Abstract
T-cell clonality estimation is important for the differential diagnosis between malignant and nonmalignant T-cell proliferation. Routinely used methods include polymerase chain reaction (PCR) analysis of T-cell receptor-γ (TCR-γ) gene rearrangements followed by Genescan analysis, polyacrylamide gel electrophoresis, or heteroduplex analysis to visualize amplification products. Here, we present a new method for the analysis after PCR of TCR-γ rearrangements using hybridization on oligonucleotide microchip. A microchip was designed to contain specific probes for all functional variable (V) and joining (J) gene segments involved in rearrangements of the TCR-γ locus. Fluorescently labeled fragments of rearranged γ-chain from patients and donors were obtained in a multiplex nested PCR and hybridized with a microchip. The results were detected using a portable microchip analyzer. Samples from 49 patients with T-cell lymphomas or leukemias and 47 donors were analyzed for T-cell clonality by microchip and single-strand conformation polymorphism analysis, which served as a standard reference method. Comparison of two techniques showed full concordance of the results. The microchip-based approach also allowed the identification of V and J gene segments involved in the particular TCR-γ rearrangement. The sensitivity of the method is sufficient to determine 10% of clonal cells in the sample.
T-cell lymphomas account for 15 to 20% of all lymphoid malignancies in Western countries. Diagnosis of T-cell lymphomas relies on clinical, morphological, and phenotypical data, although in many cases this combination is not sufficient. Diagnosis of T-cell lymphomas requires broad discrimination from skin and connective tissue diseases and other lymphoid malignancies. Furthermore, unusual clinical manifestations of T-cell lymphomas, including aplastic syndromes, high eosinophilias, and hemophagocytic syndromes, are not uncommon. Determination of T-cell clonality has important supplementary value in diagnosis of T-cell lymphomas.1,2,3
T-cell receptors (TCRs) are heterodimers consisting of either α/β or γ/δ chains. At early stages of T-cell maturation in thymus, the TCR loci are subjected to the process called V(D)J recombination. Peripheral T-cell lymphomas arise from T cells that undergo malignant transformation after most rearrangements of TCR loci are completed. Thus, detection of clonal cells with identical rearrangement favors a diagnosis of malignancy. The TCR-γ has become a favorite target for T-cell clonality assays for two reasons. First, it has a relatively simple structure. The γ-chain locus is located on chromosome 7 (7p14) and harbors 14 V gene segments, only nine of which are constantly involved in rearrangements, and five J gene segments. All Vγ gene segments are divided into four families: segments Vγ2, Vγ3, Vγ4, Vγ5, Vγ7, and Vγ8 belong to family 1 and segments Vγ9, Vγ10, and Vγ11 represent families 2, 3, and 4, respectively. The diversity (D) gene segment is absent. Second, the TCR-γ chain locus is rearranged during lymphocyte ontogeny before the α- and β-chains, so it provides a reliable target for determining T-cell clonality.3,4 The analysis of TCR-γ rearrangements is considered to be more informative for primary diagnosis of T-cell malignancy compared with analysis of other TCR loci, especially TCR-β.5,6
T-cell clonality is usually assayed by Southern blotting or the polymerase chain reaction (PCR) with subsequent detection by various techniques. Clonality analysis by Southern blotting has not found broad use in medicine because this method requires a considerable amount of high-quality DNA, is time-consuming and expensive, and usually requires radioisotopes. PCR is more simple and rapid, also allowing the use of various specimens including cytological samples and paraffin blocks.7 However, it is necessary to visualize the amplification products after PCR. Various techniques are used for this purpose: single-strand conformation polymorphism (SSCP) analysis, denaturing gradient polyacrylamide gel electrophoresis, heteroduplex analysis, and fragment analysis using the Genescan.7,8,9,10,11 The techniques also have their disadvantages, being relatively time-consuming and laborious or requiring expensive equipment.
Different approaches have been proposed to reduce labor and time of analysis. Alternative electrophoretic formats have been developed in the form of capillary electrophoresis and multichannel microchip electrophoresis.12 The electrophoretic microchip represents miniaturized format of capillary electrophoresis and dramatically reduced time of the analysis for TCR-γ gene and immunoglobulin heavy chain (IgH) gene rearrangements, compared with standard electrophoretic separation. However, this method exploited the same principle of high resolution separation of PCR-amplified fragments based on the difference in their size. A PCR assay using the LightCycler system followed by melting-curve analysis has been described to detect clonal TCR-gene rearrangements.13,14 Here, we propose an alternative method for determining T-cell clonality via analysis of the TCR-γ rearrangements. Our method combines multiplex PCR and hybridization with an oligonucleotide microchip. We have previously developed diagnostic microchips for analyzing chromosome rearrangements in leukemias.15,16 A similar approach using hybridization on oligonucleotide microarrays has been suggested recently in a padlock probe-based parallel assay for the analysis of TCR Vβ gene repertoire during immune response.17
The advantages of our approach are that the method is simple and reliable, all reactions are performed in microvolumes, the detection system is inexpensive and operator friendly, and the image analysis can be fully automated. Comparative analysis of T-cell clonality in 49 patients with T-cell lymphomas and leukemias and in the control group of 47 donors showed that the method is highly informative and suitable for clinical diagnosis of T-cell malignancies.
Materials and Methods
Patients and Samples
Forty-nine patients with various T-cell lymphoproliferative disorders were taken into the study. In all cases, the diagnosis of T-cell malignancy was verified using standard clinical, morphological, and immunophenotypic tests, and T-cell clonality was established by reference PCR with product detection by means of SSCP analysis. The control group included 47 donors without any lymphoproliferative disorder. Peripheral blood and bone marrow leukocytes or ground fresh-frozen samples of lymph nodes, spleen, and skin were analyzed. Approval was obtained from the institutional review boards of the participating institutions. All patients gave informed consent according to the Declaration of Helsinki. As a positive control, T-cell acute lymphoblastic leukemia cell line Jurkat was used, which is known to carry biallelic rearrangement-involved Vγ8 and Vγ11 gene segments.
Oligonucleotide Synthesis and Microchip Manufacturing
Oligonucleotides to be immobilized on a microchip were synthesized using an automated 394 DNA/RNA synthesizer (Applied Biosystems, Foster City, CA) by the standard phosphoroamidate method. The 3′-end of each oligonucleotide carried a spacer with a free amino group, which was introduced during synthesis with the use of 3′-amino-modifier C7 CPG 500 (Glen Research, Sterling, VA). The nucleotide sequences of the immobilized oligonucleotides are given in Table 1. The database references for genes involved in the TCR-γ rearrangements were Vγ2 (M13429), Vγ3 (M13430), Vγ4 (X13354), Vγ5 (X13355), Vγ7 (M13433), Vγ8 (M13434), Vγ9 (X15274), Vγ10 (X07206), Vγ11 (X07207), Jγ1 (M12960), Jγ2 (M12961), JγP (X58182), JγP1 (X08084), and JγP2 (M16016) (http://www.ncbi.nlm.nih.gov). Microchips were prepared by photo-inducible co-polymerization of oligonucleotides and the components of polyacrylamide gel as described previously.18 All oligonucleotide probes were spotted in duplicates.
Table 1.
Nucleotide Sequences of the Immobilized Oligonucleotides
| Gene | Oligonucleotide probe sequence |
|---|---|
| Vγ2 | 5′-CAACACAACCTTGGAGTTGTA-3′ |
| Vγ3 | 5′-ACATCCCTTGCGGTGGAGA-3′ |
| Vγ4 | 5′-ACAACGCTGGAGGTGTA-3′ |
| Vγ5 | 5′-ACACATCCTTTGAGTTGGAGA-3′ |
| Vγ7 | 5′-ACCCTGGAGTAGTAGGGGT-3′ |
| Vγ8 | 5′-AACACAACCCTGGAGTTGTA-3′ |
| Vγ9 | 5′-GTCCTGTTTCTCTACATTGTG-3′ |
| Vγ10 | 5′-CTTGATGGTAAGGATTGAAGT-3′ |
| Vγ11 | 5′-GAAGTGGAAGTGTGAGCATTT-3′ |
| Jγ1/Jγ2 | 5′-GTTGTTCCACTGCCAAAGAGTT-3′ |
| Jγp1 | 5′-GCAAATATCTTGAACCAACCAGT-3′ |
| Jγp | 5′-CTTGATTTTTTTGCCCAACTCTTG-3′ |
| Jγp2 | 5′-TGCAAACGTCTTGATCCAATCAC-3′ |
DNA Isolation and Multiplex PCR
DNA was isolated from peripheral blood, bone marrow, or biopsy specimens using the Wizard genomic DNA purification system (Promega, Madison, WI). DNA fragments required for the analysis were amplified in one tube using two-round nested multiplex PCR. Primers were selected using the Oligo 6 program (Molecular Biology Insights, Cascade, CO) and primer sequences earlier published in the study by Greiner et al19 (Table 2). In the first round, the reaction mixture (25 μl) contained 15 pmol of each primer, 67 mmol/L Tris-HCl, pH 8.6, 166 mmol/L (NH4)2SO4, 0.01% Triton X-100, 1.5 mmol/L MgCl2, 0.2 mmol/L each dNTP (Sileks, Kratovo, Russia), and 2.5 U of Taq polymerase (Sileks). Amplification was performed on a programmable thermocycler (Biometra, Göttingen, Germany) and included initial denaturation at 94°C (5 minutes) and 20 cycles of 94°C for 30 seconds, 55°C for 30 seconds, and 72°C for 30 seconds. The estimated range of product sizes for the first-round PCR reaction varies from 290 to 450 bp.
Table 2.
Nucleotide Sequences of Primers Used for Multiplex PCR
| Gene | Primer
|
|
|---|---|---|
| Designation | Sequence | |
| Round 1 | ||
| Vγ1-8 | Vγ1-8F1ex | 5′-TCTTCCAACTTGGAAGGGAGA-3′ |
| Vγ9 | Vγ9F1ex | 5′-TCTGCAACATCTGTATATTGGTATC-3′ |
| Vγ10-11 | Vγ10-11F1ex | 5′-CTGGTACCGGCAGAAACCAAA-3′ |
| Jγ1/Jγ2 | Jγ1/Jγ2R1ex | 5′-TAAACATTATTACATTATTCCAGTT-3′ |
| Jγp1/Jγp2 | Jγp1/Jγp2R1ex | 5′-TCTATCAGTTTTTCATTACTGGAAT-3′ |
| Jγp | JγpR1ex | 5′-CTCCCATCCCTTCTTTACATTGCA-3′ |
| Round 2 | ||
| Vγ1-8 | Vγ1-8F2in | Cy5-5′-GAAGGCCCCACAGCGTCTTC-3′ |
| Vγ9 | Vγ9F2in | Cy5-5′-AAGGAATCTGGCATTCCGTCAG-3′19 |
| Vγ10 | Vγ10F2in | Cy5-5′-TGTCTCAACAAAATCCGCAGCT-3′ |
| Vγ11 | Vγ11F2in | Cy5-5′-GGAAGACTAAGAAACTTGAGGT-3′ |
| Jγ1/Jγ2 | Jγ1/Jγ2R2in | 5′-TCTTCCGATACTTACCTGTGACAAC-3′ |
| Jγp1/Jγp2 | Jγp1/Jγp2R2in | 5′-GAAGTTACTATGAGCCTAGTCCCTT-3′19 |
| Jγp | JγpR2in | 5′-AAGCTTTGTTCCGGGACCAAATAC-3′19 |
Cy5, corresponding to fluorescently labeled primer. Bold font emphasizes that there is a modification with Cy5.
The product of the first PCR round (2 μl) was used as a template in the second round. The reaction mixture was essentially the same but was supplemented with 50 pmol of the fluorescently labeled Vγ-primers and 10 pmol of the nonlabeled Jγ-primers to obtain an excess of the labeled single-stranded PCR product. Amplification included initial denaturation at 94°C (5 minutes); 20 cycles of 94°C for 30 seconds, 55°C for 30 seconds, and 72°C for 30 seconds; and last synthesis at 72°C for 5 minutes. The estimated range of product sizes for the second-round PCR reaction varies from 150 to 270 bp.
Hybridization
The fluorescently labeled samples obtained in the second round of multiplex PCR were used for hybridization on the microchip. The hybridization mixture (40 μl) included 10 μl of formamide (Serva, Garden City Park, NY), 8 μl of 20× SSPE (Promega), and 22 μl of the amplificate. The mixture was heated at 95°C for 5 minutes for complete denaturation, quickly chilled on ice (2 minutes), and applied onto a microchip under hermetically attached plastic hybridization chamber and incubated at 37°C overnight. Then the hybridization chamber was removed, and the microchip was washed with 1× SSPE for 10 minutes at room temperature and dried.
Image Analysis
Fluorescent signals from cells of the microchip were detected using a portable microchip analyzer with a charge-coupled device camera and the Imageware software as described.20 As initial data, we used normalized fluorescence signals: Jm = (Im − I0)/(Bm − I0), where Im is the signal intensity per unit area of the inner part of a gel pad; Bm is the background signal, reflecting the light distribution through the microchip; I0 is the dark current in the charge-coupled device; and m is the gel pad number. Because gel pads were used on microchips in duplicates, signal intensities were averaged over two replicate spots. The average fluorescent signals are ranged according their value and N signals with maximal intensities were excluded from the total array {Jm}. N was equal to the maximal number of perfect duplexes that would be formed in the presence of a TCR-γ rearrangements in the sample in the case of biallelic rearrangement (N = 4). The signals remaining after the peak signals were removed (Jm) were used to compute the mean baseline signal <J>, its variance σ, and the threshold signal (TS) = <J> + 4 × σ. The Jm signals that exceeded the TS were considered indicative of perfect duplexes. The number and positions of perfect duplexes identified by this means reported unequivocally (owing to the microchip design) the presence and the type of TCR-γ rearrangement.
SSCP Analysis
As a reference method, PCR amplification of the TCR-γ-coding region and subsequent SSCP analysis were performed as described previously.8 Direct sequencing was performed using AB373A automated device (Applied Biosystems) and standard BigDye (Perkin-Elmer, Wellesley, MA) chemistry.
Results
Microchip Design
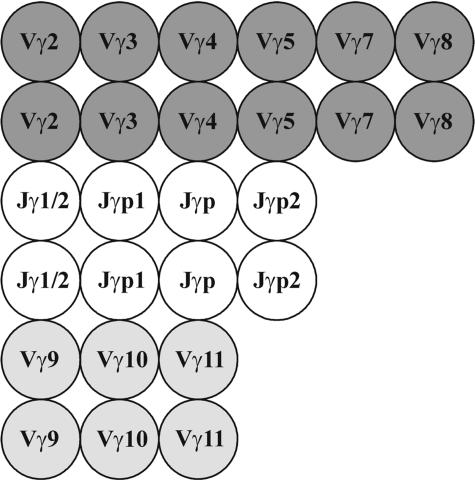
A scheme of the oligonucleotide microchip designed to identify the T-cell clonality from rearrangements of the TCR-γ locus is shown in Figure 1. The microchip contains oligonucleotide probes for all Vγ and Jγ gene segments involved in rearrangements of the TCR-γ locus, including nine probes for the Vγ (Vγ2, Vγ3, Vγ 4, Vγ5, Vγ7, Vγ8, Vγ 9, Vγ10, and Vγ11) and four probes for the Jγ gene segments (Jγ1/Jγ2, Jγp, Jγp1, and Jγp2). Individual oligonucleotide probes were designed for each Vγ and Jγ, except Jγ and Jγ2 gene segments. Because the Jγ1 and Jγ2 gene segments are highly homologous, we use a common oligonucleotide probe Jγ1/Jγ2. All oligonucleotide probes are presented as duplicate spots to increase the reproducibility of the analysis.
Figure 1.
Scheme of the microchip for detecting T-cell clonality by rearrangements of the TCR-γ locus. Gel pads with oligonucleotides complementary to sequences of the Vγ genes of family 1 (Vγ 1-8) are shown dark gray, gel pads with oligonucleotides complementary to sequences of the Vγ genes of families 2, 3, and 4 (Vγ9, Vγ10, Vγ11) are shown light gray, and gel pads with oligonucleotides complementary to sequences of the Jγ genes are shown white. Each oligonucleotide is used in duplicate on the microchip.
The PCR primers were selected to amplify the V-J fusion region of the rearranged TCR-γ locus. Germ-line γ chains are not amplified because the V and J segments are far apart before rearrangement and amplification is impossible. A single multiplex reaction was performed to amplify all possible rearrangements of the γ-chain. A consensus primer was directed to the V segments of family 1, and another consensus primer was selected for the V segments of families 3 and 4 (see Table 2). The microchip was used to detect particular V and J segments in the amplified fragment. The oligonucleotide probes were selected to hybridize specifically and efficiently to corresponding Vγ and Jγ gene segments. The number of PCR cycles required to correctly identify monoclonal rearrangements was selected experimentally. To find optimal conditions for PCR amplification, 25 control samples were tested: 15 samples from patients with T-cell lymphoma and 10 samples from donors without lymphoproliferative diseases including three samples of patients with infections. The samples were amplified for 20, 25, and 30 cycles of two-round nested PCR. The results using biochips and SSCP analysis were concordant only when 20 cycles were used in each round. With 25 and 30 cycles, the reaction reached the plateau, reducing the difference between less abundant and prevalent Vγ and Jγ fragments, so that several clonal samples would be scored as polyclonal (false-negative results).
Identification of T-Cell Clonality
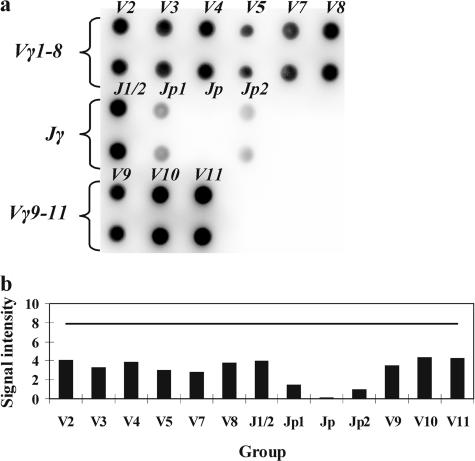
Analysis of a polyclonal T-cell population should yield signals from virtually all Vγ and Jγ gene segments under investigation. Figure 2 shows the hybridization pattern obtained with DNA of a healthy donor after amplification with the primers directed to the Vγ and Jγ gene segments. The relative signal intensities do not exceed the TS for all genes segments. The signals from all Vγ gene segments are similar in intensity; among the Jγ gene segments, the Jγ1/Jγ2 oligonucleotide probe stands out as producing somewhat more intense signals. This distribution most probably reflects the fact that the Jγ1 and Jγ2 gene segments are involved in rearrangements far more often (in more than 70% of cases) as compared with the other Jγ segments in T-lymphocyte populations.6
Figure 2.
a: Hybridization pattern obtained on the microchip with a polyclonal sample. b: Analysis of the results. The TS is shown with a horizontal line. None of the signals exceeds the TS.
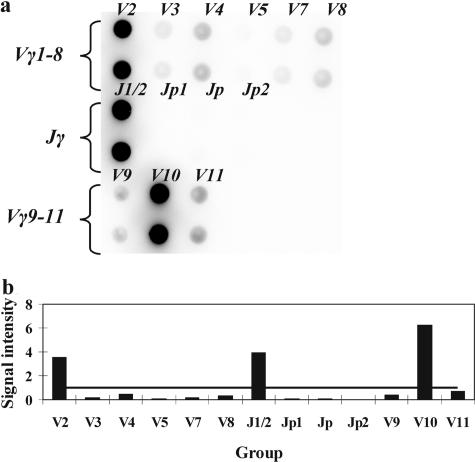
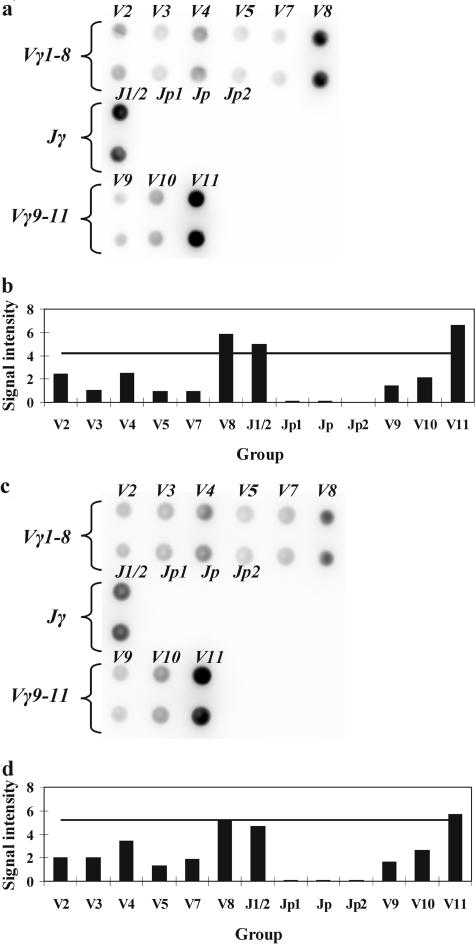
When cells with a particular combination of rearranged V-J genes prevail in a population, amplicons of one type accumulate starting from the first cycles of PCR. The labeling of DNA fragments occurs during second round of PCR parallel to amplification. Hence, it can be expected in the case of mono- or biallelic clonal rearrangement that fluorescent signals from the gel pads corresponding to one or two Vγ genes and one or two Jγ genes will be substantially more intense than signals from the other gel pads. Figure 3 shows the hybridization pattern obtained with DNA of a patient with T-cell lymphoma. Signals from the gel pads corresponding to the Vγ2, Vγ10, and Jγ1/2 gene segments exceed the TS, suggesting biallelic clonal rearrangement of the TCR-γ locus. To confirm the correct recognition of Vγ and Jγ gene segments direct sequencing was performed in several cases.
Figure 3.
a: Hybridization pattern obtained on the microchip with a clonal sample. b: Analysis of the results suggesting a clonal rearrangement of the TCR-γ locus. The TS is shown with a horizontal line. Signals from gel pads Vγ2, Vγ10, and Jγ1/2 exceed the TS and suggest a biallelic clonal rearrangement of the TCR-γ locus with the involvement of the Vγ2, Vγ10, and Jγ1/2 genes.
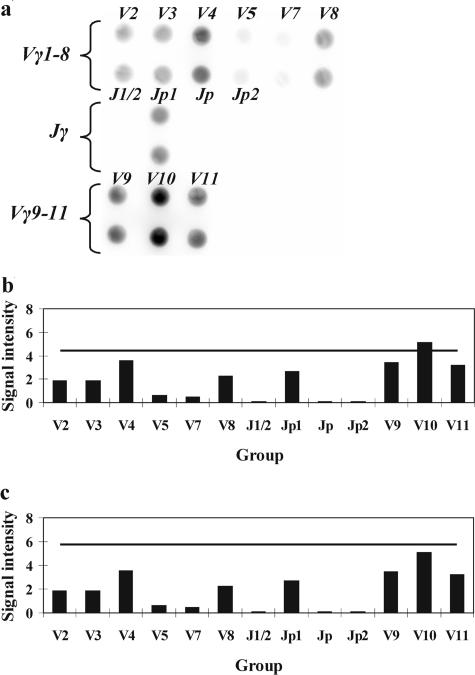
Infectious diseases such as tonsillitis, acute viral infections, and reactive lymphadenopathy may often lead to the appearance of several dominant clones in the population of T cells during normal immune response. This oligoclonality sometimes can be misdiagnosed as monoclonality, indicating lymphoproliferative malignancy. Several specimens from patients with infectious diseases were tested to make sure that the algorithm suggested for the detection of rearrangements allows avoiding false-positive results in such cases. Analysis of the hybridization pattern using as the detection limit the TS equal to <J> + 3 × σ revealed that the Vγ10 gene segment was overrepresented. The increase of the TS up to <J> + 4 × σ allows one to confidently classify the case as polyclonal one (Figure 4).
Figure 4.
a: Hybridization pattern obtained on the microchip with oligoclonal sample. b: Analysis using the threshold signal (TS) equal to <J> + 3 × σ (TS is shown with a horizontal line). c: Analysis using the TS equal to <J> + 4 × σ.
To estimate the sensitivity of our method Jurkat cells (T-cell acute lymphoblastic leukemia, a clone with Vγ8, Vγ11, and Jγ1/Jγ2) were mixed with peripheral blood leukocytes of a healthy donor at various proportions. The portion of clonal cells was 0, 5, 10, 15, 20, 30, 50, and 100%. All mixtures were tested by PCR with subsequent hybridization on microchip. The hybridization patterns for the samples with 10 and 5% of clonal cells are presented in Figure 5. In a sample with 10% of clonal cells (Figure , 5aand 5b), three signals exceeded the TS, which indicates the presence of expected biallelic rearrangement. In a sample with 5% of clonal cells only one residual signal corresponding to Vγ11 was scarcely protruded above the TS (Figure , 5cand 5d). Thus, the lower limit of sensitivity is 10% of total T-cell population. It is worth mentioning that the conclusion in favor of monoclonality can be made only when a particular Vγ-Jγ combination is discovered, not a single prevalent Vγ or Jγ segment. Therefore, the cases presented in Figure 4b and Figure 5d cannot be considered as monoclonal despite the presence of signals above the TS.
Figure 5.
Sensitivity assay. Hybridization pattern (a) and analysis of the results (b) for a sample containing 10% of Jurkat cells. Signals from gel pads Vγ8, Vγ11, and Jγ1/2 exceed the TS and suggest a biallelic clonal rearrangement of the TCR-γ locus with the involvement of the Vγ8, Vγ11, and Jγ1/2 genes. Hybridization pattern (c) and analysis of the results (d) for a sample containing 5% of Jurkat cells. Only signal from gel pad Vγ11 hardly exceeds the TS; there is no sufficient data in favor of clonal rearrangement.
Clinical Screening and Accuracy of Microchip-Based Approach
The microchip-based approach was applied for the analysis of 49 clonal samples (DNAs from patients with T-cell lymphomas or leukemias) and 47 polyclonal samples. Each sample was preliminarily tested for clonality with respect to the TCR-γ locus rearrangement by PCR-based SSCP analysis. The results obtained with clonal samples are summarized in Table 3. The incidence of biallelic rearrangements was found to be 67% using microchip-based approach and 71% using PCR-SSCP analysis. The discrepancy is attributable to the fact that microchip cannot distinguish between mono- and biallelic variants in the cases in which the same Vγ and Jγ gene segments are involved in rearrangements on homologous chromosomes (Table 3, cases 15 and 32).
Table 3.
Characteristics of Patients and the Results of Detecting T-Cell Clonality by PCR-SSCP-TCR-γ and by PCR with Subsequent Hybridization on Microchip
| No. | Age | Material | Diagnosis | PCR-SSCP-TCR-γ | PCR microchip |
|---|---|---|---|---|---|
| 1 | 71 | Blood | LGL | V1-8,V9 | V2,V9, J1/J2 |
| 2 | 35 | Spleen, FF | LGL | V1-8,V1-8 | V2,V8, Jp2 |
| 3 | 58 | Blood | LGL | V1-8,V10 | V8,V10, J1/J2 |
| 4 | 54 | Skin, FF | MF | V9 | V9, Jp2 |
| 5 | 51 | Blood | LGL | V1-8,V1-8 | V3,V5, J1/J2 |
| 6 | 54 | Blood | PTCL | V1-8,V10 | V2,V10, Jp1 |
| 7 | 59 | Blood | PTCL | V1-8,V1-8 | V7,V8, J1/J2 |
| 8 | 45 | Lymph node, FF | PTCL | V1-8,V1-8 | V7,V8, J1/J2 |
| 9 | 74 | Blood | LGL | V1-8,V1-8 | V7,V8, J1/J2 |
| 10 | 27 | Lymph node, FF | TLL | V1-8,V1-8 | V4,V8, J1/J2 |
| 11 | 69 | Blood | PTCL | V1-8,V1-8 | V2,V3, J1/J2 |
| 12 | 57 | Blood | LGL | V1-8,V1-8 | V4,V8, J1/J2 |
| 13 | 54 | Spleen, FF | PTCL | V1-8 | V2, Jp1 |
| 14 | 58 | Blood | TPLL | V1-8 | V2, J1/J2 |
| 15 | 65 | Bone marrow | LGL | V10,V10 | V10, J1/J2 |
| 16 | 21 | Tumor, FF | TLL | V1-8,V10 | V3,V10, J1/J2 |
| 17 | 28 | Pancreas, FF | TLL | V1-8,V10 | V8,V10, J1/J2 |
| 18 | 50 | Blood | SS | V1-8,V10 | V4,V10, Jp1 |
| 19 | 25 | Blood | SS | V1-8,V9 | V5,V9, J1/J2 |
| 20 | 63 | Skin, FF | MF | V10 | V10, J1/J2 |
| 21 | 48 | Blood | SS | V1-8,V9 | V4,V9, J1/J2 |
| 22 | 78 | Blood | LGL | V1-8,V1-8 | V4,V5, J1/J2 |
| 23 | 25 | Lymph node, FF | PTCL | V1-8,V10 | V3,V10, J1/J2 |
| 24 | 41 | Blood | LGL | V9 | V9, J1/J2 |
| 25 | 62 | Blood | PTCL | V1-8,V9 | V4,V9, Jp1 |
| 26 | 53 | Blood | SS | V1-8,V10 | V7,V10, J1/J2 |
| 27 | 57 | Blood | LGL | V1-8 | V8, J1/J2 |
| 28 | 54 | Blood | SS | V9,V11 | V9,V11, Jp1 |
| 29 | 55 | Blood | PTCL | V1-8,V1-8 | V4,V5, J1/J2 |
| 30 | 46 | Blood | PTCL | V1-8 | V3, J1/J2 |
| 31 | 53 | Bone marrow | TLL | V9,V11 | V9,V11, J1/J2 |
| 32 | 60 | Spleen, FF | LGL | V9,V9 | V9, J1/J2 |
| 33 | 53 | Skin, FF | MF | V1-8 | V7, J1/J2 |
| 34 | 61 | Blood | LGL | V1-8,V1-8 | V4,V5, J1/J2 |
| 35 | 59 | Blood | LGL | V1-8,V10 | V2,V10, J1/J2 |
| 36 | 64 | Blood | LGL | V9,V11 | V9,V11, J1/J2 |
| 37 | 63 | Blood | LGL | V1-8,V1-8 | V4,V5, J1/J2 |
| 38 | 30 | Blood | LGL | V10 | V10, J1/J2 |
| 39 | 48 | Blood | TPLL | V1-8,V1-8 | V2,V3, J1/J2 |
| 40 | 73 | Blood | LGL | V1-8 | V2, Jp2 |
| 41 | 57 | Blood | TPLL | V1-8,V1-8 | V2,V8, J1/J2 |
| 42 | 33 | Bone marrow | PTCL | V1-8,V10 | V2, V10, J1/J2 |
| 43 | 63 | Blood | PTCL | V10 | V10, Jp |
| 44 | 60 | Blood | LGL | V1-8 | V8, J1/J2 |
| 45 | 62 | Blood | PTCL | V1-8 | V2, J1/J2 |
| 46 | 44 | Skin, FF | MF | V1-8,V10 | V8,V10, J1/J2 |
| 47 | 50 | Blood | HSL | V9 | V9, J1/J2 |
| 48 | 53 | Blood | SS | V9,V10 | V9,V10, J1/J2 |
| 49 | 36 | Blood | SS | V1-8,V1-8 | V2,V3, J1/J2 |
SSCP, single-strand conformation polymorphism; LGL, large granular lymphocytic leukemia; MF, mycosis fungoides; PTCL, peripheral T-cell lymphoma; TLL, T-lymphoblastic lymphoma; SS, Sezary syndrome; TPLL, T-cell prolymphocytic leukemia; HSL, γδ-hepatosplenic lymphoma; FF, fresh frozen.
The frequencies at which the Vγ genes of families 1, 2, 3, and 4 and the Jγ genes are involved in clonal rearrangements of the TCR-γ locus are presented in Table 4. Among the Vγ gene segments, those of family 1 were most commonly used (total frequency 62%), whereas the lowest frequency (3.5%) was observed for the family 4 gene segment Vγ11. Among family 1 gene segments, the Vγ7 was most rarely involved in rearrangements (6% of the total rearrangements with Vγ gene segments). The other Vγ gene segments were represented at similar frequencies. As for the Jγ gene segments, the Jγ1 and Jγ2 gene segments (which are undistinguishable by our method) are most commonly used in clonal rearrangements: their frequency was 81.6% in the total rearrangements with the Jγ gene segments. All 47 DNA specimens obtained from donors without lymphoproliferative diseases were identified by hybridization on microchip as polyclonal ones (data not shown).
Table 4.
Frequencies of the Vγ Genes of Families 1, 2, 3, and 4 and the Jγ Genes in Clonal Rearrangements of the TCR Locus
| Vγ gene | Number of individual rearrangements with the involvement of this Vγ segment | Frequency |
|---|---|---|
| Vγ2 | 13 | 15.5% |
| Vγ3 | 7 | 8.3% |
| Vγ4 | 9 | 10.7% |
| Vγ5 | 6 | 7.1% |
| Vγ7 | 5 | 6% |
| Vγ8 | 12 | 14.3% |
| All Vγ1-8 genes (family 1) | ΣVγ1-8 = 52 | 62% |
| Vγ9 (family 2) | 13 | 15.5% |
| Vγ10 (family 3) | 16 | 19% |
| Vγ11 (family 4) | 3 | 3.5% |
| Total | Σ = 84 | 100% |
| Jγ gene | Number of individual rearrangements with the involvement of this Jγ segment | |
| Jγ1/Jγ2 | 40 | 81.6% |
| Jγp1 | 5 | 10.2% |
| Jγp | 1 | 2.1% |
| Jγp2 | 3 | 6.1% |
| Total | Σ = 49 | 100% |
Discussion
The unique structure of the TCR genes, which undergo a variety of rearrangements to ensure high polymorphism of TCRs makes it possible to measure T-cell clonality by detecting identical DNA fragments in an abundant and diverse pool.3,21 The rearranged TCR genes are characterized by joining and combinatorial diversities, and T-cell clonality assays take advantage of these properties. Joining diversity is related to the fact that every two rearranged loci, even those with the same V, D, and J gene segments, differ in the size of the complementarity-determining region 3 (CDR3), which is most polymorphic and is unique in each lymphocyte.22,23 Consequently, the PCR products amplified from a polyclonal population differ in size. When cells with the same rearrangement of the TCR genes prevail in a population, PCR yields one major product of a particular size.24 The TCR-γ locus lacks the D gene segment, whereas the CDR3 region joined the Vγ and Jγ gene segments is relatively low-polymorphic: the size variation is attributable to the addition or deletion of a number of nucleotides (N region) and does not exceed 20 bp; moreover, 95% of the PCR products differ in size by 5 bp. Hence, clonality assays in this case use high-resolution electrophoretic methods, such as SSCP analysis, denaturing gradient electrophoresis, and fragment analysis.7,8,9,10,11,25,26 Although quite reliable in detecting a dominant clone, these methods still yield false-positive results.
Combinatorial diversity is related to the fact that the V and J gene segments are used at random in all lymphocytes. Our method takes advantage of combinatorial diversity and makes it possible to identify the Vγ and Jγ genes involved in a particular rearrangement. The microchip contains nine oligonucleotide probes for the nine Vγ gene segments (all possible Vγ gene segments involved in rearrangements) and four probes for the five Jγ gene segments (a common probe is used for the Jγ1 and Jγ2 gene segments). Thus, the microchip distinguishes 36 variants of TCR-γ rearrangements, almost completely covering their combinatorial polymorphism.
The number of PCR cycles was selected so that the reaction did not reach the plateau. The hybridization efficiency of different oligonucleotide probes is similar as seen by hybridization patterns of polyclonal samples (Figure 2). All monoclonal samples revealed distinct peaks over the threshold line corresponding to Vγ and Jγ gene segments involved in rearrangement (Figure 3). The correct recognition of Vγ and Jγ gene segments was confirmed by direct sequencing of amplified fragments. Among polyclonal samples, the oligoclonal cases were found related to patients with infectious diseases (Figure 4). All these variants of hybridization images have full correspondence with electrophoretic patterns revealed by SSCP analysis. Thus, we can assume that the fluorescence intensity of gel pads depended on the prevalence of particular Vγ and Jγ gene segments in the lymphocyte population under study. At the same time, the reaction is not definitely quantitative, but rather semiquantitative. First, two rounds of PCR are used. Second, three independent forward primers participate in the reaction, so the efficiency of each individual reaction in the multiplex mixture may slightly differ. Because of these factors, it is not possible to quantify the amplified fragments directly by measuring the fluorescence intensities of hybridization signals. That was the reason why the microchip-based approach could not recognize the biallelic rearrangements Vγ10 and Vγ9 in cases 15 and 32, respectively (Table 3).
To estimate the accuracy of the method, we examined 49 samples of patients with lymphoproliferative disorders and 47 samples of normal donors. The results of hybridization on the microchip displayed 100% coincidence with the results obtained by other methods. The sensitivity of hybridization on the microchip, that is, the minimal portion of clonal cells that is sufficient for detecting clonality, was estimated to be ∼10%. Such sensitivity is standard for PCR-based detection of clonality. Greater sensitivity could potentially be associated with a higher rate of false-positive results. This is caused by the fact that some T-cell clones increase substantially during the normal immune response to viral infections or autoimmine diseases and are identified as dominant by amplification of the rearranged genes. For example, oligoclonality in infectious mononucleosis may be misdiagnosed as monoclonality.27,28
Our data on the prevalence of various Vγ and Jγ gene segments in malignant clones make it possible to estimate the frequencies at which the Vγ and Jγ gene segments are normally involved in rearrangements in the T-cell population. The γ-chain undergoes rearrangement before the α- and β-chains and, consequently, is rearranged in all lymphocytes regardless of whether they express the γ/δ or α/β chains. In further development, productive rearrangements of the α/β chains abolish the expression of the γ-chain. Expression of the γ-chain is preserved by only 1 to 5% of all T cells.4,29 Thus, the γ-chain is not subjected to positive (recognition of the cognate MHC) and negative (recognition of autoantigens) selection in the thymus during T-cell maturation.30,31,32 There is limited data regarding the distribution of gene segments in rearrangements of the TCR-γ chain gene. The study by Theodorou and colleagues6 demonstrated that most variable (Vγ) region rearrangements occur within the Vγ1-8 family (78%) and most joining region (Jγ) rearrangements involve the Jγ1/2 segment (64%). Recently, Lawnicki et al33 has showed a lower incidence of Vγ1-8 rearrangements (56%) and a higher incidence of Vγ9 (23%) and Vγ10 (17%). Our data are closer to this latter study giving the following rates for Vγ segment rearrangements: 62% for Vγ1-8, 15.5% for Vγ9, and 19% for Vγ10. The occurrence for Vγ11 rearrangements (3.5%) was found to be practically the same as in the study by Lawnicki et al33 (4%). Our Jγ gene segment distribution data are similar to the previous studies. Using hybridization with microchip, we can also detect the particular Vγ region of Vγ1-8 family. According to this study, rearrangements involving Vγ7 gene segment are least abundant. At the same time, the microchip-based approach cannot distinguish between bi- and monoallelic rearrangements in the cases when the same combination of Vγ and Jγ has been presented in both homologous chromosomes.
In clinical screening, we used mainly fresh frozen tissues, fresh blood, or bone marrow samples. The approach may be suitable for paraffin-embedded blocks (PEBs). The main problem of using PEBs in PCR-based diagnostics is poor DNA quality and short size of isolated DNA fragments. Widely used PCR protocols for the analysis of PEB DNA yield amplicons from 150 to 250 to 300 bp in size.8,9,10,25,26 The range of the first-round PCR product in our method varies from 290 to 450 bp. Recently, a set of control gene primers designed to amplify amplicons of 100, 200, 300, 400, and 600 bp was suggested for the assessment of the integrity and amplifiability of DNA isolated from PEBs.21 Therefore, the estimation of DNA amplifiability can be recommended when DNA from PEBs is used as a matrice in microchip-based approach.
To summarize, we developed a new method for detecting T-cell clonality with the use of microchips. Our method targets the TCR-γ locus and takes advantage of its combinatorial diversity. The virtues of the method are that the results are demonstrative and that the time of analysis is substantially reduced. Preliminary study shows that our method allows differential diagnosis of reactive and malignant T-lymphoproliferative diseases.
Acknowledgments
We thank S.V. Pan’kov for the manufacturing of biochips, A.V. Chudinov for the synthesis of fluorescent dyes, and D.V. Prokopenko and R.A. Yurasov for help in data processing.
Footnotes
Supported by the Basic Foundation for Russian Science (project no. 06-04-49771) and the United States Department of Energy (Initiatives for Proliferation Prevention grant assistance program, project no. RUC2-11036-MO-04).
References
- Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. Lyon: IARC Press; Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. World Health Organization Classification of Tumours. 2001 [Google Scholar]
- Macintyre EA, Delabesse E. Molecular approaches to the diagnosis and evaluation of lymphoid malignancies. Semin Hematol. 1999;36:373–389. [PubMed] [Google Scholar]
- Spagnolo DV, Ellis DW, Juneja S, Leong AS, Miliauskas J, Norris DL, Turner J. The role of molecular studies in lymphoma diagnosis: a review. Pathology. 2004;36:19–44. doi: 10.1080/00313020310001648404. [DOI] [PubMed] [Google Scholar]
- Alexandre D, Lefranc MP. The human gamma/delta + and alpha/beta + T cells: a branched pathway of differentiation. Mol Immunol. 1992;29:447–451. doi: 10.1016/0161-5890(92)90001-e. [DOI] [PubMed] [Google Scholar]
- Ponti R, Quaglino P, Novelli M, Fierro MT, Comessatti A, Peroni A, Bonello L, Bernengo MG. T-cell receptor gamma gene rearrangement by multiplex polymerase chain reaction/heteroduplex analysis in patients with cutaneous T-cell lymphoma (mycosis fungoides/Sezary syndrome) and benign inflammatory disease: correlation with clinical, histological and immunophenotypical findings. Br J Dermatol. 2005;153:565–573. doi: 10.1111/j.1365-2133.2005.06649.x. [DOI] [PubMed] [Google Scholar]
- Theodorou I, Raphael M, Bigorgne C, Fourcade C, Lahet C, Cochet G, Lefranc MP, Gaulard P, Farcet JP. Recombination pattern of the TCR gamma locus in human peripheral T-cell lymphomas. J Pathol. 1994;174:233–242. doi: 10.1002/path.1711740402. [DOI] [PubMed] [Google Scholar]
- Trainor KJ, Brisco MJ, Wan JH, Neoh S, Grist S, Morley AA. Gene rearrangement in B- and T-lymphoproliferative disease detected by the polymerase chain reaction. Blood. 1991;78:192–196. [PubMed] [Google Scholar]
- Sidorova IV, Nikitin EA, Peklo M, Vlasik TN, Samoilova RS, Kravchenko SK, Melikian AL, Vinogradova IE, Pivnik AV, Sudarikov AB. Use of polymerase chain reaction for determining T-cell clonality. Ter Arkh. 2003;75:48–52. [PubMed] [Google Scholar]
- Dictor M, Warenholt J, Isinger A. Resolving T-cell receptor clonality in two and genotype in four multiplex polymerase chain reactions. Haematologica. 2005;90:1524–1532. [PubMed] [Google Scholar]
- Assaf C, Hummel M, Steinhoff M, Geilen CC, Orawa H, Stein H, Orfanos CE. Early TCR-beta and TCR-gamma PCR detection of T-cell clonality indicates minimal tumor disease in lymph nodes of cutaneous T-cell lymphoma: diagnostic and prognostic implications. Blood. 2005;105:503–510. doi: 10.1182/blood-2004-06-2220. [DOI] [PubMed] [Google Scholar]
- Droese J, Langerak AW, Groenen PJ, Bruggemann M, Neumann P, Wolvers-Tettero IL, van Altena MC, Kneba M, van Dongen JJ. Validation of BIOMED-2 multiplex PCR tubes for detection of TCRB gene rearrangements in T-cell malignancies. Leukemia. 2004;18:1531–1538. doi: 10.1038/sj.leu.2403428. [DOI] [PubMed] [Google Scholar]
- Munro NJ, Snow K, Kant JA, Landers JP. Molecular diagnostics on microfabricated electrophoretic devices: from slab gel- to capillary- to microchip-based assays for T- and B-cell lymphoproliferative disorders. Clin Chem. 1999;45:1906–1917. [PubMed] [Google Scholar]
- Yang XY, Xu D, Du J, Kamino H, Rakeman J, Ratech H. Rapid detection of clonal T-cell receptor-beta gene rearrangements in T-cell lymphomas using the LightCycler-polymerase chain reaction with DNA melting curve analysis. J Mol Diagn. 2005;7:81–88. doi: 10.1016/s1525-1578(10)60012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutzmer R, Mommert S, Kiehl P, Wittmann M, Kapp A, Werfel T. Detection of clonal T cell receptor gamma gene rearrangements in cutaneous T cell lymphoma by LightCycler-polymerase chain reaction. J Invest Dermatol. 2001;116:926–932. doi: 10.1046/j.1523-1747.2001.01344.x. [DOI] [PubMed] [Google Scholar]
- Nasedkina T, Domer P, Zharinov V, Hoberg J, Lysov Y, Mirzabekov A. Identification of chromosomal translocations in leukemias by hybridization with oligonucleotide microarrays. Haematologica. 2002;87:363–372. [PubMed] [Google Scholar]
- Nasedkina TV, Zharinov VS, Isaeva EA, Mityaeva ON, Yurasov RN, Surzhikov SA, Turigin AY, Rubina AY, Karachunskii AI, Gartenhaus RB, Mirzabekov AD. Clinical screening of gene rearrangements in childhood leukemia by using a multiplex polymerase chain reaction-microarray approach. Clin Cancer Res. 2003;9:5620–5629. [PubMed] [Google Scholar]
- Banér J, Marits P, Nilsson M, Winqvist O, Landegren U. Analysis of T-cell receptor V beta gene repertoires after immune stimulation and in malignancy by use of padlock probes and microarrays. Clin Chem. 2005;51:768–775. doi: 10.1373/clinchem.2004.047266. [DOI] [PubMed] [Google Scholar]
- Rubina AY, Pan’kov SV, Dementieva EI, Pen’kov DN, Butygin AV, Vasiliskov VA, Chudinov AV, Mikheikin AL, Mikhailovich VM, Mirzabekov AD. Hydrogel drop microchips with immobilized DNA: properties and methods for large-scale production. Anal Biochem. 2004;325:92–106. doi: 10.1016/j.ab.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Greiner TC, Raffeld M, Lutz C, Dick F, Jaffe ES. Analysis of T-cell receptor-gamma gene rearrangements by denaturing gradient gel electrophoresis of GC-clamped polymerase chain reaction products: correlation with tumor-specific sequences. Am J Pathol. 1995;146:46–55. [PMC free article] [PubMed] [Google Scholar]
- Nasedkina TV, Fedorova OE, Glotov AS, Chupova NV, Samochatova EV, Maiorova OA, Zemlyakova VV, Roudneva AE, Chudinov AV, Yurasov RA, Kozhekbaeva JM, Barsky VE, Krynetskiy EY, Krynetskaia NF, Cheng C, Ribeiro RC, Evans WE, Roumyantsev AG, Zasedatelev AS. Rapid genotyping of common deficient thiopurine S-methyltransferase alleles using the DNA-microchip technique. Eur J Hum Genet. 2006;14:991–998. doi: 10.1038/sj.ejhg.5201647. [DOI] [PubMed] [Google Scholar]
- Hodges E, Williams AP, Harris S, Smith JL. T-cell receptor molecular diagnosis of T-cell lymphoma. Methods Mol Med. 2005;115:197–215. doi: 10.1385/1-59259-936-2:197. [DOI] [PubMed] [Google Scholar]
- Hughes MM, Yassai M, Sedy JR, Wehrly TD, Huang CY, Kanagawa O, Gorski J, Sleckman BP. T cell receptor CDR3 loop length repertoire is determined primarily by features of the V(D)J recombination reaction. Eur J Immunol. 2003;33:1568–1575. doi: 10.1002/eji.200323961. [DOI] [PubMed] [Google Scholar]
- Rock EP, Sibbald PR, Davis MM, Chien YH. CDR3 length in antigen-specific immune receptors. J Exp Med. 1994;179:323–328. doi: 10.1084/jem.179.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawalkar N, Ferenczi K, Jones DA, Yamanaka K, Suh KY, Sadat S, Kupper TS. Profound loss of T-cell receptor repertoire complexity in cutaneous T-cell lymphoma. Blood. 2003;102:4059–4066. doi: 10.1182/blood-2003-04-1044. [DOI] [PubMed] [Google Scholar]
- Theodorou I, Bigorgne C, Delfau MH, Lahet C, Cochet G, Vidaud M, Raphael M, Gaulard P, Farcet JP. VJ rearrangements of the TCR gamma locus in peripheral T-cell lymphomas: analysis by polymerase chain reaction and denaturing gradient gel electrophoresis. J Pathol. 1996;178:303–310. doi: 10.1002/(SICI)1096-9896(199603)178:3<303::AID-PATH475>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Scheller U, Muche JM, Sterry W, Lukowsky A. Detection of clonal T cells in cutaneous T cell lymphoma by polymerase chain reaction: comparison of mutation detection enhancement-polyacrylamide gel electrophoresis, temperature gradient gel electrophoresis and fragment analysis of sequencing gels. Electrophoresis. 1998;19:653–658. doi: 10.1002/elps.1150190507. [DOI] [PubMed] [Google Scholar]
- Malik UR, Oleksowicz L, Dutcher JP, Ratech H, Borowitz MJ, Wiernik PH. Atypical clonal T-cell proliferation in infectious mononucleosis. Med Oncol. 1996;13:207–213. doi: 10.1007/BF02990933. [DOI] [PubMed] [Google Scholar]
- Maini MK, Casorati G, Dellabona P, Wack A, Beverley PC. T-cell clonality in immune responses. Immunol Today. 1999;20:262–266. doi: 10.1016/s0167-5699(99)01472-3. [DOI] [PubMed] [Google Scholar]
- Blom B, Verschuren MC, Heemskerk MH, Bakker AQ, van Gastel-Mol EJ, Wolvers-Tettero IL, van Dongen JJ, Spits H. TCR gene rearrangements and expression of the pre-T cell receptor complex during human T-cell differentiation. Blood. 1999;93:3033–3043. [PubMed] [Google Scholar]
- McVay LD, Carding SR. Generation of human gammadelta T-cell repertoires. Crit Rev Immunol. 1999;19:431–460. [PubMed] [Google Scholar]
- Shin S, El-Diwany R, Schaffert S, Adams EJ, Garcia KC, Pereira P, Chien YH. Antigen recognition determinants of gammadelta T cell receptors. Science. 2005;308:252–255. doi: 10.1126/science.1106480. [DOI] [PubMed] [Google Scholar]
- Nishimura MI, Roszkowski JJ, Moore TV, Brasic N, McKee MD, Clay TM. Antigen recognition and T-cell biology. Cancer Treat Res. 2005;123:37–59. doi: 10.1007/0-387-27545-2_2. [DOI] [PubMed] [Google Scholar]
- Lawnicki LC, Rubocki RJ, Chan WC, Lytle DM, Greiner TC. The distribution of gene segments in T-cell receptor gamma gene rearrangements demonstrates the need for multiple primer sets. J Mol Diagn. 2003;5:82–87. doi: 10.1016/s1525-1578(10)60456-4. [DOI] [PMC free article] [PubMed] [Google Scholar]