Abstract
Recent evidence suggests that the hippocampus may have a functional role in mediating relapse to cocaine-seeking behavior. Based on the importance of the ventral CA subfields in mediating reward, the present experiment determined the effects of temporary inactivation of the ventral hippocampus on reinstatement of cocaine-seeking in a rodent model of relapse. Male, Sprague–Dawley rats self-administered i.v. cocaine (0.6 mg/kg/infusion) in the presence of discrete conditioned cues (tone + light) in daily 2-h sessions for ten days. Following seven days of extinction sessions in which neither cues nor drug were available, rats underwent four reinstatement tests in a counterbalanced, within-subjects design. Bilateral microinjections of GABA receptor agonists (baclofen/muscimol—0.1/1.0 mM) into the ventral hippocampus significantly attenuated cue-induced and cocaine-primed reinstatement compared with vehicle microinjections in the same rats. In contrast, injections just outside the ventral hippocampus did not block either form of reinstatement. Furthermore, inactivation failed to affect responding for food reinforcement, baseline extinction responding, or locomotor activity. These data indicate that the ventral hippocampus plays an important role in the relapse to cocaine-seeking behavior and may interact with key limbic structures previously implicated in cocaine addiction.
Keywords: Cocaine, Hippocampus, Reinstatement, Relapse, Self-administration
The rat hippocampus has been extensively dissociated along the dorsal-ventral gradient. In this regard, the dorsal hippocampus is defined as the 50% of hippocampal volume starting at the septal pole; whereas the ventral hippocampus is defined as the 50% of hippocampal volume starting at the temporal pole (Bannerman et al., 2004). Relative to its dorsal counterpart, the ventral hippocampus has greater output connections with the prefrontal cortex, bed nucleus of the stria terminalis, and the amygdala (Henke, 1990; Ishikawa & Nakamura, 2006), and the amygdala has extensive reciprocal connections with the ventral hippocampus (Pitkanen, Pikkarainen, Nurminen, & Ylinen, 2000). All of these structures have been implicated in stress-related behaviors, as well as cocaine-seeking behavior in the reinstatement model of relapse (Kalivas, McFarland, & See, 2003). In a recent study from our laboratory, we found that temporary inactivation of the dorsal hippocampus impaired contextual reinstatement (i.e., reinstatement of cocaine-seeking induced by occasion setting contextual cues), but not reinstatement induced by discrete cocaine-paired cues or a cocaine priming injection (Fuchs et al., 2005). In addition, Vorel, Liu, Hayes, Spector, and Gardner (2001) reported that electrical stimulation of glutamate fibers in the hippocampus induced cocaine-seeking. Black and colleagues (2004) found that lidocaine inactivation of either dorsal or ventral subiculum, the major output fibers of the hippocampus, had no effect upon cue-induced or drug-primed reinstatement. However, Sun and Rebec (2003) reported that ventral subiculum inactivation via lidocaine attenuated cue-induced and drug-primed reinstatement. Several possibilities may account for these two discrepant findings. First, differing schedules of reinforcement were used. Second, the specific site of inactivation varied between the two findings. Finally, Black, Green-Jordan, Eichenbaum, and Kantak (2004) performed additional behavioral tasks that may have affected the outcome. Importantly, inactivating the output fibers could be qualitatively different than inactivating the ventral hippocampus proper (i.e., CA subfields).
Some previous findings have suggested that activation of dopaminergic neurotransmission via the ventral hippocampus underlies general locomotor activation (Berke & Eichenbaum, 2001; Gasbarri, Sulli, & Packard, 1997). However, other evidence indicates that the ventral hippocampus mediates associative processing of discrete cues (i.e., conditioned stimuli) during conditioning. For example, Bast, Zhang, and Feldon (2001) found that inactivation of the ventral hippocampus attenuated freezing to discrete cues following delay fear conditioning. Furthermore, some models of hippocampal function suggest a dichotomy along a ‘space’ vs. ‘anxiety’ domain for the dorsal vs. ventral hippocampus (Bannerman et al., 2004), respectively. To this end, we hypothesized that inactivation of the ventral hippocampus would yield a functionally similar pattern of results as seen with amygdala inactivation (See, 2005), namely the attenuation of cue-induced, but not cocaine-primed, reinstatement. Therefore, the present study sought to determine the effects of ventral hippocampal inactivation on relapse to drug-seeking following the presentation of drug-paired cues or a drug-priming injection.
Twenty-four, male Sprague–Dawley rats (Charles River) weighing approximately 300–325 g at the start of the experiment, served as subjects. The rats were housed individually in Plexiglas chambers located in a temperature- and humidity-controlled vivarium with a reversed 12-h light–dark cycle. All rats were maintained on ~25 g food/day with ad libitum access to water. The study was conducted according to the Institute of Laboratory Animal Resources on Life Sciences Guide for the Care and Use of Laboratory Rats.
Prior to surgery, rats were initially trained to lever press for food reinforcement (Fuchs et al., 2005). Forty-eight hours following lever training, rats were anesthetized using a mixture of ketamine hydrochloride and xylazine (66 and 1.33 mg/kg, respectively), followed by equithesin (0.5 ml/kg of a solution of 9.72 mg/ml pentobarbital sodium, 42.5 mg/ml chloral hydrate, and 21.3 mg/ml magnesium sulfate heptahydrate dissolved in 44% propylene glycol, 10% ethanol solution, i.p.). Chronic indwelling catheters, prepared as described previously (Fuchs et al., 2005), were inserted into the right jugular vein and secured to surrounding tissue with sutures. The catheter ran subcutaneously and exited behind the rat’s shoulder blades. Immediately following catheter surgery, animals were placed into a stereotaxic frame (Stoelting, Wood Dale, IL). Bilateral stainless steel guide cannulae (26 gauge) were inserted in the ventral hippocampus, located 5.2 mm anteroposterior, 5.4 mm mediolateral, 5.0 mm dorsoventral. These coordinates were chosen based on previous research (Rudy & Matus-Amat, 2005) and pilot data. Surgical preparations and post-operative maintenance of the catheters have been previously described (Fuchs et al., 2005).
Animals self-administered i.v. cocaine at a dose of 0.6 mg/kg in a 50 μl infusion (cocaine HCl provided by NIDA, Research Triangle Park, NC) along a FR1 schedule of reinforcement for 10–12 consecutive days in a standard self-administration chamber (Med Associates). The house light was illuminated throughout each 2 h session. Active lever pressing resulted in a 2 s activation of the infusion pump and a 5 s presentation of the stimulus complex (CS), which consisted of a cue light above the active lever and a tone (70 dB, 4.5 kHz). Following each infusion, responding on the active lever had no consequences during a 20 s timeout period. Inactive lever presses also had no consequences, but were recorded. After the last day of self-administration, rats experienced daily 2 h extinction sessions, during which responses on either the active or inactive lever were recorded but resulted in no programmed consequences. Animals continued extinction sessions until they reached a criterion of a minimum of 7 days and ≤25 lever presses/session for two consecutive days.
Following extinction, rats underwent four reinstatement tests, in a counterbalanced, within subjects design, with a minimum of 2 days of extinction between each test. Immediately prior to each reinstatement test, the rat received either vehicle or baclofen/muscimol (B/M; 0.1/1.0 mM) bilaterally infused into the ventral hippocampus. During the first two reinstatement tests, rats were placed into the chambers for 2 h, during which the house light was illuminated and active lever presses resulted in a 5 s CS presentation in the absence of any drug reinforcement, followed by a 20 s time out period. For cocaine-primed reinstatement tests, a non-contingent dose of cocaine (10 mg/kg, i.p.) was administered 10 min prior to the rat entering the chamber for a 2 h session, during which the house light was illuminated and lever responses had no programmed consequences. After all the animals completed reinstatement testing, the rats were anesthetized, perfused, and brains dissected out. Coronal sections were cut on a vibratome and stained with cresyl violet.
Of the 24 animals that started the experiment, five were excluded due to failed cannulae or loss of catheter patency. Ten rats had bilateral ventral hippocampus placements, whereas 4 animals had cannulae located outside of the ventral hippocampus (i.e., TE cortex) and 5 animals had unilateral placements. These animals served as anatomical controls. Schematic representation of the cannulae placements and representative photomicrographs are shown in Fig. 1. The average responding on the active lever for the last 3 days of self-administration (mean ± SEM) was 46.2 ± 7.85 for the ventral hippocampus group and 45.1 ± 7.01 for the control group. The daily cocaine intake for the last 3 days of self-administration (mean ± SEM) was 19.4 ± 1.6 mg/kg/session for the ventral hippocampus group and 18.5 ± 1.0 mg/kg/session for the control group. Responding readily declined during extinction sessions, with the number of days-to-criterion (mean ± SEM) being 8.7 ± 0.54 for the ventral hippocampus group and 8.2 ± 0.31 for the control group. Finally, days-to-criterion prior to the first and second set of reinstatement tests was 2.3 ± 0.15 for the ventral hippocampus group and 2.6 ± 0.33 for the control group.
Fig. 1.
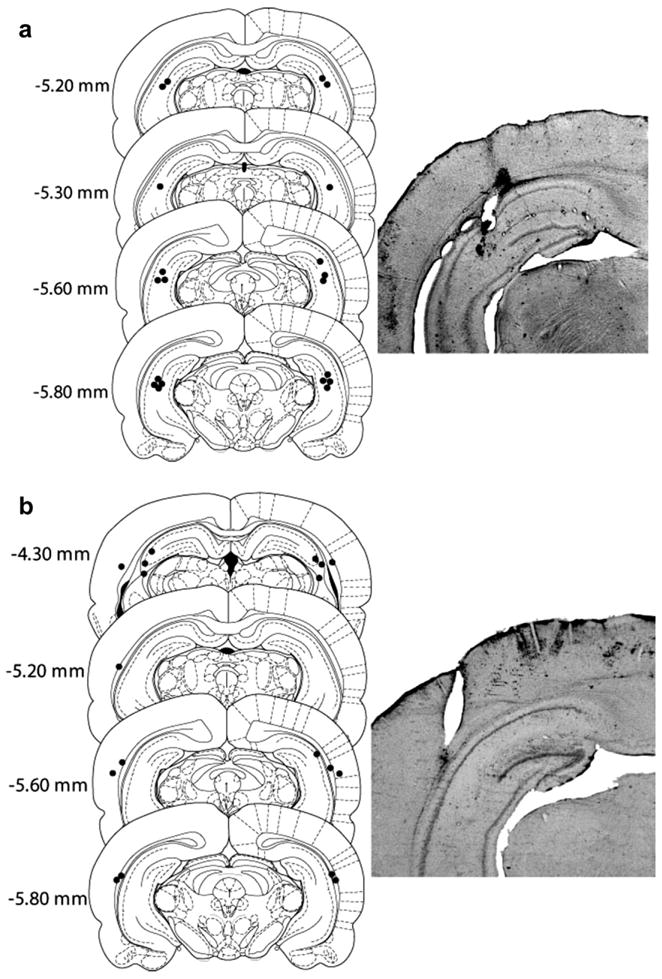
Microinfusion cannula placement within the ventral hippocampus (a) and anatomical controls (b) as verified on cresyl violet-stained sections with representative microphotographs. The symbols represent the most ventral point of the infusion cannula tract for each rat on coronal sections based on the atlas of Paxinos and Watson (1997). Numbers indicate the distance from bregma (mm).
As depicted in Fig. 2, robust conditioned-cued reinstatement occurred after vehicle infusion as measured by responding on the previously active (cocaine-paired) lever in the presence of cues for both control group (t(8) = −4.44, p < .01) and ventral hippocampus group (t(9) = −4.0, p < .01). In contrast, inactivation of the ventral hippocampus significantly attenuated conditioned-cued reinstatement only in animals with bilateral ventral hippocampus placements. A t-test for paired samples revealed no difference between vehicle and B/M treatment for the control group (t(8) =.13, p > .05); however, there was a significant difference between vehicle and B/M treatment for the ventral hippocampus group (t(9) = 2.71, p < .05). Order of drug infusion (i.e., Veh-B/M; B/M-Veh) had no effect on cue-induced reinstatement in the ventral hippocampus group, as verified by a one-way ANOVA (F(8) = 1.42, p > .05). Similar to cues, a priming injection of cocaine produced robust reinstatement after vehicle infusions for both control group (t(7) = −2.75, p < .05) and ventral hippocampus group (t(8) = −4.69, p < .01), whereas B/M inactivation significantly attenuated reinstatement in the ventral hippocampus group (t(8) = 2.97, p < .05), but not the control group (t(7) = −0.2, p > .05). As with conditioned-cued reinstatement, no order effects were noted (one-way ANOVA, F(7) = 2.85, p > .05).
Fig. 2.
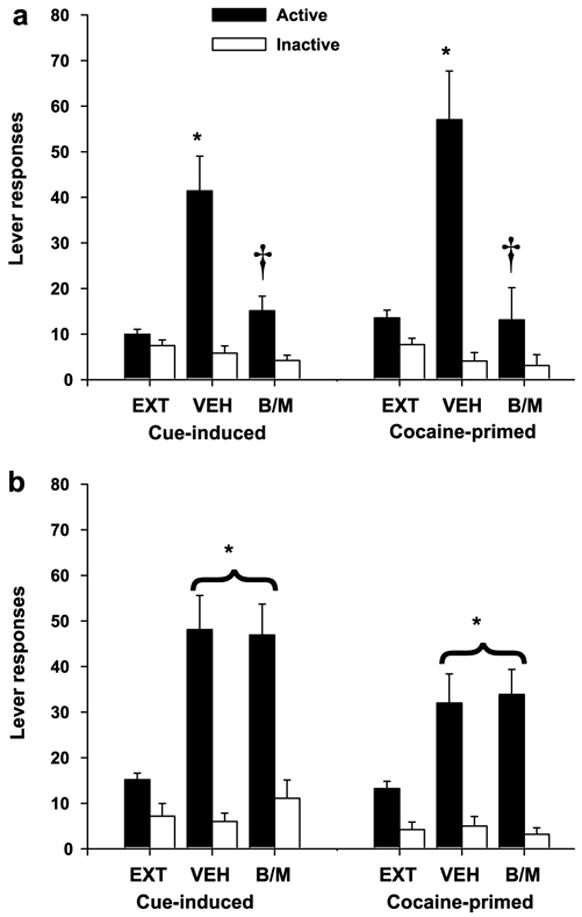
Responses on the active and inactive levers (mean ± SEM) during extinction (EXT) and conditioned cue-induced or cocaine-primed reinstatement following bilateral infusions of vehicle (VEH) or baclofen-muscimol (B/M) just prior to each test session. (a) Animals with bilateral ventral hippocampus cannulae showed significant increases in responding over EXT levels after VEH, but not B/M infusions (*p < .05, VEH significantly different from EXT; †p < .05, B/M significantly different from VEH). (b) Animals with bilateral cannulae outside of the ventral hippocampus showed significant increases in responding over EXT levels after either VEH or B/M infusions (*p < .05, significantly different from EXT).
In order to determine the selectivity of B/M inactivation of the ventral hippocampus on cocaine-seeking, we further examined the ability of B/M to alter baseline responding and the maintenance of responding for food pellets. A separate group of rats (n = 8) were catheterized and implanted with cannulae in the ventral hippocampus. Upon recovery, rats were restricted to 85–90% of their free feeding weight and trained to lever press for food pellets during daily 30-min sessions in the absence of explicit cue presentation. Once responding reached a stability criterion, rats received an infusion of B/M or vehicle in a counterbalanced, within subjects design, before the test session. Animals were then trained on cocaine self-administration as described above. After 7 days of extinction, B/M or vehicle was infused before the next extinction session. Following stable extinction responding, B/M or vehicle was infused prior to a priming injection of saline. Finally, after all operant testing was completed, we tested B/M or vehicle effects on locomotor activity following a cocaine (10 mg/kg) injection. As seen in Table 1, reversible inactivation of the ventral hippocampus had no effect upon maintenance of food-responding, baseline extinction responding, responding after a saline-priming injection, or locomotor activity.
Table 1.
Ventral hippocampus inactivation fails to affect food-seeking, baseline responding, or locomotor activity
| Test | B/M | Vehicle | t-test p value |
|---|---|---|---|
| Mean active lever responses ± SEM | |||
| Food | 321 ± 41.5 | 323.42 ± 22.3 | .35 |
| Extinction | 20.33 ± 7.99 | 7.83 ± 1.56 | .14 |
| Saline-prime | 25.5 ± 6.7 | 30.5 ± 6.1 | .49 |
| Mean distance traveled (cm) ± SEM | |||
| Locomotor activity | 726.58 ± 165.63 | 898.61 ± 201.12 | .12 |
Responses on the active lever (mean ± SEM) during maintenance of food-responding, baseline extinction responding, after a saline-priming injection, and the distance traveled during a test of locomotor activity following infusions of baclofen-muscimol (B/M) or vehicle just prior to each test session. As indicated (p-values), inactivation of the ventral hippocampus had no effect on any of these tasks.
The present results indicate that selective inhibition of the ventral hippocampus attenuates relapse to cocaine-seeking following presentation of drug-paired cues or a priming injection of cocaine itself. These data are the first to show the involvement of the ventral hippocampal CA sub-fields in relapse to drug-seeking behavior. The selective role of the ventral hippocampus is supported by: (a) the lack of effects seen in animals with cannulae located just outside of the ventral hippocampus, (b) the lack of inactivation effects during responding for food, extinction responding, responding after a saline-priming injection, and locomotor activity, and (c) our previous study that showed no effect of inactivation of the dorsal hippocampus on discrete cue-induced or cocaine-primed reinstatement (Fuchs et al., 2005).
Based on the reciprocal connections between the amygdala and ventral hippocampus (Pitkanen et al., 2000) and the involvement of the amygdala in the acquisition and reinstatement of cocaine-paired associative learning (See, 2005), we postulate interactive processing between these two structures in driving cocaine-seeking. Whereas, we did not anticipate attenuation of cocaine-seeking following the cocaine priming injection, related evidence supports the present findings. For example, NMDA stimulation of the ventral hippocampus has been shown to increase dopamine transmission in the ventral tegmental area (Gasbarri et al., 1997) and the nucleus accumbens shell (Pelag-Raibstein & Feldon, 2006), structures implicated in reinstatement of cocaine-seeking (Kalivas et al., 2003; Schmidt & Pierce, 2006). Taken together, the data suggest that the ventral hippocampus is involved in cocaine-seeking behavior extending well beyond basic motor control. One obvious source of regulation is the dopaminergic input to ventral hippocampus. Lisman and Grace (2005) have recently suggested that dopamine critically gates the amount of information processed by the CA1 subregion of the hippocampus. Given hippocampal modulation of other dopamine-innervated regions implicated in cocaine addiction, future studies may be aimed at the selective neurotransmitter modulation of the ventral hippocampus during relapse to drug-seeking.
Acknowledgments
This research was supported by National Institute on Drug Abuse grants DA010462 and DA015369, and NIH grant C06 RR015455.
References
- Bannerman DM, Rawlins JNP, McHugh SB, Deacon RMJ, Yee BK, Bast T. Regional dissociation within the hippocampus-memory and anxiety. Neuroscience and Biobehavioral Reviews. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Bast T, Zhang W, Feldon J. The ventral hippocampus and fear conditioning in rats. Experimental Brain Research. 2001;139:39–52. doi: 10.1007/s002210100746. [DOI] [PubMed] [Google Scholar]
- Berke JD, Eichenbaum HB. Drug addiction and the hippocampus. Science. 2001;294:1235. doi: 10.1126/science.294.5545.1235a. [DOI] [PubMed] [Google Scholar]
- Black YD, Green-Jordan K, Eichenbaum HB, Kantak KM. Hippocampal memory system function and the regulation of cocaine self-administration behavior in rats. Behavioral Brain Research. 2004;151:225–238. doi: 10.1016/j.bbr.2003.08.020. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RM, et al. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Sulli A, Packard MG. The dopaminergic mesencephalic projections to the hippocampal formation in the rat. Progress in Neuro-Psychopharmacological & Biological Psychiatry. 1997;21:1–22. doi: 10.1016/s0278-5846(96)00157-1. [DOI] [PubMed] [Google Scholar]
- Henke PG. Hippocampal pathway to the amygdale and stress ulcer development. Brain Research Bulletin. 1990;25:691–695. doi: 10.1016/0361-9230(90)90044-z. [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Nakamura S. Ventral hippocampal neurons project axons simultaneously to the medial prefrontal cortex and amygdala in the rat. Journal of Neurophysiology. 2006;96:2134–2138. doi: 10.1152/jn.00069.2006. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K, See RE. Psychiatric pathophysiology: Addiction. In: Tasman A, Kay J, Lieberman JA, editors. Psychiatry. 2. Vol. 1. Chichester, England: Wiley; 2003. pp. 330–337. [Google Scholar]
- Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York, NY: Academy of Sciences; 1997. [Google Scholar]
- Pelag-Raibstein D, Feldon J. Effects of dorsal and ventral hippocampal NMDA stimulation on nucleus accumbens core and shell dopamine release. Neuropharmacology. 2006;51:947–957. doi: 10.1016/j.neuropharm.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdale and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Annals of the New York Academy of Sciences. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Matus-Amat P. The ventral hippocampus supports a memory representation of context and contextual fear conditioning: Implications for a unitary function of the hippocampus. Behavioral Neuroscience. 2005;119:154–163. doi: 10.1037/0735-7044.119.1.154. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Pierce RC. Cooperative activation of D1-like and D2-like dopamine receptors in the nucleus accumbens shell is required for the reinstatement of cocaine-seeking behavior in the rat. Neuroscience. 2006;142:451–461. doi: 10.1016/j.neuroscience.2006.06.004. [DOI] [PubMed] [Google Scholar]
- See RE. Neural substrates of cocaine-cue associations that trigger relapse. European Journal of Neuroscience. 2005;526:140–146. doi: 10.1016/j.ejphar.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Sun W, Rebec GV. Lidocaine inactivation of ventral subiculum attenuates cocaine-seeking behavior in rats. Journal of Neuroscience. 2003;23:10258–10264. doi: 10.1523/JNEUROSCI.23-32-10258.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL. Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science. 2001;292:1175–1178. doi: 10.1126/science.1058043. [DOI] [PubMed] [Google Scholar]


