrophoretic velocity Vep (Veof − Veof, mms−1) of poly-L-SUCLS, poly-L-SUCILS and poly-L-SUCVS. The sign of velocity of the micelle is defined as positive when polymeric surfactant is migrated towards the negative electrode, and as negative when the same surfactant is migrated towards the positive electrode. It can be seen from Figure 3 that under moderately acidic to basic pH (6.0–9.0) and under positive polarity conditions, Veof and Vapp are fairly constant. However, the Vapp of all three polymeric surfactants turns from positive to essentially zero at pH 5.5 and then acquires negative values at pH below 5.0. The trend in Vapp of the micelle below pH 5.0 can be ascribed to significant decrease in the Veof caused by the adsorption effects of the polymeric surfactants. Furthermore, similar to the previous report of sodium dodecyl sulfate (SDS) electrophoretic behavior,11 the Vep of poly-L-SUCAAS were also found to be unaffected by variations in pH. It is surprising to note that despite the differences in morphology (Figure 2) and physical properties (Table 1) of three polymeric surfactants, there were no appreciable differences in Veof and Vapp among poly-L-SUCAAS. This similar electromigration behavior suggests that small structural variations on the polar head group of polymeric surfactants do not significantly affect Veof and Vapp.
Figure 3.
Comparison of 20 mM poly-L-SUCLS, poly-L-SUCILS and poly-L-SUCVS for dependence of electroosmotic velocity (Veof, methanol), micelle migration velocity (Vapp, dodecanophenone) and micelle electrophoretic velocity (Vep, calculated) on pH. MEKC conditions: 25 mM NH4OAc / 25 mM TEA, 25 °C, pressure injection: 50 mbar for 15s, ±20 kV applied for separations, UV detection at 214 nm.
Enantioseparation of Phenylethylamines using Experimental Design
Figure 4 shows the structure of phenylethylamines (PEAs) investigated for chiral separations. These eleven PEAs are classified according to the number of hydroxy groups present on the benzene ring. For example, class I, II and III PEAs comprise of two, one and zero hydroxy group on the benzene ring, respectively. The first screening step was to identify the variable, which have significant effects on chiral resolution (Rs). Selecting the variables and factors levels can be considered as the difficult part of the experimental design. However, by conducting the preliminary experiments and searching the appropriate literature55 one could obtain valuable information regarding the selection of variables as well the factor levels for separation in MEKC. A three-level four-factor well-balanced design from a Plackett-Burmann design56–57 was used to study the four most influential factors that maximizes chiral Rs and minimizes analysis time (AT). The structural designs shown in Table 2 was executed using poly-L-SUCLS, poly-L-SUCILS and poly-L-SUCVS at acidic pH with negative polarity and moderately acidic to neutral pH with positive polarity. The levels (−1,0,+1) of these factors were determined using poly-L-SUCLS by running individual analytes at variable conditions of buffer concentration, pH, percentage of acetonitrile (ACN) in the buffer, temperature and surfactant concentration. Using positive polarity, it was found that pH (2.0–3.0), percentage of ACN [15–25 %(v/v)], capillary temperature (15–25 °C) and surfactant concentrations (20–70 mM) were the four most common factors affecting Rs (data not shown). On the other hand, using positive polarity the variables to be evaluated were the same except the buffer concentration, which was found to have more significant effect than capillary temperature on chiral Rs. In addition, the considered factors such as, pH, %(v/v) ACN and ammonium acetate buffer were studied in the range of 6.0–7.0, 20–30 % and 15–40 mM, respectively. As response variables, resolution and elution time of the second enantiomers of each analyte (t2) of PEA enantiomers were chosen.
Figure 4.
Chemical structures of the racemic compounds studied.
Table 2.
Experimental design for separation strategy of PEAs using four factors at three levels under acidic pH conditions with negative polarity and moderately acidic to neutral pH with positive polarity.
| Exp. Design Levels | Exp. Design Levels | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (A)† | (B)‡ | |||||||||||
| Exp # | pH | ACN % (V/V) | Temp / Buffer | Micelle (mM) | pH | ACN % (V/V) | Temp (°C) | Micelle (mM) | pH | ACN % (V/V) | Buffer¶(mM) | Micelle (mM) |
| 1 | −1 | 0 | 1 | 1 | 2.0 | 15 | 25 | 70 | 6.0 | 25 | 40 | 70 |
| 2 | 0 | −1 | 0 | 1 | 2.5 | 10 | 20 | 70 | 6.5 | 20 | 25 | 70 |
| 3 | 0 | 0 | −1 | 0 | 2.5 | 15 | 15 | 45 | 6.5 | 25 | 15 | 45 |
| 4 | 1 | 0 | 0 | −1 | 3.0 | 15 | 20 | 20 | 7.0 | 25 | 25 | 20 |
| 5 | −1 | 1 | 0 | 0 | 2.0 | 20 | 20 | 45 | 6.0 | 30 | 25 | 45 |
| 6 | 1 | −1 | 1 | 0 | 3.0 | 10 | 25 | 45 | 7.0 | 20 | 40 | 45 |
| 7 | 1 | 1 | −1 | 1 | 3.0 | 20 | 15 | 70 | 7.0 | 30 | 15 | 70 |
| 8 | 0 | 1 | 1 | −1 | 2.5 | 20 | 25 | 20 | 6.5 | 30 | 40 | 20 |
| 9 | −1 | −1 | −1 | −1 | 2.0 | 10 | 15 | 20 | 6.0 | 20 | 15 | 20 |
Low pH conditions under negative polarity.
Moderately acidic to neutral pH conditions.
Buffer: Ammonium Acetate (NH4OAc).
Enantioseparation of Class I Phenylethylamines
All four analytes of class I PEAs share two common features in that they both possess two phenolic hydroxy groups as well as a chiral center bearing β-amino alcohol functionality. In particular, two of them [(±)-epinephrine and (±)-norepinephrine] are neurotransmitters58, while the other two [(±)-terbutaline and (±)-isoproterenol] are adernergeric receptor blockers.58
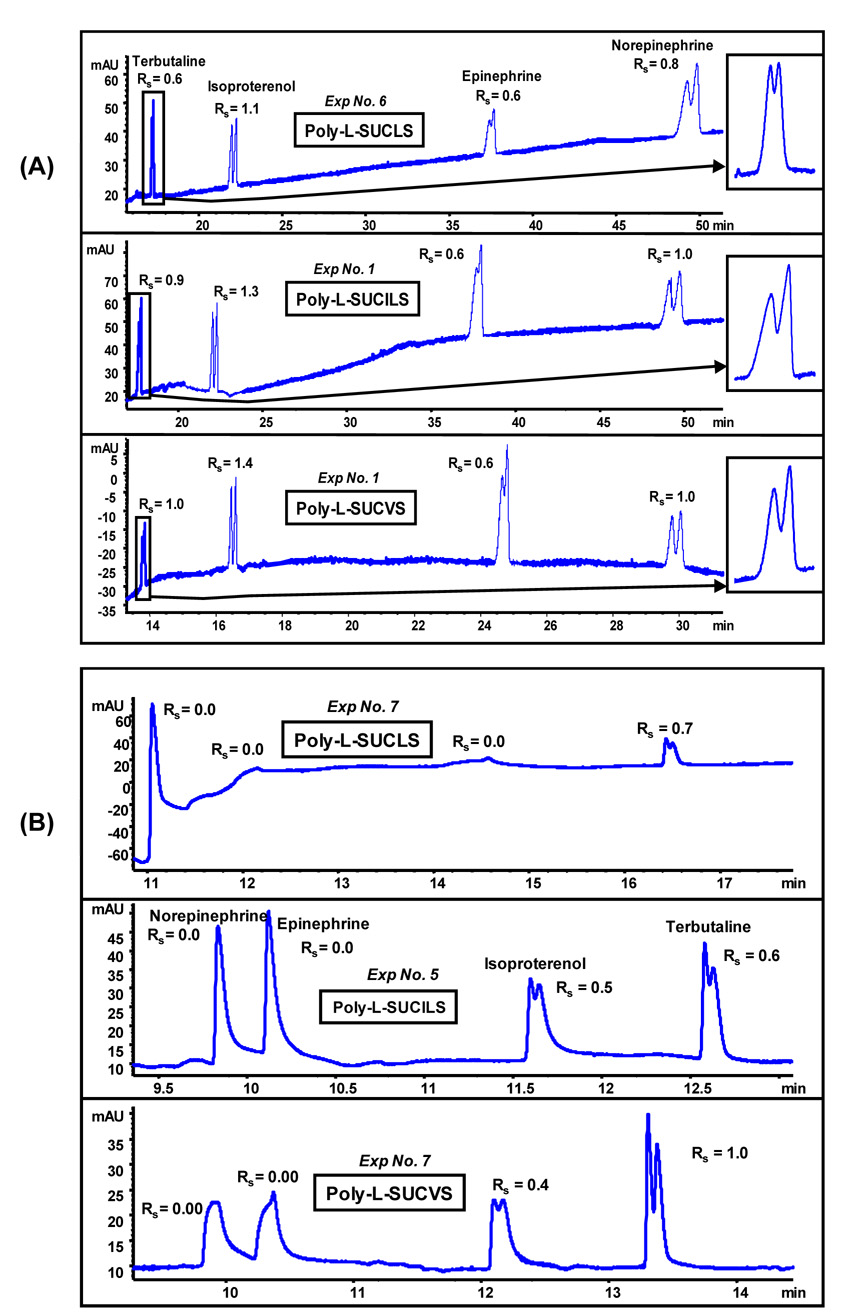
Application of the experimental design on a test mixture of four class I PEAs could allow one to determine the best polymeric sulfated surfactants. The poly-L-SUCILS showed highest enantioresolution for (±)-isoproterenol (Rs = 2.2, Exp 8), followed by poly-L-SUCLS where (±)-terbutaline was best separated (Rs = 1.5, Exp 5). Moreover, poly-L-SUCVS provided highest resolution values (Rs = 0.9 and 1.2, Exp 3) for (±)-epinephrine and (±)-norepinephrine, respectively (data not shown). Figure 5A (pH 2.0–3.0 range) and 5B (pH 6.0–7.0 range) represents the electropherograms of class I PEAs under the most suitable separation conditions that resulted in simultaneous enantioresolution of all four class I PEAs. It can be seen from the electropherograms in Figure 5 that not only enantiomeric migration order (e.g., (±)-terbutaline and (±)-isoproterenol) was reversed, but also all four PEAs eluted with opposite migration order. However, as indicated above individually all class I PEAs provided optimum Rs at different experimental design conditions. It was observed that all three polymeric surfactants behave differently in terms of chiral separation under identical experimental conditions. For instance, at low pH conditions (pH 2.0–3.0), poly-L-SUCLS provided simultaneous enantioseparation of all four class I PEAs with shortest analysis time (Figure 5A) under experimental condition number 6, while in case of poly-L-SUCILS and poly-L-SUCVS simultaneous enantioseparation was optimum under experimental condition number 1. In general moderate to higher polymeric surfactant concentrations (e.g., 45 mM and 70 mM) resulted in enantioseparation of all four class I PEAs in most of the experimental conditions (data not shown). However, at relatively lower surfactant concentration either no enantioresolutions [e.g., Rs = 0 for (±)-epinephrine and (±)-norepinephrine] or much lower enantioresolution [e.g., (±)-terbutaline and (±)-isoproterenol] were observed (data not shown). In addition, migration times were substantially higher at the lowest concentration of poly-L-SUCAAS. This trend suggests that at higher surfactants concentration the carrier capability of poly-L-SUCAAS is used as driving force not only for faster elution but also for higher enantioseparation.
Figure 5.
Comparison of (A) poly-L-SUCAAS for enantioseparation of class I PEA (0.17 mg/mL in 14:86, MeOH/H2O) at low pH under optimum conditions (see Table 3). MEKC conditions: 25 mM TEA/H3PO4, pressure injection 5 mbar for 1s, −20 kV, UV detection at 200 nm, (B) poly-L-SUCAAS for enantioseparation of class I PEA (0.25 mg/mL in MeOH/H2O) at moderately acidic to neutral pH under optimum conditions (see Table 3). MEKC conditions are same as Figure 5A except 20 °C, pressure injection of 40 mbar for 2s.
In contrast to acidic pH conditions, under moderately acidic to neutral pH range (6.0–7.0), barely any resolution was observed for (±)-isoproterenol and (±)-terbutaline. On the other hand, (±)-norepinephrine and (±)-epinephrine were never separated into enantiomers. For example, poly-L-SUCLS partially resolved terbutaline only under one condition, whereas simultaneous enantioseparation of (±)-isoproterenol and (±)-terbutaline was only achieved using poly-L-SUCILS and poly-L-SUCVS under experimental conditions 5 and 7, respectively. Nevertheless, it is clear that class I PEAs provided significantly higher enantioselectivity using all three polymeric sulfated surfactants but only under low pH conditions. One plausible explanation of higher Rs at lower pH could be due to the conformational transition of poly-L-SUCAAS from a more compact structure around pH 2.0–3.0 to a less compact structure around pH 6.0 and beyond as depicted by II/IIII ratio of pyrene emission spectrum. For example, it was observed that in case of poly-L-SUCVS polarity first slowly decreases (1.195–1.182) with the increase in pH from 2.0–4.0, and then increases (1.182–1.236) form pH 4.0–6.0, and finally remains fairly constant (1.236–1.238) from pH 6.0–8.0 (plot not shown). Similar polarity trends were also observed for poly-L-SUCLS and poly-L-SUCILS. This pH dependent conformational transition of polymeric surfactant has been reported by Chu and Thomas.59–60 Wang and Warner12 observed an opposite trend using an amide based polymeric surfactant polysodium N-undecenyl-L-valinate in which, enhanced chiral separation of (±)-laudanosine enantiomers was observed at pH 10.0 compared to pH of 8.5. In our studies, it seems that at lower pH the compact conformation of the polymeric sulfated surfactants favors the chiral interactions with the positively charged class I PEAs. In addition, separation under acidic conditions not only result in very low EOF but also increase the .effective positive charge on PEAs leading to enhanced chiral recognition.
Enantioseparation of Class II Phenylethylamines
Class II PEAs consist of three biologically active compounds (±)-synpehrine, (±)-octopamine and (±)-norphenylephrine bearing one hydroxy group on the benzene ring (Figure 4). As stated earlier for the enantioseparation of class I PEAs, all class II PEAs show maximum chiral Rs at different experimental conditions. For example, poly-L-SUCLS provided highest enantiomeric resolution (Rs = 2.0, 2.1 and 2.6) for (±)-synephrine, (±)-octopamine and (±)-nor-phenyephrine, respectively under experimental conditions 4, 5 and 8, but at the expense of longer AT (data not shown). However, longer AT was also observed with poly-L-SUCVS when maximizing the Rs factor. In contrast, executing the design on poly-L-SUCIL gave baseline Rs for the three class II PEAs without excessive AT. The overall quality of simultaneous enantioseparation was assessed based on Rs ≥ 1 for (±)-synephrine and (±)-octopamine; and Rs > 1.5 for (±)-norphenylephrine with least possible AT. Based on this criteria, Class II PEAs at low pH, showed optimum simultaneous separation under same experimental condition (Exp#1), irrespective of the type of polymeric surfactant. In addition, as noted previously for the chiral separation of class I PEAs at low pH, class II PEAs provided best Rs at most instances at higher surfactant concentrations (45–70 mM), while at 20 mM poly-L-SUCAAS concentration either no Rs or very low Rs was observed mainly due to very long AT.
Under moderately acidic to neutral pH, poly-L-SUCLS and poly-L-SUCILS provided optimum simultaneous enantioseparation under experimental condition 2, but poly-L-SUCVS provided best separation under experimental condition 1. Figure 6A show the electropherograms under acidic pH of 2.0, which resulted in overall chiral Rs ≥ 1 of all three class II PEAs using poly-L-SUCAAS. Similar to the results obtained for class I PEAs, the elution order of class II PEAs was exactly reversed in the pH range of 6.0–7.0 (Figure 6B). Again, all three stereoisomers of class II PEA were better resolved under low pH of 2.0 compared to moderately acidic pH of 6.0 or 6.5.
Figure 6.
Comparison of (A) poly-L-SUCAAS for enantioseparation of class II PEA at low pH under optimum conditions (see Table 4). Other conditions are same as Figure 5(A), (B) poly-L-SUCAAS for enantioseparation of class II PEA at moderately acidic to neutral pH under optimum conditions (see Table 4). Other conditions are same as Figure 5(B) except pressure injection of 25 mbar for 2s.
Enantioseparation of Class III Phenylethylamines
The Class III PEAs are commonly known as the ephedra alkaloids and consist of stereoisomers of (±)-ephedrine, (±)-pseudoephedrine and (±)-norephedrine. These compounds have been used to treat symptoms of cold and cough, reduce fever and induce perspiration.61 In general, the enantiomers of this class of PEAs were best resolved using either poly-L-SUCLS or poly-L-SUCLS under acidic pH, whereas under moderately acidic to neutral pH conditions, poly-L-SUCILS and poly-L-SUCVS seem to provide the maximum Rs. Furthermore, unlike several compounds of class I and class II PEAs where chiral Rs was essentially zero at lower polymeric surfactant concentrations, the class III PEA provided chiral resolution even at lower concentration of polymeric sulfated surfactants. In fact, under all experimental conditions (irrespective of pH and polarity of power supply) some chiral Rs were observed for every compound of class III PEA (data not shown).
Figure 7A and 7B show the enantioseparation of class III analytes under very acidic and moderately acidic to neutral pH conditions, respectively. As noted for the separation of class I and II PEAs, the elution order of class III PEAs was again found to be exactly reversed in the pH range of 2.0–3.0 (Figure 7A) compared to pH range of 6.0–7.0 (Figure 7B). Furthermore, it is worth mentioning that this is the only instance, where all three stereoisomers of class III PEA were resolved with similar Rs values under both acidic pH (pH 2.0) and moderately acidic to neutral pH (pH 6.0–7.0) conditions. Also, note that at low pH the peaks tend to front while at moderately acidic to neutral pH peaks tend to tail. This observation could be due to the mobility mismatch between analyte and the background electrolyte ions.
Figure 7.
Comparison of (A) poly-L-SUCAAS for enantioseparation of class III PEA at low pH under optimum conditions (see Table 5). Other conditions are same as Figure 5(A). (B) poly-L-SUCAAS for enantioseparation of class III PEA at moderately acidic to neutral pH under optimum conditions (see Table 5). Other conditions are same as Figure 5(B) except pressure injection of 25 mbar for 1s.
When comparing the chiral Rs among class I, II and III PEAs, it is very interesting to note that Rs is dramatically enhanced with decreasing substitution of phenolic hydroxy group on the benzene ring. Therefore, the chiral Rs follows the order: class I (2 hydroxy group)> class II (1 hydroxy group)>class III (no hydroxy group)]. Perhaps, the phenolic hydroxy groups on the benzene ring of PEA compete with a hydroxy group (located adjacent to the chiral center) for the hydrogen bonding interactions with highly functionalized chiral polymeric sulfated surfactants.
Application of Optimized MEKC-MS conditions for Sensitive Pseudoephedrine Assay in Human Urine Sample
In the view of the capabilities of chiral polymeric sulfated surfactants to efficiently enantioresolve PEAs sufficiently better at low pH, it seemed desirable to demonstrate the applicability of these polymers (e. g., poly-L-SUCLS) in MEKC-MS. Hence, a quantitative chiral assay for one of the PEA [e. g., (±)-pseudoephedrine] was developed in human body fluid. The (1S,2S)-(+)-pseudoephedrine is the most commonly used over-the-counter cough medicine and it has been often misused for its stimulant properties.62 Due to very similar chemical structure of the amphetamines, (±)-pseudoephedrine has also been used as a precursor for the clandistine production of methamphetamine and related illicit drugs.63 The serum half-life of (±)-pseudoephedrine is 5–8 hrs and about one-half of the dosage taken is excreted in the urine.64
Due to the aforementioned facts, we employed poly-L-SUCLS for the determination of (±)-pseudoephedrine in human urine and compared the limit of detection (LOD) at both low and high pH. A chiral MEKC-MS method development was performed on class III PEAs to obtain optimum sheath liquid and MS spray chamber parameters (data not shown). Figure 8A and B show a comparison of MEKC-MS of class III PEAs under optimum conditions. As can be seen in Fig 8A, when ammonium acetate is used in the sheath liquid, severe arcing was observed even though polymeric surfactant was employed. This observation could be due to the fact that class III PEAs are positively charged and form very strong ion pairs at low pH with the negatively charged poly-L-SUCLS. Thus the tightly bound ion-pairs are difficult to escape from the electrospray droplet, reducing sensitivity. To overcome this problem, a volatile acidic ion pairing reagent (e.g., valeric acid) was used which competes for the ion-pair formation with the positively charged analyte. Therefore, almost 3-fold higher abundance was achieved upon using valeric acid with almost no arcing and background noise (Figure 8B).
Figure 8.
Electrochromatogram comparing simultaneous MEKC separation and MS detection of class III PEA (0.17 mg/mL in 14:86 MeOH/H2O) using 25 mM poly-L-SUCLS without (A), and with (B) valeric acid in the sheath liquid. Conditions: (A) 15 mM NH4OAc / 15 mM TEA, + 20 % (v/v) ACN, 20 °C; injection, 15 mbar for 2 sec, pH 2.0 and −15 kV, 70 cm, 50 µm (I.D.), sheath liquid: 5 mM NH4OAc in MeOH/H2O (80:20, v/v), 0.5 mL/min. Spray chamber: drying gas flow 6 L/min, nebulizer pressure 4 psi, drying gas temp, 250 °C, Vcap 3000 V, fragmentor, 72 V. ESI SIM positive ions (3 ions) monitored as group SIM at m/z 166, 166, 152, (B) same as Figure 8(A) except sheath liquid is 1% (v/v) valeric acid in MeOH/H2O (80:20, v/v).
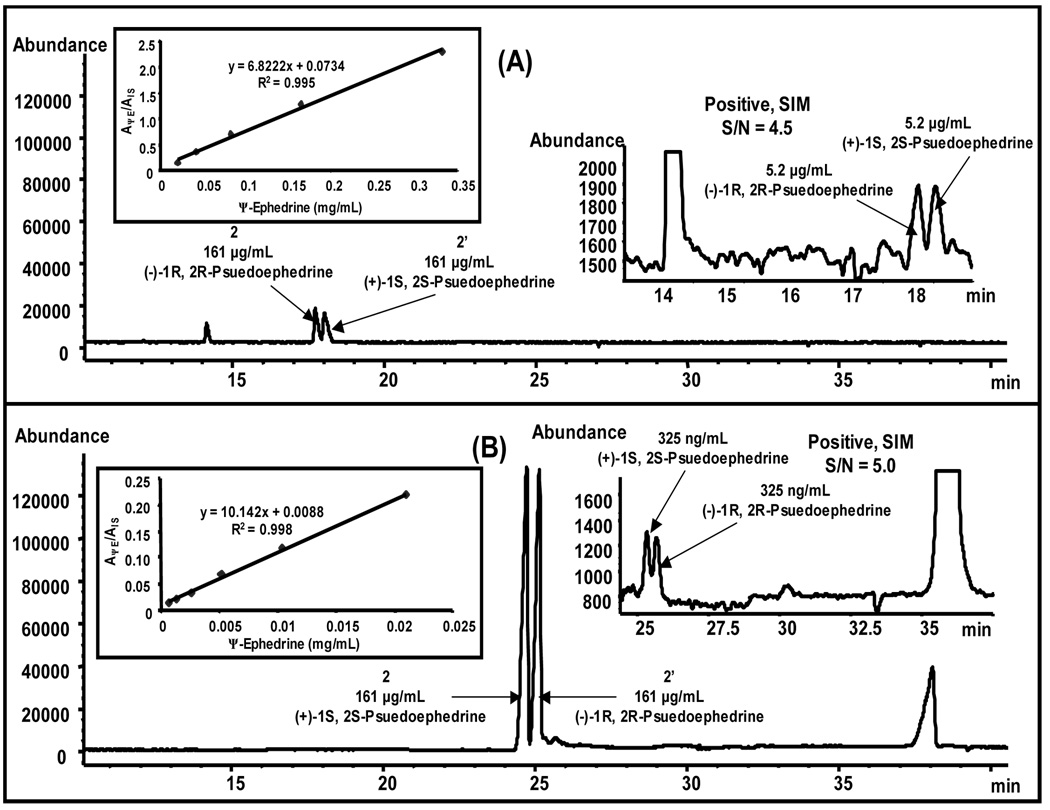
From the comparison of electropherogram in Figure 9A and 9B, it can be depicted that under similar MEKC-MS conditions except the BGE pH, the signal intensity obtained at low pH was ~ 6 fold higher compared to high pH. Consequently, from the LOD electropherograms (insets electropherograms on the right of Figure 9A and 9B), it is clear that ca. 16 times lower LOD i.e., 325 ng/mL can be achieved at low pH (pH 2.0) as compared to 5.2 µg/mL obtained at high pH (pH 8.0). This very low LOD obtained could stem from the fact that at pH 2.00 under negative polarity configuration with zero electroosmotic flow, poly-L-SUCLS migrates towards the MS detector and its carrier capability is used as a driving force for elution of (±)-pseudoephedrine. This carrier capability of poly-L-SUCLS can be attributed to the electrostatic attraction between negatively charged micelle and positively charged analyte. Thus, majority of analyte molecules migrates to the MS detection in the complexed form with the chiral micelle providing enhanced detection. Furthermore, at low pH preconcentration might also occur due to the combined effect of sweeping and the field-amplified sample stacking.65 On the other hand, at pH 8.0 under normal polarity configuration, the self-mobility of a chiral micelle is away from detector and as a result relatively less number of analyte molecules will enter into the MS detector compared to that at pH 2.0, mentioned above.
Figure 9.
Analysis of human urine spiked with (±)-psuedoephedrine enantiomers. The electropherogram [positive SIM 166 and 168 (m/z)] of human urine spiked with (±)-pseudoephedrine (2,2’) and (−)-phenylephrine (1) as IS at low pH of 2.0 (A) and high pH of 8.0 (B). Conditions are same as Figure 8(B), except 35 mM poly-L-SUCLS, sheath liquid flow rate 7.5 µL/min, pH 8.00 and +15 kV. Conditions in (B) are same as (A) except pH 2.00 and −15 kV. The insets on the right and left of Figure 9(A) and 9(B) show the enhanced region for (±)-pseudoephedrine at the LOD and calibration curves for psuedoephedrine enantiomers, respectively.
The calibration curves for human urine spiked with (±)-pseudoephedrine using (−)-phenylephine as internal standards (IS) at low and high pH are shown as inset plots on the left of Figure 9A and 9B, respectively. The value of the y-axis of the curves shows the ratio of the average peak area of (±)-pseudoephedrine to that for the IS, (−)-phenylephrine under both low and high pH conditions. The calibration curves were linear in the range of 0.65 to 21 µg/mL and 21 to 332 µg/mL for low pH (pH = 2. 0) and high pH (pH = 8.0) with good correlation of 0.995 and 0.988, respectively.
Enantioseparation of β-blockers
Figure 10A and 10B show comparison of enantioseparation of two β-blockers, (±)-atenolol and (±)-metoprolol at low and high pH respectively, under optimum conditions of poly-L-SUCLS, poly-L-SUCILS and poly-L-SUCVS. The electrokinetic chromatograms clearly shows that at low pH, (±)-atenolol and (±)-metoprolol provided longer AT but baseline resolution was always achieved, while at high pH only partial separation was obtained. The much-improved chiral resolution of the two β-blockers using any of the three polymeric surfactants at low pH is attributed to the change in conformation of the polymeric sulfated surfactant associated with the pH variations. It is also interesting to note that at low pH, hydrophilic β-blocker (±)-atenolol requires higher polymeric surfactant concentration (i.e., 35 mM), while moderately hydrophobic analyte, (±)-metoprolol requires lower polymeric surfactants concentration (i.e., 15 mM) for chiral Rs in accord to hydrophobicity of the analyte.30 Furthermore, increasing polymeric surfactant concentration decrease retention time of β-blockers using negative polarity (at pH 2.0), whereas the opposite was found to be true at high pH of 8.0 using positive polarity (data not shown).
Figure 10.
Comparison of (A) 35 mM poly-L-SUCAAS for enantioseparation of (±)-atenolol (0.25 mg/mL in 25:75, MeOH/H2O) and 15 mM poly-L-SUCAAS for enantioseparation of (±)-metoprolol (0.25 mg/mL in 25:75, MeOH/H2O). MEKC conditions: pH 2.0, 25 mM NaH2PO4 + 25 mM CH3COONa + H3PO4, 25 °C, pressure injection 5 mbar for 1s, −20 kV applied for separations, UV detection at 220 nm, (B) 25 mM poly-L-SUCAAS for enantioseparation of (±)-atenolol and (±)-metoprolol (0.25 mg/mL in MeOH/H2O). MEKC conditions: pH 8.0, 25 mM NH4OAc / 25 mM TEA, 25 °C, pressure injection of 5 mbar for 1s, +20 kV applied for separations; UV detection at 220 nm.
Enantioseparation of ±-2-(2-chlorophenoxy)propanoic acid
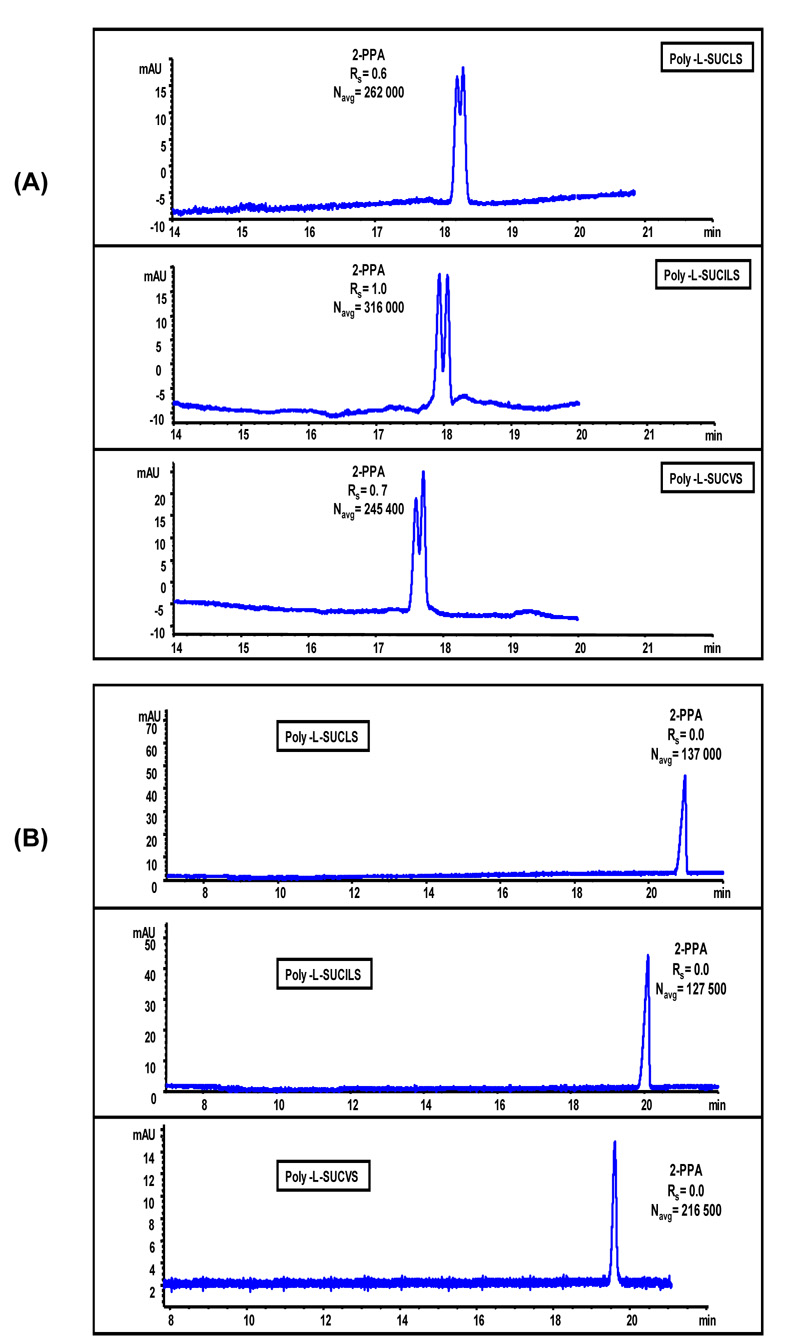
The enantiomers of (±)-2-PPA exist predominantly in the anionic form at pH ≥ 3.0 (pKa = 3.11 ± 0.1 ). This anionic chiral compound has been used for synthesis of antibiotics66 and often used as a herbicide.67 The chiral separation of (±)-2-PPA with all three poly-L-SUCAAS was compared at both low (Figure 11A) and high pH (Figure 11B). The enantiomers of (±)-2-PPA because of inherent negative charge poorly interact due to electrostatic repulsion with chiral anionic polymeric sulfated surfactants at basic pH. Therefore, as expected, no chiral resolution was obtained for (±)-2-PPA at pH 8.0. Since poly-L-SUCAAS has sulfated head group and is readily soluble in very low pH buffer, chiral separation was attempted at pH 2.0 (Fig 11A) where (±)-2-PPA is essentially neutral (pKa 3.11 ± 0.10). It can be seen in Figure 11A that partial chiral Rs of (±)-2-PPA was achieved at pH 2.0 with any of the three poly-L-SUCAAS surfactants. The successful enantioseparation of negatively charged (±)-2-PPA obtained with anionic poly-L-SUCAAS at low pH confirms that electrostatic attractive interactions does significantly contribute in the binding of charged analytes with oppositely charged polymeric surfactant. However, these interactions are not always the only major factor for chiral recognition. The hydrogen-bonding capability of the ether and the carboxylate groups in (±)-2-PPA (Figure 4) are also important in chiral discrimination using poly-L-SUCAAS surfactants. It is apparent from the electropherograms in Fig 11A that poly-L-SUCILS possessing two chiral centers provided slightly enhanced chiral Rs and N compared to poly-L-SUCLS and poly-L-SUCVS with one chiral center. However the chiral Rs of (±)-2-PPA could not be improved any further even after fine-tuning of the MEKC parameters (data not shown).
Figure 11.
Comparison of enantioseparation of ±-2-(2-chlorophenoxy)propanoic acid (±-2-PPA, 0.5 mg/mL in 50:50, MeOH/H2O) using (A) 50 mM poly-L-SUCAAS, pH 2.0. MEKC conditions are same as Figure10(A) except, 15 °C, pressure injection 50 mbar for 1s, UV detection at 200 nm, (B) 25 mM poly-L-SUCAAS, pH 8.0. MEKC conditions are same as Figure 10(B) except, pressure injection of 50 mbar for 1s, UV detection at 200 nm.
Enantioseparation of (±)-Benzoin Derivatives
Figure 12A and B show the simultaneous separation of four structurally related benzoin derivatives by using all three poly-L-SUCAAS at two different pH values, and with opposite polarity of high voltage power supply. The chiral separation of benzoin derivatives was performed to evaluate the effects of steric, hydrophobic and hydrogen-bonding factors on enantioselective interactions among these analytes and poly-L-SUCAAS. As shown in Figure 12A the most hydrophobic benzoin derivative (e. g., benzoin ethylether) elute first, where as the most hydrophilic benzoin derivative (e.g., (±)-hydrobenzoin) elute last at low pH condition under zero EOF and negative polarity of the voltage supply. On the other hand, at high pH the elution order of benzoin derivatives is exactly reversed (Figure 11B). However, under both high and low pH conditions, only (±)-hydrobenzoin could be separated into enantiomers but higher resolution was always obtained at low pH irrespective of the type of the polymeric sulfated surfactant. It seems like the structural rigidity of (±)-benzoin, (±)-benzoin methylether and (±)-benzoin ethylether due to the presence of carbonyl group completely hampers the enantioselective interactions between these analytes and poly-L-SUCAAS. Hence, the significant difference in chiral recognition is certainly due to the additional hydroxy group and less rigidity of (±)-hydrobenzoin compared to other benzoin derivatives. Again among poly-L-SUCAAS, the two chiral center bearing poly-L-SUCILS exhibited slightly higher enantioseparation of (±)-hydrobenzoin both at low and high pH.
Figure 12.
Comparison of simultaneous enantioseparation of four benzoin derivatives (0.33 mg/mL in 33:66, MeOH/H2O) using (A) 50 mM poly-L-SUCAAS, pH 3.0. MEKC conditions are same as 10(A) except, 20 °C, pressure injection 50 mbar for 1s, UV detection at 200 nm, (B) 25 mM poly-L-SUCAAS, pH 8.0. MEKC conditions are same as Figure 10(B) except, 20 °C, pressure injection of 50 mbar for 1s, UV detection at 200 nm.
Enantioseparation of (±)-PTH-amino Acids
Figure 13A and B show the chiral separation of three PTH-amino acids (AAs): (±)-PTH-tyrosine, (±)-PTH-isoleucine and (±)-PTH-tryptophan. Again, the migration order of all three PTH AAs and their respective enantiomers are exactly opposite under low and high pH conditions. At low pH (Figure 13A), using any of the three polymeric surfactant baseline resolution values were obtained for (±)-PTH-tyrosine compared to the partial resolution observed at high pH with two of the polymeric surfactants (Figure 13B). Furthermore, it is interesting to note that aromatic side chain containing PTH-amino acids (e.g., (±)-PTH-tyrosine and (±)-PTH-tryptophan) both showed inferior enantioselectivity with any of the three poly-L-SUCAAS compared to non-aromatic side chain containing PTH-amino acid (e.g., (±)-PTH-isoleucine). Similar to results obtained with (±)-2-PPA, (±)-atenolol and (±)-hydrobenzoin, at both low and high pH poly-L-SUCILS provided highest chiral resolution in most cases when compared to poly-L-SUCLS and poly-L-SUCVS. As mentioned earlier, this improved chiral separation capability of poly-L-SUCILS could be due to the presence of two chiral centers in poly-L-SUCILS, one of which is located near the surface of the micelle (on the side chain of the amino acid), easing the chiral interaction between the analyte and polymeric surfactant.
Figure 13.
Comparison of simultaneous enantioseparation of three PTH-amino acids (0.17 mg/mL in 50:50 MeOH/H2O) using (A) 15 mM poly-L-SUCAAS, pH 3.0. MEKC conditions are same as Figure 10(A) except, 20 °C, pressure injection of 50 mbar for 1s, UV detection at 269 nm, (B) 15 mM poly-L-SUCAAS, pH 8.0. MEKC conditions are same as Figure 10(B) except, pressure injection 50 mbar for 1s, UV detection at 269 nm.
Enantioseparation of (±)-Benzodiazepines
Three chiral and structurally related benzodiazepines were also separated at both low and high pH conditions employing poly-L-SUCLS, poly-L-SUCILS and poly-L-SUCVS. It is interesting to note that all three chiral benzodiazepinones show enantiomerization during chiral separation. The process of enantio-merization has been previously reported in GC, HPLC, and CE for the chiral separation of benzodiazepinones.68–70 This phenomenon often results in plateau formation and ultimately peak coalescence. The three chiral benzodiazepinones studied have similar molecular structure, differing only by the presence of chloro group on the phenyl ring and methyl group at the amide nitrogen in the benzodiazepinone skeleton (Figure 4). As noted for the other analytes, the migration order of the separated benzodiazepinones was reversed at low and high pH. At both low pH and high pH, (±)-temazepam always provided the highest resolution (irrespective of its elution order), followed by (±)-lorazepam and (±)-oxazepam using any of the three polymeric sulfated surfactants. It seems that the introduction of methyl group at the amide nitrogen in the benzodiazepinone skeleton (Figure 4) enhances the chiral interaction between the (±)-temazepam and the poly-L-SACAAS. This probably eliminates the competition for hydrogen bonding interactions between amide proton and hydroxyl proton (located next to the chiral center) on the analyte. Akbay et al. test the applicability of copolymerized surfactants obtained by polymerizing a chiral surfactant (sodium 10 undecenoyl-L-leucinate) with various molar fraction of achiral monomeric surfactant (sodium 10 undecenyl sulfate) for enantioseparation of three benzodiazepines.71 The enantiomers of (±)-temazepam provided the highest the resolution value compared to the other two benzodiazepines [(±)-oxazepam and (±)-lorazepam] at pH 8.0. However, the chiral resolution was found to decrease with increasing mole fraction of achiral sulfated surfactant in the co-polymer. In our studies, the two chiral center bearing poly-L-SUCILS provided better chiral resolution of benzodiazepinones compared to single chiral center poly-L-SUCLS and poly-L-SUCVS.
CONCLUSIONS
Three novel chiral amino acid based sulfated surfactants were synthesized and thoroughly characterized using various analytical techniques before and after polymerization. Extremely low temperature cryo-etch-HRSEM revealed tubular morphology of poly-L-SUCLS having distinct order of nanorods. In contrast, for poly-L-SUCILS, random distribution of filaments with heavy association of loosely and tightly bound water molecules was noticed. On the other hand, similar to poly-L-SUCLS, poly-L-SUCVS also showed tubular morphology but without any order of tubes.
This paper shows that enantioseparation of a small combinatorial library of PEAs using experimental design strategy resulted in optimum separation of all three classes of PEAs with minimum number of experiments. It was observed that the presence of phenolic hydroxy groups in class I and II PEAs compete with hydroxy group located next to the chiral center on the same molecule for enantioselective hydrogen bonding interactions with poly-L-SUCAAS resulting in lower chiral separation. For same two classes of PEAs, low pH chiral separation yielded enhanced Rs compared to basic pH and it is attributed to the change in conformation of micelle polymer. Enhanced MS signal abundance obtained at low pH chiral separation, led to a sensitive MEKC-MS method development of (±)-pseudoephedrine analysis in human urine. Very low LOD was obtained at pH 2.0, which is sixteen times lower as compared to the LOD obtained at pH 8.0.
Present study is the first demonstration in which successful chiral separations of a large number of structurally diverse acidic, basic and neutral racemic compounds were compared both at low and high pH conditions in MEKC using polymeric sulfated surfactants. Acidic analyte (±)-2-PPA at pH 2.0 provided chiral separation due to neutrality of negative charge, which is unattainable at basic pH due to mutual repulsion between analyte and anionic chiral selector. Basic analytes such as □-blockers [(±)-atenolol, (±)-metoprolol] provided improved chiral separation at low pH and believed to be the result of conformational transition of poly-L-SUCAAS under acidic conditions, resulting in favorable chiral interactions between □-blockers and poly-L-SUCAAS. Chiral separation of neutral analytes (e.g., benzoin derivatives) revealed the fact that poly-L-SUCAAS is highly sensitive to the structural variation of the chiral analytes. Increased rigidity (i.e., addition of carbonyl group) and alkyl substituent on hydoxy group result in total loss of chiral Rs. For another class of neutral analytes (e.g., PTH-amino acids), it is observed that aromatic ring bearing PTH-amino acids do not interact sufficiently with polymeric surfactant and result in lower chiral separation compared to aliphatic PTH-amino acids. Finally, eliminating nonenantio-selective hydrogen bonding sites (amide hydrogen) in case of benzodiazepines [e.g., (±)-temazepam] conferred maximum effect on chiral Rs compared to the other two s [(±)-oxazepam and (±)-lorazepam]. Among the three poly-L-SUCAAS investigated, poly-L-SUCILS exhibited overall the best chiral separation capability possibly due to dual chiral centers, one of which (located on the side chain of the amino acid) is very close to the micellar surface easing enantioselective interactions between chiral analyte and poly-L-SUCILS.
Figure 14.
Comparison of simultaneous enantioseparation of three benzodiazepines (0.17 mg/mL in 50:50, MeOH/H2O) using (A) 10 mM poly-L-SUCAAS, pH 3.0. MEKC conditions are same as 10(A) except 15 % (v/v) ACN was used in buffer, 17 °C, pressure injection of 25 mbar for 1s, UV detection at 200 nm, (B) 10 mM poly-L-SUCAAS, pH 8.0, 10 %ACN. MEKC conditions are same as 10(B) except, 17 °C, pressure injection 25 mbar for 1s.
ACKNOWLEDGEMENTS
This work was supported by grant from the National Institutes of Health (Grant No. 62314-02) and Petroleum Research Fund.
References
- 1.Davankov VA. Pure Appl. Chem. 1997;69:1469–1474. [Google Scholar]
- 2.Ward TJ, Hamburg DM. Anal. Chem. 2004;76:4635–4644. doi: 10.1021/ac040093t. [DOI] [PubMed] [Google Scholar]
- 3.Beesley TE, Scott RP. Chiral Chromatography. New York: John Wiley & Sons; 1999. [Google Scholar]
- 4.Aboul-Enein HY, editor. Chiral Separations by Liquid Chromatography: Theory and Applications (Chromatographic Science, Vol. 90) (Chromatographic Science) Boca Raton, FL: CRC-Press; 2003. [Google Scholar]
- 5.Gubitz G, Schmid MG, editors. Chiral Separations: Methods and Protocols (Methods in Molecular Biology) New Jersey: Humana Press; 2004. [Google Scholar]
- 6.Landers JP. Handbook of Capillary Electrophoresis. Boca Raton, FL: CRC-Press; 1997. [Google Scholar]
- 7.Khaledi MG, editor. High-Performance Capillary Electrophoresis: Theory, Techniques, and Applications. New York: Wiley-Interscience; 1998. [Google Scholar]
- 8.Weinberg R. Practical Capillary Electrophoresis. San Diego, CA: Academic Press; 2000. [Google Scholar]
- 9.Camilleri P, editor. Capillary Electrophoresis: Theory and Practice. Boca Raton, FL: CRC-Press; 1997. [Google Scholar]
- 10.Shintani J, Polonski J, editors. Handbook of Capillary Electrophoresis. London: Chapman & Hall; 1996. [Google Scholar]
- 11.Vindevogel J, Sandra P. Introduction to Micellar Electrokinetic Chromatography. Heidelberg: Huthig Pub Ltd; 1992. [Google Scholar]
- 12.Wang, Warner Anal. Chem. 1994;66:3773–3776. [Google Scholar]
- 13.Billiot EJ, Macossay JM, Thibodeaux S, Shamsi SA, Warner IM. Anal. Chem. 1998;70:1375–1381. doi: 10.1021/ac9709561. [DOI] [PubMed] [Google Scholar]
- 14.Varghese J, Cole RB. J. Chromatogr., A. 1993;652:369–376. doi: 10.1016/0021-9673(93)83255-Q. [DOI] [PubMed] [Google Scholar]
- 15.Lu W, Poon GK, Carmichael PL, Cole RB. Anal. Chem. 1996;68:668–674. doi: 10.1021/ac950786x. [DOI] [PubMed] [Google Scholar]
- 16.Schulte G, Heitmeier S, Chankvetadze B, Blaschke G. J. Chromatogr.A. 1998;800:77–82. [Google Scholar]
- 17.Cherkaoui S, Veuther J-L. J. Pharm. Biomed. Anal. 2002;27:615–626. doi: 10.1016/s0731-7085(01)00577-5. [DOI] [PubMed] [Google Scholar]
- 18.Fanali S, Desiderio C, Schule G, Hetimeier S, Strickmann D, Chankvetadze B, Blaschke G. J. Chromatogr. A. 1998;800:69–76. [Google Scholar]
- 19.Jäverfalk EM, Amini A, Westerlund D, Andrén, Per E. J. Mass Spectrom. 1998;33:183–186. [Google Scholar]
- 20.Cherkaoui S, Rudaz S, Varesio E, Veuthey J-L. Electrophoresis. 2001;22:3308–3315. doi: 10.1002/1522-2683(200109)22:15<3308::AID-ELPS3308>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka Y, Otsuka K, Terabe S. J. Chromatogr. A. 2000;875:323–330. doi: 10.1016/s0021-9673(99)01334-5. [DOI] [PubMed] [Google Scholar]
- 22.Shamsi SA. Electrophoresis. 2002;23:4036–4051. doi: 10.1002/elps.200290017. [DOI] [PubMed] [Google Scholar]
- 23.Rudaz S, Cherkaoui S, Dayer P, Fanali S, Veuthey J-L. J. Chromatogr. A. 2000;868:295–303. doi: 10.1016/s0021-9673(99)01257-1. [DOI] [PubMed] [Google Scholar]
- 24.Piirma I. Polymeric Surfactants. New York: Marcel Dekker, Inc; 1992. [Google Scholar]
- 25.Gambogi RJ, Blum FD. J. Colloid Interface Sci. 1990;140:525–534. [Google Scholar]
- 26.Shamsi SA, Palmer CP, Warner IM. Anal. Chem. 2001;73:140A–149A. doi: 10.1021/ac012412b. [DOI] [PubMed] [Google Scholar]
- 27.Rizvi SAA, Shamsi SA. Electrophoresis. 2003;24:2514–2526. doi: 10.1002/elps.200305516. [DOI] [PubMed] [Google Scholar]
- 28.Rizvi SAA, Akbay C, Shamsi SA. Electrophoresis. 2004;25:853–860. doi: 10.1002/elps.200305762. [DOI] [PubMed] [Google Scholar]
- 29.Rizvi SAA, Simons ND, Shamsi SA. Electrophoresis. 2004;25:712–722. doi: 10.1002/elps.200305774. [DOI] [PubMed] [Google Scholar]
- 30.Rizvi SAA, Shamsi SA. Electrophoresis. 2005;26:4172–4186. doi: 10.1002/elps.200500199. [DOI] [PubMed] [Google Scholar]
- 31.Hou J, Rizvi SAA, Zheng J, Shamsi SA. Electrophoresis. 2006;27:1263–1275. doi: 10.1002/elps.200500610. [DOI] [PubMed] [Google Scholar]
- 32.Tarus J, Agbaria RA, Morris K, Mwongela S, Numan A, Simuli L, Fletcher KA, Warner IM. Langmuir. 2004;20:6887–6895. doi: 10.1021/la036349s. [DOI] [PubMed] [Google Scholar]
- 33.Valle BC, Billiot FH, Shamsi SA, Zhu XF, Powe AM, Warner IM. Electrophoresis. 2004;25:743–752. doi: 10.1002/elps.200305726. [DOI] [PubMed] [Google Scholar]
- 34.Palmer CP. Electrophoresis. 2002;23:3993–4004. doi: 10.1002/elps.200290014. [DOI] [PubMed] [Google Scholar]
- 35.Billiot FH, Billiot EJ, Warner IM. J. Chromatogr. A. 2001;922:329–338. doi: 10.1016/s0021-9673(01)00865-2. [DOI] [PubMed] [Google Scholar]
- 36.Palmer CP, Terabe S. Anal. Chem. 1997;69:1852–1860. [Google Scholar]
- 37.Akbay C, Shamsi SA. Electrophoresis. 2004;25:622–634. doi: 10.1002/elps.200305763. [DOI] [PubMed] [Google Scholar]
- 38.Akbay C, Shamsi SA. Electrophoresis. 2004;25:635–644. doi: 10.1002/elps.200305764. [DOI] [PubMed] [Google Scholar]
- 39.Rundlett KL, Armstrong DW. Anal. Chem. 1996;68:3493–3497. doi: 10.1021/ac960472p. [DOI] [PubMed] [Google Scholar]
- 40.Shamsi SA. Anal. Chem. 2001;73:5103–5108. doi: 10.1021/ac0105179. [DOI] [PubMed] [Google Scholar]
- 41.Akbay C, Rizvi SAA, Shamsi SA. Anal. Chem. 2005;77:1672–1683. doi: 10.1021/ac0401422. [DOI] [PubMed] [Google Scholar]
- 42.Cifuentes A, Bartolome B, Gomez-Cordoves C. Electrophoresis. 2001;22:1561–1567. doi: 10.1002/1522-2683(200105)22:8<1561::AID-ELPS1561>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 43.Rodríguez-Delgado MA, Garcia-Montelongo FJ, Cifuentes A. Anal. Chem. 2002;74:257–260. doi: 10.1021/ac010838k. [DOI] [PubMed] [Google Scholar]
- 44.Shamsi SA, Warner IM. Electrophoresis. 1997;18:853–872. doi: 10.1002/elps.1150180604. [DOI] [PubMed] [Google Scholar]
- 45.Palmer CP, McCarney JP. Electrophoresis. 2004;25:4086–4094. doi: 10.1002/elps.200406110. [DOI] [PubMed] [Google Scholar]
- 46.Turro NJ, Yekta A. J. Am. Chem. Soc. 1978;100:5951–5952. [Google Scholar]
- 47.Omínguez A, Fernández A, González N, Iglesias E, Montenegro L. J. Chem. Educ. 1997;74:1227–1231. [Google Scholar]
- 48.Apkarian RP, Wright ER, Seredyuk VA, Eustis S, Lyon LA, Conticello VP, Menger FM. Microsc. Microanal. 2003;9(4):286–295. doi: 10.1017/S1431927603030551. [DOI] [PubMed] [Google Scholar]
- 49.Apkarian RP, Wright ER, Seredyuk VA, Eustis S, Lyon LA, Conticello VP, Menger FM. 61st Ann. Proc. Microsc. Soc. America. Microsc. & Microanal. 2003;9 Suppl 2:1542–1543. doi: 10.1017/S1431927603030551. [DOI] [PubMed] [Google Scholar]
- 50.Velegol SB, Pardi S, Li X, Velegol D, Logan BE. Langmuir. 2003;19:851–857. [Google Scholar]
- 51.Menger FM, Seredyuk VA, Apkarian RP, Wright ER. J. Am. Chem. Soc. 2002;124(42):12408–12409. doi: 10.1021/ja021025w. [DOI] [PubMed] [Google Scholar]
- 52.Talmon Y. In: Modern Characterization Methods of Surfactant Systems. Binks B, editor. Boca Raton, FL: CRC-Press; 1999. pp. 147–180. [Google Scholar]
- 53.Moroi Y. Micelles. Theoretical and Applied Aspects. New York: Plenum; 1992. [Google Scholar]
- 54.Dwars T, Paetzold E, Oehme G. Angew. Chem. Int. Ed. 2005;44:7174–7199. doi: 10.1002/anie.200501365. [DOI] [PubMed] [Google Scholar]
- 55.Vander Heyden Y, Questier F, Massart L. J. Pharm. Biomed. Anal. 1996;14:1313–1326. doi: 10.1016/s0731-7085(98)00174-5. [DOI] [PubMed] [Google Scholar]
- 56.Heyden YV, Khots MS, Massart DL. Anal. Chim. Acta. 1993;276:189–195. [Google Scholar]
- 57.Mangelings D, Tarnet I, Matthiis N, Maftouh M, Massart DL, Heyden YV. Electrophoresis. 2005;26:818–832. doi: 10.1002/elps.200410190. [DOI] [PubMed] [Google Scholar]
- 58.Silverman RB. The Organic Chemistry of Drug Design and Drug Action. San Diego, CA: Academic Press; 2004. [Google Scholar]
- 59.Chu DY, Thomas JK. Mucromorecules. 1991;24:2133–2138. [Google Scholar]
- 60.Chu DY, Thomas JK. Mucromorecules. 1991;24:22212–22216. [Google Scholar]
- 61.Chan KH, Pan RN, Hsu MC. Biomed. Chromatogr. 2005;19:337–342. doi: 10.1002/bmc.452. [DOI] [PubMed] [Google Scholar]
- 62.Ouyang J, Gao X, Baeyens WRG, Delanghe JR. Biomed. Chromatogr. 2005;19:266–271. doi: 10.1002/bmc.448. [DOI] [PubMed] [Google Scholar]
- 63.Iwata YT, Garcia A, Kanamori T, Inoue H, Kishi T, Lurie IS. Electrophoresis. 2002;23:1328–1334. doi: 10.1002/1522-2683(200205)23:9<1328::AID-ELPS1328>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 64.Simons FE, Gu X, Watson WT, Simons KJ. J Pediatr. 1996;129(5):729–734. doi: 10.1016/s0022-3476(96)70157-9. [DOI] [PubMed] [Google Scholar]
- 65.Shi W, Palmer CP. J. Sep. Sci. 2002;25:215–221. [Google Scholar]
- 66.Perron YG, Minor WF, Holdrege CT, Gottstein WJ, Godfrey JC, Crast LB, Babel RB, Cheney LC. J. Am. Chem. Soc. 1960;82:3934–3938. [Google Scholar]
- 67.Smith G, Kennard CHL, White AH. Acta Cryst. 1981;B37:275–277. [Google Scholar]
- 68.Schoetz G, Trapp O, Schurig V. Anal. Chem. 2000;72:2758–2764. doi: 10.1021/ac991439g. [DOI] [PubMed] [Google Scholar]
- 69.Trapp O, Schoetz G, Schuring V. Chirality. 2001;13:403–414. doi: 10.1002/chir.1052. [DOI] [PubMed] [Google Scholar]
- 70.Trapp O. Anal. Chem. 2006;78:189–198. doi: 10.1021/ac051655r. [DOI] [PubMed] [Google Scholar]
- 71.Akbay C, Gill NL, Agbaria RA, Warner IM. Electrophoresis. 2003;24:4209–4220. doi: 10.1002/elps.200305630. [DOI] [PubMed] [Google Scholar]