Abstract
Visceral noxious stimulation induces central neuronal plasticity changes and suggests that the c-AMP-dependent protein kinase (PKA) signal transduction cascade contributes to long-term changes in nociceptive processing at the spinal cord level. Our previous studies reported the clinical neurosurgical interruption of post synaptic dorsal column neuron (PSDC) pathway by performing midline myelotomy effectively alleviating the intractable visceral pain in patients with severe pain. However, the intracellular cascade in PSDC neurons mediated by PKA nociceptive neurotransmission was not known. In this study, by using multiple experimental approaches, we investigated the role of PKA in nociceptive signaling in the spinal cord and PSDC neurons in a visceral pain model in rats with the intracolonic injection of mustard oil. We found that mustard oil injection elicited visceral pain that significantly changed exploratory behavior activity in rats in terms of decreased numbers of entries, traveled distance, active and rearing time, rearing activity, and increased resting time when compared to that of rats receiving mineral oil injection. However, the intrathecal infusion of PKA inhibitor, H 89 partially reversed the visceral pain-induced effects. Results from Western blot studies showed that mustard oil injection significantly induced the expression of PKA protein in the lumbosacral spinal cord. Immunofluorescent staining in pre-labeled PSDC neurons showed that mustard oil injection greatly induces the neuronal profile numbers. We also found that the intrathecal infusion of a PKA inhibitor, H89 significantly blocked the visceral pain-induced phosphorylation of c-AMP –responsive element binding (CREB) protein in spinal cord in rats. The results of our study suggest that the PKA signal transduction cascade may contribute to visceral nociceptive changes in spinal PSDC pathways.
Keywords: spinal cord, post synaptic dorsal column (PSDC) neurons, protein kinase A, visceral nociception, transcription factors
1. Introduction
Visceral pain is the most common sign of acute and chronic gastrointestinal and pelvis diseases. It represents a frequent reason for patients to seek medical treatment and it is one of the most common causes of long-term suffering and persistent disability. Despite multiple therapeutic approaches, the medical community still faces a significant shortcoming in the ability to successfully relieve chronic visceral pain, especially that produced by cancer. Recently, our group discovered a critical visceral nociceptive pathway that originates from post-synaptic dorsal column (PSDC) neurons located in the central area of the spinal cord. By performing a refined and limited midline myelotomy, we were able to interrupt this pathway and relieve pain on patients with intractable cancer pain. The surgical outcomes reduced both visceral pain intensity and narcotic requirement. It confirmed that this procedure is effective in relieving intractable visceral pain (Nauta et al. 1997; Nauta et al. 2000). Since this initial discovery, several neurosurgical units around the world have used a similar procedure to treat patients with severe oncogenic visceral pain (Becker et al. 1999; Becker et al. 2002; Hu & Li 2002; Hwang et al. 2004; Kim & Kwon 2000; Vilela et al. 2001). These effective clinical procedures strongly support the solid concept that the PSDC pathway plays an important role in the transmission of visceral nociceptive information. Research in experimental animals using electrophysiological recordings, anatomic tracers and behavioral techniques has further confirmed and reinforced this transmission pathway mechanism (Ness 2000; Palecek et al. 2002; Palecek et al. 2003; Willis et al. 1999). However, the neurochemical signal transduction pathways relaying this information involved in these particular PSDC neurons in response to visceral nociceptive stimulation remains unclear.
It has been reported that the second messenger system conveys extracellular nociceptive signals from the plasma membrane into the intracellular nucleus in animal models of pain (Wu et al. 2000; Wu et al. 2001; Wu et al. 2002). As an important member of second messenger system, the intracellular PKA responds to the increased cellular levels of cAMP converted from ATP by adenylate cyclase (Sassone-Corsi 1998). The role of PKA in the nociceptive dorsal horn neurons of spinal cord in response to noxious peripheral stimulation was reported in a behavioral study (Sluka & Willis 1997). We previously reported in an electrophysiology study that the active status of PKA affects the response of primate spinothalamic tract neurons (STT) to peripheral mechanical stimuli (Lin et al. 2002). PKA was reported to regulate the expression of phosphorylation of c-AMP –responsive element binding protein (CREB) in spinal cord in rats in response to capsaicin injection (Wu et al., 2002, 2005). Another study in rats also showed that inflammatory mediators –induced hyperalgesia is dependent on the ongoing activity of PKA in spinal cord (Aley & Levine 1999). Although a study reported that another intracellular kinase in spinal cord, MAP kinase could be elicited by visceral inflammatory stimulation (Galan et al. 2003), it remains to be determined whether, PKA participate in the processing of nociception in spinal cord. The current project was designed to examine the role of PKA in effecting exploratory behavioral changes as well as the expression pattern of PKA in spinal cord and PSDC neurons in rats following visceral noxious stimulation. Finally, the effects of PKA in regulation of transcription factor, CREB protein in spinal cord during visceral nociception were also evaluated.
2. Experimental procedure
2.1 Animals
Male Sprague-Dawley rats (weighing 280–350 grams, Harlan Sprague-Dawley, Houston, Texas) were used in this study. The experimental protocol was approved by the Institutional Animal Care and Use Committee of the University of Texas Medical Branch and in accordance with the ethical guidelines of the International Association for the Study of Pain and National Institutes of Health. The visceral noxious stimulation was induced by the intracolonic injection of mustard oil (10 μl in 1000 μl mineral oil) or mineral oil (Palecek et al. 2002) under halothane anesthesia. Previous study demonstrated that intracolonic injection of mustard oil could be served as a visceral noxious stimulation since this application induces a significant inflammatory response in the mucosal area of the colon wall (Palecek et al. 2002; Palecek et al. 2003). Spinal cord tissues were collected at 30 and 60 minutes post-injection for immunoblot or immunostaining studies. To test the role of PKA on the effect of exploratory behavioral changes in rats, intrathecal administration of a PKA inhibitor was performed (Fang et al. 2002; Zhang et al. 2006).
2.2 Exploratory behavioral study
For the intrathecal administration of PKA kinase inhibitor, an intrathecal 32 gauge-catheter was implanted in the spinal subarachnoid space in anesthetized rat (Fang et al. 2003a). Briefly, a midline incision was performed to expose the suboccipital area in the animals and the catheter was gently advanced ~7.4 cm into the spinal subarachnoid space. The catheter’s tip was placed at around the estimated level of the L4/5 spinal segments. The rats were returned to the housing facility and kept for about 5 days to allow them to recover from the surgery. On the experimental day, the catheter was connected to a 1 ml syringe operated by an infusion pump and it kept a constant infusion rate of 1 μl/min for the delivery of H89. H89, a protein kinase A inhibitor ((N-[2-((3-bromophenyl)-2-propenyl)amino]ethyl)-5-isoquinoline sulfonamide, HCL, Calbiochem., San Diego, CA) and ACSF were infused. The dose of the concentration of H89 was 10 μM since it demonstrated the inhibitory effect in our previously-published results (Fang et al. 2003a; Wu et al. 2005).
The infusion of H89 was performed for 30 min. At 30-min post-infusion, the intracolonic injection of mustard oil (10 μl in 1000 μl mineral oil) or mineral oil was performed (Al Chaer et al. 1996). The exploratory activity of the rats was recorded after intracolonic injection of mustard oil. To measure the exploratory activity of the animals, two activity boxes (San Diego Instruments) was used. The boxes (40 × 40 cm2) are made from clear plastic and locomotor activity was monitored by 16 infrared beams on each of the two adjacent sides (32 total). To analyze the movement of the animal, the area of each enclosure was divided by FLEXFIELD software into 16 equal zones (4 × 4). Several parameters were measured: number of entries of the animal into a different zone, total distance traveled across the box, activity time, resting time (time when the animal is not moving), rearing activity and rearing time. The enclosures were always thoroughly cleaned both before and after each testing period of 45 min. Animals were always tested at the same time during the day (8–10 a.m.) in a separate noise-free room with a controlled temperature (70–72°F), where no people or other animals were present. The animals were brought to the room just for the test and during the test the room was kept dark, illuminated just by the monitor screen (<10 Lux). The animal activity registered by the infrared beams was monitored with a PC using FLEXFIELD software. To evaluate total activity, the results from the nine consecutive 5 min intervals was added for each animal and Means ± SEM was calculated for all animals in the group. To reduce stress and to enable precise manipulation, animals were briefly anesthetized with 2.5% halothane in oxygen during the administration of mustard oil. Mustard oil (Sigma-Fluka Com. St. Louis, MO) solution in mineral oil (2.5%, 0.1 ml) was injected via a small diameter plastic tube (PE50). Halothane anesthesia was terminated immediately after either procedure. The rats usually began to move around their cages in less than 15s. The animals were tested in the activity boxes 30 min after the end of the mustard oil application. Since the rats recover rapidly from the brief halothane anesthesia, the anesthetic does not affect the exploratory test during the 30 min period (Fang et al. 2002; Palecek et al. 2002). The enclosure was cleaned to eliminate urine and other olfactory cues from previous animals by using Cavicide and alcohol between the tests (Zhang et al. 2006).
2.3 Western blot study
The L3–L6 segments of the spinal cord tissues were collected and immediately placed into liquid nitrogen and kept in at −70 C. Then the tissue was homogenized and the concentration of total protein was tested by using a BCA kit (Pierce, Rockford, IL) and was read on a microplate reader (Sun Bioscience) (Gao et al. 2006). Equal amounts of protein (60 μg) from different group rat were loaded and size-fractionated by gel electrophoresis (SDS-PAGE) in a 4–20% Ready-Gel preparation and transferred onto a PVDF membrane (Bio-Rad, Hercules, CA). After incubating in blocking buffer, the membranes were incubated with primary polyclonal antibody to PKA catalytic unit at the Thr-197 site (Cell Signals Technology. MA) overnight at 4°C. The polyclonal antibody against PKAc does not have cross reactions with other subunits of PKA.
To study the phospho-CREB expression, the animals were intrathecally treated with H89 for 30 minutes before mustard oil injection. The rats were sacrificed at 60 minutes post-stimulation and the lumbosacral segment tissues were collected for immunoblotting. After gel electrophoresis and transferring process, the membranes were incubated with the primary polyclonal antibodies against phospho-CREB (1:1000) at the Ser-133 site (Upstate Biotechnology, Lake Placid, NY) overnight at 4°C. The phospho-CREB antibody was produced with one synthetic phospho-peptide corresponding to residues 123–136 (KRREILSRRPpSYRK) (Wu et al., 2002, 2005).
The blots were rinsed three times with PBS buffer and then incubated with HRP-conjugated anti-rabbit IgG (Santa Cruz, San Francisco, CA) in 5% non-fat milk in PBS buffer solution. The membranes were then enhanced with a chemiluminescence reagent (ECL kit, Amersham, Arlington Height, IL) and were exposed to the x-ray film. The expression of β-actin, as an internal control, was also examined with the same procedure in every group. The monoclonal antibody against β-actin was purchased from Sigma Com. (St. Louis, MO). The density of the blotted bands was acquired by using a Doc-It Gel system and analyzed by suing an AlphaEase software (Fang et al. 2003b; Wu et al. 2005). The densitometric units of bands of PKA were expressed relative to the values for β-actin. The significance of differences between groups treated with mustard oil or mineral oil was calculated and compared with the Student’s t-test (Sluka & Willis 1997). P <0.05 was considered significant. All data were expressed as mean ± S.E.M. (Fang et al. 2003b; Wu et al. 2005).
2.4 Retrograde labeling of PSDC neurons and fluorescence immunohistochemistry study
After anesthesia with sodium pentobarbital, adult male rats were placed in a stereotaxic headholder. An upper cervical laminectomy and partial removal of the occipital bone posterior to the foramen magnum exposed the dorsal column nuclei. Using a 1 μl microsyringe, a volume of 0.5 μl of Fluoro-Ruby (Molecular Probe, CA) was injected into the nucleus gracilis to label PSDC neurons (Paclecek et al., 2003). All animals were allowed 5 days to recover before the next experimental procedure or visceral stimulation. The animals were perfused with 4% paraformaldehyde in 0.1M PBS. The spinal cord (lumbosacral segments) was removed and post-fixed. The block was embedded in medium was serially sectioned (10 μm thick) in a cryostat (−20°C). Systematic random sampling was performed and three pairs of sections chosen were taken and stained with PKA antibody. Sections were incubated for 1 hr with 10% normal goat serum in PBS to block the non-specific staining. Following a rinse of PBS, the sections were incubated for overnight with the primary antibody against PKAc (Cell Signals Technology Com., MA, without cross-reaction with other subunits of PKA). The sections were rinsed in PBS and placed in Fluorescence- conjugated in goat anti-rabbit antibody (Invitrogen -Molecular Probe, CA). The sections were rinsed again with PBS and cover-slipped with mounting medium and viewed under a fluorescence microscope. Control sections for the staining consisted of the same procedure, with the omission of the antibodies. The first 10 slices processed in the segment were always used for evaluation of retrogradely labeled PSDC neuron profiles and the presence of target proteins using epi-fluorescence or confocal microscopy (Palecek et al. 2003). The number of neuronal profiles of positive PKA with PSDC neurons on each section was added to represent the total number. To assure some degree of standardization in the evaluation process, the counting of images was performed by double-blinded observers (Palecek et al. 2003). The double-labeled cells were compared between the groups. Data were analyzed for significance using a t-test and one-way analysis of variance.
3. Results
3.1 Behavioral test study
3.1.1 Effect of noxious visceral stimulation in naïve rats
after visceral noxious stimulation by intracolonic installation of mustard oil in naive rats, the exploratory activity was severely affected compared to the animals receiving mineral oil injection. The total entry activity significantly decreased from 1254 ± 206 to 439 ±130 (Figure 1A), and distance traveled decreased greatly from 2193 ± 220 to 1173 ± 197 (Figure 1B). Active time decreased from 2241 ± 190 to 1037 ± 219 (Figure 1C), whereas resting time increased from 801 ± 203 to 1837 ± 195 (Figure 1D). Rearing activity was also significantly affected by mustard oil injection. Rearing time decreased from 224 ± 42 to 68 ± 37(Figure 1E) and rearing events decreased from 335 ± 62 to 117 ± 49 (Figure 1F). The performance of statistical analysis for the data from these two provide a significant difference between the mustard oil treated and mineral oil treated groups (p<0.05 as indicated as * in Figure 1).
Figure 1.
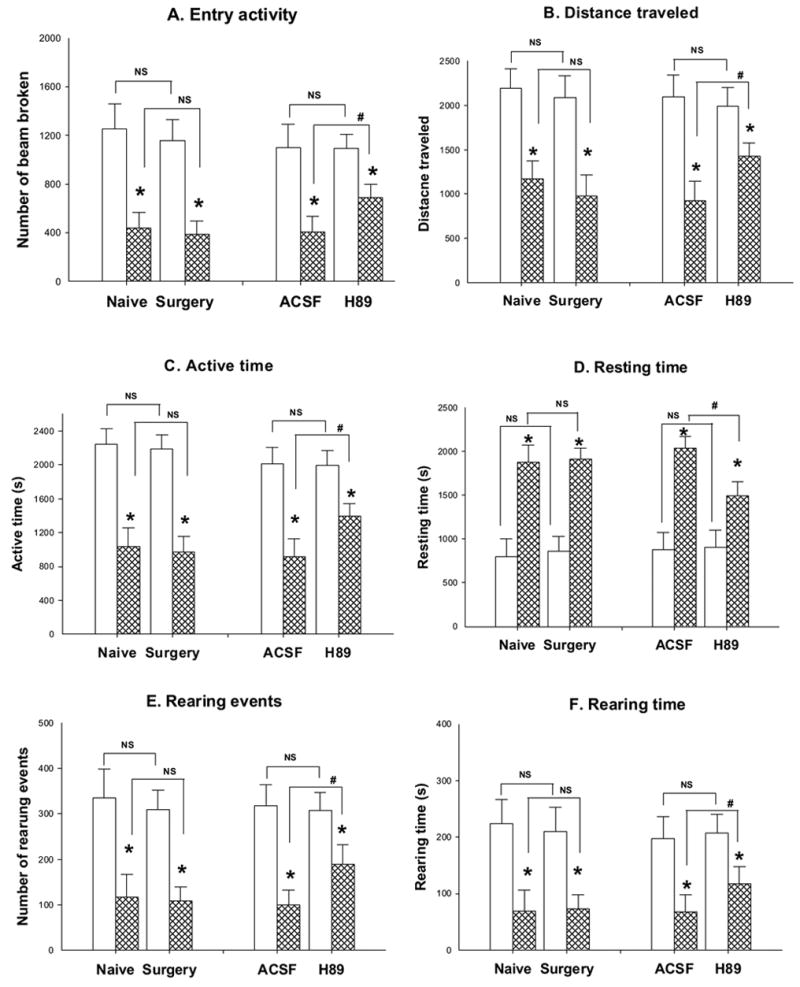
The role of PKA inhibitor, H 89 in the control of rat exploratory behavioral activity was investigated by using a computerized photo-beam monitoring FlexField system. During a consecutive period of 45 minutes (nice intervals with five minutes per interval), six parameters were measured: entry activities (number of beam broken, in Panel A), traveled distance (Panel B), active time (Panel C), resting time (Panel D), rearing events(Panel E) and rearing time (Panel D). Left panels in each individual figure show the effect of the surgical procedure on the rat’s behavioral changes after placement of an intrathecal catheter (the groups shown as Surgery) when compared to naïve rats (the groups shown as Naive) following mineral oil and mustard oil treatments. There was no significant difference in the exploratory behavioral activity between naïve rats and animals receiving the surgical procedure within the same treatment group (NS: no significant, n=5 in each group with the same treatment). However, the intracolonic injection of mustard oil (groups shown as hatched bar) results significant changes in the exploratory behavioral activity when compared to that of mineral oil treatment (groups shown as open bar, *: p< 0.05, mustard oil vs mineral oil). Mustard oil injection decreases the entry activity, traveled distance, active time, rearing events and rearing time while increases resting time. In the groups of rats receiving H89 administration intrathecally, significantly reduced the effects of mustard oil-induced changes (#: p< 0.05 in each individual panels, H 89 treatment vs ACSF treatment) without affecting that of mineral oil-treated group (NS: no significant, n=8).
3.1.2 The effect of surgery for implantation of intrathecal catheter
The surgery effect on exploratory behavior alternations was tested first in our study before we started the infusion of PKA inhibitors. The spontaneous activity of animals and all six parameters representing behavioral alternation were examined both before and the 5th day after intrathecal implantation of a plastic catheter. The motor function was demonstrated in Figure 1 and it was not impacted by the surgical procedure for the placing of the intrathecal catheter. Compared to data generated from the naive animals, the general value of exploratory behaviors of rats receiving intrathecal implantation, such as the number of total entry activity (1254 ± 206 vs 1158 ± 170 in mineral treated rats and 439 ± 130 vs 396 ± 109 in mustard oil treated group), distance traveled (2193 ± 220 vs 2087 ± 246 in mineral treated rats and 1173 ± 197 vs 981 ± 234 in mustard oil treated group), length of active time (2241 ± 190 vs 2185 ± 170 in mineral treated rats and 1037 ± 219 vs 968 ± 192 in mustard oil treated group) and rearing time (224 ± 42 vs 209 ± 43 in mineral treated rats and 68 ± 38 vs 72 ± 25 in mustard oil treated group), number of rearing events (335 ± 62 vs 309 ± 43 in mineral treated rats and 117 ± 49 vs 108 ± 31 in mustard oil treated group) and resting time (801 ± 203 vs 855 ± 170 in mineral treated rats and 1873 ± 195 vs 1915 ± 119 in mustard oil treated group) in an examined consecutive 45-minute interval were not significantly different. Within the same group treated with mineral oil or mustard oil injection, the surgical procure did not produce significant changes in these parameters (Left panels in each individual figure in Figure 1; NS: no significant, bars indicated as below labels with groups of rats of naive and received surgery ).
3.1.3 The effect of intrathecal administration of H 89, an inhibitor of PKA on exploratory behavior changes
H89, a nonselective inhibitor of PKA, was given intrathecally in a group of rats. Pretreatment with ACSF administered through the intrathecal catheter (n = 8) was done in the control group. H 89 (n = 8) was administered in the mineral oil or mustard oil treated groups. Thirty minutes after the pretreatment with agents infused through the intrathecal catheter, colonic injection was inducted in rats. Animals were put into an activity chamber 30 min after the injection to monitor the movements. Right panels in each individual group figures in Figure 1 demonstrate that the effects of intrathecal administration of H89 and ACSF on the spontaneous exploratory activity of the rats receiving noxious colonic stimulation with mustard oil as well as control agent mineral oil. In these experiments, the treatment with mustard oil produces the similar changes in the activity parameters as in naive rats which means that the decreased total activity, traveled distance, active time, resting time, rearing time and rearing events, but increased resting time when compared to the value of mineral oil-treated rats (p<0.05). However, in mustard oil-treated groups, the changed exploratory activity induced by mustard oil was reversed by intrathecal administration of PKA inhibitor, H89 (p<0.05 indicated as # in Figure 1) compared to the control group (ACSF treatment). Total activity significantly reversed from 403 ± 129 to 692 ± 103; consequently the distance traveled changed greatly from 927 ± 214 to 1429 ± 148; active time increased from 918 ± 214 to 1392 ± 148. Rearing time was also increased greatly from 68 ± 29 to 117 ± 30 and the rearing events did from 99 ± 31 to 189 ± 42 while the resting time decreased from 2038 ± 129 to 1495 ± 155 in consecutive 45-minute intervals (p<0.05 and # indicated significant in Figure 1). Meanwhile, no significant between ACSF-treated and H 89-treated groups in rats receiving mineral oil injection.
3.2 Western blotting study
The results from this study demonstrated that the PKA protein was detected by immunoblot analysis in each group of animals. The molecular weight of PKA protein was observed at the 42 KD band. Following intracolonic injection of mustard oil in two groups of rats, the expression of PKA protein showed a significant increase in the lumbosacral spinal cord at 30 and 60 time-points post-injection (Figure 2) when compared to that of rats treated with mineral oil, a control agent. Mineral oil treatment did not change the expression of PKA protein when compare to naïve rats (data not shown). The relative density of the PKA protein band significantly increased at 30 minutes (2.41 ± 1.09 vs. 5.03± 0.8; p<0.05) and 60 minutes (2.82 ± 1.47 vs. 7.29± 1.3; p<0.05) post- MO-treatment. The expression of β–actin of the same tissues was examined as a loading control.
Figure 2.
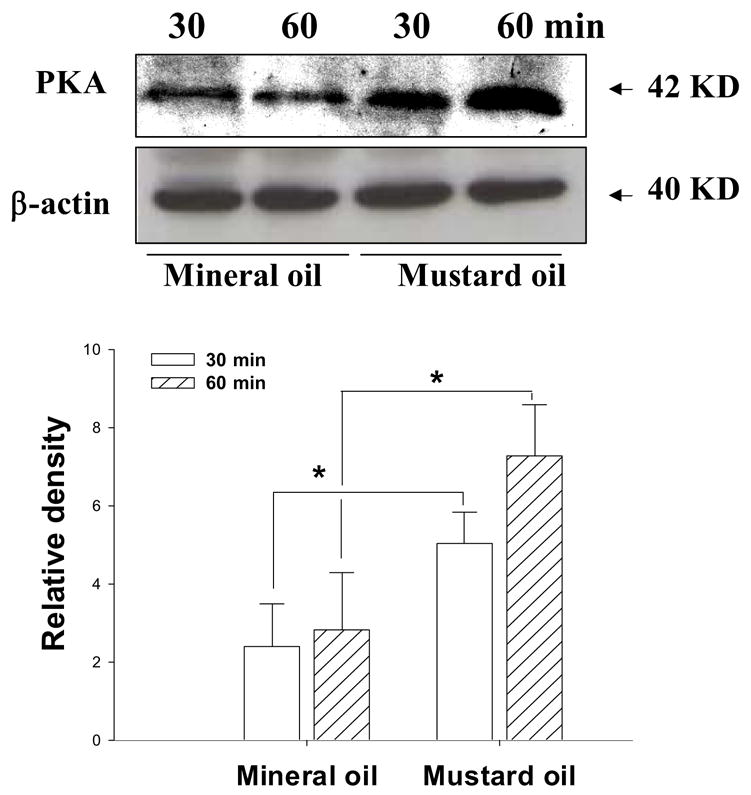
Detection of PKA protein in spinal cord tissue of rats at 30 and 60 minutes following intracolonic injection of mineral oil (as a control) and mustard oil (noxious stimulation). Upper panel: A representative picture of immunoblotted bands of PKA protein (42 KD) and β–actin (40 KD, as a loading control) from rats with different treatments. Lower panel: Bar graph summarizing the relative density of the immunoblot bands of PKA protein (as the ratio to the value of β–actin). *, p <0.05; the value from the group with mustard oil treatment vs. mineral oil treatment (n=5 in each group). Open bar, at 30 minutes post-injection; hatched bar, at 60 minutes post-injection.
3.3 Immunostaining study of PKA expression on PSDC neurons
In this study, we investigated the presence of PKA protein immunoreactivity in PSDC neurons in animals receiving visceral noxious stimulation. In these experiments, most PSDC neurons examined in lumbosacral segments showed immunoreactivity for PKA protein. The representative picture (at 30 minutes post-stimulation) is demonstrated in Figure 3. In panel A, it could be viewed that a PSDC neuron pre-labeled by the retrograde tracer and the positive PSDC neurons shows the location of PKA protein in the cytoplasma areas (Panel B in Figure 3). The yellow color in Panel C of Figure 3 shows the merged result of the previous two panels. After colon inflammation with intracolonic mustard oil injection, we have found that the profile number of examined PSDC neurons co-expressing immunopositive PKA protein significantly increased when compared to the profile number of rats treated with mineral oil (Figure 3, lower bar graph, p< 0.05). This event could be observed at the 30 minutes (26 ± 12 vs 101 ± 12) and 60 minutes (32± 14 vs 128±14) post-stimulation (Figure 3, lower bar graph).
Figure 3.
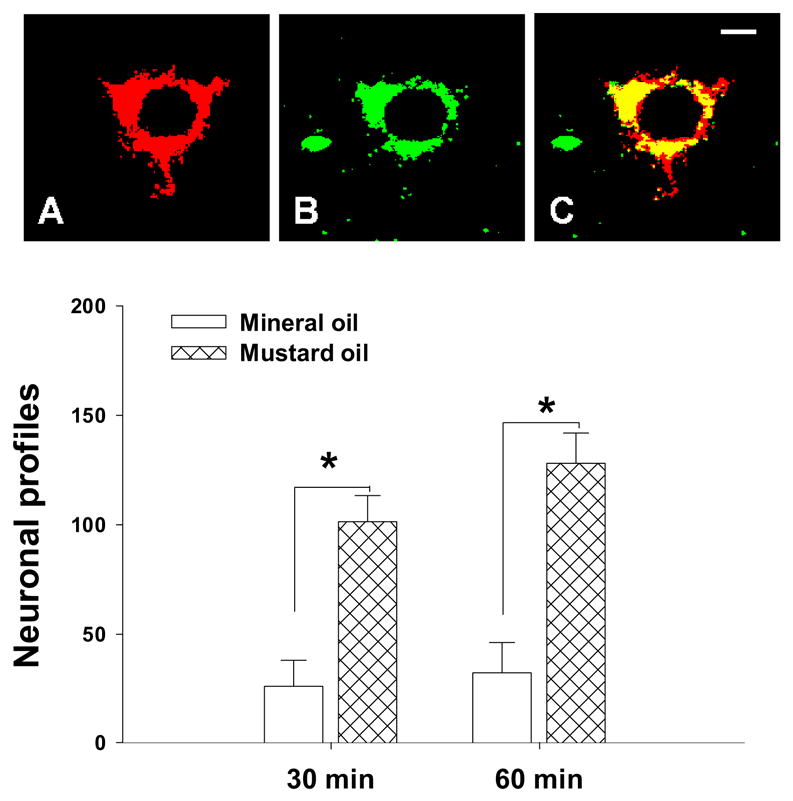
Immunofluorescent staining of PKA protein expressed on a retrogradely pre-labeled PSDC neuron collected from a rat treated with mustard oil injection (60 minutes after injection). Retrogradely labeled profile of a PSDC neuron is clearly marked by the fluorescent dye (Red color, Panel A). Immunoreactivity for PKA protein could be found on the cytoplasma (Green color, Panel B). Panel C shows the merged result (Yellow color). Lower bar graph indicated the increased cellular profiles of PKA protein in PSDC neurons from rats treated with mustard oil when compared to the value of rats treated with mineral oil. (n=6 in each group). Open bar, mineral oil treatment (as a control) group; hatched bar: mustard oil treated group. Scale bar =5 μm.
3.4 The effect of intrathecal administration of H89 on expression of phospho-CREB protein in spinal cord during visceral nociception
We also assessed the effect of protein kinase A (PKA) in mediating a transcription factor, CREB protein phosphorylation since PKA is one of the important intracellular triggers for CREB protein activation. Previous studies demonstrated that the expression of non-phosphorylated form of CREB protein do not change after noxious stimulation (Wu et al., 2005). The intrathecal administration of the selective PKA inhibitor, H89 (10 μM for 30 minutes), was performed before inducing visceral noxious stimulation with mustard oil injection in rats. The result showed that there was a significant increase in the phospho-CREB protein expression in both of ACSF- and H89- treated animals after mustard oil injection (ACSF treated group: 0.78±0.31 vs 3.17±0.25, p<0.05; H 89 treated group: 0.87±0.22 vs 1.62±0.21, p<0.05). To further evaluate the effect of H89 on phospho-CREB protein expression in response to mustard oil injection, we found that the mustard oil-induced increase in CREB protein phosphorylation was inhibited by infusion of H89 when compared to that of ACSF-treated rats (1.62±0.21 vs 3.17±0.25, p<0.05, Figure 4). The data suggest that inhibiting PKA activity could block the increased phospho-CREB protein expression in spinal cord during visceral pain state.
Figure 4.
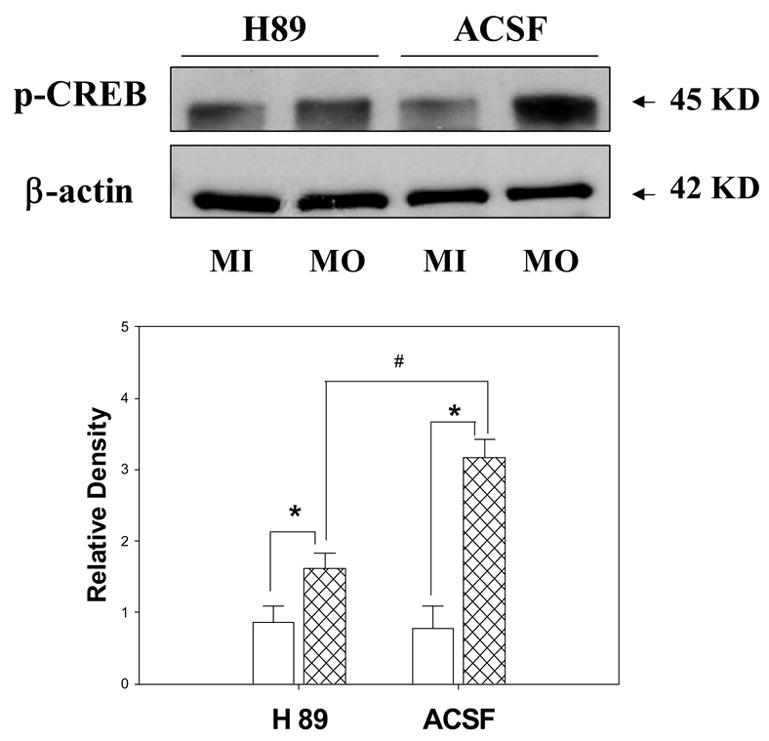
Effect of intrathecal infusion with a PKA inhibitor, H89 (for 30 minutes) on the expression of phospho-CREB protein and β-actin in spinal cord in rats at 60 minutes after visceral painful stimulation. Upper Panel Representative immunoblot bands of phospho-CREB protein (45KD) andβ-actin (42 KD) expression. Lower Panel Bar graph showing the relative density of the immunoblotting bands of phospho-CREB protein 25 (ratio to the value of β-actin). (*, p <0.05; the value from mineral oil treatment vs. mustard oil treatment in both of ACSF-treated and H89-treated groups; #, p< 0.05, the value of rats with mustard oil treatment between ACSF treatment and H89 administration; n=6 in each group). Open bar, mineral oil-treated group (labeled as MI); hatched bar, mustard oil-treated group (labeled as MO).
To study the nonspecific effects on central neurochemical changes by intracolonic MO injection, we also did some pilot Western blot studies to test the expression of PKA and phospho-CREB protein in the spinal tissue collected the from the cervical segments. However, we did not find significant changes in the protein expression of PKA and phospho-CREB between the control and the inflamed groups (data not shown).
4. Discussion
The findings in this study indicated that intracolonic mustard oil injection causes a significant change in the exploratory activity of rats with a paralleled increased expression of PKA protein as well as enhanced PKA positive neuronal profiles in PSDC cells. Furthermore, the intrathecal application of PKA inhibitor, H89, reduced the changes produced by mustard oil injection. These data collected from the multiple approaches including exploratory behavioral tests, Western blot and fluorescent immunostaining suggested a critical role of PKA in signaling nociceptive neurotransmission in the spinal cord and PSDC neurons during visceral noxious stimulation.
Following mustard oil application in the colon, the exploratory behavioral activity in rats was significantly changed due to visceral nociception. The alternations in this visceral pain model presents as similar to a previous study (Palecek et al. 2002) and are also in agreement with another investigation of exploratory activity in other pain models such as capsaicin injection, pancreatitis pain and spinal cord injured pain (Fang et al. 2002; Mills et al. 2001; Zhang et al. 2004; Zhang et al. 2006). It confirmed that the reduction of movement with a normal capacity to move results in less active time and more resting time which imply the development of visceral pain induced by mustard oil application.
As a serine/theonine protein kinase present in neurons, PKA is activated by the increased level of cyclic adenosine monophosphate (cAMP) in normal and pathological states (Taylor et al. 1990). The intracellular cAMP level regulates cellular responses by altering the interaction between the catatytic and regulatory subunits of PKA. The inactive PKA holoenzyme is activated following cAMP binds to the regulatory subunits that induce the release of two active catalytic subunits. A number of studies have shown that a role of PKA activity to regulate nociceptive behavioral reaction. Sluka et al reported that the inhibition of PKA in spinal cord significantly reduced capsaicin injection-induced secondary hyperalgesia and allodynia (Sluka et al. 1997). Another inhibitor of PKA, WIPYIDE was reported to block the PGE2 mediated inflammatory hyperalgesia (Aley & Levine 1999). In an established neuropathic pain model, H 89 showed a moderate effect on the mechanical allodynia (Hu et al. 2001). In our current study, the involvement of PKA in central visceral nociceptive neurotransmission was first demonstrated since the intrathecal administration of PKA inhibitor, H 89 showed an obvious effect in the exploratory behavioral activity in experimental animals with mustard oil injection (Figure 1). As an irritant chemical to trigger neurogenic inflammatory response in experimental animals, mustard oil applied to the peripheral tissues may induce central neurochemical changes in different models after painful stimulation (Wong et al., 2001; Kroin et al., 2004). However, it is noted that the protein expression of PKA and phospho-CREB in spinal cervical segments was not changed significantly between control and visceral pain groups. But these results do not exclude the potential possibility that nonspecific systematic effects may impact the expression of these neuronal signal molecules and it may deserve further investigations (Wong et al., 2001; Kroin et al., 2004).
It is well recognized that the PKA pathway is involved in the central and peripheral neurotransmission of nociception. Electrophysiological investigations in single dorsal root ganglion or spinal cord neurons also demonstrated that PKA play a critical role in the modulation of neuronal excitability in response to acute or chronic noxious stimulation (Hu et al. 2001; Lin et al. 2002; Zhang et al. 2002). In our previous studies on money, we reported that the sensitization of STT cells to noxious mechanical stimuli produced by Forskolin (a PKA cascade activator) could be blocked by pretreatment of the spinal cord with the PKA inhibitor, H 89 (Lin et al. 2002). H89 also blocked the increased response of primate STT cells to mechanical stimuli in capsaicin induced sensitization (Sluka et al. 1997). Another study on the chronically compressed DRG, inhibition of PKA by Rp-cAMP and H89 markedly decreased the spontaneous activity (Hu et al. 2001). The active effect of PKA on excitability of superficial dorsal horn neurons was recorded in mouse (Hu & Gereau 2003). It also reported that these PKA inhibitors completely block the exogenous cytokine, TNF-α –evoked response in most nociceptive C and Aβ fibers (Zhang et al. 2002). The results from our current experiment of enhanced PKA positive neuronal profiles in PSDC population also showed a role of PKA in visceral nociception. Combined these reports, it reveals that a PKA-mediated mechanism plays an important role in noxious signal transduction cascade.
Peripheral painful stimulation, such as mustard oil or capsaicin injection, produces an enhanced responsiveness in spinal nociceptive neurons that trigger the activation of a number of neurotransmission receptors. This event causes a large influx of calcium into the nociceptive neurons, activating multiple intracellular protein kinase cascades, such as CaM kinase II, PKC, as well as PKA kinase (Fang et al. 2002; Fang et al. 2003a; Lin et al. 2002; Sluka & Willis 1997; Wu et al. 2005). The PKA, PKC and nitric oxide synthase systems were also found to be activated following this increased calcium influx in nociceptive neurons during noxious stimulation (Fang et al. 2003a; Wu et al. 2001; Wu et al. 2005). This signaling could lead to increased intracellular accumulation of c-AMP and activation of PKA. It has been revealed that serine/theonine phosphorylation of glutamate receptors is regulated by PKA on certain amino acid residues (Fang et al. 2002; Fang et al. 2003a; Zou et al. 2002). These including that NR1 subunits of NMDA receptor and GluR1 subunits of AMPA receptors are phosphorylated by a PKA-mediated regulation mechanism. It is interesting that in PSDC neurons, the treatment of AMPA receptor antagonist, CNQX, could block the response of PSDC neurons to colonic distension (Al Chaer et al. 1996) and it suggested that AMPA receptor activation involved in the nociceptive transmission in PSDC neurons. To combined our current find of the increased expression of PKA in PSDC neurons, we could suggested that a possible role for PKA in regulation of AMPA receptor activity in PSDC neurons during visceral painful states.
Another important role for PKA in regulation of nociception in animal studies is that its function on painful stimulation-elicited gene expression through mediation of transcription factors, such as c-Fos and c-AMP responsive element binding protein (CREB) (Fang et al. 2005; Wu et al. 2000; Wu et al. 2002; Wu et al. 2005). Increased phosphorylation of CREB through the activation of glutamate receptors and the PKA, PKC and CaM kinase cascades during central sensitization was reported in several animal models of pain (Fang et al. 2005; Miyabe & Miletic 2005; Wu et al. 2005). It suggests a connection between activation of trancription factors (like CREB phosphorylation) and the molecular mechanisms mediating stimuli-induced nuclear gene regulator activation by protein kinase pathways (Fang et al. 2005; Miyabe & Miletic 2005; Sassone-Corsi et al. 1988; 1998; Wu et al. 2005). We previously found that inhibition of PKA blocked the capsaicin injection induced phosphorylation of CREB in rats (Wu et al. 2005). In the current study, we found that visceral noxious stimulation significantly induces the phosphorylation of CREB in spinal cord and H89 blocked this increase (Figure 4). The PKA-dependent molecular mechanism of regulation of gene transcription in response to peripheral painful stimuli was also seen in other models of pain (Miyabe & Miletic 2005). This mechanism may demonstrate a possible role of PKA in regulation of long-term gene expression in response to pain. In future experiments, we will investigate the intracellular mechanism of PKA to participate in the regulation of transcription factor in PSDC neurons.
In summary, the findings from our study deliver strong evidence that PKA play an important role in the neurotransmission of visceral information at the spinal cord level following colonic inflammatory/noxious stimulation. The PSDC ascending system activated by noxious visceral stimuli is involved in the active PKA in spinal cord and PSDC neurons. It seems that PKA could be considered as one of the crucial factors for signaling visceral perception in PSDC visceral pain pathway served as a development drug target for clinical visceral pain treatment.
Acknowledgments
This work was funded by Sealy Grant 2951-02, NIH P30/DK 56338-02/05, DE 15814 (LF), NIH grants NS 11255 and NS 40723 (QL). We thank Steve Schuenke for editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al Chaer ED, Lawand NB, Westlund KN, Willis WD. Pelvic visceral input into the nucleus gracilis is largely mediated by the postsynaptic dorsal column pathway. J Neurophysiol. 1996;76:2675–2690. doi: 10.1152/jn.1996.76.4.2675. [DOI] [PubMed] [Google Scholar]
- Aley KO, Levine JD. Role of protein kinase A in the maintenance of inflammatory pain. J Neurosci. 1999;19:2181–2186. doi: 10.1523/JNEUROSCI.19-06-02181.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker R, Gatscher S, Sure U, Bertalanffy H. The punctate midline myelotomy concept for visceral cancer pain control--case report and review of the literature. Acta Neurochir Suppl. 2002;79:77–78. doi: 10.1007/978-3-7091-6105-0_17. [DOI] [PubMed] [Google Scholar]
- Becker R, Sure U, Bertalanffy H. Punctate midline myelotomy. A new approach in the management of visceral pain. Acta Neurochir (Wien) 1999;141:881–883. doi: 10.1007/s007010050390. [DOI] [PubMed] [Google Scholar]
- Fang L, Wu J, Lin Q, Willis WD. Calcium-calmodulin-dependent protein kinase II contributes to spinal cord central sensitization. J Neurosci. 2002;22:4196–4204. doi: 10.1523/JNEUROSCI.22-10-04196.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L, Wu J, Lin Q, Willis WD. Protein kinases regulate the phosphorylation of the GluR1 subunit of AMPA receptors of spinal cord in rats following noxious stimulation. Brain Res Mol Brain Res. 2003a;118:160–165. doi: 10.1016/j.molbrainres.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Fang L, Wu J, Zhang X, Lin Q, Willis WD. Increased phosphorylation of the GluR1 subunit of spinal cord alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor in rats following intradermal injection of capsaicin. Neuroscience. 2003b;122:237–245. doi: 10.1016/s0306-4522(03)00526-8. [DOI] [PubMed] [Google Scholar]
- Fang L, Wu J, Zhang X, Lin Q, Willis WD. Calcium/calmodulin dependent protein kinase II regulates the phosphorylation of cyclic AMP-responsive element-binding protein of spinal cord in rats following noxious stimulation. Neurosci Lett. 2005;374:1–4. doi: 10.1016/j.neulet.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Galan A, Cervero F, Laird JM. Extracellular signaling-regulated kinase-1 and -2 (ERK 1/2) mediate referred hyperalgesia in a murine model of visceral pain. Brain Res Mol Brain Res. 2003;116:126–134. doi: 10.1016/s0169-328x(03)00284-5. [DOI] [PubMed] [Google Scholar]
- Gao Y, Gao G, Long C, Han S, Zu P, Fang L, Li J. Enhanced phosphorylation of cyclic AMP response element binding protein in the brain of mice following repetitive hypoxic exposure. Biochem Biophys Res Commun. 2006;340:661–667. doi: 10.1016/j.bbrc.2005.12.064. [DOI] [PubMed] [Google Scholar]
- Hu HJ, Gereau RW. ERK integrates PKA and PKC signaling in superficial dorsal horn neurons. II. Modulation of neuronal excitability. J Neurophysiol. 2003;90:1680–1688. doi: 10.1152/jn.00341.2003. [DOI] [PubMed] [Google Scholar]
- Hu JS, Li YJ. Clinical application of midline myelotomy to treat visceral cancer pain. Natl Med J China. 2002;82:856–867. [Google Scholar]
- Hu SJ, Song XJ, Greenquist KW, Zhang JM, LaMotte RH. Protein kinase A modulates spontaneous activity in chronically compressed dorsal root ganglion neurons in the rat. Pain. 2001;94:39–46. doi: 10.1016/S0304-3959(01)00339-6. [DOI] [PubMed] [Google Scholar]
- Hwang SL, Lin CL, Lieu AS, Kuo TH, Yu KL, Ou-Yang F, Wang SN, Lee KT, Howng SL. Punctate midline myelotomy for intractable visceral pain caused by hepatobiliary or pancreatic cancer. J Pain Symptom Manage. 2004;27:79–84. doi: 10.1016/j.jpainsymman.2003.05.005. [DOI] [PubMed] [Google Scholar]
- Kim YS, Kwon SJ. High thoracic midline dorsal column myelotomy for severe visceral pain due to advanced stomach cancer. Neurosurgery. 2000;46:85–90. [PubMed] [Google Scholar]
- Kroin JS, Ling ZD, Buvanendran A, Tuman KJ. Upregulation of spinal cyclooxygenase-2 in rats after surgical incision. Anesthesiolog. 2004;100:364–9. doi: 10.1097/00000542-200402000-00027. [DOI] [PubMed] [Google Scholar]
- Lin Q, Wu J, Willis WD. Effects of protein kinase a activation on the responses of primate spinothalamic tract neurons to mechanical stimuli. J Neurophysiol. 2002;88:214–221. doi: 10.1152/jn.2002.88.1.214. [DOI] [PubMed] [Google Scholar]
- Mills CD, Grady JJ, Hulsebosch CE. Changes in exploratory behavior as a measure of chronic central pain following spinal cord injury. J Neurotrauma. 2001;18:1091–1105. doi: 10.1089/08977150152693773. [DOI] [PubMed] [Google Scholar]
- Miyabe T, Miletic V. Multiple kinase pathways mediate the early sciatic ligation-associated activation of CREB in the rat spinal dorsal horn. Neurosci Lett. 2005;381:80–85. doi: 10.1016/j.neulet.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Nauta HJ, Hewitt E, Westlund KN, Willis WD., Jr Surgical interruption of a midline dorsal column visceral pain pathway. Case report and review of the literature. J Neurosurg. 1997;86:538–542. doi: 10.3171/jns.1997.86.3.0538. [DOI] [PubMed] [Google Scholar]
- Nauta HJ, Soukup VM, Fabian RH, Lin JT, Grady JJ, Williams CG, Campbell GA, Westlund KN, Willis WD., Jr Punctate midline myelotomy for the relief of visceral cancer pain. J Neurosurg. 2000;92:125–130. doi: 10.3171/spi.2000.92.2.0125. [DOI] [PubMed] [Google Scholar]
- Ness TJ. Evidence for ascending visceral nociceptive information in the dorsal midline and lateral spinal cord. Pain. 2000;87:83–88. doi: 10.1016/S0304-3959(00)00272-4. [DOI] [PubMed] [Google Scholar]
- Palecek J, Paleckova V, Willis WD. The roles of pathways in the spinal cord lateral and dorsal funiculi in signaling nociceptive somatic and visceral stimuli in rats. Pain. 2002;96:297–307. doi: 10.1016/S0304-3959(01)00459-6. [DOI] [PubMed] [Google Scholar]
- Palecek J, Paleckova V, Willis WD. Postsynaptic dorsal column neurons express NK1 receptors following colon inflammation. Neuroscience. 2003;116:565–572. doi: 10.1016/s0306-4522(02)00660-7. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi P. Coupling gene expression to cAMP signalling: role of CREB and CREM. Int J Biochem Cell Biol. 1998;30:27–38. doi: 10.1016/s1357-2725(97)00093-9. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi P, Visvader J, Ferland L, Mellon PL, Verma IM. Induction of proto-oncogene fos transcription through the adenylate cyclase pathway: characterization of a cAMP-responsive element. Genes Dev. 1988;2:1529–1538. doi: 10.1101/gad.2.12a.1529. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Rees H, Chen PS, Tsuruoka M, Willis WD. Inhibitors of G-proteins and protein kinases reduce the sensitization to mechanical stimulation and the desensitization to heat of spinothalamic tract neurons induced by intradermal injection of capsaicin in the primate. Exp Brain Res. 1997;115:15–24. doi: 10.1007/pl00005675. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Willis WD. The effects of G-protein and protein kinase inhibitors on the behavioral responses of rats to intradermal injection of capsaicin. Pain. 1997;71:165–178. doi: 10.1016/s0304-3959(97)03371-x. [DOI] [PubMed] [Google Scholar]
- Taylor SS, Buechler JA, Yonemoto W. cAMP-dependent protein kinase: framework for a diverse family of regulatory enzymes. Annu Rev Biochem. 1990;59:971–1005. doi: 10.1146/annurev.bi.59.070190.004543. [DOI] [PubMed] [Google Scholar]
- Vilela FO, Araujo MR, Florencio RS, Silva MA, Silveira MT. CT-guided percutaneous punctate midline myelotomy for the treatment of intractable visceral pain: a technical note. Stereotact Funct Neurosurg. 2001;77:177–182. doi: 10.1159/000064617. [DOI] [PubMed] [Google Scholar]
- Willis WD, Al Chaer ED, Quast MJ, Westlund KN. A visceral pain pathway in the dorsal column of the spinal cord. Proc Natl Acad Sci USA. 1999;96:7675–7679. doi: 10.1073/pnas.96.14.7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JK Haas DA, Hu JW. Local anesthesia does not block mustard-oil-induced temporomandibular inflammation. Anesth Anal. 2001;92:1035–40. doi: 10.1097/00000539-200104000-00043. [DOI] [PubMed] [Google Scholar]
- Wu J, Fang L, Lin Q, Willis WD. Fos expression is induced by increased nitric oxide release in rat spinal cord dorsal horn. Neuroscience. 2000;96:351–357. doi: 10.1016/s0306-4522(99)00534-5. [DOI] [PubMed] [Google Scholar]
- Wu J, Fang L, Lin Q, Willis WD. Nitric oxide synthase in spinal cord central sensitization following intradermal injection of capsaicin. Pain. 2001;94:47–58. doi: 10.1016/S0304-3959(01)00340-2. [DOI] [PubMed] [Google Scholar]
- Wu J, Fang L, Lin Q, Willis WD. The role of nitric oxide in the phosphorylation of cyclic adenosine monophosphate-responsive element-binding protein in the spinal cord after intradermal injection of capsaicin. J Pain. 2002;3:190–198. doi: 10.1054/jpai.2002.123653. [DOI] [PubMed] [Google Scholar]
- Wu J, Su G, Ma L, Zhang X, Lei Y, Li J, Lin Q, Fang L. Protein kinases mediate increment of the phosphorylation of cyclic AMP-responsive element binding protein in spinal cord of rats following capsaicin injection. Mol Pain. 2005;1:26. doi: 10.1186/1744-8069-1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JM, Li H, Liu B, Brull SJ. Acute topical application of tumor necrosis factor alpha evokes protein kinase A-dependent responses in rat sensory neurons. J Neurophysiol. 2002;88:1387–1392. doi: 10.1152/jn.2002.88.3.1387. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang X, Westlund KN. Restoration of spontaneous exploratory behaviors with an intrathecal NMDA receptor antagonist or a PKC inhibitor in rats with acute pancreatitis. Pharmacol Biochem Behav. 2004;77:145–153. doi: 10.1016/j.pbb.2003.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wu J, Lei Y, Fang L, Willis WD. Protein phosphatase 2A regulates central sensitization in the spinal cord of rats following intradermal injection of capsaicin. Mol Pain. 2006;2:9. doi: 10.1186/1744-8069-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X, Lin Q, Willis WD. Role of protein kinase A in phosphorylation of NMDA receptor 1 subunits in dorsal horn and spinothalamic tract neurons after intradermal injection of capsaicin in rats. Neuroscience. 2002;115:775–786. doi: 10.1016/s0306-4522(02)00490-6. [DOI] [PubMed] [Google Scholar]


