Abstract
Sphingobium japonicum UT26 utilizes γ-hexachlorocyclohexane (γ-HCH) as its sole source of carbon and energy. In our previous studies, we cloned and characterized genes encoding enzymes for the conversion of γ-HCH to β-ketoadipate in UT26. In this study, we analyzed a mutant obtained by transposon mutagenesis and identified and characterized new genes encoding a putative ABC-type transporter essential for the utilization of γ-HCH in strain UT26. This putative ABC transporter consists of four components, permease, ATPase, periplasmic protein, and lipoprotein, encoded by linK, linL, linM, and linN, respectively. Mutation and complementation analyses indicated that all the linKLMN genes are required, probably as a set, for γ-HCH utilization in UT26. Furthermore, the mutant cells deficient in this putative ABC transporter showed (i) higher γ-HCH degradation activity and greater accumulation of the toxic dead-end product 2,5-dichlorophenol (2,5-DCP), (ii) higher sensitivity to 2,5-DCP itself, and (iii) higher permeability of hydrophobic compounds than the wild-type cells. These results strongly suggested that LinKLMN are involved in γ-HCH utilization by controlling membrane hydrophobicity. This study clearly demonstrated that a cellular factor besides catabolic enzymes and transcriptional regulators is essential for utilization of xenobiotic compounds in bacterial cells.
γ-Hexachlorocyclohexane (γ-HCH; also called γ-BHC and lindane) is a halogenated organic insecticide that has been used worldwide. Sphingobium japonicum (formerly Sphingomonas paucimobilis) UT26 utilizes γ-HCH as the sole source of carbon and energy under aerobic conditions (21, 27, 36). S. japonicum UT26 converts γ-HCH to β-ketoadipate by means of six enzymes, LinA to LinF, through the pathway shown in Fig. 1 (12, 20, 32, 33, 37, 38). LinA and LinB, which are involved in the early steps of the degradation of γ-HCH (Fig. 1), are localized in the periplasm of this gram-negative bacterium (35). Recently we showed that 2,5-dichlorophenol (2,5-DCP), which is a by-product in the γ-HCH degradation pathway, is toxic to UT26 cells (13). These observations strongly suggested that other factors besides catabolic enzymes (e.g., localization of enzymes and detoxification of toxic metabolite) are important for the γ-HCH degradation activity of the cells. It has also been reported that other xenobiotics and their metabolites have toxic effects on bacterial cells, resulting in the reduction of cellular degrading activities (4, 31, 41).
FIG. 1.
Proposed degradation pathways of γ-HCH in S. japonicum UT26. Compounds: 1, γ-hexachlorocyclohexane (γ-HCH); 2, pentachlorocyclohexene (γ-PCCH); 3, 1,3,4,6-tetrachloro-1,4-cyclohexadiene (1,4-TCDN); 4, 1,2,4-trichlorobenzene (1,2,4-TCB); 5, 2,4,5-trichloro-2,5-cyclohexadiene-1-ol (2,4,5-DNOL); 6, 2,5-dichlorophenol (2,5-DCP); 7, 2,5-dichloro-2,5-cyclohexadiene-1,4-diol (2,5-DDOL); 8, 2,5-dichlorohydroquinone (2,5-DCHQ); 9, chlorohydroquinone (CHQ); 10, hydroquinone (HQ); 11, acylchloride; 12, γ-hydroxymuconic semialdehyde; 13, maleylacetate; 14, β-ketoadipate. GSH, glutathione (reduced form); GS-SG, glutathione (oxidized form). Square brackets show unstable materials that have yet to be detected.
Sphingomonas-related strains (sphingomonads) have broad catabolic abilities for natural and xenobiotic compounds, such as chlorinated phenols (5), polychlorinated biphenyls (42), polycyclic aromatic hydrocarbons (43), fluorene analogues (40), and azo dyes (51), and these bacterial strains are expected to have high potential for bioremediation of contaminated sites. Sphingomonads have glycosphingolipids in their outer membrane instead of lipopolysaccharides, which is characteristic of conventional gram-negative bacteria. It has been proposed that the surface hydrophobicity of sphingomonads is advantageous for the uptake and degradation of hydrophobic compounds (24). It is generally known that gram-negative bacterial outer membrane and periplasmic space play a critical role in a protective barrier that controls the influx and efflux of various solutes, organic solvents, antibiotics, and pollutants (11, 44, 47). However, studies of the cell surface and biological membrane function of sphingomonads have focused only on structural analysis of glycosphingolipids with the exception of very limited cases, e.g., a superchannel system for uptake and degradation of alginate, which consists of a ATP-binding cassette (ABC) transporter system, in Sphingomonas sp. strain A1 (15).
In this study, we identified new genes encoding a putative ABC transporter system that is required for γ-HCH utilization in S. japonicum UT26. This ABC transporter consists of four putative components, including a unique periplasmic protein and lipoprotein. The mechanism of how the ABC transporter is involved in the utilization of xenobiotics is also discussed.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. S. japonicum cells were grown at 30°C in 1/3LB (3.3 g of Bacto tryptone, 1.7 g of yeast extract, and 5 g of sodium chloride per liter) or W minimal medium (21) containing an appropriate carbon source. To test the ability to utilize γ-HCH, S. japonicum UT26 was grown on a W-medium agar plate containing 2 mM γ-HCH. Antibiotics were used at the final concentrations of 50 μg/ml for kanamycin (Km) and 20 μg/ml for tetracycline (Tc).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s)a | Source or reference |
|---|---|---|
| Strains | ||
| S. japonicum | ||
| UT26 | HCH+ Nalr | 21 |
| UT953 | UT26 TnMod-OKm-inserted mutant, Kmr HCH− | This study |
| RE1 | UT26 ΔlinKLMN Kmr HCH− | This study |
| RE2 | UT26 ΔlinK Kmr HCH− | This study |
| RE3 | UT26 ΔlinL Kmr HCH− | This study |
| RE4 | UT26 ΔlinN Kmr HCH− | This study |
| YO5 | UT26 ΔlinA | 36 |
| YO5ΔlinL | YO5 ΔlinL Kmr HCH− | This study |
| E. coli DH5α | F− φ80dlacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17 phoA supE44 thi-1 gyrA96 relA1 | 14 |
| Plasmids | ||
| pHSG397 | pMB9 replicon, Cmr | 53 |
| pUC4K | pMB9 replicon, Kmr | 54 |
| pTnMod-OKm | pMB1 replicon, mini-Tn5, Kmr | 10 |
| pKS13 | RK2 replicon; cos Mob+ Tcr | 26 |
| pKS13P | Pu promoterb | This study |
| pEX18Tc | TcrsacB oriT; suicide vector for gene replacement carrying the pUC18-derived multiple cloning sites | 18 |
| pKSR1000 | pKS13P carrying linKLMN | This study |
| pKSR1001 | pKS13P carrying linK | This study |
| pKSR1002 | pKS13P carrying linL | This study |
| pKSR1004 | pKS13P carrying linN | This study |
| pKSR1005 | pKS13P carrying linKL | This study |
| pKSR1007 | pKS13P carrying linKLM | This study |
| pKSR1008 | pKS13P carrying linLMN | This study |
| pKSR1009 | pKS13P carrying linKLN | This study |
HCH+, growth on W minimal agar containing γ-HCH; HCH−, no growth on W minimal agar containing γ-HCH.
Pu promoter, necessary for constitutive expression of the linA gene in S. paucimobilis UT26 (36).
DNA manipulations and DNA sequence analysis.
Established methods were employed for the preparation of plasmids and genomic DNAs, digestion with restriction endonucleases, ligation, agarose gel electrophoresis, and the transformation of Escherichia coli cells (30). PCR was performed with KOD-Plus DNA polymerase (TOYOBO, Osaka, Japan) or ExTaq polymerase (TAKARA, Kyoto, Japan). The nucleotide sequences were determined using an ABI PRISM 310 sequencer and ABI Prism Big Dye terminator kit (Applied Biosystems, Foster City, CA). Southern blot analysis was carried out using the conventional protocols (30) and a digoxigenin system (Roche Diagnostics, Mannheim, Germany). The nucleotide sequences were analyzed using the Genetyx program, version 12 (SDC Inc., Tokyo, Japan). Homology searches were performed using BLAST programs available at the National Center for Biotechnology Information website (http://www.ncbi.nlm.nih.gov/BLAST/). Signal peptides and lipoprotein signals were analyzed using the SignalP server (http://www.cbs.dtu.dk/services/SignalP/).
Electroporation.
The S. japonicum UT26 cells were transformed by electroporation. Cells grown on 1/3LB agar for 2 days were collected, washed three times with ice-cold 1 mM 3-morpholinepropanesulfonic acid and 10% glycerol, and mixed with DNA solution. The suspension was transferred to an electroporation cuvette with a 1-mm gap. Electroporation was conducted under the conditions of 1.8 kV, 200 Ω, and 25 μF. After the pulse, 1/3LB medium was immediately added and incubated for 2 to 10 h and spread onto a 1/3LB agar medium containing appropriate antibiotics.
Transposon mutagenesis.
Random mutagenesis of the S. japonicum UT26 genes was accomplished using pTnMod-OKm, which carries pMB1-based replication machinery in a Tn5-derived minitransposon (10). The transformation of UT26 cells by pTnMod-OKm was performed by electroporation. The subsequent screening of mutants deficient in γ-HCH utilization was performed as described previously (12).
Characterization of transposon insertion sites.
Genomic DNA prepared from a TnMod-OKm-inserted mutant, strain UT953, was digested with SphI or both BamHI and BglII. The digested DNA was self-ligated, and the self-replicating Km-resistant (Kmr) plasmids were recovered by transformation of E. coli DH5α. The plasmids thus recovered were employed to determine the sequences of the flanking regions of the TnMod-OKm insertion site using the primers that are able to anneal the regions close to the two ends of TnMod-OKm (primer up [5′-GCTGGCCTTTTGCTCAC-3′] and primer down [5′-TTGAGACACAACGTGGC-3′]).
Construction of linKLMN deletion mutants of S. japonicum UT26.
Allelic exchange mutagenesis of the S. japonicum UT26 genome was carried out by pEX18Tc, which has a sacB gene as a counterselective suicide marker (18). Approximately 1-kb regions upstream and downstream of a target gene were amplified by the PCR using the primers shown in Table S1 in the supplemental material and the UT26 genomic DNA as a template. Primer sets were designed so as to contain the EcoRI and BamHI restriction sites at the 5′ and 3′ ends, respectively, for the upstream fragments and the XbaI and HindIII restriction sites at the 5′ and 3′ ends, respectively, for the downstream fragment, respectively. The upstream and downstream fragments were sequentially cloned into the EcoRI-BamHI and XbaI-HindIII sites, respectively, of pEX18Tc, and a Kmr gene cassette amplified by the PCR from pUC4K was inserted into the BamHI-XbaI site of pEX18Tc derivatives. The resulting plasmid was introduced into S. japonicum UT26 by electroporation, and the transformants able to grow on 1/3LB plates containing 10% sucrose and Kmr transformants were selected. The expected double-crossover-mediated homologous recombination in the transformants was confirmed by the PCR.
Complementation of the linKLMN mutants.
Expression of all or part of the linKLMN cluster in the S. japonicum UT26-derived mutant strains was performed by using pKS13P as a vector. This vector was constructed by the insertion of a BglII-BamHI-flanked promoter Pu (a constitutive promoter for the expression of linA gene in strain UT26) (36) into a BamHI site of the broad-host-range vector pKS13 (26). All or part of the linKLMN cluster was amplified by the PCR using the primer sets shown in Table S1 in the supplemental material, cloned into pHSG397, and then recloned into the downstream region of Pu in pKS13P. The resulting plasmids were introduced into each mutant strain by electroporation, and the Tc-resistant (Tcr) transformants were selected on 1/3LB plate containing Tc.
γ-HCH degradation assay.
To prepare the resting cells, S. japonicum UT26 and its mutant cells were cultured in 1/3LB, collected at log phase, washed twice, and suspended in W medium. To prepare the crude extract, the cells were washed, suspended in 50 mM potassium phosphate buffer (pH 7.5) containing 10% glycerol, and disrupted by sonication using a Sonopuls GM70 (Bandelin, Berlin, Germany). Two milligrams (wet weight) of resting cells or crude extracts prepared from 2 mg cells were suspended in 5 ml of W medium with 30 μM γ-HCH and incubated at 30°C. After the reaction, the sample was mixed with an equivalent volume of ethyl acetate containing 10 μM 1,2,4-trichlorobenzene as the internal standard, and then the ethyl acetate layer was recovered and analyzed by Shimadzu GC-17A gas chromatography (GC) with an electron capture detector (ECD) and an Rtx-1 capillary column (30 m by 0.25 mm by 0.25 μm; Restek Corp.). The column temperature was increased from 100°C to 260°C at a rate of 20°C/min, and the gas flow rate was 30 ml/min.
Protein localization analysis.
Subcellular fractionation of S. japonicum and Western blot analysis for LinA and LinB were performed as described previously (35).
Sensitivity assay for γ-HCH, 2,5-DCP, and organic solvents.
S. japonicum UT26 and its mutant cells were cultured in 1/3LB agar, collected at log phase, washed in fresh 1/3LB, and diluted to approximately 5 × 101 to 5 × 105 CFU per ml in 1/3LB. A drop of cell suspension (5 μl) containing 101 to 105 cells was spotted on a 1/3LB agar plate containing 2 mM γ-HCH or 25 μM 2,5-DCP. The negative-control plate was supplemented with dimethyl sulfoxide (DMSO), which was used as a solvent for γ-HCH and 2,5-DCP. Cell growth and viability were assessed after incubation at 30°C for 2 to 5 days. To assay the sensitivity to organic solvents, the cell suspensions containing 105 cells were spotted on 1/3LB agar and allowed to dry, and each solvent was overlaid to a depth of 2 to 3 mm. Cell growth was assessed after incubation at 30°C for 2 to 5 days.
Inhibition zone bioassay.
Approximately 106 cells of a strain to be investigated were spread on 1/3LB agar. Paper disks (8-mm diameter; Advantec) were put at the center of such agar plates, and 10 μl of each reagent was spotted onto the disk. 2,5-DCP, 2,4-dichlorophenol (2,4-DCP), 2,4,5-trichlorophenol, and 2,5-dichlorohydroquinone were dissolved in DMSO, and Tc was dissolved in methanol. After incubation for more than 48 h at 30°C, the diameters of the inhibition zones surrounding the disk were measured.
Membrane permeability assay based on the uptake of hydrophobic fluorescent probes.
The membrane permeability of cells was examined using N-phenyl-1-naphthylamine (NPN) and 2′,7′-bis-(carboxyethyl)-5(6′)-carboxyfluorescein acetoxymethyl ester (BCECF-AM) as hydrophobic fluorescent probes. S. japonicum UT26 and its mutant cells were cultured in 1/3LB agar, collected at log phase, washed twice in ice-cold 50 mM HEPES (NaOH) buffer (pH 7.0), and diluted to an optical density at 660 nm (OD660) at 0.5 in the same buffer. In order to use BCDCF-AM as a hydrophobic fluorescent probe, the cells must be energized. Cells were preincubated for 2 min with 50 mM sodium succinate at room temperature before mixing with fluorescent probes. One hundred microliters of cell suspension was added to 0.9 ml of 50 mM HEPES buffer containing 10 μM NPN or 0.5 μM BCECF-AM (final concentration) in a cuvette (1-cm light path), and the fluorescence was measured immediately using a fluorescence spectrophotometer (Hitachi F-3010). NPN and BCECF were excited by light with wavelengths of 340 nm and 502 nm, and emissions were measured at 420 nm and 525 nm, respectively.
Chemicals.
γ-HCH and 2,5-DCP were purchased from Nakarai Chemical Co. (Kyoto, Japan); NPN and BCECF-AM were purchased from Wako Pure Chemical Industries (Osaka, Japan) and Merck Calbiochem (Tokyo, Japan), respectively. Stock solutions of γ-HCH and 2,5-DCP were prepared in DMSO at final concentrations of 200 mM and 20 mM, respectively.
Nucleotide sequence accession number.
The nucleotide sequence determined in this study has been deposited in the DDBJ/EMBL/GenBank databases under the accession number AB267475.
RESULTS
Identification of linKLMN as genes required for γ-HCH utilization in S. japonicum UT26.
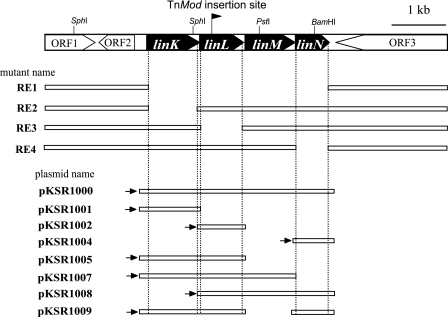
In order to analyze the γ-HCH degradation pathway in S. japonicum UT26, we carried out random transposon mutagenesis of UT26 using pTnMod-OKm, and we have isolated new UT26 mutants deficient in γ-HCH utilization (12). One of these mutants, UT953, was able to grow on a W minimal agar plate containing succinate but not on one containing γ-HCH as the sole carbon source. Obvious LinA to LinE activities in UT953 were confirmed by using GC with an ECD (data not shown). A single copy of the TnMod-OKm insertion in the UT953 genome was confirmed by Southern blot analysis (data not shown), and the UT953-derived DNA fragments containing the TnMod-OKm insertion site were recovered as plasmids in E. coli as described in Materials and Methods. Sequence analysis of the resulting plasmids revealed that the TnMod-OKm inserted in strain UT953 was located in an open reading frame (ORF) that encoded a protein homologous to putative ATP-binding protein. This ORF and three neighboring ORFs were predicted to constitute an operon (Fig. 2) and encode a putative ATP-binding cassette (ABC) transporter system as a set. These four genes were designated linK, linL, linM, and linN in that order (Fig. 2).
FIG. 2.
Organization of the linKLMN gene cluster and constructs of linKLMN mutants and plasmids for complementation. Pentagons indicate the size and orientation of the ORFs. In S. japonicum UT953, TnMod-OKm was inserted into a position shown by the small black flag. The rightward direction of the flag represents the orientation of the Kmr gene on TnMod-OKm. S. japonicum RE1, RE2, RE3 and RE4 are linKLMN deletion mutants (Table 1), and gaps between the white bars indicate regions that were deleted by homologous recombination. Plasmids pKSR1000 to pKSR1009 were used for complementation analysis (Table 1), and white bars show the insertion regions of plasmids. Rightward black arrows indicate constitutive promoter Pu (36). Mutants and plasmids were constructed as described in Materials and Methods.
To confirm the involvement of the linKLMN genes for γ-HCH utilization in S. japonicum UT26, we constructed a linKLMN deletion mutant, RE1, by allelic-exchange mutagenesis as described in Materials and Methods. S. japonicum strain RE1 was able to grow by using succinate, but not γ-HCH, as the sole carbon source. This growth defect was complemented by the introduction of plasmid carrying linKLMN but not linKL, linKLM, linLMN, or linKLN (Table 2). The linK, linL, and linN deletion mutants (RE2, RE3, and RE4, respectively) were also unable to grow on W minimal agar containing γ-HCH (Table 2). The linM deletion mutant was not obtained. The growth defects of RE3 and RE4 were complemented by the introduction of the linL and linN genes, respectively (Table 2). These results indicated that all the linKLMN genes are probably required as a set for the γ-HCH utilization in UT26. The growth defect of RE2 was not complemented by the introduction of linK (Table 2), probably due to the polar effect of the linK disruption by the insertion of the Kmr gene.
TABLE 2.
Effects of disruption and complementation of linKLMN genes on γ-HCH utilization
| Strain | Plasmid [supplied gene(s)] | Growth on γ-HCHa |
|---|---|---|
| UT26 | None | + |
| RE1 | None | − |
| pKS13P | − | |
| pKSR1005 (linKL) | − | |
| pKSR1007 (linKLM) | − | |
| pKSR1008 (linLMN) | − | |
| pKSR1009 (linKLN) | − | |
| pKSR1000 (linKLMN) | + | |
| RE2 | None | − |
| pKS13P | − | |
| pKSR1001 (linK) | − | |
| RE3 | None | − |
| pKS13P | − | |
| pKSR1002 (linL) | + | |
| RE4 | None | − |
| pKS13P | − | |
| pKSR1004 (linN) | + |
Growth on W minimal agar containing γ-HCH. Symbols: +, growth on W minimal agar containing γ-HCH; −, no growth on W minimal agar containing γ-HCH.
Sequence features of LinK, LinL, LinM, and LinN.
The linKLMN genes were predicted to encode an ABC transporter system, which is known as an active transport system, for the uptake or efflux of various molecules across the cell membrane (2, 9). The core of this ABC transporter system is formed by transmembrane (LinK homologue) and cytoplasmic ATPase (LinL homologue) components (19). LinK has six potential transmembrane-spanning domains but lacks an EAA motif, which is highly conserved in the transmembrane components of bacterial ABC importers but not exporters (2, 9). LinL is the most highly conserved among components of the ABC transporters and exhibits typical features of the ABC ATPase, i.e., Walker A and B motifs, Q loop, and H motif, which are conserved in most ATP-binding proteins, and the LSGGQ linker motif, which is specific to ABC ATPase (2, 9). LinM has a putative periplasmic signal peptide at its N-terminal 30 amino acid residues and exhibits a feature of soluble protein. LinM contains the N-terminal domain, which is similar to the mammalian cell entry (Mce) protein family in Mycobacterium tuberculosis (7, 52). LinM homologues in the database were delineated as substrate-binding proteins of ABC transporter systems or Mce-related proteins. One of the Mce family proteins, Mce1A, links with a mammalian cell invasion protein (7), but most of the Mce proteins or domains are functionally uncharacterized (52). LinN has a putative lipoprotein signal at its N-terminal portion, which is called lipobox and is cleaved by signal peptidase II (16).
Site-directed mutagenesis of LinL.
An ABC transporter operates with ATP hydrolysis provided by its ATPase component. To confirm that ATPase activity is essential for the function of LinKLMN, site-directed mutagenesis of LinL was performed. Since Glu and Gln residues of LinL, which are located near the Walker B motif (Glu188) and at the Q loop (Gln108), are highly conserved in ABC ATPases and are critical for the ATPase activity (2, 9), they were changed to Gln (E188Q) and Leu (Q108L), respectively. The introduction of the plasmid carrying these two linL mutant genes into strain RE3 did not allow the host cells to grow on the W minimal agar containing γ-HCH (Table 2).
LinKLMN suppressed the degradation of γ-HCH and the accumulation of 2,5-DCP in S. japonicum UT26.
In order to elucidate the function of linKLMN, we analyzed the γ-HCH degradation ability of linKLMN mutants in more detail by using GC with an ECD. In the whole-cell assay, S. japonicum RE1 (ΔlinKLMN strain) and RE3 (ΔlinL strain) degraded γ-HCH more rapidly than UT26 and accumulated 2,5-DCP to concentrations three- and fivefold higher, respectively, than that of strain UT26 (Fig. 3A). These phenotypes of strains RE1 and RE3 were reversed by the introduction of the wild-type linKLMN and linL genes, respectively (data not shown). When the same assay was conducted by using crude extracts, no differences were observed between UT26 and RE1 (Fig. 3B), indicating that the enzymatic activities for the γ-HCH degradation were not reduced by the disruption of linKLMN.
FIG. 3.
Degradation of γ-HCH and accumulation of 2,5-DCP in S. japonicum UT26 and its mutants. γ-HCH (30 μM) was incubated with intact cells (A) or crude extracts (B) of each strain in W medium at 30°C. The concentrations of γ-HCH and its metabolites in reaction medium were measured by GC with an ECD.
LinA and LinB, which catalyze the early reactions of γ-HCH degradation, are directly involved in the production of the dead-end metabolites 1,2,4-TCB and 2,5-DCP (Fig. 1) and are localized in the periplasmic space in S. japonicum UT26 cells (35). Cellular fractionation and Western blot analyses revealed that the localization and amount of LinA and LinB in strain RE1 were not different from those of strain UT26 (data not shown). These results indicated that LinKLMN did not affect the localization of LinA and LinB.
LinKLMN are involved in the tolerance to a metabolite(s) of γ-HCH and organic solvents.
Previously, we showed that 2,5-DCP, a dead-end metabolite of γ-HCH degradation, is toxic to S. japonicum UT26 (12). The relationship between the toxic effect of the γ-HCH metabolite(s) and the LinKLMN function was investigated. Colony formation of S. japonicum RE1 and RE3 on 1/3LB agar was drastically inhibited in the presence of γ-HCH and 2,5-DCP, and the growth defect of RE3 was recovered by the introduction of the wild-type linL gene (Fig. 4). In liquid culture, the growth of RE1 was also drastically inhibited in the presence of γ-HCH or 2,5-DCP compared to UT26 (Fig. 5A and B). To investigate the toxic effect of γ-HCH itself, we constructed a strain, YO5ΔlinL (UT26 ΔlinA ΔlinL) lacking the LinA activity. The growth of YO5ΔlinL was not inhibited in the presence of γ-HCH (data not shown), which confirmed that γ-HCH itself is not toxic to the linL mutant. These results indicated that LinKLMN play a critical role in the tolerance to the γ-HCH metabolite(s), especially to 2,5-DCP.
FIG. 4.
Assay for sensitivity of S. japonicum UT26 and its mutants to γ-HCH or 2,5-DCP. The cells at log phase cultured in 1/3LB medium were collected, washed in fresh 1/3LB medium, and diluted in 1/3LB medium, and cell suspensions (approximately 5 μl) containing 101 to 103 cells were spotted on 1/3LB agar plate with either 2 mM γ-HCH or 25 μM 2,5-DCP or with DMSO (negative control). These plates were incubated at 30°C for 5 days.
FIG. 5.
Growth curves of S. japonicum UT26 (A) and RE1 (B) in 1/3LB medium in the presence of γ-HCH or 2,5-DCP. UT26 and its mutant cells at log phase cultured in 1/3LB were collected, washed in fresh 1/3LB medium, and diluted to an OD660 of 0.05 in fresh 1/3LB with γ-HCH or 2,5-DCP. Each sample in the presence of 20 μM γ-HCH (open triangle), 6 μM 2,5-DCP (open diamond), or DMSO (negative control; filled circle), was grown at 30°C, and turbidity (OD660) was recorded by using a TVS062CA biophotorecorder (Advantec, Toyko, Japan).
To investigate whether the sensitivity of the linKLMN mutants is specific to 2,5-DCP, the sensitivities of S. japonicum UT26 and RE1 to chlorophenols and other compounds was tested. The sensitivities of strains UT26 and RE1 to chlorophenols and some chemicals were estimated using the diameter of the inhibition zone (Table 3). Insofar as we tested, only 2,5-DCP caused obviously stronger growth inhibition for RE1 than for UT26 (Table 3). Tolerance to organic solvent is estimated by measuring growth on a 1/3LB agar plate overlaid with organic solvents. The toxicity of an organic solvent correlates with its hydrophobicity as defined by the logarithm of the partitioning coefficient of a solvent in 1-octanol/water (log Pow) (44), and this log Pow value is also used to express the level of tolerance in various bacterial species and strains. UT26, but not RE1, was able to grow on 1/3LB agar overlaid with nonane (Pow of 5.5) or octane (Pow of 4.9).
TABLE 3.
Inhibition zone by chlorophenols and other chemicals
| Reagent (amt)a | Diam of inhibition zone (mm) of strainb:
|
|
|---|---|---|
| UT26 | RE1 | |
| 2,5-DCP (0.2 μmol) | NDc | >84d |
| 2,4-DCP (0.2 μmol) | ND | ND |
| 2,4-DCP (0.4 μmol) | 15 ± 1.3 | 22 ± 0.8 |
| 2,4,5-TCP (0.2 μmol) | 22 ± 7.4 | 27 ± 9.5 |
| 2,5-DCHQ (0.1 μmol) | 12 ± 0.8 | 15 ± 2.3 |
| H2O2 (2.4 μmol) | 55 ± 2.5 | 57 ± 4.1 |
| SDS (1 mg) | 29 ± 1.7 | 32 ± 0.9 |
| Tc (0.2 μg) | 62 ± 1.3 | 65 ± 0.5 |
| MeOH | ND | ND |
| DMSO | ND | ND |
Ten microliters of each reagent was applied. 2,5-DCHQ, 2,5-dichlorohydroquinone; SDS, sodium dodecyl sulfate; MeOH, methanol.
Values are means ± standard deviations from three to five independent experiments.
ND, not detected.
The diameter of a plate is 84 mm.
LinKLMN are involved in the permeability of the membrane to hydrophobic compounds.
In general, tolerance to hydrophobic compounds is highly linked to their membrane permeability (44). The rapid γ-HCH degradation in linKLMN mutants (Fig. 3A) could be explained by the increase in the rate of γ-HCH diffusion into the cells. To examine this possibility, we performed an uptake assay of hydrophobic fluorescent probes. NPN is an uncharged hydrophobic probe. Since the fluorescence of NPN is strong in phospholipid environments but weak in aqueous ones (55), it has been used to monitor the changes in bacterial membrane potential and the permeability of the outer membrane (17, 29, 48). The initial fluorescence in the linKLMN deletion mutant, RE1, suddenly increased compared with that in strain UT26, and then decreased gradually (Fig. 6A). The maximum fluorescence intensity of NPN in strain RE1 was approximately 1.5-fold greater than that in UT26 (Fig. 6A). These observations indicate that the uptake of NPN in RE1 cells is more rapid than in UT26 cells. This increase of fluorescence in RE1 was restored by the introduction of the linKLMN genes (data not shown). We also used another probe, BCECF-AM, which is nonfluorescent and cell membrane permeable. Inside the cells, BCECF-AM is easily hydrolyzed by intracellular esterase to BCECF, a nonpermeable compound that emits a strong green fluorescence (3). The rate at which BCECF fluorescence increased in RE1 was more rapid than in UT26 (Fig. 6B). These results strongly suggested that LinKLMN is involved in the permeability of the membrane to hydrophobic compounds in UT26.
FIG. 6.
Change in the fluorescence of a hydrophobic probe, NPN (A) or BCECF (B), in the presence of intact cells of S. japonicum UT26 and RE1. a.u. arbitrary units.
DISCUSSION
In this study, we identified and characterized the linKLMN genes encoding a putative ABC transporter system as an essential factor for γ-HCH utilization in S. japonicum UT26. In our previous study, we showed that the growth of strain UT26 on γ-HCH is inhibited by a γ-HCH metabolite, 2,5-DCP (13), and the results of this study strongly suggested that the putative ABC transporter is required for the tolerance to 2,5-DCP in UT26. This is the first report that clearly demonstrated the essentiality of a cellular factor besides catabolic enzymes and transcriptional regulators for the utilization of a xenobiotic compound.
The deletion of linKLMN brought about excessive γ-HCH degradation and thus an increase in the production of 2,5-DCP (Fig. 3), indicating that LinKLMN tunes the metabolic balance of γ-HCH in S. japonicum UT26 in order to prevent the accumulation of the toxic metabolite 2,5-DCP. 2,5-DCP is produced from γ-HCH by the activities of LinA and LinB (Fig. 1), which are localized in periplasm (35, 36). We presume that alteration of membrane physiology in the linKLMN mutant caused increase of γ-HCH influx into periplasm and then caused excessive γ-HCH degradation and 2,5-DCP production. Tuning the metabolic balance by enzymatic activities, regulation of the gene expression, and the copy number of genes for enzymes is most probable in cases in which xenobiotics and their metabolites are toxic for bacterial cells that can degrade them (4, 31, 41). Our study suggests that the ability of UT26 to utilize γ-HCH is established by the exquisite balance of cellular γ-HCH degradation activity and tolerance to toxic metabolites. LinKLMN are novel examples of factors tuning the metabolic balance.
The LinKLMN proteins are involved not only in tolerance to 2,5-DCP but also in tolerance to organic solvents and the permeability of the membrane to hydrophobic probes, which suggest that LinKLMN are important for membrane physiology. It has been shown that the permeability of the cell membrane to the substrate is one of the limiting factors for whole-cell catalytic activity. The toluene-utilizing bacterium Pseudomonas putida DOT-T1E is highly tolerant to organic solvents. The Ttg efflux pumps are essential for solvent tolerance in this strain (45, 46). On the other hand, several transporters are involved in the uptake of aromatic compounds, such as benzoate (8), 4-hydroxybenzoate and protocatechate (39), phthalate (6), 2,4-dichlorophenpxyacetate (28), styrene (34), and m-xylene (23). In P. putida, XylN, an outer membrane protein encoded on TOL plasmid pWW0, is presumed to be involved in the uptake of m-xylene, and the xylN deletion mutant showed lower catabolic enzyme activity than the wild-type strain did (23). However, the growth of P. putida harboring the wild-type xylN gene was inhibited by a high concentration of m-xylene, while that of xylN mutant was not (23). The functions of xylN and linKLMN were totally opposite each other in both whole-cell reaction and tolerance to substrate.
The molecular mechanism of LinKLMN is still unknown. The possibility that LinKLMN are directly involved in the membrane function which is inhibited by chlorophenols (49, 50) can be excluded, because the linKLMN mutant was not sensitive to 2,4-DCP and 2,4,5-DCP, which theoretically should have the same effect as 2,5-DCP (Table 3). One possible working hypothesis is that LinKLMN work as an efflux pump for hydrophobic compounds and are especially specific to 2,5-DCP. However, LinKLMN are not similar to other known multidrug resistance transporters, such as LmrA, an ABC-type multidrug resistance transporter of Lactococcus lactis (56). Furthermore, multidrug resistance transporters usually do not have components corresponding to LinM and LinN. Another possibility is that the LinKLMN system translocates some component(s) of the cell envelope or surface. Some transporters and lipoproteins are necessary for the biogenesis of the cell envelope (47), and LinM has in its N-terminal region an Mce domain, which has been implicated in interaction with the membrane lipid in Mycobacterium (22) or in the endoplasmic reticulum of plant cells (1). Alteration of lipid composition and membrane vesicle release are also major mechanisms for bacterial tolerance to toxic compounds (44). LinKLMN may be involved in the integrity of membrane, and thus, they can contribute to both the tolerance to some compounds and to the permeability of the membrane. In this study, we confirmed that ATPase activity is essential for LinKLMN function, but further studies are required to elucidate its detailed mechanism. The functions of LinM and LinN are especially of great interest.
The linKLMN orthologues were found in some proteobacteria, including some that have not been reported as xenobiotic or chloroaromatic-degrading bacteria. Most of the linKLMN orthologues are organized to exist as a cluster and form a putative operon. An ABC transporter system encoded by the ttg2 genes in Pseudomonas putida GM73 was identified by transposon mutagenesis to isolate a toluene-sensitive mutant (25) and is the only LinKLMN-like ABC transporter system whose function has been described, although it lacks the LinN homologue and its level of similarity to linKLM is very low. The linKLMN orthologues showing a high level of similarity (more than 60% identity at the amino acid level) were found only in sphingomonads. Most of the γ-HCH-degrading bacteria that have been reported belong to sphingomonads (27). Sphingomonads have broad catabolic abilities for xenobiotic compounds, but their resistance to hydrophobic substrates or solvents remains to be elucidated. In sphingomonad strains, the hydrophobicity of glycosphingolipid in the outer membrane has been proposed to be an advantage for the purpose of contacting hydrophobic substrates (24), but this feature is thought to be a disadvantage for protecting cells against toxic hydrophobic compounds. Actually, S. japonicum UT26 and other sphingomonads are more sensitive to 2,5-DCP than E. coli and P. putida are (13). LinKLMN and its homologues might be important for the high metabolic activity of sphingomonads toward γ-HCH or other xenobiotics.
Supplementary Material
Acknowledgments
This work was supported by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Agriculture, Forestry, and Fisheries (HC-07-2323) of Japan. R.E. was supported by a Japan Society for the Promotion of Science Research Fellowship.
Footnotes
Published ahead of print on 16 March 2007.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Awai, K., C. Xu, B. Tamot, and C. Benning. 2006. A phosphatidic acid-binding protein of the chloroplast inner envelope membrane involved in lipid trafficking. Proc. Natl. Acad. Sci. USA 103:10817-10822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biemans-Oldehinkel, E., M. K. Doeven, and B. Poolman. 2006. ABC transporter architecture and regulatory roles of accessory domains. FEBS Lett. 580:1023-1035. [DOI] [PubMed] [Google Scholar]
- 3.Bolhuis, H., H. W. van Veen, D. Molenaar, B. Poolman, A. J. Driessen, and W. N. Konings. 1996. Multidrug resistance in Lactococcus lactis: evidence for ATP-dependent drug extrusion from the inner leaflet of the cytoplasmic membrane. EMBO J. 15:4239-4245. [PMC free article] [PubMed] [Google Scholar]
- 4.Camara, B., C. Herrera, M. Gonzalez, E. Couve, B. Hofer, and M. Seeger. 2004. From PCBs to highly toxic metabolites by the biphenyl pathway. Environ. Microbiol. 6:842-850. [DOI] [PubMed] [Google Scholar]
- 5.Cassidy, M. B., H. Lee, J. T. Trevors, and R. B. Zablotowicz. 1999. Chlorophenol and nitrophenol metabolism by Sphingomonas sp. UG30. J. Ind. Microbiol. Biotechnol. 23:232-241. [DOI] [PubMed] [Google Scholar]
- 6.Chang, H. K., and G. J. Zylstra. 1999. Characterization of the phthalate permease OphD from Burkholderia cepacia ATCC 17616. J. Bacteriol. 181:6197-6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chitale, S., S. Ehrt, I. Kawamura, T. Fujimura, N. Shimono, N. Anand, S. Lu, L. Cohen-Gould, and L. W. Riley. 2001. Recombinant Mycobacterium tuberculosis protein associated with mammalian cell entry. Cell. Microbiol. 3:247-254. [DOI] [PubMed] [Google Scholar]
- 8.Collier, L. S., N. N. Nichols, and E. L. Neidle. 1997. benK encodes a hydrophobic permease-like protein involved in benzoate degradation by Acinetobacter sp. strain ADP1. J. Bacteriol. 179:5943-5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davidson, A. L., and J. Chen. 2004. ATP-binding cassette transporters in bacteria. Annu. Rev. Biochem. 73:241-268. [DOI] [PubMed] [Google Scholar]
- 10.Dennis, J. J., and G. J. Zylstra. 1998. Plasposons: modular self-cloning minitransposon derivatives for rapid genetic analysis of gram-negative bacterial genomes. Appl. Environ. Microbiol. 64:2710-2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denyer, S. P., and J. Y. Maillard. 2002. Cellular impermeability and uptake of biocides and antibiotics in gram-negative bacteria. Symp. Ser. Soc. Appl. Microbiol. 2002:35S-45S. [PubMed] [Google Scholar]
- 12.Endo, R., M. Kamakura, K. Miyauchi, M. Fukuda, Y. Ohtsubo, M. Tsuda, and Y. Nagata. 2005. Identification and characterization of genes involved in the downstream degradation pathway of γ-hexachlorocyclohexane in Sphingomonas paucimobilis UT26. J. Bacteriol. 187:847-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Endo, R., Y. Ohtsubo, M. Tsuda, and Y. Nagata. 2006. Growth inhibition by metabolites of γ-hexachlorocyclohexane in Sphingobium japonicum UT26. Biosci. Biotechnol. Biochem. 70:1029-1032. [DOI] [PubMed] [Google Scholar]
- 14.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto, W., K. Momma, Y. Maruyama, M. Yamasaki, B. Mikami, and K. Murata. 2005. Structure and function of bacterial super-biosystem responsible for import and depolymerization of macromolecules. Biosci. Biotechnol. Biochem. 69:673-692. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi, S., and H. C. Wu. 1990. Lipoproteins in bacteria. J. Bioenerg. Biomembr. 22:451-471. [DOI] [PubMed] [Google Scholar]
- 17.Helander, I. M., and T. Mattila-Sandholm. 2000. Fluorometric assessment of gram-negative bacterial permeabilization. J. Appl. Microbiol. 88:213-219. [DOI] [PubMed] [Google Scholar]
- 18.Hoang, T. T., R. R. Karkhoff-Schweizer, A. J. Kutchma, and H. P. Schweizer. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77-86. [DOI] [PubMed] [Google Scholar]
- 19.Hyde, S. C., P. Emsley, M. J. Hartshorn, M. M. Mimmack, U. Gileadi, S. R. Pearce, M. P. Gallagher, D. R. Gill, R. E. Hubbard, and C. F. Higgins. 1990. Structural model of ATP-binding proteins associated with cystic fibrosis, multidrug resistance and bacterial transport. Nature 346:362-365. [DOI] [PubMed] [Google Scholar]
- 20.Imai, R., Y. Nagata, M. Fukuda, M. Takagi, and K. Yano. 1991. Molecular cloning of a Pseudomonas paucimobilis gene encoding a 17-kilodalton polypeptide that eliminates HCl molecules from gamma-hexachlorocyclohexane. J. Bacteriol. 173:6811-6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imai, R., Y. Nagata, K. Senoo, H. Wada, M. Fukuda, M. Takagi, and K. Yano. 1989. Dehydrochlorination of gamma-hexachlorocyclohexane (gamma-BHC) by gamma-BHC-assimilating Pseudomonas paucimobilis. Agric. Biol. Chem. 53:2015-2017. [Google Scholar]
- 22.Joshi, S. M., A. K. Pandey, N. Capite, S. M. Fortune, E. J. Rubin, and C. M. Sassetti. 2006. Characterization of mycobacterial virulence genes through genetic interaction mapping. Proc. Natl. Acad. Sci. USA 103:11760-11765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kasai, Y., J. Inoue, and S. Harayama. 2001. The TOL plasmid pWW0 xylN gene product from Pseudomonas putida is involved in m-xylene uptake. J. Bacteriol. 183:6662-6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawahara, K., H. Kuraishi, and U. Zahringer. 1999. Chemical structure and function of glycosphingolipids of Sphingomonas spp. and their distribution among members of the alpha-4 subclass of Proteobacteria. J. Ind. Microbiol. Biotechnol. 23:408-413. [DOI] [PubMed] [Google Scholar]
- 25.Kim, K., S. Lee, K. Lee, and D. Lim. 1998. Isolation and characterization of toluene-sensitive mutants from the toluene-resistant bacterium Pseudomonas putida GM73. J. Bacteriol. 180:3692-3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimbara, K., T. Hashimoto, M. Fukuda, T. Koana, M. Takagi, M. Oishi, and K. Yano. 1989. Cloning and sequencing of two tandem genes involved in degradation of 2,3-dihydroxybiphenyl to benzoic acid in the polychlorinated biphenyl-degrading soil bacterium Pseudomonas sp. strain KKS102. J. Bacteriol. 171:2740-2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lal, R., C. Dogra, S. Malhotra, P. Sharma, and R. Pal. 2006. Diversity, distribution and divergence of lin genes in hexachlorocyclohexane-degrading sphingomonads. Trends. Biotechnol. 24:121-130. [DOI] [PubMed] [Google Scholar]
- 28.Leveau, J. H., A. J. Zehnder, and J. R. van der Meer. 1998. The tfdK gene product facilitates uptake of 2,4-dichlorophenoxyacetate by Ralstonia eutropha JMP134(pJP4). J. Bacteriol. 180:2237-2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loh, B., C. Grant, and R. E. Hancock. 1984. Use of the fluorescent probe 1-N-phenylnaphthylamine to study the interactions of aminoglycoside antibiotics with the outer membrane of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 26:546-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 31.McCarthy, D. L., A. A. Claude, and S. D. Copley. 1997. In vivo levels of chlorinated hydroquinones in a pentachlorophenol-degrading bacterium. Appl. Environ. Microbiol. 63:1883-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyauchi, K., Y. Adachi, Y. Nagata, and M. Takagi. 1999. Cloning and sequencing of a novel meta-cleavage dioxygenase gene whose product is involved in degradation of γ-hexachlorocyclohexane in Sphingomonas paucimobilis. J. Bacteriol. 181:6712-6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyauchi, K., S. K. Suh, Y. Nagata, and M. Takagi. 1998. Cloning and sequencing of a 2,5-dichlorohydroquinone reductive dehalogenase gene whose product is involved in degradation of γ-hexachlorocyclohexane by Sphingomonas paucimobilis. J. Bacteriol. 180:1354-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mooney, A., N. D. O'Leary, and A. D. Dobson. 2006. Cloning and functional characterization of the styE gene, involved in styrene transport in Pseudomonas putida CA-3. Appl. Environ. Microbiol. 72:1302-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagata, Y., A. Futamura, K. Miyauchi, and M. Takagi. 1999. Two different types of dehalogenases, LinA and LinB, involved in γ-hexachlorocyclohexane degradation in Sphingomonas paucimobilis UT26 are localized in the periplasmic space without molecular processing. J. Bacteriol. 181:5409-5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagata, Y., K. Miyauchi, and M. Takagi. 1999. Complete analysis of genes and enzymes for γ-hexachlorocyclohexane degradation in Sphingomonas paucimobilis UT26. J. Ind. Microbiol. Biotechnol. 23:380-390. [DOI] [PubMed] [Google Scholar]
- 37.Nagata, Y., T. Nariya, R. Ohtomo, M. Fukuda, K. Yano, and M. Takagi. 1993. Cloning and sequencing of a dehalogenase gene encoding an enzyme with hydrolase activity involved in the degradation of γ-hexachlorocyclohexane in Pseudomonas paucimobilis. J. Bacteriol. 175:6403-6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagata, Y., R. Ohtomo, K. Miyauchi, M. Fukuda, K. Yano, and M. Takagi. 1994. Cloning and sequencing of a 2,5-dichloro-2,5-cyclohexadiene-1,4-diol dehydrogenase gene involved in the degradation of γ-hexachlorocyclohexane in Pseudomonas paucimobilis. J. Bacteriol. 176:3117-3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nichols, N. N., and C. S. Harwood. 1997. PcaK, a high-affinity permease for the aromatic compounds 4-hydroxybenzoate and protocatechuate from Pseudomonas putida. J. Bacteriol. 179:5056-5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nojiri, H., H. Habe, and T. Omori. 2001. Bacterial degradation of aromatic compounds via angular dioxygenation. J. Gen. Appl. Microbiol. 47:279-305. [DOI] [PubMed] [Google Scholar]
- 41.Pérez-Pantoja, D., T. Ledger, D. H. Pieper, and B. Gonzalez. 2003. Efficient turnover of chlorocatechols is essential for growth of Ralstonia eutropha JMP134(pJP4) in 3-chlorobenzoic acid. J. Bacteriol. 185:1534-1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pieper, D. H. 2005. Aerobic degradation of polychlorinated biphenyls. Appl. Microbiol. Biotechnol. 67:170-191. [DOI] [PubMed] [Google Scholar]
- 43.Pinyakong, O., H. Habe, and T. Omori. 2003. The unique aromatic catabolic genes in sphingomonads degrading polycyclic aromatic hydrocarbons (PAHs). J. Gen. Appl. Microbiol. 49:1-19. [DOI] [PubMed] [Google Scholar]
- 44.Ramos, J. L., E. Duque, M. T. Gallegos, P. Godoy, M. I. Ramos-Gonzalez, A. Rojas, W. Teran, and A. Segura. 2002. Mechanisms of solvent tolerance in gram-negative bacteria. Annu. Rev. Microbiol. 56:743-768. [DOI] [PubMed] [Google Scholar]
- 45.Ramos, J. L., E. Duque, P. Godoy, and A. Segura. 1998. Efflux pumps involved in toluene tolerance in Pseudomonas putida DOT-T1E. J. Bacteriol. 180:3323-3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rojas, A., E. Duque, G. Mosqueda, G. Golden, A. Hurtado, J. L. Ramos, and A. Segura. 2001. Three efflux pumps are required to provide efficient tolerance to toluene in Pseudomonas putida DOT-T1E. J. Bacteriol. 183:3967-3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruiz, N., D. Kahne, and T. J. Silhavy. 2006. Advances in understanding bacterial outer-membrane biogenesis. Nat. Rev. Microbiol. 4:57-66. [DOI] [PubMed] [Google Scholar]
- 48.Sedgwick, E. G., and P. D. Bragg. 1987. Distinct phases of the fluorescence response of the lipophilic probe N-phenyl-1-naphthylamine in intact cells and membrane vesicles of Escherichia coli. Biochim. Biophys. Acta 894:499-506. [DOI] [PubMed] [Google Scholar]
- 49.Shaw, L. J., Y. Beaton, L. A. Glover, K. Killham, and A. A. Meharg. 1999. Development and characterization of a lux-modified 2,4-dichlorophenol-degrading Burkholderia sp. RASC. Environ. Microbiol. 1:393-399. [DOI] [PubMed] [Google Scholar]
- 50.Sikkema, J., J. A. de Bont, and B. Poolman. 1995. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 59:201-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stolz, A. 1999. Degradation of substituted naphthalenesulfonic acids by Sphingomonas xenophaga BN6. J. Ind. Microbiol. Biotechnol. 23:391-399. [DOI] [PubMed] [Google Scholar]
- 52.Sutcliffe, I. C., and D. J. Harrington. 2004. Lipoproteins of Mycobacterium tuberculosis: an abundant and functionally diverse class of cell envelope components. FEMS Microbiol. Rev. 28:645-659. [DOI] [PubMed] [Google Scholar]
- 53.Takeshita, S., M. Sato, M. Toba, W. Masahashi, and T. Hashimoto-Gotoh. 1987. High-copy-number and low-copy-number plasmid vectors for lacZ alpha-complementation and chloramphenicol- or kanamycin-resistance selection. Gene 61:63-74. [DOI] [PubMed] [Google Scholar]
- 54.Taylor, L. A., and R. E. Rose. 1988. A correction in the nucleotide sequence of the Tn903 kanamycin resistance determinant in pUC4K. Nucleic Acids Res. 16:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trauble, H., and P. Overath. 1973. The structure of Escherichia coli membranes studied by fluorescence measurements of lipid phase transitions. Biochim. Biophys. Acta 307:491-512. [DOI] [PubMed] [Google Scholar]
- 56.van Veen, H. W., K. Venema, H. Bolhuis, I. Oussenko, J. Kok, B. Poolman, A. J. Driessen, and W. N. Konings. 1996. Multidrug resistance mediated by a bacterial homolog of the human multidrug transporter MDR1. Proc. Natl. Acad. Sci. USA 93:10668-10672. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.