Abstract
In tissue engineering cell cultures play a crucial role besides the matrix materials for the end of substituting lost tissue functions. The cell itself is situated at the cross-roads leading to different orders of scale, from molecule to organism and different levels of function, from biochemistry to macrophysiology. Extensive in vitro investigations have dissected a vast amount of cellular phenomena and the role of a number of bioactive substances has been elucidated in the past. Further, recombinant DNA technologies allow modulation of the expression profiles of virtually all kinds of cells. However, issues of vascularization in vivo limit transferability of these observations and restrict upscaling into clinical applications. Novel in vivo models of vascularization have evolved inspired from reconstructive microsurgical concepts and they encompass axial neovascularization by means of vascular induction. This work represents a brief description of latest developments and potential applications of neovascularization and angiogenesis in tissue engineering.
Keywords: axial vascularization, arteriovenous loop, angiogenesis, in vivo bioreactors
Introduction
Cellular homeostasis and vascularization
The human body comprises on the order of 100 trillion cells, with about 260 different phenotypes, that divide, differentiate and self-assemble over time and space into an integrated system of tissues and organs [1]. Organs rely on the cell as an effector of tissue specific function, regeneration and homeostasis. While, the extracellular matrix serves as structural support, and mediates intercellular communication, a dense capillary network supplies perfusion for adequate oxygenation and nutrition as well as evacuation of waste by-products from the cell. Without vascularity cells have to rely entirely on diffusion to cover their metabolic demands.
Importance of vascularization: historical review
Greene indicated in 1961 that a cell situated further away than 200–300 μm from a capillary cannot survive. He showed that a tiny tumour implanted in the anterior ocular chamber of the guinea pig for more than a year, could not exceed a tiny dimension of 1 mm in diameter; when this tumour was heterologously reimplanted into the muscle of a rabbit where it could acquire a sufficient neocapillarization, it exploded to a sizable bulk [2]. Almost a century earlier, Barth had observed that upon autologous bone transplantation the vast majority of cells die and leave a scaffolding behind to be slowly repopulated by new host cells and an adequate new vascular network [3]. He called this process ‘creeping substitution’. At the beginning of the 1970s Folkman suggested that tumours owed their potential for unlimited growth and invasion partly to their angiogenetic capacity and focused on therapeutic modalities to counteract this process [4].
Multicellular implants in vivo
Nowadays, the issue of vascularization has gained new significance in biomedical research especially in the context of in vivo investigations with cellular assemblies. Under culture conditions survival of cells is ensured by regular medium changes whereas in vitro bioreactors are designed to provide an optimal environment. In this way a given cell population can expand and differentiate. Upon transplantation the cells rely on host vascular networks for perfusion. In a plethora of experiments cell-loaded matrices were implanted at sites of rich vascularity. Intraabdominal [5], subcutaneous [6] and intramuscular [7] implantation have been reported. These studies were based on the hypothesis that the surrounding tissue will expand its vascular network into the construct in time for an adequate amount of cells to survive and initiate physiological function. However, there are some limitations. Cells situated at central portions of the implant are condemned to death if situated further away than 200–300 μm from the nearest capillaries [2, 8]. As a consequence, multicellular implants can only function in small dimensions. Furthermore, since implantation is accompanied by an inflammatory response, the associated fibrosis of the host is likely to overrun the original cell transplants [9,10]. Controlled angiogenesis may foster the growth of the original implants and may help to control the inflammatory response. These issues of vascularization implemented the need for novel angiogenetic approaches and new in vivo models evolved with the aim to generate constructs with a dedicated neovascular network not under the immediate influence of the local environment.
Models of axial vascular induction
Development of the AV loop model
In 1979 Erol and Spira reported about their work on vascular induction by means of inserting microvascular constructs onto free skin grafts. Several vessel configurations were investigated including a flow-through vascular pedicle, a distally ligated arteriovenous pedicle as well as an arteriovenous fistula. The latter was found to possess the highest capacity of inducing and sustaining vascularization into the free skin transplant. As a result, a new tissue element was generated with a dedicated vascular network based on an arteriovenous axis. The axial vascularization of the new flap was similar to the pattern seen in tissue transplants suitable for microvascular transfer (free flaps). During the late 1980s the principle was refined and found a way into plastic surgical reconstruction under the collective designation of the so called ‘prefabricated free flaps’. This term was coined to describe a strategy of staged microsurgical transfer where a tissue element was inserted into a site of rich vascularization, usually a muscle or the forearm fascia. After the initial tissue block was ‘adopted’ by its surrounding, in terms of vascularization and perfusion, it was then elevated en bloc and transferred into the defect requiring reconstruction [11]. During the next years flap prefabrication through vascular induction was applied in several experimental and clinical settings [11–13].
In 2000 Tanaka and Morrison applied the concept on a fully synthetic dermis substitute [14]. They introduced the term ‘matrix flap’ to describe the axially vascularized bioartificial construct. In the same year they presented an augmented version of the model with a polycarbonate isolation chamber and postulated that the loop was capable of producing fibrovascular tissue de novo in the absence of any matrix [15]. In subsequent investigations it was demonstrated that extracellular matrix added into the isolation chamber could influence the formation of new tissue in quality and quantity [16]. In this experimental setting, fibrin and poly lactic-co-glycolic acid (PLGA) as well as matrigel were introduced into the chamber upon construction of the arteriovenous fistula (AV loop). Matrigel enabled growth of new vessels to a higher extent than fibrin or PLGA. In another study, the AV loop was able to induce fibrovascular growth of larger volumes when inserted into a chamber of sufficient dimensions [17]. Eventually, the loop was utilized to vascularize solid porous matrices, opening new options towards generation of bioartificial bone tissue [18,19]. In contrast to subcutaneous models of vascularization, the loop accomplished vascular growth with minimal fibrosis and polymorphonuclear infiltration [20]. In recent publications, the loop model was used for cellular approaches of tissue engineering; pancreatic cells were combined with a matrix and an AV loop for generation of pancreas-like organoids [21].
Angiogenesis in multicellular implants in vivo, the authors' experience
Surgical technique
Operations are performed on rats under inhalational anesthesia with Isoflurane. The femoral neurovascular bundle is exposed through an incision at the medial thigh. The femoral vessels are dissected from the pelvic artery in the groin to the bifurcation of the femoral artery into saphenous and popliteal arteries. After dissection of the artery and vein, a femoral venous graft is harvested from the contralateral side and interposed between the femoral vessels by microvascular anastomoses performed with an 11–0 Nylon suture (Fig. 1). The construct is placed into the Teflon chamber with the artery and vein exiting through the opening at the proximal pole. After addition of the matrix, the lid is closed and the chamber with the matrix inside is fixed onto the adductor fascia at the medial thigh with a non-absorbable polypropylene suture. Interrupted vertical mattress sutures are used for wound closure. The length of the procedure lies between 3 and 4 hrs and long-term patency of the loop varies between 85% and 88% when the operations are performed by an experienced microsurgeon.
1.
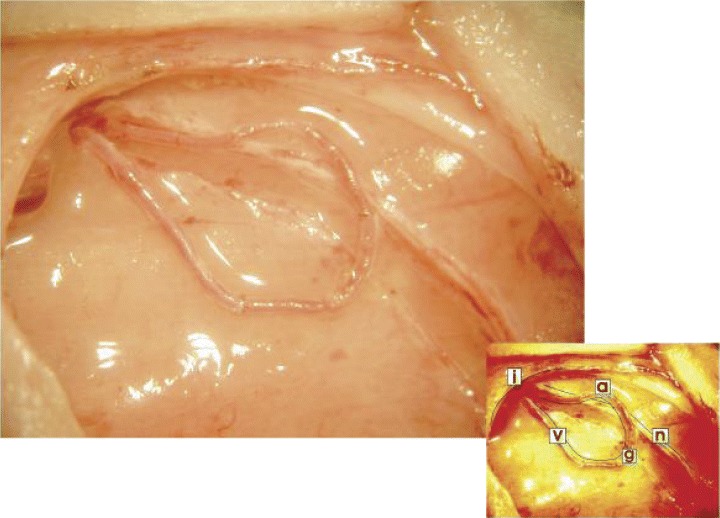
The arteriovenous fistula in situ. The femoral vein (v) and artery (a) in the left medial thigh distal to the inguinal ligament (i) are dissected from the femoral nerve (n) and a vascular graft (g) from the contralateral femoral vein is interposed by means of micro-surgical anastomosis.
Chamber
An isolation chamber separates the fibrovascular construct from the organism with exception of the arteriovenous pedicle entering and exiting the chamber from an aperture at the proximal pole. The design was developed in rat cadaver studies and during long-term experiments [19]. The chamber is made of medical grade Teflon; a biologically inert material amenable to sterilization. It is comprised by a base plate (diameter: 15 mm), under a cylindrical shell (height 6 mm diameter × 12 mm) and an upper cup (height: 2 mm × diameter: 14 mm) (Fig. 2). The basal plate has two peripheral perforations for stabilization on the fascia of the medial musculature of the thigh. The cup has a spherical form so as to minimize the risk of skin perforations in long-term investigations. A different design with ancoring stabs was applied for gel matrices to avoid dislocation of the loop outside the chamber.
2.

Schematic representation of the isolation chamber. It is comprised by a base plate (B) (diameter: 15 mm), under a cylindrical shell (height 6 mm × diameter 12 mm) (C) and an upper lid (L) (height: 2 mm × diameter: 14 mm). At the sides there are perforations for fixation of the chamber on the fascia of the medial musculature of the medial thigh.
Soft matrices
The principle was applied on a fibrin gel matrix. Kinetics of angiogenesis were greatly influenced by differences in concentrations of fibrinogen in the clot. In a fibrinogen concentration of 33 mg/ml the onset of neovascularization was delayed until day 12–14 after construction of the loop [22]. In lower concentrations (20 mg/ml) a more rapid vascular growth was accompanied by a marked fibrinolysis with disintegration of the matrix within the first 10 days. Resorption of the fibrin clot was affected by the newly formed capillaries, since in control groups without insertion of a vascular carrier into the isolation chamber, the clots remained intact throughout the length of the experiment. In this investigation we confirmed the capacity of the AV loop to generate a mature vascular network within a fibrin gel (Fig. 3). The process was defined by a gradual substitution of the clot by fibrovascular tissue. Fibrin matrices with a loose structure and low density potentiated angiogenesis but failed to maintain their structural integrity.
3.
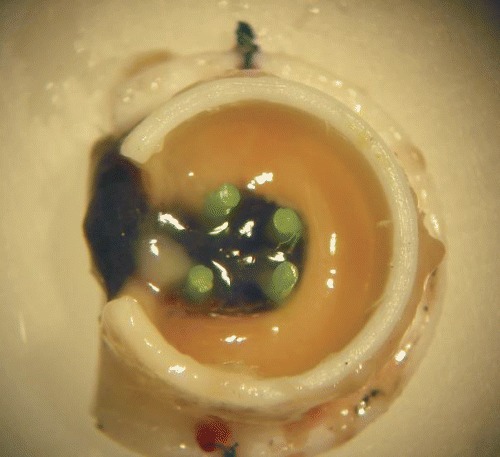
Vascularization into the fibrin clot after 2 weeks. Prior to explantation the caudal circulatory system was perfused with an India ink gel for black coloration of the capillary network. Four poly-ethylene stabs serve for stabilization of the AV loop in the isolation chamber.
Modulation
The gel matrix could serve as a carrier of modulatory substances and angiogenic growth factors were added to the fibrin clot upon implantation. Addition of VEGF-165 (0.1 ng/ml) and bFGF (0.1 ng/ml) produced a significantly enhanced angiogenic response. However, the effect of these bioactive substances was more prominent in early stages after implantation up to 2 weeks than in later evaluation intervals. Upon 4 weeks the effect of the growth factors had been equalized in terms of volume and density of the neovascular network as compared to implants devoid of growth factors (unpublished data of the authors). Evidently, late self-modulatory mechanisms effect maturation through regression of superfluous capillaries and persistence of vessels developing into a system of afferent arterioles, capillary loops and efferent venules.
Solid matrices
A biogenic matrix from bovine spongiosa with porosity between 65% and 80% and a pore size of 400–1000μm rendered acellular and non-antigenic by a standardized procedure was used. The matrix was formed to a design for optimal accommodation of the vascular axis. Different designs were tested for different configurations of the vessels [18, 23]. A central canal with an elliptical cross-section was used for bundle arrangement whereas for loop constructs a design with a circumferencial notch at the periphery of the matrix was applied (Fig. 4). The overall shape was that of a disc 9 mm in diameter and 5 mm thick. Full vascularization of the scaffold could be verified between 6 and 8 weeks post-implantation. The fibrovascular tissue in the matrix displayed a significantly lower ratio of inflammatory elements as opposed to matrices implanted subcutaneously [10]. The ratio of thrombosis in the loop did not significantly differ from ones placed into soft matrices. Under these circumstances the method was shown to be suitable for vascularized bone-like assemblies with primary stability serving as bone substitutes.
4.

Custom-made design of a biogenic processed bovine cancellous bone matrix. A scanning electron image with the discoid matrix displaying a circular notch for optimal accommodation of the AV loop is shown here. The matrix was rendered conductive prior to sputtering by means of minute copper wires. Eight canals for secondary injection of fibrin immobilized osteoblasts were included in the design.
Cell seeding
To accomplish the leap from a vascularized solid matrix to a bioartificial unit of bone tissue the matrices were secondarily loaded with primary rat osteoblasts expanded in cell culture and kept in a differentiation medium for 14 days [24]. To elucidate the fate of the cells in vivo, they were marked with a fluorescent dye prior to seeding. Matrices loaded with the same osteoblasts and implanted subcutaneously served as controls. Pre-vascularization by means of a vascular carrier significantly enhanced survival of the cells and increased transplantation efficiency [25] (Fig. 5). Differentiated osteoblasts were able to initiate osteogenesis within the construct. However, full ossification of the matrix could not be accomplished. Other modes of osteoinduction are currently under study.
5.
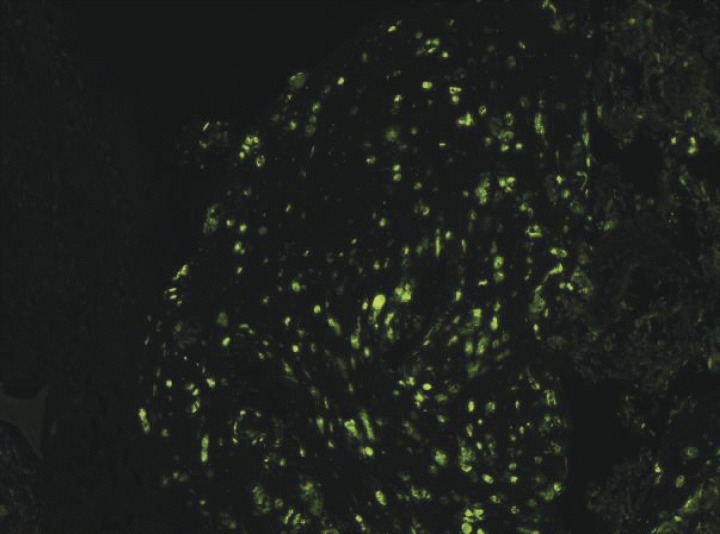
Cell survival study with CFDA marker.Transplantation of differentiated primary rat osteoblasts into the prevascularized matrices significantly enhanced initial cell survival (40 × magnification).
Evaluation methods
For evaluation of the angiogenic response in the loop several methods were used. For histology, capillaries can be visualized by injection of an India-ink gel prior to explantation (Fig. 3). Morphometric analysis of the India ink-filled lumina allows quantification of the neovascular network. Alternatively, vessels can be demonstrated by lectin GSI-B4 on formalin fixed – paraffin embedded specimens of rat tissue or CD31 (PECAM-1) on cryofixated specimens [26, 27]. Investigations on the three-dimensional morphology of the vessel bead could be performed after injection of low-viscosity resins.
Scanning electron microscopy of microvascular corrosion casts is a powerful method that allowed us to study the spatial arrangement of the capillaries with a high resolution (Fig. 6). The three dimensional distribution of angiogenetic sprouts and neocapillary branching can be readily visualized (Fig. 7).The site of a sprout displays an increased permeability.Therefore, upon injection of the resin, the heparinized saline can escape at the tip of the sprout and the resin replacing it assumes a very distinct spike-like shape.Partially filled branches can be easily distinguished due to a blunt rounded tip. (Fig. 7). Hot spots of increased angiogenesis can be localized by an increased occurance of such spikes or alternatively by ring-like structures indicating non-sprouting angiogenesis (Fig. 8). The authors were able to demonstrate direct luminal sprouting from the venous and graft segments of the AV loop. The impression of the vascular wall on the cast provides further information about the morphology of angiogenesis.Vascular segments subjected to arterial flow conditions, show a highly oriented pattern of endothelial lining with spindle like cells parallel to the flow direction. In speciments taken from implants that remained in the organism for 4 weeks or longer, signs of vascular maturation can be demonstrated. The processes of regression and persistence lead to a highly organized network of vessels of different calibre arranged in an ordered hierarchy from artery, to arteriole, to capillaries and post-capillary venules that evacuate into veins [28]. Latest advances in micro CT allow visualization of vessels with a resolution as low as 10 μm. Quantitative evaluation of three-dimensional vascular networks will soon be feasible in small animals [29].
6.

Construction of the AV loop generates a dense neovascular network. Here scanning electron microscopical images of a corrosion cast two weeks after implantation of an AV loop. Note the uniform diameter of the relatively immature neocapillaries.
7.
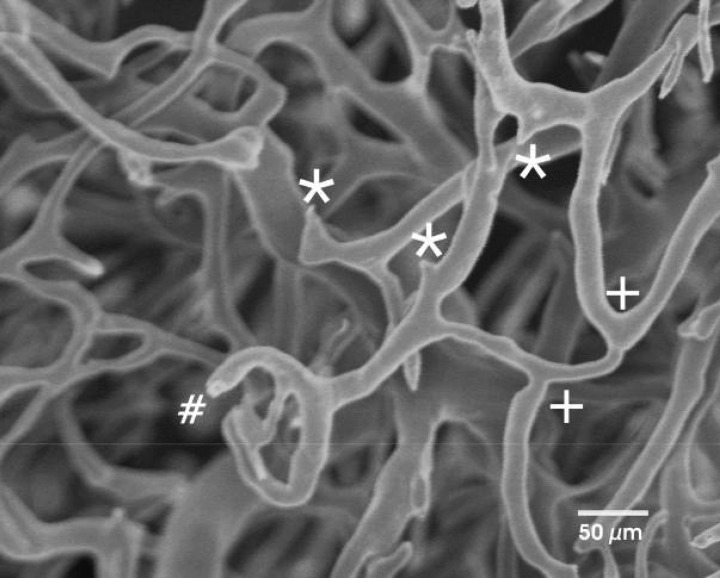
Sprouting (*) and branching (+) in the neocapillary network. Incomplete filling of the capillaries (#) is characterized by a rounded tip. Sprouts are marked by spiked tips. (Scanning electron microscopy of corrosion casts.)
8.

Non-sprouting angiogenesis or intussusceptive angiogenesis.(Scanning electron microscopy of corrosion casts.)
However, all these investigations require killing of the animal. Serial investigations are possible with inravital micro NMR [30]. Not only anatomical correlation can be visualized by tomography but dynamic studies with NMR angiography can verify patency of the vascular carrier and pinpoint the optimal time for transplantation so that adequate vascularization is warranted (Fig. 9). Recent advances with cell marking studies with hypermagnetic nanoparticles expanded the potential of NMR [31]. Intravital evaluation modalities gain importance since they reduce animal costs, improve ethical acceptability of animal experiments and bring biomedical research closer to the clinical routine.
9.

Micro-magnetic resonance angiography of the AV loop 1 weekafter implantation.
Physiology of angiogenesis in the AV loop
Flow dynamics and morphology
In the rat femoral artery mean flow rates are generally in the range of 1.5–4 ml/min [32, 33]. Flow upon systole can reach 9 ml/min. Elimination of peripheral resistance through the shunt operation can produce a further rise in the order of 4–5-fold [33]. In the femoral vein the physiological flow rate is under 1 ml/min. So, upon transfer of a femoral venous graft as an interposing segment for shunting between femoral artery and vein, the graft is exposed to at least 10-fold rise in blood flow with a corresponding rise in shear stress. Shear stress can be calculated with the Hagen – Poiseuille Law: T = 4ηQ/rπ3, where T is shear stress, η is blood viscosity, Q is flow rate in milliliters per second, and r is radius in centimetres. If blood viscosity (0.035 poise) is assumed constant and the internal radius is determined by measuring the histologic cross-sectional areas and adjusting for 30% shrinkage due to the fixation process, then it is evident that there is a 10-fold rise in shear stress on the wall of the graft.
All three segments of the loop, i.e. artery, graft and vein undergo morphological changes owing to the haemodynamic alterations. As a result of arterialization, the graft lumen diameter diminishes 70% by day 14 after its insertion into the loop [22]. Mechanical injury inflicted on the endothelium causes a denudation of the intima. A confluent endothelial lining cannot be demonstrated until 4 weeks after grafting [33].
Long-term studies have shown that although initial shunting has the form of an arteriovenous fistula, as affected by the venous graft, arteriovenous exchange after 6 weeks is partially taken over by the newly formed vascular network. The pattern of blood flow within the construct resembles that of an organoid with afferent artery, an efferent vein and a well vascularized parenchyma in between (Fig. 10).
10.
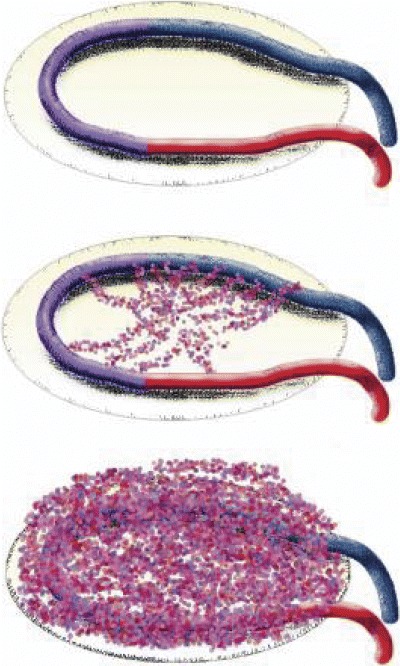
The process of neovascularization as induced by the AV loop.
The AV loop produces a vivid angiogenic response and possesses the capacity to generate a dense vascular network inside a permissive matrix. Three mechanisms are held responsible for this phenomenon: a local inflammation due to the surgical trauma on the vessels, a rise in mechanical stress on the vascular walls of the graft and the vein due to arterialization and finally gradients in ogygenation along the matrix.
A local inflammatory response secondary to the surgical trauma induces a surge of angiogenetic substances. For example, proinflammatory chemokines are known to induce upregulation VEGF from platelets and endothelial cells [34–36].
A rise in pulsatile pressure and shear stress is another factor leading to enhanced neovascularization. Insertion of a vascular graft into the arterial circulation is known to generate a rise in VEGF production from the affected endothelium both due to mechanical stimulation as well as sustained injury [36–40]. The combination of shear stress with turbulent flow present at the microvascular anastomoses is known to be a major activator of endothelial cells [41].
Gradients in partial pressure of oxygen or hypoxia within the matrix may also play a role in induction of the marked angiogenetic phenomena [42, 43].
The configuration of the AV loop is able not only to initiate but also to sustain perfusion of a construct. Remodelling and stabilization of the neovascular network are indicated by the formation of capillary loops and organization of the neovessels in a structured hierarchy [20, 44].
Molecular biology of angiogenesis in the loop
Angiogenic activity in the AV loop model indicates a shift of the angiogenic balance, as has been described in tumours. Vessels in the AV loop similar to tumor vessels have irregular morphology and are often incomplete. The structure of the vascular bed has a significant impact on the transport of nutrients and drugs into tissues. It has been suggested that normalization of the tumour vessels may improve the access of soluble mediators into tumours [45]. In analogy, both vessel number and vessel quality will contribute to optimal development of artificial tissues in the AV loop model. Optimized vessel quality may significantly increase the growth and quality of artificial tissues.
Vascular development is a carefully coordinated multi-factorial process proceeding through three major steps, regulating (1) sprouting of endothelial cells from mature vessels, (2) assembly of vessels to vascular structures and (3) maturation of the vessels and subsequent induction of quiescence (Fig. 11). Each of these steps is tightly regulated by a specific set of molecules which act on specific vascular receptor tyrosine kinases (RTK).
11.
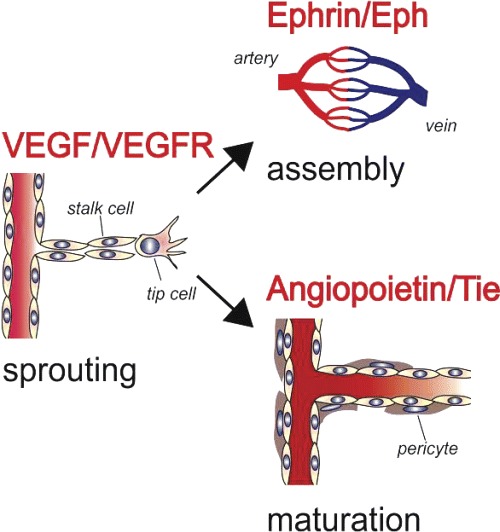
Molecular regulation of angiogenesis. Vascular development proceeds through three different steps (sprouting, assembly and maturation). Each step is regulated by a distinct set of agonists acting via specific vascular receptor tyrosine kinases (red).
Sprouting is the first step in the angiogenic cascade. The family of vascular endothelial growth factors (VEGF) is among the most potent inducers of this process [46]. The VEGF family consists of seven members, which are named VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, placenta growth factor (PIGF) and EG-VEGF (endocrine gland-derived vascular endothelial growth factor). Of these the best characterized and most important regulator of angiogenesis is VEGF-A. Four isoforms of VEGF-A are known, the secreted forms VEGF121 and VEGF165 and the membrane associated forms VEGF189 and VEGF205, which are all arising from differential RNA splicing. In the sprouting vessel gradients of VEGF-A guide the tip cells, activate proliferation of the stalk cells and inhibit apoptosis of the endothelial cells [47] (Fig. 1). The major receptor mediating the effects of VEGF-A on endothelial cells is the RTK VEGF receptor 2 (VEGF-R2, syn FLK-1(KDR).
The second step is vessel assembly and the establishment of vessel identity.These processes are regulated by the Ephrin ligands and Eph RTKs [48] (Fig. 11). Interactions of these molecules mediate cell-contact-dependent signalling. Interestingly both components can transduce signals that trigger cell responses, leading to forward and reverse signalling, via the receptor and the ligand, respectively [49]. Members of the vertebrate Eph receptor and ephrin families fall largely into two classes: GPI-anchored ephrinAs binding to EphA receptors, and transmembrane ephrinBs binding to EphB receptors and to EphA4. Studies in developing mouse embryos showed that Ephrin B2 is expressed on arteries and EphB4 on veins. The reciprocal pattern of distribution within the developing vasculature suggested that ephrin B2 and EphB4 establish arterial versus venous identity. In ephrin B2 knock-out mice angiogenesis is blocked at the primary plexus stage, suggesting that intercalation between developing arterial and venous endothelial cells is mediated by ephrin B2. Therefore, the interaction of Ephrins with their receptors likely transduces positional guidance cues on outgrowing vascular sprouts, which are critical for proper arteriovenous assembly and establishment of blood flow [48].
Paracrinely acting angiopoietins (Ang) are the ligands of the vascular RTKs Tie-1 and Tie-2 and regulate blood vessel maturation and integrity [50]. Presently four different angiopoietins (Ang-1 to -4) are known. Tie-2 is the common receptor of Ang-1 and Ang-2. Ang-1 causes vessel maturation firstly by direct effects on endothelial cells and secondly by supporting the recruitment of pericytes. Ang-2 does not induce tyrosin phosphorylation of Tie-2 and instead rather antagonizes Ang-1 binding. This activity suggests that Ang-2 is an antagonistic player of the angiopoietin/Tie-system leading to destabilization of the vasculature.
Modern vascular research opened many novel insights into the molecular mechanisms regulating the growth, differentiation and maturation of blood vessels.This provides numerous promising targets to tailor the vascular beds in AV loops specifically according to the requests of the artificial tissues produced.
Axially vascularized constructs as tools for research
Application in basic biomedical science
Recombinant cell populations
Application of recombinant DNA is nowadays an effective means of modulating the expression profiles of virtually all kinds of cells in vitro[51,52]. Several assays can reliably and reproducibly verify transfection efficiency and behaviour of cells in cultures. To assess the effect of recombinant cells on living organisms and vice versa, one has to rely on animal experiments. However, supply of oxygen and nutrients to these cells as well as evacuation of waste products, still remains a problem. After transfer of the cells, diffusion may provide for sufficient exchange but only under the premise that cells stay in immediate contact with the local tissues. Local influence might hinder evaluation of these cells in vivo[53].The AV loop with the isolation chamber can serve as an in vivo bioreactor for intravital and post-mortem studies of the implanted cells [21, 31]. Furthermore, treatment of the venous graft so as to act as a vehicle for vectors or transfected cells can broaden the span of possibilities for application of the model [54].
Modulation of angiogenesis
The process neovascularization by vascular induction displays the entire cascade of phenomena ascribed to angiogenesis, beginning from endothelial activation and proteolytic disintegration of the lamina basalis to late remodelling of the neovascular network by regression and persistence, capillary loop formation and alterations in the luminal diameter of the individual neovessels to generate an organized angial hierarchy. With the presence of an isolation chamber and the use of drug delivery technologies bioactive substances can be administered in an individual temporospatial fashion to modulate early and late phenomena of angiogenesis [55].
Hematogenous metastasis of tumours
Tumours that primarily metastasize by the hematogenous way, like sarcomas, require suitable animal models not only to dissect the pathophysio-logical process behind growth and metastasis, but also to test the efficacy of local and systemic therapeutic modalities. Until now, there are only few reliable systems for investigation of such neoplasias [56, 57]. Further investigation on lymphangiogenesis in the AV loop model might render it suitable for such studies. It is reasonable to assume that there is a time interval where the sole communication of the AV loop system with the host, will be the femoral axis serving as vascular pedicle. Appearance of remote metastases originating from tumours primarily implanted within the isolation chamber might be a valuable tool in research of cancer seeding viathe hematogenous route.
Applications in reparative medicine
Multicellular implants
“CELLS–THERE IS NO ESCAPING THEM”[58]. The combination of cells with matrices represents a major field in tissue engineering [23, 59]. The issue of vascularization in cell-loaded matrices is of cardinal importance to survival of the tissue specific cells and overall transplantation efficiency. In traditional models of extrinsic vascularization with subcutaneous, intraabdominal or intramuscular implantation, the cells depend on diffusion from the immediate surrounding for oxygenation and nutrient supply. Excellent results were achieved in experimental settings where the dimensions of the matrices were kept small and the surface-to-volume ratio was high. A sufficient portion of the cellular population survived and initiated tissue specific function [60]. In other experiments specific growth factors augmented the effect of the initially transplanted cells through recruitment of host progenitor cells [61, 62]. In a similar manner, hypoxia-related cellular death itself could initiate an apocrine release of growth factors to promote regeneration [63].
A dense capillary network preexisting in the matrix to be loaded with a tissue specific cell population, ensures a better transplantation efficiency [21, 64]. Artificial effects due to administration of growth factors could be avoided this way. Prevascularization strategies with concepts of intrinsic vascular growth might overcome some problems concerning initial homeostasis of the cellular constructs and potentiate upscaling of the experimental results into clinical dimensions.
Vascular grafts and bio-artificial vessels
Atherosclerosis is at present the most frequent cause of death in the western world. Until 2020 more patients will potentially succumb to atherosclerosis than from all other causative factors together [65]. Phenomena like intimal hyperplasia and new formation of atherosclerotic plaques limit longevity of interventional therapy and autologous vessel transplantation [66]. Generation of totally bioartificial neovessels resistant to these complications is in the epicentre of contemporary science [67, 68]. The graft segment of the AV loop model could be of value for in vivo evaluation of vascular graft disease as well as investigation of novel vascular substitutes. Recent insights into the modulation of angiogenesis may help to transfer such techniques from bench to bedside [69, 70].
Conclusion
Recently, more than 30 years after the discovery of the first tumour specific vascular growth factor [4, 71], the FDA waived anti-angiogenetic cancer chemother-apeutics through preliminary clinical trials [72]. On the antipode, during the last 10 years, research in the field of reparative medicine has focused on angiogenesis to promote the leap from bench to bedside and translate progress into clinical benefits [73]. In fact, developing strategies for generation of viable bioartificial constructs beyond the diffusion limit has been characterized as the greatest challenge facing the field of tissue engineering nowadays [74].
Although profound microsurgical skills are required, the AV loop model is close to the clinical routine; surgical angiogenesis [23] by means of vascular induction is being regularly used for flap prefabrication and osseous revascularization. It opens new horizons for investigations with cell transplantation in vivo and initial results have shown significant rise in transplantation efficiency. In the clinical setting, generation of a hybrid organoid comprised by a vascular bed of the host, and specific tissue, induced either by a differentiated cell population or by intrinsic signalling within the chamber seems under these conditions feasible.
Acknowledgments
The work was in part funded by a grant from Xue-Hong, Hans Georg Geis as well as ELAN fonds programm, University of Erlangen. The authors would like to thank Prof. Peter Greil, Institute of Materials Science, Glas and Ceramic, University of Erlangen for support with the isolation chambers as well as availability of the facilities for electron microscopy. Investigations with magnetic resonance imaging were performed by Dr. Andreas Hess, Doerenkamp-Foundation Professorship for Innovations in Animal Welfare and Consumer Protection, University of Erlangen. Finally, we would like to extend our gratitude to Prof. Lametschwandtner, University of Salzburg, Austria for guidance in techniques of corrosion casting and sharing valuable facilities and precious time with us.
References
- 1.Wade N. New York Times. New York: 2001. In tiny cells, glimpses of body's master plan. [Google Scholar]
- 2.Greene HSN. Heterologous transplantation of mammalian tumours. Exp Med. 1961;73:461. doi: 10.1084/jem.73.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barth A. Ueber histologische Befunde nach Knochenimplantationen. Arch Klin Chir. 1893;46:409–17. [Google Scholar]
- 4.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 5.Kneser U, Kaufmann PM, Fiegel HC, Pollok JM, Kluth D, Herbst H, Rogiers X. Long-term differentiated function of heterotopically transplanted hepatocytes on three-dimensional polymer matrices. J Biomed Mater Res. 1999;47:494–503. doi: 10.1002/(sici)1097-4636(19991215)47:4<494::aid-jbm5>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 6.Norrby K. in vivo models of angiogenesis. J Cell Mol Med. 2006;10:588–612. doi: 10.1111/j.1582-4934.2006.tb00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bach AD, Beier JP, Stern-Staeter J, Horch RE. Skeletal muscle tissue engineering. J Cell Mol Med. 2004;8:413–22. doi: 10.1111/j.1582-4934.2004.tb00466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moldovan NI. Angiogenesis, l'enfant terrible of vascular biology is coming of age. J Cell Mol Med. 2005;9:775–6. doi: 10.1111/j.1582-4934.2005.tb00378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wake MC, Patrick CW, Jr, Mikos AG. Pore morphology effects on the fibrovascular tissue growth in porous polymer substrates. Cell Transplant. 1994;3:339–43. doi: 10.1177/096368979400300411. [DOI] [PubMed] [Google Scholar]
- 10.Polykandriotis E, Horch RE, Arkudas A, Labanaris A, Brune K, Greil P, Bach AD, Kopp J, Hess A, Kneser U. Intrinsic versus extrinsic vascularization in tissue engineering. Adv Exp Med Biol. 2006;585:311–26. doi: 10.1007/978-0-387-34133-0_21. [DOI] [PubMed] [Google Scholar]
- 11.Khouri RK, Upton J, Shaw WW. Prefabrication of composite free flaps through staged microvascular transfer: an experimental and clinical study. Plast Reconstr Surg. 1991;87:108–15. doi: 10.1097/00006534-199101000-00017. [DOI] [PubMed] [Google Scholar]
- 12.Morrison WA, Dvir E, Doi K, Hurley JV, Hickey MJ, O'Brien BM. Prefabrication of thin transferable axial-pattern skin flaps: an experimental study in rabbits. Br J Plast Surg. 1990;43:645–54. doi: 10.1016/0007-1226(90)90184-2. [DOI] [PubMed] [Google Scholar]
- 13.Le Danvic M, Arrouvel C, Bricout N, Banzet P. [Experimental study of the neovascularization of a flap created from a vascular axial loop] Ann Chir Plast Esthet. 1988;33:377–81. [PubMed] [Google Scholar]
- 14.Tanaka Y, Tsutsumi A, Crowe DM, Tajima S, Morrison WA. Generation of an autologous tissue (matrix) flap by combining an arteriovenous shunt loop with artificial skin in rats: preliminary report. Br J Plast Surg. 2000;53:51–7. doi: 10.1054/bjps.1999.3186. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka Y, Sung KC, Tsutsumi A, Ohba S, Ueda K, Morrison WA. Tissue engineering skin flaps: which vascular carrier, arteriovenous shunt loop or arteriovenous bundle, has more potential for angiogenesis and tissue generation? Plast Reconstr Surg. 2003;112:1636–44. doi: 10.1097/01.PRS.0000086140.49022.AB. [DOI] [PubMed] [Google Scholar]
- 16.Cassell OC, Morrison WA, Messina A, Penington AJ, Thompson EW, Stevens GW, Perera JM, Kleinman HK, Hurley JV, Romeo R, Knight KR. The influence of extracellular matrix on the generation of vascularized, engineered, transplantable tissue. Ann N Y Acad Sci. 2001;944:429–42. doi: 10.1111/j.1749-6632.2001.tb03853.x. [DOI] [PubMed] [Google Scholar]
- 17.Hofer SO, Knight KM, Cooper-White JJ, O'Connor AJ, Perera JM, Romeo-Meeuw R, Penington AJ, Knight KR, Morrison WA, Messina A. Increasing the volume of vascularized tissue formation in engineered constructs: an experimental study in rats. Plast Reconstr Surg. 2003;111:1186–92. doi: 10.1097/01.PRS.0000046034.02158.EB. [DOI] [PubMed] [Google Scholar]
- 18.Kneser U, Polykandriotis E, Ohnolz J, Heidner K, Grabinger L, Euler S, Amann KU, Hess A, Brune K, Greil P, Sturzl M, Horch RE. Engineering of vascularized transplantable bone tissues: induction of axial vascularization in an osteoconductive matrix using an arteriovenous loop. Tissue Eng. 2006;12:1721–31. doi: 10.1089/ten.2006.12.1721. [DOI] [PubMed] [Google Scholar]
- 19.Kneser U, Schaefer DJ, Polykandriotis E, Horch RE. Tissue engineering of bone: the reconstructive surgeon's point of view. J Cell Mol Med. 2006;10:7–19. doi: 10.1111/j.1582-4934.2006.tb00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polykandriotis E, Horch RE, Arkudas A, Labanaris A, Brune K, Greil P, Bach AD, Kopp J, Hess A, Kneser U. Intrinsic versus extrinsic vascularization in tissue engineering. Adv Exp Med Biol. 2006:585. doi: 10.1007/978-0-387-34133-0_21. [DOI] [PubMed] [Google Scholar]
- 21.Brown DL, Meagher PJ, Knight KR, Keramidaris E, Romeo-Meeuw R, Penington AJ, Morrison WA. Survival and function of transplanted islet cells on an in vivo, vascularized tissue engineering platform in the rat: a pilot study. Cell Transplant. 2006;15:319–24. [PubMed] [Google Scholar]
- 22.Polykandriotis E, Tjiawi J, Euler S, Arkudas A, Hess A, Brune K, Greil P, Lametschwandtner A, Horch RE, Kneser U. Microvasc Res. 2007. The venous graft as an effector of early angiogenesis in a fibrin matrix. in press. [DOI] [PubMed] [Google Scholar]
- 23.Polykandriotis E, Arkudas A, Beier JP, Hess A, Greil P, Papadopoulos T, Kopp J, Bach AD, Horch RE, Kneser U. Plast Reconstr Surg. 2007. Intrinsic axial vascularization of an osteoconductive bone matrix by means of an arteriovenous vascular bundle. in press. [DOI] [PubMed] [Google Scholar]
- 24.Kneser U, Stangenberg L, Ohnolz J, Buettner O, Stern-Straeter J, Mobest D, Horch RE, Stark GB, Schaefer DJ. Evaluation of processed bovine cancellous bone matrix seeded with syngenic osteoblasts in a critical size calvarial defect rat model. J Cell Mol Med. 2006;10:695–707. doi: 10.1111/j.1582-4934.2006.tb00429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arkudas A, Beier J, Heidner K, Tjiawi J, Polykandriotis E, Srour S, Sturzl M, Horch RE, Kneser U. Tissue Eng. 2007. Axial prevascularization of porous matrices by an arteriovenous loop promotes survival and differentiation of transplanted autologous osteoblasts. in press. [DOI] [PubMed] [Google Scholar]
- 26.Rongish BJ, Hinchman G, Doty MK, Baldwin HS, Tomanek RJ. Relationship of the extracellular matrix to coronary neovascularization during development. J Mol Cell Cardiol. 1996;28:2203–15. doi: 10.1006/jmcc.1996.0212. [DOI] [PubMed] [Google Scholar]
- 27.Mattsson G, Carlsson PO, Olausson K, Jansson L. Histological markers for endothelial cells in endogenous and transplanted rodent pancreatic islets. Pancreatology. 2002;2:155–62. doi: 10.1159/000055906. [DOI] [PubMed] [Google Scholar]
- 28.Schoenenberger F, Mueller A. On the vascularization of the bovine aortic wall. Helv Physiol Pharmacol Acta. 1960;18:136–50. [PubMed] [Google Scholar]
- 29.Langheinrich AC, Ritman EL. Quantitative imaging of microvascular permeability in a rat model of lipopolysaccharide-induced sepsis: evaluation using cryostatic micro-computed tomography. Invest Radiol. 2006;41:645–50. doi: 10.1097/01.rli.0000227494.17444.64. [DOI] [PubMed] [Google Scholar]
- 30.Hess A, Stiller D, Kaulisch T, Heil P, Scheich H. New insights into the hemodynamic blood oxygenation level-dependent response through combination of functional magnetic resonance imaging and optical recording in gerbil barrel cortex. J Neurosci. 2000;20:3328–38. doi: 10.1523/JNEUROSCI.20-09-03328.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neeman M, Gilad AA, Dafni H, Cohen B. Molecular imaging of angiogenesis. J Magn Reson Imaging. 2006;25:1–12. doi: 10.1002/jmri.20774. [DOI] [PubMed] [Google Scholar]
- 32.Roer RD, Dillaman RM. Decreased femoral arterial flow during simulated microgravity in the rat. J Appl Physiol. 1994;76:2125–9. doi: 10.1152/jappl.1994.76.5.2125. [DOI] [PubMed] [Google Scholar]
- 33.Qin F, Dardik H, Pangilinan A, Robinson J, Chuy J, Wengerter K. Remodeling and suppression of intimal hyperplasia of vascular grafts with a distal arteriovenous fistula in a rat model. J Vasc Surg. 2001;34:701–6. doi: 10.1067/mva.2001.116804. [DOI] [PubMed] [Google Scholar]
- 34.Rosenkilde MM, Schwartz TW. The chemokine system–a major regulator of angiogenesis in health and disease. APMIS. 2004;112:481–95. doi: 10.1111/j.1600-0463.2004.apm11207-0808.x. [DOI] [PubMed] [Google Scholar]
- 35.Maulik N, Das DK. Potentiation of angiogenic response by ischemic and hypoxic preconditioning of the heart. J Cell Mol Med. 2002;6:13–24. doi: 10.1111/j.1582-4934.2002.tb00308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nath KA, Kanakiriya SK, Grande JP, Croatt AJ, Katusic ZS. Increased venous proinflammatory gene expression and intimal hyperplasia in an aorto-caval fistula model in the rat. Am J Pathol. 2003;162:2079–90. doi: 10.1016/S0002-9440(10)64339-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bobryshev YV, Farnsworth AE, Lord RS. Expression of vascular endothelial growth factor in aortocoronary saphenous vein bypass grafts. Cardiovasc Surg. 2001;9:492–8. doi: 10.1016/s0967-2109(01)00040-0. [DOI] [PubMed] [Google Scholar]
- 38.Ito A, Hirota S, Mizuno H, Kawasaki Y, Takemura T, Nishiura T, Kanakura Y, Katayama Y, Nomura S, Kitamura Y. Expression of vascular permeability factor (VPF/VEGF) messenger RNA by plasma cells: possible involvement in the development of edema in chronic inflammation. Pathol Int. 1995;45:715–20. doi: 10.1111/j.1440-1827.1995.tb03387.x. [DOI] [PubMed] [Google Scholar]
- 39.Dvorak HF, Detmar M, Claffey KP, Nagy JA, Van De Water L, Senger DR. Vascular permeability factor(vascular endothelial growth factor: an important mediator of angiogenesis in malignancy and inflammation. Int Arch Allergy Immunol. 1995;107:233–5. doi: 10.1159/000236988. [DOI] [PubMed] [Google Scholar]
- 40.Chien S, Li S, Shyy YJ. Effects of mechanical forces on signal transduction and gene expression in endothelial cells. Hypertension. 1998;31:162–9. doi: 10.1161/01.hyp.31.1.162. [DOI] [PubMed] [Google Scholar]
- 41.Davies PF, Remuzzi A, Gordon EJ, Dewey CF, Jr, Gimbrone MA., Jr Turbulent fluid shear stress induces vascular endothelial cell turnover in vitro. Proc Natl Acad Sci USA. 1986;83:2114–7. doi: 10.1073/pnas.83.7.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Asano Y, Ichioka S, Shibata M, Ando J, Nakatsuka T. Sprouting from arteriovenous shunt vessels with increased blood flow. Med Biol Eng Comput. 2005;43:126–30. doi: 10.1007/BF02345133. [DOI] [PubMed] [Google Scholar]
- 43.Heil M, Eitenmuller I, Schmitz-Rixen T, Schaper W. Arteriogenesis versus angiogenesis: similarities and differences. J Cell Mol Med. 2006;10:45–55. doi: 10.1111/j.1582-4934.2006.tb00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lametschwandtner A, Minnich B, Kachlik D, Setina M, Stingl J. Three-dimensional arrangement of the vasa vasorum in explanted segments of the aged human great saphenous vein: scanning electron microscopy and three-dimensional morphometry of vascular corrosion casts. Anat Rec A Discov Mol Cell Evol Biol. 2004;281:1372–82. doi: 10.1002/ar.a.20098. [DOI] [PubMed] [Google Scholar]
- 45.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 46.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 47.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–77. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heroult M, Schaffner F, Augustin HG. Eph receptor and ephrin ligand-mediated interactions during angiogenesis and tumor progression. Exp Cell Res. 2006;312:642–50. doi: 10.1016/j.yexcr.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 49.Palmer A, Klein R. Multiple roles of ephrins in morphogenesis, neuronal networking, and brain function. Genes Dev. 2003;17:1429–50. doi: 10.1101/gad.1093703. [DOI] [PubMed] [Google Scholar]
- 50.Thurston G. Role of Angiopoietins and Tie receptor tyrosine kinases in angiogenesis and lymphangiogenesis. Cell Tissue Res. 2003;314:61–8. doi: 10.1007/s00441-003-0749-6. [DOI] [PubMed] [Google Scholar]
- 51.Dugail I. Transfection of adipocytes and preparation of nuclear extracts. Methods Mol Biol. 2001;155:141–6. doi: 10.1385/1-59259-231-7:141. [DOI] [PubMed] [Google Scholar]
- 52.Benga G. Basic studies on gene therapy of human malignant melanoma by use of the human interferon beta gene entrapped in cationic multilamellar liposomes. 1. Morphology and growth rate of six melanoma cell lines used in transfection experiments with the human interferon beta gene. J Cell Mol Med. 2001;5:402–8. doi: 10.1111/j.1582-4934.2001.tb00175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin Y, Luo E, Chen X, Liu L, Qiao J, Yan Z, Li Z, Tang W, Zheng X, Tian W. Molecular and cellular characterization during chondrogenic differentiation of adipose tissue-derived stromal cells in vitro and cartilage formation in vivo. J Cell Mol Med. 2005;9:929–39. doi: 10.1111/j.1582-4934.2005.tb00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Katsube K, Bishop AT, Friedrich PF. Transduction of rabbit saphenous artery: a comparison of naked DNA, liposome complexes, and adenovirus vectors. J Orthop Res. 2004;22:1290–5. doi: 10.1016/j.orthres.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 55.Langer R. Delivery systems for angiogenesis stimulators and inhibitors. EXS. 1992;61:327–30. doi: 10.1007/978-3-0348-7001-6_52. [DOI] [PubMed] [Google Scholar]
- 56.Folberg R, Maniotis AJ. Vasculogenic mimicry. APMIS. 2004;112:508–25. doi: 10.1111/j.1600-0463.2004.apm11207-0810.x. [DOI] [PubMed] [Google Scholar]
- 57.Clarijs R, Ruiter DJ, De Waal RM. Lymphangiogenesis in malignant tumours: Does it occur. J Pathol. 2001;193:143–6. doi: 10.1002/path.808. [DOI] [PubMed] [Google Scholar]
- 58.Parenteau NL, Hardin-Young J. The use of cells in reparative medicine. Ann N Y Acad Sci. 2002;961:27–39. doi: 10.1111/j.1749-6632.2002.tb03042.x. [DOI] [PubMed] [Google Scholar]
- 59.Putnam AJ, Mooney DJ. Tissue engineering using synthetic extracellular matrices. Nat Med. 1996;2:824–6. doi: 10.1038/nm0796-824. [DOI] [PubMed] [Google Scholar]
- 60.Kruyt MC, Persson C, Johansson G, Dhert WJ, De Bruijn JD. Towards injectable cell-based tissue-engineered bone: the effect of different calcium phosphate microparticles and pre-culturing. Tissue Eng. 2006;12:309–17. doi: 10.1089/ten.2006.12.309. [DOI] [PubMed] [Google Scholar]
- 61.Vogelin E, Jones NF, Huang JI, Brekke JH, Lieberman JR. Healing of a criticalsized defect in the rat femur with use of a vascularized periosteal flap, a biodegradable matrix, and bone morphogenetic protein. J Bone Joint Surg Am. 2005;87:1323–31. doi: 10.2106/JBJS.C.00913. [DOI] [PubMed] [Google Scholar]
- 62.Perryman SV, Sylvester KG. Repair and regeneration: opportunities for carcinogenesis from tissue stem cells. J Cell Mol Med. 2006;10:292–308. doi: 10.1111/j.1582-4934.2006.tb00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hunt TR, Schwappach JR, Anderson HC. Healing of a segmental defect in the rat femur with use of an extract from a cultured human osteosarcoma cell-line (Saos-2). A preliminary report. J Bone Joint Surg Am. 1996;78:41–8. doi: 10.2106/00004623-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 64.Polykandriotis E, Arkudas A, Euler S, Beier JP, Horch RE, Kneser U. Pravaskularisationsstrategien im Tissue Engineering. Handchir Mikrochir Plast Chir. 2006;38:217–23. doi: 10.1055/s-2006-924419. [DOI] [PubMed] [Google Scholar]
- 65.World-Health-Organization. World Health Organization Report, 2000, World Health Organization: Geneva.
- 66.Motwani JG, Topol EJ. Aortocoronary saphenous vein graft disease: pathogenesis, predisposition, and prevention. Circulation. 1998;97:916–31. doi: 10.1161/01.cir.97.9.916. [DOI] [PubMed] [Google Scholar]
- 67.Roberts N, Jahangiri M, Xu Q. Progenitor cells in vascular disease. J Cell Mol Med. 2005;9:583–91. doi: 10.1111/j.1582-4934.2005.tb00490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baguneid M, Murray D, Salacinski HJ, Fuller B, Hamilton G, Walker M, Seifalian AM. Shear-stress preconditioning and tissue-engineering-based paradigms for generating arterial substitutes. Biotechnol Appl Biochem. 2004;39:151–7. doi: 10.1042/BA20030148. [DOI] [PubMed] [Google Scholar]
- 69.Horch RE. Changing paradigms in reconstructive surgery by vacuum therapy? Zentralbl Chir. 2006;131:S44–9. doi: 10.1055/s-2006-921462. [DOI] [PubMed] [Google Scholar]
- 70.Lang W, Horch RE. Distal extremity reconstruction for limb salvage in diabetic foot ulcers with pedal bypass, flap plasty and vacuum therapy. Zentralbl Chir. 2006;131:S146–50. doi: 10.1055/s-2006-921472. [DOI] [PubMed] [Google Scholar]
- 71.Byrne AM, Bouchier-Hayes DJ, Harmey JH. Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF) J Cell Mol Med. 2005;9:777–94. doi: 10.1111/j.1582-4934.2005.tb00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aggarwal S, Chu E. Current therapies for advanced colorectal cancer. Oncology. 2005;19:589–95. [PubMed] [Google Scholar]
- 73.Horch RE. Future perspectives in tissue engineering. J Cell Mol Med. 2006;10:4–6. doi: 10.1111/j.1582-4934.2006.tb00286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zandonella C. Tissue engineering: The beat goes on. Nature. 2003;421:884–6. doi: 10.1038/421884a. [DOI] [PubMed] [Google Scholar]


