Abstract
Lewy body (LB) inclusions are one of the pathological hallmarks of Parkinson’s disease (PD) and dementia with Lewy bodies (DLB). One way to better understand the process leading to LB formation and associated pathogenesis responsible for neurodegeneration in PD and DLB is to examine the content of LB inclusions. Here, we performed a proteomic investigation of cortical LBs, obtained by laser capture microdissection from neurons in the temporal cortex of dementia patients with cortical LB disease. Analysis of over 2500 cortical LBs discovered 296 proteins; of those, 17 had been associated previously with brainstem and/or cortical LBs. We validated several proteins with immunohistochemical staining followed by confocal microscopy. The results demonstrated that heat shock cognate 71 kDa protein (also known as HSC70, HSP73, or HSPA10) was indeed not only colocalized with the majority of LBs in the temporal cortex but also colocalized to LBs in the frontal cortex of patients with diffuse LB disease. Our investigation represents the first extensive proteomic investigation of cortical LBs, and it is expected that characterization of the proteins in the cortical LBs may reveal novel mechanisms by which LB forms and pathways leading to neurodegeneration in DLB and/or advanced PD. Further investigation of these novel candidates is also necessary to ensure that the potential proteins in cortical LBs are not identified incorrectly because of incomplete current human protein database.
INTRODUCTION
Lewy body (LB) inclusions are the pathologic hallmark of Parkinson’s disease (PD) and dementia with Lewy bodies (DLB) (37). Spread of LB from the brainstem to the mesocortex and eventually to the isocortex in the human brain appears to correlate with PD progression and with the development of non‐motor symptoms such as dementia and psychosis in advanced stages (10, 11, 37). PD dementia, where cortical LBs are typically found, overlaps with DLB both clinically and pathologically, and one school of thought is that these two entities are the same disease with different clinical spectra of LB diseases (33, 38, 39, 47).
One way to better understand the process leading to LB formation and the pathogenesis of LB diseases is to examine the content of LB inclusions. In fact, it was the identification of α‐synuclein (SNCA) in LBs in the substantia nigra (SN) of both sporadic and familial PD patients that established its fundamental role in PD pathogenesis (see (46) for more detailed discussion). Furthermore, Braak’s seminal article, reporting a system to stage PD, is based on the distribution of SNCA in different brain regions (11). Finally, SNCA is also the key protein that links seemly distinctively different diseases, for example, PD, DLB, multiple system atrophy, and pure autonomic failure, together under the common name of synucleinopathy (37). Thus, one would expect that a better characterization of the content of LB might shed more light on the mechanisms leading to the development of LB and to neurodegeneration in LB diseases. Indeed, extensive effort has been spent in this regard and several proteins have been identified in association with either brainstem or cortical LBs in the last few decades (37, 55). Notably, however, the majority of these proteins, for example, SNCA and ubiquitin carboxyl terminal hydrolase L1 (UCH‐L1), have been discovered through the genetics of PD and related disorders or through immunohistochemical staining of LBs in brain tissue based on the investigator’s particular hypothesis.
In contrast to the targeted and hypothesis‐driven type approach mentioned above, it has become increasingly clear that the discovery process can be greatly enhanced with a recently developed robust technique called proteomics—unbiased and extensive analysis of proteins in any given cell, organelle, or body fluid (4). In fact, we, as well as others, have recently utilized this technology to investigate novel proteins associated with SNCA in dopaminergic cells (28, 55) as well as insoluble proteins (54), plaques (34) and neurofibrillary tangles (52) associated with Alzheimer’s disease. Here, we have extended our pursuit to gain in‐depth characterization of proteins associated with LBs in human tissue. Given that only limited numbers of LBs can be found in the brainstem of patients with LB diseases, we performed our initial proteomic analysis with cortical LBs obtained by laser capture, revealing close to 300 unique proteins. We then used immunohistochemistry to confirm our finding that one of these proteins, HSC70, was indeed a novel component of cortical LBs. It is anticipated that further characterization of other candidate proteins discovered through this proteomic analysis could lead to an improved understanding of the pathogenesis of PD, DLB and associated synucleinopathy.
MATERIALS AND METHODS
Case selection for laser capture microdissection (LCM).
Six cases of DLB with cortical LBs were identified from the University of Washington Alzheimer’s Disease Research Center brain bank. Mean age at death was 74.8 with a mean postmortem interval of 7 h and a male: female gender ratio of 5:1. All six cases had a clinical history of dementia and had LBs in the brainstem, limbic system, and neocortex confirmed by SNCA immunohistochemistry. All six cases had frozen tissue available for LB capture. Frozen temporal cortex was used for LB identification and capture in all cases. This region had a high frequency of LBs observed in all cases.
LCM.
Frozen tissue (10 µm each) containing the temporal cortex were cut using a Leica CM 3050 cryostat and mounted on PALM Membrane Slides (Microlaser Technologies AG, Bernried, Germany). Sections were then fixed in cold acetone for 10 minutes, permeabilized in 0.1% Triton X‐100 for 10 minutes, and immunostained with a well‐characterized antibody to SNCA (syn 303, generous gift of J. Trojanowski and V. Lee at the University of Pennsylvania) after blocking with 3% H2O2 and 5% nonfat milk at room temperature for 45 minutes. The biotinylated secondary antibody was detected by avidin‐biotin complex (ABC kit, Vector Laboratories, Burlingame, CA, USA used at 1:100) and visualized with diaminobenzidine (DAB; Sigma, St Louis, MO, USA) and after counterstained with hematoxylin. Sections were counterstained with hematoxylin (Sigma) and dehydrated in a series of graded ethanol and stored at –80°C until used. This procedure is very similar to the method recently described by us to capture immunostained neurofibrillary tangles (52), which is also essentially identical to one used by others to capture immunostained frozen sections for mRNA analysis (18).
Laser capture microdissection (LCM) was performed with a method identical to that used to capture neurofibrillary tangles in a previous study (52). Briefly, frozen tissue sections were thawed at room temperature for 40 s and SNCA‐immunoreactive structures with the size and shape of LBs were subjected to LCM using PALM Microbeam technology under ×40 magnification. For each of five experiments, approximately 500 LBs were collected from six different autopsies, for a total of approximately 2500 LBs that were pooled for proteomic analysis. This technique, called laser pressure catapulting, uses a highly focused laser microbeam to eject tissue into the cap rather than to melt tissue with the membrane mounted to a cap via laser activation. There is no tissue contact and no heat involved in this procedure, and hence it provides optimal isolation of tissue for RNA or protein analysis (19).
Proteomic analysis using LC/MS‐MS.
LBs were extracted with 70% formic acid and sonicated gently for 1 minute. After vacuum centrifugation, samples were transferred to a NH4HCO3 buffer and reduced with iodoacetamide before being subjected to trypsin digestion overnight at 37°C as described previously (52, 55). The amount of protein in samples and completeness of digestion were estimated by silver staining aliquots of LB proteins electrophoresed in Tris‐SDS gels and compared with BSA standards prepared in parallel. Digested peptides were purified with a C18 solid phase extraction column (OasisR MCX, Milford, MT, USA), and separated by a two dimensional microcapillary high performance LC system, which integrates a strong cation‐exchange (SCX) column with two alternating reversed‐phase C18 columns (10 cm × 180 µm), followed by analysis with tandem MS (ThermoFinnigan, San Jose, CA). This technique is also known as multi‐dimensional protein identification technology or MudPIT (35). Settings for the LC‐MS/MS were as follows: 4 fractions were eluted from SCX using a binary gradient of 2%–90% solvent D (1.0 M ammonium chloride and 0.1% formic acid in 5% acetonitrile) vs. solvent C (0.1% formic acid in 5% acetonitrile). Each fraction was injected onto a reverse‐phase column automatically with the peptides being resolved using a 200 minutes binary gradient of 5%–80% solvent B (acetonitrile and 0.1% formic acid) vs. solvent A (0.1% formic acid in water). A flow rate of 160 µl/minute was used. To determine the amino acid sequence, the mass spectrometer operated in a data‐dependent MS/MS mode (a full scan mass spectrum is followed by a tandem MS), where the precursor ion was selected “on the fly” from the previous scan. An m/z ratio for an ion that had been selected for fragmentation was placed in a list and dynamically excluded for 3 minutes for further fragmentation. Proteins from the mixture were later identified automatically using the computer program SEQUEST, which searched tandem mass spectra against the International Protein Database Human v3.01. Next, the sensitivity and specificity of protein identification were determined by PeptideProphet and ProteinProphet software. ProteinProphet allows filtering of large‐scale data sets with assessment of predictable sensitivity and false‐positive identification error rates. In our study, only proteins with a high probability of accuracy (<5% error rate) were selected. All of these methods, freely accessible at the website of the Institute of Systems of Biology (http://www.systemsbiology.org/Default.aspx?pagename_proteomicssoftware), are used routinely in our lab (27, 29, 55, 56).
Triple immunofluorescent staining and confocal analysis.
This study was performed using a separate set of cases with clinical dementia and cortical LBs (3 men and 4 women with a mean age of 65 years and postmortem interval of about 8 h). Temporal cortex, frontal cortex and midbrain tissue, fixed in formalin and embedded in paraffin, were cut at 5 microns, and sections were mounted on glass slides. Sections were deparaffinized in xylene and rehydrated in graded ethanol. After microwave antigen retrieval (boiling in 0.01 M sodium citrate; pH 6.0 for 15 minutes), the slides were cooled to room temperature and rinsed in PBS. The sections were incubated in blocking solution (5% normal goat serum, 2% bovine serum albumin, and 0.1% Triton X‐100 in PBS) for 30 minutes and then incubated overnight at 4°C with primary antibodies diluted in blocking solution. The primary antibodies were SNCA (sheep anti‐SNCA polyclonal antibody, 1:500, Chemicon, Temecula, CA, USA), FASCN1 Ab‐1 (mouse anti‐fascin monoclonal antibody, 1:200, Lab Vision, Fremont, CA, USA), Connexin‐43 (rabbit anti‐connexin, 1:500, Sigma) or HSC70 (rat anti‐HSC70 monoclonal antibody, 1:200 Stressgen, Victoria, BC, Canada). Next, the sections were rinsed with PBS and incubated with secondary antibody for 2 h (Flex Fluor® 568 goat anti‐rat IgG, 1:200, Flex Fluor® 488 goat anti‐rabbit IgG, 1:200, Flex Fluor® 488 goat anti‐mouse IgG, 1:200, Flex Fluor® 568 goat anti‐sheep IgG, 1:200 and Flex Fluor® 488 goat anti‐sheep IgG, 1:200; Invitrogen Molecular Probes, Eugene, OR, USA). After rinsing, the slides were treated with 0.1% Sudan Black for 30 minutes to block auto‐fluorescence. After brief destaining in 75% ethanol and rinsing, the sections were counterstained with DAPI (Sigma) and TO‐PRO‐3 iodide (1:500) to label DNA with blue fluorescence. Images were captured using a laser scanning confocal microscope (Bio‐rad LS2000, Hercules, CA, USA). Differential interference contrast and fluorescent images were captured simultaneously using the 488 nm line from an Argon laser at 1.1% laser strength, via a 505 LP filter routed through a HFT 488 beam splitter. A Zeiss C‐Apochromat 40 × N.A. = 1.2 water immersion objective was used with a pinhole set at ∼1.4 Airy units.
RESULTS
Proteins associated with cortical Lewy bodies in patients with DLB.
Protein identification was achieved by searching MS data with SEQUEST against the International Protein Index database, followed by probability calculation using PeptideProphet and ProteinProphet. Combining the results from online MuDPIT analysis of five samples, a total of 296 proteins were identified in the LBs captured by LCM. Of these proteins, 140 were single hits whose identification should be considered provisional. This is because proteins identified by a single peptide are less likely to be replicated than those identified by multiple peptides (13). Nonetheless, all proteins are listed in the Appendices with the proteins identified by more than two peptides and single peptide separated into Appendix S1 and Appendix S2 (see Supplementary material), respectively. Functional classification of all 296 proteins is shown in Figure 1, where proteins are separated into the following categories: cytoskeleton, extracellular matrix, inflammation/immune, metabolism, neurotransmission, protein synthesis/degradation, signaling/apoptosis and unknown functions.
Figure 1.
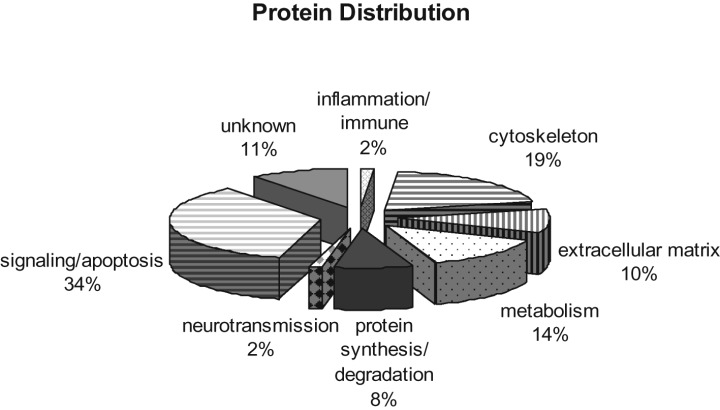
Classification of proteins identified in cortical Lewy bodies. Pie chart depicting the 296 proteins characterized by LC‐MS/MS. Functional classification of a given protein was based on the one that is best known, although typically, multiple functions may have been associated with that particular protein. Notably, a significant portion of the proteome is novel without known functions.
Among the 156 proteins identified by more than two peptides (Part A of Appendix S1), 17 have been previously reported to be associated with brainstem or cortical LBs; these include: 14‐3‐3, α‐crystallin β, α‐internexin, amyloid β A4, dynein, gelsolin, glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH), HSC70, microtubule‐associated protein (MAP; isoforms 1B, 2 and tau), neurofilament, SNCA, synaptotagmin, tubulin, ubiquitin‐activating enzyme E1, and UCH‐L1. A list of these proteins can also be found in Table 1 along with the associated references.
Table 1.
Proteins identified by proteomics that are also reported to be in Lewy bodies (LBs). Abbreviations: C = Cortical LBs; B = Brainstem LBs.
| Protein list | Location | References | |
|---|---|---|---|
| 14‐3‐3 protein | C | B | (30, 32) |
| α‐Crystallin β | B | (42) | |
| α‐Internexin | B | (12) | |
| α‐Synuclein | C | B | (49) |
| Amyloid β A4 | C | B | (31, 53) |
| Dynein | B | (22) | |
| Gelsolin | C | B | (23) |
| Glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) | B | (48) | |
| HSC70 | B | (51) | |
| Microtubule‐associated protein (MAP) isoform 1B | C | (26) | |
| Microtubule‐associated protein (MAP) isoform 2 | B | (16) | |
| Microtubule‐associated protein (MAP) isoform tau | B | (7) | |
| Neurofilament | C | B | (20, 24) |
| Synaptotagmin | B | (25) | |
| Tubulin | C | B | (6, 41) |
| Ubiquitin‐activating enzyme E1 | C | (41) | |
| Ubiquitin carboxyl‐terminal hydrolase isozyme L1 (UCH‐L1) | C | (36) | |
Validation of candidate proteins colocalizing to Lewy bodies.
It has become increasingly clear that not all proteins identified by proteomics are accurately identified, primarily due to the incomplete human protein database. In fact, our previous experience has indicated that a slight alteration in database could result in major changes in the proteins identified (1). In other words, proteins identified by proteomics need confirmation or validation with alternative means. Confirmation and validation of proteins identified by proteomic analysis of the specimen obtained by LCM is even more important, because contamination of proteins during LCM can happen. That being said, it is obviously not practical to validate all the proteins listed in Appendix S1. Consequently, we have used the following criteria in selecting the candidates for further validation; these include: (i) the identification of a protein is based on multiple peptides; (ii) there is an antibody available commercially to the protein of interest; and (iii) the candidate is involved in a process that might be important in LB disease, even though its relationship to cortical LBs is not known. With these caveats in mind, we selected connexin 43, FSCN1, and heat shock cognate 71 kDa (synonym: HSC70, HSP73, HSPA10; http://www.expasy.org/uniprot/P11142) for initial immunohistochemical validation. It should be noted that HSC70 has been recently reported as one of several heat‐shock proteins present in the SN LB of PD patients (51).
Of these three proteins, only heat shock cognate 71 kDa or HSC70 was selectively localized to cortical LBs; this is not to say that the other two proteins were not localized to LBs but rather that these were diffusely present throughout gray matter, and so they cannot be clearly interpreted as coming from laser captured LBs. It should be noted that pretreatment of tissue sections with proteinase K did not appear to improve the results. Figure 2 (upper panel) shows the triple staining results of SNCA, HSC70, and DAPI in the cortical brain tissue. While the manuscript was being prepared, a new report documented that HSC70 is colocalized to brainstem LB as determined by immunohistochemical analysis (51); thus, we repeated our triple staining in the brainstem sections, demonstrating that HSC70 was indeed a component of brainstem LB (lower panel of Figure 2). It should be noted that, although all seven cases demonstrated colocalization of the two proteins in both cortical (temporal‐cortex and isocortex) and brainstem regions, not all inclusions positive for SNCA were also positive for HSC70 (and vice versa). Furthermore, when colocalization occurred, the overlap was complete only in the cortical LBs. More specifically, in cortical LBs, we observed equal diffuse staining with both antibodies, whereas the halo part of the SN LBs was more heavily stained by the SNCA antibody (Figure 2), which is slightly different from those reported by Uryu et al (51).
Figure 2.
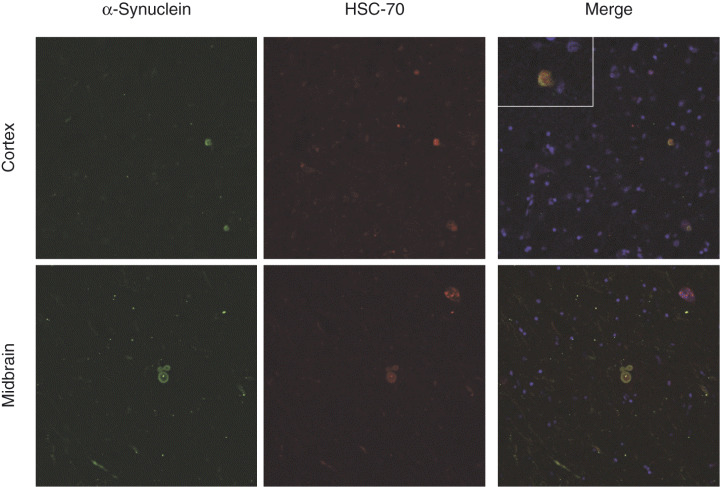
Confocal analysis of the relationship between SNCA and HSC70. Upper panel shows the triple staining results of SNCA, HSC70 and DAPI in the cortical brain sections of dementia with Lewy body (DLB) patients. Sections were stained with antibodies against SNCA and HSC70 simultaneously, along with nuclei staining (DAPI), after microwave antigen retrieval. The localization of each antibody was revealed by secondary antibodies conjugated with fluorescence probe for SNCA (green) and HSC70 (red), respectively. Yellow color in the merge pane indicates colocalization of the two antibodies. An enlarged view of a LB is shown in the inset of merged column. Lower panel shows the triple staining results of SNCA, HSC70 and DAPI in the substantia nigra of DLB patients. The experimental conditions were identical to those described for cortical sections.
DISCUSSION
In the present study, a comprehensive proteomic investigation was performed to analyze laser captured temporal cortex LBs. This analysis identified close to 300 proteins that might be components of cortical LBs. One of these proteins, HSC70, was indeed found to be colocalized to LBs by immunohistochemical staining not only in the temporal cortex but also in other neocortical regions.
Among the 156 candidate proteins identified by more than two peptides, only 17 have been reported previously to be associated with LBs. Furthermore, it needs to be stressed that some of the reported associations (eg, α‐crystallin β and α‐internexin) are only relevant to brainstem LBs, meaning that our investigation is the first to indicate that these proteins are also associated with cortical LBs. It is not known currently as to how many remaining candidate proteins are unique only to brainstem and/or cortical LBs, but a significant portion have been shown to be important in the cellular processes thought to be critical to the pathogenesis of LB disease. These include proteins involved in ubiquitin proteasomal system (UPS) responsible for degradation of proteins (eg, UCH‐L1 and ubiquitin‐activating enzyme E1) (40), proteins involved in folding and intracellular trafficking (eg, heat shock cognate 71 kDa or HSC70 and 14‐3‐3 protein) (51), and proteins involved in oxidative stress (eg, carbonyl reductase and glutathione S‐transferase M3) (2, 45). The other major category appears to be those related to synaptic transmission and vesicular transport (eg, synaptotagmin‐1, clathrin coat assembly protein, and connexin 43). It is noteworthy that synaptic dysfunction may be one of the earliest events in PD pathogenesis, as accumulating evidence has suggested that synaptic dysfunction in the striatum is typically more severe than that in the substantia nigra (9) and it occurs in preclinical PD patients (3). Finally, LBs also appeared to contain quite a few proteins related to signal transduction and apoptosis (eg, calcium/calmodulin‐dependent protein kinase, excitatory amino acid transporter and ras‐GTPase‐activating protein binding protein). It is notable that ras‐GTPase‐activating protein binding protein has been recently demonstrated to interact with LRRK2 (21), whose mutations give rise to a clinical phenotype closely resembling sporadic PD, including favorable response to L‐DOPA therapy and formation of brainstem LBs (at least in the cases secondary to the most common G2019S mutation) (43, 44). Further characterization of these proteins could be important for several reasons: (i) as cortical LBs are morphologically different from brainstem LBs, proteins unique to cortical LBs may be responsible for the morphological difference observed under the microscope; and (ii) some of these proteins may be related to LB progression from brainstem to limbic system and eventually to isocortex, which is clearly associated with the development of non‐motor symptoms seen in advanced PD patients.
Proteomics analysis, although robust and powerful in selecting candidate proteins for further studies, is associated with several caveats. First, proteins can be identified incorrectly because of incomplete database (ie, the same MS data searched against different database can give rise to quite different protein identification). Thus, as the database continues to be updated, it is expected that new proteins will show up and some will be eliminated when these MS data are analyzed again. Second, contamination can occur in LCM‐based studies. When a cellular structure (LB in this case) is outlined and captured by LCM, there is no guarantee that all the tissue included in the area in a 10 µm thick section belongs to that particular structure, as other neuronal or even glial proteins can be captured by the laser along with the targeted structures. Consequently, each candidate protein needs to be confirmed/validated before their biological roles are pursued extensively.
The validated protein, heat shock cognate 71‐kDa protein or HSC70, is a constitutively expressed member of the heat shock protein (HSP) 70 family. One recent report has noted that antibodies to HSC70 label brainstem LBs (51). Although very little is known about this protein in PD pathogenesis, HSC70 has been recognized as a key molecular chaperone that facilitates the degradation of several proteins, including mutant SOD1, in a pathway dependent on the ubiquitin proteasomal system (UPS) (15). The role of HSC70 in neurodegenerative diseases can be found in the amyotrophic lateral sclerosis model, where induction of which has been shown to protect cells from mutant SOD1 toxicity (15, 50). More recently, HSC70 has been implicated in membrane fusion and endocytosis (14), neuronal differentiation (17), oxidative stress (8), and apoptosis (5).
In conclusion, this extensive proteomic investigation of LCM captured LBs revealed close to 300 proteins and many of which were novel proteins, that is, they have never been suggested to be associated with LBs in the past. One protein, HSC70, was further validated for its true association with LBs not only in the brainstem, as previous research had suggested, but also in the neocortex. Further characterization of HSC70 and other candidate proteins in the future may provide useful clues as to the formation of LBs as well as the pathogenesis of LB diseases.
Supporting information
Appendix S2. Proteins identified by single peptide.
Supporting info item
Supporting info item
ACKNOWLEDGMENTS
The authors wish to acknowledge the generous support of the Cheng‐Mei Shaw endowment and grants from the NIH to TJM (AG05136 and NS048595) and to JZ (AG025327 and ES012703).
REFERENCES
- 1. Abdi F, Quinn JF, Jankovic J, McIntosh M, Leverenz JB, Peskind E, Nixon R, Nutt J, Chung K, Zabetian C, Samii A, Lin M, Hattan S, Pan C, Wang Y, Jin J, Zhu D, Li JY, Liu Y, Waichunas D, Montine TJ, Zhang J (2006) Detection of biomarkers with a multiplex quantitative proteomic platform in cerebrospinal fluid of patients with neurodegenerative disorders. J Alzheimers Dis 9:293–348. [DOI] [PubMed] [Google Scholar]
- 2. Adam GC, Sorensen EJ, Cravatt BF (2002) Proteomic profiling of mechanistically distinct enzyme classes using a common chemotype. Nat Biotechnol 20:805–809. [DOI] [PubMed] [Google Scholar]
- 3. Adams JR, Van Netten H, Schulzer M, Mak E, McKenzie J, Strongosky A, Sossi V, Ruth TJ, Lee CS, Farrer M, Gasser T, Uitti RJ, Calne DB, Wszolek ZK, Stoessl AJ (2005) PET in LRRK2 mutations: comparison to sporadic Parkinson’s disease and evidence for presymptomatic compensation. Brain 128:2777–2785. [DOI] [PubMed] [Google Scholar]
- 4. Aebersold R, Goodlett DR (2001) Mass spectrometry in proteomics. Chem Rev 101:269–295. [DOI] [PubMed] [Google Scholar]
- 5. Ahn SG, Kim SA, Yoon JH, Vacratsis P (2005) Heat‐shock cognate 70 is required for the activation of heat‐shock factor 1 in mammalian cells. Biochem J 392:145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alim MA, Hossain MS, Arima K, Takeda K, Izumiyama Y, Nakamura M, Kaji H, Shinoda T, Hisanaga S, Ueda K (2002) Tubulin seeds alpha‐synuclein fibril formation. J Biol Chem 277:2112–2117. [DOI] [PubMed] [Google Scholar]
- 7. Arima K, Hirai S, Sunohara N, Aoto K, Izumiyama Y, Ueda K, Ikeda K, Kawai M (1999) Cellular co‐localization of phosphorylated tau‐ and NACP/alpha‐synuclein‐epitopes in lewy bodies in sporadic Parkinson’s disease and in dementia with Lewy bodies. Brain Res 843:53–61. [DOI] [PubMed] [Google Scholar]
- 8. Bernardini C, Fantinati P, Zannoni A, Forni M, Tamanini C, Bacci ML (2004) Expression of HSP70/HSC70 in swine blastocysts: effects of oxidative and thermal stress. Mol Reprod Dev 69:303–307. [DOI] [PubMed] [Google Scholar]
- 9. Bohnen NI, Albin RL, Koeppe RA, Wernette KA, Kilbourn MR, Minoshima S, Frey KA (2006) Positron emission tomography of monoaminergic vesicular binding in aging and Parkinson disease. J Cereb Blood Flow Metab 26:1198–1212. [DOI] [PubMed] [Google Scholar]
- 10. Braak H, Del Tredici K, Bratzke H, Hamm‐Clement J, Sandmann‐Keil D, Rub U (2002) Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson’s disease (preclinical and clinical stages). J Neurol 249: (Suppl. 3):III/1–5. [DOI] [PubMed] [Google Scholar]
- 11. Braak H, Del Tredici K, Rub U, De Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211. [DOI] [PubMed] [Google Scholar]
- 12. Cairns NJ, Uryu K, Bigio EH, Mackenzie IR, Gearing M, Duyckaerts C, Yokoo H, Nakazato Y, Jaros E, Perry RH, Arnold SE, Lee VM, Trojanowski JQ (2004) alpha‐Internexin aggregates are abundant in neuronal intermediate filament inclusion disease (NIFID) but rare in other neurodegenerative diseases. Acta Neuropathol (Berl) 108:213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carr S, Aebersold R, Baldwin M, Burlingame A, Clauser K, Nesvizhskii A (2004) The need for guidelines in publication of peptide and protein identification data: Working Group on Publication Guidelines for Peptide and Protein Identification Data. Mol Cell Proteomics 3:531–533. [DOI] [PubMed] [Google Scholar]
- 14. Chang HC, Newmyer SL, Hull MJ, Ebersold M, Schmid SL, Mellman I (2002) Hsc70 is required for endocytosis and clathrin function in Drosophila. J Cell Biol 159:477–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Choi JS, Cho S, Park SG, Park BC, Lee DH (2004) Co‐chaperone CHIP associates with mutant Cu/Zn‐superoxide dismutase proteins linked to familial amyotrophic lateral sclerosis and promotes their degradation by proteasomes. Biochem Biophys Res Commun 321:574–583. [DOI] [PubMed] [Google Scholar]
- 16. D’Andrea MR, Ilyin S, Plata‐Salaman CR (2001) Abnormal patterns of microtubule‐associated protein‐2 (MAP‐2) immunolabeling in neuronal nuclei and Lewy bodies in Parkinson’s disease substantia nigra brain tissues. Neurosci Lett 306: 137–140. [DOI] [PubMed] [Google Scholar]
- 17. D’Souza SM, Brown IR (1998) Constitutive expression of heat shock proteins Hsp90, Hsc70, Hsp70 and Hsp60 in neural and non‐neural tissues of the rat during postnatal development. Cell Stress Chaperones 3:188–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fend F, Emmert‐Buck MR, Chuaqui R, Cole K, Lee J, Liotta LA, Raffeld M (1999) Immuno‐LCM: laser capture microdissection of immunostained frozen sections for mRNA analysis. Am J Pathol 154:61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fink L, Seeger W, Ermert L, Hanze J, Stahl U, Grimminger F, Kummer W, Bohle RM (1998) Real‐time quantitative RT‐PCR after laser‐assisted cell picking. Nat Med 4:1329–1333. [DOI] [PubMed] [Google Scholar]
- 20. Galvin JE, Lee VM, Baba M, Mann DM, Dickson DW, Yamaguchi H, Schmidt ML, Iwatsubo T, Trojanowski JQ (1997) Monoclonal antibodies to purified cortical Lewy bodies recognize the mid‐size neurofilament subunit. Ann Neurol 42:595–603. [DOI] [PubMed] [Google Scholar]
- 21. Gloeckner CJ, Kinkl N, Schumacher A, Braun RJ, O’Neill E, Meitinger T, Kolch W, Prokisch H, Ueffing M (2006) The Parkinson disease causing LRRK2 mutation I2020T is associated with increased kinase activity. Hum Mol Genet 15:223–232. [DOI] [PubMed] [Google Scholar]
- 22. Hafezparast M, Klocke R, Ruhrberg C, Marquardt A, Ahmad‐Annuar A, Bowen S, Lalli G, Witherden AS, Hummerich H, Nicholson S, Morgan PJ, Oozageer R, Priestley JV, Averill S, King VR, Ball S, Peters J, Toda T, Yamamoto A, Hiraoka Y, Augustin M, Korthaus D, Wattler S, Wabnitz P, Dickneite C, Lampel S, Boehme F, Peraus G, Popp A, Rudelius M, Schlegel J, Fuchs H, Hrabe de Angelis M, Schiavo G, Shima DT, Russ AP, Stumm G, Martin JE, Fisher EM (2003) Mutations in dynein link motor neuron degeneration to defects in retrograde transport. Science 300:808–812. [DOI] [PubMed] [Google Scholar]
- 23. Haltia M, Ghiso J, Wisniewski T, Kiuru S, Miller D, Frangione B (1991) Gelsolin variant and beta‐amyloid co‐occur in a case of Alzheimer’s with Lewy bodies. Neurobiol Aging 12:313–316. [DOI] [PubMed] [Google Scholar]
- 24. Hill WD, Lee VM, Hurtig HI, Murray JM, Trojanowski JQ (1991) Epitopes located in spatially separate domains of each neurofilament subunit are present in Parkinson’s disease Lewy bodies. J Comp Neurol 309:150–160. [DOI] [PubMed] [Google Scholar]
- 25. Huynh DP, Scoles DR, Nguyen D, Pulst SM (2003) The autosomal recessive juvenile Parkinson disease gene product, parkin, interacts with and ubiquitinates synaptotagmin XI. Hum Mol Genet 12:2587–2597. [DOI] [PubMed] [Google Scholar]
- 26. Jensen PH, Islam K, Kenney J, Nielsen MS, Power J, Gai WP (2000) Microtubule‐associated protein 1B is a component of cortical Lewy bodies and binds alpha‐synuclein filaments. J Biol Chem 275:21500–21507. [DOI] [PubMed] [Google Scholar]
- 27. Jin J, Hulette C, Wang Y, Zhang T, Pan C, Wadhwa R, Zhang J (2006) Proteomic Identification of a stress protein, mortalin/mthsp70/GRP75: relevance to Parkinson’s disease. Mol Cell Proteomics 5:1193–1204. [DOI] [PubMed] [Google Scholar]
- 28. Jin J, Li J, Davis J, Zhu D, Pan C, Zhang J (2006) Identification of novel proteins interacting with both a‐synuclein and DJ‐1. Mol Cell Proteomics in press. 2006 Jul 18; [Epub ahead of print. [DOI] [PubMed]
- 29. Jin J, Meredith G, Chen L, Zhou Y, Xu J, Xie F, Lockhart P, Zhang J (2005) Quantitative proteomic analysis of mitochondrial proteins: relevance to Lewy body formation and Parkinson’s disease. Mol Brain Res 24:119–138. [DOI] [PubMed] [Google Scholar]
- 30. Kawamoto Y, Akiguchi I, Nakamura S, Honjyo Y, Shibasaki H, Budka H (2002) 14‐3‐3 proteins in Lewy bodies in Parkinson disease and diffuse Lewy body disease brains. J Neuropathol Exp Neurol 61:245–253. [DOI] [PubMed] [Google Scholar]
- 31. Lantos PL, Ovenstone IM, Johnson J, Clelland CA, Roques P, Rossor MN (1994) Lewy bodies in the brain of two members of a family with the 717 (Val to Ile) mutation of the amyloid precursor protein gene. Neurosci Lett 172:77–79. [DOI] [PubMed] [Google Scholar]
- 32. Layfield R, Fergusson J, Aitken A, Lowe J, Landon M, Mayer RJ (1996) Neurofibrillary tangles of Alzheimer’s disease brains contain 14‐3‐3 proteins. Neurosci Lett 209:57–60. [DOI] [PubMed] [Google Scholar]
- 33. Leverenz JB, McKeith IG (2002) Dementia with Lewy bodies. Med Clin North Am 86:519–535. [DOI] [PubMed] [Google Scholar]
- 34. Liao L, Cheng D, Wang J, Duong DM, Losik TG, Gearing M, Rees HD, Lah JJ, Levey AI, Peng J (2004) Proteomic characterization of postmortem amyloid plaques isolated by laser capture microdissection. J Biol Chem 279:37061–37068. [DOI] [PubMed] [Google Scholar]
- 35. Link AJ, Eng J, Schieltz DM, Carmack E, Mize GJ, Morris DR, Garvik BM, Yates JR 3rd (1999) Direct analysis of protein complexes using mass spectrometry. Nat Biotechnol 17:676–682. [DOI] [PubMed] [Google Scholar]
- 36. Lowe J, McDermott H, Landon M, Mayer RJ, Wilkinson KD (1990) Ubiquitin carboxyl‐terminal hydrolase (PGP 9.5) is selectively present in ubiquitinated inclusion bodies characteristic of human neurodegenerative diseases. J Pathol 161:153–160. [DOI] [PubMed] [Google Scholar]
- 37. Lowe JS, Leigh N (2002) Disorders of movement and system degenerations. In: Greenfield’s Neuropathology. Graham DI, Lantos PL (eds), pp. 325–430. Arnold: London. [Google Scholar]
- 38. McKeith IG (2000) Spectrum of Parkinson’s disease, Parkinson’s dementia, and Lewy body dementia. Neurol Clin 18:865–902. [DOI] [PubMed] [Google Scholar]
- 39. McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez‐Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova‐Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M (2005) Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 65:1863–1872. [DOI] [PubMed] [Google Scholar]
- 40. McNaught KS, Belizaire R, Jenner P, Olanow CW, Isacson O (2002) Selective loss of 20S proteasome alpha‐subunits in the substantia nigra pars compacta in Parkinson’s disease. Neurosci Lett 326:155–158. [DOI] [PubMed] [Google Scholar]
- 41. McNaught KS, Shashidharan P, Perl DP, Jenner P, Olanow CW (2002) Aggresome‐related biogenesis of Lewy bodies. Eur J Neurosci 16: 2136–2148. [DOI] [PubMed] [Google Scholar]
- 42. Rekas A, Adda CG, Andrew Aquilina J, Barnham KJ, Sunde M, Galatis D, Williamson NA, Masters CL, Anders RF, Robinson CV, Cappai R, Carver JA (2004) Interaction of the molecular chaperone alphaB‐crystallin with alpha‐synuclein: effects on amyloid fibril formation and chaperone activity. J Mol Biol 340:1167–1183. [DOI] [PubMed] [Google Scholar]
- 43. Ross OA, Toft M, Whittle AJ, Johnson JL, Papapetropoulos S, Mash DC, Litvan I, Gordon MF, Wszolek ZK, Farrer MJ, Dickson DW (2006) Lrrk2 and Lewy body disease. Ann Neurol 59:388–393. [DOI] [PubMed] [Google Scholar]
- 44. Ross OA, Whittle AJ, Cobb SA, Hulihan MM, Lincoln SJ, Toft M, Farrer MJ, Dickson DW (2006) Lrrk2 R1441 substitution and progressive supranuclear palsy. Neuropathol Appl Neurobiol 32:23–25. [DOI] [PubMed] [Google Scholar]
- 45. Selley ML (1998) E)‐4‐hydroxy‐2‐nonenal may be involved in the pathogenesis of Parkinson’s disease. Free Radic Biol Med 25:169–174. [DOI] [PubMed] [Google Scholar]
- 46. Trojanowski JQ, Lee VM (2002) Parkinson’s disease and related synucleinopathies are a new class of nervous system amyloidoses. Neurotoxicology 23:457–460. [DOI] [PubMed] [Google Scholar]
- 47. Tsuboi Y, Dickson DW (2005) Dementia with Lewy bodies and Parkinson’s disease with dementia: are they different? Parkinsonism Relat Disord 11(Suppl. 1):S47–S51. [DOI] [PubMed] [Google Scholar]
- 48. Tsuchiya K, Tajima H, Kuwae T, Takeshima T, Nakano T, Tanaka M, Sunaga K, Fukuhara Y, Nakashima K, Ohama E, Mochizuki H, Mizuno Y, Katsube N, Ishitani R (2005) Pro‐apoptotic protein glyceraldehyde‐3‐phosphate dehydrogenase promotes the formation of Lewy body‐like inclusions. Eur J Neurosci 21:317–326. [DOI] [PubMed] [Google Scholar]
- 49. Uchikado H, Lin WL, DeLucia MW, Dickson DW (2006) Alzheimer disease with amygdala Lewy bodies: a distinct form of alpha‐synucleinopathy. J Neuropathol Exp Neurol 65:685–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Urushitani M, Kurisu J, Tateno M, Hatakeyama S, Nakayama K, Kato S, Takahashi R (2004) CHIP promotes proteasomal degradation of familial ALS‐linked mutant SOD1 by ubiquitinating Hsp/Hsc70. J Neurochem 90:231–244. [DOI] [PubMed] [Google Scholar]
- 51. Uryu K, Richter‐Landsberg C, Welch W, Sun E, Goldbaum O, Norris EH, Pham CT, Yazawa I, Hilburger K, Micsenyi M, Giasson BI, Bonini NM, Lee VM, Trojanowski JQ (2006) Convergence of heat shock protein 90 with ubiquitin in filamentous alpha‐synuclein inclusions of alpha‐synucleinopathies. Am J Pathol 168:947–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang Q, Woltjer R, Cimino P, Pan C, Montine K, Zhang J, Montine T (2005) Proteomic analysis of neurofibrillary tangles in Alzheimer Disease identifies GAPDH as a detergent‐insoluble paired helical filament tau‐binding protein. FASEB J I19: 869–871. [DOI] [PubMed] [Google Scholar]
- 53. Wischik CM, Harrington CR, Mukaetova‐Ladinska EB, Novak M, Edwards PC, McArthur FK (1992) Molecular characterization and measurement of Alzheimer’s disease pathology: implications for genetic and environmental aetiology. Ciba Found Symp 169:268–293; discussion 93–302. [DOI] [PubMed] [Google Scholar]
- 54. Woltjer RL, Cimino PJ, Boutte AM, Schantz AM, Montine KS, Larson EB, Bird T, Quinn JF, Zhang J, Montine TJ (2005) Proteomic determination of widespread detergent‐insolubility including Abeta but not tau early in the pathogenesis of Alzheimer’s disease. FASEB J 19:1923–1925. [DOI] [PubMed] [Google Scholar]
- 55. Zhou Y, Gu G, Goodlett DR, Zhang T, Pan C, Montine TJ, Montine KS, Aebersold RH, Zhang J (2004) Analysis of alpha‐synuclein‐associated proteins by quantitative proteomics. J Biol Chem 279: 39155–39164. [DOI] [PubMed] [Google Scholar]
- 56. Zhou Y, Wang Y, Kovacs M, Jin J, Zhang J (2005) Microglial activation induced by neurodegeneration: a proteomic analysis. Mol Cell Proteomics 4:1471–1479. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S2. Proteins identified by single peptide.
Supporting info item
Supporting info item


