Abstract
Lassa virus (LASV) is responsible for the deaths of thousands of people in West Africa annually. Genetic diversity among LASV strains is the highest among the Arenaviridae and represents a great challenge for vaccine development. Guinea pigs vaccinated with a ML29 reassortant vaccine experienced sterilizing immunity and complete protection when challenged on day 30 either with homologous virus or with the distantly-related Nigerian isolate. Simultaneous vaccination-challenge or challenge on day 2 after vaccination also protected 60-100% of the animals against both strains, but without sterilizing immunity. These results indicate that simultaneous replication of ML29 and LASV attenuates the virulence of LASV infection.
1. Introduction
Lassa virus (LASV) is the cause of wide-spread human infection in West Africa that occasionally develops into Lassa fever (LF) and responsible for the deaths of thousands of people each year. Based on epidemiology of the disease in Sierra Leone, Guinea, and Nigeria the “at risk” LASV sero-negative population in these countries may be as high as 59 million with an annual incidence of illness of 3 million [1]. In general population the case fatality rate is 1-2%. However, among hospitalised patients this rate may be as high as 30-50% [2]. The sizeable disease burden, numerous imported cases world-wide [3], and the possibility that LASV can be used as an agent of biological warfare [4] make a strong case for vaccine development.
A killed vaccine tested in non-human primates elicited antibodies to major structural proteins of LASV but did not protect animals against challenge [5]. Controlled clinical trials failed to show protective efficacy of human convalescent plasma [6]. In addition, there is a solid body of evidence that the clearance of LASV and recovery of LF patients is not dependent on antibody responses but is associated with cell-mediated immunity [7, 8]. Live replication-competent vaccines elicit strong cellular immune responses, confer long-term protection after a single injection, and have low manufacturing costs [9]. Two vaccines recently licensed by the FDA, FluMist (MedImmune Vaccines, Inc.) and RotaTeq (Merck & Co.), are live attenuated vaccines based on reassortant technology. We used this technology to make the LASV vaccine candidate, clone ML29 [10-12].
LASV, like other members of the Arenaviridae, has a bi-segmented (L and S) single-stranded RNA genome [13, 14], and each segment contains two genes in ambisense orientation. The L RNA encodes a large protein, L, or RNA-dependent RNA polymerase (RdRp) [15], and a small zinc-binding, Z protein [16]. The S RNA encodes the major structural proteins, nucleoprotein (NP) and glycoprotein precursor (GPC), cleaved into GP1 and GP2 [17-19]. The ML29 vaccine candidate encodes the nucleocapsid (NP) and glycoprotein (GPC) of LASV (Josiah/SL/76/H) and the Z protein and RNA polymerase (RdRp) of Mopeia virus (MOPV-AN20410), a non-pathogenic relative of LASV [10-12]. After a single inoculation, the ML29 reassortant completely protected guinea pigs against LASV-Josiah. In rhesus macaques the vaccine candidate was well attenuated and induced specific T cells capable of secreting IFN-γ in response to homologous and heterologous viruses [11].
In vitro reassortment studies with LCMV, the prototype arenavirus, showed that virulence for guinea pigs is determined by the L RNA [20]; similarly, it is likely that attenuated phenotype of ML29 is due to its MOPV-derived L segment [10, 11]. Natural reassortment between arenaviruses has not yet been described [21-23], though in vitro reassortants were well documented [10, 20, 24]. There are several examples of reassorting in the wild for the Bunyaviridae [25-27]. The existence of natural reassortment raises the concern that vaccination with an attenuated reassortant vaccine, in the presence of the virulent strain, will potentially accelerate disease spread.
LASV species represent a diverse group of viruses with overall strain variation as high as 27% at the nucleotide level [28]. LASV isolates comprise four phylogenetic lineages, three of which are found in Nigeria, a fourth in Guinea, Liberia, and Sierra Leone, and recently, a fifth lineage has been proposed for the AV strain isolated from a patient infected in Ghana or Ivory Coast [29]. The NP is the most variable gene among genes encoding major LASV antigens [28]. The highest sequence differences (base on partial NP sequences) were found between lineages II (803213/NIG/74/H) and IV (JOS/SL/76/H), 11.0-14.4%. We used these LASV isolates herein to determine whether the ML29 vaccine can protect from heterologous challenge.
Here we demonstrate that guinea pigs vaccinated with the reassortant ML29 have been completely protected from challenge with a distantly-related LASV strain. This protection seems to be associated with cell-mediated immunity that completely cleared the challenge virus from tissues. We have also shown that simultaneous replication of ML29 and LASV (Josiah/SL or 803213/NIG) resulted in attenuation of acute LASV infection such that 60-100% of the animals were protected. This current study is consistent with others [30, 31], that show that an attenuated reassortant diminishes replication of concurrent virulent viruses.
(This work was presented in part at the 14th International Symposium on HIV & Emerging Infectious Diseases, Toulon, France, June 21-23, 2006).
2. Materials and methods
2.1. Virus strains
LASV strains, Josiah/SL and LASV-803213, were obtained from Centers for Disease Control (Atlanta, GA). LCMV strain WE was received from Peter Jahrling (USAMRIID, Fort Detrick, Frederick, MD). A MOP/LAS reassortant (clone ML29) was previously described [10-12]. All work with infectious samples was performed within the maximum containment laboratory at the Southwest Foundation for Biomedical Research in San Antonio, Texas. The viruses were grown on Vero E6 cells cultured in Dulbecco's modified minimum Eagle's medium (DMEM, GIBCO-BRL) with 2% fetal calf serum (FCS, GIBCO-BRL), 1% penicillin-streptomycin, and L-glutamine (2 mM) at 37° C in 5% CO2 [11].
2. 2. Animals, challenge experiments, histology
Strain 13 guinea pigs (300-500 g, female) were purchased from USAMRIID. Animals (4-6 guinea pigs per group, Table 1) were vaccinated with clone ML29 (day 0) and challenged on day 30 (conventional immunization, groups 5-9), on day 0 (simultaneous vaccination-challenge, groups 10-12, subgroup “a”), or on day 2 after vaccination (subgroup “b”). The vaccine and challenge virus were injected subcutaneously (s.c.) into two separate sites on the back of animals in 0.5 ml of phosphate buffered saline (PBS). The LASV-Josiah strain has been considered a “homologous” challenge because the ML29 genome contains the S RNA segment derived from LAS-Josiah [10, 11], and LASV-803213/NIG strain has been considered a “heterologous” challenge because its sequence is distantly related to that of LASV-Josiah [28]. Control groups of animals were injected with PBS instead of the ML29 vaccine (groups 1-4, Table 1) or instead of challenge virus (groups 5-6). The animals were observed twice daily for clinical manifestations according to an approved scoring sheet (decreased activity, ruffled fur, loss of weight, labored breathing, hunched posture). Death or survival past 21 days was defined as an endpoint [32, 33]. Previously we showed that animals past this time point did not change survival outcome during the extended follow-up period [11]. Animals which survived on day 21 or animals which met euthanasia criteria (fever, weakness, labored breathing, >25% loss in weight) were euthanized and sacrificed for histological studies as previously described [11].
Table 1.
Results of vaccination and challenge experiments in guinea pigs
| Animal Group |
Challenged virus |
Dose, PFU |
Vacc/chal interval, days a |
No. survived/ No. infected |
Survival, % |
Day of death |
|---|---|---|---|---|---|---|
| No vaccination | ||||||
| 1. | LASV-Jo | 10e+1 | na b | 0/4 | 0 | 15-17 |
| 2. | LASV-Jo | 10e+3 | na | 0/5 | 0 | 15-16 |
| 3. | LASV-803213 | 10e+3 | na | 0/5 | 0 | 13-15 |
| 4. | LCMV-WE | 10e+3 | na | 0/5 | 0 | 13-14 |
| The ML29 conventional vaccination (challenge on day 30) | ||||||
| 5. 10e+2 | no challenge | na | na | 6/6 | 100 | na |
| 6. 10e+6 | no challenge | na | na | 6/6 | 100 | na |
| 7. 10e+3 | LASV-Jo | 10e+3 | 30 | 6/6 | 100 | na |
| 8. 10e+3 | LASV-803213 | 10e+3 | 30 | 5/5 | 100 | na |
| 9. 10e+3 | LCMV-WE | 10e+3 | 30 | 0/6 | 0 | 16-21 |
| The simultaneous vaccination/challenge experiments (challenge on day 0 and 2) | ||||||
| 10a.10e+6 | LASV-Jo | 10e+1 | 0 | 5/5 | 100 | na |
| 10b.10e+6 | LASV-Jo | 10e+1 | 2 | 3/5 | 60 | 10c, 15 |
| 11a.10e+6 | LASV-Jo | 10e+3 | 0 | 4/4 | 100 | na |
| 11b.10e+6 | LASV-Jo | 10e+3 | 2 | 4/5 | 80 | 10 c |
| 12a.10e+2 | LASV-Jo | 10e+3 | 0 | 3/4 | 75 | 14 |
| 12b.10e+2 | LASV-Jo | 10e+3 | 2 | 3/4 | 75 | 16 |
| 13. 10e+6 | LASV-803213 | 10e+3 | 0 | 3/5 | 60 | 12, 17 |
| 14. 10e+6 | LCMV-WE | 10e+3 | 0 | 0/5 | 0 | 14-16 |
Animals were s. c. vaccinated with the ML29 reassortant (day 0) and challenged simultaneously on day 0, 2, 30 after vaccination. Death or survival past 21 days was set up as an endpoint. Amino acid difference between LASV-Jo and LASV-803213 is the highest within LASV genetic lineages I-IV (9).
Non applicable.
Non-LASV-specific death (inappropriate anesthesia).
2. 3. Aspartate aminotransferase (AST) in plasma, viremia, and viral load in tissues
AST levels in plasma were measured using the Premiere Plus photometer system (Stanbio Laboratory, Inc., San Antonio, TX). Viremia and viral load in tissues were measured by quantitative RT-PCR (qRT-PCR). For detection of viral RNA in the blood of experimental animals, viral RNA was extracted according to the manufacturer's recommendations from 500 μl of EDTA treated blood using the Ribopure Blood kit (Ambion, Austin, TX). Total RNA was extracted from tissue samples that were stored in RNAlater. Briefly, 100 mg of tissue was transferred to a screw top microcentrifuge tube containing Trizol Reagent (Invitrogen, Carlsbad, CA) and a 5 mm stainless steel bead (Qiagen Inc., Valencia, CA). The tissue was then homogenized using the TissueLyser (Qiagen) homogenization system per manufacturer's recommendations. RNA was then extracted from the homogenate following the Trizol Reagent protocol described by Invitrogen. Real-time qRT-PCR was performed using an ABI PRISM 7700 Sequence Detection System (Applied Biosystems, Foster City, CA) and RNA Ultrasense one-step real-time qRT-PCR system (Invitrogen) according to manufacturer's recommendations. The primer/probe set for LASV-Josiah RNA targeted a GPC region of the S segment using LASV 36E2 and 80F2 primers [34]. The primer/probe set for LASV 803213 amplified a region of the L segment (FWD 5'- CGA CCA CAG CAA TGT TGA AG-3'; REV 5'- CCT ACC AAT TGG GAT GTG AG-3'; Probe 6FAM - TCT CTG TCT TAC AAG GAG CAA GTG – TAMRA). The qRT-PCR reaction contained 5 μM of each primer and 2.5 μM of probe for each primer/probe set in a final reaction volume of 25 μl. The reaction conditions were as follows: 50°C for 2 min, 95°C for 2 min then 40 cycles alternating between 60°C for 1 min and 95°C for 15 seconds. Standards used in qRT-PCR were generated from serial 10-fold dilutions of RNA isolated from LASV-Josiah and LASV-803123 virus stocks (10−1 PFU/ml - 10−7 PFU/ml) that were enumerated in triplicate by conventional plaque assay as previously described [11]. Sensitivity of qRT-PCR was around 100 viral RNA copies per ml of blood or per g of tissue.
2. 4. IgG ELISA, neutralization assay, western blot
IgG ELISA was performed as previously described [35]. Briefly, the ML29 antigen was prepared from serum-free culture medium of ML29-infected Vero E6 cells by ultracentrifugation in Beckman Optima L90K (SW 28, 27,000 rpm, 3 hrs). The virus was suspended in carbonate-bicarbonate buffer (pH 9.6) and 100 μl of viral antigen was used to cover wells of microtitration plates (overnight at 4° C). Serum from LASV-infected individuals [36] was used as a positive control. Neutralizing antibodies were determined by plaque reduction neutralization assay using constant doses of virus, Vero E6 cell monolayers and serial dilutions of plasma [35]. Western blotting was performed as previously described [37, 38]. Briefly, samples of the purified ML29 reassortant was resuspended in SDS-PAGE sample buffer, incubated at 100° C for 5 min and separated in 12% polyacrylamide gels. Resolved proteins were transferred to membranes and blocked overnight with 5% skim milk in PBST (0.05% Tween 20 in PBS). Blots were incubated with primary antibodies (1:100) for 1 h at room temperature, washed three times with PBST, incubated with secondary anti-rabbit IgG conjugated to alkaline phsphotase (KPL, Gaithersburg, MD) for 30 min, washed, and incubated with NBT/BCIP (Invitrogen). Developed blots were exposed to X-ray film.
2.5. Histological studies
For light microscopy, aseptically removed tissues were fixed in buffered formalin solution overnight, dehydrated, and embedded in paraffin. The tissues were sectioned and stained with hematoxylin and eosin (H&E). Tissue lesions were graded from 0 (normal) to 5 (severe) and average of tissue lesion was calculated for each experimental range.
2. 6. Data analysis
Statistical analyses and graphing were performed using the Origin 6.0 package (Microcal Software, Inc., Northampton, MA).
3. Results
3. 1. The ML29 reassortant provides complete protection from 30 day post vaccination challenge with a distantly-related Nigerian LASV isolate
Strain 13 guinea pigs are a useful small-animal model resembling human LF [32, 39]. In our experiments these animals were s.c. vaccinated with the ML29 or they received saline (as a control). The vaccinated guinea pigs were challenged on day 30 with one of two strains of LASV (Josiah/SL/76/H, 80321/NIG/74/H) or with LCMV-WE using the same route of inoculation.
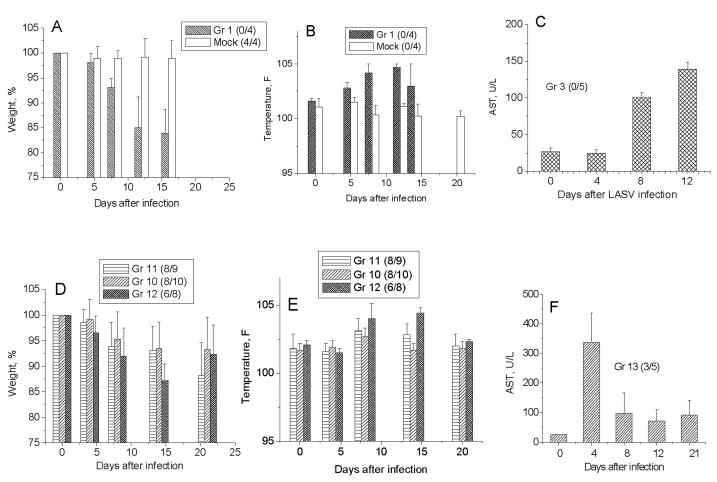
All control animals died 2 weeks after LASV infection (Table 1). The most prominent manifestations of the disease included fever, weight loss and elevated liver enzymes (Fig. 1A-C). The WE strain of LCMV also killed all guinea pigs. The animals died within 2 weeks with hind-limb paralysis immediately preceding death. All ML29-vaccinated animals were completely protected against challenge with two LASV strains, Josiah/SL (group 7) and 803213/NIG (group 8), and had no clinical manifestations of the disease. However, ML29-vaccinated animals were not protected from challenge with LCMV-WE, although vaccinated animals lived longer than animals in the control group, 16-21 days vs 13-14 days (group 9, Table 1). These observations indicate that ML29 vaccination can induce some levels of cross-protection against LCMV-WE.
Fig. 1.
Temperature, weigh and AST levels in plasma of strain 13 guinea pigs. A - C, acute LASV infection. D – F, vaccinated and challenged animals. Numbers of surviving animals to numbers of challenged animals are indicated in brackets.
3. 2. The ML29 vaccination and challenge on day 0 or day 2 protected 60-100% of animals from LASV strains
Several examples of natural reassortment between genetically-related bunyaviruses raised the concern that vaccination with an attenuated reassortant vaccine in endemic areas will affect the natural pattern of the disease and enhance pathogenicity. To address this concern we minimized vaccine-to-challenge intervals to day 0 (simultaneous challenge-vaccination) and day 2. We also used high and low doses of the ML29 vaccine and the challenge virus.
As seen in Table 1, all animals vaccinated with 1 × 106 PFU of ML29 survived after simultaneous challenge with low (group 10a) or high (group 11a) doses of LASV-Josiah. Importantly, the vaccination also protected 60% animals from challenge with high dose of the distantly-related strain, LASV-803213/NIG (group 13). Vaccination with low dose, 1 × 102 PFU of ML-29, protected 75% animals against challenge with high dose of LASV-Josiah. Vaccination two days before challenge was also effective protecting more than 60-80% of the guinea pigs against death. As seen in Table 1, two animals from group 10b and 11b (challenge on day 2) did not recover after anesthesia during blood sample collection on day 10. Low levels of viral RNA loads in tissues of these animals (not shown) suggest that the death of these animals was not LASV-associated.
Vaccination on day 0 or shortly before challenge protected animals from lethal outcome but did not protect from disease. As seen from Fig. 1D-F, vaccinated animals lost weight and had fever. However, these clinical parameters were not as severe as in the non-vaccinated group 1 and resulted in partial recovery on day 21. ML29 vaccination did not protect guinea pigs from simultaneous challenge with LCMV-WE (Table 1, group 14).
3. 3. Viremia, viral load in tissues, and humoral immune responses
Blood samples of vaccinated animals collected after challenge as well as blood samples and tissues collected at necropsy were used for virus titration and RNA isolation, for subsequent virus detection by plaque assay [11] and by qRT-PCR. In preliminary studies to facilitate and optimize rapid processing of samples in BSL4 facility we established a qRT-PCR for measurement of viral RNA concentration in plasma and tissue samples. RNA standards used in qRT-PCR were extracted from serial dilutions of infectious LASV-Josiah and LASV-803213 stocks as described in “Materials and methods”. In validation experiments (not shown) we have found a good correlation between the numbers of RNA copies and infectious titers. The ration RNA/PFU in our experiments varied from 80 to 200 confirming the previous data that each virion contains more that one copy of virus-specific sequences [14, 40]. RNA from defective particles seems also to contribute to this ratio [41]. Asper et al. [42] established the similar ration of S RNA molecules to infectious particles for AV strain of LASV.
In ML29-vaccinated animals challenged on day 30 with LASV-Josiah or with distantly-related LASV-803213/NIG (Fig. 2A, group 3 vs group 8) viremia was not detectable by conventional plaque assay or by qRT-PCR at all tested time-points, on day 4, 8, 12, 21. Co-cultivation of plasma samples with Vero cells and subsequent plaque titration did not result in virus recovery and virus was also not detected in target tissues (Fig. 2B, group 8). Therefore, this demonstrates that those animals challenged 30 days after vaccination were not only completely protected against disease but also against LASV infection suggesting that vaccination with ML29 induces sterilizing immunity.
Fig. 2.
Viremia, viral load in tissues, and humoral immune responses in vaccinated animals. A, viremia in vaccinated and control animals at different time points after challenge with LASV-803213/NIG. B, viremia and viral load in tissues of sacrificed guinea pigs. All animals died in control group (# 3). However, RNA samples were available only from blood and tissues of 3 animals. C, IgG ELISA. Plasma samples were collected at different time points from animals vaccinated with ML29 and anti-LASV IgG antibodies were determined by ELISA. D, western blotting. Plasma samples from three ML29-vaccinated animals were collected at day 42 after vaccination and incubated with antigen blots as described in Materials and Methods. Lane 1-3, blots were probed with plasma samples from vaccinated animals; lane 4, the blot was incubated with human reconvalescent anti-LASV serum [36]. NP and GP2, positions of LASV structural proteins.
It has been observed that in partially protected animals (groups 10-13, Table 1) clinical parameters were not as severe as in non-vaccinated animals (group 1) and partial recovery was seen on day 21. This was also seen in plasma AST levels in animals from group 13 (ML29 + challenge with LASV-803213/NIG on day 0) compared to nonvaccinated infected animals from group 3 (Fig. 1C, F). Two major risk factors predicting fatal outcome of LF patients are high viremia (≥103.6 TCID50/ml) and plasma AST levels (≥150 IU/ml) [6]. As seen in Fig. 2B, high viremia (>4 log10 viral RNA copies/ml) was associated with lethal outcome in group 3 and in group 13. At necropsy, in these animals viral load in target tissues even exceeded viremia levels. In surviving animals from group 13, viremia was undetectable or moderate (<3 log10 RNA/ml). The effect of vaccination on viremia (group 5 vs group 13) was statistically significant (<0.05, Student t test). In the surviving animal #26279 viremia was moderate while viral load in tissues was high and comparable to viral loads in non-surviving animals #26295 and #26297. These observations emphasize the prognostic value of LASV viremia levels in strain 13 guinea pigs.
Non-neutralizing IgG ELISA antibodies against LASV, predominantly anti-NP, were detectable at the end of the 1st week of vaccination and peaked shortly after challenge (Fig. 2C, D). As we previously showed [11], ML29 immunization induces robust T cell responses in the rodent model and in rhesus macaques. The effective virus clearance with ML29-specific T cells seems to be the main mechanism of protection during the “conventional” protocol, immunization and challenge on day 30.
3. 4. Histopathology
In spite of lethal outcome, histological lesions in experimental animals infected with LASV and in LF patients were generally mild [32, 43]. In guinea pigs, severe LASV infection was associated with interstitial pneumonia, edema, necrotizing hepatitis with hepatic vacuolization and lipidosis. Animals vaccinated with ML29 and challenged on day 30 with LASV-Josiah had no histological alterations as observed during the necropsy performed at day 70 after challenge [11]. Also, we did not see histological abnormalities in animals fully protected against LASV-803213/NIG (not shown). In partially protected animals challenged on day 0 or day 2 histological signs of acute LASV infection were observed (Table 2). Mild pathology (inflammation, necrosis, hemorrhage, and lipidosis) was seen in liver sections from vaccinated groups 10-12. In lung tissues of animals from group 11 (vaccination with 106 PFU and challenged with 103 PFU) and from group 12 (vaccination with 102 PFU and challenge with 103 PFU) signs of inflammation were even more severe that in the control group 1 (acute LASV infection). Jahlring et al. [39] also found that lung was the most “sensitive” target tissue for LASV infection in strain 13 guinea pigs. Spleen and mesenteric lymph nodes showed no significant differences in lesions between vaccinated and control animals.
Table 2.
Pathology summary of vaccination-challenge experiments
| Tissue/ Pathology |
Animal Groups: | |||
|---|---|---|---|---|
| 1 | 10a, b | 11a, b | 12a, b | |
| LIVER: | ||||
| inflammation | 1.8 a | 0.2, 1.0 | 0.7, 0.8 | 0.7, 1.6 |
| necrosis | 2.3 | 0.2, 1.5 | 0.7, 0.4 | 0.5, 2.0 |
| hemorrhage | 0.3 | 0.0, 0.0 | 0.2, 0.0 | 0.0, 0.0 |
| lipidosis | 3.0 | 0.2, 1.0 | 0.8, 2.2 | 2.5, 2.6 |
| LUNG: | ||||
| inflammation | 1.5 | 0.2, 1.0 | 3.0, 0.8 | 3.5, 3.0 |
| necrosis | 0.5 | 0.0, 0.2 | 0.6, 0.0 | 1.0, 1.0 |
| hemorrhage | 0.0 | 0.2, 0.0 | 0.0, 0.4 | 0.0, 0.0 |
| edema | 4.0 | 2.4, 0.2 | 4.3, 0.6 | 0.2, 3.0 |
| SPLEEN: | ||||
| inflammation | 0.0 | 0.0, 0.0 | 0.0, 0.0 | 0.0, 0.0 |
| necrosis | 0.5 | 0.3, 0.0 | 0.6, 0.7 | 0.7, 0.3 |
| hemorrhage | 0.0 | 0.0, 0.0 | 0.3, 0.0 | 0.2, 0.0 |
| depletion | 1.5 | 1.0, 0.7 | 1.3, 2.0 | 2.2, 2.3 |
| MLN: | ||||
| inflammation | 0.5 | 0.0, 0.2 | 0.0, 0.0 | 0.5, 0.3 |
| necrosis | 2.0 | 0.0, 0.5 | 0.5, 0.5 | 1.5, 0.3 |
| hemorrhage | 0.0 | 0.6, 0.0 | 0.0, 0.0 | 1.2, 0.0 |
| depletion | 3.0 | 1.0, 0.5 | 2.0, 2.7 | 1.5, 2.0 |
Average of tissue lesion. The extent of tissue lesions was quantified on H&E-stained sections using 5 randomly selected areas per sections and 3 sections per tissue. Grade: 0 = normal; 1 = minimal; 2 = mild; 3 = moderate; 4 = moderate/severe; 5 = severe.
Histological lesions between animals infected with LASV-Josiah and with LASV-803213/NIG were similar. Degenerative changes including hepatocellular cytoplasmic vacuolization, disruption of hepatocellular cords and sinuses with minimal individual hepatocellular necrosis and minimal increase in numbers of lymphocytes in portal areas were seen in liver sections of guinea pigs that died from LASV-803213/NIG infection. In lung, interstitial mixed cell inflammation with mild necrosis and edema were the most prominent histological alterations while tissues of protected animals had non-essential or mild alterations.
4. Discussion
The sequence variation among LASV strains is the highest among arenaviruses and this finding initially raised concern about the status of LASV as a single species [28, 44, 45]. Based on the partial NP sequences of 54 strains of LASV, Bowen et al. [28] showed that LASV isolates comprise four lineages, three of which are found in Nigeria and the fourth in Guinea, Liberia, and Sierra Leone. Recently, a fifth lineage which falls between III and IV has been proposed for the AV strain isolated from a patient infected presumably in Ghana or Ivory Coast [29]. The NP is the most variable antigen and NP protein diversity between the LASV-Josiah and LASV-803213/NIG is the highest among all LASV genetic lineages, 11.0-14.4% (at the amino acid level). In fact, the Nigerian (LP, 803213, GA391) and Sierra Leonean (Josiah) LASV strains are almost as different from each other as they are from MOPV [46]. Here we have shown that vaccination of strain 13 guinea pigs with reassortant vaccine candidate ML29 completely protected animals challenged 30 days post vaccination with LASV-Josiah and the distantly-related Nigerian strain, LASV-803213/NIG.
Reassortant analysis indicates that the S RNA of LCMV and LASV encode the major antigens eliciting protective immunity. LCMV NP expresses the immunodominant CTL epitope (NP 118-126 aa) for H-2d mice [47]. Within the epitope, a minimal tetrapeptide GVYM is identical for LCMV, LASV, MOPV and plays an important role in CTL recognition [48]. Recombinant LASV NP protein expressed in vaccinia or in Salmonella protected 33% of mice from LCMV challenge and the protection was associated with cross-reactive CTL and proliferative responses [49, 50]. Similarly, a DNA vaccine expressing LASV NP showed cross-protection against LCMV and Pichinde virus (PICV) challenges in mice; however DNA vaccination with a single epitope (LASV NP 118-126) protected mice from LCMV but not from PICV intracerebral challenge [51]. Cross-protection against LCMV challenge was also induced by LASV GP2-derived peptide, 403-417 aa, corresponding to a highly homologous sequence on the glycoprotein gene of LASV and LCMV [52]. It has been shown that cross-reactive GP2-derived epitopes overlapping with a predicted fusion peptide [53] are involved in human CD4+ T lymphocyte responses against LASV isolates [54].
We have shown recently that recombinant YF17D expressing LASV GPC derived from LASV-AV strain [29] induced incomplete protection in guinea pigs challenged with LASV-Josiah [38]. This finding suggests that LASV NP is needed to induce full protection in cross-challenge experiments. However, we have to keep in mind that LASV is a rodent-born pathogen and seems to be treated differently by immune system of rodents and non-human primates. For example, LASV NP expressed in vaccinia or in VEE-replicon particles protected 100% guinea pigs against homologous challenge [33, 55]. In contrast, non-human primates vaccinated with vaccinia expressed LASV NP developed high titers of anti-NP antibodies but protected only 27% of vaccinated animals against homologous challenge [56]. Meanwhile, it has been shown that LASV-infected individuals from endemic areas of Guinea had very strong memory CD4+ T-cell responses against the NP of LASV, which were partly strain specific and partly cross-reactive with other LASV strains [57].
Using computer-assisted algorithms, five HLA-A2.1-binding LASV GP peptides and two LASV NP peptides have been identified [58, 59]. HLA-A*0201 transgenic mice immunized with either LASV peptides GPC(42-50) or GPC(60-68) were protected against challenge with a recombinant vaccinia virus that expressed LASV GPC [59]. However, peptide-based vaccination may have severe pathological consequences in individuals recently infected with the virus or in immune individuals previously exposed, perhaps unknowingly, to the pathogen [60]. It is an exactly case for LASV infection in West Africa where 30% infections are asymptomatic and seroprevalence can be as high as 25%-55% [8, 36, 61]. In addition, healthy LASV-exposed individuals who lost their antibodies but had strong proliferative responses to LASV recombinant NP protein were also described in LF endemic areas [54].
In our experiments ML29-vaccinated animals were completely protected against LASV-Josiah/SL and LASV-803213/NIG but not against LCMV-WE challenge. Peters et al. [32] observed cross-protection between LASV-Josiah and LCMV-WE. However, in these experiments the authors used a different immunization protocol. Guinea pigs were immunized with LASV, treated with ribavirin to prevent fatal outcome, and challenged on day 75-90 with LCMV. The influence of vaccine-to-challenge intervals and virus doses on outcome of LASV challenge experiments was demonstrated in non-human primates [56].
Natural reassortment between arenaviruses has not been described so far. Such interaction was suspected but never conclusively demonstrated when LCMV was isolated from rodents captured in endemic areas of Argentine HF [62]. Experiments with Rift Valley fever virus showed that reassortants between the wild-type virus and a live attenuated ML-12 vaccine generated only phenotypes with protective activity against the disease [30]. Molecular epidemiology of infectious bursal disease virus (IBDV) genome segments A and B of a wide panel of natural and vaccine IBDV strains confirmed natural reassortment between strains. Interestingly, the reassortant that exhibited a segment from a natural virulent strain and a segment from a vaccine strain induced significantly less mortality than typical wild-type IBDV [31]. All these data together with our simultaneous vaccination-challenge experiments in guinea pigs indicate that reversion to virulence is unlikely, and further confirm that genetic reassortment with wild-type viruses during a vaccination program in endemic areas should yield attenuated variants.
Similar to the protection we observed with ML29, it has recently been reported that primates inoculated with a VSV-vectored vaccine on the day of lethal challenge are protected from Marburg HF [63]. Work in cell culture with human monocyte-derived macrophage and dendritic cells infected with LASV shows that virulent infections are poorer inducers of pro-inflammatory cytokines in comparison with non-virulent, MOPV infections [64-67], thereby linking higher interferon production to a non-virulent infection. The protection observed in simultaneous-challenge experiments may be similar in mechanism to the high dose interference phenomenon observed in murine experiments that depends on the early induction of type I interferons to activate acquired immune responses. In 1962 John Hotchin [68] described “high dose immune paralysis (HDIP),” in which mice receiving low doses of i.c. LCMV died but mice receiving high doses did not. This phenomenon has been attributed to the production of large amounts of type I interferon that suppresses virus replication [69-71]. A mechanism proposed by Borrow et al. [72] was that high doses (or immunosuppressive variants of LCMV) resulted in large numbers of infected dendritic cells, and these cells consequently produced large amounts of type I interferons that stimulated earlier development of CD8+ mediated immune response. In the mouse, an earlier cell-mediated immune response results in destruction of virus-infected antigen-presenting cells and clonal exhaustion of bystander memory T cells [73] thereby reducing viral loads and immunopathology.
Additional murine experiments have demonstrated that type I interferons confer protection by tipping the balance between viral virulence and the development of protective immunity. Mice given a lethal dose of Ebola virus i.p. could be rescued by a simultaneously-delivered s.c. dose [74] while survival did not occur in an IFN-α knockout mouse [75]. It was shown that infection via the s.c. route elicited higher levels of IFN α/β possibly due to the type of cells favored by this route of infection. A murine model in which lethal i.c. challenge with TACV could be averted by treatment with CpG ODN, a compound that stimulates IFN α/β production through TLR9, demonstrated that both innate and acquired immune mechanisms were involved in protection [76]. Thus, a link exists between direct viral effects on innate immunity and the mechanism of protection by vaccination soon after a lethal exposure.
Acknowledgements
We would like to thank Robert Geiger, Michelle Reynolds, Laurie Condel and Stacey Perez for technical support and helpful discussions. This work was supported by grant RO1-AI52367 (to I. S. L.) from the National Institutes of Health (NIH), a NIH laboratory construction grant 1C06RR12087 (SFBR, BSL-4 laboratory) and the Western Regional Centers of Excellence for Biodefense and Emerging Infectious Diseases (5 U54 AI057156-03 subcontract to J. L. P).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Richmond JK, Baglole D. Lassa fever: epidemiology, clinical features, and social consequences. BMJ. 2003;327(7426):1271–75. doi: 10.1136/bmj.327.7426.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anonymous Update on Lassa fever in West Africa. Weekly Epidemiol Record. 2005;80(10):86–88. [PubMed] [Google Scholar]
- 3.Macher AB, Wolfe MS. Historical Lassa fevers reports and 30-year clinical update. Emerg Infect Dis. 2006;12(5):835–37. doi: 10.3201/eid1205.050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borio L, Inglesby T, Peters CJ, Schmaljohn AL, Hughes JM, Jahrling PB, et al. Hemorrhagic fever viruses as biological weapons: medical and public health management. JAMA. 2002;287(18):2391–405. doi: 10.1001/jama.287.18.2391. [DOI] [PubMed] [Google Scholar]
- 5.McCormick J, Mitchell S, Kiley M, Ruo S, Fisher-Hoch S. Inactivated Lassa virus elicits a non protective immune response in rhesus monkeys. J Med Virol. 1992;31(1):1–7. doi: 10.1002/jmv.1890370102. [DOI] [PubMed] [Google Scholar]
- 6.McCormick JB, King IJ, Webb PA, Scribner CL, Craven RB, Johnson KM, et al. Lassa fever. Effective therapy with ribavirin. N Engl J Med. 1986;314(1):20–26. doi: 10.1056/NEJM198601023140104. [DOI] [PubMed] [Google Scholar]
- 7.Fisher-Hoch S, McCormick J. Towards a human Lassa fever vaccine. Rev Med Virol. 2001;11:331–41. doi: 10.1002/rmv.329. [DOI] [PubMed] [Google Scholar]
- 8.Fisher-Hoch SP, McCormick JB. Lassa fever vaccine. Expert Rev Vaccines. 2004;3(2):103–11. doi: 10.1586/14760584.3.2.189. [DOI] [PubMed] [Google Scholar]
- 9.Monath TP. Yellow fever vaccine. In: Plotkin SA, Orenstein WA, editors. Vaccines. 4th Elsevier, Inc.; 2004. pp. 1095–176. [Google Scholar]
- 10.Lukashevich IS. Generation of reassortants between African arenaviruses. Virology. 1992;188(2):600–05. doi: 10.1016/0042-6822(92)90514-p. [DOI] [PubMed] [Google Scholar]
- 11.Lukashevich IS, Patterson J, Carrion R, Moshkoff D, Ticer A, Zapata J, et al. A live attenuated vaccine for Lassa fever made by reassortment of Lassa and Mopeia viruses. J Virol. 2005;79(22):13934–42. doi: 10.1128/JVI.79.22.13934-13942.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moshkoff D, Salvato MS, Lukashevich IS. Molecular characterization of a reassortant virus derived from Lassa and Mopeia viruses. Virus Genes. 2006 Dec 2; doi: 10.1007/s11262-006-0050-3. DOI:10.1007/s11262-006-0050-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lukashevich IS, Stelmakh TA, Golubev VP, Stchesljenok EP, Lemeshko NN. Ribonucleic acids of Machupo and Lassa viruses. Arch Virol. 1984;79(34):189–203. doi: 10.1007/BF01310811. [DOI] [PubMed] [Google Scholar]
- 14.Lukashevich IS, Salvato MS. Lassa Virus Genome. Current Genomics. 2006;7(6):351–79. [Google Scholar]
- 15.Lukashevich I, Djavani M, Shapiro K, Sanchez A, Ravkov E, Nichol S, et al. The Lassa fever virus L gene: nucleotide sequence, comparison, and precipitation of a predicted 250 kDa protein with monospecific antiserum. J Gen Virol. 1997;78(3):547–51. doi: 10.1099/0022-1317-78-3-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Djavani M, Lukashevich IS, Sanchez A, Nichol ST, Salvato MS. Completion of the Lassa fever virus sequence and identification of a RING finger open reading frame at the L RNA 5′ end. Virology. 1997;235(2):414–18. doi: 10.1006/viro.1997.8722. [DOI] [PubMed] [Google Scholar]
- 17.Auperin DD, Sasso DR, McCormick JB. Nucleotide sequence of the glycoprotein gene and intergenic region of the Lassa virus S genome RNA. Virology. 1986;154(1):155–67. doi: 10.1016/0042-6822(86)90438-1. [DOI] [PubMed] [Google Scholar]
- 18.Clegg JC, Wilson SM, Oram JD. Nucleotide sequence of the S RNA of Lassa virus (Nigerian strain) and comparative analysis of arenavirus gene products. Virus Res. 1991;18(23):151–64. doi: 10.1016/0168-1702(91)90015-n. [DOI] [PubMed] [Google Scholar]
- 19.Lenz O, ter Meulen J, Klenk H-D, Seidah NG, Garten W. The Lassa virus glycoprotein precursor GP-C is proteolytically processed by subtilase SKI-1/S1P. PNAS. 2001;98(22):12701–05. doi: 10.1073/pnas.221447598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riviere Y, Ahmed R, Southern PJ, Buchmeier MJ, Oldstone MB. Genetic mapping of lymphocytic choriomeningitis virus pathogenicity: virulence in guinea pigs is associated with the L RNA segment. J Virol. 1985;55(3):704–09. doi: 10.1128/jvi.55.3.704-709.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emonet S, Lemasson JJ, Gonzalez JP, Lamballerie Xd, Charrel RN. Phylogeny and evolution of old world arenaviruses. Virology. 2006;350(2):251–57. doi: 10.1016/j.virol.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 22.Charrel RN, Lemasson JJ, Garbutt M, Khelifa R, De Micco P, Feldmann H, et al. New insights into the evolutionary relationships between arenaviruses provided by comparative analysis of small and large segment sequences. Virology. 2003;317(2):191–96. doi: 10.1016/j.virol.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 23.Archer A, Rico-Hesse R. High genetic divergence and recombination in Arenaviruses from the Americas. Virology. 2002;304(2):274–81. doi: 10.1006/viro.2002.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L, Marriott KA, Harnis DG, Aronson JF. Reassortant analysis of guinea pig virulence of Pichinde virus variants. Virology. 2001;290(1):30–38. doi: 10.1006/viro.2001.1127. [DOI] [PubMed] [Google Scholar]
- 25.Gerrard SR, Li L, Barrett AD, Nichol ST. Ngari virus is a bunyamwera virus reassortant that can be associated with large outbreaks of hemorrhagic fever in Africa. J Virol. 2004;78(16):8922–26. doi: 10.1128/JVI.78.16.8922-8926.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sall AA, Zanotto PMdA, Sene OK, Zeller HG, Digoutte JP, Thiongane Y, et al. Genetic reassortment of Rift Valley fever virus in nature. J Virol. 1999;73(10):8196–200. doi: 10.1128/jvi.73.10.8196-8200.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hewson R, Gmyl A, Gmyl L, Smirnova SE, Karganova G, Jamil B, et al. Evidence of segment reassortment in Crimean-Congo haemorrhagic fever virus. J Gen Virol. 2004;85:3059–70. doi: 10.1099/vir.0.80121-0. A. [DOI] [PubMed] [Google Scholar]
- 28.Bowen M, Rollin P, Ksiazek T, Hustad H, Bausch D, Demby A, et al. Genetic diversity among Lassa virus strains. J Virol. 2000;74(15):6992–7004. doi: 10.1128/jvi.74.15.6992-7004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gunther S, Emmerich P, Laue T, Kuhle O, Asper M, Jung A, et al. Imported Lassa fever in Germany: molecular characterization of a new Lassa virus strain. Emerg Infect Dis. 2000;6(5):466–76. doi: 10.3201/eid0605.000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saluzzo JF, Smith JF. Use of reassortant viruses to map attenuating and temperature-sensitive mutations of the Rift Valley fever virus MP-12 vaccine. Vaccine. 1990;8(4):369–75. doi: 10.1016/0264-410x(90)90096-5. [DOI] [PubMed] [Google Scholar]
- 31.Le Nouën C, Rivallan G, Toquin D, Darlu P, Morin Y, Beven V, et al. Very virulent infectious bursal disease virus: reduced pathogenicity in a rare natural segment-B-reassorted isolate. J Gen Virol. 2006;87:209–16. doi: 10.1099/vir.0.81184-0. [DOI] [PubMed] [Google Scholar]
- 32.Peters CJ, Jahrling PB, Liu CT, Kenyon RH, McKee KT, Jr, Oro JGB. Experimental studies of arenaviral hemorrhagic fevers. Curr Top Microbiol Immunol. 1987;134:5–68. doi: 10.1007/978-3-642-71726-0_2. [DOI] [PubMed] [Google Scholar]
- 33.Pushko P, Geisbert J, Parker M, Jahrling P, Smith J. Individual and bivalent vaccines based on alphavirus replicons protect guinea pigs against infection with Lassa and Ebola viruses. J Virol. 2001;75(23):11677–85. doi: 10.1128/JVI.75.23.11677-11685.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Demby AH, Chamberlain J, Brown DW, Clegg CS. Early diagnosis of Lassa fever by reverse transcription-PCR. J Clin Microbiol. 1994;32:2898–903. doi: 10.1128/jcm.32.12.2898-2903.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lukashevich IS, Rodas JD, Tikhonov II, Zapata JC, Yang Y, Djavani M, et al. LCMV-mediated hepatitis in rhesus macaques: WE but not ARM strain activates hepatocytes and induces liver regeneration. Arch Virol. 2004;149(12):2319–36. doi: 10.1007/s00705-004-0385-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lukashevich IS, Clegg JC, Sidibe K. Lassa virus activity in Guinea: distribution of human antiviral antibody defined using enzyme-linked immunosorbent assay with recombinant antigen. J Med Virol. 1993;40(3):210–17. doi: 10.1002/jmv.1890400308. [DOI] [PubMed] [Google Scholar]
- 37.Jasenosky LD, Neumann G, Lukashevich I, Kawaoka Y. Ebola virus VP40-induced particle formation and association with lipid bilayer. J Virol. 2001;75:5205–14. doi: 10.1128/JVI.75.11.5205-5214.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bredenbeek PJ, Molenkamp R, Spaan WJM, Deubel V, Marianneau P, Salvato MS, et al. A recombinant Yellow Fever 17D vaccine expressing Lassa virus glycoproteins. Virology. 2006;345(2):299–304. doi: 10.1016/j.virol.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jahrling PB, Smith S, Hesse RA, Rhoderick JB. Pathogenesis of Lassa virus infection in guinea pigs. Infect Immun. 1982;37(2):771–78. doi: 10.1128/iai.37.2.771-778.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lukashevich IS, Lemeshko NN, Stelmakh TA, Golubev VP, Stcheslyenok EP. Some biochemical properties of Lassa virus RNA and polypeptides. Med Microbiol Immunol (Berl) 1986;175(23):73–77. doi: 10.1007/BF02122419. [DOI] [PubMed] [Google Scholar]
- 41.Lukashevich IS, Trofimov NM, Golubev VP, Maryankova RF. Sedimentation analysis of the RNAs isolated from interfering particles of Lassa and Machupo viruses. Acta Virol. 1985;29(6):455–60. [PubMed] [Google Scholar]
- 42.Asper M, Sternsdorf T, Hass M, Drosten C, Rhode A, Schmitz H, et al. Inhibition of different Lassa virus strains by alpha and gamma interferons and comparison with a less pathogenic arenavirus. J Virol. 2004;78(6):3162–69. doi: 10.1128/JVI.78.6.3162-3169.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCormick JB, Fisher-Hoch SP. Lassa fever. Curr Top Microbiol Immunol. 2002;262:75–109. doi: 10.1007/978-3-642-56029-3_4. [DOI] [PubMed] [Google Scholar]
- 44.Lozano ME, Posik DM, Albarino CG, Schujman G, Ghiringhelli PD, G GC, et al. Characterization of arenaviruses using a family-specific primer set for RT-PCR amplification and RFLP analysis. Its potential use for detection of uncharacterized arenaviruses. Virus Res. 1997;49(1):79–89. doi: 10.1016/s0168-1702(97)01458-5. [DOI] [PubMed] [Google Scholar]
- 45.Bowen MD, Peters CJ, Nichol ST. Phylogenetic analysis of the Arenaviridae: patterns of virus evolution and evidence for cospeciation between arenaviruses and their rodent hosts. Mol Phylogenet Evol. 1997;8(3):301–16. doi: 10.1006/mpev.1997.0436. [DOI] [PubMed] [Google Scholar]
- 46.Clegg J. Molecular phylogeny of the Arenaviridae. Curr Top Microbiol Immunol. 2002;262:1–24. doi: 10.1007/978-3-642-56029-3_1. [DOI] [PubMed] [Google Scholar]
- 47.Whitton JL, Tishon A, Lewicki H, Gebhard J, Cook T, Salvato M, et al. Molecular analyses of a five-amino-acid cytotoxic T-lymphocyte (CTL) epitope: an immunodominant region which induces nonreciprocal CTL cross-reactivity. J Virol. 1989;63(10):4303–10. doi: 10.1128/jvi.63.10.4303-4310.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schulz M, Aichele P, Schneider R, Hansen TH, Zinkernagel RM, Hengartner H. Major histocompatibility complex binding and T cell recognition of a viral nonapeptide containing a minimal tetrapeptide. Eur J Immunol. 1991;21(5):1181–85. doi: 10.1002/eji.1830210513. [DOI] [PubMed] [Google Scholar]
- 49.Djavani M, Yin C, Xia L, Lukashevich I, Pauza C, Salvato M. Murine immune responses to mucosally delivered Salmonella expressing Lassa fever virus nucleoprotein. Vaccine. 2000;18(15):1543–54. doi: 10.1016/s0264-410x(99)00439-9. [DOI] [PubMed] [Google Scholar]
- 50.Djavani M, Yin C, Lukashevich I, Rodas J, Rai S, Salvato M. Mucosal immunization with Salmonella typhimurium expressing Lassa virus nucleocapsid protein cross-protects mice from lethal challenge with lymphocytic choriomeningitis virus. J Hum Virol. 2001;4(2):103–08. [PMC free article] [PubMed] [Google Scholar]
- 51.Rodriguez-Carreno MP, Nelson MS, Botten J, Smith-Nixon K, Buchmeier MJ, Whitton JL. Evaluating the immunogenicity and protective efficacy of a DNA vaccine encoding Lassa virus nucleoprotein. Virology. 2005;335(1):87–98. doi: 10.1016/j.virol.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 52.La Posta V, Auperin D, Kamin-Lewis R, Cole G. Cross-protection against lymphocytic choriomeningitis virus mediated by a CD4+ T-cell clone specific for an envelope glycoprotein epitope of Lassa virus. J Virol. 1993;67(6):3497–506. doi: 10.1128/jvi.67.6.3497-3506.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Glushakova SE, Lukashevich IS, Baratova A. Prediction of arenavirus fusion peptides on the basis of computer analysis of envelope protein sequences. FEBS Lett. 1990;269:145–47. doi: 10.1016/0014-5793(90)81140-j. [DOI] [PubMed] [Google Scholar]
- 54.Meulen J, Badusche M, Satoguina J, Strecker T, Lenz O, Loeliger C, et al. Old and New World arenaviruses share a highly conserved epitope in the fusion domain of the glycoprotein 2, which is recognized by Lassa virus-specific human CD4+ T-cell clones. Virology. 2004;321(1):134–43. doi: 10.1016/j.virol.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 55.Clegg JC, Lloyd G. Vaccinia recombinant expressing Lassa-virus internal nucleocapsid protein protects guinea pigs against Lassa fever. Lancet. 1987;2(8552):186–88. doi: 10.1016/s0140-6736(87)90767-7. [DOI] [PubMed] [Google Scholar]
- 56.Fisher-Hoch SP, Hutwagner L, Brown B, McCormick JB. Effective Vaccine for Lassa Fever. J Virol. 2000;74(15):6777–83. doi: 10.1128/jvi.74.15.6777-6783.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.ter Meulen J, Badusche M, Kuhnt K, Doetze A, Satoguina J, Marti T, et al. Characterization of human CD4+ T-cell clones recognizing conserved and variable epitopes of the Lassa virus nucleoprotein. J Virol. 2000;74(5):2186–92. doi: 10.1128/jvi.74.5.2186-2192.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boesen A, Sundar K, Coico R. Lassa Fever Virus Peptides Predicted by Computational Analysis Induce Epitope-Specific Cytotoxic-T-Lymphocyte Responses in HLA-A2.1 Transgenic Mice. Clin Diagn Lab Immunol. 2005;12(10):1223–30. doi: 10.1128/CDLI.12.10.1223-1230.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Botten J, Alexander J, Pasquetto V, Sidney J, Barrowman P, Ting J, et al. Identification of Protective Lassa Virus Epitopes That Are Restricted by HLA-A2. J Virol. 2006;80(17):8351–61. doi: 10.1128/JVI.00896-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu F, Feuer R, Hassett DE, Whitton JL. Peptide vaccination of mice immune to LCMV or vaccinia virus causes serious CD8+ T cell-mediated, TNF-dependent immunopathology. J Clin Invest. 2006;116(2):465–75. doi: 10.1172/JCI25608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ter Meulen J, Lukashevich I, Sidibe K, Inapogui A, Marx M, Dorlemann A, et al. Hunting of peridomestic rodents and consumption of their meat as possible risk factors for rodent-to-human transmission of Lassa virus in the Republic of Guinea. Am J Trop Med Hyg. 1996;55(6):661–66. doi: 10.4269/ajtmh.1996.55.661. [DOI] [PubMed] [Google Scholar]
- 62.Maiztegui JI, Sabattini MS, Barrera Oro JG. Activity of lymphocytic choriomeningitis virus (LCM) in the endemic area of Argentine hemorrhagic fever (AHF). I. Serological studies in rodents captured in the City of Pergamino. Medicina (B Aires) 1972;32(2):131–37. [PubMed] [Google Scholar]
- 63.Daddario-DiCaprio KM, Geisbert TW, Stroher U, Geisbert JB, Grolla A, Fritz EA, et al. Postexposure protection against Marburg haemorrhagic fever with recombinant vesicular stomatitis virus vectors in non-human primates: an efficacy assessment. Lancet. 2006;367(9520):1399–13404. doi: 10.1016/S0140-6736(06)68546-2. [DOI] [PubMed] [Google Scholar]
- 64.Baize S, Pannetier D, Faure C, Marianneau P, Marendat I, Georges-Courbot MC, Deubel V. Role of interferons in the control of Lassa virus replication in human dendritic cells and macrophages. Microbes Infect. 2006;8(5):1194–202. doi: 10.1016/j.micinf.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 65.Baize S, Kaplon J, Faure C, Pannetier D, Georges-Courbot MC, Deubel V. Lassa virus infection of human dendritic cells and macrophages is productive but fails to activate cells. J Immunol. 2004;172(5):2861–69. doi: 10.4049/jimmunol.172.5.2861. [DOI] [PubMed] [Google Scholar]
- 66.Lukashevich IS, Maryankova RF, Vladyko AS, Nashkevich N, Koleda S, Djavani M, et al. Lassa and Mopeia virus replication in human monocytes/macrophages and in endothelial cells: different effects on IL-8 and TNF-a gene expression. J Med Virol. 1999;59:552–60. [PMC free article] [PubMed] [Google Scholar]
- 67.Pannetier D, Faure C, Georges-Courbot MC, Deubel V, Baize S. Human macrophages, but not dendritic cells, are activated and produce alpha/beta Interferons in response to Mopeia virus infection. J Virol. 2004;78(19):10516–24. doi: 10.1128/JVI.78.19.10516-10524.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hotchin J. The biology of lymphocytic choriomeningitis virus: virus induced immune disease. Coldspring Harbor Symp Quant Biol. 1962;27:479–99. doi: 10.1101/sqb.1962.027.001.046. [DOI] [PubMed] [Google Scholar]
- 69.Binder D, Fehr J, Hengartner H, Zinkernagel RM. Virus-induced transient bone marrow aplasia: Major role of interferon-alpha/beta during acute infection with the noncytopathic lymphocytic choriomeningitis virus. J Exp Med. 1997;185:517–30. doi: 10.1084/jem.185.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Diebold SS, Montoya M, Unger H, Alexopoulou L, Roy P, Haswell LE, et al. Viral infection switches non-plasmacytoid dendritic cells into high interferon producers. Nature. 2003;424:324–28. doi: 10.1038/nature01783. [DOI] [PubMed] [Google Scholar]
- 71.Moskophidis D, Battegay M, Bruendler MA, Laine E, Gresser I, Zinkernagel RM. Resistance of lymphocytic choriomeningitis virus to alpha/beta interferon and to gamma interferon. J Virol. 1994;68:1951–55. doi: 10.1128/jvi.68.3.1951-1955.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Borrow P, Evans CF, Oldstone MBA. Destruction of virus-infected dendritic cells results in generalized immune suppression. J Virol. 1995;69:1059–70. doi: 10.1128/jvi.69.2.1059-1070.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zarozinkski CC, McNally JM, Lohman BL, Daniels KA, Welsh RM. Bystander sensitization to activation-induced cell death as a mechanism of virus-induced immune suppression. J Virol. 2000;74:3650–58. doi: 10.1128/jvi.74.8.3650-3658.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mahanty S, Gupta M, Paragas J, Bray M, Ahmed R, Rollin PE. Protection from lethal infection is determined by innate immune respones in a mouse model of Ebola virus infection. Virology. 2003;312:415–24. doi: 10.1016/s0042-6822(03)00233-2. [DOI] [PubMed] [Google Scholar]
- 75.Bray M. The role of the type I interferon response in the resistance of mice to filovirus infection. J Gen Virol. 2001;82(6):1365–73. doi: 10.1099/0022-1317-82-6-1365. [DOI] [PubMed] [Google Scholar]
- 76.Pedras-Vasconcelos J, Goucher D, Tonelli L, Wang V, Ito S, Verthelyi D. CpG ODN protect newborn mice from a lethal challenge with the neurotropic Tacaribe Arenavirus. J Immunol. 2006;176:4940–49. doi: 10.4049/jimmunol.176.8.4940. [DOI] [PubMed] [Google Scholar]