Abstract
1. Increasing evidence indicates that guanyl protein coupled receptors (GPCRs), including members of the vasopressin (VP) receptor family can act as homo- and heterodimers. Regulated expression and interaction of pituitary VP V1b receptor (V1bR) and corticotropin releasing hormone receptor type 1 (CRHR1) are critical for hypothalamic pituitary adrenal (HPA) axis adaptation, but it is unknown whether this involves physical interaction between these receptors.
2. Bioluminescence resonance energy transfer (BRET) experiments using V1bR and CRHR1 fused to either Renilla luciferase (Rluc) or yellow fluorescent protein (YFP) at the N-terminus, but not the carboxyl-terminus, revealed specific interaction (BRET50 = 0.39 ± 0.08, V1bR) that was inhibited by untagged V1b or CRHR1 receptors, suggesting homo- and heterodimerization. The BRET data were confirmed by coimmunoprecipitation experiments using fully bioactive receptors tagged at the aminoterminus with c-myc and Flag epitopes, demonstrating specific homodimerization of the V1b receptor and heterodimerization of the V1b receptor with CRHR1 receptors.
3. Heterodimerization between V1bR and CRHR1 is not ligand dependent since stimulation with CRH and AVP had no effect on coimmunoprecipitation. In membranes obtained from cells cotransfected with CRHR1 and V1bR, incubation with the heterologous nonpeptide antagonist did not alter the binding affinity or capacity of the receptor.
4. The data demonstrate that V1bR and CRHR1 can form constitutive homo- and heterodimers and suggests that the heterodimerization does not influence the binding properties of these receptors.
KEY WORDS: vasopressin V1b receptor, corticotropin releasing hormone type 1 receptor, dimerization, BRET, immunoprecipitation, receptor binding
INTRODUCTION
Corticotropin releasing hormone (CRH) and arginine vasopressin (AVP) are the two major stimulators of adrenocorticotropic hormone (ACTH) release from the anterior pituitary. CRH and AVP bind to specific guanyl-protein coupled receptors (GPCR) on corticotrophs, the type 1 CRH receptor (CRHR1) and the subtype V1b VP receptor (V1bR), respectively. The 415-amino acid CRHR1 receptor is coupled to the cyclic adenosine monophosphate/protein kinase A second messenger cascade through Gs (Aguilera, 1994). In contrast, the V1b receptor is 425 amino acids and coupled to phospholipase C/Ca2+ signaling pathways through Gq/11 (Liu et al., 1992; Lolait et al., 1995). There is evidence that interactions between the two regulators play an important role in modulating pituitary ACTH responsiveness according to the physiological requirement. While CRH appears to be the main regulator in rodents and human, AVP is a weak stimulant on its own but potentiates the stimulatory effect of CRH (Gillies et al., 1982). Both CRHR1 and V1bR undergo marked regulatory changes during alterations of the hypothalamo-pituitary-adrenal (HPA) axis, and the number of CRH binding sites depends largely on the prevailing levels of AVP in pituitary portal blood (Aguilera, 1994).
Studies in primary pituitary cell cultures provide additional evidence for CRH and AVP interaction in the corticotroph. Treatment of pituitary cells for 1 h with CRH increases the percentage of corticotrophs that bind AVP, without affecting the percentage of corticotrophs present in the culture (Childs et al., 1989). The reverse phenomenon also occurs; treatment of pituitary cells for 1 h with AVP increased CRH binding per corticotroph and the percentage of cells that bound CRH (Childs and Unabia, 1989). The rapidity of the effect would argue against an increase in receptor synthesis and is more consistent with transport of receptors to the plasma membrane or activation of previously unresponsive receptors. These observations may be of physiological importance since a subpopulation of corticotrophs have been identified that secrete ACTH only in the presence of both AVP and CRH (Jia et al., 1991). Perhaps, the most convincing evidence for the necessity of CRHR1 and V1bR interaction is that elimination of CRH-responsive cells using a cytotoxin prevents the stimulatory effect of AVP on proopiomelanocortin (POMC) mRNA, but not the ability of AVP to stimulate ACTH secretion (van de Pavert et al., 1997). The effect of AVP on POMC mRNA in normal cells was not mimicked by protein kinase C (PKC) activation with phorbol myristate acetate (PMA), although PMA did mimic the effect of AVP on ACTH secretion.
Functional cooperativity between the effects of CRH and AVP suggest that both receptors physically interact. Increasing evidence suggests that GPCRs are more prevalent as dimers than monomers (Kroeger et al., 2003; Bulenger et al., 2005). Heterodimerization can alter receptor trafficking and signaling compared to the individual homodimers [reviewed in (Gomes et al., 2001)]. The presence of large molecular size CRHR1 and V1b receptor forms observed by Western Blot analysis or polyacrylamide gel electrophoresis (PAGE) of radiolabeled cross-linked receptors would support the possibility that these receptors could exist as protein complexes. The predicted molecular weights (MW) of both CRHR1 and V1b receptors are 40–45 kilo Dalton (kDa). However, CRHR1 Western Blots show large molecular weight bands of 118 and 200 kDa in sheep anterior pituitaries (Green et al., 2000) and 115 kDa in rat anterior pituitaries (Castro et al., 1996). In addition, solubilization of radiolabeled CRH receptors in rat pituitary shows bands of 100–130 kDa in addition to a major band of 75 kDa (Grigoriadis and De Souza, 1988; Flores et al., 1990). In CRHR1 transfected cells, only bands ranging from 70–76 kDa have been detected (Sydow et al., 1997; Xu et al., 2001). V1b Western Blots show large MW bands in sheep anterior pituitaries [90 kDa; (Young et al., 2003)] and in human breast cancer cells [82 and 78 kDa; (North et al., 1999)], while a single band of 48 kDa, consistent with the predicted MW of the receptors, is seen in CHO cells transfected with the V1bR (Berrada et al., 2000). While these data suggest oligomerization, to date there is no direct evidence of CRHR1 and V1bR oligomerization. The aim of these studies was to investigate the possibility of homo- and heterodimerization among the vasopressin V1bR and CRHR1.
MATERIALS AND METHODS
Cell Culture
Chinese hamster ovary (CHO) cells (American Type Culture Collection, Manassas, VA) were cultured in a 5% CO2 atmosphere at 37°C and maintained in alpha-minimal essential medium (MEM) supplemented with 10% fetal bovine serum (FBS) that was heat inactivated at 56°C, 2 mM glutamine, 110 μg/mL pyruvate, 50 units/mL penicillin, and 50 μg/mL streptomycin. LVIP2.0Zc cells, containing β-galactosidase driven by a CRE-dependent promoter, provided by Drs M. Koenig and L. Mahan (NIMH, NIH, Bethesda, MD) were maintained at 5% CO2 in DMEM containing 10% FBS, 2 mM glutamine, 110 μg/mL pyruvate, 100 units/mL penicillin, 100 μg/mL streptomycin, and 25 μg/mL hygromycin. Transient transfections were performed in Opti-MEM I Reduced Serum Medium (Invitrogen, Carlsbad, CA) using Lipofectamine Plus reagent (Invitrogen) following the method suggested by the manufacturer.
Plasmid Construction
Wild-Type Receptors
The CRHR1 receptor full-length cDNA cloned into pcDNA1 was provided by Dr W. Vale (Salk Institute, La Jolla, CA). The V1b receptor full length cDNA was cloned into pAlterMAX (Promega) from the original full length clone, rap9.1V1bR (Lolait et al., 1995), provided by Dr S. Lolait, NIMH, NIH, Bethesda, MD). Expression of all genes and fusion genes was driven by CMV promoter.
Renilla Luciferase Fusion Constructs
In order to fuse the V1b receptor in frame at the C-terminus with Renilla luciferase (V1bCTRluc), the receptor sequence (minus the stop codon) was amplified by polymerase chain reaction (PCR) to introduce HindIII and BamHI restriction sites. PCR products were subcloned into a TA cloning vector (pCR2.1 topo, Invitrogen). TA clones and recipient vector (pRluc-N3, Perkin-Elmer) were digested with HindIII and BamHI and then ligated using T4 DNA ligase. The V1b receptor fused at the N-terminus with Rluc (V1bNTRluc) was produced in a similar fashion. The receptor sequence (with stop codon) was amplified by PCR to introduce XhoI and EcoRV restriction sites. PCR products were subcloned into a TA cloning vector (pCR4-TOPO, Invitrogen). TA clones and recipient vector (pRluc-C1, Perkin-Elmer) were digested with XhoI and EcoRV and then ligated using T4 DNA ligase. After each step, the accuracy of the constructs was confirmed by sequencing.
Yellow Fluorescent Protein Fusion Constructs
V1b and CRHR1 receptors were fused with yellow fluorescent protein (YFP) at the C-terminus using Gateway technology (Invitrogen). First, the V1b and CRHR1 receptors (minus the stop codon) were amplified by PCR and cloned into the pENTR Directional TOPO vector (Invitrogen). Introduction of the correct V1b and CRHR1 sequence into pENTR was verified by sequencing. Gateway LR Clonase was used to allow recombination of the V1bpENTR and CRHRpENTR vectors with the YFP-containing destination vector (pDEST504, Protein Expression Lab, NCI/NIH). Recombination was verified by restriction analysis of the V1bCTYFP and CRHRCTYFP clones. The V1b receptor was fused to YFP at the N-terminus using the same technique with the following exceptions: the stop codon was not removed and the YFP destination vector used was pDEST491 (Protein Expression Lab, NCI/NIH, Frederick, MD).
C-myc and Flag Fusion Constructs
Flag (DYKDDDDK) and c-Myc (EQKLISE) epitopes were introduced at the N-terminus of the V1b receptor. A PCR cassette containing a Sal I restriction site, the Flag tag, and N-terminus of V1b receptor was used to introduce the Flag sequence in frame with the V1b receptor in the parental V1bpAlterMAX vector (V1bFlag). The same technique was used to create the V1bmyc construct.
CRHR1 contains a cleavable signal peptide at the N-terminus. Therefore, the Flag epitope was introduced between amino acids 31 and 32 for this receptor. A 1.1 kb CRHR1 fragment was removed from its pcDNA3.1(+) backbone using Pst I and Xba I restriction enzymes and subcloned into pBluescript SK(+) (Stratagene, La Jolla, CA). A synthetic minigene was designed encoding 1-31 amino acids of CRHR1 followed by the Flag epitope and 31–42 amino acids of CRHR1. A series of oligonucleotides (54–55 nuclotides) were constructed to have 12–15 nucleotide complementary overlaps. By successive amplification and extension of oligonucleotide pairs, a second-round PCR product (∼171 bp) encoding the desired sequence was produced. In order to facilitate cloning into CRHR1(Pst I/Xba I)/pBluescript SK(+), the final PCR product was designed to contain restriction sites for Hind III and Pst I on its 5′ and 3′ ends, respectively. The synthetic gene was subcloned into pCR 2.1Topo (Invitrogen, Carlsbad, CA) for sequence verification and then digested with Hind III and Pst I to subclone into CRHR1(Pst I/Xba I)/pBluescript SK(+). The parental CRHR1/pcDNA3.1(+) vector and the new tagged CRHR1/pBluecscript SK(+) were digested with Hind III and Xba I and religated, resulting in the full-length CRHR1 with the tag introduced after amino acid 31.
Assessment of Receptor Activity
Receptor Binding Assay
[3H]AVP (1.63 TBq/mmol) and [125I]sauvagine (81.4 TBq/mmol) were purchased from PerkinElmer Life Science. AVP, sauvagine, and concavanalin A (Con A) were obtained from Sigma. The nonpeptide CRH antagonist, DMP-696, was synthesized in GlaxoSmithKline Medicinal Chemistry Department. The V1b receptor antagonist, SSR149415, was kindly provided by Dr. Claudine Serradeil-Le Gal, Sanofi Synthelabo Recherche, Toulouse, France, and ovine CRH was purchased from Bachem.
For [3H]AVP and [125I] sauvagine binding studies on tagged V1b and CRH receptors, CHO cells were transfected in 100 mm culture dishes with 4 μg of tagged V1b or CRH receptors. Twenty-four hours after transfection, cells were detached by incubation in PBS containing 5 mM EDTA, centrifuged, resuspended in binding buffer (HBSS, 20 mM Hepes, pH 7.4, 0.1% BSA and 0.2 mg/mL ConA) and aliquots containing 105 cells distributed in 96-well plates. In CRHR binding inhibition experiments, cells were incubated with 0.1 nM [125I] sauvagine at room temperature in a final volume of 200 μL of binding buffer for 120 min in the presence or absence of increasing concentrations of unlabeled sauvagine. Nonspecific binding was determined by the presence of 10 μM DMP-696. [3H]AVP saturation experiments were carried out by incubating cells with increasing radioligand concentrations (0.1–6 nM), at room temperature, for 60 min in 200 μL of binding buffer. Nonspecific binding was defined by the presence of 1 μM SSR149415.
Reactions were stopped by separating bound radioactivity by rapid filtration through GF/C filter mats presoaked in 0.5% polyethilenimmine using a cell harvester (Inotech Biosystems International). Filters were washed with 50 mM Tris-HCl, 10 mM MgCl2, 2 mM EGTA, 0.01% Triton-X100, and total bound radioactivity counted in a liquid scintillation counter (Beta counter, Packard) for 3H, or in a gamma-counter (70% efficiency) for 125I.
Membranes from CHO cells cotransfected with V1b and CRH receptor plasmids were prepared the day after cells were transfected with 2 μg of each plasmid in 75 cm2 Flasks (Nunc). Cells were harvested in phosphate buffered saline (PBS) containing 5 mM EDTA and centrifuged at 913×g for 8 min at 4°C and then resuspended in 50 mM TrisHCl, pH 7.4, containing 5 mM MgCl2, 2 mM EGTA (5 mL/Flask) and homogenized using a mechanical homogenizer (Tekmar, Cincinnati, OH). The suspension was centrifuged at 30,000×g for 30 min at 4°C. The final pellet was resuspended in 10 volumes of the same buffer and rehomogenized. Protein concentration was determined by the BCA Protein assay (Pierce, Rockford) using BSA as internal standard. Binding assays were carried out essentially as described earlier by incubating approximately 10 μg protein/well in a final volume of 200 μL of 50 mM TrisHCl, pH 7.4, 5 mM MgCl2, 2 mM EGTA and 0.1% BSA.
Radioligand binding data was analyzed by nonlinear regression analysis using GraphPad Prism 4.0 (GraphPad Software, CA, USA). Determination of K D and B max was assessed by analysis of binding saturation curves using one site binding (hyperbola) equation. Determination of IC50 for sauvagine in [125I]sauvagine binding inhibition experiments was calculated using a four-parameter logistic equation.
cAMP-Driven Beta-Galactosidase
CRH receptor activity was assessed after transient transfection into the LVIP2.0Zc cell line, which contains β-galactosidase driven by a CRE-dependent promoter (Konig et al., 1991). Transfected cells were cultured in 24-well plates and incubated with oCRH in DMEM containing 0.1% BSA. After 6 h, cells were washed with PBS and then lysed in 100 μL of 1X Reporter Lysis Buffer (Promega, Madison, WI). β-galactosidase activity was measured using a β-galactosidase enzyme assay system (Promega, Madison WI).
Inositol Phosphate Formation
Transiently transfected CHO cells were cultured in 24-well plates and labeled with 2.5 μCi/mL of myo-[3H]inositol per well for 24 h, washed with media containing 0.1% BSA and 10 mM LiCl, and then incubated for 15 min under the conditions indicated in results and figure legends. Incubations were stopped by addition of one volume of cold stop solution (1 M KOH, 18 mM sodium tetraborate, 3.8 mM EDTA, 7.6 mM NaOH) followed by neutralization with 7.5% HCl. Total inositol phosphates were separated by anion exchange chromatography as previously described (Aguilera et al., 1994) and measured in a liquid scintillation counter.
Intracellular Calcium
CHO cells were transiently transfected with V1bWT and V1b-tagged receptors and then plated to 80% confluence in black 96-well coated plates (Fisher) and incubated overnight in growth medium. Cells were washed once with loading buffer (Hank’s Balanced Salt Solution supplemented 1 mM CaCl2, 1 mM MgCl2, 0.5% BSA) and then loaded with 5 μM Indo1-AM (Invitrogen, Carlsbad, CA) in loading buffer with 1 mM probenicid for 30 min at 37°C in the dark. Cells were washed 3× with assay buffer (20 mM Hepes, 120 mM NaCl, 2 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 5 mM glucose, pH 7.4) and then left in assay buffer for 15 min in the dark at room temperature to allow hydrolysis of the AM ester. Intracellular calcium was measured using 340 nm excitation and dual wavelength emission at 390 and 480 nm using a Fluostar Optima fluorescent plate reader (BMG LabTech, Durham, NC).
Confocal Microscopy
YFP-tagged receptors were transiently transfected into CHO cells in 4-well chambered coverslips (Nunc). Twenty-four hours posttransfection, cells were fixed with 3.7% formaldehyde in PBS for 10 min at room temperature. The wells were then washed twice with PBS and stored at 4°C until visualization. Images were captured using a Zeiss 510 confocal microscope equipped with a 514 nm laser for excitation, BP530-600 emission filter, and a Plan-Apochromat 63X/1.4 oil immersion objective (Carl Zeiss, Jena, Germany).
Coimmunoprecipitation
CHO cells were transfected in 100 mm plates with receptors as described in the Results. Cells were lysed with cold Lysis Buffer (50 mM TrisHCl(pH 7.4), 2 mM EGTA, 5 mM MgCl2, 1% Triton-X100, 1× protease inhibitor cocktail [Sigma]). Lysates were then centrifuged at 16,000×g for 10 min at 4°C to remove debris and stored at −80°C. Protein determinations were made using the BCA method (Pierce). Lysates (500 ug) were precleared by incubation with protein G Sepharose beads (50 μL; Amersham) prewashed with cold lysis buffer, in a total volume of 500 μL for 1 h on ice. Beads were removed by centrifugation and antibody (anti-CRHRI/II(C-20) [2 μg; Santa Cruz], anti-Flag [4 μg; Sigma], or anti-GFP [5 μg; Santa Cruz] was added to precleared lysates and incubated with gentle rotation at 4°C overnight. Receptor-antibody complexes were isolated by addition of Protein G Sepharose beads and incubation with gentle rotation at 4°C for 1 h. Beads were carefully washed twice with 500 μL cold lysis buffer and immunoprecipitants eluted by addition of 50 μL SDS loading buffer +1 μL beta-mercaptoethanol and incubating at room temperature for 10 min. Beads were removed by centrifugation for 5 min at room temperature and 20 μL of the supernatant containing the immunoprecipitant loaded onto a 10% SDS-PAGE gel. Proteins were transferred onto PVDF membranes and blocked with 6% milk in Tris Buffered Saline/0.1% Tween 20 (TBST) for 1 h at room temp. Membranes were then incubated with primary antibody (1:1000 anti-CRHRI/II, anti-myc [Upstate], or anti-Flag; 1:200 Anti-GFP) in 6% milk/TBST for 2 h at room temperature. Membranes were washed 3× with TBST and incubated with secondary antibody (CRHRI/II, GFP: rabbit antigoat IgG-HRP; Flag, myc: rabbit antimouse IgG-HRP) in 6% milk/TBST for 1 h at room temperature. Chemiluminescence was developed with ECL+ (BioRad Laboratories).
Bioluminescence Resonance Energy Transfer
CHO cells were transiently transfected with 50–100 ng of Rluc-tagged receptor ±100 ng of YFP-tagged receptor in 12-well plates. Twenty-four hours posttransfection, wells were washed once with PBS and lifted with either Trypsin/5 mM EDTA (if no ligand stimulation is needed) or PBS/5 mM EDTA (for ligand stimulation). CHO growth media was added to inactivate trypsin (when necessary), suspension transferred to a microcentrifuge tube, and centrifuged at 1000×g for 5 min at room temperature. Cells were then washed twice with Dulbecco’s PBS (DPBS; Invitrogen, 14287-080) and resuspended in DPBS at a dilution of 2 × 106 cells/mL. After allowing the cells to recover for 1–3 h, 40 μL of cell suspension were plated in white optiplates (Perkin-Elmer) in duplicate for measurement. Rluc (480 nm emission) and YFP (530 nm emission) signals were measured before and after injection of coelenterazine h (Invitrogen, 5 μM final concentration) using a Fluostar Optima plate reader (BMG LabTech, Durham, NC). BRET was determined by the equation: BRET ratio = (530 nmexp/480 nmexp) − (530 nmbackground/480 nmbackground), where exp is cells transfected with both Rluc- and YFP-tagged receptors and background is cells transfected with Rluc-tagged receptor only. BRET ratios were compared using a one-way ANOVA with SNK posthoc analysis when indicated (SigmaStat). BRET50 values were calculated using SigmaPlot. WT competition data were analyzed with GraphPad Prism, and IC50 values for CRHRWT and V1bmyc from three independent experiments were compared by t-test.
RESULTS
Biological Activity of V1b and CRHR1-Tagged Receptors
Confocal microscopy analysis of V1bCTYFP receptors transiently transfected into CHO cells revealed predominantly plasma membrane localization (Fig. 1(A)). V1b receptors tagged at the C-terminus with either YFP or Rluc or at N-terminus with myc and Flag displayed similar biological activity as the wild-type receptor, in terms of ligand binding (Table I). Additionally, signaling properties of the V1bCTYFP, V1bCTRluc, and V1bWT receptor were similar as assessed by inositol phosphate production (data not shown) and mobilization of intracellular Ca2+ in response to AVP. AVP-induced increases in cytosolic calcium in cells transfected with N-terminus myc- and Flag-tagged V1b receptors were also similar to those of wild-type receptors (Fig. 1(B)). However, N-terminus YFP and rLuc-tagged V1b receptors displayed no binding activity (Table I). In contrast to the C-terminus construct, confocal microscopic imaging of cells transfected with V1bNTYFP revealed primarily intracellular localization (Fig. 1(A)).
Fig. 1.
Biological activity of tagged V1b and CRHR1 receptors. (A) Representative confocal images of CHO cells transfected with V1b receptor tagged at either the C-terminus or N-terminus with yellow fluorescent protein (V1bCTYFP and V1bNTYFP, respectively) or CRHR1 tagged at the C-terminus with YFP (CRHRCTYFP). (B) Representative AVP (10 nM) induced increases in intracellular calcium in CHO cells transfected with N-terminus-tagged V1b receptors with myc (V1bmyc) or Flag (V1bFlag) and C-terminus Renilla luciferase (V1bCTRluc) or YFP (V1bCTYFP), compared to wild-type receptor (V1bWT). Data points are the mean of duplicate determinations in one of three experiments. (C) CRH dose response for beta-galactosidase activity in the reporter cell line, LVIP2.0Zc, transfected with Flag- (CRHRFlag) or YFP- (CRHRCTYFP) tagged CRHR1 receptors, compared to wild-type CRHR (CRHRWT) receptor. Data points are the mean and SE of beta galactosidase activity values (absorbance) in three separate experiments.
Table I.
[3H]AVP Binding Experiments on Tagged V1b Receptors Used for BRET and Coimmunoprecipitation Experiments
| K d [3H]AVP (nM) | B max [3H]AVP (fmolrec/105 cells) | |
|---|---|---|
| V1bWT | 1.7 ± 0.3 | 10.7 ± 3.4 |
| V1bmyc | 1.6 ± 0.3 | 11.9 ± 0.9 |
| V1bFlag | 1.3 ± 0.3 | 11.1 ± 1.6 |
| V1bCTYFP | 1.5 ± 0.1 | 14.6 ± 6.9 |
| V1bCTRluc | 1.1 ± 0.3 | 12.4 ± 3.4 |
| V1bNTYFP | No specific binding | |
| V1bNTRluc | No specific binding | |
Note. Results are mean ± SEM of four dose response curves.
The CRHR1-tagged receptors were similar in characteristics to the V1b-tagged receptors. Visualization of the CRHR1CTYFP under confocal microscopy confirmed localization of the receptor at the plasma membrane (Fig. 1(A)). Addition of YFP to the C-terminus or Flag to the N-terminus of the CRHR1 receptor did not impair receptor binding (Table II). Signaling of the CRHR1 was assessed using a cell line that stably expresses a cAMP-inducible promoter driving beta-galactosidase production. There was no difference in the dose response of oCRH-stimulated beta-galactosidase activity in cells transfected with CRHRCTYFP or CRHRFlag compared to CRHR1WT receptors (Fig. 1(C)).
Table II.
[125I]Sauvagine Binding on Tagged CRHR1 Receptors Used for Coimmunoprecipitation Experiments
| IC50 sauvagine (nM) | fmol [125I]sauvagine bound/105 cells | |
|---|---|---|
| CRHR1WT | 6.5 ± 2.4 | 0.70 ± 0.29 |
| CRHRCTYFP | 7.0 ± 0.8 | 0.38 ± 0.16 |
| CRHRFlag | 8.4 ± 4.9 | 0.38 ± 0.16 |
Note. [125I]sauvagine binding was calculated as the amount of 0.1 nM radioligand specifically bound to cells. Results are mean ± SEM of four dose response curves.
V1b Receptor Homodimerization
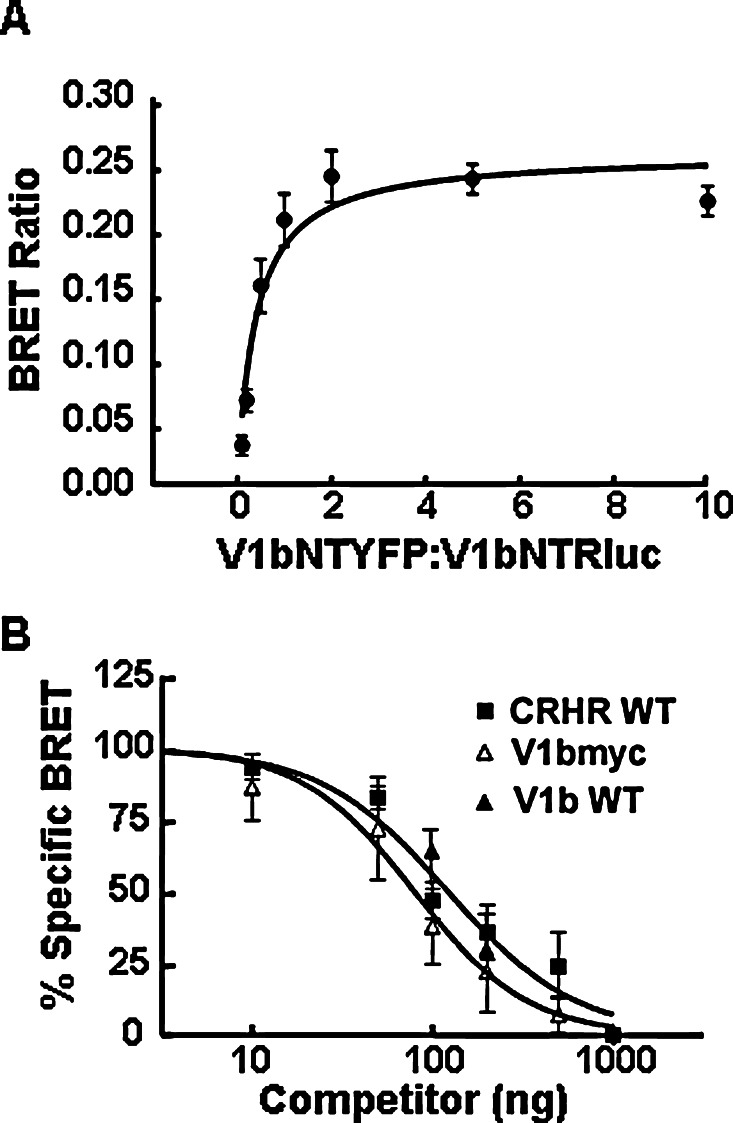
To determine whether the V1b receptors are capable of forming homodimers, we performed bioluminescence resonance energy transfer (BRET) experiments with the Rluc and YFP-tagged receptors (Fig. 2). Initial experiments showed no specific BRET signal upon cotransfecting V1bR tagged at the C-terminus with Rluc and YFP (V1bCTRluc/V1bRCTYFP). However, significant BRET signal was obtained upon cotransfecting N-terminus-tagged V1bR (V1bNTRluc/V1bNTYFP). As expected, no BRET signal was observed by cotransfecting the V1bR tagged with Rluc at the C-terminus with the V1bR tagged with YFP at the N-terminus (V1bCTRluc/V1bNTYFP), or vice versa (V1bNTRluc/V1bCTYFP). To confirm that the BRET signal obtained from the N-terminus-tagged V1b receptors is due to specific homodimerization and not simply random interaction, BRET50 calculations were determined by increasing the amount of V1bNTYFP transfected (10–1000 ng) while keeping V1bNTRluc constant (100 ng; n = 3). While random interaction would produce a linear relationship between the BRET signal and the ratio of YFP:Rluc, maximal BRET signal of 0.26 ± 0.01 was reached with V1bNTRluc over V1bNTYFP ratios as low as 2:1, which remained stable at higher levels of V1bNTYFP expression (Fig. 3(A); BRET50, 0.39 ± 0.08). Additionally, the V1bNTRluc/V1bNTYFP BRET signal was competed by the addition of increasing amounts of the V1bmyc receptor (Fig. 3(B); IC50 = 88 ± 39 ng). The wild-type V1bR added at 1:1 and 2:1 ratios (100 and 200 ng) resulted in BRET signal competition similar to that observed for V1bmyc. Since the V1bNTYFP vector was kept constant at 100 ng, the calculated IC50 corresponds to ∼1:1 ratio of V1bmyc:V1bYFP. This indicates that V1bmyc and V1bNTYFP have equal affinities for dimerization to the V1bNTRluc.
Fig. 2.
V1b receptor homodimerization assessed by bioluminescence resonance energy transfer (BRET). CHO cells were transfected with 100 ng of V1b tagged with renilla luciferase at the N- or C-terminus (V1bNTRluc or V1bCTRluc) with or without 100 ng V1b tagged with YFP at the N- or C-terminus (V1bNTYFP or V1bCTYFP). Rluc (480 nm emission) and YFP (530 nm emission) signals were measured before and after injection of coelenterazine h, using a multiplate reader. BRET was determined by the equation: BRET ratio = (530 nmexp/480 nmexp) − (530 nmbackground/480 nmbackground), where exp is cells transfected with both Rluc- and YFP-tagged receptors and background is cells transfected with Rluc-tagged receptor only. Bars represent the mean and SE of data obtained in three experiments. * P < 0.05 compared with other groups.
Fig. 3.
Specificity of V1b receptor homodimerization and V1b/CRHR1 heterodimerization. (A) BRET saturation curve performed in CHO cells transfected with 100 ng of V1bNTRluc with or without increasing amounts (10–1000 ng) of V1bNTYFP. (B) BRET competition curve. CHO cells were transfected with V1bNTRluc and V1bNTYFP (100 ng each) plus increasing amounts of either the V1bmyc (N-terminus tag) or wild-type CRHR1 (CRHR1WT) or 100 and 200 ng of wild-type V1b receptor (V1bWT). BRET ratios were measured as described in Methods and in the legend to Fig. 2. Data are represented as the mean ± SEM of data obtained in three experiments.
V1b homodimers were also detected by coimmunoprecipitation of the V1bmyc and V1bFlag receptors (Fig. 4). Western Blot analysis of protein immunoprecipitates using an anti-Flag antibody revealed a specific band of 50 kDa corresponding to the predicted nonglycolysated weight of the V1bFlag receptor. Myc immunoreactivity was readily detected in Flag immunoprecipitates of cells cotransfected with V1bmyc and V1bFlag (Fig. 4, V1bmyc+V1bFlag; n = 3), but not when mixing lysates from cells transfected with the individual tags (Fig. 4, V1bmyc and V1bFlag mix; n = 3). This indicates that the coimmunoprecipitation is due to dimerization between the V1bmyc and V1bFlag receptors and is not due to nonspecific aggregation of proteins during lysis. Similar results were obtained when the myc antibody was used to immunoprecipate the V1bmyc receptor with subsequent blotting for the V1bFlag receptor (data not shown). Stimulation of the transfected cells with 10−7 M AVP for 10 min prior to cell lysis had no effect on V1b homodimerization (Fig. 5).
Fig. 4.
Representative blot from coimmunoprecipitation of V1b receptor homodimers. Total protein lysates (500 μg) from CHO cells, untransfected, or cotransfected with N-terminus-tagged V1bFlag and V1bmyc (V1bmyc + V1bFlag), or pooled lysates from cells transfected with the individual constructs (V1bmyc & V1bFlag mix) were immunoprecipitated with Flag antibody (IP: Flag) and immunoblotted with anti-myc (IB: myc) to determine homodimerization or with anti-Flag (IB: Flag) to confirm immunoprecipitation. Coimmunoprecipitation was confirmed in three separate transfections. Specific V1b receptor bands are shown by the open arrow.
Fig. 5.
Effect of the ligand on V1b homodimerization. CHO cells were transfected with 2 μg of N-terminus-tagged V1bmyc + 2μg V1bFlag (homodimer), or transfected with either 2 μg V1bmyc + 2 μg empty vector, or 2 μg V1bFlag + 2 μg empty vector and pooled after lysis (Neg. Control). Twenty-four hours posttransfection, cells were incubated with either DMEM/0.1%BSA (Vehicle) or 10−7 M AVP (AVP), for 10 min at 37°C, prior lysis. Immunoprecipitation with anti-Flag (IP: Flag) and immunoblot with anti-myc (IB: myc, to detect homodimers) or anti-Flag (IB: Flag, to confirm immunoprecipitation) was carried out as described in Methods. The specific V1b receptor bands are shown by the open arrows.
CRHR1 Receptor Homodimerization
CRHR1 homodimers were detected by coimmunoprecipitation of the CRHRFlag and CRHRCTYFP receptors (Fig. 6). Western Blot analysis of protein immunoprecipitates using an anti-GFP antibody revealed a specific band of approximately 70 kDa corresponding to the predicted nonglycolysated weight of the CRHRCTYFP receptor. Flag immunoreactivity was readily detected in YFP immunoprecipitates of cells cotransfected with CRHRFlag and CRHRCTYFP (Fig. 6, CRHRFlag + CRHRYFP; n = 2), but not when mixing lysates from cells transfected with the individual tags (Fig. 6, CRHRFlag and CRHRYFP mix; n = 2). This indicates that the coimmunoprecipitation is due to dimerization between the CRHRFlag and CRHRCTYFP receptors and is not due to nonspecific aggregation of proteins during lysis. Similar results were obtained when the Flag antibody was used to immunoprecipitate the CRHRFlag receptor with subsequent blotting for the CRHRYFP receptor (data not shown).
Fig. 6.
Representative Western blot of tagged CRHR1 after immunoprecipitation of homodimers. Total cell protein aliquots (500 μg) from CHO cells, either from cells cotransfected with 2 μg of each CRHRFlag and CRHRCTYFP (CRHRFlag + CRHRCTYFP), or a 1:1 mix of protein from cells transfected with CRHRFlag + empty vector, or CRHRCTYFP + empty vector (CRHRFlag & CRHRCTYFP mix), or untransfected CHO cells, were immunoprecipitated with GFP antibody (IP: GFP). Immunoblots were performed with anti-Flag (IB: Flag) to determine homodimerization, and with anti-GFP (which equally cross react with YFP) (IB: GFP) to confirm immunoprecipitation. Coimmunoprecipitation was confirmed with two separate transfections. Specific bands for CRHRFlag (MW ∼45) or CRHRCTYFP bands are shown by the open arrows.
V1bR and CRHR1 Heterodimerization
Since V1b and CRHR1 receptors are coexpressed in corticotrophs of the anterior pituitary gland where they have been shown to interact at the second messenger level, we sought to determine whether the V1b receptor is capable of forming heterodimers with the CRHR1 receptor. V1bmyc (1 μg/10 cm2 plate) was transiently transfected with or without the CRHR1 WT receptor (1 μg/10 cm2 plate) into CHO cells and lysed 24 h later. Western Blot analysis of lysates immunoprecipitated with a CRHR antibody revealed multiple molecular weight bands at approximately 45, 64, and 115 kDa. The 45 kDa band is consistent with the predicted molecular weight of the nonglycosylated receptor, whereas the 64 kDa band is likely representative of the glycosylated receptor. The larger 115 kDa band may represent an SDS-resistant oligomer. These molecular weights are consistent with previous reports (Castro et al., 1996; Sydow et al., 1997; Xu et al., 2001). Myc immunoreactivity was detected in CRHR immunoprecipitants from cells cotransfected with V1bmyc and CRHR1 WT, but not in samples containing a mixture of lysates of V1bmyc and CRHR1 WT transfected in separate plates, demonstrating that coimmunoprecipitation is not due to nonspecific aggregation of proteins during lysis (Fig. 7(A)). Similar results were obtained when the myc antibody was used to immunoprecipitate the V1bmyc receptor with subsequent blotting for the CRHR receptor (data not shown). Similar to the V1b receptor homodimer, stimulation with 10−7 M oCRH + 10−7 M AVP for 10 min prior to cell lysis did not affect V1b/CRHR1 heterodimerization (data not shown).
Fig. 7.
Representative blots of V1b/CRHR1 coimmunoprecipitation. (A) Aliquots containing 500 μg of lysates from CHO cells cotransfected with 2 μg V1bmyc + 2 μg CRHRWT (V1bmyc + CRHR WT), or a 1:1 mixture of proteins from cells individually transfected with V1bmyc + 2 μg empty vector or 2 μg CRHR1WT + 2 μg empty vector (V1bmyc and CRHR WT mix), or untransfected cells were immunoprecipitated with anti-CRHR antibody (IP: CRHR). Immunoblot with anti-myc (IB: myc) was performed to determine heterodimerization, and with anti-CRHR (IB: CRHR) to confirm immunoprecipitation. Coimmunoprecipitation was confirmed in three separate transfections. (B) V1b/CRHR1 heterodimerization specificity was determined by competition with increasing amounts (0–2 μg) of wild-type V1b receptor in cells cotransfected with 1 μg each of V1bmyc and CRHR1WT. A 1:1 mixture of proteins from cells transfected individually with either V1bmyc or CRHR1WT, and untransfected CH) cells were used as negative controls. Coimmunoprecipitation was confirmed in two separate transfections performed in duplicate.
To demonstrate the specificity of the V1b/CRHR heterodimer, increasing amounts of V1bWT receptor were cotransfected to compete for V1bmyc/CRHR dimerization. Addition of V1bWT at a 1:1 ratio with V1bmyc was sufficient to reduce myc immunoreactivity in CRHR immunoprecipitants and no myc immunoreactivity was present at a 2:1 ratio despite similar levels of CRHR receptor pulldown in each sample (Fig. 7(B)). This suggests that the untagged V1b WT receptor was able to compete with the V1bmyc receptor for CRHR dimerization and is consistent with specific heterodimerization. To further confirm the ability of V1b receptors to form heterodimers with CRHR1 receptors, increasing amounts of CRHR1WT (10–1000 ng) were cotransfected with V1bNTRluc and V1bNTYFP (100 ng each) in order to compete with V1b homodimer BRET signal. Increasing CRHR1 WT receptor decreased the BRET obtained between the V1bNTRluc and V1bNTYFP vectors with an IC50 of 130 ± 24 ng, supporting the view that the CRHR1 receptor forms heterodimers with the V1b receptor (Fig. 3). There was no significant difference between the IC50 for V1bmyc and CRHR1 competition of the V1b homodimer, suggesting that the V1b receptor is equally likely to form a heterodimer with CRHR1 as a homodimer.
Effect of V1b/CRHR1 Heterodimerization on Ligand Binding
To determine whether heterodimerization of V1b and CRHR1 receptors changes the binding properties of the individual receptors, membrane-rich fractions from CHO cells cotransfected with V1b and CRHR1 receptors were tested for their ability to bind [3H]AVP or [125I]sauvagine in the presence or absence of saturating concentrations of the heterologous agonist or antagonist ligand. The number of V1bR and CRHR1 binding sites in membranes from cotransfected cells was about 250 and 500 fmol/mg protein, respectively, similar to those seen in rat pituitary membranes. The binding of [3H]AVP in the presence of the CRHR1 agonist, oCRH, or nonpeptide antagonist, DMP-696, was almost identical to that in control cotransfected membranes, in terms of receptor affinity and B max. Similarly, [125I]sauvagine binding was similar in the presence and absence of saturating concentrations of the V1b agonist, AVP, or nonpeptide antagonist, SSR149415 (Fig. 8 or Table III). This data demonstrates that ligand-induced conformational changes in the V1b/CRHR1 heterodimer do not influence the binding affinity for the heterologous ligand.
Fig. 8.
Effect of heterologous ligand on the binding properties of CRHR1/V1bR heterodimers. (A) [3H]AVP binding saturation curves in the presence and in the absence of 10 μM of CRH or the nonpeptide CRHR1 antagonist, DMP-696. Binding curves were performed by incubation of 10 μg membrane protein from CHO cells cotransfected with V1b and CRH receptor plasmids (2 ug DNA of each in 75 cm2) with 0.1 to 6 nM of [3H]AVP, in the absence or presence or the V1b receptor antagonist, 1 μM SSR149415 (nonspecific), at room temperature for 60 min. (B) Binding saturation curves for [125I]Sauvagine, in the presence and in the absence of AVP, or 1 μM of the nonpeptide V1b receptor antagonist, SSR149415. Binding curves were performed by incubating 10 μg of membrane protein from cotransfected CHO cells with [125I]sauvagine and increasing concentrations of unlabeled sauvagine, for 1 h at room temperature (nonspecific binding was measured in the presence of 10 μM DMP-696. Data points are the mean of duplicate incubations in a representative experiment.
Table III.
[3H]AVP and [125I]Sauvagine Binding on Membranes Prepared From CHO Cells Cotransfected with V1bmyc and CRHR1 Receptors
| K d (nM) | B max (fmol/mg protein) | |
|---|---|---|
| [3H]AVP binding | ||
| Control | 0.6 ± 0.2 | 265 ± 58 |
| Plus DMP-696 1 μM | 0.4 ± 0.2 | 279 ± 59 |
| Plus CRH 100 nM | 0.5 ± 0.1 | 212 ± 64 |
| [125I]Sauvagine binding | ||
| Control | 0.3 ± 0.1 | 472 ± 51 |
| Plus SSR149415 1 μM | 0.3 ± 0.1 | 502 ± 77 |
| Plus AVP 20nM | 0.2 ± 0.1 | 460 ± 40 |
Note. Data shown are mean ± SEM of three binding saturation curves.
DISCUSSION
It is well recognized that G-protein-coupled receptors are able to form homo- and heterodimeric complexes (Kroeger et al., 2001; Bulenger et al., 2005). This has been shown for most studied receptors, including, the GABAB1 and GABAB2 receptors (Kaupmann et al., 1998), δ and κ opioid (Jordan et al., 2000), μ and δ-opioid (George et al., 2000), adenosine A1 and dopamine D1 (Gines et al., 2000), angiotensin AT1 and bradykinin B2 (AbdAlla et al., 2000), AT1 and AT2, somatostatin 1 and somatostatin 5 (Rocheville et al., 2000b), somatostatin 5 and dopamine D2 (Rocheville et al., 2000a), as well as somatostatin 2 and μ opioid receptors (Pfeiffer et al., 2002). The established functional interactions between CRHR1 and V1bR prompted us to investigate the possibility that the actions of these receptors involve physical coupling. The present study, using BRET and coimmunoprecipitation techniques, demonstrates that vasopressin V1bR and CRHR1 are capable of forming constitutive homo- and heterodimers and that this interaction does not affect the binding properties of the receptors. While homo- and heterodimerization of V1b and CRHR1 was demonstrated by coimmunoprecipitation studies using fully bioactive CRHR1 and V1b-tagged receptors, the ability of these receptors to dimerize was independent of ligand–receptor interaction and bioactivity of the receptor.
In contrast to the report by Robert et al. (2005), we were unable to detect V1bR homodimers using C-terminal fusion constructs. It is noteworthy that the V1bCTYFP used in the current study contains a considerably longer sequence between the end of the V1bR and the beginning of the YFP compared to the Robert study (Robert et al., 2005). Therefore, it is possible that the conformation of our V1bCTYFP did not allow sufficient proximity for energy transfer. It is also possible that an intact C-terminus of the V1b receptor is required for dimerization and that C-terminus tag could interfere with the process. However, this is unlikely since we have noted that oxytocin receptor (OTR)/V1bR heterodimers are detectable by BRET when V1bCTRluc is cotransfected with OTRCTYFP, suggesting that C-terminus-tagged V1b receptor can heterodimerize (unpublished observation).
The similar IC50 displayed by the V1bR and CRHR1 to compete V1bRNTluc-V1bRNTYFP dimers indicates a similar affinity of the V1bR for another V1bR or the CRHR1, and suggests that they have similar probability to form homodimers or heterodimers. These competition experiments suggest that under physiological conditions, the proportion of homodimers and heterodimers depends on the relative expression of each of the receptor. Since the binding assays in transfected cells revealed V1bR and CRHR1 levels in the range of that in the anterior pituitary, the competition of these receptors for homo- and heterodimerization could have functional consequences in conditions such as chronic stress in which marked downregulation of CRHR1 is associated with V1bR upregulation (Aguilera, 1994).
While it has been proposed that homo- and heterodimers can be assembled either during biosynthesis or at the plasma membrane level, most experimental evidence seems to indicate that dimers are formed in the endoplasmic reticulum. The ability of ligands to regulate dimer formation in some receptors has suggested that dimers are formed at the plasma membrane. For example, exposure to ligand has been shown to increase, e.g., beta adrenergic, somatostatin, and TRH receptors (Hebert et al., 1996; Patel et al., 2002; Zhu et al., 2002), or decrease, e.g., opioid receptors (Cvejic and Devi, 1997), dimerization. However, ligand-induced changes in conformation of the receptor could account for changes in BRET by altering the position of receptor tags or in immunoprecipitation efficiency by modifying access of antibodies to the epitopes (Ayoub et al., 2002). The probability of receptors to homo- or heterodimerize in the endoplasmic reticulum is likely to depend on their relative affinity for each other and the relative synthesis rate of the receptors involved. The present data supports the possibility that CRHR1 and V1bR dimers are assembled during synthesis upon insertion in the endoplasmic reticulum, as shown for other receptors such as the GABAB, β2-adrenergic, muscarinic, V1a, V2, and oxytocin receptors as examples (Salahpour et al., 2000; Zeng and Wess, 2000; Calver et al., 2001; Terrillon et al., 2004). First, the major bands in the Western Blot after coimmunoprecipitation of dimers correspond to the molecular weight of immature, nonglycosylated CRH and V1b receptors. Second, the lack of effect of the peptide ligands, CRH and AVP, on dimer formation suggests that a stable intermolecular interaction is already established when the receptors reach the cell surface. Finally, the specific BRET signal obtained for the V1b homodimer using N-terminal tags, despite the confocal microscopy demonstration that aminoterminus YFP-tagged V1b receptors remained intracellular, provides additional evidence for dimer formation prior to reaching the plasma membrane. This is consistent with a recent report demonstrating homodimerization of V1b mutant receptors which are retained in the endoplasmic reticulum (Robert et al., 2005). For the GABAB receptors 1 and 2, alpha 1B- and 1D- adrenergic receptors, and for taste receptors, it has been shown that heterodimerization is a prerequisite for plasma membrane insertion of the receptors (Kaupmann et al., 1998; Nelson et al., 2002; Hague et al., 2004). Since in these experiments it was not possible to prevent dimerization, it is not clear whether physical interaction between the receptors is required for cell surface expression of CRHR1 and V1b receptors. Overall, these data strongly suggest that homo- and heterodimerization of V1b and CRHR1 is constitutive and not a regulated process.
An important question is the physiological implications of V1bR and CRHR1 dimerization. While it has been reported that some G-protein-coupled receptors can function as monomers, in many cases heterodimerization appears to alter the functional characteristics of the receptor (Kroeger et al., 2001; Bulenger et al., 2005). In the case of the GABAB receptor, dimerization is required for receptor trafficking to the plasma membrane and function (White et al., 1998). In cases such as the adrenergic, opiate-somatostatin, somatostatin-dopamine, angiotensin II-bradykinin, dimerization appears to alter pharmacological properties of receptors (Jordan et al., 2000; Rocheville et al., 2000a; Breit et al., 2004; Zhu et al., 2005). The present experiments found no obvious effect of dimerization on the pharmacological properties of V1b and CRHR1. The inability of the heterologous agonist or antagonist to alter receptor capacity or affinity strongly suggests that V1bR-CRHR1 heterodimerization does not alter the binding properties of the receptors. Similar, lack of effect of dimerization on receptor binding has been reported for the CRHR1 homodimer, somatostatin-opioid heterodimers, and V2-oxytocin heterodimers (Pfeiffer et al., 2002; Terrillon et al., 2003; Kraetke et al., 2005). However, the role of dimerization on CRH and AVP signaling is still unclear. Consistent with previous reports in anterior pituitary cells, which coexpress endogenous V1b and CRHR1 (Abou-Samra et al., 1987; Bilezikjian et al., 1987), AVP potentiated the CRH-induced cAMP production in CHO cells cotransfected with both receptors (data not shown). However, it is not possible to attribute the modulatory effect of AVP to dimerization without using means to prevent physical interaction between the receptors. In these experiments, all attempts to prevent dimerization (V1bR mutation CC334AA or cotransfection with V1bR fragments, including the C-terminus, the 7th transmembrane domain, and the 3rd intracellular loop) were unsuccessful, thus no conclusions can be drawn on the consequences of dimerization on receptor signaling. In fact, it is more likely that the potentiating effect of AVP on CRH-stimulated cAMP can be explained solely through interactions at the signaling level, since the effect can be mimicked by activation of PKC by phorbol esters bypassing the V1b receptor (Abou-Samra et al., 1987). In the present experiments, the agonist and the heterologous nonpeptide antagonist were used in receptor binding assays to induce changes in receptor conformation while obviating effects of second messenger signaling unrelated to dimerization. The inability of these compounds to alter CRHR1 and V1bR binding in cells cotransfected with both receptors supports the view that dimerization does not influence the signaling properties of the receptor. The possibility that V1bR and CRHR1 homo- or heterodimerization may lead to changes in receptor trafficking and/or desensitization remains to be investigated. Effects of dimerization on receptor trafficking have been shown for the somatostatin-opioid, somatostatin 1-somatostatin 5, TRHR1-TRHR2, and vasopressin V2-oxytocin receptor pairs (Rocheville et al., 2000b; Hanyaloglu et al., 2002; Pfeiffer et al., 2002; Terrillon et al., 2003).
In summary, we show that vasopressin V1bR and CRHR1 are capable of forming homo- and heterodimers in a ligand-independent manner and that this interaction does not affect the binding properties of the receptors. Elucidation of the physiological significance of V1b and CRHR1 homo- and heterodimerization will require a closer examination of the subcellular distribution, trafficking, desensitization, and resensitization of the receptors following exposure to ligand.
ACKNOWLEDGMENTS
The authors would like to thank Hans Zingg and Dominic Devost (McGill University, Montreal, Canada) for the oxytocin receptor fusion constructs and helpful advice, Dr Claudine Serradeil-LeGal (Sanofi-Synthelabo, Toulouse, France) for providing the nonpeptide V1bR antagonist, SSR149415 and Ying Liu, SEP, NICHD, for her valuable help. This research was supported by the Intramural Research Program, NICHD, NIH. S.F.Y. is supported by the Pharmacology Research Associate Training Program of the National Institute of General Medical Sciences, NIH, Bethesda, MD.
REFERENCES
- AbdAlla, S., Lother, H., and Quitterer, U. (2000). AT1-receptor heterodimers show enhanced G-protein activation and altered receptor sequestration. Nature407:94–98. [DOI] [PubMed] [Google Scholar]
- Abou-Samra, A. B., Harwood, J. P., Manganiello, V. C., Catt, K. J., and Aguilera, G. (1987). Phorbol 12-myristate 13-acetate and vasopressin potentiate the effect of corticotropin-releasing factor on cyclic AMP production in rat anterior pituitary cells: Mechanisms of action. J. Biol. Chem.262:1129–1136. [PubMed] [Google Scholar]
- Aguilera, G. (1994). Regulation of pituitary ACTH secretion during chronic stress. Front. Neuroendocrinol.15:321–350. [DOI] [PubMed] [Google Scholar]
- Aguilera, G., Pham, Q., and Rabadan-Diehl, C. (1994). Regulation of pituitary vasopressin receptors during chronic stress: Relationship to corticotroph responsiveness. J. Neuroendocrinol.6:299–304. [DOI] [PubMed] [Google Scholar]
- Ayoub, M. A., Couturier, C., Lucas-Meunier, E., Angers, S., Fossier, P., Bouvier, M., and Jockers, R. (2002). Monitoring of ligand-independent dimerization and ligand-induced conformational changes of melatonin receptors in living cells by bioluminescence resonance energy transfer. J. Biol. Chem.277:21522–21528. [DOI] [PubMed] [Google Scholar]
- Berrada, K., Plesnicher, C. L., Luo, X., and Thibonnier, M. (2000). Dynamic interaction of human vasopressin/oxytocin receptor subtypes with G protein-coupled receptor kinases and protein kinase C after agonist stimulation. J. Biol. Chem.275:27229–27237. [DOI] [PubMed] [Google Scholar]
- Bilezikjian, L. M., Blount, A. L., and Vale, W. W. (1987). The cellular actions of vasopressin on corticotrophs of the anterior pituitary: Resistance to glucocorticoid action. Mol. Endocrinol.1:451–458. [DOI] [PubMed] [Google Scholar]
- Breit, A., Lagace, M., and Bouvierm, M. (2004). Hetero-oligomerization between beta2- and beta3-adrenergic receptors generates a beta-adrenergic signaling unit with distinct functional properties. J. Biol. Chem.279:28756–28765. [DOI] [PubMed] [Google Scholar]
- Bulenger, S., Marullo, S., and Bouvier, M. (2005). Emerging role of homo- and heterodimerization in G-protein-coupled receptor biosynthesis and maturation. Trends Pharmacol. Sci.26:131–137. [DOI] [PubMed] [Google Scholar]
- Calver, A. R., Robbins, M. J., Cosio, C., Rice, S. Q., Babbs, A. J., Hirst, W. D., Boyfield, I., Wood, M. D., Russell, R. B., Price, G. W., Couve, A., Moss, S. J., and Pangalos, M. N. (2001). The C-terminal domains of the GABA(b) receptor subunits mediate intracellular trafficking but are not required for receptor signaling. J. Neurosci.21:1203–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro, M. G., Morrisonm, E., Perone, M. J., Brown, O. A., Murray, C. A., Ahmed, I., Perkins, A. V., Europe-Finner, G., Lowenstein, P. R., and Linton, E. A. (1996). Corticotrophin-releasing hormone receptor type 1: Generation and characterization of polyclonal antipeptide antibodies and their localization in pituitary cells and cortical neurones in vitro. J. Neuroendocrinol.8:521–531. [DOI] [PubMed] [Google Scholar]
- Childs, G. V., and Unabia, G. (1989). Activation of protein kinase C and L calcium channels enhances binding of biotinylated corticotropin-releasing hormone by anterior pituitary corticotropes. Mol. Endocrinol.3:117–126. [DOI] [PubMed] [Google Scholar]
- Childs, G. V., Westlund, K. N., and Unabia, G. (1989). Characterization of anterior pituitary target cells for arginine vasopressin: including cells that store adrenocorticotropin, thyrotropin-beta, and both hormones. Endocrinology125:554–559. [DOI] [PubMed] [Google Scholar]
- Cvejic, S., and Devi, L. (1997). Dimerization of the delta opioid receptor: Implication for a role in receptor internalization. J. Biol. Chem.272:26959–26964. [DOI] [PubMed] [Google Scholar]
- Flores, M., Carvallo, P., and Aguilera, G. (1990). Physicochemical characterization of corticotrophin releasing factor receptor in rat pituitary and brain. Life Sci.47:2035–2040. [DOI] [PubMed] [Google Scholar]
- George, S. R., Fan, T., Xie, Z., Tse, R., Tam, V., Varghese, G., and O’Dowd, B. F. (2000). Oligomerization of mu- and delta-opioid receptors. Generation of novel functional properties. J. Biol. Chem.275:26128–26135. [DOI] [PubMed] [Google Scholar]
- Gillies, G. E., Linton, E. A., and Lowry, P. J. (1982). Corticotropin releasing activity of the new CRF is potentiated several times by vasopressin. Nature299:355–357. [DOI] [PubMed] [Google Scholar]
- Gines, S., Hillion, J., Torvinen, M., Le Crom, S., Casado, V., Canela, E. I., Rondin, S., Lew, J. Y., Watson, S., Zoli, M., Agnati, L. F., Verniera, P., Lluis, C., Ferre, S., Fuxe, K., and Franco, R. (2000). Dopamine D1 and adenosine A1 receptors form functionally interacting heteromeric complexes. Proc. Natl. Acad. Sci. USA.97:8606–8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes, I., Jordan, B. A., Gupta, A., Rios, C., Trapaidze, N., and Devi, L. A. (2001). G protein coupled receptor dimerization: implications in modulating receptor function. J. Mol. Med.79:226–242. [DOI] [PubMed] [Google Scholar]
- Green, J. L., Figueroa, J. P., Massman, G. A., Schwartz, J., and Rose, J. C. (2000). Corticotropin-releasing hormone type I receptor messenger ribonucleic acid and protein levels in the ovine fetal pituitary: Ontogeny and effect of chronic cortisol administration. Endocrinology141:2870–2876. [DOI] [PubMed] [Google Scholar]
- Grigoriadis, D. E., and De Souza, E. B. (1988). The brain corticotropin-releasing factor (CRF) receptor is of lower apparent molecular weight than the CRF receptor in anterior pituitary. Evidence from chemical cross-linking studies. J. Biol. Chem.263:10927–10931. [PubMed] [Google Scholar]
- Hague, C., Uberti, M. A., Chen, Z., Hall, R. A., and Minneman, K. P. (2004). Cell surface expression of alpha1D-adrenergic receptors is controlled by heterodimerization with alpha1B-adrenergic receptors. J. Biol. Chem.279:15541–15549. [DOI] [PubMed] [Google Scholar]
- Hanyaloglu, A. C., Seeber, R. M., Kohout, T. A., Lefkowitz, R. J., and Eidne, K. A. (2002). Homo- and hetero-oligomerization of thyrotropin-releasing hormone (TRH) receptor subtypes. Differential regulation of beta-arrestins 1 and 2. J. Biol. Chem.277:50422–50430. [DOI] [PubMed] [Google Scholar]
- Hebert, T. E., Moffett, S., Morello, J. P., Loisel, T. P., Bichet, D. G., Barret, C., and Bouvier, M. (1996). A peptide derived from a beta2-adrenergic receptor transmembrane domain inhibits both receptor dimerization and activation. J. Biol. Chem.271:16384–16392. [DOI] [PubMed] [Google Scholar]
- Jia, L. G., Canny, B. J., Orth, D. N., and Leong, D. A. (1991). Distinct classes of corticotropes mediate corticotropin-releasing hormone- and arginine vasopressin-stimulated adrenocorticotropin release. Endocrinology128:197–203. [DOI] [PubMed] [Google Scholar]
- Jordan, B. A., Cvejic, S., and Devi, L. (2000). Opioids and their complicated receptor complexes. Neuropsychopharmacology23:S5–S18. [DOI] [PubMed] [Google Scholar]
- Kaupmann, K., Malitschek, B., Schuler, V., Heid, J., Froestl, W., Beck, P., Mosbacher, J., Bischoff, S., Kulik, A., Shigemoto, R., Karschin, A., and Bettler, B. (1998). GABA(B)-receptor subtypes assemble into functional heteromeric complexes. Nature396:683–687. [DOI] [PubMed] [Google Scholar]
- Konig, M., Mahan, L. C., Marsg, J. W., Fink, J. S., and Brownstein, M. J. (1991). Method for identifying ligands that bind to cloned Gs- or Gi-coupled receptors. Mol. Cell. Neurosci.2:331–337. [DOI] [PubMed] [Google Scholar]
- Kraetke, O., Wiesner, B., Eichhorst, J., Furkert, J., Bienert, M., and Beyermann, M. (2005). Dimerization of corticotropin-releasing factor receptor type 1 is not coupled to ligand binding. J. Recept. Signal Transduct. Res.25:251–276. [DOI] [PubMed] [Google Scholar]
- Kroeger, K. M., Hanyaloglu, A. C., Seeber, R. M., Miles, L. E., and Eidne, K. A. (2001). Constitutive and agonist-dependent homo-oligomerization of the thyrotropin-releasing hormone receptor. Detection in living cells using bioluminescence resonance energy transfer. J. Biol. Chem.276:12736–12743. [DOI] [PubMed] [Google Scholar]
- Kroeger, K. M., Pfleger, K. D., and Eidne, K. A. (2003). G-protein receptor oligomerization in neuroendocrine pathways. Front. Neuroendocrinol. 24:254–278. [DOI] [PubMed] [Google Scholar]
- Liu, J. P., Engler, D., Funder, J. W., and Robinson, P. J. (1992). Evidence that the stimulation by arginine vasopressin of the release of adrenocorticotropin from the ovine anterior pituitary involves the activation of protein kinase C. Mol. Cell. Endocrinol.87:35–47. [DOI] [PubMed] [Google Scholar]
- Lolait, S. J., O’Carroll, A. M., Mahan, L. C., Felder, C. C., Button, D. C., Young, W. S., 3rd, Mezey, E., and Brownstein, M. J. (1995). Extrapituitary expression of the rat V1b vasopressin receptor gene. Proc. Natl. Acad. Sci. USA.92:6783–6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, G., Chandrashekar, J., Hoon, M. A., Feng, L., Zhao, G., Ryba, N. J., and Zuker, C. S. (2002). An amino-acid taste receptor. Nature416:199–202. [DOI] [PubMed] [Google Scholar]
- North, W. G., Fay, M. J., and Du, J. (1999). MCF-7 breast cancer cells express normal forms of all vasopressin receptors plus an abnormal V2R. Peptides20:837–842. [DOI] [PubMed] [Google Scholar]
- Patel, R. C., Kumar, U., Lamb, D. C., Eid, J. S., Rocheville, M., Grant, M., Rani, A., Hazlett, T., Patel, S. C., Gratton, E., and Patel, Y. C. (2002). Ligand binding to somatostatin receptors induces receptor-specific oligomer formation in live cells. Proc. Natl. Acad. Sci. USA.99:3294–3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer, M., Koch, T., Schroder, H., Laugsch, M., Hollt, V., and Schulz, S. (2002). Heterodimerization of somatostatin and opioid receptors cross-modulates phosphorylation, internalization, and desensitization. J. Biol. Chem.277:19762–19772. [DOI] [PubMed] [Google Scholar]
- Robert, J., Auzan, C., Ventura, M. A., and Clauser, E. (2005). Mechanisms of cell-surface rerouting of an ER-retained mutant of the vasopressin V1b/V3 receptor by a pharmacological chaperone. J. Biol. Chem.280:2300–2308. [DOI] [PubMed] [Google Scholar]
- Rocheville, M., Lange, D. C., Kumar, U., Patel, S. C., Patel, R. C., and Patel, Y. C. (2000a). Receptors for dopamine and somatostatin: formation of hetero-oligomers with enhanced functional activity. Science288:154–157. [DOI] [PubMed] [Google Scholar]
- Rocheville, M., Lange, D. C., Kumar, U., Sasi, R., Patel, R. C., and Patel, Y. C. (2000b). Subtypes of the somatostatin receptor assemble as functional homo- and heterodimers. J. Biol. Chem.275:7862–7869. [DOI] [PubMed] [Google Scholar]
- Salahpour, A., Angers, S., and Bouvier, M. (2000). Functional significance of oligomerization of G-protein-coupled receptors. Trends Endocrinol. Metab.11:163–168. [DOI] [PubMed] [Google Scholar]
- Sydow, S., Radulovic, J., Dautzenberg, F. M., and Spiess, J. (1997). Structure–function relationship of different domains of the rat corticotropin-releasing factor receptor. Mol. Brain Res.52:182–193. [DOI] [PubMed] [Google Scholar]
- Terrillon, S., Barberis, C., and Bouvier, M. (2004). Heterodimerization of V1a and V2 vasopressin receptors determines the interaction with beta-arrestin and their trafficking patterns. Proc. Natl. Acad. Sci. USA.101:1548–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrillon, S., Durroux, T., Mouillac B., Breit, A., Ayoub, M. A., Taulan, M., Jockers, R., Barberis, C., and Bouvier, M. (2003). Oxytocin and vasopressin V1a and V2 receptors form constitutive homo- and heterodimers during biosynthesis. Mol. Endocrinol.17:677–691. [DOI] [PubMed] [Google Scholar]
- van de Pavert, S. A., Clarke, I. J., Rao, A., Vrana, K. E., and Schwartz, J. (1997). Effects of vasopressin and elimination of corticotropin-releasing hormone-target cells on pro-opiomelanocortin mRNA levels and adrenocorticotropin secretion in ovine anterior pituitary cells. J. Endocrinol.154:139–147. [DOI] [PubMed] [Google Scholar]
- White, J. H., Wise, A., Main, M. J., Green, A., Fraser, N. J., Disney, G. H., Barnes, A. A., Emson, P., Foord, S. M., and Marshall, F. H. (1998). Heterodimerization is required for the formation of a functional GABA(B) receptor. Nature396:679–682. [DOI] [PubMed] [Google Scholar]
- Xu, G., Rabadan-Diehl, C., Nikodemova, M., Wynn, P., Spiess, J., and Aguilera, G. (2001). Inhibition of corticotropin releasing hormone type-1 receptor translation by an upstream AUG triplet in the 5′ untranslated region. Mol. Pharmacol.59:485–492. [DOI] [PubMed] [Google Scholar]
- Young, S. F., Smith, J. L., Figueroa, J. P., and Rose, J. C. (2003). Ontogeny and effect of cortisol on vasopressin-1b receptor expression in anterior pituitaries of fetal sheep. AJP—Regul. Integr. Comp. Physiol.284:R51–R56. [DOI] [PubMed] [Google Scholar]
- Zeng, F., and Wess, J. (2000). Molecular aspects of muscarinic receptor dimerization. Neuropsychopharmacology23:S19–S31. [DOI] [PubMed] [Google Scholar]
- Zhu, C., Cook, L. B., and Hinkle, P. M. (2002). Dimerization and phosphorylation of thyrotropin-releasing hormone receptors are modulated by agonist stimulation. J. Biol. Chem.277:28228–28237. [DOI] [PubMed] [Google Scholar]
- Zhu, W., Zeng, X., Zheng, M., and Xiao, R. P. (2005). Heterodimerization of beta1- and beta2-adrenergic receptor subtypes optimizes beta-adrenergic modulation of cardiac contractility. Circ. Res.97:244–251. [DOI] [PubMed] [Google Scholar]