Abstract
Allopregnanolone (ALLO) and androsterone (ADT) are naturally occurring 3α-hydroxysteroids that act as positive allosteric regulators of γ-aminobutyric acid type A receptors. In addition, ADT activates nuclear farnesoid X receptor and ALLO activates pregnane X receptor. At least with respect to γ-aminobutyric acid type A receptors, the biological activity of ALLO and ADT depends on the 3α-hydroxyl group and is lost upon its conversion to either 3-ketosteroid or 3β-hydroxyl epimer. Such strict structure-activity relationships suggest that the oxidation or epimerization of 3α-hydroxysteroids may serve as physiologically relevant mechanisms for the control of the local concentrations of bioactive 3α-hydroxysteroids. The exact enzymes responsible for the oxidation and epimerization of 3α-hydroxysteroids in vivo have not yet been identified, but our previous studies showed that microsomal nicotinamide adenine dinucleotide-dependent short-chain dehydrogenases/reductases (SDRs) with dual retinol/sterol dehydrogenase substrate specificity (RoDH-like group of SDRs) can oxidize and epimerize 3α-hydroxysteroids in vitro. Here, we present the first evidence that microsomal nicotinamide adenine dinucleotide-dependent 3α-hydroxysteroid dehydrogenase/epimerase activities are widely distributed in human tissues with the highest activity levels found in liver and testis and lower levels in lung, spleen, brain, kidney, and ovary. We demonstrate that RoDH-like SDRs contribute to the oxidation and epimerization of ALLO and ADT in living cells, and show that RoDH enzymes are expressed in tissues that have microsomal 3α-hydroxysteroid dehydrogenase/epimerase activities. Together, these results provide further support for the role of RoDH-like SDRs in human metabolism of 3α-hydroxysteroids and offer a new insight into the enzymology of ALLO and ADT inactivation.
3α-Hydroxysteroids are formed endogenously as natural products of cholesterol metabolism. Some of these compounds exhibit potent regulatory properties. For example, a C21 3α-hydroxysteroid allopregnanolone (ALLO, 3α-hydroxy-5α-pregnan-20-one) binds to γ-aminobutyric acid type A (GABAA) receptors with high affinity and potentiates γ-aminobutyric acid-evoked chloride ion channel conductance (1–3). Other actions of ALLO include the induction of myelin formation (4, 5), promotion of neuronal survival (6, 7), and delay of the onset and severity of neurodegenerative pathology in a mouse model of Niemann-Pick’s disease (8). As suggested recently, the latter effect may be due to ALLO activation of pregnane X receptors (9).
Similarly to ALLO, a C19 3α-hydroxysteroid androsterone (ADT, 3α-hydroxy-5α-androstan-17-one), which is the major metabolite of testosterone and the first androgen to be identified (10), acts as a positive allosteric regulator of GABAA receptors, and exhibits anticonvulsant properties (11). In addition to regulating the membrane GABAA receptors, ADT appears to function as a direct activator of the nuclear farnesoid X receptor (12), suggesting a role for ADT in modulation of cholesterol, lipid, and glucose metabolism (13).
At least with respect to GABAA receptors, the biological potencies of both ALLO and ADT are determined by the functional group at carbon 3. The oxidation of 3α-hydroxyl group to 3-ketone group results in a loss of biological activity (14). Furthermore, the 3β-epimers of both compounds, epiallopregnanolone (epiALLO) and epiandrosterone (epi-ADT), are not only inactive (10, 15), but epiALLO is known to act as a functional antagonist of ALLO at GABAA receptors (16–20). Such strict structure-activity relationships suggest that the oxidation and epimerization of 3α-hydroxysteroids might serve as physiologically relevant mechanisms for the regulation of their physiological actions.
In vitro, the oxidation of 3α-hydroxysteroids can be catalyzed by two types of enzymes: the cytosolic aldo-keto reductases (AKRs) and the membrane-bound short-chain dehydrogenases/reductases (SDRs). Although bidirectional in vitro, the NADP+-dependent AKRs function in the reductive direction in living cells and are thought to be primarily responsible for the reduction of 3-ketosteroids to 3α-hydroxysteroids (21). On the other hand, the oxidation of 3α-hydroxysteroids to 3-ketosteroids is thought to be carried out by the members of the SDR superfamily of proteins (22–28). Some of these enzymes, specifically retinol/sterol dehydrogenase (RoDH)-like SDRs, can also catalyze the conversion of ADT and ALLO to their respective 3β-epimers in vitro (23–25).
RoDH-like SDRs are bifunctional nicotinamide adenine dinucleotide (NAD+)-dependent microsomal enzymes that recognize both retinoids (thus the name RoDH for retinol dehydrogenase) and 3α-hydroxysteroids as substrates (29). Humans have four microsomal SDRs with a 3α-hydroxysteroid dehydrogenase (3α-HSD) activity: RoDH-4 (28), RoDH-like 3α-HSD (RL-HSD) (22, 23, 25), retinol dehydrogenase-like (RDHL, also known as nonhepatic 3α-HSD (24)], and 11-cis-retinol dehydrogenase (11-cis-RDH) (30). Two of these enzymes, RL-HSD and RDHL, exhibit both a 3α-HSD and a 3(α→β)-hydroxysteroid epimerase (3(α→β)-HSE) activities in vitro (23, 25).
Few studies that have been carried out thus far have shown that the microsomal NAD+-dependent 3α-HSD activity exists in rat brain (31–33) and in human lung (34). These studies examined the activity of microsomes in the reverse direction, the reduction of 3-ketosteroids [5α-dihydroprogesterone (DHP) or 5α-dihydrotestosterone (DHT)] to their corresponding alcohol forms in the presence of reduced nicotinamide adenine dinucleotide (NADH). Under these conditions, epimerization of the 3α-hydroxyl group to 3β-hydroxyl group could not have been detected. To date, it remains unknown whether human or animal tissues possess a microsomal 3(α→β)-HSE activity and whether the tissue 3α-HSD/3(α→β)-HSE activity could be due to the presence of RoDH-like SDR dehydrogenases/epimerases. Furthermore, it has not yet been established whether RoDH-like SDRs can oxidize or epimerize ALLO and ADT in the cellular environment. To address these questions, we investigated the distribution and levels of microsomal 3α-HSD/(α→β)-HSE activity in human tissues, examined relative potencies of SDR enzymes as 3α-HSDs and 3(α→β)-HSEs in living cells, and established whether RoDH-like SDRs are expressed in tissues that possess the microsomal NAD+-dependent 3α-HSD/3(α→β)-HSE activities.
Materials and Methods
Isolation of the light membrane fractions from human tissues
Frozen samples of testis, lung, ovary, kidney, heart, spleen, and skeletal muscle were obtained from the Anatomical Gift Foundation and stored at −80 C. Human brain samples were provided by the Alzheimer Disease Center of the University of Kansas Medical Center. Frozen tissues were homogenized in 50 mM Tris-acetate buffer, pH 7.4, 1 mM dithiothreitol, 0.1 mM EDTA, 0.25 M sucrose. Cellular debris and mitochondria were removed by centrifugation at 1,000 × g and 10,000 × g, respectively, for 15 min each. The light membrane fraction was pelleted by centrifugation at 105,000 × g for 2 h through a 0.6 M sucrose cushion and resuspended in 90 mM potassium phosphate, pH 7.4, 40 mM KCl, 0.1 mM EDTA, 1 mM dithiothreitol, 20% glycerol. Protein concentration was determined by Lowry et al. (35) using BSA as a standard.
Activity assays
The light membrane fractions of human tissues were incubated with tritiated steroids in the presence of 1 mM NAD+ at 37 C for various times as indicated. All reactions were carried out in 90 mM potassium phosphate, pH 7.4, and 40 mM KCl (reaction buffer) in siliconized glass tubes as described previously (28). Commercially available radiolabeled steroids (NEN Life Science Products, Boston, MA) (~40–60 Ci/mmol each) were diluted with cold steroids (Steraloids Inc., Newport, RI; and Sigma, St. Louis, MO) dissolved in dimethyl sulfoxide (Me2SO). The 125μl reactions (final concentration of Me2SO < 1%) were started with the addition of membranes. The reactions were stopped by adding 25 volumes of methylene chloride and placed on ice, followed by centrifugation. The organic layer was evaporated under a stream of nitrogen and dissolved in 40 μl of methylene chloride. Steroids were separated by development in toluene:acetone (4:1) on silica gel thin-layer chromatography (TLC) plates (Sigma). TLC plates containing 3H-labeled steroids were exposed to PhosphorImager tritium screen (GE Healthcare Life Sciences, Piscataway, NJ) overnight, and the intensity of the bands was calculated using ImageQuanT 5.0 program. In addition, TLC plates were cut into 1-cm wide sections, which were then counted in scintillation fluid (Bio-Safe II; Research Products International Corp., Mt. Prospect, IL). Products of each reaction were identified by comparison with reference steroids. A control without added cofactor was included with each sample. Sf9 insect cell microsomes containing recombinant enzymes were used as positive controls for 3α-HSD activity.
Preparation of human embryonic kidney (HEK) 293 cells stably transfected with human RoDH-like SDRs
For measurements of RoDH-like SDR activities in the cells, the cDNAs for RoDH-4 and RL-HSD were stably transfected into HEK293 cells (ATCC, Manassas, VA). The cDNA for RoDH-4 was cloned into a eukaryotic expression vector pIRESneo (Clontech, Mountain View, CA), which was cleaved with BstXI and blunt-ended with T4 DNA polymerase, then digested with BamHI. RoDH-4 cDNA previously cloned into BglII and XbaI restriction sites of pVL1392 vector (28) was cleaved on the 3′ end with XbaI endonuclease. The cleaved end was blunt-ended with T4 DNA polymerase, and the cDNA was excised from the pVL1392 vector by cleaving its 5′ end with BglII endonuclease. RoDH-4 cDNA with one sticky end (BglII site) and one blunt end was ligated into the BamHI site (compatible with BglII) and the blunt site of pIRESneo.
To prepare expression vector for RL-HSD, the corresponding cDNA previously cloned into the BamHI and EcoRI restriction sites of pVL1393 vector (23) was cleaved on the 3′ end with EcoRI endonuclease followed by blunt-ending with T4 DNA polymerase, and then excised from pVL1393 vector by cleaving on the 5′ end with BamHI endonuclease. RL-HSD cDNA with one sticky end (BamHI site) and one blunt end was ligated into the matching sites of pIRESneo prepared as described above. All expression constructs were verified by sequencing.
pIRES vectors containing RoDH cDNAs were transfected into HEK293 cells using Lipofectamine and Plus Reagent in Opti-MEM medium as suggested by the manufacturer (Invitrogen, Carlsbad, CA). Control cells were prepared by transfecting empty pIRESneo vector into HEK293 cells. Forty-eight hours after transfection, the cells received fresh MEM supplemented with 10% horse serum and antibiotic G418 (0.4 mg/ml). After 2 wk postplating, independent G418-resistant cell foci were isolated with cloning rings, detached with trypsin-EDTA, and transferred to 96-well multiwell dishes. The cloned cell lines were expanded in MEM containing G418 (0.4 mg/ml). HEK293 cells stably transfected with RDHL were obtained from Dr. D. P. Uzunov (Neuroscience Research, Novartis Institutes for BioMedical Research, Novartis Pharma AG, WSJ-386.3.264002, Basel, Switzerland) (36).
Cells stably expressing RoDH-like SDRs were incubated with tritiated ALLO, ADT, or DHP for various times as indicated. DHP was synthesized enzymatically by incubating 1 μM radiolabeled ALLO with 10–20 μg of RoDH-4-expressing Sf9 microsomes in the presence of 1 mM NAD+ in 1 ml for 30 min at 37 C and purified by TLC.
The apparent Km value of RoDH-4 for the oxidation of ALLO was determined at a fixed NAD+ (1 mM) concentration and six concentrations of ALLO between 0.0625 and 1.0 μM using microsomal preparation of RoDH-4 expressed in Sf9 cells as described previously (28). The amount of enzyme was adjusted so that the product formed was less than 10% of the amount of substrate within the 15-min reaction time and was linearly proportional to the amount of microsomes added. A baseline value obtained in the absence of added cofactor was subtracted from each experimental data point.
Western blot analysis and immunohistochemistry
Polyclonal antisera were raised in rabbits against the N-terminal fragment of RoDH-4 (amino acids 22–104), the C-terminal fragment of RoDH-4 (amino acids 159–304), the N-terminal fragment of RL-HSD (amino acids 22–104), and the N-terminal fragment of 11-cis-RDH (amino acids 22–103). In addition, polyclonal antiserum against the protein-specific peptide ERMKQSWKEAPKHIKETYGQQY of RL-HSD was raised in chickens by Cocalico Biologicals Inc. (Reamstown, PA) Affinity-purified chicken IgY fraction was obtained using peptide coupled to agarose.
For Western blot analysis, microsomal proteins extracted from tissue samples were separated in 12% denaturing polyacrylamide gel, and transferred to Hybond-P membrane (Amersham Pharmacia Biotech, Piscataway, NJ). Protein was detected using ECL Western blotting analysis system (Amersham Pharmacia Biotech) according to the manufacturer’s instructions. The rabbit polyclonal primary antisera were used at a 1:3000 to 1:5000 dilution, chicken anti-peptide antiserum was used at a 1:500 dilution. The antisera were diluted in 3% BSA, 20 mM Tris, pH 7.6, 137 mM NaCl, and 0.1% Tween 20. Visualization was performed using horseradish peroxidase-conjugated antirabbit antibodies (at 1:10,000 dilution) and ECL Western blotting detection reagents (Amersham Pharmacia Biotech).
For immunohistochemical analysis, the Hybrid-Ready Human Neural Tissue Slides from Novagen (Madison, WI) were deparaffinized by three 5-min washes in different containers of xylenes. Xylenes were removed by two 5-min washes in 100% ethanol, and endogenous tissue peroxidase activity was inactivated by incubating the slides with 3% H2O2 in absolute methanol (1:4) for 5 min. Tissue was rehydrated by a series of 5-min washes in containers with decreasing concentrations of ethanol (95%, 70%, 50%, and 30%) and washed twice in PBS. Nonspecific antibody binding was blocked by incubation for 30 min at room temperature in PBS containing 10% of goat serum. Sections were incubated overnight at 4 C in primary anti-RoDH-4 antibodies diluted 1:100 in PBS with 0.2% Triton X-100 (PBS-TX). The next day, sections were washed three times in PBS-TX for 5 min each, and then incubated for 1 h in goat antirabbit secondary antibody conjugated to horseradish peroxidase (1:50; Jackson ImmunoResearch, West Grove, PA) in PBS-TX at room temperature. Sections were washed for 5 min in PBS-TX, followed by two 5 min washes in 0.1 M Tris-saline. Antigen-antibody complexes were visualized by incubation in 3,3′-diaminobenzidine in 0.1 M Tris-saline containing 0.001% H2O2 for 10–15 min, and then rinsed in PBS for 5 min and in distilled water for 3 min. Sections were dehydrated through graded ethanol, cleared in xylene, and mounted with Permount (Fisher Scientific, Pittsburgh, PA). Histochemical controls included incubations with preimmune serum and omission of the primary antiserum. Assays were repeated on three to five occasions; results of these experiments agreed, attesting to the consistency of the method. Substitution of immune serum for preimmune serum or omission of primary antiserum resulted in no specific staining.
Results
Distribution of microsomal 3α-hydroxysteroid dehydrogenase/epimerase activity in human tissues
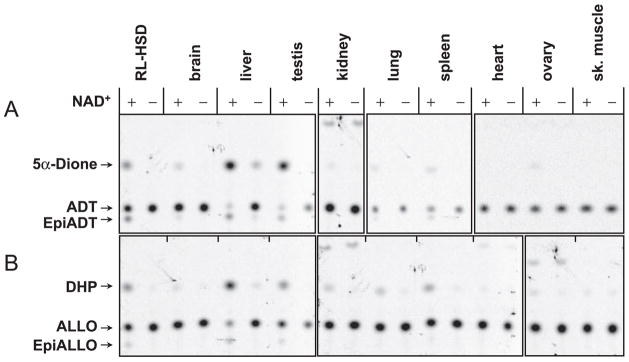
To determine whether human tissues possess microsomal 3α-HSD/3(α→β)-HSE activities, light membrane fractions were isolated from eight different human tissues and incubated with two different 3α-hydroxysteroids, ALLO and ADT, in the presence or absence of NAD+. Reaction products were analyzed by TLC. As can be seen on radiochromatogram in Fig. 1, the products of 3α-hydroxyl oxidation of both ALLO and ADT were observed in all reactions except those that contained membranes from heart and skeletal muscle. The 3α-hydroxyl oxidation depended on the addition of NAD+ because little or no product was formed in its absence. To adjust for the great range of activity levels among tissues, different amounts of microsomes were used for different tissue samples. The amount of radioactivity associated with the product generated by samples with the lowest activity was at least 5-fold greater than the background radioactivity of the flanking plate segments.
Fig. 1.
Distribution of the NAD+-dependent microsomal 3α-HSD/3(α→β)-HSE activity in human tissues. The light membrane fractions were incubated in the presence of 1 mM NAD+ and 1 μM ADT (A) or 1 μM ALLO (B) for 1 h at 37 C. Ten micrograms of liver and testis membranes were used in the reaction with ADT and 2.5 micrograms were used in the reaction with ALLO. For all other tissues, the reactions contained 62 μg of the membrane protein. Control samples contained the same amount of protein but lacked the cofactor. A sample with recombinant RL-HSD was included as a positive control for the 3α-HSD activity. It should be noted that different postmortem collection times could differentially influence enzyme activities in various tissues, although the activities of recombinant RoDH-like SDRs in microsomal preparations are rather stable.
The highest levels of 3α-HSD activity were detected in liver and testis (Table 1). Spleen and lung had at least 140-fold lower activities (Table 1), and ovary, kidney, and brain displayed approximately three orders of magnitude lower activities than liver or testis (Table 1). These results demonstrated that many human tissues contain microsomal NAD+-dependent 3α-HSD activity and that its levels vary greatly among tissues.
TABLE 1.
NAD+-dependent microsomal 3α-HSD activity of human tissues
| Tissue | 3α-HSD activity (pmol·min−1mg−1)
|
|
|---|---|---|
| ADT | ALLO | |
| Liver | 3400 ± 270 | 4000 ± 300 |
| Testis | 520 ± 20 | 550 ± 37 |
| Lung | 5.6 ± 0.3 | 1.8 ± 0.1 |
| Spleen | 7.7 ± 1.7 | 5.3± 0.2 |
| Brain | 1.4 ± 0.3 | 0.8 ± 0.1 |
| Kidney | 0.4 ± 0.1 | 2.3 ± 0.3 |
| Ovary | 2.0 ± 0.3 | 1.0 ± 0.2 |
| Heart | ~0.1 | ~0.3 |
| Skeletal muscle | ~0.1 | ~0.1 |
The 3α-HSD activities were measured using 1 μM ADT or 1 μM ALLO as substrates in the presence of 1 mM NAD+. The amount of microsomes was adjusted so that no more than 10% of the substrate was utilized during the reaction time.
In addition to 3-ketone products, some of the tissue microsomes produced 3β-epimers of ALLO and ADT. ADT was actively epimerized by microsomes from liver, testis, lung, spleen, and brain, whereas epimerization of ALLO was detected in the reactions with liver, testis, and spleen microsomes. Importantly, the 3(α→β)-HSE activity required NAD+ as a cofactor and was detected only in those reactions that also produced 3-ketosteroids, suggesting that epimerization occurs via 3-ketosteroids as intermediate products. These results demonstrated for the first time that human tissue microsomes possess an NAD+-dependent 3(γ→β)-HSE activity.
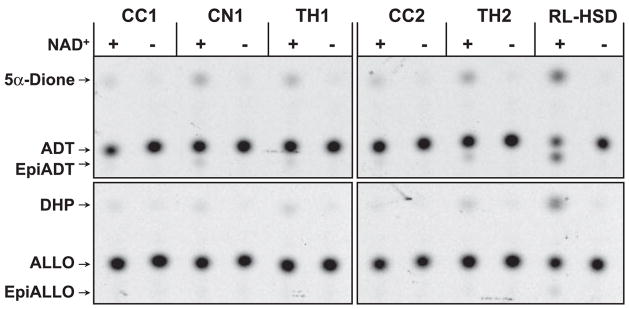
Because of great interest in the regulation of biosynthesis and degradation of 3α-hydroxysteroids in the brain (37), we examined the microsomal 3α-HSD/3(α→β)-HSE activities of human brain in more detail. It was reported that the NAD+-dependent 3α-HSD activity exhibits a region-specific distribution in rat brain (33); therefore, samples from three different regions of human brain were used for analysis. ADT was oxidized and epimerized in all three brain regions, but the relative activities appeared to be higher in caudate nucleus and thalamus than in corpus callosum (Fig. 2). Similarly, ALLO was oxidized by microsomes from all three regions, but thalamus microsomes appeared to be the most active. Some epimerization of ALLO was also detected, although it was weaker compared with epimerization of ADT. Thus, analysis of brain regions from two donors confirmed that 3α-hydroxysteroids can be oxidized and epimerized by human brain microsomes in the presence of NAD+ and suggested that both 3α-HSD and 3(α→β)-HSE activities may be distributed in a brain region-specific manner.
Fig. 2.
The NAD+-dependent microsomal 3α-HSD/3(α→β)-HSE activity of human brain. The light membrane fractions from corpus callosum (CC), caudate nucleus (CN), and thalamus from two different donors (TH1 and TH2) were incubated with 1 μM ADT or 1 μM ALLO in the presence or absence of 1 mM NAD+ at 37 C for 2 h. The amount of protein used in each reaction was as follows: 140 μg CC1, 340 μg CN1, 160 μg TH1, 190 μg CC2, and 90 μg TH2. Caudate nucleus from donor 2 is shown in Fig. 1.
Characterization of the 3α-HSD/3(α→β)-HSE activities of human RoDH-like SDRs in living cells
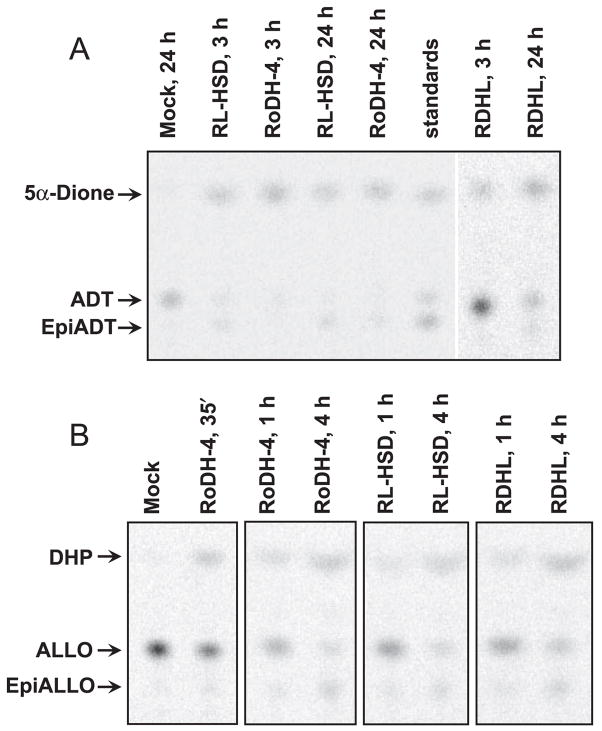
To determine whether RoDH-like enzymes are capable of oxidizing and epimerizing ALLO and ADT in living cells, we prepared cell lines stably transfected with each enzyme and incubated the cells with either ALLO or ADT. With ADT as substrate, there was a clear difference in the products of ADT metabolism depending on the enzyme expressed in the cells (Fig. 3A). RL-HSD-transfected cells quickly produced significant amounts of both androstanedione (5α-dione) and epiADT. By 24 h, most of the ADT in these cells had been metabolized (Fig. 3A). In contrast, RoDH-4 and RDHL-transfected cells produced primarily 5α-dione and little if any epiADT (Fig. 3A). These results indicated that metabolism of ADT in living cells transfected with various RoDH-like enzymes correlated well with the in vitro activities of the respective enzymes (23–25, 28).
Fig. 3.
Radiochromatogram of the products of RL-HSD, RoDH-4, and RDHL enzymatic activities in living cells. HEK293 cells stably transfected with SDR cDNAs (RoDH-4, RL-HSD, RDHL) or with empty vector (Mock) were incubated with either 1 μM ADT (A) or 1 μM ALLO (B) for indicated times. Reaction products were extracted and analyzed by TLC.
Interestingly, metabolism of ALLO in the cells was different from that of ADT. As expected, ALLO was oxidized to DHP only in the cells transfected with one of RoDH enzymes but not in mock-transfected cells (Fig. 3B). However, in contrast to enzyme-specific epimerization of ADT, all stably transfected cell lines produced epiALLO (Fig. 3B). Previous in vitro studies suggested that only two of the enzymes, RL-HSD and RDHL, can epimerize ALLO to epiALLO (23–25). Kinetic analysis of RoDH-4 carried out in this study showed that, although RoDH-4 is a highly active ALLO dehydrogenase (the apparent Km value of 0.43 ± 0.08 μM and the Vmax value of 19.0 ± 1.5 nmol · min−1mg−1 of microsomal protein), this enzyme does not display an appreciable ALLO epimerase activity. A similar conclusion has been reached with respect to 11-cis-RDH, except 11-cis-RDH appeared to be less active as ALLO dehydrogenase than RoDH-4 (data not shown). Therefore, it was surprising that significant epimerization of ALLO nevertheless was observed in cells stably transfected with RoDH-4. This observation suggested that HEK293 cells might contain an endogenous enzyme(s) that can reduce DHP produced by all RoDH enzymes to epiALLO, but not 5α-dione to epiADT.
To test this hypothesis, mock-transfected and RoDH-transfected cells were incubated with either DHP or 5α-dione, and the reaction products were analyzed by TLC. As shown in Fig. 4, there was a clear difference in the cellular metabolism of DHP vs. 5α-dione. 5α-Dione was converted to epiADT in RL-HSD-transfected cells, but not in mock-transfected cells (Fig. 4A), whereas DHP was converted to epiALLO in all cell lines examined including the mock-transfected cells (Fig. 4B). The rate of DHP reduction to epiALLO by mock-transfected cells (6 pmol · min−1 ·mg−1) was 10-fold greater than the rate of 5α-dione reduction to epiADT. This observation confirmed that HEK293 cells contain an endogenous enzyme that strongly prefers DHP over 5α-dione as a substrate. Furthermore, this enzyme appeared to specifically reduce DHP to 3β-hydroxyl epimer (epiALLO) because no production of 3α-hydroxy epimer (ALLO) could be detected.
Fig. 4.
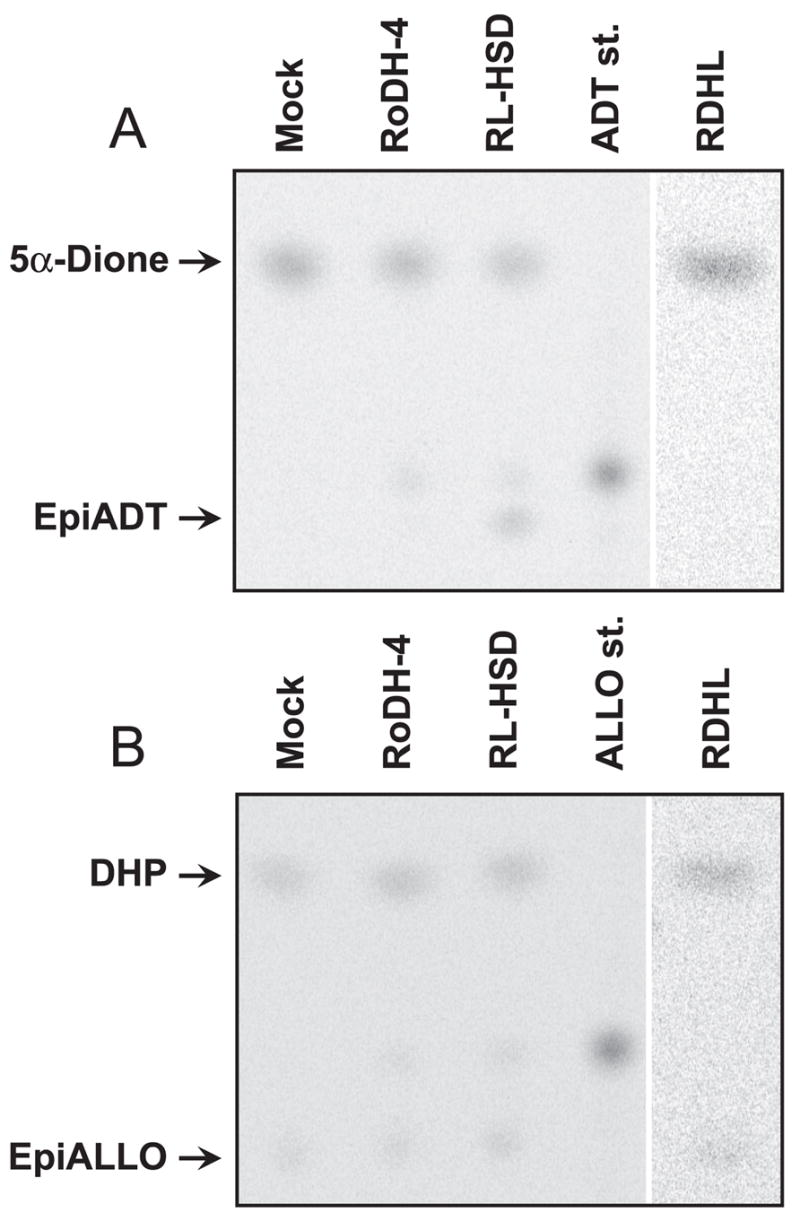
Metabolism of DHP and 5α-Dione in cells stably transfected with RL-HSD, RoDH-4, and RDHL. The cells were incubated with 0.58 μM 5α-Dione for 1 h (A) or with 0.72 μM DHP for 3.5 h (B). The products were extracted and analyzed by radiochromatography. ALLO st., ALLO standard; ADT st., ADT standard.
To obtain additional information regarding the identity of the DHP 3β-hydroxysteroid oxidoreductase (3β-HSOR) in HEK293 cells, we determined its subcellular localization and cofactor preference. The activity was found in both the cytosolic and microsomal fractions (Fig. 5). Importantly, unlike microsomal RoDHs, both types of endogenous 3β-HSOR activities preferred NADPH as a cofactor and little or no activity was detected with NADH. Hence, these enzymes could not have contributed to the NAD+-dependent epimerization of ADT or ALLO observed with human tissue microsomes in experiments described in Fig. 1.
Fig. 5.
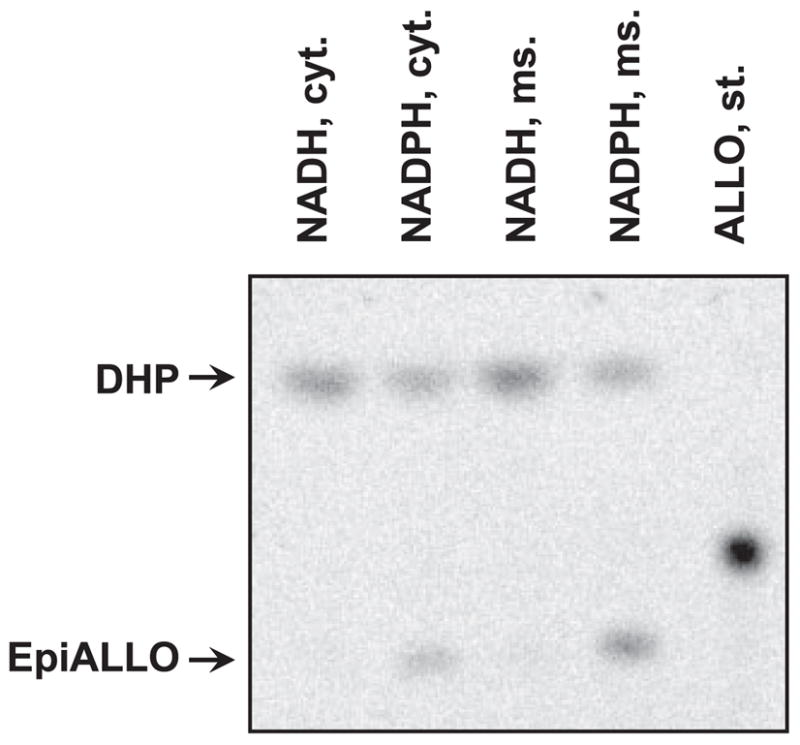
Subcellular localization and cofactor preference of the endogenous 3β-HSOR activity of HEK293 cells. Mock-transfected HEK293 cells were fractionated to obtain the cytosolic and microsomal fractions. Equal portions (1/10) of each fraction were used in activity assays. These corresponded to 135 μg of cytosolic and 30 μg of microsomal protein per reaction. Samples were incubated with 0.55 μM DHP and 1 mM NADH or reduced nicotinamide adenine dinucleotide phosphate (NADPH) for 4 h at 37 C. Cyt., Cytosol; ms., microsomes; st., standard.
To determine whether any of the RoDH enzymes can convert DHP to epiALLO in the cells and thus contribute to epimerization of ALLO, we measured the rates of DHP reduction to epiALLO by the stably transfected cell lines. These assays revealed that cells transfected with RoDH-4 or RDHL reduced DHP at a rate similar to that of mock-transfected cells, suggesting that neither RoDH-4 nor RDHL contribute significantly to the overall rate of DHP conversion to epiALLO. In contrast, cells transfected with RL-HSD had a 2-fold higher rate of DHP reduction than mock-transfected cells (12 vs. 6 pmol · min−1 · mg−1). Furthermore, with 5α-dione as substrate, the rate of 3β-epimer (epiADT) formation in RL-HSD-transfected cells was as high as 37 pmol · min−1 · mg−1 compared with only 0.6 pmol · min−1 · mg−1 for mock-transfected cells (Fig. 3B). This suggested that RL-HSD could act as a 3β-HSOR in living cells. However, its 3β-HSOR activity would be distinctively different from that of the endogenous microsomal or cytosolic DHP 3β-HSOR, because RL-HSD would use NADH as a cofactor.
In summary, analysis of RoDH activities in the cells indicated that RL-HSD could contribute to epimerization of ADT and ALLO in vivo, whereas RoDH-4, RDHL, and 11-cis-RDH would contribute primarily to 3α-hydroxyl oxidation of these steroids.
Expression of RoDH-like SDRs in human tissues
To determine whether RoDH-like SDRs are expressed in tissues with microsomal 3α-HSD/3(α→β)-HSE activity, we examined the expression pattern of RoDH-like proteins in human tissues. Five different antibody preparations generated by our laboratory were available for this study: rabbit polyclonal antibodies against the N-terminal fragments of RoDH-4, RL-HSD, and 11-cis-RDH; rabbit polyclonal antibodies against the C-terminal fragment of RoDH-4; and chicken antibodies against a peptide specific for RL-HSD.
Specificity of the antibodies was characterized using recombinant proteins expressed in Sf9 cells. Antibodies against the N-terminal fragment of RL-HSD (Fig. 6A, lane 3) cross-reacted with RoDH-4 because of the high sequence conservation between the two proteins in this region (88% amino acid identity). These antibodies also reacted weakly with RDHL, but did not react with 11-cis-RDH (Fig. 6A). Antibodies against the C-terminal region of RoDH-4 (Fig. 6B) and the N-terminal region of 11-cis-RDH (data not shown) were specific for the respective proteins. Interestingly, side-by-side Western blot analysis revealed that of RoDH-like proteins have different electrophoretic mobility in SDS-PAGE, suggesting that they can be also distinguished based on their electrophoretic mobility. 11-cis-RDH was the fastest moving protein (data not shown), followed by RL-HSD, RDHL, and RoDH-4 (Fig. 6).
Fig. 6.
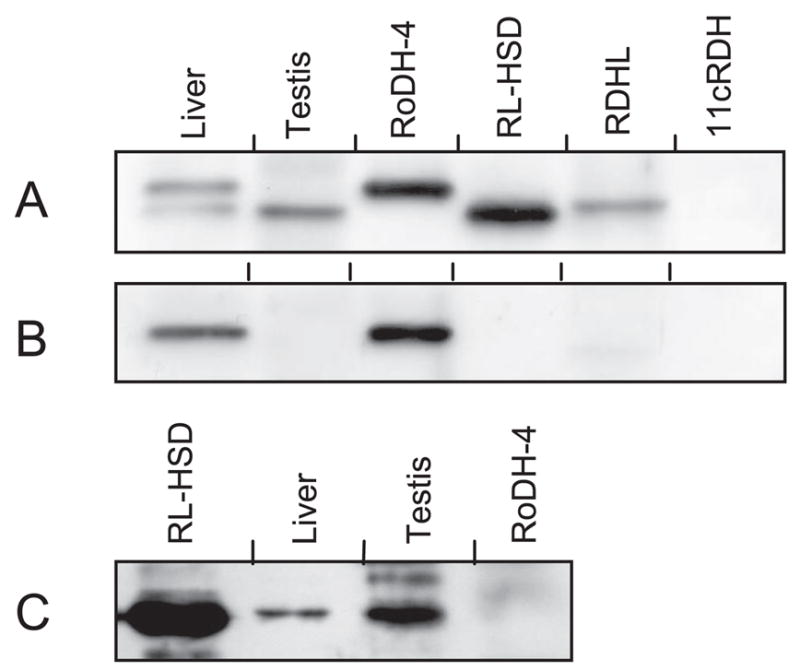
Characterization of antibodies and Western blot analysis of liver and testis. A, Antibodies against the N-terminal fragment of RL-HSD at a 1:3000 dilution. B, Antibodies against the C-terminal fragment of RoDH-4 at a 1:5000 dilution. C, Antibodies against the peptide specific for RL-HSD. The amount of protein was as follows: liver, 5 μg; testis, 16 μg; RoDH-4 microsomes, 1 μg; RL-HSD microsomes, 0.5 μg; RDHL microsomes, 9 μg; 11-cis-RDH (11cRDH) microsomes, 4.5 μg.
Western blot analysis of RoDH protein expression was carried out using the light membrane fractions from the same human tissues that were examined for 3α-HSD activity above. Consistent with their cross-reactivity, antibodies against the conserved N-terminal domains of RL-HSD and RoDH-4 recognized two protein bands in liver. Electrophoretic mobility of the lower band was identical to that of RL-HSD, whereas electrophoretic mobility of the upper band was identical to RoDH-4 (Fig. 6A), indicating that liver contains both of these proteins. The identities of these bands were further confirmed using RoDH-4-specific (Fig. 6B) and RL-HSD-specific (Fig. 6C) antibodies. In contrast to liver, only one protein band was detected in testis. This protein band was identified as RL-HSD, based on its characteristic electrophoretic mobility and recognition by RL-HSD-specific anti-peptide antibodies (Fig. 6C). Expression levels of RoDH-like SDRs in other tissues were below the detection limit of Western blot analysis.
RoDH expression in human brain was investigated by immunohistochemistry. This was done using protein-specific antibodies against either the C terminus of RoDH-4 or the N terminus of 11-cis-RDH, because these antibodies were the most specific and sensitive of all available antibody preparations. Anti-peptide antibodies specific for RL-HSD proved to be ineffective for immunohistochemical analysis of either brain or liver tissue sections.
Staining with RoDH-4-specific antiserum revealed that RoDH-4 was localized in neurons of human cerebellum, diencephalon, and cerebral cortex (Fig. 7). Similar neuronal expression pattern of RoDH-4 was observed in human thalamus, spinal cord, pons, medulla oblongata, and hippocampus (data not shown). 11-cis-RDH expression was also detected in neurons of human hippocampus and thalamus. Thus, both RoDH-4 and 11-cis-RDH were found to be expressed in the human brain and, therefore, could contribute to the 3α-hydroxyl oxidation of neurosteroids.
Fig. 7.
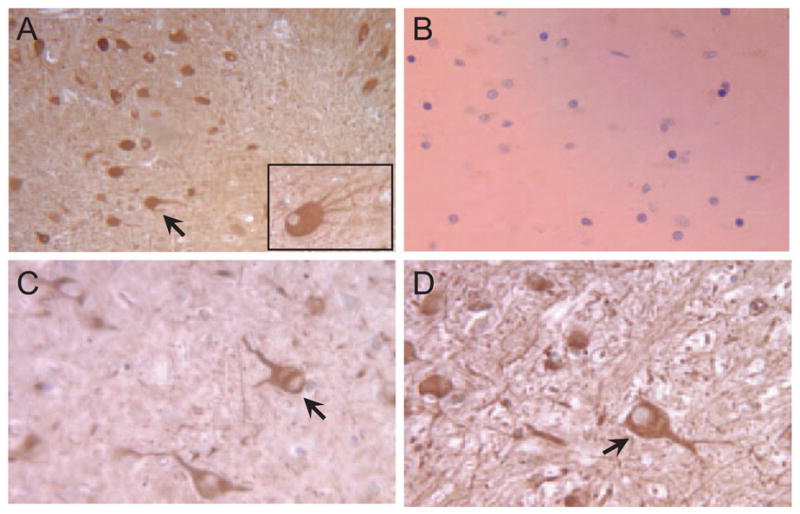
Immunolocalization of RoDH-4 in human brain. A, Cerebellum, × 100 magnification; inset at the bottom of the figure represent another field at a higher magnification (× 400), showing neuron-specific immunostaining in more detail. B, Preimmune serum, × 400. C, Cerebral cortex, × 400. D, Diencephalon, × 400. Note immunopositive staining in neurons (marked by arrows).
Discussion
In this study, we analyzed the distribution of the NAD+-dependent microsomal 3α-HSD activity in human tissues and demonstrated, for the first time, that microsomes from some tissues possess not only a 3α-HSD but also an NAD+-dependent 3(α→β)-HSE activity. The requirement for NAD+ as a cofactor for both the dehydrogenase and epimerase reactions and the obligatory appearance of a 3-ketosteroid in each reaction where epimerization was observed suggested that epimerization occurred in two steps, the oxidation of 3α-hydroxyl group followed by the reduction of 3-ketone group to 3β-hydroxyl group, much like it has been described recently for 3(α→β) epimerization of vitamin D (38).
Based on our current knowledge, such two-step epimerization of ADT and ALLO can be carried out in vitro only by the members of RoDH-like group of SDRs and, specifically, by RL-HSD and RDHL (23–25). No other microsomal enzyme has been shown to oxidize or epimerize 3α-hydroxysteroids in the presence of NAD+. RL-HSD has much lower Km values for ADT (0.23 μM) and ALLO (0.24 μM) than RDHL (24 and 5 μM, respectively), and thus, it is likely that RL-HSD is the primary enzyme responsible for epimerization of these compounds by human tissue microsomes under the conditions of the assay. The catalytic efficiency of RL-HSD for the oxidation/epimerization of ADT is 2.5-fold higher than that for ALLO (23). This might explain why epimerization of ALLO occurs less efficiently and, therefore, is detectable in fewer tissues than epimerization of ADT. This also suggests that NAD+-dependent epimerization of ADT may be a good marker of RL-HSD activity.
The highest levels of 3(α→β)-HSE activity are observed in liver and testis, which also contain high levels of RL-HSD protein. Although RL-HSD protein is undetectable in other tissues by western blotting, Northern blot analysis shows that RL-HSD is expressed in human lung and spleen, both of which possess the microsomal ADT epimerase activity, suggesting the presence of catalytically active RL-HSD protein. Previously, we reported that RL-HSD is expressed in many areas of human brain, but the distribution of the message across brain areas appeared to be uneven, being higher in caudate nucleus and thalamus and lower in corpus callosum (23). This study showed that human brain microsomes exhibit ADT epimerase activity, which is also higher in caudate nucleus and thalamus and lower in corpus callosum. Thus, the distribution of microsomal 3(α→β)-HSE activity in the brain and in other human tissues is in agreement with the expression pattern of RL-HSD.
Uneven distribution of ALLO metabolizing enzymes across brain areas has been implicated in the regulation of the local concentrations of ALLO (33), which would affect GABAA receptor conductivity in a region-specific manner. It has been shown that the levels of ALLO and DHP vary in different regions of human brain (39). The results of this study suggest that uneven distribution of the 3(α→β)-HSE activity could create different local concentrations of not only DHP but also of epiALLO, providing a mechanism for further fine-tuning of GABAA receptor conductivity by regulating the ratio between ALLO and its functional antagonist epiALLO.
In recent years, there has been an increase in recognition of potential physiological significance of epiALLO. Elevated levels of epiALLO were found in women with chronic fatigue syndrome (40). On the other hand, patients with panic disorder were reported to have lower than normal concentrations of epiALLO but greater concentrations of ALLO (41–43). Opposite changes in the ratio of ALLO/epiALLO were found in patients with major depression (44–47) and premenstrual syndrome (48). Thus, disequilibrium between ALLO and epiALLO appeared to be associated with certain psychopathologies, suggesting that controlled formation of epiALLO may be physiologically important.
Although RDHL, RoDH-4, and 11-cis-RDH are themselves not efficient as epimerases, their 3α-HSD activity may contribute to epimerization of 3α-hydroxysteroids by providing 3-ketosteroids for the NADP+-dependent 3β-HSORs identified in this study for the first time. This pathway could play a role specifically in epimerization of ALLO, because, as shown in the present study, there are cytosolic and microsomal NADP+-dependent 3β-HSORs that can reduce DHP to epiALLO. The identity of the NADP+-dependent DHP 3β-HSORs is currently unknown, but it is possible that some members of the AKR family of proteins (49) could catalyze DHP reduction in the cytosol, whereas the microsomal NADP+-dependent reduction of DHP could be carried out by some members of the SDR protein superfamily (50). In support of the latter notion, NADP+-dependent microsomal 3β-HSORs have been described in rat, mouse, and hamster, and an NADP+-dependent 3β-HSOR activity has been demonstrated in human liver (51). Together, the NAD+-dependent 3α-HSDs and the NADP+-dependent DHP reductases could regulate the local concentrations of ALLO and epi-ALLO in a brain-region specific manner, depending on their expression pattern in the brain.
Overall, the expression pattern of RDHL, RoDH-4, and 11-cis-RDH is consistent with their role in brain 3α-hydroxysteroid metabolism. In the present study, RoDH-4 and 11-cis-RDH were both localized by immunohistochemistry in several areas of human brain, and the expression of RDHL mRNA in brain has been reported previously (24). In addition to brain, these enzymes may also contribute to 3α-HSD metabolism in a number of other tissues. RoDH-4 was shown to be highly expressed in the liver, RDHL message was detected at high levels in trachea and at lower levels in colon, lymph node, bone marrow, and placenta (24), whereas 11-cis-RDH message was detected at some level in most tissues (30). Importantly, as shown here, all human RoDH-like SDRs can oxidize or epimerize ADT and ALLO in living cells, indicating that these enzymes can function as 3α-HSDs/3(α→β)-HSE under physiologically relevant conditions.
In support of the role of RoDH-like enzymes in human 3α-hydroxysteroid metabolism, RL-HSD has been recently identified as the major oxidative 3α-HSD that converts inactive 3α-androstanediol to a potent androgen dihydrotestosterone in human prostate (52). Interestingly, in the case of 3α-androstanediol, RoDH-like SDRs convert a less potent compound to a more potent compound, whereas in the case of ALLO or ADT, RoDH activity results in a decrease of their biological potencies at GABAA receptors. This observation suggests that the physiological outcome of RoDH activities will be determined by the availability of specific substrates and molecular targets present in specific types of cells and tissues. Identification of RoDH-like SDRs as steroid molecular switches, which could have an impact on the regulation of GABAA, androgen, farnesoid, and pregnane receptors, makes them important targets for potential pharmacological interventions.
Acknowledgments
We thank Dr. Doncho P. Uzunov (Neuroscience Research, Novartis Institutes for BioMedical Research) for providing HEK293 cell line stably transfected with RDHL. We are also very grateful to Luan D. Dao, graduate student in the Department of Biochemistry and Molecular Genetics, University of Alabama at Birmingham, for his enthusiastic help in preparation of brain immunostaining images.
This work was supported by the National Institute on Alcohol Abuse and Alcoholism Grant AA12153 to N.Y.K.
Abbreviations
- ADT
Androsterone, 3α-hydroxy-5α-androstan-17-one
- AKR
aldo-keto reductase
- ALLO
allopregnanolone, 3α-hydroxy-5α-pregnan-20-one
- 11-cis-RDH
11-cis-retinol dehydrogenase
- DHP
5α-dihydroprogesterone, 5α-pregnan-3, 20-dione
- 5α-Dione
androstanedione, 5α-androstan-3,17-dione
- EpiALLO
epiallopregnanolone, 3β-hydroxy-5α-pregnan-20-one
- GABAA
γ-aminobutyric acid type A
- HEK
human embryonic kidney
- 3α-HSD
3α-hydroxysteroid dehydrogenase
- HSE
hydroxysteroid epimerase
- 3β-HSOR
3β-hydroxysteroid oxidoreductase
- NAD
nicotinamide adenine dinucleotide
- NADH
reduced nicotinamide adenine dinucleotide
- RDHL
RDH-like
- RL-HSD
RoDH-like 3α-HSD
- RoDH
retinol/sterol dehydrogenase
- SDR
short-chain dehydrogenase/reductase
- TLC
thin-layer chromatography
Footnotes
Present address for S.V.C.: Center for Matrix Biology, Vanderbilt University Medical Center, S-3223 Medical Center North, 1161 21st Avenue South, Nashville, Tennessee 37232-2372.
Present address for A.L.C.: Kirksville College of Osteopathic Medicine, 800 West Jefferson Street, Kirksville, Missouri 63501.
Present address for N.V.K.: Department of Veterinary Biosciences, College of Veterinary Medicine, University of Illinois at Urbana-Champaign, 3635 Veterinary Medicine Basic Sciences Building, 2001 South Lincoln Avenue, Urbana, Illinois 61802.
Present address for K.S.S.: Department of Laboratory Medicine and Pathology, University of Minnesota Medical School, 420 Delaware Street SE, Room K-107 Diehl Hall, Minneapolis, Minnesota 55455.
Endocrinology is published monthly by The Endocrine Society (http://www.endosociety.org), the foremost professional society serving the endocrine community.
Disclosure Statement: The authors have nothing to disclose.
References
- 1.Paul SM, Purdy RH. Neuroactive steroids. FASEB J. 1992;6:2311–2322. [PubMed] [Google Scholar]
- 2.Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- 3.Lambert JJ, Belelli D, Hill-Venning C, Peters JA. Neurosteroids and GABAA receptor function. Trends Pharmacol Sci. 1995;16:295–303. doi: 10.1016/s0165-6147(00)89058-6. [DOI] [PubMed] [Google Scholar]
- 4.Baulieu EE, Schumacher M. Progesterone as a neuroactive neurosteroid, with special reference to the effect of progesterone on myelination. Hum Reprod. 2000;15:1–13. doi: 10.1093/humrep/15.suppl_1.1. [DOI] [PubMed] [Google Scholar]
- 5.Schumacher M, Weill-Engerer S, Liere P, Robert F, Franklin RJ, Garcia-Segura LM, Lambert JJ, Mayo W, Melcangi RC, Parducz A, Suter U, Carelli C, Baulieu EE, Akwa Y. Steroid hormones and neurosteroids in normal and pathological aging of the nervous system. Prog Neurobiol. 2003;71:3–29. doi: 10.1016/j.pneurobio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Brinton RD. The neurosteroid 3α-hydroxy-5α-pregnan-20-one induces cytoarchitectural regression in cultured fetal hippocampal neurons. J Neurosci. 1994;14:2763–2774. doi: 10.1523/JNEUROSCI.14-05-02763.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciriza I, Azcoitia I, Garcia-Segura LM. Reduced progesterone metabolites protect rat hippocampal neurones from kainic acid excitotoxicity in vivo. J Neuroendocrinol. 2004;16:58–63. doi: 10.1111/j.1365-2826.2004.01121.x. [DOI] [PubMed] [Google Scholar]
- 8.Griffin LD, Gong W, Verot L, Mellon SH. Niemann-Pick type C disease involves disrupted neurosteroidogenesis and responds to allopregnanolone. Nat Med. 2004;10:704–711. doi: 10.1038/nm1073. [DOI] [PubMed] [Google Scholar]
- 9.Langmade SJ, Gale SE, Frolov A, Mohri I, Suzuki K, Mellon SH, Walkley SU, Covey DF, Schaffer JE, Ory DS. Pregnane X receptor (PXR) activation: a mechanism for neuroprotection in a mouse model of Niemann-Pick C disease. Proc Natl Acad Sci USA. 2006;103:13807–13812. doi: 10.1073/pnas.0606218103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kochakian CD. History, chemistry and pharmacodynamics of anabolic-androgenic steroids. Wien Med Wochenschr. 1993;143:359–363. [PubMed] [Google Scholar]
- 11.Kaminski RM, Marini H, Kim WJ, Rogawski MA. Anticonvulsant activity of androsterone and etiocholanolone. Epilepsia. 2005;46:819–827. doi: 10.1111/j.1528-1167.2005.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang S, Lai K, Moy FJ, Bhat A, Hartman HB, Evans MJ. The nuclear hormone receptor farnesoid X receptor (FXR) is activated by androsterone. Endocrinology. 2006;147:4025–4033. doi: 10.1210/en.2005-1485. [DOI] [PubMed] [Google Scholar]
- 13.Caron S, Cariou B, Staels B. FXR: More than a bile acid receptor? Endocrinology. 2006;147:4022–4024. doi: 10.1210/en.2006-0701. [DOI] [PubMed] [Google Scholar]
- 14.Purdy RH, Morrow AL, Blinn JR, Paul SM. Synthesis, metabolism, and pharmacological activity of 3α-hydroxy steroids which potentiate GABA-receptor-mediated chloride ion uptake in rat cerebral cortical synaptoneurosomes. J Med Chem. 1990;33:1572–1581. doi: 10.1021/jm00168a008. [DOI] [PubMed] [Google Scholar]
- 15.Harrison NL, Majewska MD, Harrington JW, Barker JL. Structure-activity relationships for steroid interaction with the γ-aminobutyric acid A receptor complex. J Pharmacol Exp Ther. 1987;241:346–353. [PubMed] [Google Scholar]
- 16.Gee KW, Chang WC, Brinton RE, McEwen BS. GABA-dependent modulation of the Cl− ionophore by steroids in rat brain. Eur J Pharmacol. 1987;136:419–423. doi: 10.1016/0014-2999(87)90317-7. [DOI] [PubMed] [Google Scholar]
- 17.Wang MD, Bäckström T, Landgren S. The inhibitory effects of allopregnanolone and pregnanolone on the population spike, evoked in the rat hippocampal CA1 stratum pyramidale in vitro, can be blocked selectively by epiallopregnanolone. Acta Physiol Scand. 2000;169:333–341. doi: 10.1046/j.1365-201x.2000.00744.x. [DOI] [PubMed] [Google Scholar]
- 18.Bäckström T, Wahlström G, Wahlström K, Zhu D, Wang MD. Isoallopregnanolone; an antagonist to the anaesthetic effect of allopregnanolone in male rats. Eur J Pharmacol. 2005;512:15–21. doi: 10.1016/j.ejphar.2005.01.049. [DOI] [PubMed] [Google Scholar]
- 19.Bitran D, Hilvers RJ, Kellogg CK. Anxiolytic effects of 3α-hydroxy-5α[β]-pregnan-20-one: endogenous metabolites of progesterone that are active at the GABAA receptor. Brain Res. 1991;561:157–161. doi: 10.1016/0006-8993(91)90761-j. [DOI] [PubMed] [Google Scholar]
- 20.Lundgren P, Strömberg J, Bäckström T, Wang M. Allopregnanolone-stimulated GABA-mediated chloride ion flux is inhibited by 3β-hydroxy-5α-pregnan-20-one (isoallopregnanolone) Brain Res. 2003;982:45–53. doi: 10.1016/s0006-8993(03)02939-1. [DOI] [PubMed] [Google Scholar]
- 21.Bauman DR, Steckelbroeck S, Penning TM. The roles of aldo-keto reductases in steroid hormone action. Drug News Perspect. 2004;17:563–578. doi: 10.1358/dnp.2004.17.9.872570. [DOI] [PubMed] [Google Scholar]
- 22.Biswas MG, Russell DW. Expression cloning and characterization of oxidative 17β- and 3α-hydroxysteroid dehydrogenases from rat and human prostate. J Biol Chem. 1997;272:15959–15966. doi: 10.1074/jbc.272.25.15959. [DOI] [PubMed] [Google Scholar]
- 23.Chetyrkin SV, Hu J, Gough WH, Dumaual N, Kedishvili NY. Further characterization of human microsomal 3α-hydroxysteroid dehydrogenase. Arch Biochem Biophys. 2001;386:1–10. doi: 10.1006/abbi.2000.2203. [DOI] [PubMed] [Google Scholar]
- 24.Chetyrkin SV, Belyaeva OV, Gough WH, Kedishvili NY. Characterization of a novel type of human microsomal 3α-hydroxysteroid dehydrogenase: unique tissue distribution and catalytic properties. J Biol Chem. 2001;276:22278–22286. doi: 10.1074/jbc.M102076200. [DOI] [PubMed] [Google Scholar]
- 25.Huang X-F, Luu-The V. Molecular characterization of a first human 3(α→β)-hydroxysteroid epimerase. J Biol Chem. 2000;275:29452–29457. doi: 10.1074/jbc.M000562200. [DOI] [PubMed] [Google Scholar]
- 26.He XY, Wegiel J, Yang SY. Intracellular oxidation of allopregnanolone by human brain type 10 17β-hydroxysteroid dehydrogenase. Brain Res. 2005;1040:29–35. doi: 10.1016/j.brainres.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 27.He XY, Wegiel J, Yang YZ, Pullarkat R, Schulz H, Yang SY. Type 10 17β-hydroxysteroid dehydrogenase catalyzing the oxidation of steroid modulators of γaminobutyric acid type A receptors. Mol Cell Endocrinol. 2005;229:111–117. doi: 10.1016/j.mce.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 28.Gough WH, VanOoteghem S, Sint T, Kedishvili NY. cDNA cloning and characterization of a new human microsomal NAD+-dependent dehydrogenase that oxidizes all-trans retinol and 3α-hydroxysteroids. J Biol Chem. 1998;273:19778–19785. doi: 10.1074/jbc.273.31.19778. [DOI] [PubMed] [Google Scholar]
- 29.Belyaeva OV, Kedishvili NY. Comparative genomic and phylogenetic analysis of short-chain dehydrogenases/reductases with dual retinol/sterol substrate specificity. Genomics. 2006;88:820–830. doi: 10.1016/j.ygeno.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Wang J, Chai X, Eriksson U, Napoli JL. Activity of human 11-cis-retinol dehydrogenase (Rdh5) with steroids and retinoids and expression of its mRNA in extra-ocular human tissue. Biochem J. 1999;338:23–27. [PMC free article] [PubMed] [Google Scholar]
- 31.Krause JE, Karavolas HJ. Pituitary 5α-dihydroprogesterone 3α-hydroxysteroid oxidoreductases. Subcellular location and properties of NADH- and NADPH-linked activities. J Biol Chem. 1980;255:11807–11814. [PubMed] [Google Scholar]
- 32.Krause JE, Karavolas HJ. Subcellular location of hypothalamic progesterone metabolizing enzymes and evidence for distinct NADH- and NADPH-linked 3α-hydroxysteroid oxidoreductase activities. J Steroid Biochem. 1980;13:271–280. doi: 10.1016/0022-4731(80)90005-9. [DOI] [PubMed] [Google Scholar]
- 33.Li X, Bertics PJ, Karavolas HJ. Regional distribution of cytosolic and particulate 5α-dihydroprogesterone 3α-hydroxysteroid oxidoreductases in female rat brain. J Steroid Biochem Mol Biol. 1997;60:311–318. doi: 10.1016/s0960-0760(96)00195-1. [DOI] [PubMed] [Google Scholar]
- 34.Blomquist CH, Lima PH, Hotchkiss JR. Inhibition of 3α-hydroxysteroid dehydrogenase (3α-HSD) activity of human lung microsomes by genistein, daidzein, coumestrol and C(18)-, C(19)- and C(21)-hydroxysteroids and ketosteroids. Steroids. 2005;70:507–514. doi: 10.1016/j.steroids.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 35.Lowry OH, Rosebrough NH, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 36.Schule C, Romeo E, Uzunov DP, Eser D, di Michele F, Baghai TC, Pasini A, Schwarz M, Kempter H, Rupprecht R. Influence of mirtazapine on plasma concentrations of neuroactive steroids in major depression and on 3α-hydroxysteroid dehydrogenase activity. Mol Psychiatry. 2006;11:261–272. doi: 10.1038/sj.mp.4001782. [DOI] [PubMed] [Google Scholar]
- 37.Mellon SH, Griffin LD. Neurosteroids: biochemistry and clinical significance. Trends Endocrinol Metab. 2002;13:35–43. doi: 10.1016/s1043-2760(01)00503-3. [DOI] [PubMed] [Google Scholar]
- 38.Higashi T, Sakajiri K, Shimada K. Analysis of C-3 epimerization in (24R)-24,25-dihydroxyvitamin D3 catalyzed by hydroxysteroid dehydrogenase. J Pharm Biomed Anal. 2004;36:429–436. doi: 10.1016/j.jpba.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 39.Bixo M, Andersson A, Winblad B, Purdy RH, Bäckström T. Progesterone, 5α-pregnane-3,20-dione and 3α-hydroxy-5α-pregnane-20-one in specific regions of the human female brain in different endocrine states. Brain Res. 1997;764:173–178. doi: 10.1016/s0006-8993(97)00455-1. [DOI] [PubMed] [Google Scholar]
- 40.Murphy BE, Abbott FV, Allison CM, Watts C, Ghadirian AM. Elevated levels of some neuroactive progesterone metabolites, particularly isopregnanolone, in women with chronic fatigue syndrome. Psychoneuroendocrinology. 2004;29:245–268. doi: 10.1016/s0306-4530(03)00026-x. [DOI] [PubMed] [Google Scholar]
- 41.Ströhle A, Romeo E, di Michele F, Pasini A, Yassouridis A, Holsboer F, Rupprecht R. GABAA receptor-modulating neuroactive steroid composition in patients with panic disorder before and during paroxetine treatment. Am J Psychiatry. 2002;159:145–147. doi: 10.1176/appi.ajp.159.1.145. [DOI] [PubMed] [Google Scholar]
- 42.Ströhle A, Holsboer F. Stress responsive neurohormones in depression and anxiety. Pharmacopsychiatry. 2003;36(Suppl 3):S207–S214. doi: 10.1055/s-2003-45132. [DOI] [PubMed] [Google Scholar]
- 43.Ströhle A, Romeo E, di Michele F, Pasini A, Hermann B, Gajewsky G, Holsboer F, Rupprecht R. Induced panic attacks shift γaminobutyric acid type A receptor modulatory neuroactive steroid composition in patients with panic disorder: preliminary results. Arch Gen Psychiatry. 2003;60:161–168. doi: 10.1001/archpsyc.60.2.161. [DOI] [PubMed] [Google Scholar]
- 44.Romeo E, Ströhle A, di Michele F, Spaletta G, Hermann B, Holsboer F, Pasini A, Rupprecht R. Effects of antidepressant treatment on neuroactive steroids in major depression. Am J Psychiatry. 1998;155:910–913. doi: 10.1176/ajp.155.7.910. [DOI] [PubMed] [Google Scholar]
- 45.Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, Guidotti A. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci USA. 1998;95:3239–3244. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ströhle A, Romeo E, Hermann B, di Michele F, Spaletta G, Pasini A, Holsboer F, Rupprecht R. Concentrations of 3α-reduced neuroactive steroids and their precursors in plasma of patients with major depression and after clinical recovery. Biol Psychiatry. 1999;45:274–277. doi: 10.1016/s0006-3223(98)00328-x. [DOI] [PubMed] [Google Scholar]
- 47.Ströhle A, Pasini A, Romeo E, Hermann B, Spalletta G, di Michele F, Holsboer F, Rupprecht R. Fluoxetine decreases concentrations of 3α,5α-tetra-hydrodeoxycorticosterone (THDOC) in major depression. J Psychiatr Res. 34:183–186. doi: 10.1016/s0022-3956(00)00006-6. [DOI] [PubMed] [Google Scholar]
- 48.Wang M, Seippel L, Purdy RH, Bäckström T. Relationship between symptom severity and steroid variation in women with premenstrual syndrome: study on serum pregnenolone, pregnenolone sulfate, 5α-pregnane-3,20-dione and 3α-hydroxy-5α-pregnan-20-one. J Clin Endocrinol Metab. 1996;81:1076–1082. doi: 10.1210/jcem.81.3.8772579. [DOI] [PubMed] [Google Scholar]
- 49.Steckelbroeck S, Oyesanmi B, Jin Y, Lee SH, Kloosterboer HJ, Penning TM. Tibolone metabolism in human liver is catalyzed by 3α/3β-hydroxysteroid dehydrogenase activities of the four isoforms of the aldo-keto reductase (AKR)1C subfamily. J Pharmacol Exp Ther. 2006;316:1300–1309. doi: 10.1124/jpet.105.091587. [DOI] [PubMed] [Google Scholar]
- 50.Simard J, Ricketts ML, Gingras S, Soucy P, Feltus FA, Melner MH. Molecular biology of the 3β-hydroxysteroid dehydrogenase/δ5-δ4 isomerase gene family. Endocr Rev. 2005;26:525–582. doi: 10.1210/er.2002-0050. [DOI] [PubMed] [Google Scholar]
- 51.Pirog EC, Collins DC. Metabolism of dihydrotestosterone in human liver: importance of 3α- and 3β-hydroxysteroid dehydrogenase. J Clin Endocrinol Metab. 1999;84:3217–3221. doi: 10.1210/jcem.84.9.5963. [DOI] [PubMed] [Google Scholar]
- 52.Bauman DR, Steckelbroeck S, Williams MV, Peehl DM, Penning TM. Identification of the major oxidative 3α-hydroxysteroid dehydrogenase in human prostate that converts 5α-androstane-3α,17β-diol to 5α-dihydrotestosterone: a potential therapeutic target for androgen-dependent disease. Mol Endocrinol. 2006;20:444–458. doi: 10.1210/me.2005-0287. [DOI] [PubMed] [Google Scholar]