Abstract
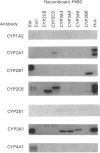
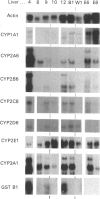
Cytochrome P450s play a central role in the metabolism and disposition of an extremely wide range of drugs and chemical carcinogens. Individual differences in the expression of these enzymes may be an important determinant in susceptibility to adverse drug reactions, chemical toxins and mutagens. In this paper, we have measured the relative levels of expression of cytochrome P450 isoenzymes from eight gene families or subfamilies in a panel of twelve human liver samples in order to determine the individuality in their expression and whether any forms are co-regulated. Isoenzymes were identified in most cases on Western blots based on the mobility of authentic recombinant human cytochrome P450 standards. The levels of the following P450 proteins correlated with each other: CYP2A6, CYP2B6 and a protein from the CYP2C gene subfamily, CYP2E1 and a member of the CYP2A gene subfamily, CYP2C8, CYP3A3/A4 and total cytochrome P450 content. Also, the levels of two proteins in the CYP4A gene subfamily were highly correlated. These correlations are consistent with the relative regulation of members of these gene families in rats or mice. In addition, the level of expression of specific isoenzymes has also been compared with the rate of metabolism of a panel of drugs, carcinogens and model P450 substrates. These latter studies demonstrate and confirm that the correlations obtained in this manner represent a powerful approach towards the assignment of the metabolism of substrates by specific human P450 isoenzymes.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Seilman S., Amelizad Z., Oesch F., Wolf C. R. Identification of human cytochromes P-450 analogous to forms induced by phenobarbital and 3-methylcholanthrene in the rat. Biochem J. 1985 Dec 15;232(3):869–876. doi: 10.1042/bj2320869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adesnik M., Atchison M. Genes for cytochrome P-450 and their regulation. CRC Crit Rev Biochem. 1986;19(3):247–305. doi: 10.3109/10409238609084657. [DOI] [PubMed] [Google Scholar]
- Ames B. N., Durston W. E., Yamasaki E., Lee F. D. Carcinogens are mutagens: a simple test system combining liver homogenates for activation and bacteria for detection. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2281–2285. doi: 10.1073/pnas.70.8.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames B. N., Mccann J., Yamasaki E. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test. Mutat Res. 1975 Dec;31(6):347–364. doi: 10.1016/0165-1161(75)90046-1. [DOI] [PubMed] [Google Scholar]
- Aoyama T., Korzekwa K., Nagata K., Gillette J., Gelboin H. V., Gonzalez F. J. Estradiol metabolism by complementary deoxyribonucleic acid-expressed human cytochrome P450s. Endocrinology. 1990 Jun;126(6):3101–3106. doi: 10.1210/endo-126-6-3101. [DOI] [PubMed] [Google Scholar]
- Back D. J., Tjia J. F., Karbwang J., Colbert J. In vitro inhibition studies of tolbutamide hydroxylase activity of human liver microsomes by azoles, sulphonamides and quinolines. Br J Clin Pharmacol. 1988 Jul;26(1):23–29. doi: 10.1111/j.1365-2125.1988.tb03359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball S. E., Forrester L. M., Wolf C. R., Back D. J. Differences in the cytochrome P-450 isoenzymes involved in the 2-hydroxylation of oestradiol and 17 alpha-ethinyloestradiol. Relative activities of rat and human liver enzymes. Biochem J. 1990 Apr 1;267(1):221–226. doi: 10.1042/bj2670221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaune P. H., Kremers P. G., Kaminsky L. S., De Graeve J., Albert A., Guengerich F. P. Comparison of monooxygenase activities and cytochrome P-450 isozyme concentrations in human liver microsomes. Drug Metab Dispos. 1986 Jul-Aug;14(4):437–442. [PubMed] [Google Scholar]
- Burke M. D., Thompson S., Elcombe C. R., Halpert J., Haaparanta T., Mayer R. T. Ethoxy-, pentoxy- and benzyloxyphenoxazones and homologues: a series of substrates to distinguish between different induced cytochromes P-450. Biochem Pharmacol. 1985 Sep 15;34(18):3337–3345. doi: 10.1016/0006-2952(85)90355-7. [DOI] [PubMed] [Google Scholar]
- Carmichael J., Forrester L. M., Lewis A. D., Hayes J. D., Hayes P. C., Wolf C. R. Glutathione S-transferase isoenzymes and glutathione peroxidase activity in normal and tumour samples from human lung. Carcinogenesis. 1988 Sep;9(9):1617–1621. doi: 10.1093/carcin/9.9.1617. [DOI] [PubMed] [Google Scholar]
- Distlerath L. M., Reilly P. E., Martin M. V., Davis G. G., Wilkinson G. R., Guengerich F. P. Purification and characterization of the human liver cytochromes P-450 involved in debrisoquine 4-hydroxylation and phenacetin O-deethylation, two prototypes for genetic polymorphism in oxidative drug metabolism. J Biol Chem. 1985 Jul 25;260(15):9057–9067. [PubMed] [Google Scholar]
- Eichelbaum M., Gross A. S. The genetic polymorphism of debrisoquine/sparteine metabolism--clinical aspects. Pharmacol Ther. 1990;46(3):377–394. doi: 10.1016/0163-7258(90)90025-w. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Forrester L. M., Neal G. E., Judah D. J., Glancey M. J., Wolf C. R. Evidence for involvement of multiple forms of cytochrome P-450 in aflatoxin B1 metabolism in human liver. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8306–8310. doi: 10.1073/pnas.87.21.8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez F. J. Molecular genetics of the P-450 superfamily. Pharmacol Ther. 1990;45(1):1–38. doi: 10.1016/0163-7258(90)90006-n. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J., Skoda R. C., Kimura S., Umeno M., Zanger U. M., Nebert D. W., Gelboin H. V., Hardwick J. P., Meyer U. A. Characterization of the common genetic defect in humans deficient in debrisoquine metabolism. Nature. 1988 Feb 4;331(6155):442–446. doi: 10.1038/331442a0. [DOI] [PubMed] [Google Scholar]
- Gough A. C., Miles J. S., Spurr N. K., Moss J. E., Gaedigk A., Eichelbaum M., Wolf C. R. Identification of the primary gene defect at the cytochrome P450 CYP2D locus. Nature. 1990 Oct 25;347(6295):773–776. doi: 10.1038/347773a0. [DOI] [PubMed] [Google Scholar]
- Greenlee W. F., Poland A. An improved assay of 7-ethoxycoumarin O-deethylase activity: induction of hepatic enzyme activity in C57BL/6J and DBA/2J mice by phenobarbital, 3-methylcholanthrene and 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Pharmacol Exp Ther. 1978 Jun;205(3):596–605. [PubMed] [Google Scholar]
- Guengerich F. P. Oxidation of 17 alpha-ethynylestradiol by human liver cytochrome P-450. Mol Pharmacol. 1988 May;33(5):500–508. [PubMed] [Google Scholar]
- Guengerich F. P. Roles of cytochrome P-450 enzymes in chemical carcinogenesis and cancer chemotherapy. Cancer Res. 1988 Jun 1;48(11):2946–2954. [PubMed] [Google Scholar]
- Gulati P., Skett P. Interactions of insulin, thyroxine and dexamethasone with growth hormone in the control of steroid metabolism by isolated rat hepatocytes. Biochem Pharmacol. 1989 Nov 15;38(22):4061–4067. doi: 10.1016/0006-2952(89)90687-4. [DOI] [PubMed] [Google Scholar]
- Gustafsson J. A., Stenberg A. Irreversible androgenic programming at birth of microsomal and soluble rat liver enzymes active on androstene-3,17-dione and 5alpha-androstane-3alpha,17beta-diol. J Biol Chem. 1974 Feb 10;249(3):711–718. [PubMed] [Google Scholar]
- Hall M., Forrester L. M., Parker D. K., Grover P. L., Wolf C. R. Relative contribution of various forms of cytochrome P450 to the metabolism of benzo[a]pyrene by human liver microsomes. Carcinogenesis. 1989 Oct;10(10):1815–1821. doi: 10.1093/carcin/10.10.1815. [DOI] [PubMed] [Google Scholar]
- Hayes J. D., Kerr L. A., Cronshaw A. D. Evidence that glutathione S-transferases B1B1 and B2B2 are the products of separate genes and that their expression in human liver is subject to inter-individual variation. Molecular relationships between the B1 and B2 subunits and other Alpha class glutathione S-transferases. Biochem J. 1989 Dec 1;264(2):437–445. doi: 10.1042/bj2640437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson C. J., Scott A. R., Yang C. S., Wolf C. R. Testosterone-mediated regulation of mouse renal cytochrome P-450 isoenzymes. Biochem J. 1990 Mar 15;266(3):675–681. doi: 10.1042/bj2660675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howie A. F., Forrester L. M., Glancey M. J., Schlager J. J., Powis G., Beckett G. J., Hayes J. D., Wolf C. R. Glutathione S-transferase and glutathione peroxidase expression in normal and tumour human tissues. Carcinogenesis. 1990 Mar;11(3):451–458. doi: 10.1093/carcin/11.3.451. [DOI] [PubMed] [Google Scholar]
- Hussey A. J., Hayes J. D., Beckett G. J. The polymorphic expression of neutral glutathione S-transferase in human mononuclear leucocytes as measured by specific radioimmunoassay. Biochem Pharmacol. 1987 Nov 15;36(22):4013–4015. doi: 10.1016/0006-2952(87)90472-2. [DOI] [PubMed] [Google Scholar]
- Hussey A. J., Stockman P. K., Beckett G. J., Hayes J. D. Variations in the glutathione S-transferase subunits expressed in human livers. Biochim Biophys Acta. 1986 Nov 7;874(1):1–12. doi: 10.1016/0167-4838(86)90094-4. [DOI] [PubMed] [Google Scholar]
- Juvonen R. O., Shkumatov V. M., Lang M. A. Purification and characterization of a liver microsomal cytochrome P-450 isoenzyme with a high affinity and metabolic capacity for coumarin from pyrazole-treated D2 mice. Eur J Biochem. 1988 Jan 15;171(1-2):205–211. doi: 10.1111/j.1432-1033.1988.tb13777.x. [DOI] [PubMed] [Google Scholar]
- Kawano S., Kamataki T., Yasumori T., Yamazoe Y., Kato R. Purification of human liver cytochrome P-450 catalyzing testosterone 6 beta-hydroxylation. J Biochem. 1987 Sep;102(3):493–501. doi: 10.1093/oxfordjournals.jbchem.a122081. [DOI] [PubMed] [Google Scholar]
- Kimura S., Hardwick J. P., Kozak C. A., Gonzalez F. J. The rat clofibrate-inducible CYP4A subfamily. II. cDNA sequence of IVA3, mapping of the Cyp4a locus to mouse chromosome 4, and coordinate and tissue-specific regulation of the CYP4A genes. DNA. 1989 Sep;8(7):517–525. doi: 10.1089/dna.1.1989.8.517. [DOI] [PubMed] [Google Scholar]
- Kitteringham N. R., Lambert C., Maggs J. L., Colbert J., Park B. K. A comparative study of the formation of chemically reactive drug metabolites by human liver microsomes. Br J Clin Pharmacol. 1988 Jul;26(1):13–21. doi: 10.1111/j.1365-2125.1988.tb03358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronbach T., Fischer V., Meyer U. A. Cyclosporine metabolism in human liver: identification of a cytochrome P-450III gene family as the major cyclosporine-metabolizing enzyme explains interactions of cyclosporine with other drugs. Clin Pharmacol Ther. 1988 Jun;43(6):630–635. doi: 10.1038/clpt.1988.87. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lang M. A., Juvonen R., Järvinen P., Honkakoski P., Raunio H. Mouse liver P450Coh: genetic regulation of the pyrazole-inducible enzyme and comparison with other P450 isoenzymes. Arch Biochem Biophys. 1989 May 15;271(1):139–148. doi: 10.1016/0003-9861(89)90264-6. [DOI] [PubMed] [Google Scholar]
- Lewis A. D., Hickson I. D., Robson C. N., Harris A. L., Hayes J. D., Griffiths S. A., Manson M. M., Hall A. E., Moss J. E., Wolf C. R. Amplification and increased expression of alpha class glutathione S-transferase-encoding genes associated with resistance to nitrogen mustards. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8511–8515. doi: 10.1073/pnas.85.22.8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A. Y., West S. B. Multiplicity of mammalian microsomal cytochromes P-45. Pharmacol Rev. 1979 Dec;31(4):277–295. [PubMed] [Google Scholar]
- McManus M. E., Burgess W. M., Veronese M. E., Huggett A., Quattrochi L. C., Tukey R. H. Metabolism of 2-acetylaminofluorene and benzo(a)pyrene and activation of food-derived heterocyclic amine mutagens by human cytochromes P-450. Cancer Res. 1990 Jun 1;50(11):3367–3376. [PubMed] [Google Scholar]
- Meehan R. R., Barlow D. P., Hill R. E., Hogan B. L., Hastie N. D. Pattern of serum protein gene expression in mouse visceral yolk sac and foetal liver. EMBO J. 1984 Aug;3(8):1881–1885. doi: 10.1002/j.1460-2075.1984.tb02062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan R. R., Forrester L. M., Stevenson K., Hastie N. D., Buchmann A., Kunz H. W., Wolf C. R. Regulation of phenobarbital-inducible cytochrome P-450s in rat and mouse liver following dexamethasone administration and hypophysectomy. Biochem J. 1988 Sep 15;254(3):789–797. doi: 10.1042/bj2540789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan R. R., Gosden J. R., Rout D., Hastie N. D., Friedberg T., Adesnik M., Buckland R., van Heyningen V., Fletcher J., Spurr N. K. Human cytochrome P-450 PB-1: a multigene family involved in mephenytoin and steroid oxidations that maps to chromosome 10. Am J Hum Genet. 1988 Jan;42(1):26–37. [PMC free article] [PubMed] [Google Scholar]
- Meier U. T., Dayer P., Malè P. J., Kronbach T., Meyer U. A. Mephenytoin hydroxylation polymorphism: characterization of the enzymatic deficiency in liver microsomes of poor metabolizers phenotyped in vivo. Clin Pharmacol Ther. 1985 Nov;38(5):488–494. doi: 10.1038/clpt.1985.213. [DOI] [PubMed] [Google Scholar]
- Meier U. T., Meyer U. A. Genetic polymorphism of human cytochrome P-450 (S)-mephenytoin 4-hydroxylase. Studies with human autoantibodies suggest a functionally altered cytochrome P-450 isozyme as cause of the genetic deficiency. Biochemistry. 1987 Dec 15;26(25):8466–8474. doi: 10.1021/bi00399a065. [DOI] [PubMed] [Google Scholar]
- Miles J. S., McLaren A. W., Forrester L. M., Glancey M. J., Lang M. A., Wolf C. R. Identification of the human liver cytochrome P-450 responsible for coumarin 7-hydroxylase activity. Biochem J. 1990 Apr 15;267(2):365–371. doi: 10.1042/bj2670365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscow J. A., Townsend A. J., Goldsmith M. E., Whang-Peng J., Vickers P. J., Poisson R., Legault-Poisson S., Myers C. E., Cowan K. H. Isolation of the human anionic glutathione S-transferase cDNA and the relation of its gene expression to estrogen-receptor content in primary breast cancer. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6518–6522. doi: 10.1073/pnas.85.17.6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert D. W., Negishi M. Multiple forms of cytochrome P-450 and the importance of molecular biology and evolution. Biochem Pharmacol. 1982 Jul 15;31(14):2311–2317. doi: 10.1016/0006-2952(82)90523-8. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Nelson D. R., Coon M. J., Estabrook R. W., Feyereisen R., Fujii-Kuriyama Y., Gonzalez F. J., Guengerich F. P., Gunsalus I. C., Johnson E. F. The P450 superfamily: update on new sequences, gene mapping, and recommended nomenclature. DNA Cell Biol. 1991 Jan-Feb;10(1):1–14. doi: 10.1089/dna.1991.10.1. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. II. SOLUBILIZATION, PURIFICATION, AND PROPERTIES. J Biol Chem. 1964 Jul;239:2379–2385. [PubMed] [Google Scholar]
- Okey A. B. Enzyme induction in the cytochrome P-450 system. Pharmacol Ther. 1990;45(2):241–298. doi: 10.1016/0163-7258(90)90030-6. [DOI] [PubMed] [Google Scholar]
- Raunio H., Syngelmä T., Pasanen M., Juvonen R., Honkakoski P., Kairaluoma M. A., Sotaniemi E., Lang M. A., Pelkonen O. Immunochemical and catalytical studies on hepatic coumarin 7-hydroxylase in man, rat, and mouse. Biochem Pharmacol. 1988 Oct 15;37(20):3889–3895. doi: 10.1016/0006-2952(88)90070-6. [DOI] [PubMed] [Google Scholar]
- Reilly P. E., Thompson D. A., Mason S. R., Hooper W. D. Cytochrome P450IIIA enzymes in rat liver microsomes: involvement in C3-hydroxylation of diazepam and nordazepam but not N-dealkylation of diazepam and temazepam. Mol Pharmacol. 1990 May;37(5):767–774. [PubMed] [Google Scholar]
- Relling M. V., Aoyama T., Gonzalez F. J., Meyer U. A. Tolbutamide and mephenytoin hydroxylation by human cytochrome P450s in the CYP2C subfamily. J Pharmacol Exp Ther. 1990 Jan;252(1):442–447. [PubMed] [Google Scholar]
- Seidegård J., Pero R. W., Miller D. G., Beattie E. J. A glutathione transferase in human leukocytes as a marker for the susceptibility to lung cancer. Carcinogenesis. 1986 May;7(5):751–753. doi: 10.1093/carcin/7.5.751. [DOI] [PubMed] [Google Scholar]
- Shaw P. M., Barnes T. S., Cameron D., Engeset J., Melvin W. T., Omar G., Petrie J. C., Rush W. R., Snyder C. P., Whiting P. H. Purification and characterization of an anticonvulsant-induced human cytochrome P-450 catalysing cyclosporin metabolism. Biochem J. 1989 Nov 1;263(3):653–663. doi: 10.1042/bj2630653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T., Misono K. S., Guengerich F. P. Human liver microsomal cytochrome P-450 mephenytoin 4-hydroxylase, a prototype of genetic polymorphism in oxidative drug metabolism. Purification and characterization of two similar forms involved in the reaction. J Biol Chem. 1986 Jan 15;261(2):909–921. [PubMed] [Google Scholar]
- Simmons D. L., Kasper C. B. Genetic polymorphisms for a phenobarbital-inducible cytochrome P-450 map to the Coh locus in mice. J Biol Chem. 1983 Aug 25;258(16):9585–9588. [PubMed] [Google Scholar]
- Song B. J., Gelboin H. V., Park S. S., Tsokos G. C., Friedman F. K. Monoclonal antibody-directed radioimmunoassay detects cytochrome P-450 in human placenta and lymphocytes. Science. 1985 Apr 26;228(4698):490–492. doi: 10.1126/science.2580351. [DOI] [PubMed] [Google Scholar]
- Tjia J. F., Back D. J., Breckenridge A. M. Calcium channel antagonists and cyclosporine metabolism: in vitro studies with human liver microsomes. Br J Clin Pharmacol. 1989 Sep;28(3):362–365. doi: 10.1111/j.1365-2125.1989.tb05439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P. P., Beaune P., Kaminsky L. S., Dannan G. A., Kadlubar F. F., Larrey D., Guengerich F. P. Purification and characterization of six cytochrome P-450 isozymes from human liver microsomes. Biochemistry. 1983 Nov 8;22(23):5375–5383. doi: 10.1021/bi00292a019. [DOI] [PubMed] [Google Scholar]
- Waxman D. J., Attisano C., Guengerich F. P., Lapenson D. P. Human liver microsomal steroid metabolism: identification of the major microsomal steroid hormone 6 beta-hydroxylase cytochrome P-450 enzyme. Arch Biochem Biophys. 1988 Jun;263(2):424–436. doi: 10.1016/0003-9861(88)90655-8. [DOI] [PubMed] [Google Scholar]
- Wolf C. R., Miles J. S., Gough A., Spurr N. K. Molecular genetics of the human cytochrome P-450 system. Biochem Soc Trans. 1990 Feb;18(1):21–24. doi: 10.1042/bst0180021. [DOI] [PubMed] [Google Scholar]
- Wolf C. R., Miles J. S., Seilman S., Burke M. D., Rospendowski B. N., Kelly K., Smith W. E. Evidence that the catalytic differences of two structurally homologous forms of cytochrome P-450 relate to their heme environment. Biochemistry. 1988 Mar 8;27(5):1597–1603. doi: 10.1021/bi00405a031. [DOI] [PubMed] [Google Scholar]
- Wolf C. R., Oesch F. Isolation of a high spin form of cytochrome P-450 induced in rat liver by 3-methylcholanthrene. Biochem Biophys Res Commun. 1983 Mar 16;111(2):504–511. doi: 10.1016/0006-291x(83)90335-2. [DOI] [PubMed] [Google Scholar]
- Wrighton S. A., Ring B. J., Watkins P. B., VandenBranden M. Identification of a polymorphically expressed member of the human cytochrome P-450III family. Mol Pharmacol. 1989 Jul;36(1):97–105. [PubMed] [Google Scholar]
- Zanger U. M., Hauri H. P., Loeper J., Homberg J. C., Meyer U. A. Antibodies against human cytochrome P-450db1 in autoimmune hepatitis type II. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8256–8260. doi: 10.1073/pnas.85.21.8256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaphiropoulos P. G., Mode A., Norstedt G., Gustafsson J. A. Regulation of sexual differentiation in drug and steroid metabolism. Trends Pharmacol Sci. 1989 Apr;10(4):149–153. doi: 10.1016/0165-6147(89)90167-3. [DOI] [PubMed] [Google Scholar]
- de Waziers I., Cugnenc P. H., Yang C. S., Leroux J. P., Beaune P. H. Cytochrome P 450 isoenzymes, epoxide hydrolase and glutathione transferases in rat and human hepatic and extrahepatic tissues. J Pharmacol Exp Ther. 1990 Apr;253(1):387–394. [PubMed] [Google Scholar]