Abstract
Many stroke research groups utilize the model of middle cerebral artery occlusion induced by insertion of an intraluminal thread, owing to its pragmatism and reliability of cerebral infarct generation. However, seventy five percent of stroke cases result from a thromboembolic event and ten percent from occlusive atherothrombosis in situ. Here, we characterize a mouse model of repeated thromboembolic stroke, which closely mimics the intravascular pathophysiology of arterial thrombus generation from an atherosclerotic plaque, and subsequent release of a thrombus into the cerebral circulation as an embolus. Common carotid artery thrombosis (CCAT) was induced photochemically leading to non-occlusive platelet aggregation in C57/BL6 male mice (n = 35), and was followed by mechanical assistance to facilitate release of the thrombus (MRT) and thus promote embolism. Six experimental groups, differing by changes in the surgical protocol, were used for the purpose of determining which such procedure yielded the most reliable and consistent brain infarct volumes with the lowest mortality at 3 days after surgery. The group which best satisfied these conditions was a double insult group which consisted of animals that underwent CCAT for 2 minutes by means of argon laser irradiation (514.5 nm) at an intensity of ca. 130 W/cm2, with concomitant injection of erythrosin B (EB) (35 mg/kg infused over those same two minutes), followed by MRT one minute later; the entire procedure was repeated 24 hours later. This group showed a percent of brain lesion volume of 15 ± 4 % (mean ± SD) with no associated 3-day mortality. Compared to a single insult group which sustained a percent brain lesion volume of 7 ± 3 %, there was a statistically significant (p<0.05) increase in the volume of infarction in the double-insult group.
Keywords: Stroke animal models, thromboembolic stroke, photochemical damage
INTRODUCTION
The annual incidence of symptomatic stroke in the United States is estimated to be above 700,000 (Thom et al, 2006). With approximately 150,000 fatalities per year, stroke is the third leading cause of death in the United States. Additionally, 11 million subclinical strokes occur each year in this country (Leary et al, 2003). Cerebral vessel occlusions account for approximately 85% of all strokes, while the remaining 15% arise from intracerebral bleeding (Rosamond et al, 1999). Ischemic stroke has a 1-month mortality rate of 15% and a 1-year mortality rate of almost 30% (Sacco et al., 1982). Embolisms, either arterio-arterial or cardiogenic, account for 75% of all cerebral vessel occlusions and constitute the most frequent cause of focally impeded blood flow within the brain (Mergenthaler et al., 2004).
Current knowledge of the pathophysiology of ischemic stroke is based largely on research utilizing animal models of mechanically induced intra- and/or extracerebral arterial occlusion. These models are of three types: focal ischemia, global ischemia, or focal with subsequent global ischemia. Such models include blocking blood flow by advancement of an intraluminal thread into the middle cerebral artery (MCA) (Koizumi et al., 1986), transection of the MCA (Tamura et al., 1981), or infusion of foreign bodies (i.e., micro/macro spheres) (Bralet et al., 1979; Miyake et al., 1993; Mayzel-Oreg et al., 2003; Garriets et al., 2003). Additionally, models of thromboembolic stroke have also been developed; these have relied on the infusion of autologous (Kudo et al., 1982; Kaneko et al., 1985; Beech et al., 2001) or heterologous clots (Overgaard et al., 1992; Zhang et al, 1997; Kilic et al. 1998; Zhang et al., 2005) into the cerebral circulation. It is important to note that the latter are produced outside the vessel and are then secondarily inserted into brain vasculature. In contrast, photochemical sensitization of common carotid artery thrombosis (CCAT) generates a multifocal model of thromboembolic stroke that may more accurately mimic several physiological features of clinical stroke (Futrell et al., 1988). Briefly, this laser-driven photochemical technique causes direct peroxidation of the endothelial membrane by singlet molecular oxygen, the sole photoproduct when erythrosin B or rose Bengal is used as the representative Type II photosensitizing dye (Watson et al., 1987). The resultant photochemically induced endothelial damage specifically attracts platelets, when then degranulate and self-sensitize a chain process of aggregation. This generates a large but non-occlusive platelet thrombus within the selected CCA, which emits a steady shower of emboli which upon enlodgment downstream create multiple embolic infarcts (Dietrich et al., 1993a and 1993b).
Photochemically induced thrombus generation relies on a functioning hemostatic system interacting with arterial endothelium rendered thrombogenic by the photochemical reaction. Also, previous studies on acute blood-brain barrier (BBB) consequences following MCA photothrombosis suggested that endothelial-platelet interactions at the vascular site of injury are responsible for the release of neurohumoral factors which contribute to the acute opening of the BBB (Dietrich et al, 1988). Therefore, this model features the important role that platelets and their secretory products may have in contributing to neuronal injury via thromboembolism (Joseph et al., 1991). On the other hand, in a recent clinical study, histological examination of thrombo- and atheroemboli retrieved from the MCA or intracranial internal carotid artery of patients with acute ischemic stroke showed that no two such emboli were similar in overall appearance, but demonstrated a distinctive pattern, comprised of layers of “serpentine” fibrin/platelet bands interspersed with accumulations of trapped erythrocytes (Marder et al, 2006). This particular photochemical method of generating thrombi does not generate fibrin at the injury site (i.e., the CCA). However, once the thrombi dislodge and are flowing downstream as emboli, shear injury to the endothelial wall upon embolus enlodgement has the potential to generate further platelet aggregation with involvement of fibrin.
Prior investigations in rats evaluated the histopathological outcome of single or repeated thromboembolic episodes at various time intervals (Danton et al., 2002b). One of the most prominent findings of these studies was that exposure to a prior thromboembolic event put the brain at risk for hemorrhagic infarction after a second embolic exposure. Additional investigations indicated that delayed upregulation of the endothelial nitric oxide pathway in distal arteries could account for some of the vascular deficits in the vulnerability of the brain to embolic insults resulting from CCAT (Danton et al., 2002a). Embolism from CCAT is characterized by small emboli traveling from a proximal site to lodge in a distal circulatory bed unprepared by chronic ischemia. Embolic Infarcts of this kind are abrupt in onset, with a maximal flow deficit from the start. Progressive worsening generally does not occur.
While this embolic model mimics clinical pathophysiology, there are difficulties with inter-subject reproducibility and consistency. Just as human patients with similar atherosclerotic disease may have different-sized infarcts in various locations, rodents that undergo non-occlusive arterial injury may not necessarily sustain the same degree of cerebral damage. Variations in embolus path and in vascular anatomy between individuals and species, blood flow changes, and recanalization time may alter the location and size of embolic infarcts (Maeda et al., 1998; Liebeskind, 2005).
To resolve this issue, parameters for a murine embolic model that reliably yields more consistent infarcts were developed. Furthermore, the effects of repeated embolic episodes on infarct size and character were evaluated. Herein we characterize a mouse model of photochemically induced thromboembolic stroke, in which rapid damage to the carotid vascular endothelium generates thrombi which subsequently embolize into the distal cerebrovasculature. Different approaches were performed, including single CCAT generation, double CCAT, and CCAT associated with subsequent mechanical assistance to release the formed thrombus at different time points.
MATERIALS AND METHODS
Animals
Thirty five (n=35) male mice (C57BL/6J, Jackson Laboratory, Bar Harbor, ME) weighing 19–25 g and 12–16 weeks old were used in this study. Mice were provided with a standard diet and tap water ad libitum. All animal procedures followed the National Institutes of Health Guides of the care and use of laboratory animals and were approved by the University of Miami Animal Care and Use Committee.
Surgical preparation and photochemically mediated embolic stroke injury
At 24 hours prior to surgery, animals were anesthetized with 3% isoflurane and anesthesia was maintained with 1.5 % isoflurane and a mixture of oxygen 30% and nitrous oxide 70%. The animals were placed in supine position with a rectal probe connected to a heating pad to maintain body temperature at 37 ± 0.5 °C. In preparation for surgery, a commercial product for hair removal (# 0660, American International Industries, Los Angeles, CA) was applied to the left inguino-femoral area and the midline of the neck from the lower jaw to the sternum. Five minutes later, the depilatory cream was removed; a subcutaneous injection of 30 ml/kg of normal saline (NS) was administered to ensure proper hydration of animals before surgery. Mice were returned to their cages without food but with water ad libitum. Once animals recovered from the anesthesia, they would further clean and groom themselves, leaving the planned site of surgery in pristine condition for surgery the following day. This simple maneuver decreased post-surgical wound infection dramatically.
On the day of surgery, animals were anesthetized as described before, and with the animals in supine position a 0.5 mm incision was made in the left inguinal area. Blunt dissection of the femoral vein was performed and a 32G catheter was inserted, advanced 4 mm, and secured using 6-0 nylon sutures. The surgical cavity was filled with normal saline at room temperature. Afterward, a rostro-caudal midline incision was extended from the lower jaw to the inferior aspect of the sternal manubrium. The right common carotid artery (CCA), the proximal portion of the adjacent internal carotid artery (ICA) and of the external carotid artery (ECA) were dissected by blunt technique.
A 6-0 nylon suture was used to permanently ligate the ECA, in order to direct emboli to the cerebral vasculature. A Transonic Doppler flow probe (Model 0.5 VB, Transonic Systems, Ithaca, NY) coupled with a temperature probe was placed on the right CCA approximately 3 mm proximal to its bifurcation, and the surgical cavity was filled with Signa® Gel (Parker Laboratories, Inc, Fairfield, NJ) and normal saline in approximately a 1:1 ratio. The probe was connected to a flowmeter (Transonic Model T206, Ithaca, NY) and interpreted with a computerized data acquisition program (Power Lab version 3.4.5, ADI Instruments, Colorado Springs, CO). Isoflurane was decreased to 1% and the CCA blood flow was monitored for 10 to 15 minutes.
CCAT was produced via the photochemical method using Erythrosin B (EB) as the photosensitizing dye (Danton et al, 2002a). EB (#190449, ICN Biomedicals, Inc, Irvine, CA) was dissolved at 2 mg/100 ml into saline and injected with an infusion pump (PHD2000, Harvard, Holliston, MA) via the femoral venous catheter at a rate of 17.5 mg/kg/min for two minutes (total dose was 35mg/kg). Simultaneously, a tunable argon laser (Model Innova 70-4, Coherent, Inc, Fremont, CA) operating at 514.5 nm with a power of 165 mW was focused through a 5× beam expander with focus range 1.2M to ∞ (# NT55-577, Edmund Industrial Optics, Barrington, NJ) placed 68.5 cm from the laser source. The laser beam was then redirected perpendicularly onto the subject CCA (ca. 400 μm in diameter) by a mirror.
In addition, a technique which involved gentle mechanical assistance in the release of the thrombus formed within the CCA was employed. Briefly, a few minutes (see below) after CCAT was performed, the subject’s irradiated CCA was gently tapped for one or two seconds with a cotton swab. This “MRT” technique caused a more consistent and controllable release of the newly formed thrombus into the bloodstream and toward the cerebral vasculature.
Animal Groups
Mice were assigned to one of six groups. Mice in group 1 (n = 5) were subjected to CCAT until one of the following conditions was met: total occlusion of the CCA as evidenced by Doppler blood flow measurement, ten minutes of laser irradiation had elapsed, or until the temperature within the surgical cavity reached 37.9 °C. The first standard was selected because when the vessel is occluded, there is no need to further irradiate it. The second standard of ten minutes of laser irradiation was established as a mean to limit the dose of laser received by an animal if occlusion was not attained. For this study, every animal had its CCA occluded before this time point. The third standard, i.e., temperature within the surgical cavity reaching 37.9°C, was established because it was considered that beyond this temperature, platelet aggregation would not be generated by direct photochemical damage of the endothelium but rather by protein denaturation secondary to thermal injury.
A typical recording of blood flow through the CCA prior to, during, and after irradiation is illustrated in Fig. 1A. After the laser was shut off, partial and pale discoloration of the subject CCA would be seen at the site of irradiation owing to the newly formed thrombus. Fifteen minutes later, MRT was performed and consequently the discoloration of the CCA vanished and the typical bright red color of the CCA was again observed. Surgical wounds were then sutured and mice were returned to their cages with water only, ad libitum. To control for the secondary insult procedure, a sham surgery was performed 24 hours later (but without CCA laser irradiation), animals were returned to their cages, and food and water ad libitum were reinstituted until sacrifice 72 hours later.
Figure 1. Doppler blood flow recordings of the common carotid artery (CCA).

*1 = Pre-irradiation period (laser is off); *2 = period of irradiation (laser is on with concomitant 2 minute infusion of EB); *3 = Post-irradiation period (laser is off). (A) Typical blood flow recording of common carotid artery thrombosis (CCAT) until occlusion. Total occlusion of the right CCA of this animal was attained after approximately four minutes and fifteen seconds of irradiation. Note that the laser is shut off once this occlusion is evidenced and that, in this case, there is a brief period of reperfusion before reocclusion. This phenomenon was seen in most of the animals subjected to occlusive CCAT. (B) Typical right CCA blood flow recording of an animal subjected to two minutes of laser irradiation. Changes in blood flow are subtle; there is slight hyperemia during the post-irradiation period.
Mice in group 2 (n = 6) also underwent an initial CCAT until one of the above-mentioned conditions was met. Fifteen minutes after shutting off the laser, the Doppler probe was removed along with the fluid within the surgical cavity, and MRT was performed. Twenty four hours later, mice underwent a second CCAT procedure exactly as before, but this time the laser beam was focused 2 mm distal to the original site of injury. Following the same parameters, 15 minutes after total occlusion MRT was performed at the site of discoloration. Animals were then returned to their cages with food and water, and sacrificed 72 hours later.
Based on 3-day survival and preliminary histopathological results it was predicted that the procedures outlined for the two previous groups would yield large infarct volumes and possibly high mortality. It was therefore decided to attempt to limit the size of the stroke in order to reduce mortality. Consequently, for group 3 animals (n=7) only a single occlusive CCAT procedure was performed until one of the three conditions was met, and instead of performing MRT at 15 minutes post-CCAT, it was performed at one minute. No additional procedure was performed and animals were then returned to their cages and sacrificed 72 hours later.
To continue with the effort of limiting the size of the cerebral infarcts generated, mice in group 4 (n = 5) were again subjected to a single CCAT with the laser irradiation limited to only two minutes, disregarding all of the three conditions outlined before. Fig. 1B shows a typical common carotid artery blood flow pattern in animals subjected to 2-min laser irradiation. No other procedure was performed, including MRT, and animals were returned to their cages and sacrificed 72 hours later. In order to examine the effect of MRT on infarct volume when using this tactic, group 5 (n = 6) animals underwent the same single CCAT procedure for two minutes. MRT was then performed one minute afterward. Animals were returned to their cages and sacrificed 72 hours later.
Finally, it was decided to evaluate the effect of repeated insults (CCAT) separated from each other by 24 hours with the inclusion of MRT. Previous studies from our laboratory had shown that repeated CCAT separated by 24 hours led to increased infarct size in rats (Danton et al, 2002b) without increasing associated mortality. Mice in group 6 (n = 6) were therefore subjected to the CCAT procedure for 2 minutes. MRT was performed one minute afterward. Mice were then returned to their cages and 24 hours later the same procedure was performed, except that this time the laser was focused 2 mm distal to the initial site of the original irradiation on the CCA. Animals were again returned to their cages and sacrificed 72 hours later. A summary of the different animal groups is outlined in Table 1.
Table 1.
Summary of animal groups
| GROUP | n | 1st CCAT (time of irradiation) | Mean time for CCA occlusion | MRT @ | 24 hour mortality | 2nd CCAT | 2nd MRT @ | Average time for CCA occlusion | 72 hour mortality | Overall mortality |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5 | Until one of three conditions was met* | 3 min 40 sec | 15 min | 0% | Sham | 15 min | No occlusion obtained | 40% (2/5) | 40% |
| 2 | 6 | Until one of three conditions was met* | 4 min 4 sec | 15 min | 33% (2/6) | Until one of three conditions was met* | 15 min | 2 min 50 sec | 75% (3/4) | 83% |
| 3 | 7 | Until one of three conditions was met* | 3 min 51 sec | 1 min | 0% | N/A | N/A | N/A | 0% | 0% |
| 4 | 5 | 2 min | No occlusion | None | 0% | N/A | N/A | N/A | 0% | 0% |
| 5 | 6 | 2 min | No occlusion | 1 min | 0% | N/A | N/A | N/A | 0% | 0% |
| 6 | 6 | 2 min | No occlusion | 1 min | 0% | 2 min | 1 min | No occlusion | 0% | 0% |
Total occlusion of right common carotid artery (CCA) as evidenced by Doppler flow measurement, or cessation of irradiation after 10 minutes, or when the temperature within the surgical cavity reached 37.9 °C. N/A = indicates that this procedure was not performed on a particular group of animals. MRT @ = indicates elapsed time from common carotid artery thrombosis (CCAT) to when mechanical assistance in the release of the thrombus (MRT) was performed.
Physiology
Physiological variables measured for animals in each of the groups included rectal temperature, temperature within the cervical surgical cavity, Doppler flow measurement of the CCA, and time under inhaled anesthesia via face mask instead of mechanical ventilation. Once it was established which group yielded the proposed goal in terms of brain infarct reproducibility, a separate group of animals (n=5) was used for the sole purpose of analyzing physiological parameters before and after surgery. For this group of animals, core temperature, mean arterial blood pressure, pH, pCO2, and pO2 were within normal values (Table 2).
Table 2.
Physiological parameters of a selected group of animals
| Temperature | MABP (mmHg) | pH | PCO2 | PO2 | |
|---|---|---|---|---|---|
| First CCAT+MRT | |||||
| Pre | 36.95±0.10 | 94.92±3.86 | 7.42±0.03 | 37.70±3.23 | 129.95±20.52 |
| Post | 37±0.14 | 101.55±2.90 | 7.41±0.03 | 37.70±0.03 | 133.98±20.03 |
| Second CCAT+MRT | |||||
| Pre | 36.88±0.13 | 92.5±1.29 | 7.44±0.02 | 39.21±15.57 | 156.17±10.14 |
| Post | 36.98±0.10 | 97.5±3.11 | 7.41±0.03 | 37.75±11.53 | 148.20±22.77 |
Animals (n=5) were subjected to common carotid artery thrombosis (CCAT) followed by mechanical assistance in the release of the thrombus (MRT). None of these animals were included in any of the analyzed groups. MABP = Mean Arterial Blood Pressure.
Histological Procedures
Three days after their last surgical procedure, mice were reanesthetized and perfusion-fixed with saline for 3 minutes followed by FAM, a mixture of 40% formaldehyde, glacial acetic acid, and methanol (1:1:8 by volume) for 30 minutes. Brains were removed and postfixed in FAM for at least 72 hours more. Brains were then embedded in paraffin. The tissue was sectioned coronally; fifteen micron thick sections were cut and mounted at 30 micron intervals and stained with hematoxylin and eosin for histopathological examination, and for the determination of infarct cross-sectional areas and volume integration.
Histological controls
EB solution was infused into three animals via their femoral vein; none of them presented cerebral histopathological changes when sacrificed 72 hours later. Additionally, three mice subjected to direct CCA irradiation with the laser at 165 mW of peak power for 10 min, but without concomitant administration of EB, showed no cerebral histopathological changes.
Quantitative assessment
Brain volume was measured by tracing the contour of five distinct Bregma levels which were +1.7, +0.14, −1.22, −2.7, and −3.64 (Paxinos and Franklin, 2001). These were chosen for some of their distinct anatomical landmarks which made identification of the appropriate Bregma level easier. The Z position of each brain section was registered in the serial section manager provided by the software. Built-in algorithms allowed collection of information needed for the estimation of volumes of the brain. This analysis was done by means of a workstation which consisted of a motorized stage, a video camera, and Neurolucida software (Microbrightfield Inc., Williston, VT). Similarly, the infarcted areas of the brain were confined within Bregma levels +1.7 and −3.64. To ensure the accurate calculation of infarct volumes, approximately 150 sections per brain were reviewed and approximately 40 of those sections were analyzed and measured. We were also able to reconstruct as 3-D geometric solids the brain, its infarcts, and the distribution of infarcts arising from focal embolism within the brain.
Statistical analysis
Histopathological data were expressed as means ± standard deviation (SD). Because of variability of total brain size from animal to animal and to compensate for the space-occupying effect of brain edema, the percent of brain lesion volume (%BLV) was calculated as the sum of volumes of individual infarcts in the right hemisphere divided by total brain volume. This expressed the percentage of infarcted tissue with respect to total brain volume. %BLV of groups with similar CCAT protocols were compared using one way ANOVA followed by Tukey’s test. Groups 1, 2, and 3 were compared with respect to each other as well as with groups 4, 5, and 6.
RESULTS
Histopathological findings
In contrast with previous work on photochemically induced embolic stroke in rats (Danton et al, 2002a,b), the single CCAT procedure in mice produced brain infarcts in every animal. Fig. 2 illustrates the distribution of infarct volumes at four distinct Bregma levels. A few (1–2) very small contralateral cortical infarcts were also seen in many animals. The number of ipsilateral infarcts ranged from six to twenty per animal. Furthermore, infarcts were reliably found in the striatum, hippocampus, and various portions of all cortical layers, occasionally extending into the subcortical white matter. Infarcts varied in size but were often large and found in the MCA territory (Fig. 2). Areas of neuronal dropout in infarcted tissue were visible (Fig. 3A). A clearly demarcated transition between normal and diseased tissue could be observed (Fig 3B). Just as previously described for rats in a similar stroke model (Urrea et al., 2004), many of the larger lesions evidenced eosinophilic cell bodies and pyknotic nuclei scattered among the necrotic neuropil. Selective neuronal necrosis and frank infarction were frequently seen in the hippocampus (Fig. 3C). Additionally, areas of focal infarction were frequently found in the thalamus (Fig. 3D). Areas of focal hemorrhage and areas of thrombosis were also consequences of CCAT (Fig. 4).
Figure 2. Hematoxylin-Eosin (H & E)-stained tissue from an injured mouse from group 3.
Insets magnify regions of interest. Arrows within insets highlight evidence of infarction and associated tissue breakdown. (A) Bregma level (BL) +1.5. (B) BL +0.14. (C) BL −1.22. (D) BL −2.7. Hippocampal infarcts are seen in (C) and (D).
Figure 3. H & E staining of different areas from an injured brain.

(A) Cortical infarct at Bregma Level (BL) +1.42 showing area of non-necrosed tissue but with neuronal dropout (*). (B) Cortical infarct at BL −1.9; the transition from injured (superior aspect of picture) to normal (inferior aspect) tissue is shown. (C) Infarct in CA1, subiculum, and dentate gyrus at BL −2.7. (D) Thalamic infarct in subventricular area at BL −0.94. 3v = Third ventricle.
Figure 4. Vascular consequences of CCAT.
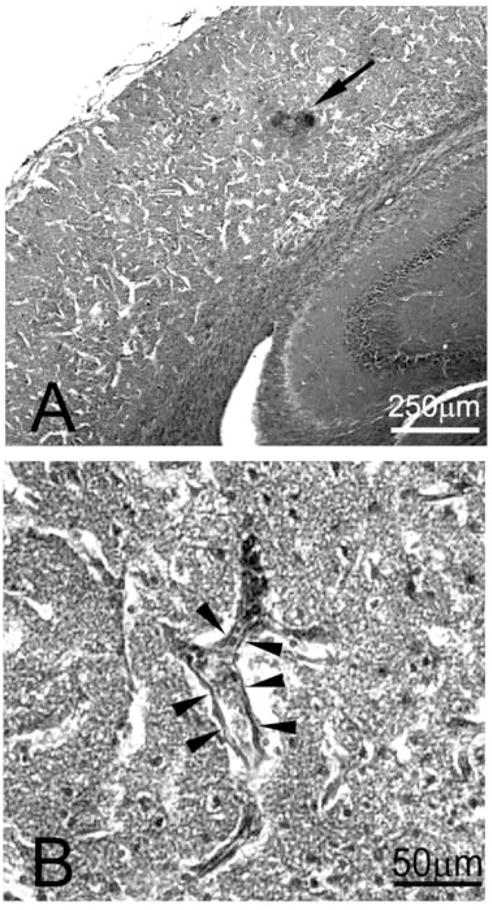
H&E staining of brain from a mouse subjected to CCAT. (A) Cortex at Bregma level −2.6; arrow points towards area of focal hemorrhage (dark spots). White streaks in cortex correspond to tissue breakdown. (B) Higher magnification of a cortical infarct. Arrowheads point to endothelial cells which line a thrombosed vessel wall.
Survival and infarct volume patterns among the different groups
Differences in mortality for group 1 (single CCAT+MRT) and group 2 (double CCAT+MRT) at 24 hours after the first insult was 0% vs. 33% respectively. The reason for this was not precisely determined, but hemorrhagic transformation could have accounted for this phenomenon. Additionally, overall mortality in these same groups was extremely high (40% vs. 83% respectively) and animals commonly died prior to the 72 hour intended survival period, making the double insult procedure more lethal. Nonetheless, infarct volumes were obtained from those animals which did manage to reach the 72 hour time point of sacrifice, and their infarct volumes were well above 30% of total brain volume. The thrombus released from the CCA after 15 minutes of occlusion generated lethal damage and evidence of severe brain swelling, and thus it was hypothesized that the clot was too well organized and too large at the time it was dislodged from the CCA. The embolus probably stayed in the MCA and did not travel to distal vascular beds. Subsequent groups were then designed to decrease the mortality. Hence, the time of irradiation and time between total occlusion and MRT were modified to produce less lethal but more consistently observable infarcts within the designated time frame.
Group 3 animals received the same laser irradiation time as the previous two; however, MRT was performed one minute after total occlusion. This simple change allowed every animal to survive until sacrifice at 72 hours. The ipsilateral hemisphere displayed a number of ischemic and sometimes hemorrhagic infarcts. The resulting infarct volumes varied greatly from animal to animal. Percent of brain lesion volume obtained was 25 ± 12% (mean percentage of infarcted tissue ± SD). Statistical differences between groups 1, 2, and 3 were nonsignificant.
To determine the effect of not performing MRT and reducing the time of laser irradiation, group 4 animals were subjected only to a single CCAT for two minutes. The result was a %BLV of 10 ± 5% (Fig. 5). Standard deviation was 50% of the mean, clearly indicating a very high variability.
Figure 5. Percent brain lesion volume (%BLV), expressed as the percentage of infarcted tissue with respect to total brain volume (±SD).

* = p < 0.05, denotes statistically significant difference in %BLV between single common carotid artery thrombosis followed by mechanical assistance in the release of the thrombus (CCAT+MRT) (group 5) and double CCAT+MRT (group 6) animals. CCAT among these groups of animals was sustained for 2 minutes followed by MRT a minute later (if applied to surgical protocol).
For Group 5 animals (single CCAT+MRT), the %BLV was smaller but more variable in size. The mean of the %BLV was 7 ± 3%. This result indicated that variability was still within a very broad range.
Group 6 (double CCAT+MRT) animals were the most consistent in that the %BLV was less scattered about the mean when compared to all the other groups evaluated in this investigation. %BLV in this group averaged 15 ± 4%.
When comparing group 4 (single CCAT) with group 5 animals (single CCAT + MRT), there was a trend for group 4 animals to show larger infarcts that more commonly extended into the subcortical regions. However, statistical significance was not achieved. On the other hand, when comparing group 5 (single CCAT + MRT) with group 6 (double CCAT + MRT), a statistically significant difference in %BLV was achieved, with p < 0.05 (Fig. 5).
DISCUSSION
We have demonstrated that the paradigm which dictates an increased vulnerability of the postthrombotic brain to a second thromboembolic insult is valid in the strain of mice used for this study. There was a statistically significant difference (p<0.05) in the increase of infarct volume from a single CCAT+MRT to a double CCAT+MRT procedure. This newly described model incorporates ipsilateral ECA ligation, 2 minutes of laser irradiation of the CCA at a peak power of 165 mW (yielding an average intensity of 130 W/cm2) with concomitant infusion of 35 mg/kg of EB, followed by MRT one minute later.
It is important to note that the %BLV within the double insult group of infarcts examined at 72 hours following the last CCAT was less scattered about the mean. By day 3, the infarcts were presumed to be stable by means of the penumbra being incorporated into the core of damaged tissue, thus accounting for less intergroup and intragroup variability. Our previous experience with the double-intervention embolic model in rat showed that a later embolic insult was disproportionately devastating to the brain compared to the pathological effects of an initial embolic insult. In mice, however, the results of the second surgical intervention (required to produce a delayed embolic insult) were more severe than the initial embolic insult, but not disproportionately so. For rats, a previous study on Wistar rats (Danton et al., 2002b) determined that a second embolic insult at 24 hours from the initial one would generate an increase in infarct volume by at least 23-fold. On the other hand, in the strain of mice used for this study, the increase in infarct volume from a single two minute CCAT+MRT (group 5) to a double two minute CCAT+MRT (group 6) was approximately two-fold. This disparity between rats and mice might be explained by the already mentioned differences in anatomy among species and strains, but more importantly, to the implementation of MRT. With the latter, one single CCAT+MRT in mice suffices to generate significant infarct volumes as opposed to a single CCAT in rats. Additionally, double CCAT in rats generated an absolute infarct volume of 22 ± 13 (mm3 ± SD); thus, the SD was over 50% of the mean, indicating a very high variability. For this mouse study, double CCAT+MRT animals had an SD below 27%. Reducing injury variability is crucial when experimenting with any animal model, particularly when putting to test potential neuroprotective strategies and evaluating reduction in infarct volume as an outcome.
Ligation of the ECA was performed as a means to effectively ensure that emboli generated at the CCA would flow toward cerebral arteries instead of neck and facial arteries. Additionally, regulation of temperature within the cervical surgical cavity was of utmost importance. Utilizing only normal saline (NS) to fill the surgical cavity resulted in a temperature below 34 °C. In contrast, using gel and NS in a 1:1 ratio maintained cavity temperature approximately 1.5°C below that of the core temperature of the animal, but still above 34°C. There have been several reports of the effect of systemic hypothermia on platelet function and enzymatic activity of coagulation factors (Dietrich et al., 1987; Johnston et al., 1994; Wolberg et al, 2004). However, our experience of this would suggest that not only does systemic hypothermia alter platelet function, but also localized hypothermia may have an effect on platelet physiology as evidenced by inefficient thrombus and embolus generation when the cervical cavity temperature was below 34 °C (when using NS as the sole liquid to fill it).
The murine intraluminal stroke model has been used extensively during the past fifteen years. However, previous studies have reported that this model induces hyperthermia (> 39° C). Such an increase in body temperature is attributed to hypothalamic damage caused by the obstruction of the hypothalamic artery as it originates from the distal ICA (Li et al, 1999; Reglodi et al, 2000). It is accepted from clinical and experimental studies that hyperthermia increases infarct volume and worsens outcome (Reglodi et al, 2000; Yanamoto et al., 2001). A technique used for the generation of stroke while avoiding damage to the hypothalamus is direct microsurgical occlusion of the MCA (Yamashita et al., 1997). However, this technique requires performing a craniotomy, and thus it is a more invasive procedure which can alter intracranial pressure, hemodynamics and local brain temperature and may cause parenchyma and vascular injuries (Gerriets et al., 2003). Although no effort was made to avoid injury to the hypothalamus, hyperthermia was not evidenced in any of the animals in this study at 24 hours after the first thromboembolic insult or at 72 hours after the second insult, indicating that there probably was no direct damage to the thermoregulatory areas.
Other techniques for stroke generation in rats have involved induction of focal cerebral ischemia by intracarotid embolization with viscous silicone (Lauer et al., 2002), injection of macrospheres (Gerriets et al., 2003), and injection of microspheres (Bralet et al., 1979; Miyake et al., 1993). However, these models rely on the administration of foreign bodies which have the sole purpose of blocking blood flow and thus the important role played by platelets in the initial phase of ischemic stroke is overlooked. In addition, such models require the administration of heparin, heparinized saline, and/or the use of heparinized tubing for the insertion of the foreign bodies. The use of heparin can further alter the physiopathology of an evolving stroke, and therefore should be considered as an intervention. Heparin was not used in any of the animals subjected to the different surgical protocols.
In experimental stroke, mutant mice are increasingly used to elucidate complex sequences of molecular events which are believed to be involved in the manifestations of ischemic injury (Kinouchi et al., 1991; Huang et al., 1996; Connolly et al., 1997; Bonventre et al., 1997; Asahi et al., 2000; Manley et al., 2000; Naus et al., 2001; Tehraninan et al., 2006;). The majority of the work is based on models that are straightforward in their approach to generating focal cerebral ischemia, such as the intraluminal thread model. Most of these studies focus on proving basic concepts and there is limited discussion of how these results relate to human thromboembolic stroke. In addition, direct photothrombosis of cerebral vessels induces simultaneous vasogenic (extracellular) edema and cytotoxic (intracellular) edema (Dietrich et al, 1987; van Bruggen et al, 1992; Lee et al, 1996). Unlike direct photothrombotic stroke performed on cerebral vessels, it is hypothesized that since this embolic stroke model has its origin in the CCA, it does not generate the high incidence of vasogenic edema associated with rapid loss of endothelial integrity of a brain vessel. On the other hand, reperfusion-like phenomena have also been described in a photochemically induced thrombotic occlusion model in rats and its role in the progress of brain damage has been outlined (Takamatsu et al., 1998; Watson et al, 2002); similar vascular perturbations may be present in mice as well. Data presented in this study show evidence of hemorrhagic foci that would indicate direct vascular damage due to embolic events. The observation that a second embolic insult to the thrombosed brain increases infarct size suggests similar thrombotic mechanisms in both the rat and mouse CCAT models.
Typically, human strokes are small in size, ranging from 28–80 cm3 (Carmichael, 2005). The percentage of infarcted tissue in proportion to the total volume of the ipsilateral affected hemisphere corresponds to 4.5 – 14% (Lyden et al, 1994; NINDS, 2000). This percentage is increased to 39 – 47% in the presence of malignant human brain infarction (Oppenheim et al, 2000; Foerch et al, 2004), but the latter only accounts for approximately 10% of human stroke cases. The majority of the mouse middle cerebral occlusion (MCAo) models do not reflect the usual cases of human stroke, in that the bulk of recent publications using this model note infarcts comprising between 21 to 45 % of the ipsilateral hemisphere (Belayev et al, 1999; Boutin et al, 2001; McColl et al, 2004). With regard to infarct volume, these models are in fact more similar to human malignant infarction (Carmichael, 2005). When percent hemispheric lesion volume (lesion volume/ipsilateral hemispheric volume) was applied to the results of this present study, the infarcted tissue ratio was approximately 14% for the single insult group (single CCAT+MRT, group 5) and 30% for the double insult group (double CCAT+MRT separated by 24 hours, group 6), closer to the values obtained when examining the size of infarction from human populations. Furthermore, although no comparison to human cortical infarcts was attempted in this study, a previous study demonstrated the histopathological relevance of photochemically induced brain infarcts to human ischemic disease (Pevsner et al., 2001).
The MRT technique was employed for the first time in a photochemical model of stroke in mice after carefully observing that the photochemically generated thrombus in the CCA, although fragmenting into smaller emboli, was still partially adherent to the arterial wall, until its release was aided by the MRT technique. To our knowledge, there is no documentation in the medical literature of a human subject suffering a stroke from “tapping” a diseased atheromatous plaque on a carotid artery. Nonetheless, a risk of neurological complications (including stroke) has been indicated for those patients who present a clinically evident carotid bruit and are to receive carotid massage as part of their diagnostic workup for syncope (Sarasin et al., 2002; Morillo et al., 2004). However, with this in mind, this model is not intended to be relevant solely to such a reduced human population, but rather relevant to thromboembolic stroke populations in general which, as stated previously, account for 75% of cerebral vessel occlusions. The clinical importance of the model here presented lies then in the similarity to the events present in human disease following an arterial embolism. This model can be summed up as a real-time model that generates autologous platelet thrombi intravascularly via photochemical generation at the CCA, and then with the MRT technique, such thrombi can be embolized at a predetermined time. The emboli then flow downstream to the brain vasculature, thus generating reasonably consistent infarct volumes. This model can be used to study human neuroprotective strategies, and pathophysiological mechanisms of repetitive thromboembolic events, including inflammatory and apoptotic pathways.
Acknowledgments
Support and Acknowledgements: The authors thank Dr. Carlos Urrea, Dr. George Lotocki, Dr. Beata Frydel, Mrs. Ofelia Alonso, Mrs. Gladys Ruenes, and Mr. David Siqueira for technical assistance, and Dr. Kyle Padgett and Dr. Coleen Atkins for critical comments on the manuscript. Additionally, the authors thank Dr. Helen Bramlett for her assistance with statistical analysis. Supported by NIH grant NS 27127 and by American Heart Association predoctoral fellowship 0515078B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- NINDS; The National Institute of Neurological Disorders and Stroke (NINDS) rt-PA Stroke Study Group. Effect of intravenous recombinant tissue plasminogen activator on ischemic stroke lesion size measured by computed tomography. Stroke. 2000;31:2912–2919. doi: 10.1161/01.str.31.12.2912. [DOI] [PubMed] [Google Scholar]
- Asahi M, Asahi K, Jung JC, del Zoppo GJ, Fini ME, Lo EH. Role for matrix metalloproteinase 9 after focal cerebral ischemia: effects of gene knockout and enzyme inhibition with BB-94. J Cereb Blood Flow Metab. 2000;20:1681–1689. doi: 10.1097/00004647-200012000-00007. [DOI] [PubMed] [Google Scholar]
- Beech JS, Williams SC, Campbell CA, Bath PM, Parsons AA, Hunter AJ, Menon DK. Further characterisation of a thromboembolic model of stroke in the rat. Brain Res. 2001;895:18–24. doi: 10.1016/s0006-8993(00)03331-x. [DOI] [PubMed] [Google Scholar]
- Belayev L, Busto R, Zhao W, Fernandez G, Ginsberg MD. Middle cerebral artery occlusion in the mouse by intraluminal suture coated with poly-L-lysine: neurological and histological validation. Brain Res. 1999;833:181–190. doi: 10.1016/s0006-8993(99)01528-0. [DOI] [PubMed] [Google Scholar]
- Bonventre JV, Huang Z, Taheri MR, O’Leary E, Li E, Moskowitz MA, Sapirstein A. Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2. Nature. 1997;390:622–625. doi: 10.1038/37635. [DOI] [PubMed] [Google Scholar]
- Borsello T, Clarke PG, Hirt L, Vercelli A, Repici M, Schorderet DF, Bogousslavsky J, Bonny C. A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Nat Med. 2003;9:1180–1186. doi: 10.1038/nm911. [DOI] [PubMed] [Google Scholar]
- Boutin H, LeFeuvre RA, Horai R, Asano M, Iwakura Y, Rothwell NJ. Role of IL-1alpha and IL-1beta in ischemic brain damage. J Neurosci. 2001;21:5528–5534. doi: 10.1523/JNEUROSCI.21-15-05528.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bralet AM, Beley A, Beley P, Bralet J. Brain edema and blood-brain barrier permeability following quantitative cerebral microembolism. Stroke. 1979;10:34–38. doi: 10.1161/01.str.10.1.34. [DOI] [PubMed] [Google Scholar]
- Carmichael ST. Rodent models of focal stroke: size, mechanism, and purpose. NeuroRx. 2005;2:396–409. doi: 10.1602/neurorx.2.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly ES, Jr, Winfree CJ, Prestigiacomo CJ, Kim SC, Choudhri TF, Hoh BL, Naka Y, Solomon RA, Pinsky DJ. Exacerbation of cerebral injury in mice that express the P-selectin gene: identification of P-selectin blockade as a new target for the treatment of stroke. Circ Res. 1997;81:304–310. doi: 10.1161/01.res.81.3.304. [DOI] [PubMed] [Google Scholar]
- Danton GH, Prado R, Truettner J, Watson BD, Dietrich WD. Endothelial nitric oxide synthase pathophysiology after nonocclusive common carotid artery thrombosis in rats. J Cereb Blood Flow Metab. 2002a;22:612–619. doi: 10.1097/00004647-200205000-00013. [DOI] [PubMed] [Google Scholar]
- Danton GH, Prado R, Watson BD, Dietrich WD. Temporal profile of enhanced vulnerability of the postthrombotic brain to secondary embolic events. Stroke. 2002b;33:1113–1119. doi: 10.1161/hs0402.105554. [DOI] [PubMed] [Google Scholar]
- Diehl KH, Hull R, Morton D, Pfister R, Rabemampianina Y, Smith D, Vidal JM, van de Vorstenbosch C. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J Appl Toxicol. 2001;21:15–23. doi: 10.1002/jat.727. [DOI] [PubMed] [Google Scholar]
- Dietrich WD, Dewanjee S, Prado R, Watson BD, Dewanjee MK. Transient platelet accumulation in the rat brain after common carotid artery thrombosis. An 111In-labeled platelet study. Stroke. 1993a;24:1534–1540. doi: 10.1161/01.str.24.10.1534. [DOI] [PubMed] [Google Scholar]
- Dietrich WD, Prado R, Halley M, Watson BD. Microvascular and neuronal consequences of common carotid artery thrombosis and platelet embolization in rats. J Neuropathol Exp Neurol. 1993b;52:351–360. doi: 10.1097/00005072-199307000-00002. [DOI] [PubMed] [Google Scholar]
- Dietrich WD, Prado R, Watson BD, Nakayama H. Middle cerebral artery thrombosis: acute blood-brain barrier consequences. J Neuropathol Exp Neurol. 1988;47:443–451. doi: 10.1097/00005072-198807000-00005. [DOI] [PubMed] [Google Scholar]
- Dietrich WD, Watson BD, Busto R, Ginsberg MD, Bethea JR. Photochemically induced cerebral infarction. I. Early microvascular alterations. Acta Neuropathol (Berl) 1987;72:315–325. doi: 10.1007/BF00687262. [DOI] [PubMed] [Google Scholar]
- Faraday N, Rosenfeld BA. In vitro hypothermia enhances platelet GPIIb-IIIa activation and P-selectin expression. Anesthesiology. 1998;88:1579–1585. doi: 10.1097/00000542-199806000-00022. [DOI] [PubMed] [Google Scholar]
- Foerch C, Otto B, Singer OC, Neumann-Haefelin T, Yan B, Berkefeld J, Steinmetz H, Sitzer M. Serum S100B predicts a malignant course of infarction in patients with acute middle cerebral artery occlusion. Stroke. 2004;35:2160–2164. doi: 10.1161/01.STR.0000138730.03264.ac. [DOI] [PubMed] [Google Scholar]
- Futrell N, Watson BD, Dietrich WD, Prado R, Millikan C, Ginsberg MD. A new model of embolic stroke produced by photochemical injury to the carotid artery in the rat. Ann Neurol. 1988;23:251–257. doi: 10.1002/ana.410230307. [DOI] [PubMed] [Google Scholar]
- Gerriets T, Li F, Silva MD, Meng X, Brevard M, Sotak CH, Fisher M. The macrosphere model: evaluation of a new stroke model for permanent middle cerebral artery occlusion in rats. J Neurosci Methods. 2003;122:201–211. doi: 10.1016/s0165-0270(02)00322-9. [DOI] [PubMed] [Google Scholar]
- Gladstone DJ, Black SE, Hakim AM. Toward wisdom from failure: lessons from neuroprotective stroke trials and new therapeutic directions. Stroke. 2002;33:2123–2136. doi: 10.1161/01.str.0000025518.34157.51. [DOI] [PubMed] [Google Scholar]
- Huang Z, Huang PL, Ma J, Meng W, Ayata C, Fishman MC, Moskowitz MA. Enlarged infarcts in endothelial nitric oxide synthase knockout mice are attenuated by nitro-L-arginine. J Cereb Blood Flow Metab. 1996;16:981–987. doi: 10.1097/00004647-199609000-00023. [DOI] [PubMed] [Google Scholar]
- Johnston TD, Chen Y, Reed RL., 2nd Functional equivalence of hypothermia to specific clotting factor deficiencies. J Trauma. 1994;37:413–417. doi: 10.1097/00005373-199409000-00014. [DOI] [PubMed] [Google Scholar]
- Joseph R, Tsering C, Grunfeld S, Welch KM. Platelet secretory products may contribute to neuronal injury. Stroke. 1991;22:1448–1451. doi: 10.1161/01.str.22.11.1448. [DOI] [PubMed] [Google Scholar]
- Kaneko D, Nakamura N, Ogawa T. Cerebral infarction in rats using homologous blood emboli: development of a new experimental model. Stroke. 1985;16:76–84. doi: 10.1161/01.str.16.1.76. [DOI] [PubMed] [Google Scholar]
- Kilic E, Hermann DM, Hossmann KA. A reproducible model of thromboembolic stroke in mice. Neuroreport. 1998;9:2967–2970. doi: 10.1097/00001756-199809140-00009. [DOI] [PubMed] [Google Scholar]
- Kinouchi H, Epstein CJ, Mizui T, Carlson E, Chen SF, Chan PH. Attenuation of focal cerebral ischemic injury in transgenic mice overexpressing CuZn superoxide dismutase. Proc Natl Acad Sci U S A. 1991;88:11158–11162. doi: 10.1073/pnas.88.24.11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer N, Thomalla G, Soennichsen J, Fiehler J, Knab R, Kucinski T, Zeumer H, Rother J. Magnetic resonance imaging and clinical patterns of patients with ‘spectacular shrinking deficit’ after acute middle cerebral artery stroke. Cerebrovasc Dis. 2005;20:285–290. doi: 10.1159/000087926. [DOI] [PubMed] [Google Scholar]
- Koizumi J, Yoshida Y, Nakazwa T, Ooneda G. Experimental studies of ischemic brain edema. I. A new experimental model of cerebral embolism in rats in which recirculation can be introduced in the ischemic area. Jpn J Stroke. 1986;8:1–8. [Google Scholar]
- Lauer KK, Shen H, Stein EA, Ho KC, Kampine JP, Hudetz AG. Focal cerebral ischemia in rats produced by intracarotid embolization with viscous silicone. Neurol Res. 2002;24:181–190. doi: 10.1179/016164102101199594. [DOI] [PubMed] [Google Scholar]
- Leary MC, Saver JL. Annual incidence of first silent stroke in the United States: a preliminary estimate. Cerebrovasc Dis. 2003;16:280–285. doi: 10.1159/000071128. [DOI] [PubMed] [Google Scholar]
- Lee VM, Burdett NG, Carpenter A, Hall LD, Pambakian PS, Patel S, Wood NI, James MF. Evolution of photochemically induced focal cerebral ischemia in the rat. Magnetic resonance imaging and histology. Stroke. 1996;27:2110–2118. doi: 10.1161/01.str.27.11.2110. discussion 2118–2119. [DOI] [PubMed] [Google Scholar]
- Li F, Omae T, Fisher M. Spontaneous hyperthermia and its mechanism in the intraluminal suture middle cerebral artery occlusion model of rats. Stroke. 1999;30:2464–2470. doi: 10.1161/01.str.30.11.2464. discussion 2470–2461. [DOI] [PubMed] [Google Scholar]
- Liebeskind DS. Collaterals in acute stroke: beyond the clot. Neuroimaging Clin N Am. 2005;15:553–573. x. doi: 10.1016/j.nic.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Lyden PD, Zweifler R, Mahdavi Z, Lonzo L. A rapid, reliable, and valid method for measuring infarct and brain compartment volumes from computed tomographic scans. Stroke. 1994;25:2421–2428. doi: 10.1161/01.str.25.12.2421. [DOI] [PubMed] [Google Scholar]
- Maeda K, Hata R, Hossmann KA. Differences in the cerebrovascular anatomy of C57black/6 and SV129 mice. Neuroreport. 1998;9:1317–1319. doi: 10.1097/00001756-199805110-00012. [DOI] [PubMed] [Google Scholar]
- Manley GT, Fujimura M, Ma T, Noshita N, Filiz F, Bollen AW, Chan P, Verkman AS. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med. 2000;6:159–163. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- Marder VJ, Chute DJ, Starkman S, Abolian AM, Kidwell C, Liebeskind D, Ovbiagele B, Vinuela F, Duckwiler G, Jahan R, Vespa PM, Selco S, Rajajee V, Kim D, Sanossian N, Saver JL. Analysis of thrombi retrieved from cerebral arteries of patients with acute ischemic stroke. Stroke. 2006;37:2086–2093. doi: 10.1161/01.STR.0000230307.03438.94. [DOI] [PubMed] [Google Scholar]
- Mayzel-Oreg O, Omae T, Kazemi M, Li F, Fisher M, Cohen Y, Sotak CH. Microsphere-induced embolic stroke: an MRI study. Magn Reson Med. 2004;51:1232–1238. doi: 10.1002/mrm.20100. [DOI] [PubMed] [Google Scholar]
- Mergenthaler P, Dirnagl U, Meisel A. Pathophysiology of stroke: lessons from animal models. Metab Brain Dis. 2004;19:151–167. doi: 10.1023/b:mebr.0000043966.46964.e6. [DOI] [PubMed] [Google Scholar]
- Miyake K, Takeo S, Kaijihara H. Sustained decrease in brain regional blood flow after microsphere embolism in rats. Stroke. 1993;24:415–420. doi: 10.1161/01.str.24.3.415. [DOI] [PubMed] [Google Scholar]
- Morillo CA, Baranchuk A. Current Management of Syncope: Treatment Alternatives. Curr Treat Options Cardiovasc Med. 2004;6:371–383. doi: 10.1007/s11936-004-0021-8. [DOI] [PubMed] [Google Scholar]
- Naus CC, Ozog MA, Bechberger JF, Nakase T. A neuroprotective role for gap junctions. Cell Commun Adhes. 2001;8:325–328. doi: 10.3109/15419060109080747. [DOI] [PubMed] [Google Scholar]
- Oppenheim C, Samson Y, Manai R, Lalam T, Vandamme X, Crozier S, Srour A, Cornu P, Dormont D, Rancurel G, Marsault C. Prediction of malignant middle cerebral artery infarction by diffusion-weighted imaging. Stroke. 2000;31:2175–2181. doi: 10.1161/01.str.31.9.2175. [DOI] [PubMed] [Google Scholar]
- Overgaard K, Sereghy T, Boysen G, Pedersen H, Diemer NH. Reduction of infarct volume and mortality by thrombolysis in a rat embolic stroke model. Stroke. 1992;23:1167–1173. doi: 10.1161/01.str.23.8.1167. discussion 1174. [DOI] [PubMed] [Google Scholar]
- Pevsner PH, Eichenbaum JW, Miller DC, Pivawer G, Eichenbaum KD, Stern A, Zakian KL, Koutcher JA. A photothrombotic model of small early ischemic infarcts in the rat brain with histologic and MRI correlation. J Pharmacol Toxicol Methods. 2001;45:227–233. doi: 10.1016/s1056-8719(01)00153-8. [DOI] [PubMed] [Google Scholar]
- Reglodi D, Somogyvari-Vigh A, Maderdrut JL, Vigh S, Arimura A. Postischemic spontaneous hyperthermia and its effects in middle cerebral artery occlusion in the rat. Exp Neurol. 2000;163:399–407. doi: 10.1006/exnr.2000.7367. [DOI] [PubMed] [Google Scholar]
- Rosamond WD, Folsom AR, Chambless LE, Wang CH, McGovern PG, Howard G, Copper LS, Shahar E. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30:736–743. doi: 10.1161/01.str.30.4.736. [DOI] [PubMed] [Google Scholar]
- Sacco RL, Wolf PA, Kannel WB, McNamara PM. Survival and recurrence following stroke. The Framingham study. Stroke. 1982;13:290–295. doi: 10.1161/01.str.13.3.290. [DOI] [PubMed] [Google Scholar]
- Sarasin FP, Louis-Simonet M, Carballo D, Slama S, Rajeswaran A, Metzger JT, Lovis C, Unger PF, Junod AF. Prospective evaluation of patients with syncope: a population-based study. Am J Med. 2001;111:177–184. doi: 10.1016/s0002-9343(01)00797-5. [DOI] [PubMed] [Google Scholar]
- Saver JL, Johnston KC, Homer D, Wityk R, Koroshetz W, Truskowski LL, Haley EC. Infarct volume as a surrogate or auxiliary outcome measure in ischemic stroke clinical trials. The RANTTAS Investigators. Stroke. 1999;30:293–298. doi: 10.1161/01.str.30.2.293. [DOI] [PubMed] [Google Scholar]
- Stoll G, Bendszus M. Inflammation and atherosclerosis: novel insights into plaque formation and destabilization. Stroke. 2006;37:1923–1932. doi: 10.1161/01.STR.0000226901.34927.10. [DOI] [PubMed] [Google Scholar]
- Takamatsu H, Kondo K, Ikeda Y, Umemura K. Neuroprotective effects depend on the model of focal ischemia following middle cerebral artery occlusion. Eur J Pharmacol. 1998;362:137–142. doi: 10.1016/s0014-2999(98)00773-0. [DOI] [PubMed] [Google Scholar]
- Tamura A, Graham DI, McCulloch J, Teasdale GM. Focal cerebral ischaemia in the rat: 1. Description of technique and early neuropathological consequences following middle cerebral artery occlusion. J Cereb Blood Flow Metab. 1981;1:53–60. doi: 10.1038/jcbfm.1981.6. [DOI] [PubMed] [Google Scholar]
- Tehranian R, Rose ME, Vagni V, Griffith RP, Wu S, Maits S, Zhang X, Clark RS, Dixon CE, Kochanek PM, Bernard O, Graham SH. Transgenic mice that overexpress the anti-apoptotic Bcl-2 protein have improved histological outcome but unchanged behavioral outcome after traumatic brain injury. Brain Res. 2006;1101:126–135. doi: 10.1016/j.brainres.2006.05.049. [DOI] [PubMed] [Google Scholar]
- Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, Zheng ZJ, Flegal K, O’Donnell C, Kittner S, Lloyd-Jones D, Goff DC, Jr, Hong Y, Adams R, Friday G, Furie K, Gorelick P, Kissela B, Marler J, Meigs J, Roger V, Sidney S, Sorlie P, Steinberger J, Wasserthiel-Smoller S, Wilson M, Wolf P. Heart disease and stroke statistics--2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- Urrea C, Danton GH, Bramlett HM, Dietrich WD. The beneficial effect of mild hypothermia in a rat model of repeated thromboembolic insults. Acta Neuropathol (Berl) 2004;107:413–420. doi: 10.1007/s00401-004-0827-1. [DOI] [PubMed] [Google Scholar]
- Valeri CR, Feingold H, Cassidy G, Ragno G, Khuri S, Altschule MD. Hypothermia-induced reversible platelet dysfunction. Ann Surg. 1987;205:175–181. doi: 10.1097/00000658-198702000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bruggen N, Cullen BM, King MD, Doran M, Williams SR, Gadian DG, Cremer JE. T2- and diffusion-weighted magnetic resonance imaging of a focal ischemic lesion in rat brain. Stroke. 1992;23:576–582. doi: 10.1161/01.str.23.4.576. [DOI] [PubMed] [Google Scholar]
- Watson BD, Prado R, Veloso A, Brunshwig JP, Dietrich WD. Cerebral blood flow restoration and reperfusion injury after ultraviolet Laser-facilitated middle cerebral artery recanalization in rat thrombotic stroke. Stroke. 2002;33:428–434. doi: 10.1161/hs0202.102730. [DOI] [PubMed] [Google Scholar]
- Williams GR, Jiang JG, Matchar DB, Samsa GP. Incidence and occurrence of total (first-ever and recurrent) stroke. Stroke. 1999;30:2523–2528. doi: 10.1161/01.str.30.12.2523. [DOI] [PubMed] [Google Scholar]
- Wolberg AS, Meng ZH, Monroe DM, 3rd, Hoffman M. A systematic evaluation of the effect of temperature on coagulation enzyme activity and platelet function. J Trauma. 2004;56:1221–1228. doi: 10.1097/01.ta.0000064328.97941.fc. [DOI] [PubMed] [Google Scholar]
- Yamashita K, Busch E, Wiessner C, Hossmann KA. Thread occlusion but not electrocoagulation of the middle cerebral artery causes hypothalamic damage with subsequent hyperthermia. Neurol Med Chir (Tokyo) 1997;37:723–727. doi: 10.2176/nmc.37.723. discussion 727–729. [DOI] [PubMed] [Google Scholar]
- Yanamoto H, Nagata I, Niitsu Y, Zhang Z, Xue JH, Sakai N, Kikuchi H. Prolonged mild hypothermia therapy protects the brain against permanent focal ischemia. Stroke. 2001;32:232–239. doi: 10.1161/01.str.32.1.232. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Chopp M, Zhang RL, Goussev A. A mouse model of embolic focal cerebral ischemia. J Cereb Blood Flow Metab. 1997;17:1081–1088. doi: 10.1097/00004647-199710000-00010. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Ding G, Jiang Q, Zhang RL, Zhang X, Gan WB, Chopp M. A model of mini-embolic stroke offers measurements of the neurovascular unit response in the living mouse. Stroke. 2005;36:2701–2704. doi: 10.1161/01.STR.0000190007.18897.e3. [DOI] [PubMed] [Google Scholar]



