I. Introduction
Vitamin A (retinol) has long been considered important for the maintenance of the immune system, but its role in antibody production is still being uncovered. Antibody production, the hallmark of a successful response to vaccination, is, indeed, the only proven mechanism which vaccines protect against infectious disease (Beverley, 2002; Del Giudice, 2003). This review focuses on studies, in the past decade, on the effects of providing vitamin A (VA) or its active metabolite, retinoid acid (RA), during the inductive phase of the antibody response in vivo. After discussing the rationale for the topics selected, the chapter then considers: 1) the effect of VA supplementation on the response to immunization in children; 2) experimental studies addressing mechanisms by which VA and/or RA may affect antibody production in vivo; and 3) innate immune cells and factors regulated by VA and RA that may affect immunization outcome; and is followed by 4) a discussion of factors that may account for differences observed in human and animals studies of VA supplementation and the response to immunization. For other reviews addressing VA deficiency and infection, and morbidity and mortality outcomes in VA supplementation studies, see (Semba, 2000; Stephensen, 2001; Villamor and Fawzi, 2005).
II. Rationale for interest in VA supplementation and antibody production
Vitamin A deficiency in young children is associated with increased morbidity and mortality, especially from measles and diarrheal diseases (Beaton et al., 1994; Sommer and West, 1996). It has been estimated that improving VA status in children at risk of deficiency will reduce mortality by 23% (Beaton et al., 1994), and avert >240,00 deaths per year (Ching et al., 2000). The reduction in morbidity and mortality by VA is widely attributed to a decreased severity of infectious diseases (Beaton et al., 1994; Semba, 1999; Villamor and Fawzi, 2005). In 1994, WHO established a policy of integrating VA supplementation as a part of the Expanded Program of Immunization (EPI) in countries where VA deficiency is still prevalent (World Health Organization, 1994). For 6- to 12-month old infants, it is recommended that 100,000 IU of VA (equivalent to 30 mg of retinol) be given along with measles immunization, and for infants under 6 months of age, 25,000 IU of VA along with diphtheria-pertussis-tetanus (DPT) vaccines. Therefore, many children worldwide are now receiving VA along with immunizations against poliovirus, measles, diphtheria, pertussis, and tetanus. The primary goal has been to provide enough VA to protect against VA deficiency for a period of 4 to 6 months (Sommer and West, 1997; Underwood, 1995). However, it is also possible that VA might promote the vaccine antibody response. To date, this has been tested relatively sporadically and only in trials much smaller than the mortality trials that led up the to policy to provide VA with immunization. Because co-treatment with VA at the time of immunization is widely practiced today, it is important to understand the effect that VA supplementation may have on the response to immunization. As discussed in the section below, only a few of the studies that have measured the antibody response to vaccination have shown evidence of a benefit of VA, but neither have they shown reduced titers or other evidence of unintended effects.
Animal experiments have demonstrated that an adequate level of vitamin A is necessary to mount an efficient antibody response to many antigens; however, previous studies have also revealed that VA-deficient animals are capable of producing a strong antibody response to some antigens (Ross, 1996a ;Ross, 1996b). Thus, the requirement for VA may be better considered as conditional, as it apparently differs with and depends on the type of immune stimulus employed. Antigens that require T-cell help [T-cell dependent (TD), antigens, such as tetanus toxoid and cellular antigens] in the initiation phase of the antibody response, or antigens that, while considered T-cell independent are nonetheless regulated by T cells (TI-2 antigens, such as polysaccharides), were poorly immunogenic in VA-deficient animals, while TI-1 antigens [lipopolysaccharides (LPS)] were strongly immunogenic in VA-deficient animals as well as controls (Arora and Ross, 1994; Ross, 1996a; Ross, 1996b). Indeed, in response to some types of antigenic challenge, VA deficient animals have produced higher titers of certain antibodies. For example, anti-influenza IgG was elevated in VA-deficient mice infected with influenza virus (Stephensen, Moldoveanu and Gangopadhyay, 1996). These data provide evidence that the antibody response is dysregulated by VA deficiency, rather than simply impaired. It is encouraging that VA-deficient animals remain to some extent immunocompetent, as this suggests that when they are provided with VA, the “machinery” for a competent immune response is present, and although dysregulated, it may potentially be re-regulated to produce normal adaptive immune responses without significant delay.
Retinoic acid, a natural bioactive metabolite of VA (Ross, 2006), is well known as a hormone capable of promoting the differentiation of a wide variety of cell types, and as a regulator of many physiological processes. Animal experiments have demonstrated that supplementation with VA or RA in vivo can stimulate the antibody response to vaccination, even in normal, non-VA deficient animals (Cui, Moldoveanu and Stephensen, 2000; DeCicco, Youngdahl and Ross, 2001; Ma, Chen and Ross, 2005). Therefore, VA and/or RA could potentially be useful as immunological stimuli, but their effects need to be better understood at the cellular and whole-body levels. Little is known about the underlying mechanisms, especially related to lymphocyte activation and differentiation, through which supplemental VA or RA may stimulate antibody production. A successful antibody response requires a finely timed collaboration amongst several types of cells: antigen-presenting cells (dendritic cells, macrophages, and B-cells), T-helper (Th) cells; cytokine-producing cells [natural killer (NK) cells, and NK-T cells], and naïve B-cells capable of developing into plasmacytes and then antibody-secreting plasma cells, or memory B cells required for long-lived immunity and protection against a potential future encounter with the infectious agent for which the vaccine has been designed. An area of significant interest regarding VA and RA is whether, alone and when combined with other immune stimuli, they are effective in augmenting the humoral response to vaccination.
The past decade in immunology research has been remarkable for the greatly increased understanding of the role of innate immunity in shaping the outcome of adaptive immune responses. It had long been recognized that bacterial cell wall components –LPS, lipotechoic acid and other components – as well as viral nucleic acids, provide “danger signals” to mammalian cells, and often are potent immune stimuli (Janeway and Medzhitov, 2002;Matzinger, 2002). These bacterial and viral components are typically composed of repeating subunit structures, which Janeway and coworkers (Janeway and Medzhitov, 2002) termed “Pathogen-Associated Molecular Patterns,” or PAMPs, and these agents have now been shown to be ligands for Toll-like receptors [TLR family molecules (Janeway and Medzhitov, 2002;Takeda and Akira, 2005)], or other “pattern recognition receptors” (PRR) on the surface of mammalian cells. The interaction of PAMPs with PPRs triggers potent innate immune responses, including production of cytokines and oxidants, which are critical in the first line of defense against many microbial pathogens. It is now recognized that cells and factors considered part of the innate immune system, that typically act very early and are relatively nonspecific in the course of infection or immune stimulation, have a strong impact on cells of the adaptive immune system – B-cells and T-cells – that are activated more slowly but sustained over time to produce humoral immunity and the memory response that is critical to successful vaccination.
VA and RA have been shown to affect some of the functions of macrophages, NK cells, and other cells of the innate immune system in earlier studies [reviewed in (Ross, 1996b)], and to stimulate the functions of dendritic cells in culture (Hengesbach and Hoag, 2004). The exposure of cells of the innate immune system to VA and RA at the time of antigen exposure (priming in vivo) may stimulate the initial phase of antibody production and, if memory to antigen is formed, affect the response to vaccination over a much longer term. It therefore is also important to understand how retinoids affect the innate immune system, to better understand the regulation of adaptive immunity.
III. VA and the response to immunization in children
Several studies in the last decade have added to the literature on VA supplementation and immunity in young children. The effect of VA given with measles immunization on serum antibody titers and seroconversion percentage was studied in a randomized controlled trial in 395 infants, 9-12 month-old, in India (Cherian et al., 2003). Previous studies in 6-9 month old infants [see references in (Cherian et al., 2003)] had shown no enhanced response to measles immunization in one study, while in two others the antibody response was higher but serconversion rates were similar, and in another study antibody titer and seroconversion were increased in VA-supplemented infants. Supplemented with 100,000 IU of VA (30 mg retinol) did not affect the rate of seroconversion, but rates were 99% in both the supplemented and unsupplemented groups, so any additional effect could not have been detected. Antibody titers at 1 and 6 months post-vaccination did not differ, nor did the proportion of infants with titers considered protective for measles at 6 months post-vaccination. The authors commented that most infants in this study were breast fed, which would be expected to provide some protection against VA deficiency. However, VA did not enhance the response of low weight-for-age infants in the study, a group that may be considered more vulnerable to VA deficiency.
A small prospective study of 89 healthy breast-fed infants, randomized into 4 groups, examined VA supplementation, alone and combined with vitamin E, on the response to DPT immunization (Kutukculer et al., 2000). Infants were given 30,000 IU of VA (9 mg retinol) for 3 days just after each immunization, at 2, 3 and 4 months, and they received a booster immunization (without supplementation) at 16-18 months. Vitamin A levels were determined and pre- and post-immunization and antibody titers were measured. No significant differences were found for serum VA, vitamin E, or anti-tetanus antibody titers at 2, 5 and 16 months. However, visual inspection of the data indicates that titers at 5 months were, on average, about 25% higher in the two groups that received VA, with and without vitamin E. Overall, this small study did not find a benefit of VA on the anti-tetanus antibody response, consistent with two earlier small studies. However, an earlier larger study had reported lower levels of anti-tetanus IgG in VA-deficient children. Together, the studies support either no observable benefit on immunization against tetanus, or possibly a small benefit in some studies.
Bahl et al. (2002) reported on the effect of VA supplementation, given at EPI contacts, on the antibody response to oral polio vaccine (OPV) in Indian infants in a community with a significant rate of stunting and a high prevalence of subclinical VA deficiency (37% of children 1-5 years old with serum retinol <0.7 μmol/L). Thus it was believed that VA deficiency may exist prior to 6 months of age in this community, and therefore providing VA before 6 months of age might prove beneficial. Three hundred ninety-nine infants received OPV at 6, 10 and 14 weeks of age, and half (n=194) of the infants received 25, 000 IU of VA (7.5 mg retinol) with each immunization. The mothers also received 60 mg of retinol 18-38 days post-partum. OPV titers were determined to poliovirus type 1, type 2 and type 3 12 weeks after the third immunization. VA supplemented infants had a significantly higher geometric mean antibody titer against OPV type 1 (relative risk ratio 1.55; 95% CI, 1.03-2.31), while the number of infants with protective titers against poliovirus was also increased. No differences, however, were observed for responses to type 2 or type 3 poliovirus immunization. The authors concluded that VA did not interfere with the response to any of the three types of poliovirus, while the response to type 1 poliovirus was enhanced. Although it is unknown why the beneficial response was limited to type 1 poliovirus, the authors noted that the proportion of infants in the placebo group with protective antibody titers was lower (71%) for type 1 poliovirus than for either type 2 or type 3 poliovirus (93 and 80%, respectively), while VA increased the percent of infants with protective titers against type 1 poliovirus to 82%. Thus it may be that the effect of VA supplementation is limited to those antigens which tend to be less effective, and for which the response rate of the untreated group is low.
The effect of VA given simultaneously with measles immunization was examined in a study of 462 children in Guinea-Bissau who were randomized to receive either a two-dose schedule of measles vaccine at the ages of 6 months and 9 months (n=150 infants) or one dose of measles vaccine at age 9 months (n=312 infants), the more common age for immunization in developing countries (Benn et al., 1997). Children were followed up to the age of 18 months when serum measles titers were determined. The rate of seroconversion was 98% among children who received two doses of vaccine. Neither the percetage of seroconversion nor geometric mean titer of anti-measles antibodies differed in children receiving VA compared with children receiving no supplement. Among children receiving only one dose of measles vaccine at age 9 months, seroconversion was also high, 95%. In this group, antibody titer was significantly higher in the children, and especially in boys, who received VA (relative risk ratio 1.52, CI, 1.22-1.88). Benn et al. (1997) interpreted their study as providing no indication that simultaneous administration of measles vaccine and vitamin A supplements has a negative effect on measles immunity. Among the children who had received two doses of measles vaccine at the ages of 6 months and 9 months, VA had no significant effect, while among children receiving only one dose of measles vaccine at age 9 months of age, 100,000 IU of VA increased antibody concentrations, especially for boys.
These children were then followed up and re-examined when they reached age 6-8 years of age (Benn et al., 2002). At that time, fewer of the previously VA-supplemented children had non-protective antibody concentrations (P =0.0095), and among children with protective antibody levels, VA-supplemented children tended to have higher antibody titers (P=0.09). This results suggests the effect of VA on measles vaccine given at age 9 months may results in longer protection, although titer levels were not higher.
Wieringa et al. (2004) examined the effect of VA, or other micronutrients, administered in vivo, on whole blood cytokine production measured ex vivo, in a small study of 59 Indonesian infants without evidence of infection. Whole blood was treated with phytohemagglutinin and LPS, incubated at 37°C for 10 h, and cytokine concentrations were measured. Serum retinol, zinc and iron were measured as indicators of micronutrient status. The VA deficient and non-deficient groups had serum retinol concentrations of 0.49 and 0.88 μmol/L, respectively. Interferon (IFN)γ production was lower (P<0.05) in the VA deficient group, while IL-12 production showed a similar mean difference, but was not significant, and neither IL-10 nor IL-6 showed any differences. VA did not affect leukocyte counts or differential blood cell counts. The lower production of IFNγ would be consistent with a reduced type 1 cytokine response; however, in mice, VA deficient CD4+ T-cells were reported to secrete more IFNγ per cell (Carman and Hayes, 1991), which was reduced RA. Since LPS is a ligand of TLR4 (Janeway and Medzhitov, 2002; Takeda and Akira, 2005), the IFNγ could have been produced by non-T cells, such as innate immune cells bearing TLR4 on their surface. Although this study was small, it is interesting for its exploration of a possible mechanism whereby VA might affect the balance of Th1: Th 2 cytokine production.
IV. Experimental studies of VA or RA supplementation and antibody production in vivo
A. Experimental models
Experiments to examine the effects of VA or RA on antibody production in vivo have been conducted in several animal models. Animal models of VA deficiency provide a means to assess the effects of nutritional repletion with VA, or the effects due specifically to RA in the absence of significant levels of retinol when RA is given as a treatment. It is well appreciated that most, if not all, of the biological effects of VA outside of vision are attributed to RA, and RA, while not stored, is able to reverse the growth impairment and normalize epithelial functions in retinol-depleted animals (Dowling and Wald, 1960), so long as it is supplied continously (Lamb, Apiwatanaporn and Olson, 1974). VA adequate animal models provide a means to assess the potential immunostimulatory or immunoinhibitory activity of supplemental VA, or of RA used to simulate a therapeutic treatment, on antibody production and/or regulatory cytokines. Additionally, combinations of VA or RA together with other immune stimuli are of interest. For example, our laboratory has investigated the effects of VA or RA combined with LPS, now known as a ligand for TLR4, and of VA combined with tumor necrosis factor (TNF)-α, a ligand for several receptors of the TNF-α family that are critical for immune regulation. Each of these combinations increased the antibody response to tetanus toxoid in VA-deficient rats, and also elevated the level of anti-tetanus antibodies above the normal level in VA-adequate rats (Ross, 2000). We have also combined VA and RA with PIC, a double-stranded RNA that is a mimetic of double-stranded RNA viruses and a ligand of TLR3 (Janeway and Medzhitov, 2002;Takeda and Akira, 2005). PIC has been studied by others and ourselves for its ability to elicit type I IFNs and activate NK cells, but it is now known that PIC-stimulated dendritic cells and peripheral blood mononuclear cells produce an array of cytokines, including TNF-α and chemokines (Re and Strominger, 2004). As described below, PIC and RA are strong and sometimes synergistic regulators of antibody production. Factors like LPS, TNF-α and PIC are most often considered “pro-inflammatory,” yet LPS (from normal commensal microflora), and TNF and related molecules are necessary for a normal immune response. The concept of employing TLR ligands such as PIC, CpG, or other PAMPs as vaccine adjuvants, or of incorporating cytokine-expressing vectors such as IL-12 with vaccines, is now receiving serious attention (Del Giudice, 2003; Marciani, 2003; Schijns and Tangeras, 2005).
It is important to bear in mind that in the nutritional model of VA deficiency, deficiency develops gradually, generally over a course of weeks or longer, and thus changes in bone marrow hematopoiesis, and lymphoid organ cellularity may already be apparent, along with other physiological-biochemical changes. Retinoids are known to be important for the maintenance of stromal cell interactions with neighboring cells, and a dysfunction of cell-cell interactions may develop insidiously, as VA deficiency becomes more severe. Conversely, the response to treatment with VA and RA can be very rapid, as both forms of the vitamin are rapidly absorbed from the intestine, even in the VA-deficient state (Ross and Zolfaghari, 2004), and changes in the expression of some retinoid-responsive genes can be detected within hours after administration of RA to VA-deficient animals (Wang, Zolfaghari and Ross, 2001; Zolfaghari and Ross, 2002; Zolfaghari et al., 2002). Thus, the context of supplementation studies, especially the extent of deficiency prior to treatment, is likely to be an important factor in the outcomes observed.
B. RA treatment and antibody production in a VA-deficient model
Antibody responses to tetanus toxoid, a TD antigen, had previously been shown to be reduced during VA deficiency, and restored by supplementation with VA [reviewed in (Ross, 1996b;Ross, 2000)]. In a study to test whether RA alone, and RA combined with PIC, can increase antigen-specific antibody production, VA-deficient rats were immunized with tetanus toxoid and treated orally with all-trans-RA, PIC, or the combination at the time of primary immunization (DeCicco et al., 2000). After the primary response has subsided, all rats were reimmunized with tetanus toxoid; however, no additional RA or PIC was administered. VA-deficient rats produced low primary anti-tetanus IgG response (∼20% of the VA-adequate control group) and a very low secondary anti-tetanus IgG response (<10% of the VA-adequate control group). The primary response was increased by RA alone and by PIC alone. The primary response was increased much more strongly, in a synergistic manner, by RA+ PIC (P<0.0001). Interestingly, the secondary response was equally augmented by RA + PIC, even though these treatments were given only at the time of first immunization (priming). PIC alone, however, did not promote the secondary anti-tetanus response, suggesting that despite an effect of PIC (or cytokines elicited by the binding of PIC to TLR3), on the primary immune response, the development of memory cells was not increased by PIC. However, when PIC was combined with RA the anti-tetanus memory response was increased strongly and synergistically. These results suggest that RA is required for the differentiation and/or maintenance of memory T and memory B cells, while PIC alone is not sufficient to induce or maintain these populations. In combination, however, the augmentation of antibody production is both stronger than for either agent alone, and long-lasting.
Several cytokine mRNAs were measured in the spleen of VA-deficient rats to explore possible mechanisms for the differences in antibody production. In VA-deficient rats (DeCiccoet al., 2000), mRNA levels were low for interleukin (IL)-2 receptor-β, interferon regulatory factor-1, a downstream factor in IFN-regulated gene transcription, and signal transducer and activator of transcription (STAT)-1, a mediator of type I and type II IFN signal transduction and transcriptional activation. In contrast, RA together with PIC increased the expression of each of these factors (P<0.0001 versus controls). Conversely, in VA deficiency, IL-12 and IL-10 mRNAs were both elevated, but reduced towards normal levels by RA. As noted later, an elevation in Th 1/type 1 cytokines, either absolutely or in relationship to Th 2/type 2 cytokines, has been consistently observed in the VA deficient state, and the IL-12 results, but not the IL-10 results in this study, support this conclusion. RA normalized this cytokine mRNA imbalance by down-regulating IL-12 mRNA to the level in VA-adequate controls. Because retinol levels were very low in the VA-deficient group throughout this experiment, the down-regulation of type 1 responses in rats supplemented with RA is apparently directly due to this retinoid. Overall, this study provided support for the notion that RA together with PIC could be a promising combination for stimulating specific immunity in vivo.
C. RA treatment and antibody production in VA-adequate models
A study of similar design was conducted in non-immunocompromised rats of normal VA status to assess the effects of RA, given at a dose resembling a chemotherapeutic regimen, on TD antibody production (DeCicco, Youngdahl and Ross, 2001). Rats were treated with 100 μg RA ± 20 μg PIC on day 1, with continued administration of 100 μg of RA daily for 11 days, after which antibody production, changes in lymphocyte populations, and cell proliferation were evaluated. In another study conducted for just 21 hours, early changes in lymphocyte populations and gene expression were measured. Similar to previous results in VA-deficient rats, the combination of RA + PIC significantly potentiated anti-tetanus IgG levels in VA-adequate rats. This combination also increased the numbers of B cells and MHC class II+ cells in spleen and lymph nodes, determined by flow cytometry, and the number of NK cells in spleen and blood (see section V). RA + PIC significantly increased the levels of IL-10, IL-12, and STAT-1 mRNA, and STAT-1 protein, suggestive of heightened immune stimulation. Because tetanus toxoid is a TD antigen, the proliferative response of T-cells ex vivo was further studied after short-term treatments administered in vivo, as a model for the early stages of T-cell activation in vivo. RA combined with PIC significantly increased T-cell proliferation stimulated by anti-CD3/phorbol myristyl acetate + IFNα ex vivo. These changes in antibody production, cell distribution, cytokine gene expression, and T-cell proliferation suggest that the combination of RA + PIC stimulates humoral and cell-mediated immunity. It was interesting, however, that the strong synergy between RA and PIC on anti-tetanus antibody production was not apparent in the VA-sufficient rat model.
To further investigate the potential for RA and PIC to promote immunity in the VA-adequate state, studies were conducted in adult mice immunized with tetanus toxoid and treated with RA and/or PIC at priming (Ma, Chen and Ross, 2005). Three independent studies of short and long duration were conducted to evaluate early responses to treatment and long-term outcomes on antibody titers and memory formation. Anti-tetanus IgG isotypes were measured to further assess the effect of treatment on Th 1/type 1 immunity, associated with higher IgG2a responses, and Th 2/type 2 immunity associated with higher IgG1 production. Whereas RA and PIC differentially regulated both primary and secondary anti-TT IgG isotypes, the combination of RA+ PIC stimulated the highest level of total anti-TT IgG (Figure 1A, 1B). Concomitantly, the ratio of IgG1 to IgG2a was similar to that of the control group, indicating that the combination of RA+PIC promoted a higher but normally balanced response.
Figure 1.
Primary and secondary (memory) anti-tetanus antibody responses of adult (A, B) and neonatal (C,D) mice treated at the time of initial dose (priming) with retinoic acid (RA, given orally), polyriboinosinic acid: polyribocytidylic acid (PIC, i.p.), or both RA+PIC. For determination of the memory response, animals were re-immunized with tetanus toxoid without additional treatment with RA or PIC. Data from Ma et al., 2005 and Ma and Ross, 2005.
Antibody production was strongly associated with type 1/type 2 cytokine gene expression, assessed as IFNγ and IL-12 mRNA as indicators of type 1 response, and IL-4 and IL-12 mRNA as indicators for type 2 response. Whereas RA reduced type 1 cytokines (IFNγ and IL-12), PIC enhanced both type 1 and type 2 cytokines and cytokine-related transcription factors (T-bet, GATA-4). Despite the presence of PIC, the IL-4:IFNγ ratio was significantly elevated by RA, indicative of skewing towards Th-2/type 2 immunity in RA-treated mice. In addition, RA and/or PIC modulated the level of expression of costimulatory molecules involved in B-cell activation, CD80/CD86, an effect that was evident 3 days after antigen priming. Overall, RA, PIC and RA + PIC rapidly and differentially increased the anti-tetanus Ig response. However, the greatest and yet well-balanced response was achieved with RA + PIC, which resulted in a robust, durable and proportionate increase in all anti-TT IgG isotypes. Because no further treatment with RA or PIC was given after the primary immunization, the heightened secondary antibody response in RA+PIC-treated mice (Fig. 1B) must be attributable to re-activation of immune memory, which was augmented by RA, and RA+ PIC, during the primary response.
D. RA supplementation in a neonatal model
Neonates are highly susceptible to infectious diseases (Kovarik and Siegrist, 1998). In clinical trials in newborn babies, VA supplementation on days 1 and 2 after birth reduced neonatal mortality in the first two months of life, especially in infants of low birth weight (Rahmathullah et al., 2003). The vitamin A status, in terms of liver VA reserves and plasma retinol of newborns, even full-term infants of well-nourished mothers, is low as compared to that of older children and adults. Thus, neonates might well be considered physiologically deficient in VA deficient, or as of marginal vitamin A status (Ross, 2005). Neonates are known to respond poorly to conventional vaccines due to immaturity of the immune system (Siegrist, 2001), and this has stimulated a search for better adjuvants for neonatal vaccines. As indicated in Table 1, the pattern of immune deficiency reported for neonates (Marshall-Clarke et al., 2000) resembles the pattern observed in VA deficient adult rats and mice (Ross, 1996a), with low responses to TD and polysaccharide (TI-2) antigens, but a relatively normal response to TI-1 (LPS-type) antigens.
Table I. Characteristics of antibody responses in murine adult and neonatal models, and in adult VA-deficient rats.
| Antigen type | Adults1 | Neonates1 | VA-Deficient adult rats2 |
|---|---|---|---|
| T-cell independent type 1 (TI-1) | ++ | ++ | +++ |
| T-cell independent type 2 (TI-2) | ++ | + | +/- |
| T-cell dependent (TD), total Ig | Weak | ||
| Isotype switching | +++ | Weak | Weak |
| Affinity maturation | +++ | Poor | ND |
| Heterogeneity | +++ | Restricted | ND |
From (Marshall-Clarke et al., 2000)
From (DeCicco et al., 2000; Ross, 1996a; Ross, 1996b).
ND, not determined
Because RA and PIC successfully promoted the antibody response of both VA-deficient rats (DeCicco et al., 2000) and non-VA deficient adult rats (DeCicco, Youngdahl and Ross, 2001) and mice (Ma, Chen and Ross, 2005), we hypothesized that RA, PIC and both RA+PIC in combination would promote a stronger anti-tetanus response in neonatal mice. No previous studies of RA on the immune system in this age group had been reported. We modeled the treatments in our neonatal study (Ma and Ross, 2005) on our previous study of RA + PIC given to adult mice (Ma, Chen and Ross, 2005), scaling the doses of RA and PIC for neonatal mice based on body weight. Early-life treatments with RA and/or PIC were well tolerated, as indicated by equal growth rates in all groups. As was observed in adult mice, RA, PIC, and RA+ PIC stimulated the primary anti-tetanus IgG response in neonatal mice (control < RA < PIC < RA+PIC, Fig. 1C). Neonates were maintained on a normal diet after weaning and then, on day 40, they were re-immunized as young adult rats, without any further treatment with RA, PIC, or RA+ PIC, to assess the formation of the anti-tetanus memory response. Treatment with RA at priming resulted in a durable increase in anti-tetanus IgG, which was about 4 times than that of the control group (Fig. 1D). PIC, a potent adjuvant in adult mice, also elevated the neonatal primary anti-tetanus IgG response, and induced tetanus-specific IFNγ; however, PIC alone failed to benefit the memory response (Fig. 1D). The combination of RA+ PIC was more potent than either agent alone in elevating both primary and secondary anti-tetanus IgG responses, as well as all IgG isotypes. Nevertheless, the titer of the memory anti-tetanus response of mice that were treated at neonatal age with RA, PIC and RA + PIC, during the priming phase of the immune response, was about half that of the memory response of mice primed as adults and treated comparably. Therefore, although anti-tetanus immunity was stimulated by RA + PIC in neonatal mice, the formation of B and T cell memory was lower, compared to that of adult mice.
E. Cytokine production and Th1:Th2 antibody isotype balance
VA deficiency was shown previously to be associated with a predominance of type 1 cytokines (Carman and Hayes, 1991; Carman, Smith and Hayes, 1989; Wiedermann et al., 1993). In recent studies of VA supplementation and RA treatment, several investigators measured cytokines, IgG isotypes, or both, that are considered signatures of type 1 and type 2 responses. A consistent finding across studies has been a relative increase in type 2 immunity compared to type 1 immunity after treatment with VA or RA, which has been observed either as an increase in the level of Th2/type 2 cytokines, or as a decrease in Th1/type 1 cytokines. In either case, the ratio of Th2 to Th1 cytokines, and/or IgG isotypes, was increased by retinoid treatment. This pattern is robust, as it has been observed in various models of immunization and infection. A predominance of type 2 response was reported in a study of mice immunized intramuscularly with a DNA vector expressing human choronic gonadotrophin, in which IgG production to the expressed antigen was monitored over the course of several weeks, during which mice were treated with or without RA continuously (Yu et al., 2005), so that exposure to RA was much higher in this study. The ratio of IgG1/IgG2a plasma antibody levels was somewhat increased in the RA-treated mice, suggesting skewing towards a Th2 response occurred in this model of immunization. In a study of respiratory infection, rather than immunization, in a mouse model of influenza, two groups of mice were fed high VA diet either before and continuing after influenza infection, or beginning at the time of infection to simulate the adjuvant therapy previously used in clinical trials (Cui, Moldoveanu and Stephensen, 2000). The production of IFNγ, a Th1 cytokine, was lower in the group fed the high level of VA continuously compared with the control group, whereas the production of IL-10, a Th2 cytokine, was higher. No differences in disease symptoms were found among the three groups. The authors noted that high-dose VA supplements may enhance Th2-mediated responses, which are beneficial in the case of extracellular bacterial and parasitic infections, and IgA-mediated responses to mucosal infections (Cui, Moldoveanu and Stephensen, 2000).
In the immunization study discussed above in neonatal mice, TT-specific lymphocyte proliferation and type 1/type 2 cytokine production, measured as IL-5 and IFNγ in cell culture supernatants after restimulation with tetanus toxoid, were significantly augmented by the prior in vivo treatment with RA, PIC or the combination of RA + PIC. RA alone selectively increased anti-TT IgG1 and IL-5, resulting in skewing towards a type 2 response. Additionally, in a 3-day study, RA and PIC significantly modulated the maturation and/or differentiation of neonatal B cells, natural killer (NK)/NKT cells (see next section), and antigen-presenting cells. These results imply that RA rapidly affects cell populations while effectively promoting predominantly type 2 responses in neonatal mice, an age group in which type 2 immunity is inherently predominant (Marshall-Clarke et al., 2000). However, when RA was combined with PIC as a nutritional-immunological intervention, the production of anti-tetanus IgG isotypes was well balanced, robust, and durable into adulthood, and both type 1 and type 2 cytokine responses were increased.
In summary, several experimental models are consistent in showing that adaptive immune responses are elevated by VA or RA, but skewed in the type 2 direction. Retinoids combined with immune stimuli like PIC, however, stimulated a quantitatively higher level of immune response in which, as shown in both adult and neonatal mice. Qualitatively, the balance of Th1:Th2 cytokines and the production of IgG isotypes were similar to that of VA-adequate animals.
V. Innate immune cells and factors regulated by VA and RA, that may affect immunization outcome
Natural killer (NK) cells are important effector cells of the innate immune system, and also important regulators of adaptive immunity (Lanier, 2005; Papamichail et al., 2004). NK cells are produced in bone marrow and enter blood as relatively immature cells, which then can rapidly mature under the influence of various cytokines, especially type I interferons (α/β), released early after viral infection, and IL-2, IL-12, and IL-18 which act synergistically to increase the activation state of NK cells and increase their cytotoxic activity against tumor cells (Baxevanis, Gritzapis and Papamichail, 2003). Unlike cytotoxic T cells, NK cells do not require prior sensitization by exposure to the target cell to become cytotoxic. Activated NK cells produce various cytokines and thus play an important immunomodulatory role in the production of antibodies. The marker for NK cells, NK1.1 in mice and NKR-P1 on human and rat cells (Lanier, Chang and Phillips, 1994), is also expressed on a subset of T-lymphocytes, the NKT cells (Bendelac et al., 1997; Kronenberg, 2005). NK cells are known as an early source of IFNγ, whereas NKT cells have been shown to rapidly secrete IL-4 and IL-10, as well as IFNγ. Understanding the development, functionality and activation potential of NK cells and NKT cells is currently of great interest both in relationship to antibody production and tumor immunity.
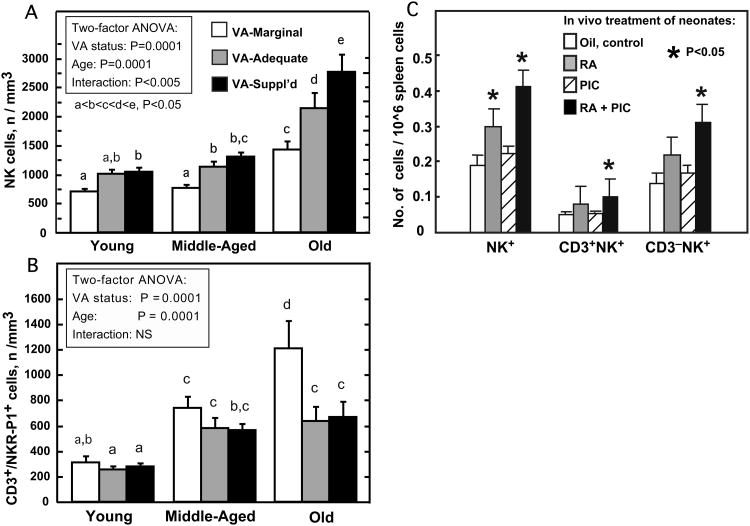
NK and NKT cells in spleen and peripheral blood were investigated in a study of aging rats fed diets that differed only in VA contents (marginal, adequate, and supplemented) from the time of weaning, throughout their life time (Dawson et al., 1999; Dawson and Ross, 1999). The study was designed to cover a wide range of VA consumption, but to exclude states of clinically evident VA deficiency or toxicity. Rats fed these diets showed distinct differences in VA status, ranging from a state of marginal VA deficiency (depleted liver VA stores, reduced serum retinol, but normal growth, which became progressively lower as the rats aged), to normal VA status (normal plasma retinol, and tissue VA reserves that gradually accumulated with age), to a state of excessive VA accumulation in the VA-supplemented group (elevated plasma retinol and tissues stores, which increased with age, but no overt toxicity) (Dawson et al., 1999; Dawson and Ross, 1999). To quantify NK and NKT cells, PBMC and splenocytes were co-stained with antibodies against NKR-P1 and CD3, and analyzed by flow cytometry. Marginal VA status was associated with a reduction in the number of NK cells in peripheral blood and a lower percentage of NK cells compared total PBMCs (Dawson et al., 1999). The reduction in NK cells in VA-marginal rats is consistent with previous reports of low NK cells and reduced cytotoxicity in VA-deficient rats (Zhao, Murasko and Ross, 1994). Conversely, VA supplementation and aging increased the percentage and number of NK cells above the values in VA-adequate rats (Dawson et al., 1999). Overall, the percentage of NK cells was significantly reduced in VA-marginal rats and increased in VA-supplemented rats, and the effect of diet was greater in old-aged rats (Fig. 2A). In contrast to NK cells, the percentage and number of NKT cells were both increased in peripheral blood of VA-marginal rats, but NKT cells did not differ between VA-adequate and VA-supplemented rats (Dawson and Ross, 1999) (Fig. 2B). The proportion of CD3+ cells (total T cells) expressing NKR-P1 increased significantly with age, consistent with the higher proportion of these cells among total T cells in adult humans compared to infants [15-40% in adults vs. <5% in infants (Lanier, Chang and Phillips, 1994)]. Overall in this long-term study in rats, marginal VA deficiency significantly increased the absolute number and the proportion of NKT cells relative to NK cells (Dawson et al., 1999; Dawson and Ross, 1999), especially as rats aged, and the marginal VA status became more tenuous (with serum declining from 0.97, to 0.63, to 0.38 in VA-marginal young, middle-aged, and old rats, respectively) (Dawson et al., 1999). Given the cytokine-producing function of NKT cells, it is tempting to speculate that an increase in NKT cells could be a compensatory mechanism that helps to counteract a reduced overall capacity for cytokine production in VA deficiency. However, it was reported earlier that IFNγ production is elevated in splenocytes of VA-deficient mice (Carman and Hayes, 1991). An increase in the production of IFNγ by activated NKT cells might explain, in part, the dysregulated antibody responses of VA-deficient mice and rats. However, IFNγ production was not observed to differ in this study of VA-marginal, adequate and VA-supplemented aging rats, which may suggest that retinol must be nearly completely depleted before IFNγ production becomes dysregulated.
Figure 2.
Innate immune cells in rats and mice are regulated by dietary VA and acute treatment with RA. In a study of long-term VA status (A, B), rats were fed either a VA-marginal, VA-adequate, or VA-supplemented diet from weaning until the age indicated (Young, 2-3 months old; Middle-Aged, 8-10 months old; Old, 18-22 months old). A. Natural Killer (NK) cells in peripheral blood were decreased in rats fed VA-marginal diet and increased in rats fed VA-supplemented diet, while age was also a factor for NK cell number. Data from Dawson et al., 1999. B. Natural Killer T-cells (NKT cells) in peripheral blood were regulated by diet in a reciprocal manner compared to NK cells; age was also a factor for NKT cells. Data from Dawson and Ross, 1999. The % of NKT cells was inversely correlated with the ratio of CD4:CD8 T cells in peripheral blood (not shown, Dawson and Ross, 1999). C. Neonatal mice were treated in a short-term (3 days after priming with tetanus toxoid) study with RA (days -1. 0, 1 and 2 before cells were analyzed on day 3), PIC (day 0 only) or both RA+PIC. Total NK1.1+ cells, CD3+NK1.1+ (NKT), and CD3-NK1.1+ (NK cells) were analyzed by flow cytometry after double staining with fluorescently-labeled anti-NK1.1 and anti-CD3 antibodies. Data from Ma and Ross, 2005.
NK cell cytolytic activity in spleen and blood, measured as lysis of Yac-1 target cells, was proportional to the number of NK cells, and was therefore lower in VA-marginal rats, and in older rats. NK cell lytic efficiency (activity per NK cell) also fell with age but it was not affected by VA status. All groups showed a similar increase in NK cell lytic function when peripheral blood cells were incubated with IFNα (Dawson et al., 1999), a cytokine known to be released early in the response to viruses and to be a potent activator of NK cells (Asselin-Paturel and Trinchieri, 2005). This result suggests that the cell surface receptor activated by IFNα and the signaling pathways involved in NK cell proliferation and increased cytotoxicity are intact and functionally equivalent in rats of different ages and VA status. Although IL-2 production by peripheral blood mononuclear cells did not differ significantly with VA status, IL-2 production by splenocytes was lower in VA-marginal rats in each age group (Dawson et al., 1999). Overall, this study identified VA status as a factor in maintaining the NK to NKT cell ratio, and suggested that even marginal VA deficiency, especially with advancing age, is a risk factor for reduced NK cell function. Unfortunately, it was unknown at the time this study was conducted that a substantial proportion of the lymphocytes residing in the liver are NKT cells (Wick, Leithauser and Reimann, 2002), and thus this population was not analyzed. It would be of interest to characterize liver NKT cells in relationship to VA status because NKT cells are implicated in the rapid production of cytokines, especially IFNγ and IL-4, which are both important in regulating adaptive immune responses, and as NKT cells in the liver may be important in the surveillance of virally-infected or abnormal cells passing through the liver sinusoids (Kawamura et al., 1999;Wick, Leithauser and Reimann, 2002).
In short-term studies of VA-adequate rats treated with RA, coincident with immunization with tetanus toxoid (DeCicco, Youngdahl and Ross, 2001). RA alone, in an 11-day study, did not significantly increase the NK cell population in spleen or blood, but did increase the number of NK-cells induced by PIC, known to stimulate NK cell proliferation. In a more comprehensive study of the lymphocyte populations in VA-adequate adult mice (Ma, Chen and Ross, 2005), RA was not a significant factor for the number of NK1.1+ NK cells, but, as in adult VA-adequate rats, RA further increased the positive effect of PIC on the NK cell population. RA also was a positive regulator of the CD3+ NK1.1+ (NKT) population, and it increased the NKT to NK cell ratio. Furthermore, the NKT to NK cell ratio in adult mouse spleen was positively correlated with the ratio of IL-4 to IFNγ mRNAs in the same tissue, which was higher in RA-treated mice, suggesting that an elevation of NKT cells could be a factor in the increase in Th2/type 2 antibody production (ratio of IgG1 to IgG2a) in the same animals (Ma, Chen and Ross, 2005), which is likely to be driven in part by IL-4. As discussed above, the ability of RA to promote type 2 immune responses has been a consistent finding in a number of studies. In neonatal mice treated at the time of antigen priming with RA and/or PIC (Ma and Ross, 2005), the population of NK1.1+ cells in the spleen was less than half that in adult spleen (NK cells, 2.08 vs. 4.33%; NKT cells, 0.7 vs. 1.36%, respectively). Yet even with these small numbers, it was observed that RA combined with PIC rapidly increased the percentage and number of splenic NK and NKT cells (Fig. 2C). The changes in cell populations observed in normal rats (DeCicco, Youngdahl and Ross, 2001) and mice (Ma and Ross, 2005; Ma, Chen and Ross, 2005) after short-term treatment with RA are likely to be due to a rapid release of cells already near maturation, or to changes in cell-surface molecules that could result in changes in the number of cells in certain compartments. It is interesting that RA has been shown to influence the homing of mouse T-cells to the gut (Iwata et al., 2004), through expression of integrins and other factors.
VI. Discussion and perspectives
As the review above indicates, currently there is only scattered evidence for a positive effect on VA on antibody production in children, whereas, in animals, the evidence for a positive effect of VA in VA-deficient animals, and of RA, in both VA-deficient and VA-sufficient animals, is quite consistent. Several factors could possibly account for these differences and each should be considered, including: species differences; the VA status of the host at the time of immunization; the timing of the dose; and the nature of the vaccine or antigen administered.
Although species differences are possible, it seems unlikely that humans and rodents differ in a fundamental way in their response to VA, or to vaccination, because these species are similar in their transport and metabolism of VA, they have mostly similar lymphocyte populations, and many of their genes are homologous.
The VA status of the host at the time of immunization could be a factor, as most animal studies of VA deficiency have been conducted after the animals have reached a state of nearly complete depletion of retinol, with low serum retinol (typically <0.2 μmol/L) and exhaustion of liver VA reserves. In the human studies, VA deficiency has been defined at a higher level of serum retinol (as high as <0.7 μmol/L), and mostly healthy, and often breast fed, children have been studied. It is therefore possible that an effect of VA supplementation on antibody production in children was not apparent in most of the studies because the VA status of the children enrolled was not low enough for differences between the VA-supplemented and placebo groups to have been discerned. This interpretation is supported by the results of an earlier animal study in VA-adequate neonatal rats, in which VA supplementation neither increased nor decreased the anti-tetanus antibody response, although it was evident that the neonates responded to the immunization and produced a memory response (Gardner and Ross, 1995). It is interesting that the study of Bahl et al. (2002), in which 6-month old children from a community with a high prevalence of low serum VA were immunized with OPV, showed a significant effect of VA for serum titers against OPV type 1. The VA status of these children may have been more tenuous than that of children in other studies where VA supplementation was given with immunization and the antibody response was measured.
The timing of the dose may also be a significant factor. In most of the human studies of VA and immunization, and in the WHO/EPI (World Health Organization, 1994) strategy for using immunization contacts to deliver VA and eliminate VA deficiency, VA has been, or is, administered at high dosage on a periodic basis. In animal studies, VA has been incorporated at a higher than usual level into the diet (Cui, Moldoveanu and Stephensen, 2000), or if provided as an oral supplement, usually given more than once. These differences may be significant because, although a single large dose of VA can quickly restore plasma retinol to a normal level and replenish liver reserves, it does not provide VA continuously for absorption from the intestines. In comparison, a diet enriched in VA, or oral VA supplements given in smaller divided doses, would not only restore plasma retinol to a normal level and replenish liver reserves, but would also provide retinol substrate for formation of retinyl esters in the intestine, which are released bound to chylomicrons. VA is also, to some extent, oxidized to RA in the intestines. While most chylomicron-associated retinyl ester is taken up by the liver, a proportion of the newly absorbed chylomicron retinyl ester is taken up into extra-hepatic tissues, especially tissues that express lipoprotein lipase and are active in the metabolism of chylomicron triglycerides. Additionally, some of the newly absorbed VA is oxidized in the intestine and absorbed as RA into the portal system (Ross, 2006). Newly absorbed VA and intestinally formed metabolites may have a metabolic fate different from that of retinol bound to retinol-binding protein. It was shown in studies of chylomicron metabolism that chylomicron-associated VA is taken up, in an apparently transient manner, by bone marrow (Hussain et al., 1989a; Hussain et al., 1989b). However, the implications of this uptake process for hematopoiesis and immune function have not been studied. It is also interesting that, among several large-scale community trials on the effect of VA on child mortality, the study showing the largest reduction in all-cause mortality, 54% (Rahmathullah et al., 1990), delivered VA as a weekly dose at a level near the recommended dietary allowance. This reduction in mortality was more than twice the average reduction of 23% for 8 studies combined (Beaton et al., 1994), most of which delivered VA to children as periodic large-dose supplements. The delivery of VA as periodic supplements using vaccination contacts is convenient, but it may be that smaller, more frequent doses, or dietary improvement (Underwood and Smitasiri, 1999), provide benefits that are missed with larger infrequent doses.
Another difference between the human and animal studies that could be important is the form of the immunizing dose. The goal of experiments in animals is to demonstrate potential effects and mechanisms, and thus studies are often designed to optimize the researcher's ability to discriminate differences. In most of the animal studies of VA and immunity, the immunizing dose has been provided without additional adjuvants. In contrast, vaccines for humans have undergone optimization to safely produce strong antibody responses, and most contain proprietary or known adjuvants (Beverley, 2002; Del Giudice, 2003). It thus may be that the vaccines used in human studies already contain enough extra “help,” due to the adjuvants they contain, to promote a strong antibody response. In animals, the addition of bacterial LPS, TNF-α (Arora and Ross, 1994), or PIC (DeCicco, Youngdahl and Ross, 2001; DeCicco et al., 2000; Ma and Ross, 2005; Ma, Chen and Ross, 2005) significantly increased antibody production in both VA-deficient and VA-adequate animal models, and also reduced the difference in antibody response due to differences in VA status. In the study of antibody production in children immunized with OPV, it is interesting that percentage seroconversion was high for OPV types 2 and 3 (Bahl et al., 2002), even in children who did not receive VA, while VA increased the response to OPV type 1, for which the rate of seroconversion was the lowest. Similarly, the seroconversion response to measles immunization was relatively low, and in this study VA was effective in at least some subgroups of children, (Benn et al., 1997), and may have promoted protection over a longer period of time (Benn et al., 2002). If some vaccines promote a strong response regardless of VA, this may explain why an effect of VA was not consistently evident in all studies. Therefore, it may be that the experimental animal models correctly predict the positive impact of VA on antibody production, but that this impact is only evident in children if the response to the vaccine is not already strong.
Treating animals with RA provides another way to explore the impact of VA on the immune system. The model is also relevant to the human condition because RA and other retinoids, due to their ability to induce cell differentiation, are used therapeutically in the treatment of leukemias, other cancers and dermatological diseases (Altucci and Gronemeyer, 2001). In animals given RA, rather than VA itself, the physiological controls that otherwise regulate and limit the conversion of VA to RA are by-passed. The results of several animal studies have demonstrated the potential of RA, at a well-tolerated therapeutic level, to augment antibody production. Therefore, these results suggest that RA could be useful in the treatment of some forms of immunodeficiency. The tendency of VA and RA to promote Th2/type 2 responses may be beneficial in the response to certain types of pathogens and infectious diseases, but not to others. However, when RA is combined with other agents, such as PIC, that promote a Th1/type 1 response, a higher and well-balanced antibody response can be achieved. These results suggest that combination therapies in which RA is co-administered with other immune stimuli could offer a range of possibilities for modulating the magnitude and the type of antibody response elicited by various vaccines.
Acknowledgments
I thank all of the researchers whose projects over the years have made important contributions to the ideas discussed in this review. Supported by NIH grant DK-41479, and the Dorothy Foehr Huck Chair.
Abbreviations (defined on first use)
- CI
confidence interval
- DPT
diphtheria, pertussis, tetanus
- EPI
Expanded Programme on Immunization
- IFN
interferon
- IU
international unit
- LPS
lipopolysaccharide
- OPV
oral polio vaccine
- NK
natural killer
- NKT
natural kill T-(Cell)
- PBMC
peripheral blood mononuclear cell(s)
- PIC
polyriboinosinic acid: polyribocytidylic acid
- RA
retinoic acid
- TD
T-cell dependent
- TI
T-cell independent
- TNF
tumor necrosis factor
- VA
vitamin A
- WHO
World Health Organization
References
- Altucci L, Gronemeyer H. The promise of retinoids to fight against cancer. Nat Rev. 2001;1:181–193. doi: 10.1038/35106036. [DOI] [PubMed] [Google Scholar]
- Arora D, Ross AC. Antibody response against tetanus toxoid is enhanced by lipopolysaccharide or tumor necrosis factor-alpha in vitamin A-sufficient and -deficient rats. Am J Clin Nutr. 1994;59:922–928. doi: 10.1093/ajcn/59.4.922. [DOI] [PubMed] [Google Scholar]
- Asselin-Paturel C, Trinchieri G. Production of type I interferons: plasmacytoid dendritic cells and beyond. J Exp Med. 2005;202:461–465. doi: 10.1084/jem.20051395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahl R, Bhandari N, Wahed MA, Kumar GT, Bhan MK, Vitami WCIL. Vitamin A supplementation of women postpartum and of their infants at immunization alters breast milk retinol and infant vitamin A status. J Nutr. 2002;132:3243–3248. doi: 10.1093/jn/132.11.3243. [DOI] [PubMed] [Google Scholar]
- Baxevanis CN, Gritzapis AD, Papamichail M. In vivo antitumor activity of NKT cells activated by the combination of IL-12 and IL-18. J Immunol. 2003;171:2953–2959. doi: 10.4049/jimmunol.171.6.2953. [DOI] [PubMed] [Google Scholar]
- Beaton GH, Martorell R, Aronson KA, Edmonston B, McCabe G, Ross AC, Harvey B. Vitamin A supplementation and child morbidity and mortality in developing countries. Food Nutr Bull. 1994;15:282–289. [Google Scholar]
- Bendelac A, Rivera MN, Park SH, Roark JH. Mouse CD1-specific NK1 T cells: Development, specificity, and function. Annu Rev Immunol. 1997;15:535–562. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- Benn CS, Aaby P, Balé C, Olsen J, Michaelsen KF, George E, Whittle H. Randomised trial of effect of vitamin A supplementation on antibody response to measles vaccine in Guinea-Bissau, west Africa. Lancet. 1997;350:101–106. doi: 10.1016/S0140-6736(96)12019-5. [DOI] [PubMed] [Google Scholar]
- Benn CS, Balde A, George E, Kidd M, Whittle H, Lisse IM, Aaby P. Effect of vitamin A supplementation on measles-specific antibody levels in Guinea-Bissau. Lancet. 2002;359:1313–1314. doi: 10.1016/S0140-6736(02)08274-0. [DOI] [PubMed] [Google Scholar]
- Beverley PCL. Immunology of vaccination. British Medical Bulletin. 2002;62:15–28. doi: 10.1093/bmb/62.1.15. [DOI] [PubMed] [Google Scholar]
- Carman JA, Hayes CE. Abnormal regulation of IFN-g secretion in vitamin A deficiency. J Immunol. 1991;147:1247–1252. [PubMed] [Google Scholar]
- Carman JA, Smith SM, Hayes CE. Characterization of a helper T-lymphocyte defect in vitamin A deficient mice. J Immunol. 1989;142:388–393. [PubMed] [Google Scholar]
- Cherian T, Varkki S, Raghupathy P, Ratnam S, Chandra RK. Effect of Vitamin A supplementation on the immune response to measles vaccination. Vaccine. 2003;21:2418–2420. doi: 10.1016/s0264-410x(03)00060-4. [DOI] [PubMed] [Google Scholar]
- Ching P, Birmingham M, Goodman T, Sutter R, Loevinsohn B. Childhood mortality impact and costs of integrating vitamin A supplementation into immunization campaigns. Am J Pub Health. 2000;90:1526–1529. doi: 10.2105/ajph.90.10.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui DM, Moldoveanu Z, Stephensen CB. High-level dietary vitamin A enhances T-helper type 2 cytokine production and secretory immunoglobulin A response to influenza A virus infection in BALB/c mice. J Nutr. 2000;130:1132–1139. doi: 10.1093/jn/130.5.1132. [DOI] [PubMed] [Google Scholar]
- Dawson HD, Li NQ, DeCicco KL, Nibert JA, Ross AC. Chronic marginal vitamin A status reduces natural killer cell number and function in aging Lewis rats. J Nutr. 1999;129:1510–1517. doi: 10.1093/jn/129.8.1510. [DOI] [PubMed] [Google Scholar]
- Dawson HD, Ross AC. Chronic marginal vitamin A status affects the distribution and function of T cells and natural T calls in aging Lewis rats. J Nutr. 1999;129:1782–1790. doi: 10.1093/jn/129.10.1782. [DOI] [PubMed] [Google Scholar]
- DeCicco KL, Youngdahl JD, Ross AC. All-trans-retinoic acid and polyriboinosinic:polyribocytidylic acid in combination potentiate specific antibody production and cell-mediated immunity. Immunology. 2001;104:341–348. doi: 10.1046/j.1365-2567.2001.01317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCicco KL, Zolfaghari R, Li NQ, Ross AC. Retinoic acid and polyriboinosinic acid:polyribocytidylic act synergistically to enhance the antibody response to tetanus toxoid during vitamin A deficiency: possible involvement of interleukin-2 receptor beta, signal transducer and activator of transcription-1, and interferon regulatory factor-1. J Inf Dis. 2000;182:S29–S36. doi: 10.1086/315908. [DOI] [PubMed] [Google Scholar]
- Del Giudice G. Vaccination strategies. An overview Vaccine. 2003;21:S2/83–S82/88. doi: 10.1016/s0264-410x(03)00205-6. [DOI] [PubMed] [Google Scholar]
- Dowling JE, Wald G. The biological function of vitamin A acid. Proc Natl Acad Sci USA. 1960;46:587–608. doi: 10.1073/pnas.46.5.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner EM, Ross AC. Immunologic memory is established in nursling rats immunized with tetanus toxoid, but is not affected by concurrent supplementation with vitamin A. Am J Clin Nutr. 1995;62:1007–1012. doi: 10.1093/ajcn/62.5.1007. [DOI] [PubMed] [Google Scholar]
- Hengesbach LM, Hoag KA. Physiological concentrations of retinoic acid favor myeloid dendritic cell development over granulocyte development in cultures of bone marrow cells from mice. J Nutr. 2004;134:2653–2659. doi: 10.1093/jn/134.10.2653. [DOI] [PubMed] [Google Scholar]
- Hussain MM, Mahley RW, Boyles JK, Fainaru M, Brecht WJ, Lindquist PA. Chylomicron-chylomicron remnant clearance by liver and bone marrow in rabbits. J Biol Chem. 1989a;264:9571–9582. [PubMed] [Google Scholar]
- Hussain MM, Mahley RW, Boyles JK, Lindquist PA, Brecht WJ, Innerarity TL. Chylomicron metabolism. Chylomicron uptake by bone marrow in different animal species. J Biol Chem. 1989b;264:17931–17938. [PubMed] [Google Scholar]
- Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- Kawamura T, Seki S, Takeda K, Narita J, Ebe Y, Naito M, Hiraide H, Abo T. Protective effect of NK1.1+ T cells as well as NK cells against intraperitoneal tumors in mice. Cell Immunol. 1999;193:219–225. doi: 10.1006/cimm.1999.1477. [DOI] [PubMed] [Google Scholar]
- Kovarik J, Siegrist CA. Immunity in early life. Immunol Today. 1998;19:150–152. doi: 10.1016/s0167-5699(97)01230-9. [DOI] [PubMed] [Google Scholar]
- Kronenberg M. Toward an understanding of NKT cell biology: Progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- Kutukculer N, Akil T, Egemen A, Kurugöl Z, Aksit S, Özmen D, Turgan N, Bayindir O, Çaglayan S. Adequate immune response to tetanus toxoid and failure of vitamin A and E supplementation to enhance antibody response in healthy children. Vaccine. 2000;18:2979–2984. doi: 10.1016/s0264-410x(00)00097-9. [DOI] [PubMed] [Google Scholar]
- Lamb AJ, Apiwatanaporn P, Olson JA. Induction of rapid, synchronous vitamin A deficiency in the rat. J Nutr. 1974;104:1140–1148. doi: 10.1093/jn/104.9.1140. [DOI] [PubMed] [Google Scholar]
- Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- Lanier LL, Chang C, Phillips JH. Human NKR-P1A. A disulfide-linked homodimer of the C-type lectin superfamily expressed by a subset of NK and T lymphocytes. J Immunol. 1994;153:2417–2428. [PubMed] [Google Scholar]
- Ma Y, Ross AC. The anti-tetanus immune response of neonatal mice is augmented by retinoic acid combined with polyriboinosinic:polyribocytidylic acid. Proc Natl Acad Sci USA. 2005;102:13556–13561. doi: 10.1073/pnas.0506438102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YF, Chen QY, Ross AC. Retinoic acid and polyriboinosinic: polyribocytidylic acid stimulate robust anti-tetanus antibody production while differentially regulating type 1/type 2 cytokines and lymphocyte populations. J Immunol. 2005;174:7961–7969. doi: 10.4049/jimmunol.174.12.7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciani DJ. Vaccine adjuvants: role and mechanisms of action in vaccine immunogenicity. Drug Disc Today. 2003;8:934–943. doi: 10.1016/s1359-6446(03)02864-2. [DOI] [PubMed] [Google Scholar]
- Marshall-Clarke S, Reen D, Tasker L, Hassan J. Neonatal immunity: how well has it grown up? Immunol Today. 2000;21:35–41. doi: 10.1016/s0167-5699(99)01548-0. [DOI] [PubMed] [Google Scholar]
- Matzinger P. An innate sense of danger. Ann N Y Acad Sci. 2002;961:341–342. doi: 10.1111/j.1749-6632.2002.tb03118.x. [DOI] [PubMed] [Google Scholar]
- Papamichail M, Perez SA, Gritzapis AD, Baxevanis CN. Natural killer lymphocytes: biology, development, and function. Canc Immunol Immunother. 2004;53:176–186. doi: 10.1007/s00262-003-0478-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmathullah L, Tielsch JM, Thulasiraj RD, Katz J, Coles C, Devi S, John R, Prakash K, Sadanand AV, Edwin N, Kamaraj C. Impact of supplementing newborn infants with vitamin A on early infant mortality: community based randomised trial in southern India. Br Med J. 2003;327:254–257. doi: 10.1136/bmj.327.7409.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmathullah L, Underwood BA, Thulasiraj RD, Milton RC, Ramaswamy K, Rahmathullah R, Babu G. Reduced mortality among children in southern India receiving a small weekly dose of Vitamin A. N Engl J Med. 1990;323:929–935. doi: 10.1056/NEJM199010043231401. [DOI] [PubMed] [Google Scholar]
- Re F, Strominger JL. Heterogeneity of TLR-induced responses in dendritic cells: from innate to adaptive immunity. Immunobiology. 2004;209:191–198. doi: 10.1016/j.imbio.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Ross AC. The relationship between immunocompetence and vitamin A status. In: Sommer A, West KP Jr, editors. Vitamin A deficiency: health, survival, and vision. Oxford University Press, Inc.; New York: 1996a. pp. 251–273. [Google Scholar]
- Ross AC. Vitamin A deficiency and retinoid repletion regulate the antibody response to bacterial antigens and the maintenance of natural killer cells. Clin Immunol Immunopathol. 1996b;80:S36–S72. doi: 10.1006/clin.1996.0143. [DOI] [PubMed] [Google Scholar]
- Ross AC. Vitamin A, retinoids and immune responses. In: Livrea MA, editor. Vitamin A and retinoids: an update of biological aspects and clinical applications. Birkhèuser Verlag; Basel: 2000. pp. 83–95. [Google Scholar]
- Ross AC. Introduction to vitamin A: a nutritional and life cycle perspective. In: Packer L, Obermüller-Jevic U, Kraemer K, Sies H, editors. Carotenoids and Retinoids Molecular Aspects and Health Issues. AOCS Press; Champaign, IL: 2005. pp. 23–41. [Google Scholar]
- Ross AC. Vitamin A and carotenoids. In: Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, editors. Modern Nutrition in Health and Disease. William & Wilkins; Baltimore: 2006. pp. 319–431. [Google Scholar]
- Ross AC, Zolfaghari R. Regulation of hepatic retinol metabolism: Perspectives from studies on vitamin A status. J Nutr. 2004;134:269S–275S. doi: 10.1093/jn/134.1.269S. [DOI] [PubMed] [Google Scholar]
- Schijns VE, Tangeras A. Vaccine adjuvant technology: from theoretical mechanisms to practical approaches. Dev Biol (Basel) 2005;121:127–134. [PubMed] [Google Scholar]
- Semba RD. Vitamin A as “anti-infective” therapy, 1920-1940. J Nutr. 1999;129:783–791. doi: 10.1093/jn/129.4.783. [DOI] [PubMed] [Google Scholar]
- Semba RD. Vitamin A and infectious diseases. In: Livrea MA, editor. Vitamin A and retinoids: An update of biological aspects and clinical applications. Birkhèuser Verlag; Basel: 2000. pp. 97–108. [Google Scholar]
- Siegrist CA. Neonatal and early life vaccinology. Vaccine. 2001;19:3331–3346. doi: 10.1016/s0264-410x(01)00028-7. [DOI] [PubMed] [Google Scholar]
- Sommer A, West KP., Jr . Vitamin A Deficiency: Health, Survival, and Vision. Oxford University Press, Inc.; New York: 1996. [Google Scholar]
- Sommer A, West KP., Jr The duration of the effect of vitamin A supplementation. Am J Pub Health. 1997;87:467–469. doi: 10.2105/ajph.87.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephensen CB. Vitamin A, infection, and immune function. Annu Rev Nutr. 2001;21:167–192. doi: 10.1146/annurev.nutr.21.1.167. [DOI] [PubMed] [Google Scholar]
- Stephensen CB, Moldoveanu Z, Gangopadhyay NN. Vitamin A deficiency diminishes the salivary immunoglobulin A response and enhances the serum immunoglobulin G response to influenza A virus infection in BALB/c mice. J Nutr. 1996;126:94–102. doi: 10.1093/jn/126.1.94. [DOI] [PubMed] [Google Scholar]
- Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- Underwood BA. Editorial: the timing of high-dose vitamin A supplementation to children. Am J Pub Health. 1995;85:1200–1201. doi: 10.2105/ajph.85.9.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood BA, Smitasiri S. Micronutrient malnutrition: Policies and programs for control and their implications. Annu Rev Nutr. 1999;19:303–324. doi: 10.1146/annurev.nutr.19.1.303. [DOI] [PubMed] [Google Scholar]
- Villamor E, Fawzi WW. Effects of vitamin A supplementation on immune responses and correlation with clinical outcomes. Clin Microbiol Rev. 2005;18:446–464. doi: 10.1128/CMR.18.3.446-464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zolfaghari R, Ross AC. Cloning of rat cytochrome P450RAI (CYP26) cDNA and regulation of hepatic CYP26 gene expression by retinoic acid in vivo. FASEB J. 2001;15:A602. doi: 10.1016/S0003-9861(02)00043-7. [DOI] [PubMed] [Google Scholar]
- Wick MJ, Leithauser F, Reimann J. The hepatic immune system. Crit Rev Immunol. 2002;22:47–103. [PubMed] [Google Scholar]
- Wiedermann U, Hanson LÅ, Kahu H, Dahlgren UI. Aberrant T-cell function in vitro and impaired T-cell dependent antibody response in vivo in vitamin A-deficient rats. Immunology. 1993;80:581–586. [PMC free article] [PubMed] [Google Scholar]
- Wieringa FT, Dijkhuizen MA, West CE, van der Ven-Jongekrijg J, van der Meer JW. Reduced production of immunoregulatory cytokines in vitamin A- and zinc-deficient Indonesian infants. Eur J Clin Nutr. 2004;58:1498–1504. doi: 10.1038/sj.ejcn.1601998. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO EPI/GEN/94.9. WHO; Geneva: 1994. Using immunization contacts as the gateway to eliminating vitamin A deficiency – a policy document. [Google Scholar]
- Yu S, Xia M, Xu W, Chu Y, Wang Y, Xiong S. All-trans retinoic acid biases immune response induced by DNA vaccine in a Th2 direction. Vaccine. 2005;23:5160–5167. doi: 10.1016/j.vaccine.2005.06.019. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Murasko DM, Ross AC. The role of vitamin A in natural killer cell cytotoxicity, number and activation in the rat. Nat Immun. 1994;13:29–41. [PubMed] [Google Scholar]
- Zolfaghari R, Ross AC. Lecithin:retinol acyltransferase expression is regulated by dietary vitamin A and exogenous retinoic acid in the lung of adult rats. J Nutr. 2002;132:1160–1164. doi: 10.1093/jn/132.6.1160. [DOI] [PubMed] [Google Scholar]
- Zolfaghari R, Wang Y, Sancher A, Chen Q, Ross AC. Cloning and molecular expression analysis of large and small lecithin:retinol acyltransferase mRNAs in the liver and other tissues of adult rats. Biochem J. 2002;368:621–631. doi: 10.1042/BJ20020918. [DOI] [PMC free article] [PubMed] [Google Scholar]