Abstract
We developed a recombinant defective adenovirus with an insert of gene encoding extracellular domain of mouse Flt3L (Ad-mFlt3L) under control of cytomegalovirus promoter to investigate the biological efficacy of Flt3L in combination with chemotherapeutical drug, 5-FU, in eliciting an effective anti-cancer immunity in mouse hepatoma and colon cancer model systems. The constructed Ad-mFlt3L efficiently infected hepatoma and colon cancer cells both in vitro and in vivo, leading to a high production of mFlt3L proteins in association with accumulation of DCs, NK cells and lymphocytes in local tumor tissues. Administration of Ad-mFlt3L can protect bone marrow injury caused by 5-Fu and stimulates proliferation and maturation of lymphocytes, APCs and NKs. Intratumoral injection of Ad-mFlt3L followed by an intraperitoneal administration of 5-Fu significantly inhibited tumor growth and cured established tumors. Adenovirus mediated Flt3L gene therapy synergies with chemotherapeutic drug, 5-Fu, in elicitation of long-lasting antitumor immunity. The tumor specific immunity can be adoptively transferred into naïve animals successfully by transfusion of CD3+CD8+ T cells from the treated mice. The data suggests that adenovirus mediated Flt3L gene therapy in combination with 5-Fu chemotherapy may open a new avenue for development of anti-cancer chemogenetherapy.
Keywords: Hepatoma, Colon cancer, 5-Fu, Flt3L, Immunity
Introduction
Flt3L is an essential molecule in the differentiation and maturation of early murine and human hematopoietic precursor/stem cells [10, 12, 16]. A unique biological function of Flt3L is able to induce dendritic cells and NK cells to accumulation in lymphoid and non-lymphoid tissues, which is critical for induction of host immune responses mediated by immune cells and antibodies [5, 14, 20]. Importantly, Flt3L also stimulates activation of NK cells and increases the number of functional mature NK cells in vivo [6, 13, 19]. Because both DCs and NK cells play a role in stimulating host immune system, systemic administration of Flt3L is effective in induction of antitumor immunity that protects against tumor challenges and mediates the regression of established tumors [1, 17, 21]. Administration of either soluble Flt3L protein or tumor cells transfected with Flt3L gene and expressing Flt3L molecules on cell surfaces only cause transient tumor regression in immunogenic tumor models. These treated animals usually developed recurrent tumors after the termination of treatment [22]. Though modification of tumor cells both in vitro and in vivo with Flt3L-expressing vectors could also generate immunogenic tumor cells, the current studies are still unable to support the hypothesis that the modified tumor cells in this manner can serve as therapeutic vaccines to treat established large tumors, most likely because of various “escape” mechanisms [18]. Recently, a promising alternative approach has been reported in which local administration of Flt3L attracted NK cell and DCs into tumor tissues and caused the regression of subcutaneously growing tumors in combination with low dose radiotherapy [2]. An ideal therapy for treatment of cancers should be effective both in destroying tumor cells and in the induction of antitumor immunity. Because of its unique biological activities, Flt3L is considered as a potential candidate to enhance host anticancer immunity following a routine chemotherapy and radiotherapy, which usually cause serious bone marrow injury and immune suppression. To further determine this hypothesis, we investigated the efficacy of adenovirus-mediated Flt3L gene therapy in combination with 5-Fu in treatment of hepatoma and colon carcinoma in syngeneic animal model systems.
Materials and methods
Tumor cell lines and animals
SMCC-1 cell line is a chemical-induced colon carcinoma cell line derived from C57BL/6 mice. The following were purchased from American Tissue Culture Collection (ATCC, Manassas, VA, USA) and routinely maintained in our laboratory: Hepa1-6, a liver carcinoma cell line; EL4, a lymphoma cell line also derived from C57BL/6 mice; an NK-sensitive YAC-1 cell line; a human embryonic renal cell line, 293. Ten- to 12-week-old C57BL/6 mice were obtained from the Planned Parenthood Research Institute, Shanghai, People’s Republic of China. All animals in this study were housed in pathogen-free conditions and were maintained in accordance with guideline of the Committee on Animals of the Second Military Medical University.
Construction of Ad-mFL and treatment of established tumors in vivo
Ad-mFL and Ad-GFP were constructed and generated as reported previously [11]. SMCC-1 and hepa 1-6 cells in exponential growth phase were harvested and each 4 × 106 cells in a volume of 100 μl of PBS were inoculated subcutaneously into the right lateral flank of syngeneic C57BL/6 mice. When the tumors reached 0.8–1 cm in diameter (15 days after inoculation), 1 × 109 efu of Ad-mFL or Ad-GFP, diluted in 0.1 ml saline buffer (PBS) or PBS alone was intratumorally injected two times (day 1 and 3). After last intratumoral injection, each group was followed by intraperitoneal administration of 5-Fu at a dose of 10 mg/kg for 3 days. The tumor size was measured in two perpendicular diameters with precision calipers every second day and calculated as described previously [8]. To investigate if injection of Ad-mFL into the tumors followed by 5-Fu treatment is effective in eliminating tumors, which grew within a short distance from the treated tumors, two groups of mice, 12 for each group, were inoculated with either SMCC-1 or hepa1-6 cells individually. Each animal received two injections both in the right and left lateral flanks. When tumors reached 0.5 cm size on both sides, the same procedure was applied to tumor growing in left lateral flanks. Tumor development in both sides was monitored. To determine long-term immunity, mice treated with 5-Fu plus Ad-mFL with the specific rejection of SMCC-1 or hepa1-6 tumor challenges were maintained for 4 months. The animals were divided into two groups and re-challenged with 4 × 106 parental hepa1-6 cells or SMCC-1 cells, respectively. Tumor development was determined by visual observation. Mice treated with Ad-GFP vectors were used as control group.
Generation of CTLs and adoptive transfer experiments
For generation of tumor-specific CTLs, the naïve and treated mice were killed and their spleens were removed aseptically by a routine surgical procedure and single cell suspensions were prepared as described previously [21]. The cytotoxic activity of splenocytes from treated and untreated animals was determined using the 51Cr release assay. NK cell killing activity was measured by a standard procedure as reported previously [23]. For the adoptive transfer experiments, the 2 × 107 CTLs were injected intravenously into groups of naïve mice. On the seventh day following adoptive transfer of splenocytes, the animals were challenged by subcutaneous injection either with 2 × 106 parental hepa1-6 cells or 2 × 106 SMCC-1 cells into their right flanks. Tumor developments were monitored daily and on the 120th day following tumor inoculation, all mice were histopathologically examined for tumor formation in different organs. For depletion experiments, groups of mice were treated with purified rat monoclonal antibodies to mouse CD4 (GK1.5), CD8 (Lyt2), NK cells (4D11) or a control rat monoclonal antibody to IgG1 (4G6) as described previously [8].
Immunohistochemistry
Tissues were obtained from the different groups of mice and fixed with 10% buffered formalin. After embedding in paraffin, 4-μm sections were cut and stained with hematoxylin and eosin. Finally, the sections were routinely examined under a microscope. For immunohistochemical staining, tumor tissues were embedded in Tissue-Tek OCT compound and frozen in liquid nitrogen. Tissues were sectioned on a cryostat at 5–7 μm and fixed in prechilled acetone for 10 min. After rinsing in PBS, endogenous peroxidase activity was quenched with 3% hydrogen peroxide in methanol (v/v) and the slides were rinsed again in PBS. Nonspecific binding was blocked by incubation in PBS containing 1% BSA and 1.5% normal goat serum for 20 min. Subsequently, Monoclonal antibodies to mouse CD4, CD8, NK cells and isotype-matched control antibody at a dilution of 1:100 with PBS were added to tissues for 1 h at room temperature within a humidified chamber. After two washes in PBS, the sections were incubated with peroxidase-conjugated anti-rat IgG secondary antibody for 30 min. Diaminobenzidine were applied as substrate for the peroxidase reaction and color reaction was developed for 6 min. Slides were counterstained with Mayer’s hematoxylin and images were digitized using a Sony Imager for data analysis. The cell infiltration was scored for the number of positive cells in ten randomly chosen high power field by three observers. No immunological staining was scored as (−); 0–10 positive cells as (+); 10–20 positive cells as (++) and >20 positive cells as (+++).
ELISpot assay and hematopoiesis analysis
Spleen cells from each treated or control animal were co-cultured in 96-well plates at 2 × 105 cells/well with Ad-mFL transfected, mock-vector transfected, parental Hepa1-6 cells or SMCC-1 cells at 2 × 104 cells/well for ELISpot assay as reported previously. For investigation of hematopoietic functions of Ad-FL in the treated mice, Bone marrow derived CD34+ cells were isolated from the treated or untreated animals as described previously [16]. Approximately 90% of enriched cell population was CD34+ cells and these cells were plated in 0.5 ml complete medium in 24-well plates. Cytokines were added at the following concentrations: 2 U/ml erythropoietin (EPO), 10 ng/ml IL-3, 20 ng/ml granulocyte-macrophage colony-stimulating factor (GM-CSF). The plates were incubated at 37°C in a 5% CO2 incubator and total colony numbers were counted on day 14. Extra-bone marrow hematopoiesis was evaluated by measuring the number of peripheral white blood cells (WBC) and platelets and by weighting the spleens of the treated animals.
Results
Tumor regression induced by local injection of Ad-mFL combined with 5-Fu
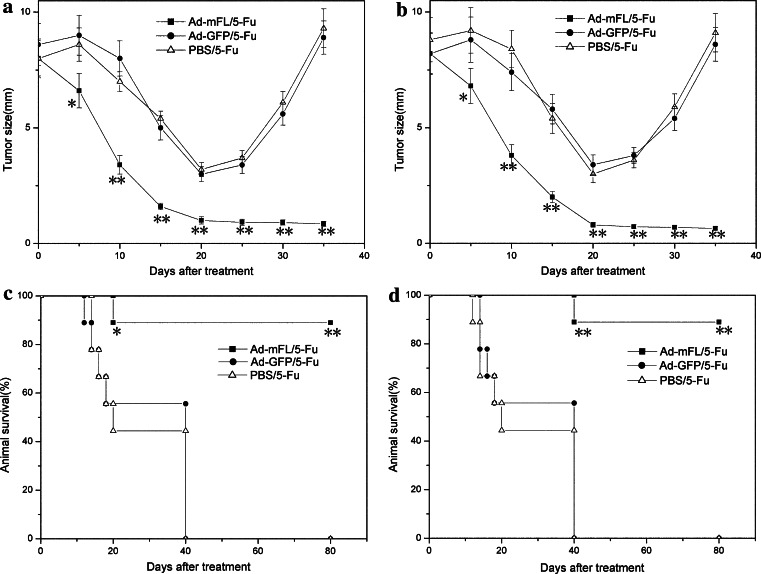
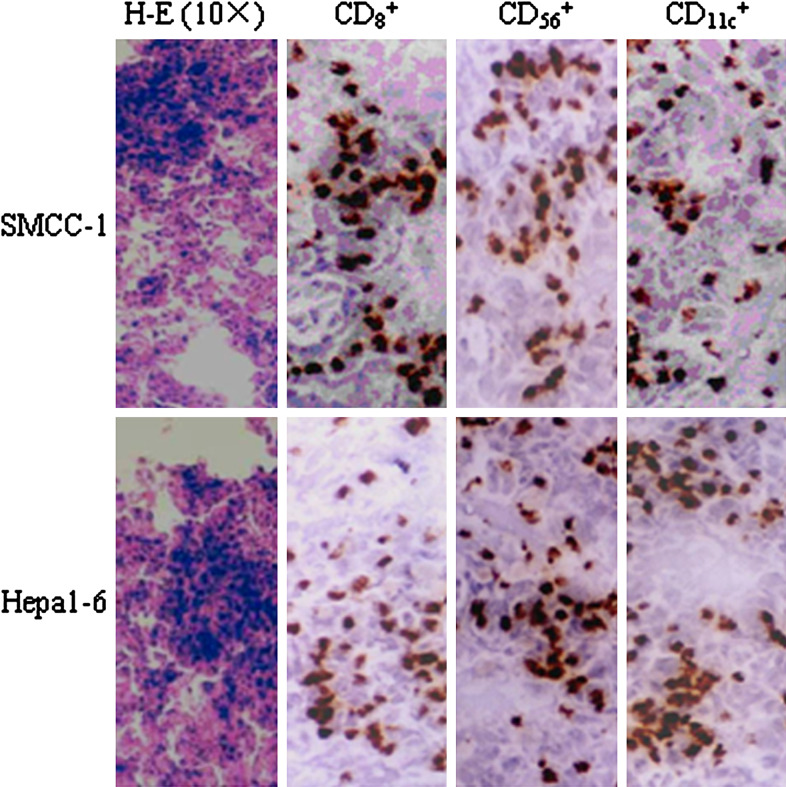
To further determine whether intratumoral injection of Ad-mFL combined with a systemic administration of 5-Fu is effective in eradicating the established mouse hepatoma and colon cancer of a large size, either hepa 1-6 or SMCC-1 tumor cell lines was subcutaneously injected into syngeneic C57BL/6 mice as reported previously [7]. On day 15, animals with tumors about 1 cm in diameter were injected with Ad-mFL, Ad-GFP (a control adenovirus) or saline buffer (PBS) followed by three injections of 5-Fu intraperitoneally at a dose of 10 mg/kg. Treatment of animals with Ad-mFL in combination with 5-Fu induced regression of hepa1-6 cells-derived tumors in 9 of 20 mice and SMCC-1 cells-derived colon tumors in 12 of 20 mice on day 20 (Fig. 1a, b), and led to a complete eradication of animals bearing either hepatoma and colon cancer within 42 days after the termination of treatment. In contrast, animals bearing hepa 1-6 tumors treated with either Ad-GFP or PBS followed by injection of 5-Fu at the same dose developed large tumors and died within 38 days. No tumor-bearing animals survived for more than 40 days in the groups that received Ad-GFP plus 5-Fu or PBS plus 5-Fu. Treatment of tumor-bearing mice with this combinatory protocol greatly prolonged the survival time of the animals in comparison with the control groups (Fig. 1c, d). More importantly, injection of Ad-mFL into tumors in left lateral flanks following 5-Fu treatment was effective in inducing complete regression of syngeneic tumors growing on right lateral flanks. No tumor development was observed after 4 months following the treatment. This suggests that tumor regression induced by this approach is associated with induction of effective immunity against parent tumors. The inhibition of tumor development is accompanied by tumor necrosis and a lymphocyte infiltration. The majority of infiltrating cells in the treated tumors were CD3+CD8 + T cells and NK cells indicated by immunohistochemistry (Fig. 2).
Fig. 1.
Treatment of mouse hepatoma and colon carcinoma with Ad-mFL in combination with 5-Fu. 4 × 106 SMCC-1 or Hepa1-6 cells in a volume of 100 μl of PBS were inoculated subcutaneously into the right lateral flank of syngeneic C57BL/6 mice, six groups, 20 per group. When the tumors reached 0.8–1 m in diameter (15 days after inoculation), 1 × 109 fu of Ad-mFL or Ad-GFP, diluted in 0.1 l saline buffer (PBS) or PBS alone were intratumorally injected for two times (day 1 and 3). After last intratumoral injection, each group was followed by intraperitoneal administration of 5-Fu at a dose of 10mg/kg for 3 days. Tumor developments were monitored and animal survival was calculated. a Tumor size of SMCC-1 colon carcinoma; b tumor size of Hepa1-6 hepatoma; c survival of animals bearing SMCC-1 colon carcinoma; d survival of animals bearing Hepa1-6 hepatoma. These experiments were repeated from three times with comparable results. *P ≤ 0.05, **P ≤ 0.01. Student’s t test for a and b or Fisher’s exact test for c and d
Fig. 2.
Infiltration of T lymphocytes, NK cells and Dendritic cells in tumor tissues treated with Ad-mFL in combination with 5-Fu. Animals were treated by different protocols. At the end of observation period, mice were killed and paraffin-embedded tumor sections were prepared. CD8+ T cells, CD56+ NK cells and CD11c+ DC were stained by a routine IHC protocol. A strong infiltration (+++) of CD8+ T cells, NK cells and DCs were observed
Enhancement of hematopoiesis in 5-Fu treated animals by Ad-mFL genetherapy
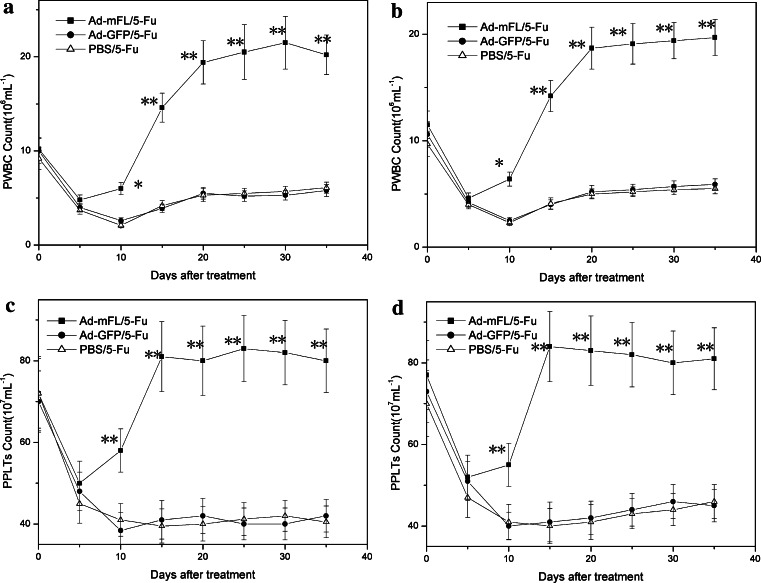
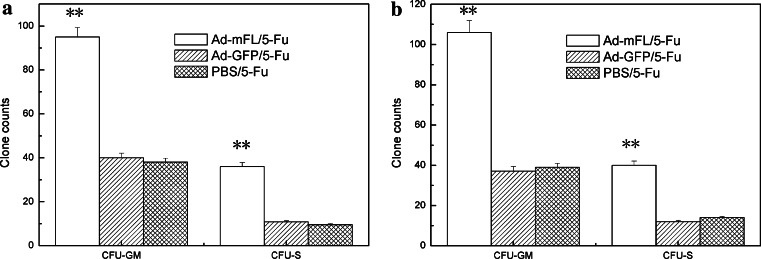
To determine whether Ad-mFL therapy is effective in protecting bone marrow injury induced by administration of 5-Fu, bone marrow hematopoiesis was evaluated by colony-forming assay in the treated mice. Administration of 5-Fu induced severe bone marrow hypoplasia. The number of peripheral blood WBCs and platelets (PLTs) in 5-Fu treated mice dropped from 9.2 ± 1.2 × 106/ml and 72 ± 9.1 × 107/ml before treatment to 1.7 ± 0.35 × 106 and 41 ± 6.7 × 107/ml at the seventh day after administration of 5-Fu and continuous to keep these low levels until day15 following treatment. Injection of Ad-mFL significantly enhanced the recovery of WBCs and PLTs, which started to increase at day 7 and reached normal levels on day 12. On day 15, the total numbers of WBCs and PLTs in Ad-mFL treated animals were 2.5-fold higher than that in PBS treated and Ad-GFP treated mice (Fig. 3). To further investigate whether Ad-mFL therapy can facilitate hematopoiesis in 5-Fu-impaired bone marrow, bone marrow cells were harvested from the mice treated with different protocols on day 7 and analyzed for the numbers of granulocyte-macrophage colony-forming unite (CFU-GM) and stem cell colony-forming unite (CFU-S). The number of myeloid progenitors in Ad-mFL-treated mice was 2.7-fold higher and the number of stem cell progenitors was 3.1-fold higher than in 5-Fu treated controls (Fig. 4). Thus, administration of Ad-mFL was not only stimulated the normal bone marrow, but also, promoted the bone marrow hematopoiesis, which had been impaired by chemotherapeutic drug, 5-Fu.
Fig. 3.
Recovery of 5-Fu induced reduction of PWBC and PLTs by Ad-mFL. The peripheral WBC counts and PLT counts of the mice treated with different protocols (twenty mice per group) were measured every day. a PWBC counts of SMCC-1 xenografted mice; b PBWC counts of Hepa1-6 xenografted mice; c PPLTs counts of SMCC-1 xenografted mice; d PPLTs counts of Hepa1-6 xenografted mice. The results are the representative data from three comparable experiments. *P ≤ 0.05, **P ≤ 0.01 (Student’s t test)
Fig. 4.
Enhancement of bone marrow damage induced by 5-Fu. Bone marrow cells from the mice treated with different protocols on day 7 and analyzed for the numbers of granulocyte-macrophage colony-forming unite (CFU-GM) and stem cell colony-forming unite (CFU-S). a CFU-GM and CFU-S of SMCC-1 xenografted mice; b CFU-GM and CFU-S of Hepa1-6 xenografted mice. These experiments are representative data from three comparable experiments, five mice per group. *P ≤ 0.05, **P ≤ 0.01 (Student’s t test)
Induction of anti-tumor immunity with specificity against parental tumors
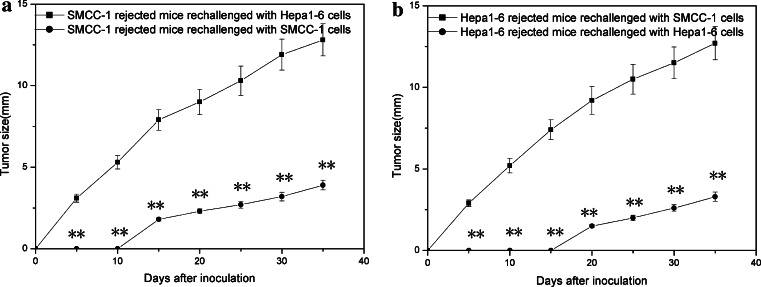
To investigate whether the mice developed a long-lasting antitumor immunity following Ad-mFL treatment in combination with 5-Fu, we re-challenged the mice with complete tumor regression after treatment with parental and syngeneic tumor cell lines. The second challenge was conducted in the left lateral flank with 4 × 106 parental cells or unrelated control tumor cells, respectively, on 80th day after the initial tumor rejection. The mice treated with Ad-mFL plus 5-Fu were able to prevent identical parental tumor challenging and the tumors failed to grow in all of the animals, whereas the tumor still grew progressively with a similar speed in the animals with rejection of unrelated tumors as control group (Fig. 5). The majority of T cells from spleen and lymph nodes of the mice treated with Ad-mFL plus 5-Fu are CD3 +CD8 +CD25+ T cells indicated by flowcytometry analysis. These T cells showed significantly higher proliferating responses to identical parental tumor cells and no proliferative responses were observed when irradiated unrelated tumor cells were used as stimulators. In a parallel experiment, spleen cells from the treated animals had significantly killing activities on the tumor cell lines identical to rejected tumors and a YAC-1 cell line, but not to unrelated tumor cell lines (Fig. 6).
Fig. 5.
Induction of long-lasting anti-tumor specific immunity by Ad-mFL plus 5-Fu. Animals with complete regression of SMCC-1 or hepa1-6 xenografted tumors induced by administration of Ad-mFL in combination with 5-Fu therapy were maintained for 4 months. The animals were then divided into two groups, five per group, and re-challenged with 4 × 106 parental Hepa1-6 or SMCC-1 cells, respectively. Tumor developments were monitored. These experiments were repeated for three times with similar results. *P ≤ 0.05, **P ≤ 0.01 (Student’s t test)
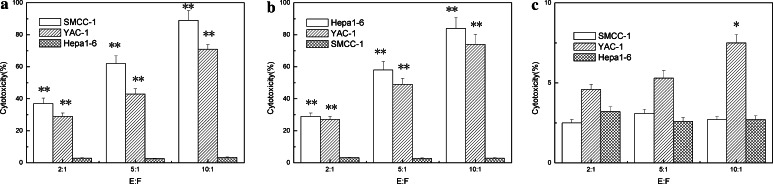
Fig. 6.
Cytotoxicity assay of spleen cells from the treated animals. The purified splenic cells were co-cultured with γ-irradiated (5,000 rad) parental hepa1-6 or SMCC-1 cells for 9 days in complete RPMI-1640 medium supplemented with 10% fetal bovine serum, 1% l-glutamine and 20 μm/ml recombinant IL-2. The cytotoxic activity of splenocytes from treated and untreated animals was determined using the 51Cr release assay. NK cell killing activity was measured by a standard procedure as reported previously. a Splenocytes from five mice with regression of SMCC-1 tumors; b splenocytes from five mice with regression of Hepa1-6 tumors; c Splenocytes from five naïve mice. The results are the representative data from three comparable experiments. *P ≤ 0.05, **P ≤ 0.01 (Student’s t test)
Establishment of effective immunity in naïve mice by adoptive transfer of splenocytes
To further determine whether induced immune response is mediated by T cells and NKs, 5 × 107 CD3+ CD4+ T cells or 5 × 107 CD3+CD8+ T cells obtained from the treated animals, which had rejected with either hepa1-6 or SMCC-1 tumors, were adoptively transferred into naïve animals as reported previously [15]. Adoptive transfer of 5 × 107 CD3+CD8+ T cells from the mice, which had rejected hepa1-6 tumors, only significantly prevented hepa 1-6 tumor challenging and had no effect against SMCC-1 tumor challenging. In contrast, adoptive transfer of T cells from the mice rejected SMCC-1 tumors only specifically prevent identical tumor challenging but not hepa1-6 tumors (Table 1). The mice that received the same amount of naïve T cells showed no antitumoral activity against tumor cell challenging. These data demonstrated that adoptive transfer of the CD3+CD8+ T cells developed in the treated animals, but not CD3+CD4+ T cells, protected against a subsequent challenge with the identical tumor cells in syngeneic naïve animals. Depletion of CD3+CD8+ T cell subpopulation with anti-CD8 monoclonal antibody, but not CD3+CD4+ or NKs with anti-CD4 or anti-NK antibody, completely abrogated antitumoral immunity elicited by this adoptive transfer approach.
Table 1.
The treated mice were killed and spleens were removed aseptically by a routine surgical procedure and single cell suspensions were prepared as described previously
| Adoptive transfer cell type | Inoculation cell type | |
|---|---|---|
| SMCC-1 | Hepa1-6 | |
| Adoptive transfer splenocytes of SMCC-1 rejection mice | ||
| CD3+CD8+ | 0/5 | 5/5 |
| CD3+CD4+ | 4/5 | 5/5 |
| Adoptive transfer splenocytes of Hepa1-6 rejection mice | ||
| CD3+CD8+ | 5/5 | 1/5 |
| CD3+CD4+ | 5/5 | 5/5 |
The 5 × 107 CTLs were injected intravenously into groups of naive mice, five per group. On the seventh day following adoptive transfer of splenocytes, the animals were challenged by subcutaneous injection either with 4 × 106 parental cells or 4 × 106 syngeneic tumor cells into the right flank. Tumor growth was monitored. These results are the representative data from three comparable experiments. P ≤ 0.01 (χ2 test)
Discussion
Cancer is one of the most common causes of death in the world. The conventional therapies, such as chemotherapy, radiotherapy and surgical approach, are routinely used for treatment of malignancies clinically. Despite of aggressive therapies, recurrence and metastasis of tumors still remain a major problem in cancer therapy. Thus, current attempts to prevent tumor recurrence/metastasis and improve survival of the patients must include strategies that can specifically destroy tumor cells and induce anti-tumor immunity. Chemotherapeutic drug, 5-Fu, is effective in killing majority of tumor cells, especially for adenocarcinoma. However, clinical efficacy of 5-Fu is dose-dependant and is restrictively limited by side effects following dose increasing. The bone marrow damage is one of serious side effects of 5-Fu, which induced host immune suppression and leads to, in some cases, termination of the treatment [4]. It is an urgent need clinically to minimize damage of host immune system during large dose of chemotherapy. Our previous data showed that administration of soluble FL protein and local injection of Ad-mFL was effective in eliciting host immune responses, which is able to cure tumors with a small size [21]. Here, we reported that intratumoral injection of Ad-mFL in combination with 5-Fu had a synergistic efficacy in treatment of colon and liver cancers in mice, which are incurable when treated with either 5-Fu or Ad-FL alone. The toxicity of 5-Fu to hematopoietic stem cells and immune cells can be effectively protected by administration of Ad-mFL, which is a key foundation for induction of antitumor immunity during a therapeutic dose of 5-Fu treatment. The bone marrow and immune cells in peripheral organs in the mice treated with 5-Fu in combination with Ad-mFL maintained normal functions as compared to naïve animals. These results strongly suggest that Ad-mFL functions as an immune adjuvant to enhance host immune response, which is also effective even in the animals with serious bone marrow damage during 5-Fu treatment.
Our results lead us to question the mechanism(s) that causes stronger antitumor immunity of Ad-mFL when used in combination with 5-Fu. The mice received a combination of 5-Fu and Ad-mFL have obvious tumor necrosis and activation of DCs and NK cells. These DCs could then uptake and present tumor antigen(s) released by the apoptotic tumor cells killed by 5-Fu to T cells and elicit an antigen specific anti-tumor immunity. This hypothesis is also strongly supported by a majority infiltration of DCs and NKs in tumor tissues in the treated animals but not in that in the animals treated with 5-Fu or Ad-mFL alone. The infiltrated NKs have a strong killing activity on tumor cells indicted by in vitro cytotoxicity assay and directly kill tumor cells. The tumor antigens released by killed tumor cells can be up-taken and presented by the infiltrated DCs, which stimulate host immunity effective in leading regression of tumors. More importantly, chemotherapeutic drug, 5-Fu, is routinely used for the treatment of liver and colon cancers clinically and prolongs the survival of patients with these cancers. As showed in this study, administration of 5-Fu alone could also inhibit tumor growth following the treatment. However, tumors were rapidly growing after 20 days when host bone marrow and immune systems became impaired due to side effects of 5-Fu. Administration of Ad-mFL protects bone marrow damage caused by 5-Fu and enhances recovery of the impaired host immune systems, which contribute to elicitation of anti-tumor immunity and prevents tumor recurrences after termination of 5-Fu treatment. The mechanism(s) by which Ad-mFL synergizes with 5-Fu in the curing of liver and colon cancers in these syngeneic model systems might be in part due to the maintenance of normal functions of DCs and NKs by soluble Flt3L produced by Ad-mFL transfected tumor cells during 5-Fu treatment.
Animals with tumor regression following this treatment procedure specifically protect parental tumor challenge but did not protect against challenge with syngeneic tumor cell lines. In vivo data is consistent with the in vitro data showing that splenocytes from mice treated with Ad-mFL in combination with 5-Fu proliferated vigorously in vitro when identical parental hepa1-6 cells or SMCC-1 tumor cells were used as stimulators. No proliferating responses were observed when other syngeneic tumor cells were used as stimulators. The majority of responder splenocytes was IFN-γ producing CD3+CD4+ T cells in ELISpot assay indicting a Th 1 response was induced. The CTLs generated from the treated mice showed a specific killing activity on parental tumor cells and YAC-1 cells, a NK sensitive cell line. These data suggest that immunity elicited by this approach is tumor specific and mainly mediated by both CD3+CD8+ and NK cells. More importantly, this antigen specific immunity is long lasting and could be detected after 200 days following the treatment, which could be also transferred into naïve animals successfully by adoptively transferring spleen cells from the treated animals. The explanation for this effectively adoptive transfer may be associated with memory T cells and DCs in the transferred spleen cells, which have been sensitized and present the tumor antigens on their cell surfaces. When these cells were adoptively transferred into naïve animals, the functioning memory T cells and DCs could expend in the animals and directly involve immune response against tumor challenge by the well-defined mechanisms reported previously [3, 9].
Our data thus indicate that administration of Ad-mFL in combination with 5-Fu can effectively elicit an antitumor immune response in mouse liver cancer and colon cancer model systems, which are both preventive and curative. In association with the immune responses, DCs and NKs recruitment to the tumor tissues occurred, which may facilitate tumor necrosis and enhance tumor antigen presentation. The induced long-lasting immunity is mediated by both T cells and NK cells. In addition to the simplicity and potential clinical expediency, this approach might provide a novel strategy for treatment of liver cancer and colon cancer and might have a broad clinical application for different tumors in combination with other therapies.
Acknowledgments
This work was supported in part by the grants from National Natural Science Foundation of China, Shanghai Commission of Science and Technology, E-Institutes of Shanghai Universities Immunology Division and Ministry of Science and Technology of China (973 and 863 projects) as well as a special grant from Shanghai Pudong Bureau of Science and Technology of China.
Abbreviations
- Flt3L
Fms-like tyrosine kinase 3 ligand
- NK
Natural killer
- HCC
Hepatocellular carcinoma
- 5-Fu
5-Fluorouracil
- MEM
Minimal essential medium
- Ad-mFL
Adenovirus with an insert of gene encoding extracellular domain of mouse Flt3L
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- FBS
Fetal bovine serum
- CMV
Cytomegalovirus
- EFU
Expression-forming unit
- CFU
Colony-forming unit
- BFU
Burst-forming unit
- MOI
Multiplicity of infection
- IHC
Immunohistochemistry
Footnotes
Sheng Hou, Geng Kou, and Xiaoqiang Fan are contribute equally to this paper.
Reference
- 1.Borges L, Miller RE, Jones J, Ariail K, Whitmore J, Fanslow W, Lynch DH. Synergistic action of fms-like tyrosine kinase 3 ligand and CD40 ligand in the induction of dendritic cells and generation of antitumor immunity in vivo. J Immunol. 1999;163(3):1289–1297. [PubMed] [Google Scholar]
- 2.Chakravarty PK, Alfieri A, Thomas EK, Beri V, Tanaka KE, Vikram B, Guha C. Flt3-ligand administration after radiation therapy prolongs survival in a murine model of metastatic lung cancer. Cancer Res. 1999;59(24):6028–6032. [PubMed] [Google Scholar]
- 3.Darcy PK, Haynes NM, Snook MB, Trapani JA, Cerruti L, Jane SM, Smyth MJ. Redirected perforin-dependent lysis of colon carcinoma by ex vivo genetically engineered CTL. J Immunol. 2000;164(7):3705–3712. doi: 10.4049/jimmunol.164.7.3705. [DOI] [PubMed] [Google Scholar]
- 4.Djazayeri K, Szilvassy Z, Peitl B, Nemeth J, Nagy L, Kiss A, Szabo B, Benko I. Accelerated recovery of 5-fluorouracil-damaged bone marrow after rosiglitazone treatment. Eur J Pharmacol. 2005;522(1–3):122–129. doi: 10.1016/j.ejphar.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 5.Drakes ML, Lu L, Subbotin VM, Thomson AW. In vivo administration of flt3 ligand markedly stimulates generation of dendritic cell progenitors from mouse liver. J Immunol. 1997;159(9):4268–4278. [PubMed] [Google Scholar]
- 6.Fernandez NC, Lozier A, Flament C, Ricciardi-Castagnoli P, Bellet D, Suter M, Perricaudet M, Tursz T, Maraskovsky E, Zitvogel L. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med. 1999;5(4):405–411. doi: 10.1038/7403. [DOI] [PubMed] [Google Scholar]
- 7.Guo YJ, Che XY, Shen F, Xie TP, Ma J, Wang XN, Wu SG, Anthony DD, Wu MC. Effective tumor vaccines generated by in vitro modification of tumor cells with cytokines and bispecific monoclonal antibodies. Nat Med. 1997;3(4):451–455. doi: 10.1038/nm0497-451. [DOI] [PubMed] [Google Scholar]
- 8.Guo Y, Wu M, Chen H, Wang X, Liu G, Li G, Ma J, Sy MS. Effective tumor vaccine generated by fusion of hepatoma cells with activated B cells. Science. 1994;263(5146):518–520. doi: 10.1126/science.7507262. [DOI] [PubMed] [Google Scholar]
- 9.Gyobu H, Tsuji T, Suzuki Y, Ohkuri T, Chamoto K, Kuroki M, Miyoshi H, Kawarada Y, Katoh H, Takeshima T, Nishimura T. Generation and targeting of human tumor-specific Tc1 and Th1 cells transduced with a lentivirus containing a chimeric immunoglobulin T-cell receptor. Cancer Res. 2004;64(4):1490–1495. doi: 10.1158/0008-5472.CAN-03-2780. [DOI] [PubMed] [Google Scholar]
- 10.Hannum C, Culpepper J, Campbell D, McClanahan T, Zurawski S, Bazan JF, Kastelein R, Hudak S, Wagner J, Mattson J, et al. Ligand for FLT3/FLK2 receptor tyrosine kinase regulates growth of haematopoietic stem cells and is encoded by variant RNAs. Nature. 1994;368(6472):643–648. doi: 10.1038/368643a0. [DOI] [PubMed] [Google Scholar]
- 11.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95(5):2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lyman SD, James L, Vanden Bos T, de Vries P, Brasel K, Gliniak B, Hollingsworth LT, Picha KS, McKenna HJ, Splett RR, et al. Molecular cloning of a ligand for the flt3/flk-2 tyrosine kinase receptor: a proliferative factor for primitive hematopoietic cells. Cell. 1993;75(6):1157–1167. doi: 10.1016/0092-8674(93)90325-K. [DOI] [PubMed] [Google Scholar]
- 13.Lynch DH, Andreasen A, Maraskovsky E, Whitmore J, Miller RE, Schuh JC. Flt3 ligand induces tumor regression and antitumor immune responses in vivo. Nat Med. 1997;3(6):625–631. doi: 10.1038/nm0697-625. [DOI] [PubMed] [Google Scholar]
- 14.Maraskovsky E, Brasel K, Teepe M, Roux ER, Lyman SD, Shortman K, McKenna HJ. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J Exp Med. 1996;184(5):1953–1962. doi: 10.1084/jem.184.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazzolini G, Qian C, Narvaiza I, Barajas M, Borras-Cuesta F, Xie X, Duarte M, Melero I, Prieto J. Adenoviral gene transfer of interleukin 12 into tumors synergizes with adoptive T cell therapy both at the induction and effector level. Hum Gene Ther. 2000;11(1):113–125. doi: 10.1089/10430340050016201. [DOI] [PubMed] [Google Scholar]
- 16.McKenna HJ, de Vries P, Brasel K, Lyman SD, Williams DE. Effect of flt3 ligand on the ex vivo expansion of human CD34+ hematopoietic progenitor cells. Blood. 1995;86(9):3413–3420. [PubMed] [Google Scholar]
- 17.Peron JM, Esche C, Subbotin VM, Maliszewski C, Lotze MT, Shurin MR. FLT3-ligand administration inhibits liver metastases: role of NK cells. J Immunol. 1998;161(11):6164–6170. [PubMed] [Google Scholar]
- 18.Sang H, Pisarev VM, Munger C, Robinson S, Chavez J, Hatcher L, Parajuli P, Guo Y, Talmadge JE. Regional, but not systemic recruitment/expansion of dendritic cells by a pluronic-formulated Flt3-ligand plasmid with vaccine adjuvant activity. Vaccine. 2003;21(21–22):3019–3029. doi: 10.1016/S0264-410X(03)00143-9. [DOI] [PubMed] [Google Scholar]
- 19.Shaw SG, Maung AA, Steptoe RJ, Thomson AW, Vujanovic NL. Expansion of functional NK cells in multiple tissue compartments of mice treated with Flt3-ligand: implications for anti-cancer and anti-viral therapy. J Immunol. 1998;161(6):2817–2824. [PubMed] [Google Scholar]
- 20.Steptoe RJ, Fu F, Li W, Drakes ML, Lu L, Demetris AJ, Qian S, McKenna HJ, Thomson AW. Augmentation of dendritic cells in murine organ donors by Flt3 ligand alters the balance between transplant tolerance and immunity. J Immunol. 1997;159(11):5483–5491. [PubMed] [Google Scholar]
- 21.Wang H, Dai J, Hou S, Qian W, Li B, Ma J, Fan X, Zhao J, Yang S, Sang H, Yang Q, Wang R, Guo Y. Treatment of hepatocellular carcinoma with adenoviral vector-mediated Flt3 ligand gene therapy. Cancer Gene Ther. 2005;12(9):769–777. doi: 10.1038/sj.cgt.7700843. [DOI] [PubMed] [Google Scholar]
- 22.Wang A, Braun SE, Sonpavde G, Cornetta K. Antileukemic activity of Flt3 ligand in murine leukemia. Cancer Res. 2000;60(7):1895–1900. [PubMed] [Google Scholar]
- 23.Ye Z, Hellstrom I, Hayden-Ledbetter M, Dahlin A, Ledbetter JA, Hellstrom KE. Gene therapy for cancer using single-chain Fv fragments specific for 4-1BB. Nat Med. 2002;8(4):343–348. doi: 10.1038/nm0402-343. [DOI] [PubMed] [Google Scholar]