Abstract
Crying is a universal vocalization in human infants, as well as in the infants of other mammals. Little is known about the neural structures underlying cry production, or the circuitry that mediates a caregiver’s response to cry sounds. In this review, the specific structures known or suspected to be involved in this circuit are identified, along with neurochemical systems and hormones for which evidence suggests a role in responding to infants and infant cries. In addition, evidence that crying elicits parental responses in different mammals is presented. An argument is made for including ‘crying’ as a functional category in the vocal repertoire of all mammalian infants (and the adults of some species). The prevailing neural model for crying production considers forebrain structures to be dispensable. However, evidence for the anterior cingulate gyrus in cry production, and this structure along with the amygdala and some other forebrain areas in responding to cries is presented.
Keywords: mammals, vocalization, behavior, brain, evolution
1. Introduction
Describing the neural circuitry that mediates cry production and the neural circuitry in the listener that detects, analyzes and leads to a response to the cries are the two themes of this paper. These two circuits, along with the crying behavior, specifically the acoustic signals, comprise a functional network. This entire behavioral and neural assemblage constitutes a communication network that probably evolved together and which I will refer to as the ‘mammalian cry circuit.’ A working hypothesis widely accepted in the field is that crying arose early in mammalian evolution as an adaptation for the requirement of maternal contact and sustenance of an infant, where the need to re-establish contact between mother and infant was essential for infant survival in those instances when contact was lost. I wish to endorse the view that the neural circuitry that mediates the calling and response also evolved early in mammalian evolution, and, after diverging into circuitry underlying two classes of cries, has remained largely unchanged to the present day. As behavior doesn’t leave a fossil record (nor do the detailed circuits of the brain), these hypotheses are based on a comparative analysis of the signals, the responses to them, and a comparison of the neuroanatomy known to be involved in vocal production and the auditory processing of these signals.
2. Historical Perspective
Research on crying has a long history. Charles Darwin devoted a section of his book on the expression of emotions [20] to ‘weeping,’ equating this term to ‘crying’ because, as he pointed out, the term ‘weep’ comes from the Anglo-Saxon ‘wop,’ the primary meaning of which is ‘outcry.’ With the advent of the sound spectrograph, attention turned to the cry sounds of human infants and describing their acoustic characteristics. A series of papers by J. Lind, J. Bosma and H. Truby [58] described the cry of newborn infants along with the vocal tract and respiratory gestures associated with their production from a clinical perspective. Thereafter followed a monograph devoted to an analysis of human infant cry sounds, providing descriptive terminology of acoustic features that persist to this day [118]. Over the next decade and a half, a number of papers addressed crying from clinical and comparative perspectives. Much of this work was brought together in volumes devoted entirely to infant crying [3, 55]. Reviews pertaining to critical acoustic features of crying related to developmental or clinical issues can be found in these volumes. The use of vocalizations as a measure of the distress arising from social separation has become widespread and much of the literature devoted to these calls (hereafter, ‘crying’) is summarized in this article. This research has not been without its critics. Noteworthy was a paper by Blumberg and Sokoloff [8], who attempted to discredit claims that findings based on the most widely used animal model, the laboratory rat, were a valid measure of emotional expression, but were, instead, a byproduct of reflexive abdominal compression. Their assertion was skillfully and eloquently put to rest in a rejoinder by Panksepp [86].
Research on the mechanisms underlying crying and cry responding began considerably more recently. Perhaps the first investigator to examine the neurochemical control of crying in mammals was J. P. Scott (reviewed in [104]), who used dogs as experimental animals. Scott was also the first to conclude that separation distress was not physiologically equivalent to the emotion of anxiety, an important insight as it suggests that the neural pathways underlying the crying associated with social separation are distinct from neural pathways mediating behavioral manifestations of anxiety. A symposium devoted to the physiological control of mammalian vocalizations featured crying in humans and animal models [76]. One paper in that volume summarized the extensive work of Jaak Panksepp and colleagues on the neurochemical and neural control of ‘distress vocalizations’ (separation calls or cries) in a number of animal species [90], including the first published identification of brain substrates implicated in production of cries in guinea pigs (based on Herman’s 1979 unpublished dissertation [36]) and chickens (based on Bishop’s 1984 unpublished dissertation [7]). A symposium devoted to affective neuroscience produced a discussion of critical concepts regarding the analysis of separation-distress systems in the brain [88]. More recent papers are reviewed in the following sections.
3. What is ‘crying’?
Some investigators have taken the position that tearful emissions mark crying in humans and, as only humans produce tears, only humans, as a consequence, cry. In this paper, it is the emissions from the vocal tract, not the lachrymal gland, that are of interest.
Descriptions of vocal repertoires in non-human mammals typically do not include a category called ‘crying.’ Instead, infant vocalizations often are characterized as ‘distress calls’ or ‘separation calls’ when an infant is separated from its caregiver, a context that can result in extensive vocalizations. Contextually, human infants also produce extensive vocalizations when distressed or separated, and most observers readily accept that these sounds comprise a bout of ‘crying.’ Careful analysis of crying in human infants has shown that, while there is variability in the acoustic structure of cries according to the level of distress and some evidence in support of individual differences, the attributes that make up cry sounds are readily agreed upon and have been extensively documented in the literature (reviewed, e.g., by [51] and [111]). It seems reasonable to adopt a similar approach to the sounds of non-human mammalian infants, wherein the category of vocalizations made by distressed or separated infants would also be referred to as ‘crying.’ This would still enable a more detailed breakdown into cry sub-types (as is occasionally done with human cry sounds), where appropriate.
4. Infant cries of mammals in comparative perspective
Many mammalian infants have been found to make characteristic sounds when distressed. Aside from the contextual similarities, there can be added the structural similarities between the cries of human and non-human mammalian infants. One research group studying domestic cats considered kittens’ cries to be essentially the same sounds, acoustically and functionally, as the cries of human infants [16]. Studies of the ‘separation calls’ (i.e., cries) of mammalian infants across several mammalian orders have documented acoustic characteristics with a common theme. An analysis of the cries of a range of mammals suggested that they shared a basic acoustic structure [73].
However, acoustic analyses suggest that two basic types of infant cries are produced. One type consists of sounds that are relatively long in duration and rich in acoustic content. Another type, less common, consists of brief click-like transients. The two types appear to be characteristic of species, species-groups and possibly larger taxa. (See [79] for a list of primate examples of both types; also see Fig. 1). In some species it has been reported that both types co-occur. The first class appears to be characteristic of the majority of mammals, including some that are predominantly nocturnal. The ‘click’ class is found in a small number of species which also are largely nocturnal, and may represent a separate evolutionary history. One example of the click class would be the infant calls of Garnett’s greater bush baby, Otolemur garnettii, a prosimian primate [5, 6]. In studies of captive individuals, all infants made similar clicks. Very young infants made clicks when alone or appeared distressed. At older ages, infants made clicks in apparent dialogue with their mothers, who emitted short growls [5]. In a related species of prosimian primate, the Lesser Bushbaby (Galago moholi), Mascagni and Doyle [67] reported that infants are capable of producing click-like distress calls from the earliest age. The main function of this call appeared to alert and guide the mother to the infant, in those instances where contact between mother and infant was lost.
Figure 1.
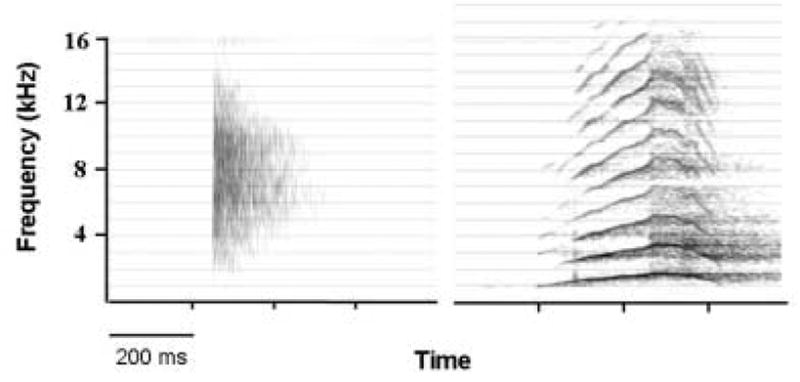
Spectrographic examples of infant cries in two primate species. These illustrate the two types of infant cry in mammals. Left) Click emitted by Garnett’s greater bushbaby (Otolemur garnetti) after being deposited on a branch by its mother at 4 days of age. Right) Coo (‘tonal’ cry) following brief maternal separation in rhesus macaque (Macaca mulatta) at 14 days of age.
Newman [71], in pointing out the structural similarities of the separation-induced cries of human and many non-human primate infants, argued that these were essentially the same sounds. Where differences in certain structural parameters occurred, these could largely be accounted for by differences in the size of the vocalizer, such that relatively larger infants made cries that were longer in duration and lower in fundamental frequency as a consequence of the more massive vocal folds and larger lung capacity. Moreover, Newman [77] reported that examples of both Old World (macaque) and New World (marmoset) primate lineages shared the same basic developmental course in terms of their crying behavior. This convergent ontogenetic course, together with the broadly similar acoustic structure of the cries of most primates, suggests either that cry acoustic structure arose early in primate evolution and has remained little changed, or that there has been a large degree of convergent evolution toward an acoustic structure that is highly adaptive in a variety of habitats and social settings. One way to resolve these alternative hypotheses is to examine the underlying brain mechanisms mediating cry production and perception. Evidence that the same neural circuitry underlies crying in a wide range of mammals would support the idea that an evolutionarily conserved system arose early and has remained largely unchanged. It would also validate the use of nonhuman mammals as models for learning about the cry circuit in humans.
5. The neural mechanisms underlying crying
The literature on the neural basis of infant crying in humans is, not surprising, both sparse and largely inferential. However, a neural model of infant crying might be called the ‘brainstem model’ as it concludes that no structures rostral to the midbrain are required, at least during the first few months of an infant’s life. This view appears to have arisen, in part, from reports that anencephalic infants cry. The precise nature of such crying appears to be lacking. Some information comes from evaluation of a hydranencephalic infant, in which the cerebral hemispheres were largely absent, although the basal ganglia, cerebellum and brainstem were present and without any gross abnormalities. This infant was reported to have a shrill cry at 30 hours post-partum and at 6 weeks of age the cry was shrill and weak [2]. Another study [80], in this case of a true anencephalic infant, reported that the infant “cried weakly but otherwise like any other infant (p. 389).” A post-mortem examination of the brain revealed that there was no brain tissue above the mesencephalic level, including the lack of a recognizable thalamus. A number of studies have been conducted in animals that provide evidence in support of a brainstem model of crying. Kyuhou and Gemba [50] found in guinea pigs that the species-specific isolation call could be elicited by electrical stimulation of the rostral periaqueductal gray (PAG) of the midbrain. In a study of infant rats, precollicular decerebrations of 8 day-old rats did not eliminate ultrasonic vocalizations given in response to social isolation and cooling [68]. The authors concluded (p. 1114): “The present results constitute proof that neural circuits at or caudal to the level of the midbrain are sufficient for organizing the week-old rat’s vocal response to cooling and isolation.”
Perhaps the earliest experimental evidence that brainstem structures can elaborate a cry in the absence of the forebrain came from a study in cats [65]. Cats were decerebrated just behind the diencephalon. Electrodes were placed on the cut surface of the midbrain or inserted deep into the caudal brainstem. Cries were elicited from the rostral midbrain, including PAG and extending through the dorsal part and into the lateral and ventral portions of the tegmental area.
In intact adult squirrel monkeys, ‘chirping’ (a category of vocalizations that includes the species-specific cry given when separated) could be elicited in the brainstem caudal to the diencephalon in the caudal periaqueductal gray and along the caudal spinothalamic tract [43]. In awake, intact macaque monkeys, recording with microelectrodes from the PAG found a close association between unit activity and vocalization. In one female, in which the predominant vocalizations were ‘coos’ (the species-specific isolation call), a cluster of units in the PAG were associated with vocalization [52].
Chemical stimulation of the brainstem has successfully evoked vocalization in several studies. Injection of D,L-homocysteic acid (an excitatory amino acid) into the caudal PAG of unanesthetized, precollicular decerebrated cats resulted in voiced sounds (howl, mew, growl) [126]. In cats, it is possible to evoke mewing sounds in adults by electrically stimulating a region in the ventrolateral pons [21]. Brain stimulation studies of the PAG or adjacent tegmentum in adult mammals have, for the most part, found that crying is rarely produced, even in species such as the squirrel monkey, where ‘crying’ (isolation calls) continues into adulthood. One exception to this, however, is a study with anesthetized guinea pigs [90]. There, electrical stimulation of sites within and adjacent to the PAG produced ‘distress vocalizations’ (judged by the authors to be equivalent to separation-induced vocalizations). In a study using adult squirrel monkeys, Newman and MacLean [75] found that bilateral lesions of the ventral PAG and adjacent midbrain tegmentum eliminated nearly entirely the production of spontaneous isolation calls, although other calls appeared to be unaffected. This same study reported that bilateral lesions of the thalamic tegmentum resulted in production of abnormal isolation calls, without changing the motivation to vocalize.
In humans, studies of brain function during crying do not exist. However, in adults, a PET study found that during voiced speech (which shares some laryngeal control mechanisms with crying), blood flow increased in the PAG, as well as the cerebellar vermis and parts of the thalamus [103]. This study also found activation in cortical regions (see below).
6. Forebrain mechanisms of cry production
As mentioned above, the existing model for cry production is essentially a brainstem model, with no role for the cortex, and with limited evidence for other structures within the cerebral hemispheres playing a role in manipulating crying behavior. However, one cortical region is now recognized to be involved in vocal production, and some evidence in nonhuman primates supports its role in crying. The region in question is the cingulate gyrus, also referred to as ‘limbic cortex’, particularly in non-primate mammals where its differentiation is less well developed. As outlined by MacLean [62], the thalamo-cingulate pathway is one of the three major divisions of the limbic system, the others being the amygdalar and septal divisions. MacLean reasoned that since the thalamo-cingulate division had no clear counterpart in non-mammalian vertebrates, it probably served functions that were unique to mammals. Among the functions he considered were infant crying and mother-infant communication [61, 63]. Evidence for a role of the cingular cortex in vocalization comes from a variety of sources. Smith [110], working with macaque monkeys lightly anesthetized with ether, found that electrical stimulation of the exposed surface of the anterior cingulate gyrus resulted in complex movements and autonomic changes. Smith’s observations deserve repeating in their entirety (p. 242–3):”The most striking response obtained from the rostral cingular cortex is vocalization, identical to that which the animal makes under the usual conditions of laboratory existence.” Later, he goes on to say (p. 243):”The sounds emitted run nearly the whole gamut which the monkey is capable of producing and are impossible to describe in writing. While the low-pitched guttural sound is the one most frequently obtained, it gives place at times to higher pitched cooing sounds, at other times to cries, soft and plaintive, such as the animals make at feeding time.” Anatomically, the electrically responsive cortex fell within Brodmann’s area 24, a region that is cytoarchitectonically agranular and without clear cortical layers. Area 23, comprising the caudal portion of the cingulate gyrus and cytoarchitectonically distinct in having granularity and layering, was unresponsive to electrical stimulation. The ‘cooing sounds’ mentioned in this paper are likely acoustically similar to the cry sounds of rhesus infants (see [32] for a detailed acoustic analysis), hence Smith’s study represents perhaps the first indication that the anterior cingulate region is part of the cry circuit, at least in nonhuman primates. In an extensive study involving lightly anesthetized monkeys (macaques and guenons), cats and dogs, Kaada [44] found that vocalizations could be evoked in about half of the monkeys (but none of the cats or dogs) by stimulating the anterior ends of the limbic and hippocampal gyri. The effective region was quite localized, being limited to “an area in the forward upper portion of the limbic gyrus, and to banks of the cingulate sulcus” (p.164). As was the case with Smith’s 1945 study, the vocalizations resembled those heard from monkeys daily in their home cage, and varied from low cooing sounds to high pitched cries. Bilateral ablation of motor cortex was without effect on the vocalization evoked by stimulating limbic or hippocampal cortex. In 1967, Robinson [99] published the first detailed study of electrically evoked vocalization in awake primates. In his study, the subjects were male macaques, chaired and restrained so that electrodes could be lowered systematically and a wide area of the brain covered. Notable was the finding that positive stimulation sites were found in and near the anterior cingulate gyrus. The typical evoked vocalization from this region was characterized as “… a soft, high-pitched descending call similar to the separation call…(p. 349).” Soon thereafter, Jürgens and Ploog [43] did a similar study in awake squirrel monkeys. In this case, the neuroanatomically identified positive sites for vocalization were supported with audiospectrographic displays of the evoked vocalizations. The results were broken down by type of vocalization evoked and the associated neuroanatomical sites from which each vocal sub-type was evoked. Their ‘chirping calls’ category, including the species-specific separation call, could be evoked by stimulation in the anterior cingulate gyrus (and the subcallosal cortex immediately ventral to it), the midline thalamus, rostral hippocampus, caudal periaqueductal gray, and along the caudal spinothalamic tract.
Lesioning studies of the anterior cingulate gyrus support the brain stimulation studies cited above. MacLean and Newman [64] reported that ablation of the rostral midline cortex, including areas 24, 25 and 12 (as defined by Rosabal [100]) resulted in long-lasting elimination or profound reduction in production of separation-induced cries in adult squirrel monkeys. Ablation of the overlying neocortex had only transient effect on separation call production. Calls produced in other contexts, including feeding, physical restraint and alarm, appeared to be unaffected. In a series of experiments aimed at testing the role of the cerebral cortex in controlling monkey phonation, it was found that ablations of transitional parieto-occipital cortex and temporal association cortex had no effect on conditioned vocalizations, whereas bilateral removal of the anterior cingulate and subcallosal gyrus resulted in loss of the ability to produce conditioned vocalizations [114]. In all subjects, the call emitted at the end of the conditioning process was a prolonged coo. Interpretation of the effects of cingulate damage remains uncertain, given that cingulate lesions affect the overall social interaction with familiar conspecifics [33].
One other forebrain structure implicated in crying is the amygdala. One study, using infant rhesus macaques, demonstrated that bilateral ablations led to changes in cry structure suggestive of a blunted affect [78]. Another study in which neonatal ablations of the amygdala was performed and infants tested shortly after weaning, found that lesioned animals screamed less and failed to show a preference for their mother in tests involving dyadic interactions with the mother or another female [4].
No study has been performed on brain activation during crying in human infants. However, given the likelihood that similar laryngeal action is involved in both crying and production of voiced sounds in adults, a PET study describing cortical activation during production of voiced speech is of interest. Shulz, et al. [103] found significant elevation of regional blood flow in the inferior operculum of the prefrontal gyrus, the medial prefrontal cortex and the anterior cingulate cortex in voiced but not during whispered speech.
7. Neurochemical control of crying
Given the lack of widespread acceptance of ‘crying’ as a functional category in animal communication, it is not surprising that the neural basis of crying from a comparative perspective has been correspondingly neglected. Nevertheless, if one accepts that the distress calls of separated infants represent crying, then a literature on the neurochemistry of crying is rather extensive. A seminal paper titled ‘The Neurochemical Control of Crying’ [89] dealt with behavioral pharmacological studies of domestic chicks. As will be reviewed below, many of the same neurochemical systems shown to mediate distress calling (‘crying’) in chicks are also active in mammals, but the importance of a particular neural system found only in mammals (the thalamo-cingulate division of the limbic system of MacLean) will limit this review to the mammalian literature for the most part. The neurochemical system most clearly associated with crying is the opiate system. Panksepp, et al. [87] reviewed the literature for an opioid effect on separation-induced crying in puppies, young guinea pigs and chicks. These authors made a clear case for recognizing social attachment, and its vocal concomitants when separation between an infant and its attachment figure occurs, as a primary emotion. As they state (p. 473):” This reaction [crying] to separation is immediate, reflex-like and consistent across different animals, and its expression appears to require no previous learning.” They state further (p. 473): “Because of the vigor and ubiquity of this emotional response to social separation, it seems likely that social motivation is a direct manifestation of innate neural circuits which are as spontaneously responsive as those which govern other basic motivated behavior patterns such as feeding and drinking.” Morphine (classical opiate agonist) and naloxone (opiate antagonist) have been widely used in experimental studies, and a reduction in crying following morphine which is reversible or blocked by naloxone is taken as evidence for the activation of an endogenous opiatergic system. Subsequent experiments with guinea pigs demonstrated that centrally evoked distress cries could be modulated by externally administered naloxone, in that the drug resulted in an increase in rate of calling for a given set of stimulation parameters [37]. Other investigators have provided additional evidence for an important role for opiates in modulating the vocal response to social separation. Kalin, et al. [45] found that morphine significantly reduced isolation-induced cries in rhesus macaque infants, naloxone significantly increased the cries over control (no drug) levels, and that naloxone blocked the morphine effect. Preweanling rat pups, along with their importance as developmental models in other studies, have been found to exhibit an opioid-sensitive crying response when separated from littermates and dam. As there are several sub-types of opioid receptors in the brain (morphine and naloxone working at the mu receptor), studies have attempted to determine which receptor subtypes are involved in rat pup isolation calling. Carden, et al. [17] found that ligands that are agonists to mu, delta and kappa opioid receptors all effect crying rate in rat pups. However, while the mu and delta agonists suppressed crying, the kappa agonist actually increased crying. The authors concluded that the three receptor subtypes may work in concert or in opposition to each other, or may function independently within individual time frames. Precisely where in the brain these opioid-based changes in crying occur is a topic of great interest and importance. Based on a review of the literature, Panksepp, et al. [91] concluded that the highest brain region elaborating social loss is the cingulate gyrus and related connections. With this in mind, it is of considerable interest that opiate receptor density in the limbic cortex of rats (which includes the cingulate gyrus) is high [56]. Similarly, high levels of opiate receptor were found in the cingulate cortex of rhesus macaque monkeys [123].
Another neurochemical receptor system implicated in infant crying is the alpha-2 adrenoreceptor system. In a non-human primate, Harris and Newman [34] found that the alpha2-adrenoreceptor agonist clonidine reduced isolation calls in adults in a dose-dependent manner. This effect was reversed by the alpha2-adrenoreceptor antagonist yohimbine, but not by the alpha1-adrenoreceptor antagonist prazosin. Another study [69] confirmed the increased crying following administration of a kappa receptor agonist, and found that an alpha-2 adrenoreceptor antagonist, yohimbine, attenuated kappa agonist-induced crying increases, as well as attenuating crying increases due to an alpha-2 adrenoreceptor agonist, clonidine. The authors concluded that a shared mechanism underlies the effects of clonidine and the κ-agonist on infant rat pup crying. Kehoe [47], working with rat pups, likewise implicated alpha2-adrenergic mechanisms in isolation-induced crying. Here, clonidine exerts different effects depending on the age of the infant. At 10 days of age, clonidine produced a dose-dependent increase in crying, whereas at 17 days, clonidine had a differential effect, increasing crying at some doses, but reducing crying at other doses.
Other neurochemical systems have been implicated in regulating isolation call production, e.g., [9, 42, 46 and 122]. These include the benzodiazepine receptor complex, as well as cholinergic and serotonergic pathways. A review of some of the evidence for neurochemical control of vocalizations in anxiety-provoking circumstances has been presented by Newman [72]. In addition, the neuropeptides vasopressin (AVP) and oxytocin (OT) have attracted a lot of interest, due to the evidence that they are involved in regulating social attachment behavior in rodents and non-human primates [39, 41, 42, 49, 70, 92, 120, 121].
The question of where neuropeptides and neurotransmitter receptor ligands exert their action in the brain is of paramount importance. Both oxytocin and vasopressin have been localized to the amygdala, both with respect to receptors and to the effects of these neuropeptides on specific neuronal populations, e.g., [38] and [125]. Oxytocin in the medial amygdala has also been implicated in the processes underlying early social recognition in mice [27].
8. Crying in the ear of the beholder: the perceptual side of the cry circuit
Crying is typically a loud and conspicuous behavior. Given by a helpless infant would put the infant at great risk for discovery and predation were it not for the fact that the infant’s mother or other caregiver hears and responds to the cries, as well. Since the mother usually has a better notion of where the crying infant is than a potential predator does, and is also likely to respond more quickly to the cries in a noisy environment where there are lots of other competing sounds, this crying and retrieval behavioral circuit has likely been strongly selected for over the course of mammalian evolution. A number of studies have been conducted on portions of the mammalian auditory pathways with respect to species-specific vocalization responses. In cats, medial geniculate (auditory thalamic area) nucleus units were found responsive to the kitten isolation call, with approximately 30% in the caudal division responding only to the vocal stimulus and not to other auditory stimuli [15]. In the cat auditory cortex, kitten meow evoked responses were found in a number of multi-unit records. However, responses in general were stronger to low- or high-frequency harmonics of the natural call than to the call, itself [30]. In squirrel monkeys, the species-specific isolation call evokes responses from single units in the auditory cortex. However, responses were mainly to specific temporal components of the vocal stimulus [74, 124].
9. Crying and parental behavior
The internal neural substrates mediating parenting behavior have been examined, although much of this work has been done only in laboratory rat females. An in-depth review of the literature on the neural substrates of rat maternal behavior is beyond the scope of this paper. However, some of this work is of relevance to the present paper, as infant vocal behavior has been used as a measure of lesion effects on maternal behavior. First, however, the evidence that infant cries elicit maternal (parental) behavior needs to be examined.
Mother rats (Rattus norvegicus) approach and maintain proximity to a pup that is emitting cries (ultrasonic vocalizations) significantly more than do virgin rats [25]. This study showed that a warm, silent pup together with recorded ultrasounds was required to elicit approach of maternal or virgin females, the former remaining in proximity of the pup for a significantly longer period of time. A subsequent experiment [26] demonstrated that odors from the pup were important in a mother seeking a pup and maintaining close proximity. Thus, based on these experiments, the pup cries were insufficient, by themselves, to elicit parental behavior in mothers but did appear to activate the initial seeking behavior. Similar experiments with mice have shown the importance of infant cries on maternal responsiveness, e.g., [82] (and conversely, the importance of maternal attentiveness on the amount of infant crying [19]). Some information is available on the acoustic dimensions of rodent pup ultrasounds in eliciting maternal responding. Ehret and Haack [24] found that synthesized tones of a limited range of durations and major frequency components above 40 kHz elicited responses as strong as the natural ultrasonic calls of mouse pups. The neural circuitry underlying this response appears to be lateralized to the left hemisphere [23].
Evidence for caregiver responses to crying exists in other mammals. In humans, where crying has long been a subject of interest in the developmental psychology and pediatric literature, e.g. [51], [111] and [119], the responsiveness of the mother to an infant’s cry has been studied in detail. In non-human primates, playback studies have shown that a squirrel monkey mother recognizes its infant, based solely on the individual acoustic characteristics of its cries [115]. Similarly, mothers responded to the calls of their own infant more than to the calls of an unrelated infant in Japanese macaques [109]. In domestic sheep, ewes and lambs exchange bleats, apparently playing a role in facilitating recognition each other and distinguishing the mother/infant pair from other ewes and lambs [117]. In sheep, a simple frequency code distinguishes the calls of different mothers and infants [105]. In other herd-living mammals, infants likewise recognize their mothers based on the acoustic structure of their vocalizations (e.g., fur seals [18]). In bottlenose dolphins, mothers and offspring engage in vocal exchanges using ‘signature whistles,’ highly stable calls wherein the detailed acoustic structure of male (but not female) offspring whistles match those of the mother [102].
10. The neural circuitry underlying cry perception
The neural structures mediating recognition of cries in humans have not been studied extensively, but some information is available. An fMRI study of maternally experienced women provided evidence of activation by the cries of human neonates in several brain areas, notably the anterior cingulate cortex [59], as well as other forebrain structures [60]. A similar study using infant cries tested parents and nonparents. Paradoxically, this study showed that the anterior cingulate gyrus had decreased activity in women, whether parents or not. Parents of both sexes showed a greater activation in the amygdala to cries than to laughter [106]. One study in which cry sounds were produced by adult actors, found that the right amygdala was activated to a greater extent than the corresponding region in the left hemisphere [101]. In non-human mammals, some information is available regarding the specific structures in the brain related to maternal responsiveness to crying. Many of the earlier studies, involving assessing the behavioral effects of experimental destruction or inactivation of specific neural structures in rodents, identified the medial preoptic area (mpoa) as an essential part of the circuit and important in a range of behavioral elements making up maternal care of infants (reviewed in [83] and [84]). Other studies, using expression of the c-fos gene, confirmed the importance of the mpoa, as well as other structures implicated in the activation and inhibition of maternal behavior (e.g., [108, 112]). A recent review [29] has provided more detail regarding which of the various components associated with maternal behavior in rodents can be assigned to specific neural circuits. However, none of the studies specifically identified the circuitry involved in detecting and discriminating infant cries, or the circuitry underlying the activation of caregiver responses to the cries.
11. Neurochemistry of cry perception
With respect to the neurochemical control of listeners’ responses to crying, the literature lacks specificity on this issue. However, there have been studies of the role of opiates and, more recently, of oxytocin, on infant retrieval. Studies by Bridges and Grimm [10, 31] showed that morphine disrupted the onset and quality of maternal responsiveness, an effect that concurrent treatment with naloxone prevented. Subsequently, it was determined that c-fos activity in the mpoa of lactating rats was also reduced by morphine and restored by naloxone [113]. With respect to oxytocin, its effects on maternal behavior were tested directly by administering oxytocin into the cerebral ventricles of intact virgin rats. Nearly half of the subjects displayed full maternal behavior towards foster pups. Saline- or vasopressin-treated animals showed no maternal behavior. The oxytocin-induced maternal behavior was facilitated by concomitant administration of estradiol benzoate, to simulate the hormonal status of females in estrus [92]. Oxytocin in the nucleus accumbens has been associated with parental vigor in caring for pups in the prairie vole [85]. In rats, parturition activates FosB and c-fos, two immediate early genes, in cells expressing oxytocin receptors as well as in oxytocinergic neurons [57]. Some question has been raised regarding whether oxytocin is essential for normal maternal behavior, given that OT-knockout mice display normal maternal behavior [81]. In humans, oxytocin administration was found to reduce activation in the amygdala to fear-evoking visual stimuli [49]. No one, as yet, appears to have done a similar study using cry stimuli.
12. Relationships between infant crying and parental hormones
It is widely accepted that maternal hormones associated with parturition and lactation play an important role in activating and maintaining parental behavior, essential in the mother who must maintain milk production and accept the nursing infant. Considerable evidence in animal studies has determined that these hormonal changes exert their effects on specific areas of the brain. Recent reviews of these studies can be found in Leckman and Herman [53] and Kinsley and Lambert [48]. Of particular interest is prolactin, a peptide prohormone produced in the anterior pituitary (as well as other tissues) associated with, among other things, milk production in the mammary glands. In the context of the present paper, it has been shown that exposure to rat pup ultrasounds causes a dramatic increase in prolactin, as well as maternal behaviors in lactating mother rats [35]. Several papers by Bridges and colleagues have established that centrally administered prolactin (and related placental lactogenic peptides) stimulates maternal behavior in steroid-primed female rats [11, 12, 13, 66]. None of these studies specifically focused on responsiveness to infant crying in response to the prolactin administration. While evidence for prolactin release associated with infant crying in human mothers appears to be lacking, human fathers do show a relationship between their emotional responses to infant crying and prolactin levels [28]. One study has reported that crying increases the temperature of the lactating breast [116]. Prolactin has also been associated with infant carrying in nonhuman primates. Prolactin levels are elevated after infant carrying in marmoset fathers [22], as well as in parentally inexperienced males and females [97]. Further, bromocriptine, a dopamine agonist, lowers serum prolactin and disrupts parental behavior in marmosets [98]. However, this latter finding has been called into question [1]. A final area of consideration is the relationship between steroid hormones, particularly estrogen, in responding to cries. Lee, et al. [54] showed that mother rats will bar-press to gain access to pups. Similarly, Pryce (reviewed in [93]) showed that female marmosets will bar-press to gain access to infants, even when the only stimulus is a recording of infant crying. The relationship between these behaviors and estrogen is indirect, but, at least in marmosets (and the related red-bellied tamarin), estrogen levels correlate with level of parental care [94].
13. Linkage of the cry circuit to other vocal networks
The cry circuit represents, at least from my perspective, the evolutionarily earliest form of vocal communication network in mammals. Following the emergence of the neural substrates mediating mothers’ responding to crying infants by retrieval, and perhaps in roughly this order in evolutionary history, came circuits mediating mothers calling back to their crying infants, followed by the emergence of other vocal signals that fostered closer range communication between mothers and their infants, and by circuits for calling that promoted integrity within larger social groupings. The neural substrates that mediate these different levels of vocal engagement have scarcely begun to be studied. It seems more likely that incorporation of parts of the cry circuit into other vocal circuits that promote affiliative interactions has occurred, rather than for each of these sub-types of communication network to have evolved a separate set of circuit elements. However, that remains an issue that will probably be unresolvable with present methods.
14. Suggestions for future studies
Much remains to be done on defining the mammalian cry circuit. Many types of studies that are part of the large field of cry research can contribute. With respect to hormonal modulation of the responsiveness to cries, evidence exists in humans [28] and non-human primates [94, 95, 96] that hormonal status influences responsiveness to cries. This work supplements the extensive evidence using rodents as research subjects that hormonal status has a profound influence on maternal responsiveness to infants, some of which was reviewed earlier in this paper. There is the temptation to focus strictly on the cry circuit as a stand-alone entity in the brain. However, given the strong evidence that caregiver-infant interactions utilize several sensory modalities (particularly, from a comparative perspective, olfaction) it will be important to study the interaction of different modalities in brain circuits related to the perceptual side of the cry circuit. The production side presents somewhat different challenges. The infant cry circuit doubtless changes with development. The nature of the changes, in terms of gene activation and neural architecture, will be technically difficult but conceptual pretty straightforward to determine. Various issues related to the neurochemical control of crying remain to be resolved, including the potentiation of crying when the mother is nearby (e.g., [107]). The growing availability of molecular genetic techniques applied to behavioral phenotypes opens up a huge research area for study, particularly with respect to the role of specific genes in regulating crying and cry responsivity, and the interaction of genetic and environmental factors, e.g., [14]. It is my hope that the present review will serve a useful role in focusing attention on the importance of the cry circuit in mammalian evolution, and help to stimulate a range of future studies.
15. Discussion and Conclusions
I have reviewed the evidence identifying which neural structures and related neurochemical systems are part of the ‘cry circuit,’ i.e., the circuitry underlying the production of cry sounds and the circuitry in the listener that is activated upon hearing cries leading to a behavioral response. Working out the complete circuit is far from finished, but a beginning outline of the schematic (on the production side) would include the anterior cingulate gyrus (in particular that part in front of and beneath the genu of the corpus callosum), midline thalamic structures connected to this cortical region (comprising the thalamo-cingulate division of the limbic system of MacLean), possibly the amygdala, portions of the periaqueductal gray and adjacent tegmental regions of the midbrain, an as-yet poorly defined region in the pons near the spino-thalamic tract, and a portion of the nucleus ambiguus (containing the motor neurons innervating the laryngeal muscles). On the receiver side, the amygdala, medial preoptic area, cingular gyrus, and portions of temporal lobe auditory cortex are all probably involved, but how they are wired up and under what conditions they become active are unclear. A summary of the relevant structures is presented in Table 1. As to neurochemical systems implicated in the circuit, the evidence is strongest for the opiatergic system, followed by oxytocin pathways, in both production and receptive parts of the circuit. ‘Crying,’ as a behavior, is argued to be a ubiquitous mammalian trait, found in all mammalian infants and in the adults of some species. Recognition of the widespread existence and importance of this behavior in the evolution of mammals and in the survival of mammalian infants may generate a more intensive investigation of the neuroanatomical basis of crying than presently exists. Ideally, this will provide benefits to the health of human infants, as well as provide greater insight into an important functional circuit of the mammalian brain.
Table 1.
Brain Structures in the Mammalian Cry Circuit
| Neural Structure | Production (P) Responding (R) | Primary Sources | Additional Sources | Species
Rodent (R) Primate (P) Cat (C) Human (H) |
|---|---|---|---|---|
| Periaqueductal Gray (midbrain) | P | 38, 45, 47, 58 | 17, 68, 80, 114 | R, P, C |
| Thalamic Tegmentum | P | 68 | P | |
| Amygdala | P, R | 3, 70, 91, 95 | 23 | P, R, H |
| Medial Preoptic Area | R | 75, 76 | R | |
| Anterior Cingulate Gyrus | P, R | 39, 57, 99, 103 | 38, 52, 89 | P, H |
| Medial Geniculate nucleus | R | 12 | C | |
| Auditory Cortex | R | 26, 67, 112 | C, P |
Acknowledgments
This paper is dedicated to the memory of Prof. Dr. Detlev Ploog, who died at the age of 85 on December 7, 2005. He contributed significantly to my training in neuroethology and was an inspiration to many in the field. I wish to thank Dr. Jaak Panksepp, who reviewed an earlier version of the manuscript, and Dr. Michelle Becker, who prepared Figure 1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Almond RE, Brown GR, Keverne EB. Suppression of prolactin does not reduce infant care by parentally inexperienced male common marmosets (Callithrix jacchus) . Horm Behav. 2006;49:673–680. doi: 10.1016/j.yhbeh.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 2.Barnet A, Bazelon M, Zapella M. Visual and auditory function in an hydranencephalic infant. Brain Res. 1966;2:351–360. doi: 10.1016/0006-8993(66)90004-7. [DOI] [PubMed] [Google Scholar]
- 3.Barr RG, Hopkins B, Green JA, editors. Clinics in Developmental Medicine no. 152. Cambridge University Press; Cambridge United Kingdom: 2000. Crying as a Sign, Symptom and Signal. [Google Scholar]
- 4.Bauman MD, Lavenex P, Mason WA, Capitanio JP, Amaral DG. The development of mother-infant interactions after neonatal amygdala lesions in rhesus monkeys. J Neurosci. 2004;24:711–721. doi: 10.1523/JNEUROSCI.3263-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becker ML. Vocal communication in the small-eared bushbaby (Otolemur garnettii): Morphology, sound structure, and social context (Doctoral dissertation, The University of Memphis, 2000) Diss Abstr Int-B. 2001;61(11):6169. [Google Scholar]
- 6.Becker ML, Buder EH, Ward JP. Spectrographic description of vocalizations in captive Otolemur garnettii. Int J Primatol. 2003;24:415–446. [Google Scholar]
- 7.Bishop P. Unpublished doctoral dissertation. Bowling Green State University; Bowling Green Ohio: 1984. Brain and opiate modulation of avian affective vocalizations. [Google Scholar]
- 8.Blumberg MS, Sokoloff G. Do infant rats cry? Psych Rev. 2001;108:83–95. doi: 10.1037/0033-295x.108.1.83. [DOI] [PubMed] [Google Scholar]
- 9.Branchi I, Campolongo P, Alleva E. Scopolamine effects on ultrasonic vocalization emission and behavior in the neonatal mouse. Behav Brain Res. 2004;151:9–16. doi: 10.1016/S0166-4328(03)00277-8. [DOI] [PubMed] [Google Scholar]
- 10.Bridges RS, Grimm CT. Reversal of morphine disruption of maternal behavior by concurrent treatment with the opiate antagonist naloxone. Science. 1982;218:166–168. doi: 10.1126/science.7123227. [DOI] [PubMed] [Google Scholar]
- 11.Bridges RS, Freemark MS. Human placental lactogen infusions into the medial preoptic area stimulate maternal behavior in steroid-primed, nulliparous female rats. Horm Behav. 1995;29:216–226. doi: 10.1006/hbeh.1995.1016. [DOI] [PubMed] [Google Scholar]
- 12.Bridges RS, Numan M, Ronsheim PM, Mann PE, Lupini CE. Central prolactin infusions stimulate maternal behavior in steroid-treated, nulliparous female rats . Proc Natl Acad Sci USA. 1990;87:8003–8007. doi: 10.1073/pnas.87.20.8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bridges RS, Robertson MC, Shiu RP, Sturgis JD, Henriquez BM, Mann PE. Central lactogenic regulation of maternal behavior in rats: steroid dependence, hormone specificity, and behavioral potencies of rat prolactin and rat placental lactogen I. Endocrinology. 1997;138:756–763. doi: 10.1210/endo.138.2.4921. [DOI] [PubMed] [Google Scholar]
- 14.Brunelli SA. Selective breeding for an infant phenotype: rat pup ultrasonic vocalization (USV) Behav Genet. 2005;35:53–65. doi: 10.1007/s10519-004-0855-6. [DOI] [PubMed] [Google Scholar]
- 15.Buchwald J, Dickerson L, Harrison J, Hinman C. Medial geniculate body responses to cat cries. In: Syka J, Masterton RB, editors. Auditory Pathway. New York: Plenum Publishing Corp; 1988. pp. 319–322. [Google Scholar]
- 16.Buchwald JS, Shipley C, Altafullah I, Hinman C, Harrison J, Dickerson L. The feline isolation call. In: Newman JD, editor. The Physiological Control of Mammalian Vocalization. New York: Plenum Press; 1988. pp. 119–135. [Google Scholar]
- 17.Carden SE, Barr GA, Hofer MA. Differential effects of specific opioid receptor agonists on rat pup isolation calls. Dev Brain Res. 1991;62:17–22. doi: 10.1016/0165-3806(91)90185-l. [DOI] [PubMed] [Google Scholar]
- 18.Charrier I, Mathevon M, Jouventin P. Vocal signature recognition of mothers by fur seal pups. Anim Behav. 2003;65:543–550. [Google Scholar]
- 19.D’Amato FR, Scalera E, Sarli C, Moles A. Pups call, mothers rush: does maternal responsiveness affect the amount of ultrasonic vocalizations in mouse pups? Behav Genet. 2005;35:103–112. doi: 10.1007/s10519-004-0860-9. [DOI] [PubMed] [Google Scholar]
- 20.Darwin C. The Expression of the Emotions in Man and Animals. University of Chicago Press; Chicago: 1965. [Google Scholar]
- 21.De Lanerolle NC, Lang FF. Functional neural pathways for vocalization in the domestic cat. In: Newman JD, editor. The Physiological Control of Mammalian Vocalization. New York: Plenum Press; 1988. pp. 21–41. [Google Scholar]
- 22.Dixson AF, George L. Prolactin and parental behaviour in a male New World primate. Nature. 1982;299:551–553. doi: 10.1038/299551a0. [DOI] [PubMed] [Google Scholar]
- 23.Ehret G. Left hemisphere advantage in the mouse brain for recognizing ultrasonic communication calls. Nature. 1987;325:249–251. doi: 10.1038/325249a0. [DOI] [PubMed] [Google Scholar]
- 24.Ehret G, Haack B. Ultrasound recognition in house mice: key-stimulus configuration and recognition mechanism. J Comp Physiol A. 1982;148:245–251. [Google Scholar]
- 25.Farrell WJ, Alberts JR. Stimulus control of maternal responsiveness to Norway rat (Rattus norvegicus) pup ultrasonic vocalizations. J Comp Psychol. 2002;116:297–307. doi: 10.1037/0735-7036.116.3.297. [DOI] [PubMed] [Google Scholar]
- 26.Farrell WJ, Alberts JR. Maternal responsiveness to infant Norway rat (Rattus norvegicus) ultrasonic vocalizations during maternal behavior cycle and after steroid and experiential induction regimens . J Comp Psychol. 2002a;116:286–296. doi: 10.1037/0735-7036.116.3.286. [DOI] [PubMed] [Google Scholar]
- 27.Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fleming AS, Corter C, Stallings J, Steiner M. Testosterone and prolactin are associated with emotional responses to infant cries in new fathers. Horm Behav. 2002;42:399–413. doi: 10.1006/hbeh.2002.1840. [DOI] [PubMed] [Google Scholar]
- 29.Gammie SC. Current models and future directions for understanding the neural circuitries of maternal behavior in rodents. Behav Cog Neurosci Rev. 2005;4:119–135. doi: 10.1177/1534582305281086. [DOI] [PubMed] [Google Scholar]
- 30.Gehr DD, Komiya H, Eggermont JJ. Neuronal responses in cat primary auditory cortex to natural and altered species-specific calls. Hearing Res. 2000;150:27–42. doi: 10.1016/s0378-5955(00)00170-2. [DOI] [PubMed] [Google Scholar]
- 31.Grimm CT, Bridges RS. Opiate regulation of maternal behavior in the rat. Pharmacol Biochem Behav. 1983;19:609–616. doi: 10.1016/0091-3057(83)90336-2. [DOI] [PubMed] [Google Scholar]
- 32.Hammerschmidt K, Newman JD, Champoux M, Suomi SJ. Changes in rhesus macaque ‘coo’ vocalizations during early development. Ethology. 2000;106:873–886. [Google Scholar]
- 33.Handland KA, Rushworth MFS, Gaffan D, Passingham RE. The effect of cingulated lesions on social behaviour and emotion. Neuropsychologia. 2003;41:919–931. doi: 10.1016/s0028-3932(02)00325-1. [DOI] [PubMed] [Google Scholar]
- 34.Harris JC, Newman JD. Mediation of separation distress by α2-adrenergic mechanisms in a non-human primate. Brain Res. 1987;410:353–356. doi: 10.1016/0006-8993(87)90337-4. [DOI] [PubMed] [Google Scholar]
- 35.Hashimoto H, Saito TR, Furudate S, Takahashi KW. Prolactin levels and maternal behavior induced by ultrasonic vocalizations in the rat pup . Exp Anim. 2001;50:307–312. doi: 10.1538/expanim.50.307. [DOI] [PubMed] [Google Scholar]
- 36.Herman BH. Unpublished doctoral dissertation. Bowling Green State University; Bowling Green Ohio: 1979. An exploration of brain social attachment substrates in guinea pigs. [Google Scholar]
- 37.Herman BH, Panksepp J. Ascending endorphin inhibition of distress vocalization. Science. 1981;211:1060–1062. doi: 10.1126/science.7466377. [DOI] [PubMed] [Google Scholar]
- 38.Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308:245–248. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- 39.Insel TR. A neurobiological basis of social attachment. Am J Psychiatry. 1997;154:726–735. doi: 10.1176/ajp.154.6.726. [DOI] [PubMed] [Google Scholar]
- 40.Insel T, Miller L, Gelhard R, Hill J. Rat pup ultrasonic isolation calls and the benzodiazepine receptor. In: Newman JD, editor. The Physiological Control of Mammalian Vocalization. New York: Plenum Press; 1988. pp. 331–342. [Google Scholar]
- 41.Insel TR. Oxytocin—a neuropeptide for affiliation: evidence from behavioral, receptor autoradiographic, and comparative studies. Psychoneuroendocrinology. 1992;17:3–35. doi: 10.1016/0306-4530(92)90073-g. [DOI] [PubMed] [Google Scholar]
- 42.Insel TR, Young LJ. Neuropeptides and the evolution of social behavior. Curr Opin Neurobiol. 2000;10:784–789. doi: 10.1016/s0959-4388(00)00146-x. [DOI] [PubMed] [Google Scholar]
- 43.Jürgens U, Ploog D. Cerebral representation of vocalization in the squirrel monkey. Exp Brain Res. 1970;10:532–554. doi: 10.1007/BF00234269. [DOI] [PubMed] [Google Scholar]
- 44.Kaada BR. Somatomotor, autonomic and electrocorticographic responses to electrical stimulation of ‘rhinencephalic’ and other structures in primates, cat and dog. Acta Physiol Scand. 1951;24 (Suppl 83):3–285. [PubMed] [Google Scholar]
- 45.Kalin NH, Shelton SE, Barksdale CM. Opiate modulation of separation-induced distress in non-human primates. Brain Res. 1988;440:285–292. doi: 10.1016/0006-8993(88)90997-3. [DOI] [PubMed] [Google Scholar]
- 46.Kalin NH, Shelton SE, Barksdale CM. Separation distress in infant rhesus monkeys: effects of diazepam and Ro 15–1788. Brain Res. 1987;408:192–198. doi: 10.1016/0006-8993(87)90371-4. [DOI] [PubMed] [Google Scholar]
- 47.Kehoe P. Ontogeny of adrenergic and opioid effects on separation vocalizations in rats. In: Newman JD, editor. The Physiological Control of Mammalian Vocalization. New York: Plenum Press; 1988. pp. 301–320. [Google Scholar]
- 48.Kinsley CH, Lambert KG. The maternal brain. Sci Amer. 2006;294:72–79. doi: 10.1038/scientificamerican0106-72. [DOI] [PubMed] [Google Scholar]
- 49.Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, Gruppe H, Mattay VS, Gallhofer B, Meyer-Lindenberg A. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kyuhou S-I, Gemba H. Two vocalization-related subregions in the midbrain periaqueductal gray of the guinea pig. NeuroReport. 1998;9:1607–1610. doi: 10.1097/00001756-199805110-00064. [DOI] [PubMed] [Google Scholar]
- 51.LaGasse LL, Neal AR, Lester BM. Assessment of infant cry: acoustic cry analysis and parental perception. Ment Retard Dev Dis Res Rev. 2005;11:83–93. doi: 10.1002/mrdd.20050. [DOI] [PubMed] [Google Scholar]
- 52.Larson CR. On the relation of PAG neurons to laryngeal and respiratory muscles during vocalization in the monkey. Brain Res. 1991;552:77–86. doi: 10.1016/0006-8993(91)90662-f. [DOI] [PubMed] [Google Scholar]
- 53.Leckman JF, Herman AE. Maternal behavior and developmental psychopathology . Biol Psychiatry. 2002;51:27–43. doi: 10.1016/s0006-3223(01)01277-x. [DOI] [PubMed] [Google Scholar]
- 54.Lee A, Clancy S, Fleming AS. Mother rats bar-press for pups: effects of lesions on the mpoa and limbic sites on maternal behavior and operant responding for pup-reinforcement . Behav Brain Res. 1999;100:15–31. doi: 10.1016/s0166-4328(98)00109-0. [DOI] [PubMed] [Google Scholar]
- 55.Lester BM, Boukydis CFZ. Infant Crying: Theoretical and Research Perspectives. Plenum Press; New York: 1985. [Google Scholar]
- 56.Lewis ME, Pert A, Pert CB, Herkenham M. Opiate receptor localization in rat cerebral cortex. J Comp Neurol. 1983;216:339–358. doi: 10.1002/cne.902160310. [DOI] [PubMed] [Google Scholar]
- 57.Lin SH, Kiyohara T, Sun B. Maternal behavior: activation of the central oxytocin receptor system in parturient rats? NeuroReport. 2003;14:1439–1444. doi: 10.1097/00001756-200308060-00007. [DOI] [PubMed] [Google Scholar]
- 58.Lind J, editor. Acta Paeditrica Scandinavica. Almqvist and Wiksells; Uppsala: 1965. Newborn Infant Cry. Supplement 163. [Google Scholar]
- 59.Lorberbaum JP, Newman JD, Horwitz AR, Dubno JR, Lydiard RB, Hamner MB, Bohning DE, George MS. A potential role for thalamocingulate circuitry in human maternal behavior. Biol Psychiatry. 2002;51:431–445. doi: 10.1016/s0006-3223(01)01284-7. [DOI] [PubMed] [Google Scholar]
- 60.Lorberbaum JP, Newman JD, Dubno JR, Horwitz AR, Nahas Z, Teneback CC, Bloomer CW, Bohning DE, Vincent D, Johnson MR, Emmanuel N, Brawman-Mintzer O, Book SW, Lydiard RB, Ballenger JC, George MS. Feasibility of using fMRI to study mothers responding to infant cries. Depression Anxiety. 1999;10:99–104. doi: 10.1002/(sici)1520-6394(1999)10:3<99::aid-da2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 61.MacLean PD. Evolution of audiovocal communication as reflected by the therapsid-mammalian transition and the limbic thalamocingulate division. In: Newman JD, editor. The Physiological Control of Mammalian Vocalization. New York: Plenum Press; 1988. pp. 185–201. [Google Scholar]
- 62.MacLean PD. The Triune Brain in Evolution: Role in Paleocerebral Functions. New York: Plenum Press; 1990. p. 672. [DOI] [PubMed] [Google Scholar]
- 63.MacLean PD. Brain evolution relating to family, play, and the separation call. Arch Gen Psychiatry. 1985;42:405–417. doi: 10.1001/archpsyc.1985.01790270095011. [DOI] [PubMed] [Google Scholar]
- 64.MacLean PD, Newman JD. Role of midline frontolimbic cortex in production of the isolation call of squirrel monkeys. Brain Res. 1988;450:111–123. doi: 10.1016/0006-8993(88)91550-8. [DOI] [PubMed] [Google Scholar]
- 65.Magoun HW, Atlas D, Ingersoll EH, Ranson SW. Associated facial, vocal and respiratory components of emotional expression: an experimental study. J Neurol Psychopathol. 1937;17:241–255. doi: 10.1136/jnnp.s1-17.67.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mann PE, Bridges RS. Lactogenic hormone regulation of maternal behavior. Prog Brain Res. 2001;133:251–262. doi: 10.1016/s0079-6123(01)33019-4. [DOI] [PubMed] [Google Scholar]
- 67.Mascagni O, Doyle GA. Infant distress vocalizations on the Southern African Lesser Bushbaby (Galago moholi) Int J Primatol. 1993;14:41–60. [Google Scholar]
- 68.Middlemis-Brown JE, Johnson ED, Blumberg MS. Separable brainstem and forebrain contributions to ultrasonic vocalizations in infant rats. Behav Neurosci. 2005;119:1111–1117. doi: 10.1037/0735-7044.119.4.1111. [DOI] [PubMed] [Google Scholar]
- 69.Nazarian A, Krall CM, Osburn JR, McDougall SA. Ultrasonic vocalizations of preweanling rats: involvement of both a2-adrenoreceptor and κ-opioid receptor systems . Eur J Pharmacol. 2001;415:165–171. doi: 10.1016/s0014-2999(01)00849-4. [DOI] [PubMed] [Google Scholar]
- 70.Nelson EE, Panksepp J. Brain substrates of infant-mother attachment: contributions of opioids, oxytocin, and norepinephrine. Neurosci Biobehav Rev. 1998;22:437–452. doi: 10.1016/s0149-7634(97)00052-3. [DOI] [PubMed] [Google Scholar]
- 71.Newman JD. The infant cry of primates: An evolutionary perspective. In: Lester BM, Boukydis CFZ, editors. Infant Crying. New York: Plenum Publishing Corp; 1985. pp. 307–323. [Google Scholar]
- 72.Newman JD. Vocal manifestations of anxiety and their pharmacological control. In: File SE, editor. Psychopharmacology of Anxiolytics and Antidepressants, International Encyclopedia of Pharmacology and Therapeutics, Section 136. New York: Pergamon Press; 1991. pp. 251–260. [Google Scholar]
- 73.Newman JD. The primate isolation call: a comparison with precocial birds and non-primate mammals. In: Rogers LJ, Kaplan G, editors. Comparative Vertebrate Cognition: Are Primates Superior to Non-Primates? New York: Kluwer Academic/Plenum Publishers; 2004. pp. 171–187.pp. 171–187. [Google Scholar]
- 74.Newman JD, Wollberg Z. Responses of single neurons in the auditory cortex of squirrel monkeys to variants of a single call type. Exp Neurol. 1973;40:821–824. doi: 10.1016/0014-4886(73)90116-7. [DOI] [PubMed] [Google Scholar]
- 75.Newman JD, MacLean PD. Effects of tegmental lesions on the isolation call of squirrel monkeys. Brain Res. 1982;232:317–329. doi: 10.1016/0006-8993(82)90276-1. [DOI] [PubMed] [Google Scholar]
- 76.Newman JD, editor. The Physiological Control of Mammalian Vocalization. Plenum Press; New York: 1988. [Google Scholar]
- 77.Newman JD. Vocal ontogeny in macaques and marmosets: convergent and divergent lines of development. In: Zimmermann E, Newman JD, Jürgens U, editors. Current Topics in Primate Vocal Communication. New York: Plenum Press; 1995. pp. 73–97. [Google Scholar]
- 78.Newman JD, Bachevalier J. Neonatal ablations of the amygdala and inferior temporal cortex alter the vocal response to social separation in rhesus macaques. Brain Res. 1997;758:180–186. doi: 10.1016/s0006-8993(97)00212-6. [DOI] [PubMed] [Google Scholar]
- 79.Newman JD. The primate isolation call and the evolution and physiological control of human speech. In: Wind J, Chiarelli B, Bichakjian B, Nocenti A, editors. Language Origin: A Multidisciplinary Approach, NATO ASI Series D. Vol. 61. Dordrecht: Kluwer Academic Publishers; 1992. pp. 301–321. [Google Scholar]
- 80.Nielsen JM, Sedgwick RP. Instincts and emotions in an anencephalic monster. J Nerv Ment Dis. 1949;110:387–394. doi: 10.1097/00005053-194911050-00003. [DOI] [PubMed] [Google Scholar]
- 81.Nishimori K, Young LJ, Guo Q, Wang Z, Insel TR. Oxytocin is required for nursing but is not essential for parturition or reproductive behavior. Proc Natl Acad Sci USA. 1996;93:11699–11704. doi: 10.1073/pnas.93.21.11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Noirot E. Selective priming of maternal responses by auditory and olfactory cues from mouse pups. Dev Psychobiol. 1969;2:273–276. doi: 10.1002/dev.420020413. [DOI] [PubMed] [Google Scholar]
- 83.Numan M. Neural basis of maternal behavior in the rat. Psychoneuroendocrinology. 1988;13:47–62. doi: 10.1016/0306-4530(88)90006-6. [DOI] [PubMed] [Google Scholar]
- 84.Numan M. A neural circuit analysis of maternal behavior in the rat. Acta Paediatr Suppl. 1994;397:19–28. doi: 10.1111/j.1651-2227.1994.tb13261.x. [DOI] [PubMed] [Google Scholar]
- 85.Olazabal DE, Young LJ. Oxytocin receptors in the nucleus accumbens facilitate ‘spontaneous’ maternal behavior in adult female prairie voles. Neuroscience. 2006;141:559–568. doi: 10.1016/j.neuroscience.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 86.Panksepp J. Can anthropomorphic analyses of separation cries in other animals inform us about the emotional nature of social loss in humans? Comment on Blumberg and Sokoloff (2001) Psych Rev. 2003;110:376–388. doi: 10.1037/0033-295x.110.2.376. [DOI] [PubMed] [Google Scholar]
- 87.Panksepp J, Herman BH, Vilberg T, Bishop P, DeEskinazi FG. Endogenous opioids and social behavior. Neurosci Biobehav Rev. 1980;4:473–487. doi: 10.1016/0149-7634(80)90036-6. [DOI] [PubMed] [Google Scholar]
- 88.Panksepp J, Newman JD, Insel TR. Critical conceptual issues in the analysis of separation-distress systems of the brain. In: Strongman KT, editor. Intern Rev Studies Emotion 2. J. Wiley; Chichester: 1992. pp. 51–72. [Google Scholar]
- 89.Panksepp J, Meeker R, Bean NJ. The neurochemical control of crying. Pharmacol Biochem Behav. 1980;12:437–443. doi: 10.1016/0091-3057(80)90050-7. [DOI] [PubMed] [Google Scholar]
- 90.Panksepp J, Normansell L, Herman B, Bishop P, Crepeau L. Neural and neurochemical control of the separation distress call. In: Newman JD, editor. The Physiological Control of Mammalian Vocalization. New York: Plenum Press; 1988. pp. 263–299. [Google Scholar]
- 91.Panksepp J, Siviy SM, Normansell LA. Brain opioids and social emotions. In: Reite M, Fields T, editors. The Psychobiology of Attachment and Separation. New York: Academic Press; 1985. pp. 3–49. [Google Scholar]
- 92.Pedersen CA, Prange AJ., Jr Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin . Proc Natl Acad Sci USA. 1979;76:6661–6665. doi: 10.1073/pnas.76.12.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pryce CR. The regulation of maternal behaviour in marmosets and tamarins. Behav Processes. 1993;30:201–224. doi: 10.1016/0376-6357(93)90133-C. [DOI] [PubMed] [Google Scholar]
- 94.Pryce CR, Abbott DH, Hodges JK, Martin RD. Maternal behaviour is related to prepartum urinary oestradiol levels in red-bellied tamarin monkeys. Physiol Behav. 1988;44:717–726. doi: 10.1016/0031-9384(88)90052-2. [DOI] [PubMed] [Google Scholar]
- 95.Pryce CR, Dobeli M, Martin RD. Effects of sex steroids on maternal motivation in the common marmoset (Callithrix jacchus): development and application of an operant system with maternal reinforcement. J Comp Psychol. 1993;107:99–115. doi: 10.1037/0735-7036.107.1.99. [DOI] [PubMed] [Google Scholar]
- 96.Pryce CR, Mutschler T, Dobeli M, Nievergelt C, Martin RD. Prepartum sex steroid hormones and infant-directed behaviour in primiparous marmoset mothers (Callithrix jacchus). In: Pryce CR, Martin RD, Skuse D, editors. Motherhood in Human and nonhuman Primates, 3rd Schultz-Biegert Symposium; Kartause Ittingen. Basel: Karger; 1994. 1995. pp. 78–86. [Google Scholar]
- 97.Roberts RL, Jenkins KT, Lawler T, Jr, Wegner FH, Newman JD. Bromocriptine administration lowers serum prolactin and disrupts parental responsiveness in common marmosets (Callithrix j. jacchus) Horm Behav. 2001;39:106–112. doi: 10.1006/hbeh.2000.1639. [DOI] [PubMed] [Google Scholar]
- 98.Roberts RL, Jenkins KT, Lawler T, Wegner FH, Norcross JL, Bernhards DE, Newman JD. Prolactin levels are elevated after infant carrying in parentally inexperienced common marmosets. Physiol Behav. 2001;72:713–720. doi: 10.1016/s0031-9384(01)00430-9. [DOI] [PubMed] [Google Scholar]
- 99.Robinson BW. Vocalization evoked from the forebrain in Macaca mulatta. Physiol Behav. 1967;2:345–354. [Google Scholar]
- 100.Rosabal F. Cytoarchitecture of the frontal lobe of the squirrel monkey. J Comp Neurol. 1967;130:87–108. doi: 10.1002/cne.901300202. [DOI] [PubMed] [Google Scholar]
- 101.Sander K, Scheich H. Auditory perception of laughing and crying activates human amygdala regardless of attentional state. Cog Brain Res. 2001;12:181–198. doi: 10.1016/s0926-6410(01)00045-3. [DOI] [PubMed] [Google Scholar]
- 102.Sayigh LS, Tyack PL, Wells RS, Scott MD. Signature whistles of free-ranging bottlenose dolphins Tursiops truncates: stability and mother-offspring comparisons. Behav Ecol Sociobiol. 1990;26:247–260. [Google Scholar]
- 103.Schulz GM, Varga M, Jeffires K, Ludlow CL, Braun AR. Functional neuroanatomy of human vocalization: an H215O PET study. Cerebral Cortex. 2005;15:1835–1847. doi: 10.1093/cercor/bhi061. [DOI] [PubMed] [Google Scholar]
- 104.Scott JP. Effects of psychotropic drugs on separation distress in dogs. Neuropsychopharmacology; Proc. IX Congress, CINP, Excerpta Medica Amsterdam; Paris. 1974. pp. 735–745. [Google Scholar]
- 105.Searby A, Jouventin P. Mother-lamb acoustic recognition in sheep: a frequency coding. Proc Roy Soc Lond B. 2003;270:1765–1771. doi: 10.1098/rspb.2003.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Seifritz E, Esposito F, Neuhoff JG, Luthi A, Mustovic H, Dammann G, Bardeleben Uv, Radue EW, Cirillo S, Tedeschi G, Di Salle F. Differential sex-independent amygdala response to infant crying and laughing in parents versus nonparents. Biol Psychiatry. 2003;54:1367–1375. doi: 10.1016/s0006-3223(03)00697-8. [DOI] [PubMed] [Google Scholar]
- 107.Shair HN, Brunelli SA, Hofer MA. Lack of evidence for mu-opioid regulation of a socially mediated separation response. Physiol Behav. 2005;83:767–777. doi: 10.1016/j.physbeh.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 108.Sheehan TP, Cirrito J, Numan MJ, Numan M. Using c-fos immunocytochemistry to identify forebrain regions that may inhibit maternal behavior in rats. Behav Neurosci. 2000;114:337–352. doi: 10.1037//0735-7044.114.2.337. [DOI] [PubMed] [Google Scholar]
- 109.Shizawa Y, Nakamichi M, Hinobayashi T, Minami T. Playback experiment to test maternal responses of Japanese macaques (Macaca fuscata) to their own infant’s call when the infants were four to six months old. Behav Processes. 2005;68:41–46. doi: 10.1016/j.beproc.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 110.Smith WK. The functional significance of the rostral cingular cortex as revealed by its responses to electrical excitation. J Neurophysiol. 1945;8:241–255. [Google Scholar]
- 111.Soltis J. The signal functions of early infant crying. Behav Brain Sci. 2004;27:443–490. [PubMed] [Google Scholar]
- 112.Stack EC, Balakrishnan R, Numan MJ, Numan M. A functional neuroanatomical investigation of the role of the medial preoptic area in neural circuits regulating maternal behavior. Behav Brain Res. 2002;131:17–36. doi: 10.1016/s0166-4328(01)00370-9. [DOI] [PubMed] [Google Scholar]
- 113.Stafisso-Sandoz G, Polley D, Holt E, Lambert KG, Kinsley CH. Opiate disruption of maternal behavior: morphine reduces, and naloxone restores, c-fos activity in the medial preoptic area of lactating rats. Brain Res Bull. 1998;45:307–313. doi: 10.1016/s0361-9230(97)00375-4. [DOI] [PubMed] [Google Scholar]
- 114.Sutton D, Larson C, Lindeman RC. Neocortical and limbic lesion effects on primate phonation. Brain Res. 1974;71:61–75. doi: 10.1016/0006-8993(74)90191-7. [DOI] [PubMed] [Google Scholar]
- 115.Symmes D, Biben M. Maternal recognition of individual infant squirrel monkeys from isolation call playbacks. Am J Primatol. 1985;9:39–46. doi: 10.1002/ajp.1350090105. [DOI] [PubMed] [Google Scholar]
- 116.Vuorenkoski V, Wasz-Hockert O, Koivisto E, Lind J. The effect of cry stimulus on the temperature of the lactating breast of primipara: A thermographic study. Experientia. 1969;25:1286–1287. doi: 10.1007/BF01897502. [DOI] [PubMed] [Google Scholar]
- 117.Walser ES, Walters E, Hague P. Vocal communication between ewes and their own and alien lambs. Behaviour. 1982;81:140–151. [Google Scholar]
- 118.Wasz-Höckert O, Lind J, Vuorenkoski V, Partanen T, Valanne E. Clinics in Developmental Medicine. Vol. 29. Spastics International Medical Publications; Lavenham Suffolk: 1968. The infant cry. A spectrographic and auditory analysis. [Google Scholar]
- 119.Winberg J. Mother and newborn baby: mutual regulation of physiology and behavior—a selective review. Dev Psychobiol. 2005;47:217–229. doi: 10.1002/dev.20094. [DOI] [PubMed] [Google Scholar]
- 120.Winslow JT, Insel TR. Social status in pairs of male squirrel monkeys determines the behavioral response to central oxytocin administration. J Neurosci. 1991;11:2032–2038. doi: 10.1523/JNEUROSCI.11-07-02032.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Winslow JT, Hearn EF, Ferguson J, Young LJ, Matzuk MM, Insel TR. Infant vocalization, adult aggression, and fear behavior of an oxytocin null mutant mouse. Horm Behav. 2000;37:145–155. doi: 10.1006/hbeh.1999.1566. [DOI] [PubMed] [Google Scholar]
- 122.Winslow JT, Insel TR. Serotonergic modulation of the rat pup isolation call: studies with 5HT1 and 5HT2 subtype-selective agonists and antagonists. Psychopharmacology. 1991;105:513–520. doi: 10.1007/BF02244372. [DOI] [PubMed] [Google Scholar]
- 123.Wise SP, Herkenham M. Opiate receptor distribution in the cerebral cortex of the rhesus monkey. Science. 1982;218:387–389. doi: 10.1126/science.6289441. [DOI] [PubMed] [Google Scholar]
- 124.Wollberg Z, Newman JD. Auditory cortex of squirrel monkey: response patterns of single cells to species-specific vocalizations. Science. 1972;175:212–214. doi: 10.1126/science.175.4018.212. [DOI] [PubMed] [Google Scholar]
- 125.Young LJ, Toloczko D, Insel TR. Localization of vasopressin (V1a) receptor binding and mRNA in the rhesus monkey brain. J Neuroendocrinol. 1999;11:291–297. doi: 10.1046/j.1365-2826.1999.00332.x. [DOI] [PubMed] [Google Scholar]
- 126.Zhang SP, Davis PJ, Bandler R, Carrive P. Brain stem integration of vocalization: role of the midbrain periaqueductal gray. J Neurophysiol. 1994;72:1337–1356. doi: 10.1152/jn.1994.72.3.1337. [DOI] [PubMed] [Google Scholar]


