Abstract
Serotonin [5-hydroxytryptamine (5HT)] is a vasoconstrictor that also acts as a developmental signal early in embryogenesis. The 5HT transporter (SERT) on the membranes of the placental trophoblast cells controls 5HT levels in the maternal bloodstream to maintain stable transplacental blood flow and simultaneously provide 5HT to the embryo. The 5HT uptake rate of placental SERT is important for both the mother and the developing embryo. The impact of glucose on the placental SERT system during diabetic pregnancy is not known. The present in vitro study investigated this important issue in human placental choriocarcinoma (JAR) cells that were cultured for 24–96 h in a medium containing either 5.5 (physiologic concentration) or 25 mmol/L D-glucose (diabetic-like concentration). The 5HT uptake rates of the cultured cells were not altered at exogenous D-glucose concentrations in the range of 5.5–15 mmol/L, but were decreased significantly at a diabetic-like concentration (≥25 mmol/L). To understand better the role of glucose on the placental 5HT system, we first characterized SERT in JAR cells at different cell-cycle phases and then determined the expression levels of SERT on the plasma membrane and in the intracellular pools of JAR cells at the late-S and G2 phases, where the uptake rates were decreased 73% under diabetic-like glucose concentrations. Finally, the importance of self-association of SERT molecules was examined. In JAR cells co-expressing Flag- and myc-tagged SERT, myc-antibody precipitated 70% of Flag-SERT, indicating that a large percentage of SERT proteins exist as oligomers in situ. Under diabetic conditions, myc-antibody no longer precipitated Flag-SERT, suggesting a disruption in the aggregation of SERT molecules. Therefore, we propose that under uncontrolled diabetic conditions, glucose down-regulates 5HT uptake rates of placental SERT by interfering with its functional expression in a cell-cycle-dependent manner.
Keywords: 5HT transporter, in vitro diabetic model, placenta, serotonin uptake
Diabetes is a complex disease, exhibiting different types and degrees of pathology. The major symptom of diabetes is hyperglycemia (Renold et al. 1977). During the course of pregnancy, hyperglycemia-associated alterations induce developmental tissue and organ abnormalities through largely unexplored pathways and mechanisms (Brownlee 2001).
During early embryogenesis, even before serotonergic neurons appear, serotonin (5-hydroxytryptamine; 5HT), as a neurotransmitter and as a mitogen (McGeer and McGeer 1973; Hansson et al. 1999), acts as a growth regulatory signal in the developing nervous system (Walther and Bader 1999) by activating 5HT receptors and downstream signal transduction pathways (Lauder et al. 1988; Liu and Lauder 1992; Lauder 1993). The age-dependent expression of 5HT transporter (SERT) mRNA indicates that during early neurulation, there is direct access of maternal 5HT to the embryo via the SERT system expressed on the yolk sac and placenta (Balkovetz et al. 1989). The appearance of embryonic 5HT prior to trypthophan hydroxylase (McGeer and McGeer 1973; Walther and Bader 1999), which is required in the pathway of 5HT synthesis, suggests that embryos are provided with this important neurotransmitter through the maternal blood via uptake by the placental SERT (McGeer and McGeer 1973; Walther and Bader 1999; Hendricks et al. 2003). Genetic and pharmacological disruption of 5HT signaling during pregnancy causes adverse effects, including high blood pressure in the mother and neuroanatomical abnormalities in the fetus. Altered levels of 5HT cause brain defects and delay the onset of neuronal differentiation along the pathways where 5HT fibers grow (Lauder et al. 1988; Liu and Lauder 1992; Lauder 1993; Vitalis, et al. 1998; Cases et al. 1996; Salichon et al. 2001). SERT inhibitors, such as cocaine and amphetamines (Prasad et al. 1994; Ramamoorthy et al. 1995), prevent the transporter-mediated placental clearance of 5HT and are involved in the pathogenesis of clinical symptoms in the developing fetus. Additionally, a direct relationship between plasma 5HT levels and SERT uptake rate has been demonstrated in several studies, such as those in SERT knock-out mice (Bengel et al. 1998), polymorphisms in the SERT-linked promoter region, and SERT inhibitors (Heils et al. 1996; Lesch et al. 1996; Greenberg et al. 1999). SERT on the membranes of the trophoblast cells regulates extracellular 5HT levels and prevents the vasoconstriction in the placental vascular bed, thereby securing a stable blood flow to the embryo (Ramamoorthy et al. 1993). Therefore, placental SERT plays a major role during pregnancy for both the mother and the developing embryo (Kekuda et al. 2000). Diabetes (types 1 and 2), existing prior to pregnancy and associated with hyperglycemia, is related to an increased incidence of congenital malformations (Hansson et al. 1999). However, the impact of glucose on placental SERT during diabetic pregnancy is not known. The present study investigated the effect of glucose at a diabetic-like concentration on the SERT system in human placental choriocarcinoma (JAR) cells and demonstrated that diabetic glucose concentrations disrupt the aggregation of SERT proteins in the membrane, thereby impairing optimal SERT function.
Materials and methods
Materials
Expression vectors, cell culture materials, and Lipofectamine 2000 were from Invitrogen (Carlsbad, CA, USA). Insulin-free fetal bovine serum (FBS) was provided by Gemini Bio-Products (Woodland, CA, USA). Micro BCA Protein Assay Kit, enhanced chemiluminescence (ECL) western blotting system, streptavidin-agarose beads, NHS-SS-biotin [sulfosuccinimidyl 2-(biotinamido) ethyl-1,3-dithio-propionate] and immuno-pure horseradish peroxidase (HRP)-conjugated streptavidin were from Pierce (Rockford, IL, USA). Mouse monoclonal anti-myc-antibody was from Chemicon International (Temecula, CA, USA). Mouse monoclonal-Flag, peroxidase-conjugated donkey-anti-mouse and -rabbit secondary antibodies were from Sigma-Aldrich (St Louis, MO, USA). JAR cells were provided by American Type Culture Collection (Manassas, VA, USA). Protein A sepharose beads and non-immune rabbit serum were purchased from Zymed Co. (San Francisco, CA, USA). High-affinity cocaine analog, 0.1 μmol/L 2β-carbomethoxy-3-tropane (β-CIT) was provided by the National Institute of Mental Health.
Reverse transcription polymerase chain reaction analysis
Total RNA was extracted from 28-h post-transfected cells using the guanidine thiocyanate/acid phenol method as indicated by the manufacturer. Contaminating genomic DNA was removed with RNase-free DNase. cDNA was synthesized with Superscript II RNase H-Reverse Transcriptase as indicated by the manufacturer. PCR was performed in the advanced Px2 personal thermal cycler (Thermo Hybaid, MA, USA). Reactions contained 10 μL 1.5 mmol/L MgCl2 BioMix sample, 0.5 μL 20 pmol/μL forward primer, 0.5 μL 20 pmol/μL reverse primer, and 1 μL cDNA. After initial denaturation at 94°C for 2 min, 35 cycles of amplification were completed by denaturation for 30 s at 94°C, annealing at temperature specified for each pair of primers for 30 s, and extension for 30 s at 72 °C. To compare the relative quantity of the reverse transcription polymerase chain reaction (RT-PCR) reactions, the transcription level of β-actin, a ‘housekeeping’ gene, was used as a control. Reactions without cDNA were used as negative control. Results were visualized by gel electrophoresis in 1% agarose in TAE buffer (Tris–HCl, acetic acid, EDTA, pH 8.0) and stained with ethidium bromide. Reactions were repeated at least twice.
Plasmids, constructs, and cell-line expression systems
The SERT was subcloned into EcoRV + XbaI and Kpn1/Not1 sites of the expression vectors pcDNA3, placing rat SERT (rSERT) expression under dual control of the cytomegalovirus (CMV) and T7 RNA polymerase promoters suitable for expression in JAR cells. A myc or Flag epitope was tagged to the amino and carboxyl termini of SERT as described previously (Ozaslan et al. 2003).
JAR cells were maintained in Roswell Park Memorial Institute (RPMI) medium supplemented with 10% FBS, 2 mmol/L L-glutamine, penicillin, and streptomycin. FBS contains 4.3 micro-international units/mL insulin.
A diabetic condition is defined by a blood glucose concentration greater than 250 mg/dL (13.8 mmol/L). Thus, the 5HT uptake rate of SERT was measured in JAR cells cultured in RPMI medium supplemented with insulin-free FBS (Gemini Bio-Products) and either 5.5 mmol/L (physiologic concentration) or 25 mmol/L (diabetic-like concentration) D-glucose (Hahn et al. 1998; Ogura et al. 1999).
To measure the whole-cell surface expression and the self-association ability of the transporter, these cells were transiently transfected with rSERT constructs using a 1: 3 ratio of Lipofectamine 2000 reagent in opti-MEM I (Invitrogen, Carlsbad, CA, USA). Cells were used in biotinylation, western blotting, and immunoprecipitation assays 24 h post-transfection.
SERT antibody
Proteintech Group Inc. (Chicago, IL, USA) prepared SERT-antibody with a synthetic peptide corresponding to the last 26 amino acids from SERT’s C-terminal (586–630) (Ozaslan et al. 2003). The synthetic peptide sequence (586–630) is a highly conserved region across different SERT species, but divergent from other gene family members (Blakely et al. 1991). We purified this antibody using standard affinity purification via peptide-sepharose procedures (Ozaslan et al. 2003).
Western blotting analysis
Cells were solubilized in phosphate-buffered saline (PBS) containing 0.44% SDS, 1 mmol/L phenylmethylsulfonyl fluoride (PMSF), and protease inhibitor mixture (PIM). The PIM contained 5 mg/mL pepstatin and 50 mg/mL leupeptin; and 5 mg/mL aprotinin was included with each lysis buffer (Kilic and Rudnick 2000), which also contained the alkylating agent N-ethylmaleimide (NEM), at a final concentration of 5 mmol/L to prevent oxidation and formation of non-specific disulfide bonds during lysis and to retain the native monomeric structures in the gel (Kocabas et al. 2003). Samples were analyzed by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and transferred to the nitrocellulose membrane. Immunoblot analysis was performed, first with SERT antibody (diluted 1: 400) and then with HRP-conjugated anti-rabbit secondary antibody at a dilution of 1:5000. The signals were visualized using an ECL western blotting detection system. Blots were visualized under a VersaDoc 1000 gel visualization and analysis system (Bio-Rad Laboratories, Hercules, CA, USA).
Cell surface biotinylation
Cell surface expression levels of the transporters were detected using non-cleavable biotin coupled to highly reactive N-hydroxy-succinimide ester, NHS-SS-biotin, membrane-impermeant biotinylation reagent. Following transfection, cells were biotinylated with the membrane-impermeant biotinylating reagent, NHS-SS-biotin, as described previously (Kilic and Rudnick 2000; Kilic et al. 2003). This reagent selectively modifies only the external lysine residues on membrane proteins. All SERT proteins used in this study, with or without epitope tagging, contained the same number of external lysine residues and were expected to react equally with NHS-SS-biotin. Following biotinylation, cells were treated with 100 mmol/L glycine to complete quenching of the unreacted NHS-SS-biotin and lysed in TBS containing 1% SDS, 1% Triton X-100 (PerkinElmer, Waltham, MA, USA), and PIM/PMSF. The biotinylated proteins were recovered with an excess amount of streptavidin-agarose beads during overnight incubation. Biotinylated proteins were eluted in sample buffer separated on SDS–PAGE, transferred to nitrocellulose, and detected with anti-SERT antibody as previously described (Ozaslan et al. 2003).
Co-immunoprecipitation
Protein–protein interaction was demonstrated by co-expressing two different protein forms, myc-SERT and Flag-SERT, in JAR cells at a 1: 1 ratio. Following transfection, JAR cells were treated with 10 mmol/L NEM for 30 min. Cells were lysed in immunoprecipitation buffer [55 mmol/L triethylamine (pH 7.5), 111 mmol/L NaCl, 2.2 mmol/L EDTA, and 0.44% SDS + 1% Triton X-100, 1 mmol/L PMSF, PIM] (Kilic and Rudnick 2000; Kocabas et al. 2003) that also contained 5 mmol/L NEM. Cell lysates were first pre-cleared by incubation with non-immune rabbit serum and protein A for 1 h and then centrifuged. The pre-cleared lysate was combined with an equal volume of a 1: 1 slurry of rabbit-anti-mouse protein A sepharose (RAM-PAS) beads, as previously described, for primary immunoprecipitation with polyclonal Flag-antibody (Kilic and Rudnick 2000) and then mixed overnight at 4°C. The lysate immune complexes were recovered by brief centrifugation in a bench-top microfuge, washed several times in high- and low-salt immunoprecipitation buffers, and eluted in Laemmli sample buffer [50 mmol/L Tris–HCl, pH 6.8, 2% SDS, 0.1% bromophenol blue, 10% glycerol, and 1% β-mercaptoethanol (MSH)]. For the non-reducing condition, 5 mmol/L NEM was included in the Laemmli sample buffer containing no MSH. Samples were separated on a 10% SDS–PAGE. After electrophoresis, gels were analyzed by immunoblotting with monoclonal anti-myc-antibodies (diluted 1: 1000) and then with HRP-conjugated anti-mouse secondary antibody at a dilution of 1: 10 000 to demonstrate self-association. The signals were developed with the ECL western blotting detection system, and the blots were visualized under a VersaDoc 1000 gel visualization and analysis system.
Quantitation of human SERT expression
Cells were solubilized in PBS containing 0.44% SDS, 1 mmol/L PMSF, and PIM. The PIM contained 5 mg/mL pepstatin and 50 mg/mL leupeptin; and 5 mg/mL aprotinin was included with each lysis buffer (Kilic and Rudnick 2000). A standard curve was generated by separating different amounts (in a broad range 0–25 μg protein) of cell protein from human SERT (hSERT) expressing Chinese Hamster ovary cell extracts on 8% SDS–PAGE and analyzed by immunoblotting with anti-SERT antibody (diluted 1: 400), and then with HRP-conjugated anti-rabbit secondary antibody at a dilution of 1: 5000 (Kilic and Rudnick 2000). The relative amounts of SERT constructs were determined in every experiment within a quantitative western blot analysis. The integrated density values of the major bands corresponding to 90 kD were quantitated by densitometry using a VersaDoc 1000 analysis system (Bio Rad Lab).
Transport assay
JAR cells in 24-well culture plates were assayed by incubating with 20.5 nmol/L [1,2-3H (N)]-5HT (3400 cpm/pmol) in PBS/CM for 10 min. The intact cells were washed quickly with ice-cold PBS, lysed in SDS, transferred to scintillation vials, and radioactivity was determined (Kilic and Rudnick 2000). Background accumulation of 3H-5HT that occurred independent of SERT was measured in the same experiment by treating JAR cells with 0.1 μmol/L β-CIT and the resulting value was subtracted from each experimental value. 0.1 μmol/L β-CIT totally inactivates the 5HT uptake rate of SERT (Wall et al. 1993). In parallel, the protein concentration for each well was determined using the Micro BCA Protein Assay Kit (Pierce).
Cell-cycle analysis
JAR cell DNA synthesis was monitored by pulse labeling for 30 min with (1 μCi/mL) [3H]-thymidine. The cells (1 × 106/100 mm plate) were washed twice with cold PBS. The cells were then scrapped harvested with a rubber policeman into 1 mL PBS. The suspension was homogenized by sonication, and a 0.2-mL aliquot was mixed with 1 mL 10% cold trichloroacetic acid (TCA). The insoluble material was collected on a 0.45-μm Millipore filter (Millipore, Billerica, MA, USA), washed with cold TCA and counted in Optifluor with a scintillation counter.
For DNA analysis, cells were fixed in 70% ethanol, washed in PBS, resuspended in 1 mL of PBS containing 1 mg/mL RNase and 50 mg/mL propidium iodide, incubated for 20 min in the dark at 20–25°C, and analyzed by flow cytometry using a FACS Calibur (Becton Dickinson, Mountain View, CA, USA). The data were analyzed using the ModFit DNA analysis program (Verity Software House, Topsham, ME, USA).
Data analysis
Non-linear regression fits of experimental and calculated data were performed with Origin, which uses the Marquardt–Levenberg non-linear least squares curve-fitting algorithm. Each figure shows a representative experiment that was performed at least twice. The statistical analysis given in the Results section is from multiple experiments. Data with error bars represent the mean ± standard deviation (SD) for triplicate samples. A two-way independent factorial analysis of variance was conducted to investigate 5HT uptake rate of SERT differences in insulin and glucose categories. ANOVA results, presented in Table 1, showed significant differences between the presence of insulin and the various levels of glucose for 5HT uptake rate of SERT.
Table 1.
A two-way independent factorial analysis of variance was conducted to investigate 5HT uptake rate of SERT differences in insulin and glucose categories
| Source | Type III sum of squares | df | Mean square | F | p-value | Partial η2 |
|---|---|---|---|---|---|---|
| Insulin | 0.009 | 1 | 0.009 | 338.702 | 0.000 | 0.909 |
| Glucose | 0.218 | 5 | 0.044 | 1626.443 | 0.000 | 0.996 |
| Insulin and glucose | 0.012 | 5 | 0.002 | 87.791 | 0.000 | 0.928 |
| Error | 0.001 | 34 | 2.68E-005 | |||
| Total | 1.453 | 46 |
Results
SERT in JAR cells with D-glucose at diabetic-like concentration and without insulin
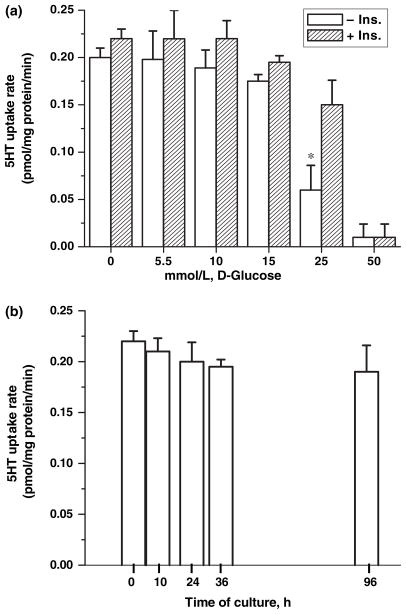
Human placental JAR cells endogenously express SERT and have proven to be very useful in studies on the regulatory aspects of the placental transporter (Balkovetz et al. 1989). Utilizing JAR cells in this study, the mechanism by which high glucose contributes to the characteristics of placental SERT was investigated. JAR cells were incubated in RPMI containing FBS with or without insulin and D-glucose at different concentrations (5.5, 10, 15, and 25 mmol/L) for 24 h. The 5HT uptake rates of these cells were measured (Fig. 1a). ANOVA results, presented in Table 1, showed a significant difference between the presence of insulin and the various levels of glucose for SERT uptake rate [F(5,34) = 87.79, p < 0.001, partial η2 = 0.928] (Fig. 1a). Simple effects examining mean differences in the insulin and no-insulin groups at each level of glucose were completed. These data demonstrate no significant difference between the insulin and no-insulin groups when no glucose is present or at the 50 mmol/L level of glucose; however, there was significantly slower SERT uptake rate in the insulin condition compared to the no-insulin condition for the intermediate glucose conditions (5.5, 10, 15, and 25 mmol/L).
Fig. 1.
Characterization of SERT in JAR cells with glucose at different concentrations. (a) JAR cells were cultured in RPMI supplemented with 10% FBS with/without insulin and D-glucose at different concentrations, 5.5, 10, 15, 25 mmol/L. The [3H]-5HT uptake rate in intact cells was measured as described in the Methods section. Results are expressed as a percentage of uptake after pre-treatment without glucose (percentage of control). Significant differences in 5HT uptake rates of JAR cells between 25 mmol/L glucose and physiological levels of glucose were observed in the absence of insulin, as shown by asterisks (*, p < 0.001). A two-way independent factorial analysis of variance was conducted to investigate 5HT uptake rate of SERT differences in insulin and glucose categories; ANOVA results, presented in Table 1. (b) The time-course effect of 5.5 mmol/L D-glucose on SERT in JAR cells cultured in insulin-free RPMI supplemented with 5.5 mmol/L D-glucose for 0, 10, 24, 36, for 96 h was also measured. Rates of uptake are expressed as means of SD values of triplicate determinations from three independent experiments.
D-glucose in the range of 5.5–15 mmol/L did not alter the 5HT uptake rates. However, JAR cells exposed to 25 mmol/L D-glucose in the presence or absence of insulin exhibited decreased 5HT uptake rate of 32% or 70%, respectively.
The impact of insulin alone on the 5HT system of JAR cells was tested by culturing them in full RPMI media supplemented with or without insulin containing FBS. 5HT uptake rates appeared not to be altered in these cells (data are not shown).
To examine the possibility that the exogenous 5.5 mmol/L D-glucose (normoglycemia) has a time-dependent effect on the placental 5HT system, JAR cells were cultured in RPMI media supplemented with insulin-free FBS. However, even after 96 h incubation, the 5HT uptake rates of these cells were not altered by D-glucose at physiological concentration in the absence of insulin (Fig. 1b). To capture the adverse effect of glucose on placental SERT, the functional characteristics of SERT in cell-cycle phases of JAR cells were analyzed.
Cell-cycle analysis of JAR cells in the presence of 25 mmol/L D-glucose
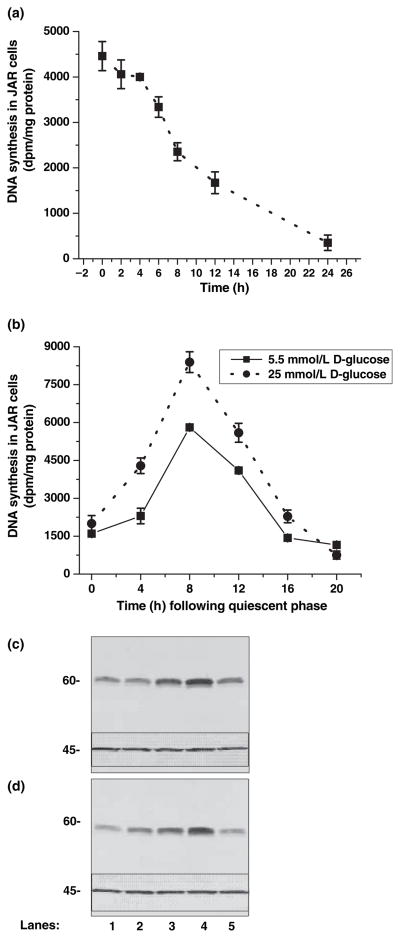
At the end of 12 h serum deprivation, JAR cells were rendered quiescent (Fig. 2a). Then, the time course for the phases of the cell cycle was determined based on DNA synthesis which was measured by 3H-thymidine incorporation of DNA (Fig. 2a). The rate of DNA synthesis confirmed that quiescent JAR cells enter S- and G2-phase after 8 and 16 h, respectively, during incubation in insulin-free full media supplemented with or without 25 mmol/L glucose (Panel B). Also, these data demonstrate that there is a 60% increase in overall DNA synthesis of JAR cells if they enter the cell cycle in media supplemented with 25 mmol/L D-glucose and insulin-free FBS (Table 1).
Fig. 2.
Cell-cycle analysis of JAR cells. JAR cells were brought into the quiescent phase through serum deprivation and incubated in [3H]-thymidine to measure the DNA synthesis as described in the Methods section (a). To estimate the times for the phases during the cell cycle, quiescent cells were taken back to the insulin-free full medium supplemented with 5.5 (dotted line) or 25 mmol/L D-glucose (solid line). (b) The estimated time of the phases in the cell cycle is summarized in Table 2. The results reported here are the mean and SD of three separate experiments. Quiescent JAR cells were incubated in insulin-free full RPMI medium containing 5 mmol/L (c) or with 25 mmol/L D-glucose-supplemented RPMI medium (d); after 4, 8, 12, 16, or 24 h (lanes 1–5, respectively), a group of cells was harvested and prepared for western blotting with cyclin B-antibody. Actin was used as a loading control (inset in the figures 45 kD). The immunoblots are representative of at least three independently performed experiments.
Cells in mitosis contain a dominant regulatory factor known as the maturation promoting factor, which consists of two polypeptides, CDK1 and cyclin B (Dunphy et al. 1988). Cyclin B is utilized in our studies to monitor the phases of the JAR cell cycle as a second approach to 3H-thymidine assay. Quiescent JAR cells were incubated in full media in the presence of either 5 or 25 mmol/L glucose (Figs 2c and d, respectively); every 4 h, a group of cells was harvested and prepared for western blotting with cyclin B antibody. The expression of cyclin B shows that JAR cells enter late-S and G2 phases after around 10 and 16 h of incubation in full media, respectively. Expression of actin was also monitored as the control of our experimental setting. The expression of actin in the inset of Fig. 2 was not altered between the phases, showing the equal amounts of protein loaded in each well.
5HT uptake rate of SERT in high-glucose-supplemented JAR cells at different phases of the cell cycle
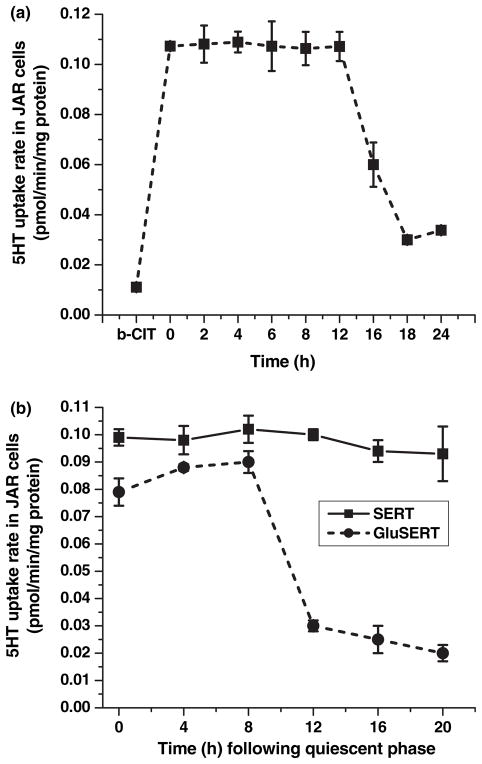
To determine the 5HT uptake rates at different phases of the cell cycle, JAR cells were rendered to the quiescent stage by serum starvation; and every 4 h, a group of cells was harvested and assayed for 5HT uptake rates. During the first 12 h of serum starvation, the 5HT uptake rates of JAR cells were not changed, but after 12 h, 5HT uptake rates decreased significantly (Fig. 3a). Background accumulation of 3H-5HT, which occurred independent of SERT, was measured in the same experiment by treating the JAR cells with the high-affinity cocaine analog, 0.1 μmol/L β-CIT (Wall et al. 1993). The accumulation was not subtracted from each point, but shown on the graph (Fig. 3a, the first point). Placental SERT appeared to be functionally active in the quiescent cells.
Fig. 3.
5HT Uptake profile of hSERT in JAR cells at different phases with 5.5 or 25 mmol/L D-glucose. [3H]-5HT uptake was measured in intact JAR cells placed into the quiescent phase through serum deprivation (a) The quiescent JAR cells were entered into the cell-cycle phases in full RPMI supplemented with 10% FBS (without insulin) and 5.5 or 25 mmol/L D-glucose. The 5HT uptake rate of SERT was monitored in these cells every 4 h as described in the Methods section (b) Rates of uptake are expressed as means of SD values of triplicate determinations from three independent experiments.
The 5HT uptake rates were monitored in JAR cells which entered the cell cycle in insulin-free FBS containing full RPMI media supplemented with 5.5 mmol/L glucose (Fig. 3b). The uptake profile of JAR cells was not paralleled to the rate of DNA synthesis. However, if quiescent JAR cells entered the cell cycle in the presence of 25 mmol/L D-glucose and in the absence of insulin, the 5HT uptake rate of SERT was diminished by 73% after 12 h incubation, at late-S and G2 phases (Fig. 3b, dotted line).
Therefore, we investigated whether glucose at diabetic-like concentrations has an impact on the SERT system by altering the whole cell expression and/or the density on the plasma membrane and/or the self-association ability of transporter molecules.
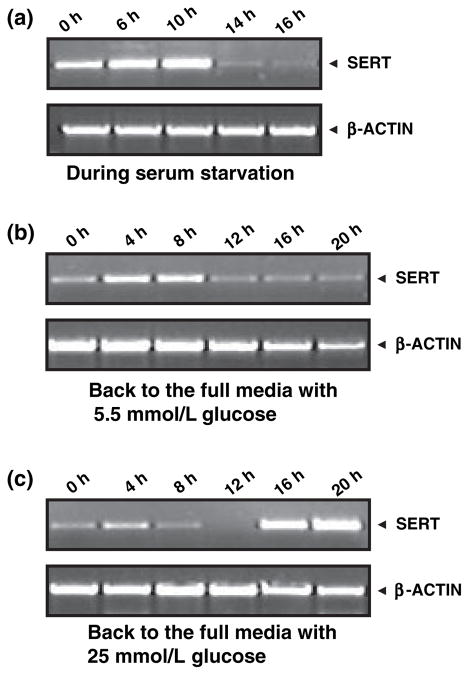
Relative SERT mRNA levels in phases of JAR cell cycle
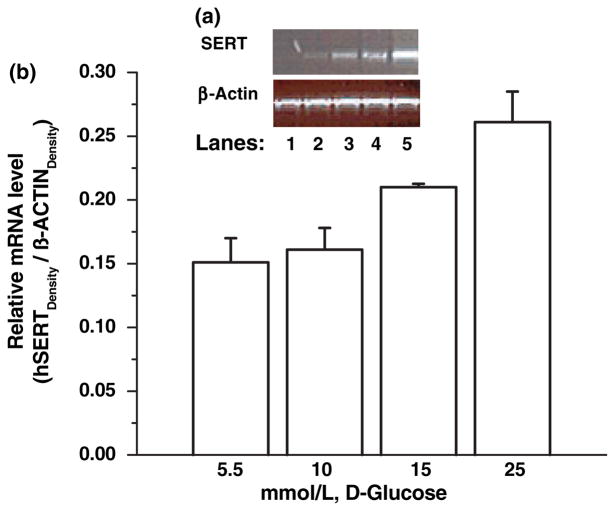
Initially, the relative SERT mRNA levels were determined in JAR cells treated with different concentrations (5.5, 10, 15, 25 mmol/L) of D-glucose for 24 h (Fig. 4a, lanes 2–5). Reaction without cDNA (lane 1) was the negative control. Bands were analyzed semi-quantitatively as the ratio of each band to the transcriptional level of β-actin (Fig. 4b). SERT mRNA levels in 25 mmol/L glucose-treated JAR cells increased, compared with the control conditions (5 mmol/L D-glucose).
Fig. 4.
Concentration-dependent effect of exogenous D-glucose on relative SERT mRNA in JAR cells. JAR cells were cultured in RPMI supplemented with 10% FBS without insulin and D-glucose at different concentrations, 5.5, 10, 15, 25 mmol/L (lanes 2–5). The relative SERT mRNA levels were measured. Reactions without cDNA (lane 1) or only H2O (not shown in the figure) were used as negative controls. (a) shows the representative of four different experiments. The band densities were calculated as the ratio of each band to the transcription level of β-actin (b) Relative mRNA levels are expressed as means of SD values of triplicate determinations from three independent experiments.
Next, this effect of glucose on transcriptional level expression of SERT in the cell-cycle phases of placental cells was captured. The SERT mRNA level expression was measured in quiescent JAR cells (Fig. 5a). Then, the quiescent cells were incubated in insulin-free FBS containing full media supplemented with 5.5 or 25 mmol/L glucose. Their SERT mRNA was extracted, and RT-PCR was performed (Figs 5b and c).
Fig. 5.
Relative SERT mRNA levels in different JAR cell-cycle phases. SERT mRNA levels were monitored by semi-quantitative RT-PCR analysis during serum starvation (a) and the entrance of quiescent cells into various phases of the JAR cell cycle in the presence of 5.5 mmol/L (b) or with 25 mmol/L D-glucose (c) containing full RPMI supplemented with 10% FBS without insulin. The images are representatives of at least four to five independently performed experiments.
Finally, we addressed whether the increase in mRNA expression levels is associated with the protein levels. SERT protein expression levels in whole-cell and on the JAR cell membrane at G2 phase of the cell cycle were monitored in the presence of glucose at physiological or diabetic-like concentrations.
Whole-cell and plasma membrane expression of hSERT in JAR cells entered into G2phase with 5.5 or 25 mmol/L D-glucose-containing media
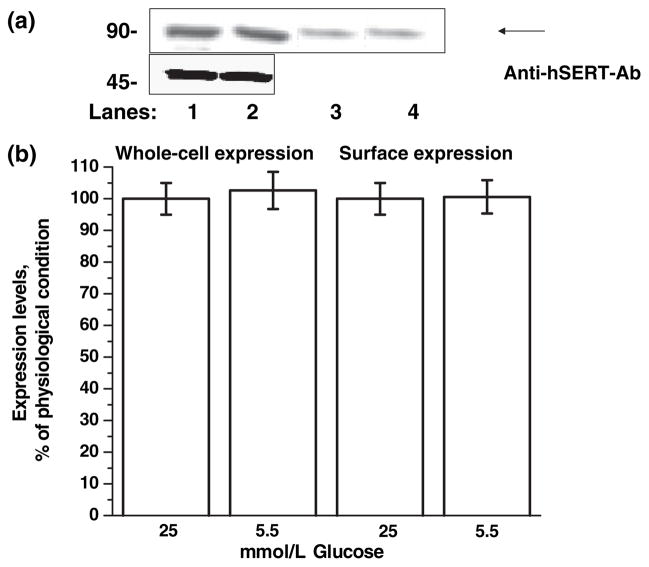
A possible explanation for the decreased 5HT uptake rates is that the distribution of transporters between cell-surface and intracellular locations may be altered by 25 mmol/L D-glucose at G2 phase. To test this possibility, the whole-cell and plasma membrane expressions of SERT were determined in JAR cells, which had entered G2 phase in insulin-free full media supplemented with 25 or 5.5 mmol/L D-glucose by biotinylation of surface proteins followed by western blot analysis (Fig. 6a). The quantitative analysis of western blot results showed no differences between the whole-cell and surface expression of SERT proteins at physiological and diabetic-like glucose concentrations (Fig. 6b).
Fig. 6.
Whole-cell and surface expression of hSERT in JAR cells entered into the G2phase with or without 25 mmol/L D-glucose-containing full medium. (a) For whole-cell expression, JAR cells entered the G2 phase in insulin-free full RPMI supplemented with 5.5 (lanes 2 and 4) or 25 mmol/L D-glucose (lanes 1 and 3); 50 μL cell lysate was separated by SDS–PAGE and visualized by western blotting, and the integrated density values were determined by densitometry. The standard curve for quantification of hSERT expression was prepared as described previously (Kilic and Rudnick 2000). For cell-surface expression, JAR cells entered the G2 phase in insulin-free full RPMI medium supplemented with 5.5 (lane 3) or 25 mmol/L glucose (lane 4); then the intact cells were biotinylated. The labeled cell surface proteins were separated and visualized as above. The immunoblots are representatives of three independently performed experiments. (b) The results from western blotting are shown above a summary of combined data from three densitometric scans. All lanes contain protein recovered from the same number of cells equivalent to 30% of one well from a confluent 6-well dish. Three wells of each condition were pooled and an aliquot of this mixture was run on the gel.
The self-associated form of SERT is the most favorable state for its optimum rate of 5HT uptake (Schmid et al. 2001). Therefore, next, the ability of SERT molecules to associate with each other in JAR cells cultured in diabetic-like conditions was tested.
Self-association of SERT in high-glucose-supplemented JAR cells
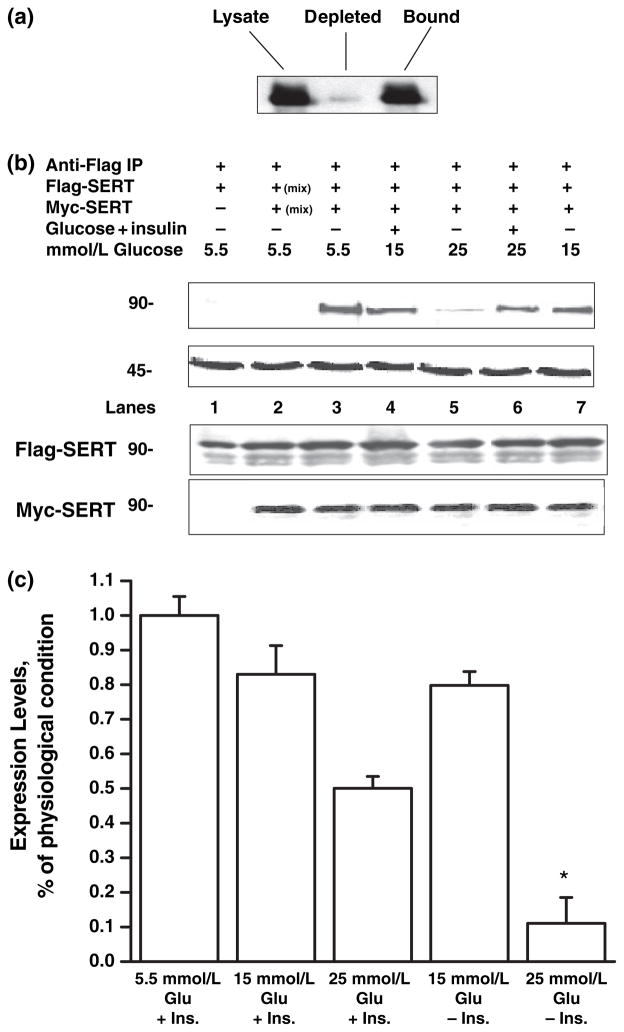
Before studying the impact of glucose on the self-association ability of placental SERT, first the significance of the self-association of SERT molecules in JAR cells was addressed. The extent of myc-SERT depletion from the cell lysate on immunoprecipitation of Flag-SERT was measured. Lysate from JAR cells expressing a 1: 1 mixture of the two forms of SERT constructs was first pre-cleared and a sample of the pre-cleared lysate was separated by SDS–PAGE and was assayed by western blotting with anti-myc-antibodies. An equivalent sample of the same lysate was analyzed after treatment with an amount of protein A beads and Flag-antibody that was independently determined to maximally precipitate myc-SERT from the mixture (Fig. 7a). From three independent experiments, the precipitation removed 70% ± 2% of the myc-SERT from solution. No myc-SERT was detected in the precipitate if Flag-antibody was replaced with pre-immune rabbit serum.
Fig. 7.
Self-association ability of SERT proteins under different glucose concentrations. (a) The significance of self-association of SERT molecules in JAR cells was tested. The extent of myc-SERT depletion from the cell lysate on immunoprecipitation of Flag-SERT was measured. Lysate from JAR cells expressing a 1: 1 mixture of myc-SERT/Flag-SERT was first pre-cleared and a sample of the pre-cleared lysate was separated by SDS–PAGE and was assayed by western blotting with anti-myc-antibodies (lysate). An equivalent sample of the same lysate was analyzed after treatment with an amount of protein A beads and Flag-antibody that was independently determined to maximally precipitate myc-SERT from the mixture (depleted). From three independent experiments, the precipitation removed 70 ± 2% of the myc-SERT from solution (bound). (b) JAR cells were co-transfected with SERT-Flag and myc-SERT constructs in a 1: 1 ratio where indicated. Transfection medium was replaced with insulin-free full RPMI containing 5.5, 15, or 25 mmol/L D-glucose (lanes 3, 7, 5). The impact of insulin in FBS on exogenous glucose was tested with co-immunoprecipitation of myc-SERT/Flag-SERT expressing JAR cells. Cells were cultured and co-transfected as described in the text. Following transfection, cells were incubated in full RPMI supplemented with 5.5 or 25 mmol/L glucose (lanes 4 and 6, respectively). After 24 h, co-transfected cells were harvested, solubilized, and treated with mouse anti-myc-antibody coated RAM-PAS beads. The immunoprecipitates were separated and blotted with polyclonal anti-Flag-antibodies. Control experiments in which only SERT-Flag transfected JAR cells were subjected to the same procedure did not reveal a visible band (lane 1) in the immunoblot. Another control experiment was the analysis of lysates from D-glucose-treated JAR cells expressing myc-SERT or Flag-SERT individual constructs which were mixed prior to immunoprecipitation (lane 2). Equal amounts of samples from the pre-cleared cell lysates prior to immunoprecipitation were resolved on two identical SDS–PAGE and analyzed by western blot. One of the blots was probed with anti-myc-antibody, while the other blot was probed with anti-Flag- antibody as the control for the whole-cell expressions of these two forms of SERT. The positions of molecular weight standards are indicated (in kD). After immune complexes were recovered by brief centrifugation, equal amounts of supernatant were resolved on SDS–PAGE and analyzed by western blotting with anti-actin-antibody as a loading control. The inset in the representative blots shows that all lanes contain protein recovered from the same number of cells equivalent to 30% of one 100-mm culture plate. The immunoblots are representatives of three independently performed experiments. (c) The results from the western blots are shown above a summary of combined data from three densitometric scans. All lanes contain protein recovered from the same number of cells equivalent to 30% of one 100-mm culture dish. *p < 0.001.
Then, the impact of glucose on the self-association ability of placental SERT in the presence or absence of insulin was examined. JAR cells were seeded in full RPMI supplemented with insulin-free FBS. On the following day, cells were transfected with myc-SERT/Flag-SERT (1: 1 ratio) or only one of the constructs. Following a 7-h incubation, the transfection media was changed to full RPMI containing insulin-free FBS and 5.5, 15, or 25 mmol/L D-glucose (Fig. 7b, lanes 3, 7 and 5, respectively). Cells were lysed and immunoprecipitation was performed with polyclonal Flag-antibody, as described in the Methods section (Kilic and Rudnick 2000). The lysate immune complexes were eluted and analyzed by western blotting with monoclonal myc-antibody. Probing the Flag-bound proteins with myc-antibody produced one major band at 90 kD, indicating an association between SERT proteins from the cells treated with 5.5 and 15 mmol/L glucose (Fig. 7a, lanes 3 and 7), but not with 25 mmol/L glucose (lane 5).
When the JAR cells, co-transfected with myc-SERT/Flag-SERT constructs, were incubated in full RPMI-supplemented FBS with insulin and 15 or 25 mmol/L D-glucose (Fig. 7b, lanes 4 and 6, respectively), a 90-kD major band representing the myc-SERT was recovered from the cells. The density of each band at 90 kD was measured and normalized to the band which appeared in lane 3, where the JAR cells were treated with glucose at physiological concentration (Fig. 7c). The quantification of the bands at 90 kD showed that insulin partially overruled the impact of 25 mmol/L glucose on the self-association of SERT (lane 6).
As controls for non-specific precipitation of myc-SERT with Flag-SERT, the immunoprecipitation analysis of JAR cells expressing only Flag-SERT was performed (Fig. 7b, lane 1). No protein band was labeled, demonstrating that myc-SERT did not bind non-specifically to protein-A beads. Our next control was the same analysis using lysates from D-glucose-treated JAR cells expressing myc-SERT or Flag-SERT individual constructs which were mixed prior to immunoprecipitation (lane 2). No interaction was detected, indicating that the interaction occurs only when both proteins are co-expressed in the same cell and not promoted during protein solubilization. Both SERT constructs, Flag- and myc-SERT, were equally expressed, and their co-expression did not affect the expression level of SERT (Fig. 7b, lane 2). Expressions of actin, Flag-SERT, and myc-SERT were also monitored as the control of our experimental setting. After immune complexes were recovered by brief centrifugation, equal amounts from supernatant were resolved on SDS–PAGE and western blotting with anti-actin-, Flag-, or myc-antibody. As shown by the representative blots in the inset of Fig. 7b, the expressions of these proteins were not altered between glucose and/or insulin treatment at different concentrations, showing the equal amounts of protein loaded in each well.
Discussion
The brush border membrane of the placental syncytiotrophoblasts is directly exposed to the maternal circulation and forms the main barrier to maternal–fetal transport systems which are responsible for the transfer of nutrients from mother to fetus (Smith et al. 1992). SERT on placental syncytiotrophoblastic cells (Prasad et al. 1994; Ramamoorthy et al. 1995; Bengel et al. 1998; Kekuda et al. 2000) plays an important role in clearing 5HT from the intervillous space and maintaining 5HT levels for the embryo via uteroplacental blood flow (Ganapathy and Leibach 1994, 1995). In the intervillous space, 5HT enables the uterine arteries to open and maintain optimal blood circulation in the uteroplacental vascular bed from mother to fetus (Lauder et al. 1988; Liu and Lauder 1992; Lauder 1993). Therefore, alterations in 5HT uptake rates and the expression of placental SERT are suggested to be involved in the pathogenesis of clinical symptoms in the mother and the developing fetus (Lauder et al. 1988; Liu and Lauder 1992; Lauder 1993).
Diabetes is a major contributing factor to the development of many of the complications of pregnancy, particularly impaired fetal growth. Although the importance of 5HT on embryogenesis, fetal growth, and development is recognized (Ye et al. 1998; Fukumoto et al. 2005), under uncontrolled diabetic conditions, the impact of glucose on the uptake rate of placental SERT was not known before our studies. 25 mmol/L D-glucose in cell culture media without insulin-containing serum has been used as a model system to mimic diabetes-like or uncontrolled diabetic conditions (Hahn et al. 1998; Weiss et al. 2001). Using this in vitro model system, the effect of uncontrolled diabetic conditions on the placental SERT system was investigated. Uncontrolled diabetic conditions lowered the 5HT uptake rates of JAR cells by 70%, compared with 5HT uptake rates under physiological conditions. These findings suggest that glucose under in vitro uncontrolled diabetic conditions alters the number of functional transporters, which becomes the limiting factor.
To understand better the mechanism by which glucose exerts its effect on the functional expression of placental SERT under uncontrolled diabetic conditions, SERT in JAR cells at different phases of the cell cycle were characterized. 3H-thymidine incorporation of DNA with flow-cytometry is commonly used to determine the phases of the cell cycle. It is known that glucose under uncontrolled diabetic conditions increases the activity of thymidine kinase, the rate-limiting enzyme of thymidine incorporation (Swenne 1990). The cell cycle is regulated by cyclin-dependent kinases. Therefore, cyclin B was also monitored in determining the phases of the cell cycle. Both data on cyclin B and 3H-thymidine incorporation of DNA with flow-cytometry agreed on the time-length of phases in cycling JAR cells. Then, the 5HT uptake rates of JAR cells at different phases of the cell cycle were determined. Our data demonstrated that under physiological conditions, the 5HT uptake rates of placental SERT (i) did not change as parallel to the rate of DNA synthesis; (ii) decreased by 70% at G2 phase. These findings imply that 5HT uptake rates of SERT were not altered in different phases of the cell cycle; glucose down-regulates SERT in JAR cells at the late-S and G2 phases. In general, alterations in the 5HT influx occur as a result of changes in some intrinsic factors, such as lower protein and surface expression or changed transporter capacity.
While determining the phases of the cell cycle, it was noted that there was a 60% increase in DNA synthesis of JAR cells under uncontrolled diabetic conditions. Therefore, first, the mRNA level expressions of SERT in intact JAR cells at different phases of the cell cycle were monitored. Similarly, under uncontrolled diabetic conditions, the SERT mRNA levels appeared to increase at the G2 phase. Therefore, we wanted to confirm whether this effect is associated with protein expression levels or whether it is a general impact of glucose on the transcriptional level expression of some proteins under diabetic-like conditions. The densities of transporters on the plasma membrane and in the whole-cell lysate were measured, and SERT expression was found not to undergo diabetic hyperglycemia-associated alterations.
In searching for the mechanism by which hyperglycemia contributes to the rate of placental SERT, next, the self-association ability of placental SERT under uncontrolled diabetic conditions was addressed. Oligomerization is reported as a common phenomenon among the transporters of biogenic amine neurotransmitters. This emphasizes the importance of oligomerization on the uptake rates of transporters (Kilic and Rudnick 2000; Kocabas et al. 2003). Furthermore, the self-associated form is the preferred, and most efficient, conformation for SERT molecules (Schmid et al. 2001). SERT has been reported to be a homo-oligomer, based in part on co-immunoprecipitation studies of myc- and Flag-tagged SERT constructs (Kilic and Rudnick 2000). In cells expressing a mixture of myc-SERT/Flag-SERT constructs, the SERT construct which is vulnerable to chemical modification is protected (Kilic and Rudnick 2000). This finding supports the notion that the two constructs are physically associated in the membrane. The demonstration of fluorescence resonance energy transfer between different green fluorescent proteins fused to the N-termini of SERT molecules also supports the idea of homo-oligomerization (Schmid et al. 2001). Therefore, our next step was to evaluate the impact of 25 mmol/L D-glucose on the self-association of placental SERT molecules in full RPMI medium supplemented with insulin-free FBS. The high extent of Flag-SERT co-precipitated with myc-SERT (70%) suggests that self-association is a major modification of SERT molecules in JAR cells. However, under uncontrolled diabetic conditions, the self-association of SERT molecules was reduced by 88%. Based on this finding, we hypothesize that glucose at diabetic-concentrations may play a role in altering 5HT uptake rates of placental SERT by interfering with the self-association ability of transporter molecules. Therefore, we propose that under uncontrolled diabetic conditions, glucose down-regulates 5HT uptake rates of placental SERT by interfering with its functional expression in a cell-cycle-dependent manner. Ultimately, our data suggest that under uncontrolled diabetic conditions, the 5HT levels in transplacental blood become higher and the delivery of 5HT to the embryo becomes lower – both undesirable outcomes for the health of the mother and the growing embryo.
5-Hydroxytryptamine plays an important role in regulating the development of the embryo and is provided by placental SERT until the embryo begins to synthesize its own 5HT. Therefore, it is important to investigate the consequences of a depleted 5HT flow from a diabetic mother to the embryo. 5HT regulates the developmental processes of neurons (Liu and Lauder 1992), the migration of the cranial neural crest cells that produce most of the mesenchyme of the cranio-facial primordia (Hansson et al. 1999), and it may have other related effects within the central nervous system. The identification of an ETS domain transcription factor, Pet-1 (Hendricks et al. 1999, 2003), has made it possible to study the factors and molecular mechanisms that control the development of serotonergic neurons. The Pet-1 ETS factor is a precise marker of developing and adult 5HT neurons and is expressed shortly before 5HT appears in the hindbrain. Thus, in Pet-1 knock-out mice, the majority of 5HT neurons fail to differentiate (Hendricks et al. 1999, 2003). Pet-1 is now accepted as an early and precise marker of 5HT neurons and functions specifically in the differentiation and maintenance of these neurons.
It is important to explore the patterning and organization of 5HT in the embryos of diabetic rats by addressing how early these embryos start producing 5HT and Pet-1, as an indicator for the formation of serotonergic neurons. Additionally, trypthophan hydroxylase mRNA levels should be determined in time-course experiments to identify the first endogenous production of 5HT in an embryo of diabetic rats, to elucidate the impact of hyperglycemia on the pattern of embryonic 5HT and correlate the underlying effect of placental SERT. Further work is needed to elucidate the impact of high glucose on 5HT uptake by placental SERT in the early development of embryos in diabetic pregnancies.
Table 2.
Cell cycle analysis of JAR cells
| Incubation time (hour) following serum starvation | Cell cycle phases |
3H-thymidine incorporation (dpm/mg protein) |
mRNA SERT/β-actin |
5HT uptake rate (pmol/mg protein/min) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| −Glucose | +Glucose | %↑ | −Glucose | +Glucose | %↑ | −Glucose | +Glucose | %↓ | ||
| 0 | G0/G1 | 1800 ± 112 | 2000 ± 318 | 11 | 0.406 ± 0.04 | 0.415 ± 0.01 | 2.2 | 0.099 ± 0.003 | 0.079 ± 0.005 | 20 |
| 4 | G1 | 2390 ± 308 | 3800 ± 307 | 61 | 0.438 ± 0.02 | 0.582 ± 0.07 | 33 | 0.098 ± 0.005 | 0.088 ± 0.001 | 10 |
| 8 | S | 5008 ± 111 | 8391 ± 411 | 68 | 0.622 ± 0.02 | 0.740 ± 0.08 | 19 | 0.10 ± 0.005 | 0.090 ± 0.004 | 10 |
| 16 | Late-S | 1431 ± 121 | 2399 ± 251 | 68 | 0.501 ± 0.09 | 0.832 ± 0.01 | 66 | 0.094 ± 0.004 | 0.025 ± 0.005 | 73 |
| 20 | G2 | 1154 ± 49 | 2000 ± 158 | 73 | 0.494 ± 0.03 | 0.870 ± 0.01 | 76 | 0.094 ± 0.01 | 0.020 ± 0.003 | 79 |
All results are an accumulation of at least three experiments. Data represent mean ± SD of three or more independent experiments, unless otherwise noted. 3H-thymidine incorporation, transport, and mRNA measurements were performed as described under Materials and methods section. The columns showing the increases/decreases are based on the percent effect of glucose on individual measurements.
Acknowledgments
We would like to gratefully acknowledge Dr. Jennifer Peszka (Hendrix College, Conway, AR, USA) for her assistance in the statistical analysis of our data. This work was supported by grants (to F. Kilic) from the NICHD/National Institutes of Health (Grant R03 HD053477-01), National Alliance for Research on Schizophrenia and Depression.
Abbreviations used
- β-CIT
2β-carbomethoxy-3-tropane
- CMV
cytomegalovirus
- ECL
enhanced chemiluminescence
- FBS
fetal bovine serum
- HRP
horseradish peroxidase
- hSERT
human SERT
- 5HT
5-hydroxytryptamine
- JAR
placental choriocarcinoma
- NEM
N-ethyl-maleimide
- PBS
phosphate-buffered saline
- PIM
protease inhibitor mixture
- PMSF
phenylmethylsulfonyl fluoride
- RAM-PAS
rabbit-anti-mouse protein A sepharose
- RPMI
Roswell Park Memorial Institute
- rSERT
rat SERT
- RT-PCR
reverse transcription polymerase chain reaction
- SERT
5HT transporter
- SDS–PAGE
sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- TCA
trichloroacetic acid
References
- Balkovetz DF, Tiruppathi C, Leibach FH, Mahesh VB, Ganapathy V. Evidence for an imipramine-sensitive serotonin transporter in human placental brush-border membranes. J Biol Chem. 1989;264:2195–2198. [PubMed] [Google Scholar]
- Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, Mossner R, Westphal H, Lesch K. Altered brain serotonin homeostasis and locomotor insensitivity to 3,4-methylenedioxymethamphetamine (“Ecstasy”) in serotonin transporter-deficient mice. Mol Pharmacol. 1998;53:649–655. doi: 10.1124/mol.53.4.649. [DOI] [PubMed] [Google Scholar]
- Blakely RD, Berson HE, Fremeau RT, Jr, Caron MC, Peek MM, Priace HK, Bradley CC. Cloning and expression of a functional serotonin transporter from rat brain. Nature. 1991;354:66–70. doi: 10.1038/354066a0. [DOI] [PubMed] [Google Scholar]
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- Cases O, Vitalis T, Seif I, De Maeyer E, Sotelo C, Gaspar P. Lack of barrels in the somatosensory cortex of monoamine oxidase A-deficient mice: role of a serotonin excess during the critical period. Neuron. 1996;16:297–307. doi: 10.1016/s0896-6273(00)80048-3. [DOI] [PubMed] [Google Scholar]
- Dunphy WG, Brizuela L, Beach D, Newport J. The Xenopus cdc2 protein is a component of MPF, a cytoplasmic regulator of mitosis. Cell. 1988;54:423–431. doi: 10.1016/0092-8674(88)90205-x. [DOI] [PubMed] [Google Scholar]
- Fukumoto T, Blakely R, Levin M. Serotonin transporter function is an early step in left-right patterning in chick and frog embryos. Dev Neurosci. 2005;27:349–363. doi: 10.1159/000088451. [DOI] [PubMed] [Google Scholar]
- Ganapathy V, Leibach FH. Placental biogenic amines and their transporters. In: Ramasaastry BV, editor. Placental Toxicology. CRC Press; Boca Raton: 1995. pp. 161–174. [Google Scholar]
- Ganapathy V, Leibach FH. Human placenta: a direct target for cocaine action. Placenta. 1994;15:785–795. doi: 10.1016/s0143-4004(05)80181-x. [DOI] [PubMed] [Google Scholar]
- Greenberg BD, Tolliver TJ, Huang S-J, Li Q, Bengel D, Murphy DL. Genetic variation in the serotonin transporter promoter region affects serotonin uptake in human blood platelets. Am J Med Genet. 1999;88:83–87. [PubMed] [Google Scholar]
- Hahn T, Barth S, Weiss U, Mosgoeller W, Desoye G. Sustained hyperglycemia in vitro down-regulates the GLUT1 glucose transport system of cultured human term placental trophoblast: a mechanism to protect fetal development? FASEB J. 1998;12:1221–1231. doi: 10.1096/fasebj.12.12.1221. [DOI] [PubMed] [Google Scholar]
- Hansson SR, Mezey E, Hoffman BJ. Serotonin transporter messenger RNA expression in neural crest-derived structures and sensory pathways of the developing rat embryo. Neuroscience. 1999;89:243–265. doi: 10.1016/s0306-4522(98)00281-4. [DOI] [PubMed] [Google Scholar]
- Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, Lesch KP. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Hendricks TJ, Fyodorov DV, Wegman LJ, Lelutiu NB, Pehek EA, Yamamoto B, Silver J, Weeber EJ, Sweatt JD, Deneris ES. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003;37:233–247. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- Hendricks T, Francis N, Fyodorov D, Deneris ES. The ETS domain factor Pet-1 is an early and precise marker of central serotonin neurons and interacts with a conserved element in serotonergic genes. J Neuroscience. 1999;19:10348–10356. doi: 10.1523/JNEUROSCI.19-23-10348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kekuda R, Leibach FH, Furesz TC, Smith CH, Ganapathy V. Polarized distribution of interleukin-1 receptors and their role in regulation of serotonin transporter in placenta. J Pharmacol Exp Ther. 2000;292:1032–41. [PubMed] [Google Scholar]
- Kilic F, Rudnick G. Oligomerization of 5HT transporter and its functional consequences. Proc Natl Acad Sci USA. 2000;97:3106–3111. doi: 10.1073/pnas.060408997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilic F, Murphy D, Rudnick G. A Human 5HT transporter mutation causes constitutive activation of transport activity. Mol Pharmacol. 2003;64:4–12. doi: 10.1124/mol.64.2.440. [DOI] [PubMed] [Google Scholar]
- Kocabas A, Rudnick G, Kilic F. Functional consequences of Homo- But Not Hetero-Oligomerization between transporters for the biogenic amine neurotransmitters. J Neurochem. 2003;85:1513–1520. doi: 10.1046/j.1471-4159.2003.01793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauder JM, Tamir H, Sadler TW. Serotonin and morphogenesis. I. Sites of serotonin uptake and -binding protein immunoreactivity in the midgestation mouse embryo. Development. 1988;102:709–720. doi: 10.1242/dev.102.4.709. [DOI] [PubMed] [Google Scholar]
- Lauder JM. Neurotransmitters as growth regulatory signals: role of receptors and second messengers. Trends Neurosci. 1993;16:233–240. doi: 10.1016/0166-2236(93)90162-f. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Liu JP, Lauder JM. S-100 beta and insulin-like growth factor-II differentially regulate growth of developing serotonin and dopamine neurons in vitro. J Neurosci Res. 1992;33:248–256. doi: 10.1002/jnr.490330208. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Neurotransmitter synthetic enzymes. Prog Neurobiol. 1973;2:69–117. doi: 10.1016/0301-0082(73)90007-5. [DOI] [PubMed] [Google Scholar]
- Ogura K, Sakata M, Yamaguchi M, Kurachi H, Murata Y. High concentration of glucose decreases glucose transporter-1 expression in mouse placenta in vitro and in vivo. J Endocrinol. 1999;160:443–452. doi: 10.1677/joe.0.1600443. [DOI] [PubMed] [Google Scholar]
- Ozaslan D, Wang S, Ahmed B, Bene A, Kocabas AM, Kilic F. Glycosyl modification facilitates homo- and hetero-oligomerization of serotonin transporter. A specific role for the sialic acid residues. J Biol Chem. 2003;278:43991–44000. doi: 10.1074/jbc.M306360200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Prasad PD, Leibach FH, Mahesh VB, Ganapathy V. Human placenta as a target organ for cocaine action: interaction of cocaine with the placental serotonin transporter. Placenta. 1994;15:267–278. doi: 10.1016/0143-4004(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Baumen AL, Moore KR, Han H, Yang-Fen T, Chang AS, Ganapathy V, Blakely RD. Anti-depressant- and cocaine-sensitive human serotonin transporter: molecular cloning, expression, and chromosomal localization. Proc Natl Acad Sci USA. 1993;90:2542–2546. doi: 10.1073/pnas.90.6.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy JD, Ramamoorthy S, Leibach FH, Ganapathy V. Antidepressant- and cocaine-sensitive human the 5HT transporter: molecular cloning, expression, and chromosomal localization. Am J Obstet Gynecol. 1995;173:1782–1787. [Google Scholar]
- Renold AE, Stauffacher W, Cahill GF. Diabetes Mellitus. In: Stanbury JB, Wyngaarden JB, Fredricson DS, editors. The Metabolic Basis of Inherited Disease. McGraw-Hill; New York: 1977. [Google Scholar]
- Salichon N, Gaspar P, Upton AL, Picaud S, Hanoun N, Hamon M, De Maeyer E, Murphy DL, Mossner R, Lesch KP, Hen R, Seif I. Excessive activation of serotonin (5-HT) 1B receptors disrupts the formation of sensory maps in monoamine oxidase a and 5-ht transporter knock-out mice. J Neurosci. 2001;21:884–896. doi: 10.1523/JNEUROSCI.21-03-00884.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid JA, Scholze P, Kudlacek O, Freissmuth M, Singer EA, Sitte HH. Oligomerization of the human 5HT transporter and of the rat GABA transporter 1 visualized by fluorescence resonance energy transfer microscopy in living cells. J Biol Chem. 2001;276:3805–3810. doi: 10.1074/jbc.M007357200. [DOI] [PubMed] [Google Scholar]
- Smith CH, Moe AJ, Ganapathy V. Nutrient transport pathways across the epithelium of the placenta. Annu Rev Nutr. 1992;12:183–206. doi: 10.1146/annurev.nu.12.070192.001151. [DOI] [PubMed] [Google Scholar]
- Swenne I. Islet cell thymidine kinase activity as indicator of islet cell proliferation in rat pancreas. Diabetes. 1990;39:70–5. doi: 10.2337/diacare.39.1.70. [DOI] [PubMed] [Google Scholar]
- Vitalis T, Cases O, Callebert J, Launay JM, Price DJ, Seif I, Gaspar P. Effects of monoamine oxidase A inhibition on barrel formation in the mouse somatosensory cortex: determination of a sensitive developmental period. J Comp Neurol. 1998;393:169–184. doi: 10.1002/(sici)1096-9861(19980406)393:2<169::aid-cne3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Wall SC, Innis RB, Rudnick G. Binding of the cocaine analog 2 beta-carbomethoxy-3 beta-(4-[125I]iodophenyl)tropane to serotonin and dopamine transporters: different ionic requirements for substrate and 2 beta-carbomethoxy-3 beta-(4-[125I]iodophe-nyl)tropane binding. Mol Pharmacol. 1993;43:264–270. [PubMed] [Google Scholar]
- Walther DJ, Bader M. Serotonin synthesis in murine embryonic stem cells. Mol Brain Res. 1999;68:55–63. doi: 10.1016/s0169-328x(99)00046-7. [DOI] [PubMed] [Google Scholar]
- Weiss U, Cervar M, Puerstner P, Schmut O, Haas J, Mauschitz R, Arikan G, Desoye G. Hyperglycemia in vitro alters the proliferation and mitochondrial activity of the choriocarcinoma cell lines BeWo, JAR and JEG-3 as models for human first-trimester trophoblast. Diabetologia. 2001;44:209–219. doi: 10.1007/s001250051601. [DOI] [PubMed] [Google Scholar]
- Ye W, Shimamura K, Rubenstein JL, Hynes MA, Rosenthal A. FGF and Shh signals control dopaminergic and serotonergic cell fate in the anterior neural plate. Cell. 1998;93:755–766. doi: 10.1016/s0092-8674(00)81437-3. [DOI] [PubMed] [Google Scholar]