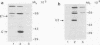Abstract
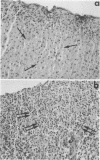
Glycosylated, membrane-associated E1 (58-kDa) and E2 (47- to 49-kDa) rubella virus proteins and unglycosylated nucleoprotein C (33 kDa), from separately expressed vaccinia virus recombinants, were injected into golden Syrian hamsters. Rubella virus E1 and E2 glycoproteins consistently induced an organ-specific autoimmune disease, autoimmune lymphocytic hypophysitis, which was evidenced by the induction of autoantibodies against pituitary cells and by lymphocytic infiltration of the pituitary. Neonatal thymectomy prevented the disease. In contrast, rubella virus nucleoprotein C did not induce either autoantibodies against pituitary cells or lymphocytic infiltration of the pituitary. This finding raises the possibility that virus-specific protein itself can induce an organ-specific autoimmune disease in certain circumstances.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asa S. L., Bilbao J. M., Kovacs K., Josse R. G., Kreines K. Lymphocytic hypophysitis of pregnancy resulting in hypopituitarism: a distinct clinicopathologic entity. Ann Intern Med. 1981 Aug;95(2):166–171. doi: 10.7326/0003-4819-95-2-166. [DOI] [PubMed] [Google Scholar]
- Clarke D. M., Loo T. W., Hui I., Chong P., Gillam S. Nucleotide sequence and in vitro expression of rubella virus 24S subgenomic messenger RNA encoding the structural proteins E1, E2 and C. Nucleic Acids Res. 1987 Apr 10;15(7):3041–3057. doi: 10.1093/nar/15.7.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinami R. S., Oldstone M. B. Amino acid homology between the encephalitogenic site of myelin basic protein and virus: mechanism for autoimmunity. Science. 1985 Nov 29;230(4729):1043–1045. doi: 10.1126/science.2414848. [DOI] [PubMed] [Google Scholar]
- GOUDIE R. B., PINKERTON P. H. Anterior hypophysitis and Hashimoto's disease in a young woman. J Pathol Bacteriol. 1962 Apr;83:584–585. [PubMed] [Google Scholar]
- Gonatas N. K., Howard J. C. Inhibition of experimental allergic encephalomyelitis in rats severely depleted of T cells. Science. 1974 Nov 29;186(4166):839–841. doi: 10.1126/science.186.4166.839. [DOI] [PubMed] [Google Scholar]
- Hobman T. C., Gillam S. In vitro and in vivo expression of rubella virus glycoprotein E2: the signal peptide is contained in the C-terminal region of capsid protein. Virology. 1989 Nov;173(1):241–250. doi: 10.1016/0042-6822(89)90240-7. [DOI] [PubMed] [Google Scholar]
- Isenberg D. A., Shoenfeld Y., Walport M., Mackworth-Young C., Dudeney C., Todd-Pokropek A., Brill S., Weinberger A., Pinkas J. Detection of cross-reactive anti-DNA antibody idiotypes in the serum of systemic lupus erythematosus patients and of their relatives. Arthritis Rheum. 1985 Sep;28(9):999–1007. doi: 10.1002/art.1780280907. [DOI] [PubMed] [Google Scholar]
- Kalkkinen N., Oker-Blom C., Pettersson R. F. Three genes code for rubella virus structural proteins E1, E2a, E2b and C. J Gen Virol. 1984 Sep;65(Pt 9):1549–1557. doi: 10.1099/0022-1317-65-9-1549. [DOI] [PubMed] [Google Scholar]
- Leppard K., Totty N., Waterfield M., Harlow E., Jenkins J., Crawford L. Purification and partial amino acid sequence analysis of the cellular tumour antigen, p53, from mouse SV40-transformed cells. EMBO J. 1983;2(11):1993–1999. doi: 10.1002/j.1460-2075.1983.tb01690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margalit H., Spouge J. L., Cornette J. L., Cease K. B., Delisi C., Berzofsky J. A. Prediction of immunodominant helper T cell antigenic sites from the primary sequence. J Immunol. 1987 Apr 1;138(7):2213–2229. [PubMed] [Google Scholar]
- Oker-Blom C., Ulmanen I., Käriäinen L., Pettersson R. F. Rubella virus 40S genome RNA specifies a 24S subgenomic mRNA that codes for a precursor to structural proteins. J Virol. 1984 Feb;49(2):403–408. doi: 10.1128/jvi.49.2.403-408.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B. Molecular mimicry and autoimmune disease. Cell. 1987 Sep 11;50(6):819–820. doi: 10.1016/0092-8674(87)90507-1. [DOI] [PubMed] [Google Scholar]
- Ortiz-Ortiz L., Weigle W. O. Cellular events in the induction of experimental allergic encephalomyelitis in rats. J Exp Med. 1976 Sep 1;144(3):604–616. doi: 10.1084/jem.144.3.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak C. Y., Cha C. Y., Rajotte R. V., McArthur R. G., Yoon J. W. Human pancreatic islet cell specific 38 kilodalton autoantigen identified by cytomegalovirus-induced monoclonal islet cell autoantibody. Diabetologia. 1990 Sep;33(9):569–572. doi: 10.1007/BF00404146. [DOI] [PubMed] [Google Scholar]
- Shoenfeld Y., Segol G., Segol O., Neary B., Klajman A., Stollar B. D., Isenberg D. A. Detection of antibodies to total histones and their subfractions in systemic lupus erythematosus patients and their asymptomatic relatives. Arthritis Rheum. 1987 Feb;30(2):169–175. doi: 10.1002/art.1780300207. [DOI] [PubMed] [Google Scholar]
- Singh V. K., Kalra H. K., Yamaki K., Abe T., Donoso L. A., Shinohara T. Molecular mimicry between a uveitopathogenic site of S-antigen and viral peptides. Induction of experimental autoimmune uveitis in Lewis rats. J Immunol. 1990 Feb 15;144(4):1282–1287. [PubMed] [Google Scholar]
- Sobrinho-Simões M., Brandão A., Paiva M. E., Vilela B., Fernandes E., Carneiro-Chaves F. Lymphoid hypophysitis in a patient with lymphoid thyroiditis, lymphoid adrenalitis, and idiopathic retroperitoneal fibrosis. Arch Pathol Lab Med. 1985 Mar;109(3):230–233. [PubMed] [Google Scholar]
- Srinivasappa J., Saegusa J., Prabhakar B. S., Gentry M. K., Buchmeier M. J., Wiktor T. J., Koprowski H., Oldstone M. B., Notkins A. L. Molecular mimicry: frequency of reactivity of monoclonal antiviral antibodies with normal tissues. J Virol. 1986 Jan;57(1):397–401. doi: 10.1128/jvi.57.1.397-401.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyka K. V., Drachman D. B., Griffin D. E., Pestronk A., Winkelstein J. A., Fishbeck K. H., Kao I. Myasthenia gravis. Study of humoral immune mechanisms by passive transfer to mice. N Engl J Med. 1977 Jan 20;296(3):125–131. doi: 10.1056/NEJM197701202960301. [DOI] [PubMed] [Google Scholar]
- Vladutiu A. O., Rose N. R. Transfer of experimental autoimmune thyroiditis of the mouse by serum. J Immunol. 1971 Apr;106(4):1139–1142. [PubMed] [Google Scholar]
- Yoon J. W., Lesniak M. A., Fussganger R., Notkins A. L. Genetic differences in susceptibility of pancreatic beta cells to virus-induced diabetes mellitus. Nature. 1976 Nov 11;264(5582):178–180. doi: 10.1038/264178a0. [DOI] [PubMed] [Google Scholar]
- Yoon J. W., Onodera T., Notkins A. L. Virus-induced diabetes mellitus. XV. Beta cell damage and insulin-dependent hyperglycemia in mice infected with coxsackie virus B4. J Exp Med. 1978 Oct 1;148(4):1068–1080. doi: 10.1084/jem.148.4.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J. W. The role of viruses and environmental factors in the induction of diabetes. Curr Top Microbiol Immunol. 1990;164:95–123. doi: 10.1007/978-3-642-75741-9_6. [DOI] [PubMed] [Google Scholar]
- Zamvil S. S., Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]
- van Eden W., Holoshitz J., Nevo Z., Frenkel A., Klajman A., Cohen I. R. Arthritis induced by a T-lymphocyte clone that responds to Mycobacterium tuberculosis and to cartilage proteoglycans. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5117–5120. doi: 10.1073/pnas.82.15.5117. [DOI] [PMC free article] [PubMed] [Google Scholar]